Density Functional Study of Electrocatalytic Carbon Dioxide Reduction in Fourth-Period Transition Metal–Tetrahydroxyquinone Organic Framework
Abstract
:1. Introduction
2. Calculation Details
3. Result and Discussion
3.1. Analysing the TM-THQ Structure
3.2. Stability of TM-THQ
3.3. Selectivity of TM-THQ Catalytic Converters towards CO2 Reduction (CO2RR) and Hydrogen Evolution Reaction (HER)
3.4. Potential Pathways and Adsorption Energies
3.5. Electrochemical CO2 Reduction Pathways
3.5.1. Significant Generation of CO Products
3.5.2. Significant Generation of HCHO Products
3.5.3. HCOOH as the Predominant Product of Formation
3.5.4. CH4 as Main Catalyst Product
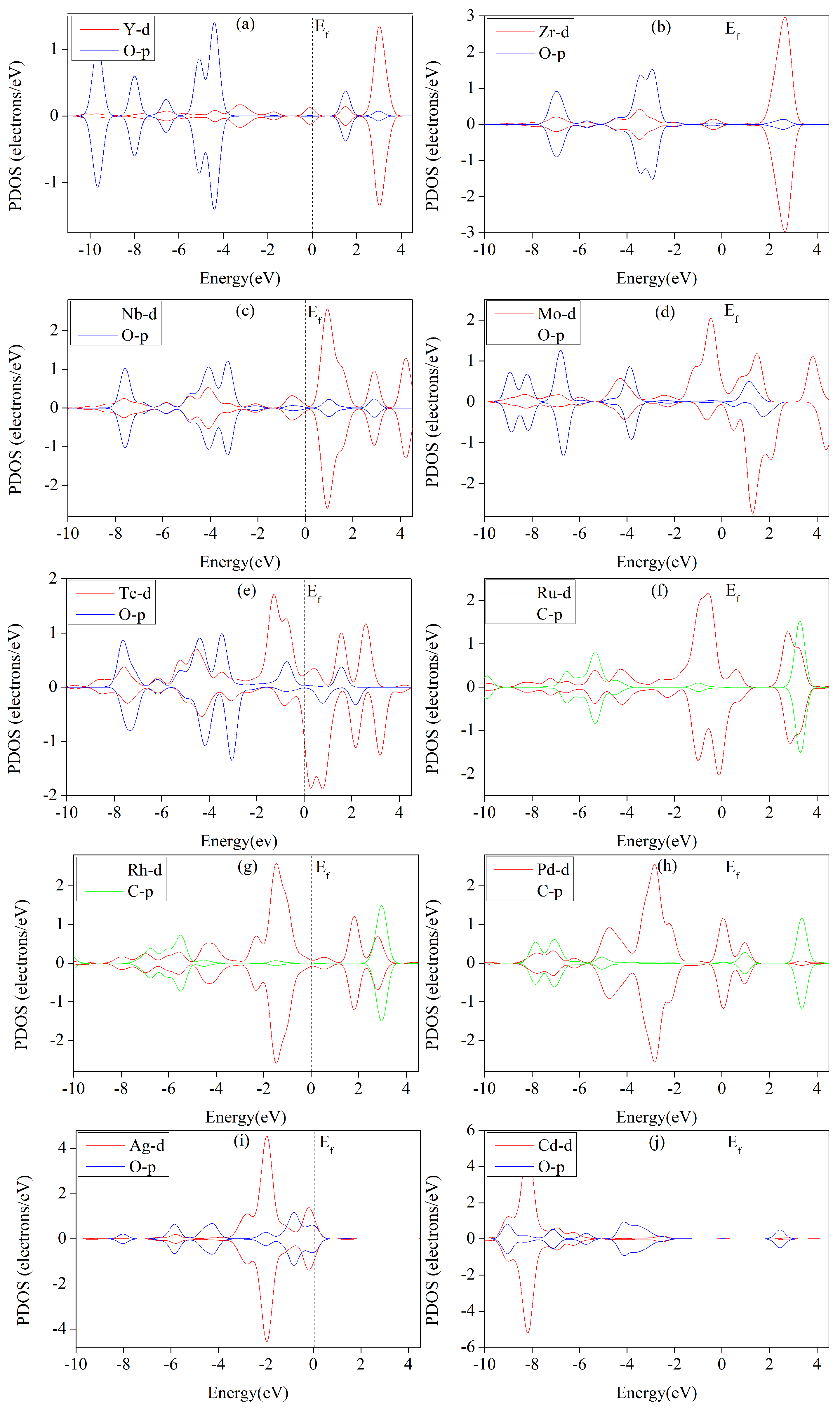
3.6. Analysis of Electronic Structures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, E.; Wang, T.; Yu, K.; Liu, J.; Chen, W.; Li, A.; Rong, H.; Lin, R.; Ji, S.; Zheng, X.; et al. Bismuth Single Atoms Resulting from Transformation of Metal-Organic Frameworks and Their Use as Electrocatalysts for CO2 Reduction. J. Am. Chem. Soc. 2019, 141, 16569–16573. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dai, X.; Yao, T.; Chen, W.; Wang, X.; Wang, J.; Yang, J.; Wei, S.; Wu, Y.; Li, Y. Ionic Exchange of Metal-Organic Frameworks to Access Single Nickel Sites for Efficient Electroreduction of CO2. J. Am. Chem. Soc. 2017, 139, 8078–8081. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Kim, Y.; Hwang, Y.J.; Won, D.H. Progress in development of electrocatalyst for CO2 conversion to selective CO production. Carbon Energy 2020, 21, 72–98. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Gao, Y.; Gong, C.; Addicoat, M.A.; Heine, T.; Wöll, C.; Sun, L. Highly oriented MOF thin film-based electrocatalytic device for the reduction of CO2 to CO exhibiting high faradaic efficiency. J. Mater. Chem. 2016, 4, 15320–15326. [Google Scholar] [CrossRef]
- Deng, P.; Yang, F.; Wang, Z.; Chen, S.; Zhou, Y.; Zaman, S.; Xia, B. Metal-Organic Frameworks-derived Carbon Nanorods Encapsulated Bismuth Oxides for Rapid and Selective CO2 Electroreduction to Formate. Angew. Chem. 2020, 59, 10807–10813. [Google Scholar] [CrossRef] [PubMed]
- Graciani, J.; Mudiyanselage, K.; Xu, F.; Baber, A.E.; Evans, J.; Senanayake, S.D.; Stacchiola, D.J.; Liu, P.; Hrbek, J.; Sanz, J.F.; et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 2014, 345, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, T.; Huang, H.H.; Wang, J.; Zhong, D.C.; Lu, T. A Dinuclear Cobalt Cryptate as a Homogeneous Photocatalyst for Highly Selective and Efficient Visible-Light Driven CO2 Reduction to CO in CH3CN/H2O Solution. Angew. Chem. 2017, 56, 738–743. [Google Scholar] [CrossRef]
- Schuchmann, K.; Müller, V. Direct and Reversible Hydrogenation of CO2 to Formate by a Bacterial Carbon Dioxide Reductase. Science 2013, 342, 1382–1385. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, Y.; Peng, Y.; Huang, Z.; Ma, Q.; Zhang, H. Two-dimensional metal-organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef]
- Wu, H.B.; Lou, X.W.D. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: Promises and challenges. Sci. Adv. 2017, 3, eaap9252. [Google Scholar] [CrossRef]
- Wu, D.; Guo, Z.; Yin, X.; Pang, Q.; Tu, B.; Zhang, L.; Wang, Y.; Li, Q. Metal–Organic Frameworks as Cathode Materials for Li–O2 Batteries. Adv. Mater. 2014, 26, 3258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, Z.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Batteries. Joule 2018, 2, 2235–2259. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.T.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen eVolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Deng, X.; Li, R.; Wu, S.; Wang, L.; Hu, J.; Ma, J.; Jiang, W.; Zhang, N.; Zheng, X.; Gao, C.; et al. Metal-Organic Framework Coating Enhances the Performance of Cu2O in Photoelectrochemical CO2 Reduction. J. Am. Chem. Soc. 2019, 141, 10924. [Google Scholar] [CrossRef] [PubMed]
- Trickett, C.A.; Helal, A.; Al-Maythalony, B.A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. The chemistry of metal–organic frameworks for CO2 capture, regeneration and conversion. Nat. Rev. Mater. 2017, 2, 17045. [Google Scholar] [CrossRef]
- Zhou, H.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.H.; Bushuyev, O.S.; Li, J.; de Luna, P.; Seifitokaldani, A.; Dinh, C.T.; de Arquer, F.P.G.; Wang, Y.; Liang, Z.; Proppe, A.H.; et al. Metal-Organic Frameworks Mediate Cu Coordination for Selective CO2 Electroreduction. J. Am. Chem. Soc. 2018, 140, 11378–11386. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Qiu, M.; Zhang, Y.; Zhu, Y.S.; Liu, L.; Sun, Y.; Bu, X.; Zhang, J.; Lin, Q. Acid and Base Resistant Zirconium Polyphenolate-Metalloporphyrin Scaffolds for Efficient CO2 Photoreduction. Adv. Mater. 2018, 30, 1704388. [Google Scholar] [CrossRef]
- Xu, H.Q.; Hu, J.; Wang, D.; Li, Z.; Zhang, Q.; Luo, Y.; Yu, S.; Jiang, H. Visible-Light Photoreduction of CO2 in a Metal-Organic Framework: Boosting Electron-Hole Separation via Electron Trap States. J. Am. Chem. Soc. 2015, 137, 13440. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, J.; Lu, W.; Sun, L.B.; Sculley, J.P.; Balbuena, P.B.; Zhou, H. Porous materials with pre-designed single-molecule traps for CO2 selective adsorption. Nat. Commun. 2013, 4, 1538. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xu, H.; Yue, Y.; Guo, Z.; Yu, J.; Chen, Z.; Gao, J.; Yang, Y.; Qian, G.; Chen, B. A luminescent mixed-lanthanide metal-organic framework thermometer. J. Am. Chem. Soc. 2012, 134, 3979–3982. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yang, D.; Liu, H.; Sun, X.; Chen, C.; Bi, J.; Liu, J.; Wu, H.; Han, B. Hollow metal organic framework-mediated in-situ architecture of copper dendrites for enhanced CO2 electroreduction. Angew. Chem. 2020, 59, 8896–8901. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Li, L.; Sheveleva, A.M.; Han, X.; Li, J.; Liu, L.; Tuna, F.; McInnes, E.J.L.; Han, B.; Yang, S.; et al. Electro-reduction of carbon dioxide at low over-potential at a metal–organic framework decorated cathode. Nat. Commun. 2020, 11, 5464. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Song, J.; Xi, S.; Du, Y.; Wang, J.; Huang, Z.; Xu, Z.J.; Wang, X. Boosting Electrochemical CO2 Reduction on Metal-Organic Frameworks via Ligand Doping. Angew. Chem. 2019, 58, 4041–4045. [Google Scholar] [CrossRef] [PubMed]
- Majidi, L.; Ahmadiparidari, A.; Shan, N.; Misal, S.N.; Kumar, K.; Huang, Z.; Rastegar, S.; Hemmat, Z.; Zou, X.; Zapol, P.; et al. 2D Copper Tetrahydroxyquinone Conductive Metal–Organic Framework for Selective CO2 Electrocatalysis at Low Overpotentials. Adv. Mater. 2021, 33, 2004393. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yang, T.; Li, P.; Wei, Z. DFT study on ORR catalyzed by bimetallic Pt-skin metals over substrates of Ir, Pd and Au. Nano Mater. Sci. 2021, 5, 287–292. [Google Scholar] [CrossRef]
- Zeng, X.; Xiao, C.; Liao, L.; Tu, Z.J.; Lai, Z.; Xiong, K.; Wen, Y. Two-Dimensional (2D) TM-Tetrahydroxyquinone Metal–Organic Framework for Selective CO2 Electrocatalysis: A DFT Investigation. Nanomaterials 2022, 12, 4049. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the Dmol(3) approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Delley, B. Hardness conserving semilocal pseudopotentials. Phys. Rev. B 2002, 66, 155125. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, C.; Du, A.; Dou, S.X.; Li, Z. In-plane graphene/boron-nitride heterostructures as an efficient metal-free electrocatalyst for the oxygen reduction reaction. Nanoscale 2016, 8, 14084–14091. [Google Scholar] [CrossRef]
- Qin, G.; Du, A.; Sun, Q. A theoretical insight into a feasible strategy for the fabrication of borophane. Phys. Chem. Chem. Phys. PCCP 2018, 20, 16216–16221. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Cui, Q.; Yun, B.; Sun, L.; Du, A.; Sun, Q. High capacity and reversible hydrogen storage on two dimensional C2N monolayer membrane. Int. J. Hydrog. Energy 2018, 43, 9895–9901. [Google Scholar] [CrossRef]
- Back, S.; Yeom, M.S.; Jung, Y. Active Sites of Au and Ag Nanoparticle Catalysts for CO2 Electroreduction to CO. ACS Catal. 2015, 5, 5089–5096. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Logadóttir, Á.; Nørskov, J.K. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 2005, 319, 178–184. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Zeng, X.; Liao, L.; Wang, M.; Wang, H. Rare-earth metal-N6 centers in porous carbon for electrocatalytic CO2 reduction. Phys. Chem. Chem. Phys. PCCP 2023, 25, 20381–20394. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Yang, L.; Ganz, E. Electrocatalytic reduction of CO2 by two-dimensional transition metal porphyrin sheets. J. Mater. Chem. A 2019, 7, 11944–11952. [Google Scholar] [CrossRef]
- Zeng, X.; Liao, L.; Wang, M.; Wang, H. Density functional calculation of two-dimensional transition metal-hexaiminotriphenylene (TM-HITP) electrocatalytic CO2 reduction. Catal. Sci. Technol. 2023, 13, 5351–5364. [Google Scholar] [CrossRef]
- Zeng, X.; Liao, L.; Yu, Q.; Wang, M.; Wang, H. Theoretical Prediction of Electrocatalytic Reduction of CO2 Using a 2D Catalyst Composed of 3d Transition Metal and Hexaamine Dipyrazino Quinoxaline. Chem. Eur. J 2023, 16, e202302232. [Google Scholar]
- Zeng, X.; Tu, Z.J.; Yuan, Y.; Liao, L.; Xiao, C.; Wen, Y.; Xiong, K. Two-Dimensional Transition Metal-Hexaaminobenzene Monolayer Single-Atom Catalyst for Electrocatalytic Carbon Dioxide Reduction. Nanomaterials 2022, 12, 4005. [Google Scholar] [CrossRef]
- Shi, C.; Chan, K.; Yoo, J.S.; Nørskov, J.K. Barriers of Electrochemical CO2 Reduction on Transition Metals. Org. Process. Res. Dev. 2016, 20, 1424–1430. [Google Scholar] [CrossRef]
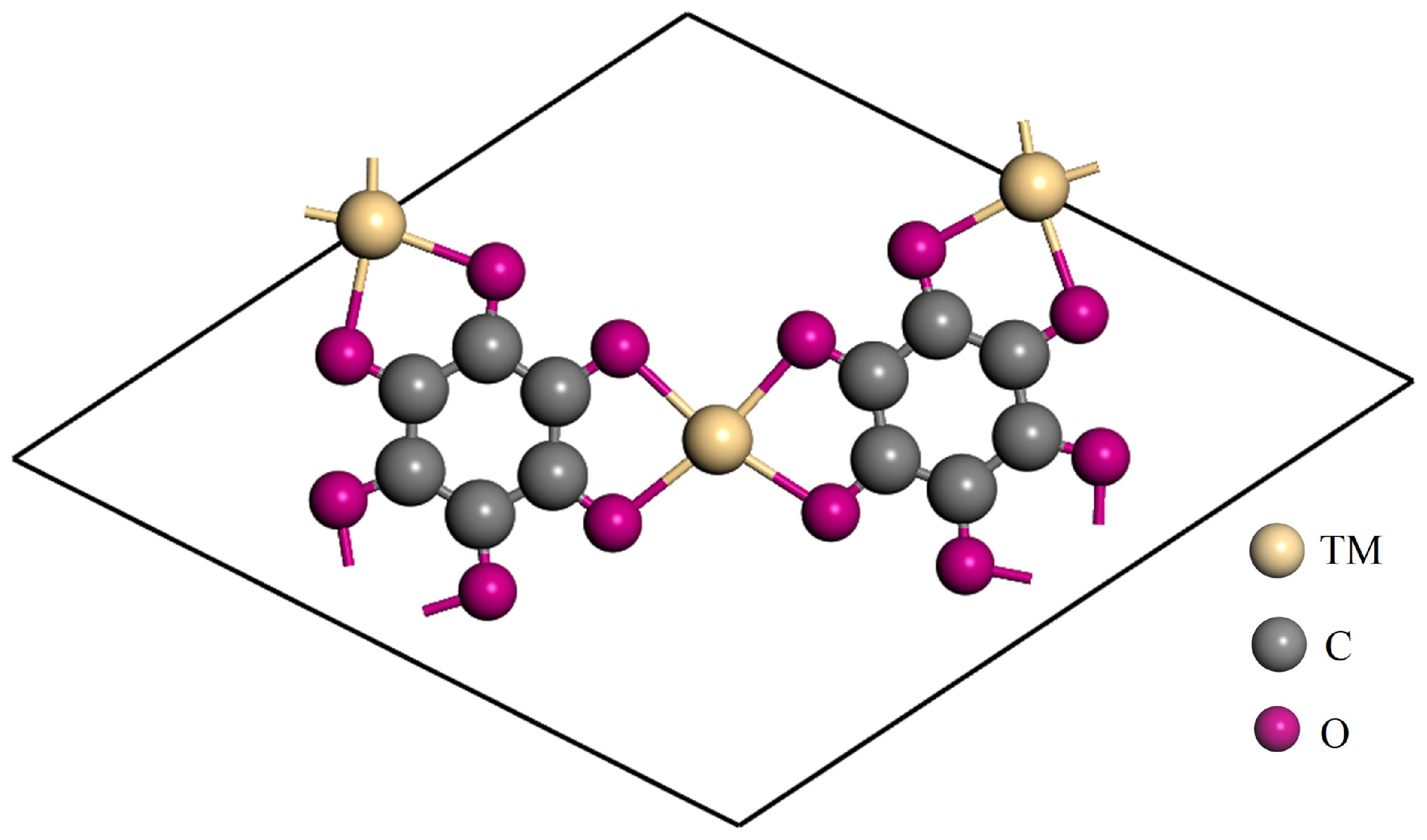


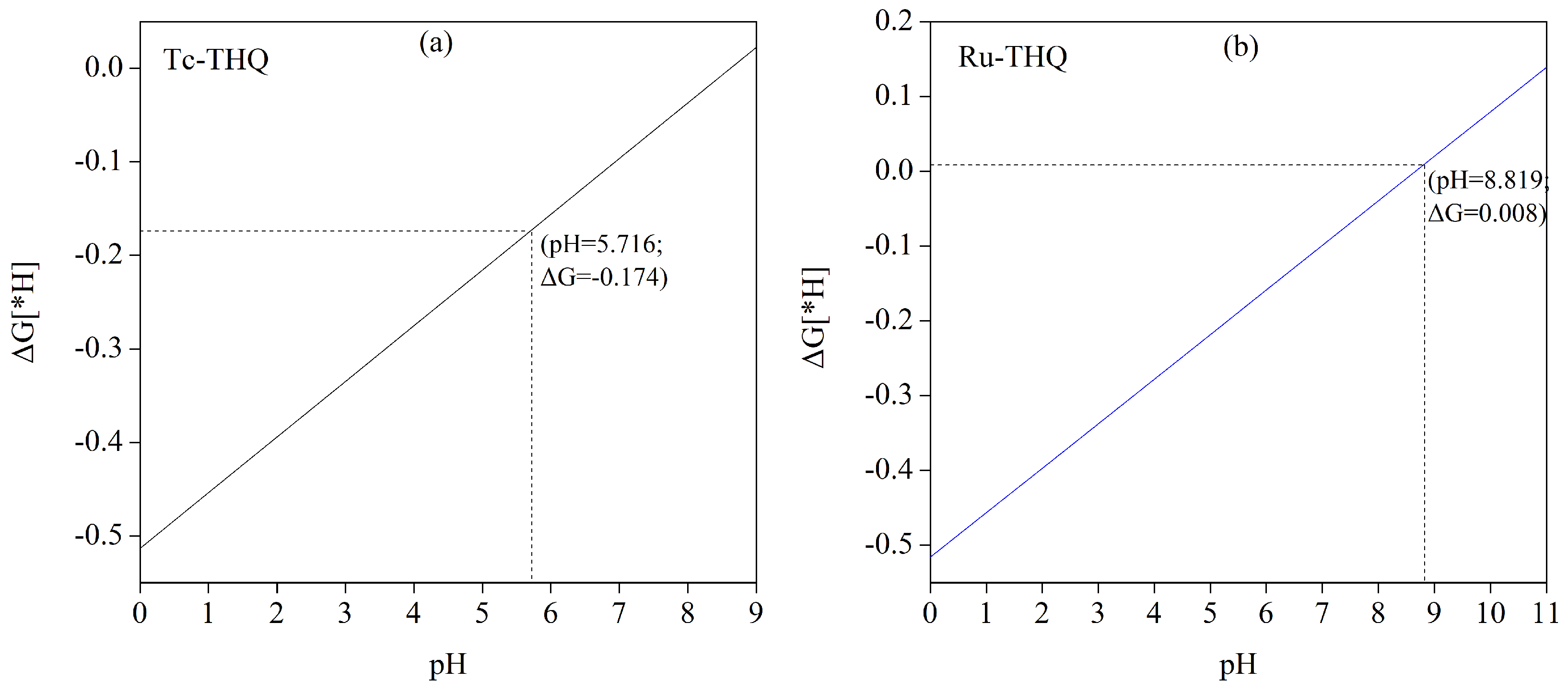



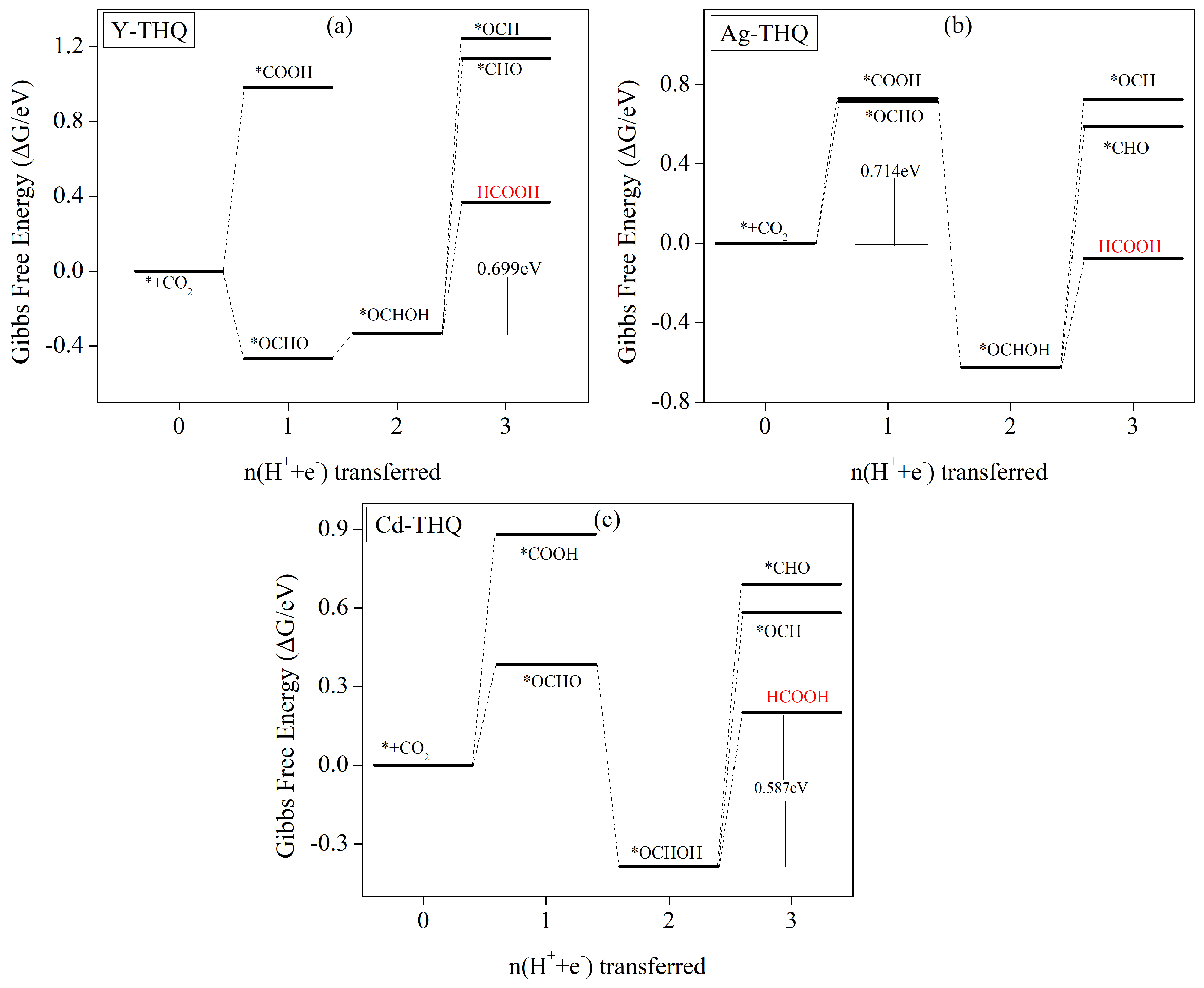
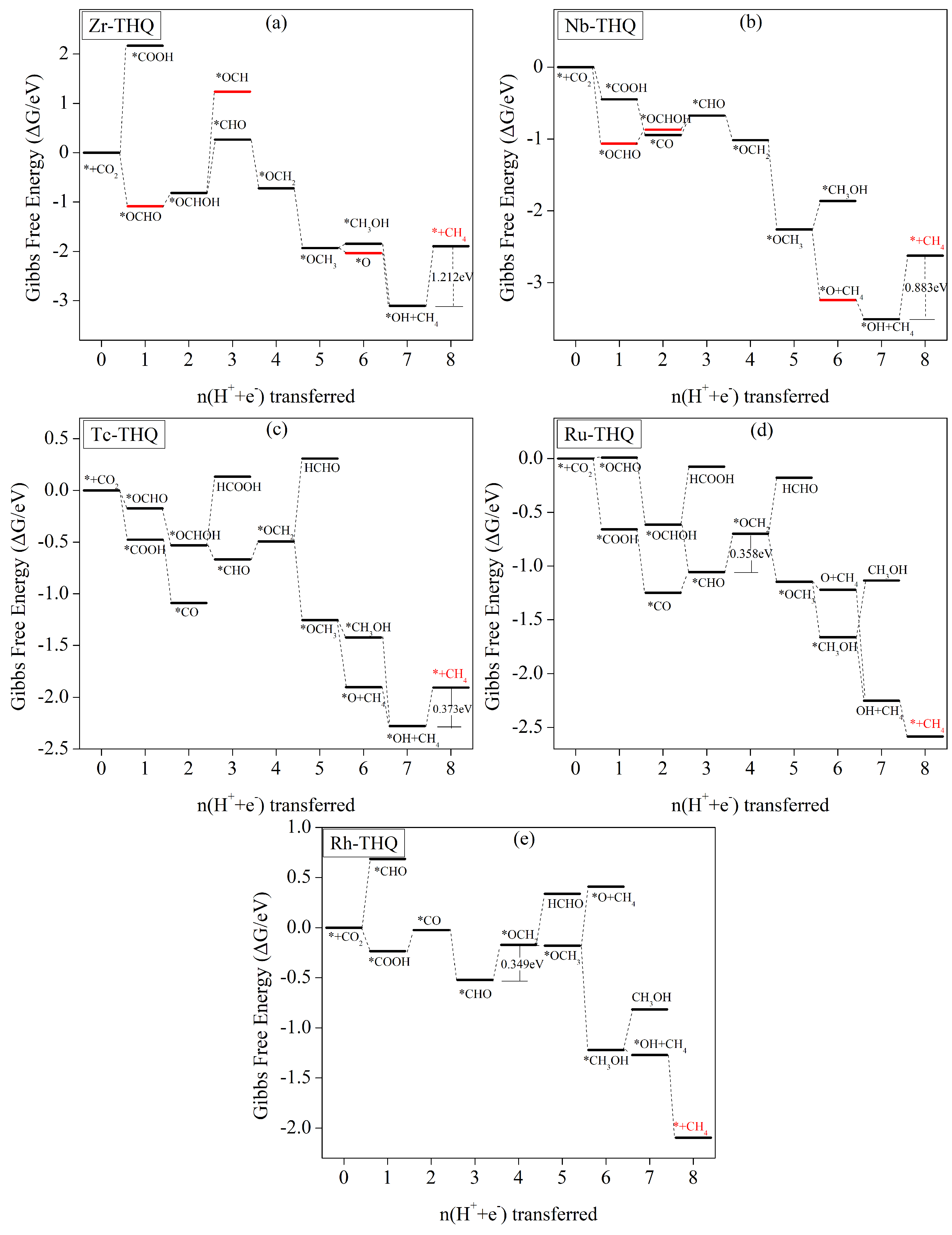
| TM-THQ | /e | Spin-TM | /e | /Å |
|---|---|---|---|---|
| Y | 1.0963 | 0.000 | −0.2936 | 2.235 |
| Zr | 1.0686 | 0.000 | −0.2719 | 2.060 |
| Nb | 0.8625 | 0.000 | −0.2487 | 2.022 |
| Mo | 0.7139 | 2.459 | −0.2192 | 2.034 |
| Tc | 0.4140 | 0.000 | −0.1657 | 2.017 |
| Ru | 0.4486 | 0.000 | −0.1808 | 2.023 |
| Rh | 0.3776 | 0.000 | −0.1726 | 2.028 |
| Pd | 0.5272 | 0.000 | −0.2074 | 2.076 |
| Ag | 0.4393 | 0.000 | −0.2046 | 2.345 |
| Cd | 0.6934 | 0.000 | −0.2380 | 2.254 |
| TM-HAB | Major Product | Rate-Determining Step | ||
|---|---|---|---|---|
| Y | HCOOH | * | 0.699 | 0.949 |
| Zr | CH4 | * | 1.212 | 1.043 |
| Nb | CH4 | * | 0.883 | 0.714 |
| Mo | HCHO | 0.718 | 0.788 | |
| Tc | CH4 | 0.373 | 0.204 | |
| Ru | CH4 | 0.358 | 0.189 | |
| Rh | CH4 | 0.348 | 0.179 | |
| Pd | CO | 0.564 | 0.67 | |
| Ag | HCOOH | 0.714 | 0.964 | |
| Cd | HCOOH | * | 0.587 | 0.837 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Zeng, X.; Xiao, Y.; Ruan, W.; Xiong, K.; Lai, Z. Density Functional Study of Electrocatalytic Carbon Dioxide Reduction in Fourth-Period Transition Metal–Tetrahydroxyquinone Organic Framework. Molecules 2024, 29, 2320. https://doi.org/10.3390/molecules29102320
Wen Y, Zeng X, Xiao Y, Ruan W, Xiong K, Lai Z. Density Functional Study of Electrocatalytic Carbon Dioxide Reduction in Fourth-Period Transition Metal–Tetrahydroxyquinone Organic Framework. Molecules. 2024; 29(10):2320. https://doi.org/10.3390/molecules29102320
Chicago/Turabian StyleWen, Yufeng, Xianshi Zeng, Yanan Xiao, Wen Ruan, Kai Xiong, and Zhangli Lai. 2024. "Density Functional Study of Electrocatalytic Carbon Dioxide Reduction in Fourth-Period Transition Metal–Tetrahydroxyquinone Organic Framework" Molecules 29, no. 10: 2320. https://doi.org/10.3390/molecules29102320
APA StyleWen, Y., Zeng, X., Xiao, Y., Ruan, W., Xiong, K., & Lai, Z. (2024). Density Functional Study of Electrocatalytic Carbon Dioxide Reduction in Fourth-Period Transition Metal–Tetrahydroxyquinone Organic Framework. Molecules, 29(10), 2320. https://doi.org/10.3390/molecules29102320






