Coordinative Compounds Based on Unsaturated Carboxylate with Versatile Biological Applications
Abstract
:1. Introduction
2. Coordinative Compounds with Unsaturated Carboxylate Developed for Antimicrobial Applications
2.1. Coordinative Compounds with Antimicrobial Activity on Planktonic Strains
2.1.1. Complexes with Acrylate and N-Based Heterocycles
- (a)
- Complexes with mixed ligands—acrylate and imidazole/alkyl-imidazole
- (b)
- Complexes with mixed ligands—acrylate and benzimidazole/alkylbenzimidazole
- (c)
- Complexes with mixed ligands—acrylate and 2,2′-bipyridine complexes
- (d)
- Complexes with mixed ligands—acrylate and pyrazole/pyrazole derivatives
2.1.2. Complexes with Methacrylate/Methacrylate Derivatives and Different N-Donor Ligands
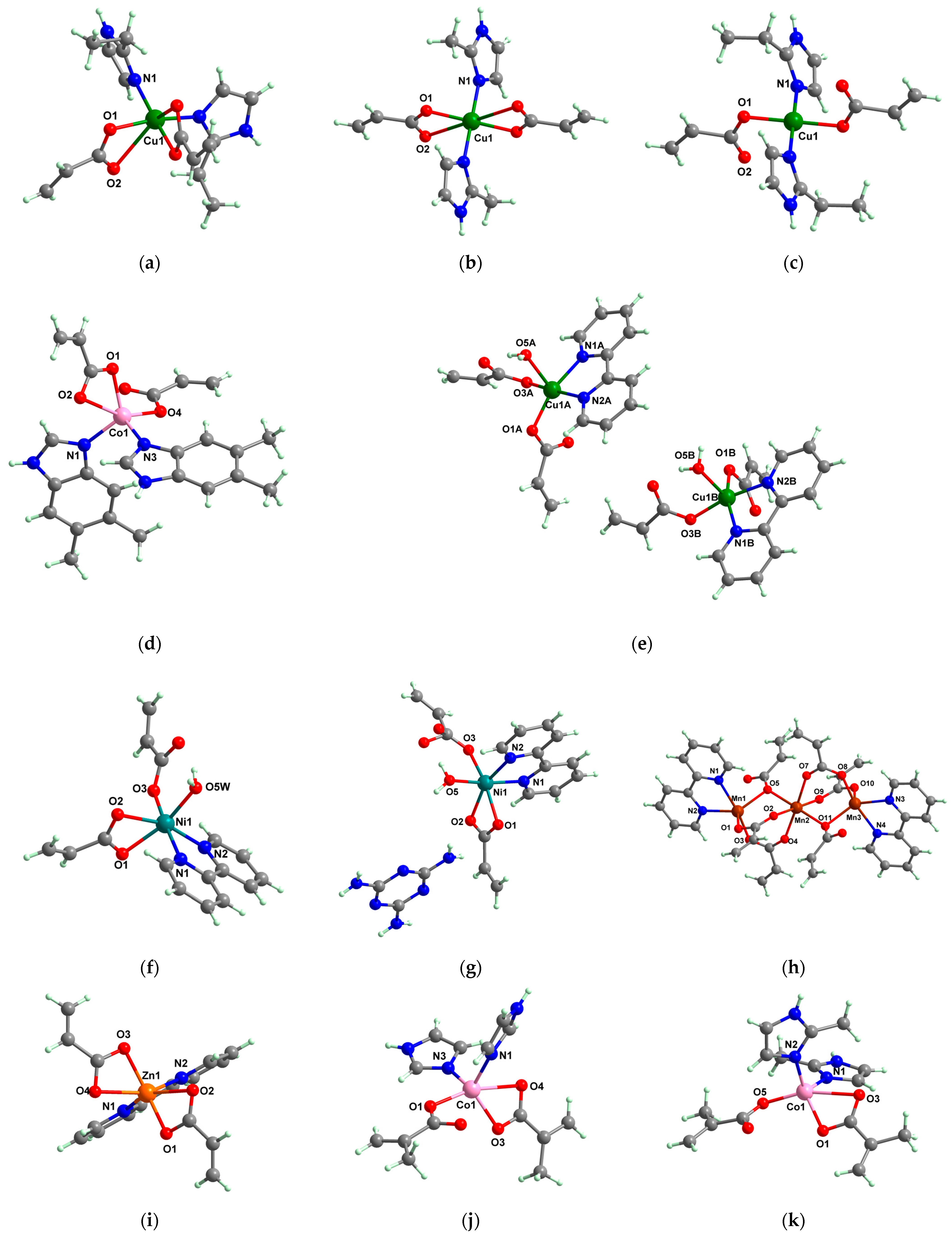
2.1.3. Complexes with Cinnamate/Cinnamate Derivatives and Different N-Donor Ligands
| Compound Formulation | Unsaturated Carboxylate Ligand | Auxiliary Ligand | Biological Activity | Ref. |
|---|---|---|---|---|
 cis-[Cu(acr)2(2-EtIm)2] 4 | acrylate (chelate) * | 2-ethylimidazole | ABA **: B. subtilis (IZD = 22 mm) | [32] |
 trans-[Cu(acr)2(2-MeIm)2] 5 | acrylate (chelate) * | 2-methylimidazole | ABA: E. faecium (IZD = 30 mm), B. subtilis (IZD = 35 mm), S. aureus (IZD = 18 mm) | [32] |
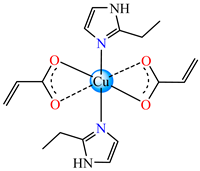 trans-[Cu(acr)2(2-EtIm)2] 6 | acrylate (unidentate semicoordination) * | 2-ethylimidazole | ABA: B. subtilis (IZD = 27 mm) P. aeruginosa (IZD = 18 mm) | [32] |
 [Co(acr)2(HBzIm)2]∙0.5H2O 8 | acrylate | benzimidazole | ABA: E. faecium E5 (MIC = 62.5 μg∙mL−1), B. subtilis ATCC 6683 (MIC = 62.5 μg∙mL−1), E. coli ATCC 25922 (MIC = 62.5 μg∙mL−1), S. aureus (MIC = 62.5 μg∙mL−1) AFA ***: C. albicans (MIC = 62.5 μg∙mL−1) | [33] |
 [Cu2(acr)4(HBzIm)2] 9 | acrylate | benzimidazole | ABA: S. aureus ATCC 6538 (MIC = 31.25 μg∙mL−1), B. subtilis 6683 (MIC = 62.5 μg∙mL−1), E. faecium E5 (MIC = 62.5 μg∙mL−1), E. coli ATCC 25922 (MIC = 62.5 μg∙mL−1) AFA: C. albicans 1760 (MIC = 62.5 μg∙mL−1) | [34] |
 [Cu(acr)2(HBzIm)2(H2O)]∙(H2O) 10 | acrylate | benzimidazole | ABA: S. aureus ATCC 6538 (MIC = 62.5 μg∙mL−1), B. subtilis 6683 (MIC = 125 μg∙mL−1), E. faecium E5 (MIC = 62.5 μg∙mL−1), E.coli ATCC 25922 (MIC = 125 μg∙mL−1) | [34] |
 [Co(acr)2(2-MeBzIm)2]∙0.5H2O 13 | acrylate | 2-methylbenzimidazole | ABA: E. faecium E5 (MIC = 62.5 μg∙mL−1), B. subtilis ATCC 6683 (MIC = 31.25 μg∙mL−1), S. aureus (MIC = 31.25 μg∙mL−1) E. coli ATCC 25922 (MIC = 31.25 μg∙mL−1) AFA: C. albicans (MIC = 62.5 μg∙mL−1) | [33] |
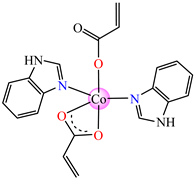
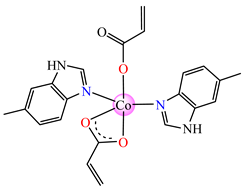 [Co(acr)2(5-MeBzIm)2] 14 | acrylate | 5-methylbenzimidazole | ABA: E. faecium E5 (MIC = 62.5 μg∙mL−1), B. subtilis ATCC 6683 (MIC = 62.5 μg∙mL−1), S. aureus (MIC = 31.25 μg∙mL−1), E. coli ATCC 25922 (MIC = 62.5 μg∙mL−1) AFA: C. albicans (MIC = 62.5 μg∙mL−1) | [33] |
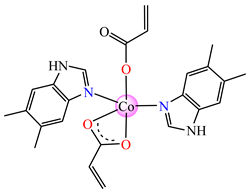 [Co(acr)2(5,6-Me2BzIm)2] 16 | acrylate (unidentate + chelate) * | 5,6-dimethylbenzimidazole | ABA: E. faecium E5 (MIC = 62.5 μg∙mL−1), B. subtilis ATCC 6683 (MIC = 31.25 μg∙mL−1), E. coli ATCC 25922 (MIC = 31.25 μg∙mL−1), S. aureus (MIC = 31.25 μg∙mL−1) AFA: C. albicans (MIC = 31.25 μg∙mL−1) | [33] |
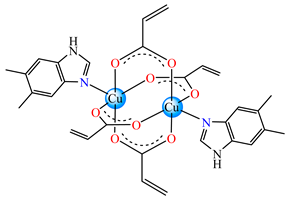 [Cu2(acr)4(5,6-Me2BzIm)2] 17 | acrylate | 5,6-dimethylbenzimidazole | ABA: MRSA 1263 (MIC = 250 μg∙mL−1) | [36] |
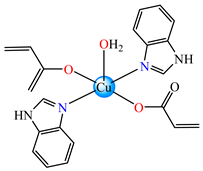 [Cu(acr)2(5,6-Me2BzIm)2(H2O)]∙H2O 18 | acrylate | 5,6-dimethylbenzimidazole | ABA: E. coli (MIC = 125 μg∙mL−1), K. pneumoniae (MIC = 125 μg∙mL−1), MRSA 1263 (MIC = 125 μg∙mL−1), B. subtilis (MIC = 125 μg∙mL−1) | [36] |
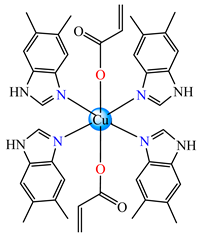 [Cu(acr)2(5,6-Me2BzIm)4] 19 | acrylate | 5,6-dimethylbenzimidazole | ABA: S. aureus (MIC = 250 μg∙mL−1), MRSA 1263 (MIC = 250 μg∙mL−1) | [36] |
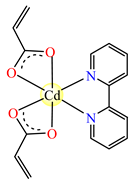 [Cd(acr)2(2,2′-bipy)]∙1.5H2O 21 | acrylate | 2,2′-bipyridine | ABA: Shigella sp. (MIC = 256 μg∙mL−1), Acinetobacter boumani (MIC = 128 μg∙mL−1), P. aeruginosa 1700 (MIC = 256 μg∙mL−1), S. aureus MRSA (MIC = 256 μg∙mL−1) AFA: C. albicans (MIC = 256 μg∙mL−1) | [37] |
 [Cu(acr)2(2,2′-bipy)(H2O)] 22 | acrylate (unidentate) * | 2,2′-bipyridine | ABA: E. coli (MIC = 128 μg∙mL−1) AFA: C. albicans (MIC = 128 μg∙mL−1) | [38] |
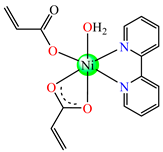 [Ni(acr)2(2,2′-bipy)(H2O)] 23 | acrylate (unidentate + chelate) | 2,2′-bipyridine | AFA: C. albicans (MIC = 128 μg∙mL−1) | [38] |
 [Ni(acr)2(2,2′-bipy)(H2O)]∙MA 24 | acrylate (unidentate + chelate) | 2,2′-bipyridine | ABA: S. aureus ATCC 25923 (MIC = 70 μg∙mL−1) | [39] |
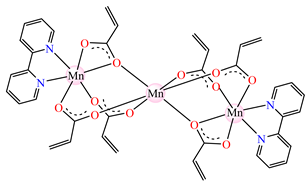 [Mn3(acr)6(2,2′-bipy)2] 25 [Mn3(acr)6(2,2′-bipy)2] 25 | acrylate (bridge through one or two oxygen atoms) * | 2,2′-bipyridine | ABA: E. coli (MIC = 256 μg∙mL−1) AFA: C. albicans (MIC = 128 μg∙mL−1) | [38] |
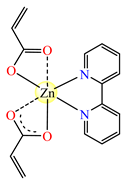 [Zn(acr)2(2,2′-bipy)]∙H2O 26 | acrylate (chelate) * | 2,2′-bipyridine | ABA: S. aureus (MIC = 128 μg∙mL−1) AFA: C. albicans (MIC = 128 μg∙mL−1) | [38] |
 [Cd(acr)2(phen) (H2O)] 27 | acrylate | 1,10-phenantroline | ABA: Acinetobacter boumani (MIC = 64 μg∙mL−1), P. aeruginosa 1700 (MIC = 256 μg∙mL−1), S. aureus MRSA (MIC = 256 μg∙mL−1) AFA: C. albicans (MIC = 256 μg∙mL−1) | [37] |
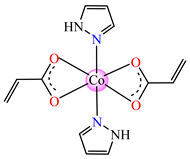 [Co(acr)2(Hpz)2] 28 | acrylate | 1H-pyrazole | ABA: B. subtilis (MIC = 125 μg∙mL−1) | [40] |
 [Co(acr)2(3-MeHpz)2] 29 | acrylate | 3-methyl-1H-pyrazole | ABA: B. subtilis (MIC = 125 μg∙mL−1) | [40] |
 [Co(acr)2(4-MeHpz)2] 30 | acrylate | 4-methyl-1H-pyrazole | ABA: B. subtilis (MIC = 125 μg∙mL−1) | [40] |
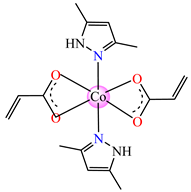 [Co(acr)2(dmpz)2] 31 | acrylate | 3,5-dimethyl-1H-pyrazole | ABA: B. subtilis (MIC = 125 μg∙mL−1) | [40] |
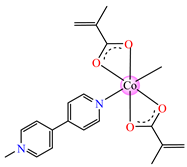 [Co(Macr)2(4,4′-bipy)]∙0.5H2O 32 | methacrylate | 4,4′-bipyridine | ABA: S. aureus (MIC = 125 μg∙mL−1), P. aeruginosa (MIC = 125 μg∙mL−1), E. coli ESBL 1576 (MIC = 31.25 μg∙mL−1), E. coli ATCC 25922 (MIC = 31.25 μg∙mL−1) | [42] |
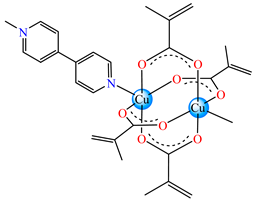 [Cu(Macr)2(4,4′-bipy)]∙0.5H2O 33 | methacrylate | 4,4′-bipyridine | ABA: P. aeruginosa (MIC = 125 μg∙mL−1), E. coli ESBL 1576 (MIC = 62.5 μg∙mL−1), E. coli ATCC 25922 (MIC = 62.5 μg∙mL−1) | [42] |
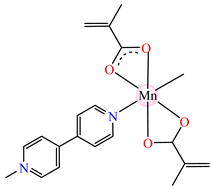 [Mn(Macr)2(4,4′-bipy)] 34 | methacrylate | 4,4′-bipyridine | ABA: S. aureus (MIC = 125 μg∙mL−1), P. aeruginosa (MIC = 125 μg∙mL−1), E. cloacae (MIC = 250 μg∙mL−1), E. coli ESBL 1576 (MIC = 31.25 μg∙mL−1), E. coli ATCC 25922 (MIC = 62.5 μg∙mL−1) | [42] |
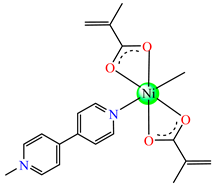 [Ni(Macr)2(4,4′-bipy)]∙1.5H2O 35 | methacrylate | 4,4′-bipyridine | ABA: S. aureus (MIC = 31.25 μg∙mL−1), P. aeruginosa (MIC = 250 μg∙mL−1), E. coli ESBL 1576 (MIC = 31.25 μg∙mL−1), E. coli ATCC 25922 (MIC = 62.5 μg∙mL−1) | [42] |
 [Zn(Macr)2(4,4′-bipy)]∙0.5H2O 36 | methacrylate | 4,4′-bipyridine | ABA: S. aureus (MIC = 125 μg∙mL−1), P. aeruginosa (MIC = 125 μg∙mL−1), E. cloacae (MIC = 250 μg∙mL−1), E. coli ESBL 1576 (MIC = 31.25 μg∙mL−1), E. coli ATCC 25922 (MIC = 62.5 μg∙mL−1) | [42] |
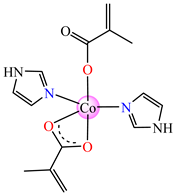 [Co(Macr)2(HIm)2] 37 | methacrylate (unidentate + chelate) * | imidazole | ABA: E. coli ATCC 8739 (MIC = 31.2 μg∙mL−1), P. aeruginosa ATCC 1671 (MIC = 62.5 μg∙mL−1), S. aureus ATCC 6538 (MIC = 15.6 μg∙mL−1), E. faecalis ATCC 29212 (MIC = 31.2 μg∙mL−1) AFA: C. albicans ATCC 26790 (MIC = 7.8 μg∙mL−1) | [43] |
 [Co(Macr)2(2-MeIm)2] 38 | methacrylate (unidentate + chelate) * | 2-methylimidazole | ABA: E. coli ATCC 8739 (MIC = 31.2 μg∙mL−1), P. aeruginosa ATCC 1671 (MIC = 15.6 μg∙mL−1), S. aureus ATCC 6538 (MIC = 15.6 μg∙mL−1), E. faecalis ATCC 29212 (MIC = 31.2 μg∙mL−1) | [43] |
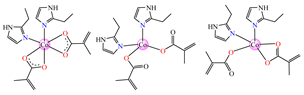 [Co(Macr)2(2-EtIm)2] 39 [Co(Macr)2(2-EtIm)2] 39 | methacrylate (unidentate + chelate in left unit; unidentate in middle unit; chelate in right unit) * | 2-ethylimidazole | ABA: E. coli ATCC 8739 (MIC = 125 μg∙mL−1), P. aeruginosa ATCC 1671 (MIC = 31.2 μg∙mL−1), S. aureus ATCC 6538 (MIC = 15.6 μg∙mL−1), E. faecalis ATCC 29212 (MIC = 62.5 μg∙mL−1) AFA: C. albicans ATCC 26790 (MIC = 15.6 μg∙mL−1) | [43] |
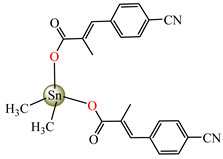 [Me2Sn(cpma)2] 40 | 3-(4-cyanophenyl)-2-methylacrylate | methyl | ABA: S. aureus (IZD = 20 mm), E. coli (IZD = 20 mm), Bortedella bronchiseptica (IZD = 25 mm), Micrococcus luteus (IZD = 20 mm) AFA: A. fumigatus (PGI = 65%) | [44] |
 [Bu2Sn(cpma)2] 41 | 3-(4-cyanophenyl)-2-methylacrylate | n-butane | ABA: S. aureus (IZD = 20 mm), E. coli (IZD = 20 mm), Bortedella bronchiseptica (IZD = 25 mm) AFA: A. flavus (PGI = 50%), A. fumigatus (PGI = 55%), Fusarium solani (PGI = 95%) | [44] |
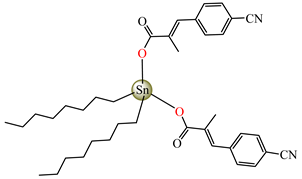 [Oct2Sn(cpma)2] 42 | 3-(4-cyanophenyl)-2-methylacrylate | n-octane | ABA: E. coli (IZD = 16 mm), Bortedella bronchiseptica (IZD = 10 mm) | [44] |
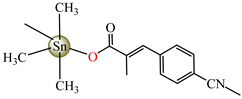 [Me3Sn(cpma)]n43 | 3-(4-cyanophenyl)-2-methylacrylate (unidentate) * | methyl | ABA: S. aureus (IZD = 20 mm), E.coli (IZD = 25 mm), Bortedella bronchiseptica (IZD = 30 mm) AFA: A. flavus (PGI = 96%), A. niger (PGI = 100%), A. fumigatus (PGI = 100%), Fusarium solani (PGI = 100%) | [44] |
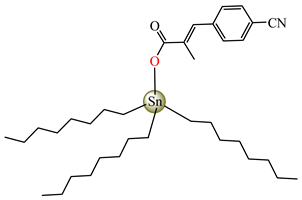 [Bu3Sn(cpma)] 44 | 3-(4-cyanophenyl)-2-methylacrylate | butyl | ABA: S. aureus (IZD = 22 mm), E. coli (IZD = 23 mm), Bortedella bronchiseptica (IZD = 25 mm), Micrococcus luteus (IZD = 20 mm) AFA: A. niger (PGI = 58%), A. fumigatus (PGI = 75%), Fusarium solani (PGI = 75%) | [44] |
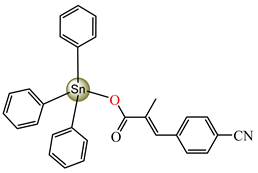 [Ph3Sn(cpma)] 45 | 3-(4-cyanophenyl)-2-methylacrylate (bidentate chelate) * | phenyl | ABA: S. aureus (IZD = 30 mm), E. coli (IZD = 20 mm), Bortedella bronchiseptica (IZD = 20 mm), Micrococcus luteus (IZD = 27 mm) AFA: A. flavus (PGI = 98%), A. niger (PGI = 97%), A. fumigatus (PGI = 100%), Fusarium solani (PGI = 96%) | [44] |
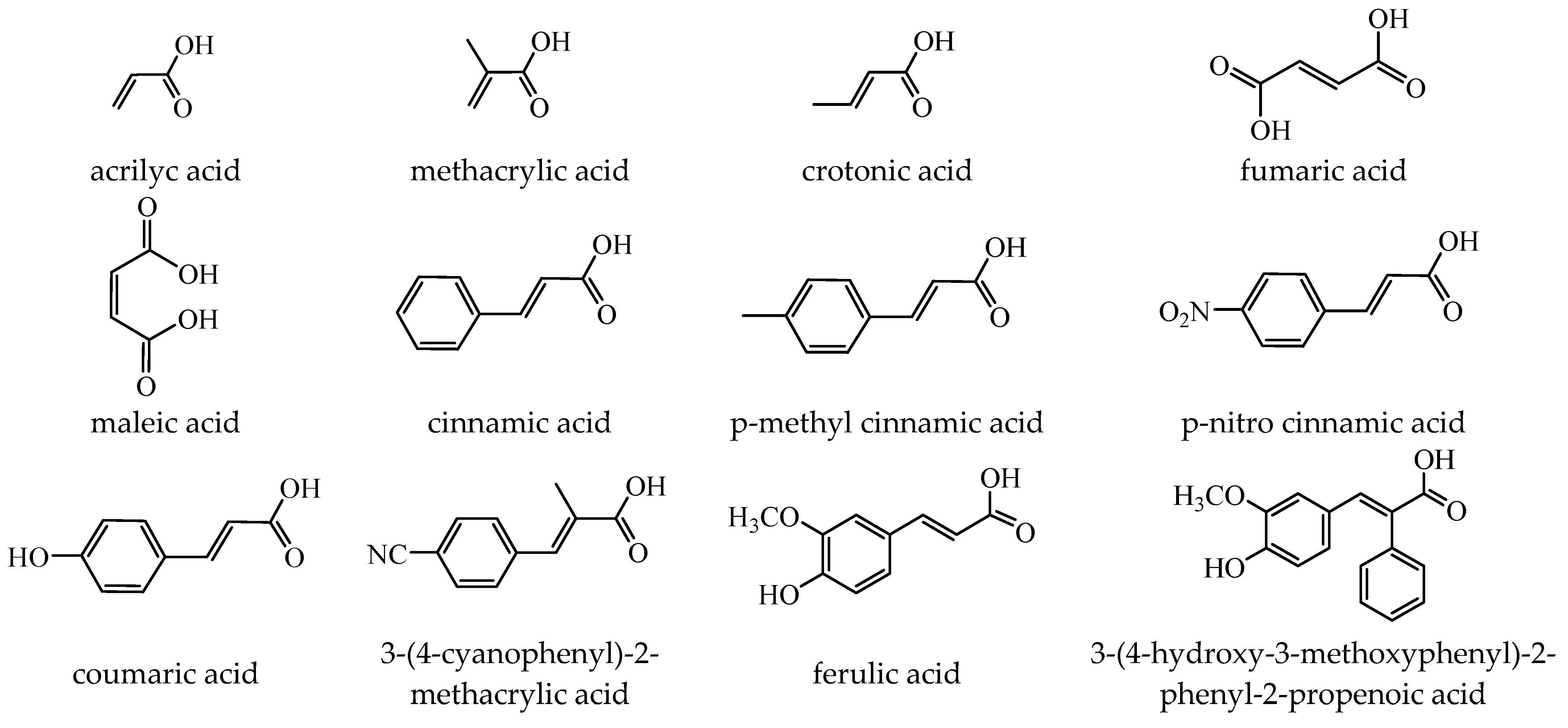
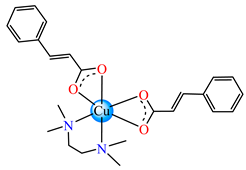 [Cu(cin)2(tmeda)]∙0.7H2O 46 | cinnamate (unidentate) * | N,N,N’,N’-tetramethylenediamine | ABA: Bacillus spizizenii (MIC = 10 μg∙mL−1), S. aureus (MIC = 25 μg∙mL−1) | [47] |
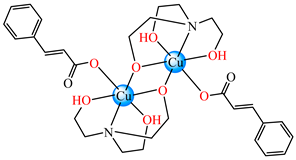 [Cu2(cin)2(tea)](H2O) 47 [Cu2(cin)2(tea)](H2O) 47 | cinnamate (unidentate) * | triethanolamine | ABA: S. aureus (MIC = 25 μg∙mL−1) | [48] |
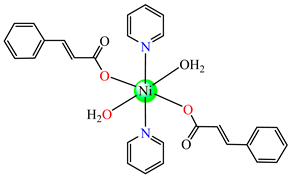 trans-[Ni(cin)2(py)2(H2O)2] 48 | cinnamate (unidentate) * | pyridine | ABA: Micrococcus luteus (IZD = 25 mm) | [49] |
 trans,cis-[Ni(mcin)2(2,2′-bipy)(H2O)2] 49 trans,cis-[Ni(mcin)2(2,2′-bipy)(H2O)2] 49 | p-methylcinnamate (unidentate) * | 2,2′-bipyridine | ABA: S. aureus (IZD = 20 mm) | [49] |
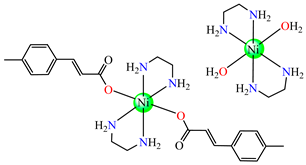 {trans-[Ni(mcin)2(en)2][Ni(en)2(H2O)2](mcin)∙H2O} 50 {trans-[Ni(mcin)2(en)2][Ni(en)2(H2O)2](mcin)∙H2O} 50 | p-methylcinnamate (unidentate) * | ethylenediamine | ABA: Micrococcus luteus (IZD = 21 mm) | [49] |
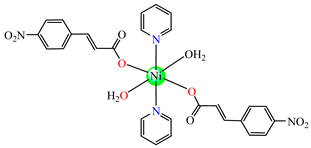 trans-[Ni(ncin)2(py)2(H2O)2] 51 trans-[Ni(ncin)2(py)2(H2O)2] 51 | p-nitrocinnamate (unidentate) * | pyridine | ABA: Micrococcus luteus (IZD = 21 mm), B. subtilis (IZD = 18 mm) | [49] |
 [Me3Sn(hmpp)2]n54 | 3-(4-hydroxy-3-methoxyphenyl)-2-phenylpropenoate | methyl | ABA: E. coli (IZD = 15 mm), B. subtilis (IZD = 15 mm), P. aeruginosa (IZD = 18 mm) | [58] |
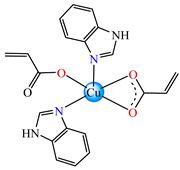 [Cu(acr)2(HBzIm)2] 60 | acrylate | benzimidazole | AFA: C. albicans 1760 (MIC = 31.25 μg∙mL−1) | [34] |
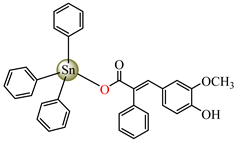
 [Bu3Sn(hmpp)] 61 | 3-(4-hydroxy-3-methoxyphenyl)-2-phenylpropenoate | butyl | AFA: A. flavus (PGI = 70%), Microsporum canis (PGI = 65%) | [58] |
 [Ph3Sn(hmpp)] 62 | 3-(4-hydroxy-3-methoxyphenyl)-2-phenylpropenoate | phenyl | AFA: Microsporum canis (PGI = 60%) | [58] |
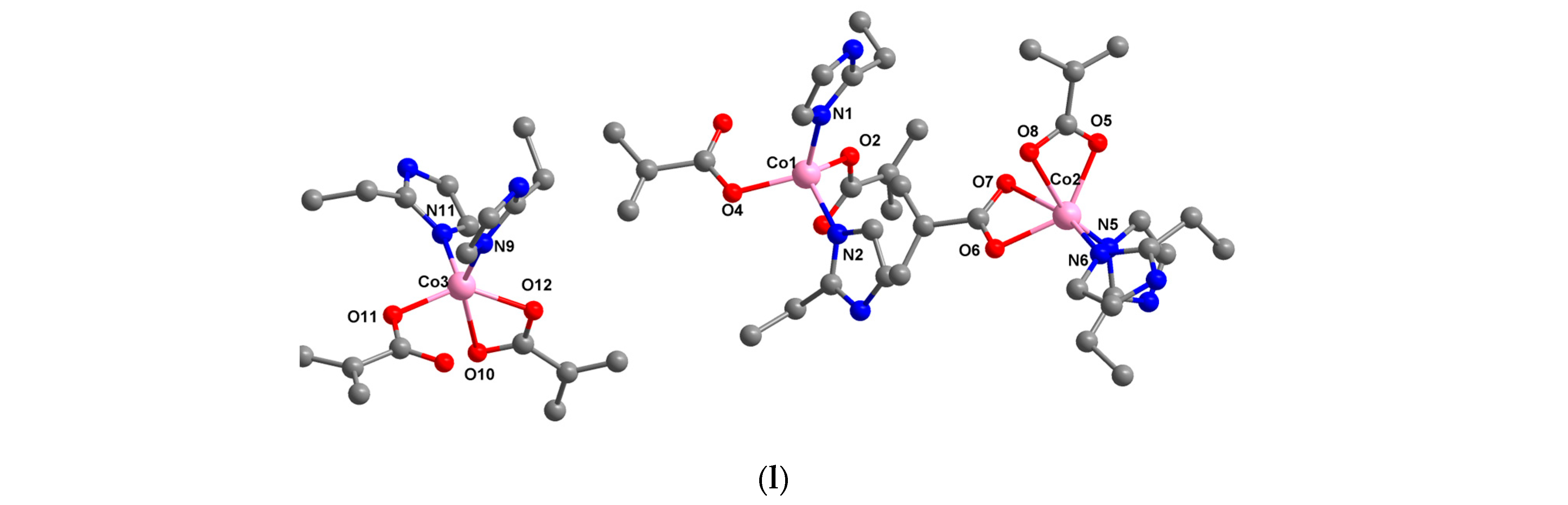
2.1.4. Complexes with Maleate and Different Heterocyclic Amine
2.2. Coordinative Compounds with Unsaturated Carboxylate with Activity on Bacterial Biofilm
2.3. Coordinative Compounds with Unsaturated Carboxylate with Antifungal Properties
3. Coordinative Compounds with Unsaturated Carboxylate Developed for Antitumor Applications
3.1. Coordinative Compounds with Unsaturated Carboxylate with Antitumor Activity
| Compound Structure */Formulation | Unsaturated Carboxylate Ligand | Auxiliary Ligand | Biological Activity | Ref. |
|---|---|---|---|---|
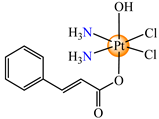 cis,trans,cis-[Pt(NH3)2(OH)(cin)Cl2] (64) | cinnamate | ammonia, hydroxyl, chloride | HeLa, HCT116 (p53 positive and p53 non-expressing), MDA-MB-231, MCF-7, rhabdomyosarcoma human cells (IC50 in micromolar range) | [73] |
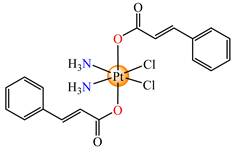 cis,trans,cis-[Pt(NH3)2(cin)2Cl2] (65) | cinnamate | ammonia, chloride | HeLa, HCT116 (p53 positive and p53 non-expressing), MDA-MB-231, MCF-7, rhabdomyosarcoma human cells (IC50 in submicromolar range) | [73] |
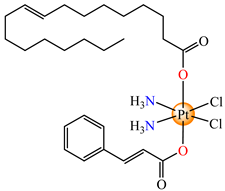 cis,trans,cis-[Pt(NH3)2(cin)(ole)Cl2] (66) | cinnamate, oleate | ammonia, chloride | MCF-7, T47D, MDA 453 and SK-BR-3 breast cancer cells (IC50 in micromolar range) | [79] |
 cis,trans,cis-[Pt(NH3)2(cin)(val)Cl2] (67) | cinnamate | ammonia, valproate, chloride | human lung (A549), breast (MCF-7), hepatocellular (HepG-2), bladder (5637), mice bladder (MB49) and breast (4T1) carcinoma (IC50 in submicromolar range) | [80] |
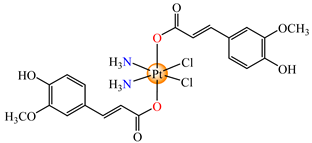 cis,trans,cis-[Pt(NH3)2(fer)2Cl2] (68) | ferulate | ammonia, chloride | lung carcinoma (A549 and A549/DDP) (IC50 in submicromolar range) | [81] |
 [Bu3Sn(fer)] (69) | ferulate | butyl | HCT116, HT-29 and Caco-2 (IC50 in nanomolar range) | [82] |
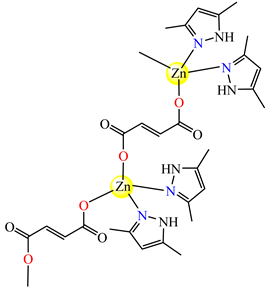 {[Zn2(μ-fum)2(Hdmpz)4]∙3H2O}n (77) | fumarate (bridge) * | 3,5-dimethylpyrazole | DL (IC50 in micromolar range) | [86] |
3.2. Coordinative Compounds with Unsaturated Carboxylate with Antitumor Activity on Resistant Cells
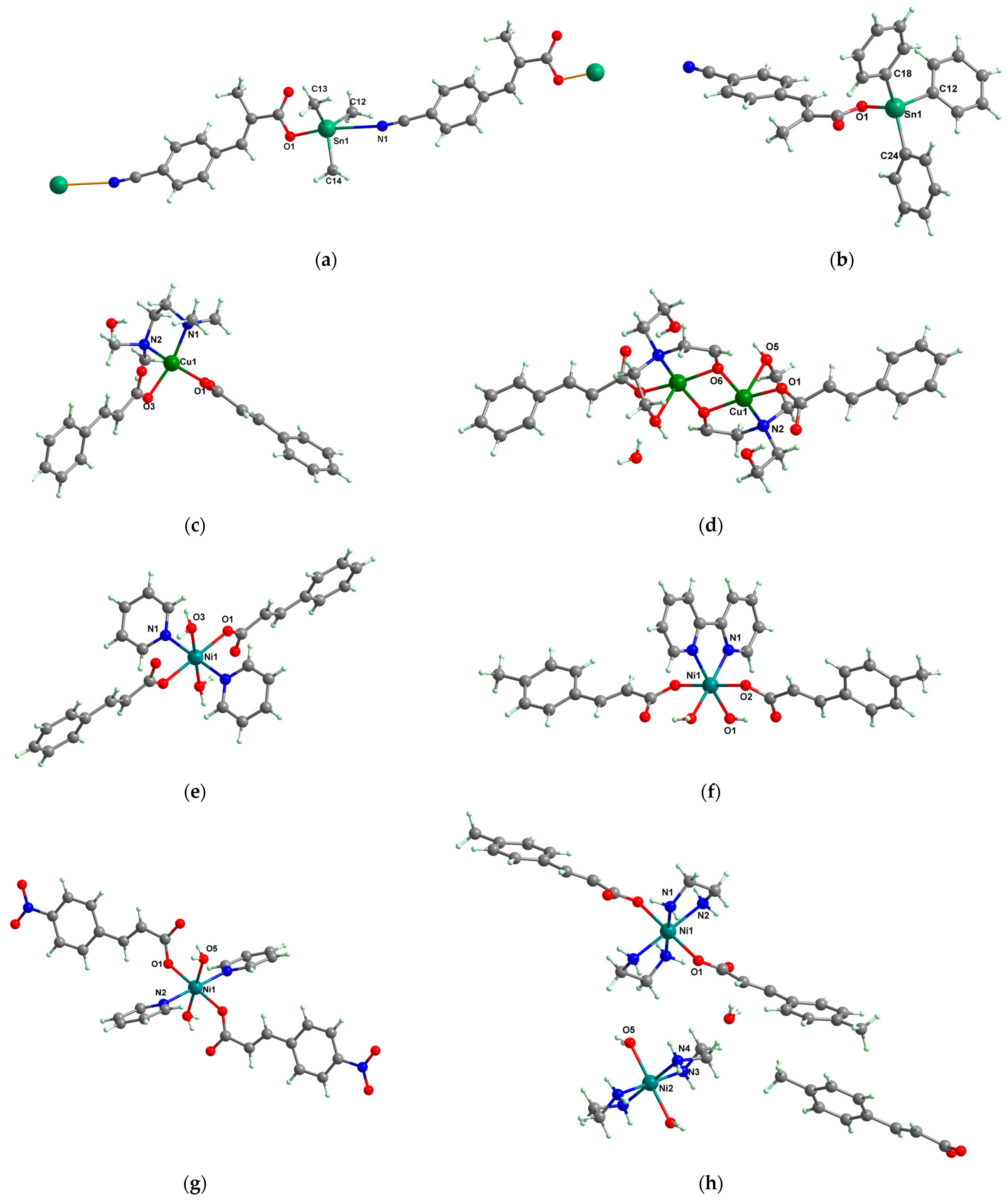
4. Coordinative Compounds with Unsaturated Carboxylate with DNA-Intercalative Abilities and Antioxidant Activity
| Compound Structure */Formulation | Unsaturated Carboxylate Ligand | Auxiliary Ligand | Biological Activity | Ref. |
|---|---|---|---|---|
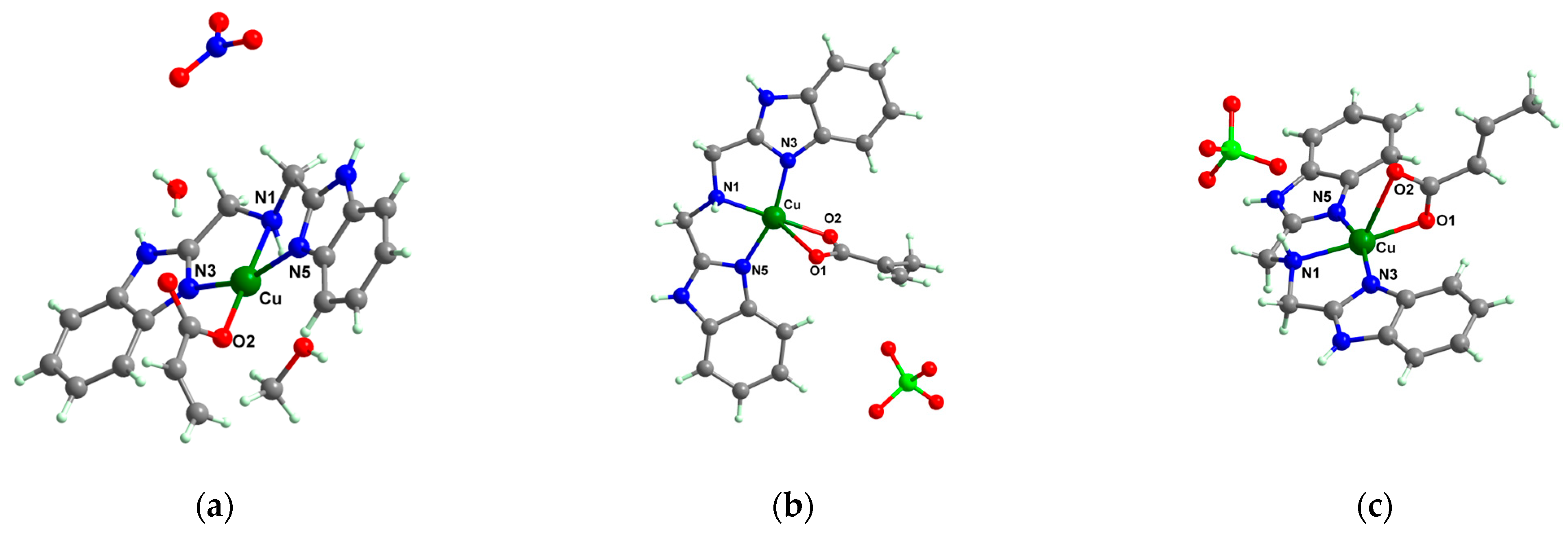
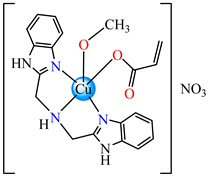 [Cu(acr)(bba)(CH3O)]NO3∙H2O 79 | acrylate (unidentate) * | bis(2-benzimidazolylmethyl)amine, methanol | DNA binding, superoxide radical scavenger (IC50 = 1.55 mM) | [96] |
 [Cu(macr)(bba)]ClO480 | methacrylate (chelate) * | bis(2-benzimidazolylmethyl)amine | DNA binding, superoxide radical scavenger (IC50 = 0.87 mM) | [96] |
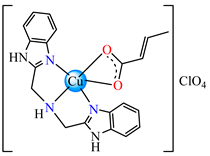 [Cu(crot)(bba)]ClO481 | crotonate (chelate) * | bis(2-benzimidazolylmethyl)amine | DNA binding; superoxide radical scavenger (IC50 = 1.27 mM | [96] |
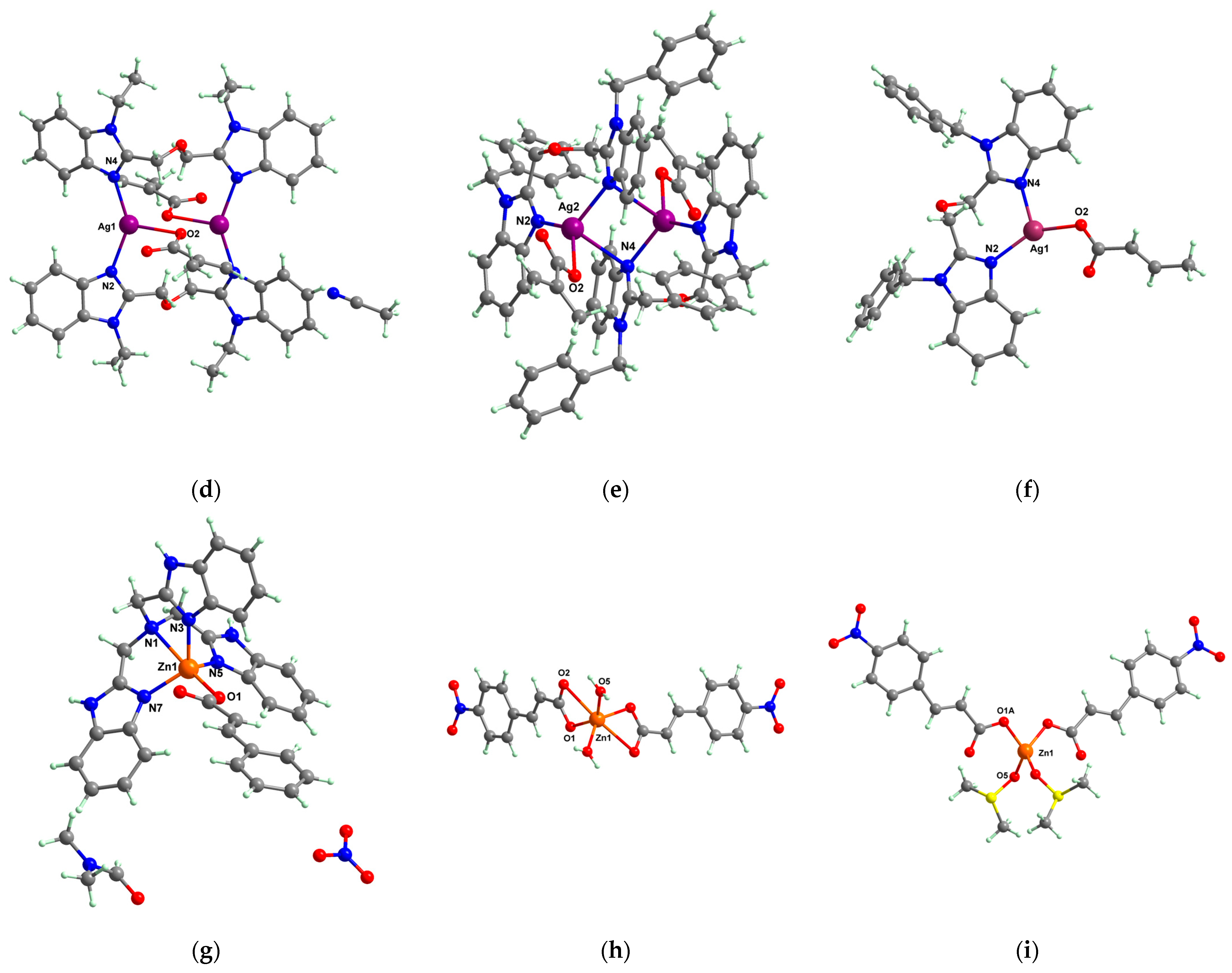
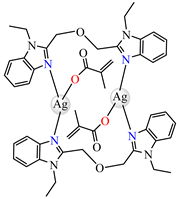 [Ag2(macr)2(etobb)2]∙CH3CN 82 | methacrylate (unidentate) * | 1,3-bis(1-ethylbenzimidazol-2-yl)-2- oxapropane | DNA binding | [98] |
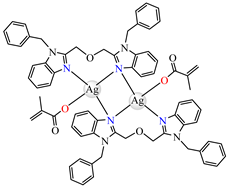 [Ag(macr)(bobb)] 83 | methacrylate (unidentate) * | 1,3-bis(1-benzylbenzimidazol-2-yl)-2- oxapropane | DNA binding; hydroxyl radical scavenger | [98] |
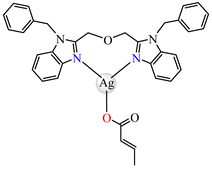 [Ag(crot)(bobb)] 85 | crotonate (unidentate) * | 1,3-bis(1-benzylbenzimidazol-2-yl)-2- oxapropane | DNA binding; hydroxyl radical scavenger | [99] |
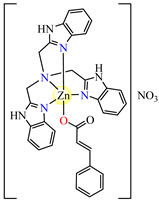 [Zn(cin)(tbima)]NO3∙DMF 88 | cinnamate (unidentate) * | tris(2-benzimidazylmethyl)amine | DNA binding; hydroxyl radical scavenger | [102] |
 [Zn(ncin)2(H2O)2] 89 [Zn(ncin)2(H2O)2] 89 | p-nitro cinnamate (chelate) * | water | DNA binding | [103] |
 [Zn(ncin)2(DMSO)2] 90 | p-nitro cinnamate(unidentate) * | dimethylsulfoxide | DNA binding | [103] |
5. Conclusions
6. Further Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | antibacterial activity |
| AFA | antifungal activity |
| aobb | 1,3-bis(1-allylbenzimidazol-2-yl)-2-oxopropane |
| apy | aminopyridine |
| ATCC | American Type Culture Collection |
| bba | bis(2-benzimidazolylmethyl)amine |
| bebt | 1,3-bis(1-ethylbenzimidazol-2-yl)-2-thiapropane |
| bipy | bipyridine |
| bobb | 1,3-bis(1-benzylbenzimidazol-2-yl)-2-oxapropane |
| Bu | n-butyl |
| DMF | N,N-dimethylformamide |
| dmpz | 3,5-dimethyl-1H-pyrazole |
| DMSO | dimethylsulfoxide |
| en | ethylenediamine |
| Et | ethyl |
| EtIm | ethylimidazole |
| Etobb | 1,3-bis(1-ethylbenzimidazol-2-yl)-2-oxapropane |
| H2cou | coumaric acid |
| H2fer | ferulic acid |
| H2fum | fumaric acid |
| H2mal | maleic acid |
| Hacr | acrylic acid |
| HBzIm | benzimidazole |
| Hcin | cinnamic acid |
| HClcin | chlorocinnamic acid |
| Hcpma | 3-(4-cyanophenyl)-2-methylacrylic acid |
| Hcrot | crotonic acid |
| Hhmpp | 3-(4-hydroxy-3-methoxyphenyl)-2-phenylpropenoic acid |
| HIm | imidazole |
| Hicemac | 2-isocyanatoethyl methacrylic acid |
| Hmacr | methacrylic acid |
| Hmcin | methylcinnamic acid |
| HMeOcin | methoxy cinnamic acid |
| Hncin | para-nitro cinnamic acid |
| Hole | oleic acid |
| Hpaa | 2-phenyl-3-thiophen-2-yl-acrylic acid |
| Hpz | 1H-pyrazole |
| hqn | hydroxyquinoline |
| Htaa | 3-thiophen-2-yl-acrylic acid |
| Hval | valproic acid |
| iqn | isoquinoline |
| IZD | inhibition zone diameter |
| MBEC | minimum biofilm eradication concentration |
| Me | methyl |
| Me2BzIm | dimethylbenzimidazole |
| MeBzIm | methylbenzimidazole |
| MeHpz | methyl-1H-pyrazole |
| MeIm | methylimidazole |
| MIC | minimum inhibitory concentration |
| mlm | melamine |
| PAA | polyacrylates |
| PGI | percentage growth inhibition |
| Ph | phenyl |
| phen | phenantroline |
| PMAA | polymethacrylic acid |
| PMMA | poly(methyl)methacrylates |
| PPF | poly(propylene)fumarate |
| py | pyridine |
| qn | quinoline |
| ROS | reactive oxygen species |
| tbima | tris(2-benzimidazylmethyl)amine |
| tea | triethanolamine |
| tmeda | N,N,N’,N’-tetramethylenediamine |
References
- Serrano-Aroca, A.; Deb, S. (Eds.) Acrylic-Based Materials for Biomedical and Bioengineering Applications; Acrylate Polymers for Advanced Applications; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Yuan, Z.; Han, H.; Li, T.; Li, L.; Guo, X. Chitosan cross-linked poly(acrylic acid) hydrogels: Drug release control and mechanism. Colloids Surf. B 2017, 152, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Dunne, N.; Tzagiollari, A.; Sahebalzamani, M.; Dunne, T.J. Acrylic cements for bone fixation in joint replacement. In Woodhead Publishing Series in Biomaterials, Joint Replacement Technology, 3rd ed.; Revell, P., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 213–262. [Google Scholar] [CrossRef]
- Corsaro, C.; Neri, G.; Santoro, A.; Fazio, E. Acrylate and Methacrylate Polymers’ Applications: Second Life with Inexpensive and Sustainable Recycling Approaches. Materials 2022, 15, 282. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Young, A.M. Antibacterial adhesives for bone and tooth repair. In Woodhead Publishing Series in Biomaterials, Joining and Assembly of Medical Materials and Devices; Zhou, Y., Breyen, M., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 491–513. [Google Scholar] [CrossRef]
- Cai, Z.; Wan, Y.; Becker, M.; Long, Y.-Z.; Dean, D. Poly(propylene fumarate)-based materials: Synthesis, functionalization, properties, device fabrication and biomedical applications. Biomaterials 2019, 208, 45–71. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; De Santis, R.; Gloria, A.; Barbaro, K.; Altigeri, A.; Fadeeva, I.V.; Rau, J.V. Modification of PMMA Cements for Cranioplasty with Bioactive Glass and Copper Doped Tricalcium Phosphate Particles. Polymers 2020, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Saboktakin, M.R.; Tabatabaie, R.; Maharramov, A.; Ramazanov, M.A. Synthesis and rheological properties of poly(methyl methacrylate)/polymethacrylic acid nanocomposites as denture resins. Compos. B Eng. 2011, 42, 851–855. [Google Scholar] [CrossRef]
- Corsaro, C.; Neri, G.; Mezzasalma, A.M.; Fazio, E. Weibull Modeling of Controlled Drug Release from Ag-PMA Nanosystems. Polymers 2021, 13, 2897. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Barboza, A.; Fang, L.; Ribeiro, J.; Cuevas-Suarez, C.; Moraes, R.; Lund, R. Physicomechanical, optical, and antifungal properties of polymethyl methacrylate modified with metal methacrylate monomers. J. Prosthet. Dent. 2021, 125, 706.e1–706.e6. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.; Ren, L.; Tang, C. Metal-containing and related polymers for biomedical applications. Chem. Soc. Rev. 2016, 45, 5232–5263. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Rabinskiy, L.N.; Kydralieva, K.A.; Uflyand, I.E. Recent advances in metallopolymer-based drug delivery systems. RSC Adv. 2019, 9, 37009–37051. [Google Scholar] [CrossRef]
- Basak, S.; Bandyopadhyay, A. Tethering smartness to the metal containing polymers—Recent trends in the stimuli-responsive metal containing polymers. J. Organomet. Chem. 2021, 956, 122129. [Google Scholar] [CrossRef]
- Bäumer, N.; Matern, J.; Fernández, G. Recent progress and future challenges in the supramolecular polymerization of metal-containing monomers. Chem. Sci. 2021, 12, 12248. [Google Scholar] [CrossRef]
- Metwally, A.M.; Mohamed, H.I.; ElKhawaga, H.A.; Ibrahim, S.M.; Reda, L.M. Novel bioactive copper and nickel polymeric complexes: Synthesis, characterization, antimicrobial activity, and DFT calculations. J. Appl. Polym. Sci. 2023, 141, e55037. [Google Scholar] [CrossRef]
- Pomogailo, A.; Rozenberg, A.; Dzhardimalieva, G. Polymer nanocomposites on the base of metal carboxylates. Adv. Mater. Sci. 2001, 1, 19–27. [Google Scholar]
- Tabasi, H.; Babaei, M.; Abnous, K.; Taghdisi, S.M.; Saljooghi, M.; Ramezani, A.S.; Alibolandi, M. Metal–polymer-coordinated complexes as potential nanovehicles for drug delivery. J. Nanostruct. Chem. 2021, 11, 501–526. [Google Scholar] [CrossRef]
- Schrader, S.M.; Botella, H.; Vaubourgeix, J. Reframing antimicrobial resistance as a continuous spectrum of manifestations. Curr. Opin. Microbiol. 2023, 72, 102259. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.D.; Lee, R.E. Editorial overview: Recent advances in antimicrobial drug discovery and resistance. Curr. Opin. Microbiol. 2023, 71, 102242. [Google Scholar] [CrossRef]
- Ye, J.; Chen, X. Current Promising Strategies against Antibiotic-Resistant Bacterial Infections. Antibiotics 2023, 12, 67. [Google Scholar] [CrossRef]
- Wahab, S.; Salman, A.; Khan, Z.; Khan, S.; Krishnaraj, C.; Yun, S.-I. Metallic Nanoparticles: A Promising Arsenal against Antimicrobial Resistance—Unraveling Mechanisms and Enhancing Medication Efficacy. Int. J. Mol. Sci. 2023, 24, 14897. [Google Scholar] [CrossRef] [PubMed]
- Barbu, I.C.; Gheorghe-Barbu, I.; Grigore, G.A.; Vrancianu, C.O.; Chifiriuc, M.C. Antimicrobial Resistance in Romania: Updates on Gram-Negative ESCAPE Pathogens in the Clinical, Veterinary, and Aquatic Sectors. Int. J. Mol. Sci. 2023, 24, 7892. [Google Scholar] [CrossRef]
- Rees, T.W.; Ho, P.-Y.; Hess, J. Recent Advances in Metal Complexes for Antimicrobial Photodynamic Therapy. ChemBioChem 2023, 24, e202200796. [Google Scholar] [CrossRef]
- Tasnim, N.T.; Ferdous, N.; Rumon, M.M.H.; Shakil, M.S. The Promise of Metal-Doped Iron Oxide Nanoparticles as Antimicrobial Agent. ACS Omega 2024, 9, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Olar, R.; Badea, M.; Chifiriuc, M.C. Metal Complexes—A Promising Approach to Target Biofilm Associated Infections. Molecules 2022, 27, 758. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Gupta, A.; Upadhye, V.; Singh, S.C.; Sinha, R.P.; Häder, D.-P. Therapeutic Strategies against Biofilm Infections. Life 2023, 13, 172. [Google Scholar] [CrossRef] [PubMed]
- Diban, F.; Di Lodovico, S.; Di Fermo, P.; D’Ercole, S.; D’Arcangelo, S.; Di Giulio, M.; Cellini, L. Biofilms in Chronic Wound Infections: Innovative Antimicrobial Approaches Using the In Vitro Lubbock Chronic Wound Biofilm Model. Int. J. Mol. Sci. 2023, 24, 1004. [Google Scholar] [CrossRef]
- Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm Formation and Control of Foodborne Pathogenic Bacteria. Molecules 2023, 28, 2432. [Google Scholar] [CrossRef] [PubMed]
- Juszczuk-Kubiak, E. Molecular Aspects of the Functioning of Pathogenic Bacteria Biofilm Based on Quorum Sensing (QS) Signal-Response System and Innovative Non-Antibiotic Strategies for Their Elimination. Int. J. Mol. Sci. 2024, 25, 2655. [Google Scholar] [CrossRef] [PubMed]
- Zafer, M.; Mohamed, G.; Ibrahim, S.; Ghosh, S.; Bornman, C.; Elfaky, M. Biofilm-mediated infections by multidrug-resistant microbes: A comprehensive exploration and forward perspectives. Arch. Microbiol. 2024, 206, 101. [Google Scholar] [CrossRef] [PubMed]
- Vlaicu, I.D.; Olar, R.; Vasile Scăețeanu, G.; Silvestro, L.; Maurer, M.; Stănică, N.; Badea, M. Thermal, spectral and biological investigation of new nickel complexes with imidazole derivatives. J. Therm. Anal. Calorim. 2018, 134, 503–512. [Google Scholar] [CrossRef]
- Vlaicu, I.D.; Borodi, G.; Vasile Scăețeanu, G.; Chifiriuc, M.C.; Măruțescu, L.; Popa, M.; Stefan, M.; Mercioniu, I.F.; Maurer, M.; Daniliuc, C.; et al. X-ray, crystal structure, geometric isomerism, and antimicrobial activity of new copper (II) carboxylate complexes with imidazole derivatives. Molecules 2018, 23, 3253. [Google Scholar] [CrossRef]
- Vlaicu, I.D.; Olar, R.; Maxim, C.; Chifiriuc, M.C.; Bleotu, C.; Stănică, N.; Vasile Scăețeanu, G.; Dulea, C.; Avram, S.; Badea, M. Evaluating the biological potential of some new cobalt (II) complexes with acrylate and benzimidazole derivatives. Appl. Organomet. Chem. 2019, 33, e4976. [Google Scholar] [CrossRef]
- Badea, M.; Vlaicu, I.D.; Olar, R.; Constand, M.; Bleotu, C.; Chifiriuc, M.C.; Măruțescu, L.; Lazăr, V.; Grecu, M.N.; Marinescu, D. Thermal behaviour and characterisation of new biologically active Cu(II) complexes with benzimidazole as main ligand. J. Therm. Anal. Calorim. 2014, 118, 1119–1133. [Google Scholar] [CrossRef]
- Olar, R.; Vlaicu, I.D.; Chifiriuc, M.C.; Bleotu, C.; Stănică, N.; Vasile Scăețeanu, G.; Silvestro, L.; Dulea, C.; Badea, M. Thermal behaviour of new nickel (II) complexes with unsaturated carboxylates and heterocyclic N-donor ligands. J. Therm. Anal. Calorim. 2017, 127, 731–741. [Google Scholar] [CrossRef]
- Vlaicu, I.D.; Constand, M.; Olar, R.; Marinescu, D.; Grecu, M.N.; Lazăr, V.; Chifiriuc, M.C.; Badea, M. Thermal stability of new biologic active copper (II) complexes with 5,6-dimethylbenzimidazole. J. Therm. Anal. Calorim. 2013, 113, 1369–1377. [Google Scholar] [CrossRef]
- Badea, M.; Olar, R.; Marinescu, D.; Lazar, V.; Chifiriuc, C.; Vasile, G. Thermal behaviour of new biological active cadmium mixed ligand complexes. J. Therm. Anal. Calorim. 2009, 97, 781–785. [Google Scholar] [CrossRef]
- Vasile Scăețeanu, G.; Chifiriuc, M.C.; Bleotu, C.; Karmezan, C.; Măruțescu, L.; Daniliuc, C.; Maxim, C.; Calu, L.; Olar, R.; Badea, M. Synthesis, structural characterization, antimicrobial activity, and in vitro biocompatibility of new unsaturated carboxylate complexes with 2,2’-bipyridine. Molecules 2018, 23, 157. [Google Scholar] [CrossRef] [PubMed]
- Olar, R.; Daniliuc, C.G.; Vasile Scăețeanu, G.; Cerc Korošec, R.; Čelan Korošin, N.; Chifiriuc, M.C.; Badea, M. Structural and antimicrobial characterization of co-crystal [Ni(bpy)(acr)2(H2O)]∙MA. Crystals 2022, 12, 1078. [Google Scholar] [CrossRef]
- Vlaicu, I.D.; Olar, R.; Marinescu, D.; Lazăr, V.; Badea, M. Physico-chemical and thermal characterization of new Co(II) complexes with pyrazole derivatives. J. Therm. Anal. Calorim. 2013, 113, 1337–1343. [Google Scholar] [CrossRef]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Razzaghi, M.; Ismail, A.F.; Rama Krishna, S.; Berto, F. Polymethyl Methacrylate-Based Bone Cements Containing Carbon Nanotubes and Graphene Oxide: An Overview of Physical, Mechanical, and Biological Properties. Polymers 2020, 12, 1469. [Google Scholar] [CrossRef] [PubMed]
- Vasile, G.; Olar, R.; Marinescu, D.; Kriza, A.; Măruțescu, L.; Chifiriuc, M.C.; Lazăr, V.; Badea, M. Thermal study of new biologic active complexes with mixed ligands. J. Therm. Anal. Calorim. 2013, 111, 1783–1790. [Google Scholar] [CrossRef]
- Fudulu, A.; Olar, R.; Maxim, C.; Scăețeanu Vasile, G.; Bleotu, C.; Matei, L.; Chifiriuc, M.C.; Badea, M. New cobalt (II) complexes with imidazole derivatives: Antimicrobial efficiency against planktonic and adherent microbes and in vitro cytotoxicity features. Molecules 2021, 26, 55. [Google Scholar] [CrossRef]
- Tariq, M.; Ali, S.; Shah, N.A.; Muhammad, N.; Tahir, M.N.; Khalid, N. Catalytic, biological and DNA interaction studies of 3-(4-cyanophenyl)-2-methylmethacrylate organotin (IV) carboxylates derivatives: Synthesis, spectroscopic characterization and X-ray structures. Inorg. Chim. Acta 2013, 405, 444–454. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnmic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef] [PubMed]
- Drakontaeidi, A.; Pontiki, E. Multi-Target-Directed Cinnamic Acid Hybrids Targeting Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 582. [Google Scholar] [CrossRef]
- Batool, S.S.; Gilani, S.R.; Zainab, S.S.; Tahir, M.N.; Harrison, W.; Haider, M.S.; Syed, Q.; Mazhar, S.; Shoaib, M. Synthesis, crystal structure, thermal studies and antimicrobial activity of a mononuclear Cu(II)-cinnamate complex with N,N,N’, N’-tetramethylenediamine as co-ligand. Polyhedron 2020, 178, 114346. [Google Scholar] [CrossRef]
- Kondratenko, Y.; Zolotarev, A.A.; Ignatyev, I.; Ugolkov, V.; Kochina, T. Synthesis, crystal structure and properties of copper (II) complexes with triethanolamine and carboxylic acids (succinic, salicylic, cinnamic). Trans. Met. Chem. 2020, 45, 71–81. [Google Scholar] [CrossRef]
- Begum, R.; Rehman, M.; Shahid, K.; Haider, A.; Iqbal, M.; Tahir, M.N.; Ali, S. Synthesis, structural elucidation, DNA-binding and biological activity of nickel (II) mixed ligand carboxylate complexes. J. Mol. Struct. 2021, 1242, 130801. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. p-Coumaric acid kills bacteria through dual damage mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Li, J.; Zhao, N.; Xu, R.; Li, G.; Dong, H.; Wang, B.; Li, Z.; Fan, M.; Wei, X. Deciphering the antibacterial activity and mechanism of p-coumaric acid against Alicyclobacillus acidoterrestris and its application in apple juice. Int. J. Food Microbiol. 2022, 378, 109822. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Pavăl, N.-E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.-C.; Amăriucăi-Mantu, D.; Stoleru, V. Future Antimicrobials: Natural and Functionalized Phenolics. Molecules 2023, 28, 1114. [Google Scholar]
- Kalinowska, M.; Mazur, L.; Piekut, J.; Rzączyńska, Z.; Laderiere, B.; Lewandowski, W. Synthesis, crystal structure, spectroscopic properties, and antimicrobial studies of a zinc (II) complex of p-coumaric acid. J. Coord. Chem. 2013, 66, 334–344. [Google Scholar] [CrossRef]
- Kalinowska, M.; Piekut, J.; Bruss, A.; Follet, C.; Sienkiewicz-Gromiuk, J.; Swislocka, R.; Rzaczynska, Z.; Lewandowski, W. Spectroscopic (FT-IR, FT_raman, 1H, 13C NMR, UV/VIS), thermogravimetric and antimicrobial studies of Ca(II), Mn(II), Cu(II), Zn(II) and Cd(II) complexes of ferulic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 122, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Van Kerk, G.J.M.D.; Luijten, J.G.A. Investigations on organo-tin compounds. III. The biocidal properties of organo-tin compounds. J. Appl. Chem. 1954, 4, 314–319. [Google Scholar] [CrossRef]
- Debnath, P.; Debnath, P.; Roy, M.; Sieroń, L.; Maniukiewicz, W.; Aktar, T.; Maiti, D.; Novikov, A.S.; Misra, T.K. Novel Organotin(IV) Complexes of 2-[4-Hydroxy-3-((2-hydroxyethylimino)methyl)phenylazo]benzoic Acid: Synthesis, Structure, Noncovalent Interactions and In Vitro Antibacterial Activity. Crystals 2022, 12, 1582. [Google Scholar] [CrossRef]
- Dhingra, N.; Singh, J.B.; Singh, H.L. Synthesis, spectroscopy, and density functional theory of organotin and organosilicon complexes of bioactive ligands containing nitrogen, sulfur donor atoms as antimicrobial agents: In vitro and in silico studies. Dalton Trans. 2022, 51, 8821–8831. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Khan, B.A. Organotin (IV) carboxylates of substituted α-cinnamic acid, RnSn(OCOC(R2)=CHR1)4−n: Synthesis, spectroscopic characterization and biological evaluation. Heteroat. Chem. 2015, 26, 417–425. [Google Scholar] [CrossRef]
- Arciszewska, Z.; Gama, S.; Kalinowska, M.; Swiderski, G.; Swisłocka, R.; Gołebiewska, E.; Naumowicz, M.; Worobiczuk, M.; Cudowski, A.; Pietryczuk, A.; et al. Caffeic acid/Eu(III) complexes: Solution equilibrium studies,structure characterization and biological activity. Int. J. Mol. Sci. 2022, 23, 888. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Xu, D.-J.; Wu, J.-Y.; Chiang, M. Synthesis and crystal structure of a binuclear maleato copper (II) complex with phenantroline. J. Chem. Crystallogr. 2005, 35, 615–619. [Google Scholar] [CrossRef]
- Wiehl, L.; Schreurer, J.; Haussühl, E. Crystal structure of triaqua-1,10-phenantroline-nickel (II) maleate dihydrate, Ni(H2O)3(C12H8N2)(C4H2O4)∙2H2O. Z. Kristallogr. NCS 2008, 223, 82–84. [Google Scholar] [CrossRef]
- Savchenkov, A.; Grigoriev, M.; Udivankin, P.; Pushkin, D.; Serezhkina, L. Maleate ions as ligands in crystal structures of coordination compounds, including two uranyl complexes. Polyhedron 2017, 127, 331–336. [Google Scholar] [CrossRef]
- Ferrer-Luque, C.M.; Arias-Moliz, M.T.; Gonzalez-Rodriguez, M.P.; Baca, P. Antimicrobial activity of maleic acid and combinations of cetrimide with chelating agents against Enterococcus Faecalis biofilm. J. Endod. 2010, 36, 1673–1675. [Google Scholar] [CrossRef]
- Islam, S.; Hossain, B.; Reza, Y. Antimicrobial studies of mixed ligand transition metal complexes of maleic acid and heterocyclic amine bases. J. Med. Sci. 2003, 3, 289–293. [Google Scholar] [CrossRef]
- Devereux, M.; McCann, M.; Leo, V.; Geraghty, M.; McKee, V.; Wikaira, J. Synthesis and fungitoxic activity of manganese (II) complexes of fumaric acid: X-ray crystal structures of [Mn(fum)(bipy)(H2O)] and [Mn(Phen)2(H2O)2](fum)∙4H2O (fumH2=fumaric acid; bipy=2,2’-bipyridine; phen=1,10-phenantroline). Polyhedron 2000, 19, 1205–1211. [Google Scholar] [CrossRef]
- Li, Z.Y.; Shen, Q.-H.; Mao, Z.-W.; Tan, C.-P. Rising interest in the development of metal complexes in cancer immunotherapy. Chem. Asian J. 2022, 17, e202200270. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Paprocka, R.; Szadkowska, M.W.; Janciauskiene, S.; Kosmalski, T.; Kulik, M.; Basa, A.H. Latest developments in metal complexes as anticancer agents. Coord. Chem. Rev. 2022, 452, 214307. [Google Scholar] [CrossRef]
- Roberts, N.B.; Alqazzaz, A.; Hwang, J.R.; Qi, X.; Keegan, A.D.; Kim, A.J.; Winkles, J.A.; Woodworth, G.F. Oxaliplatin disrupts pathological features of glioma cells and associated macrophages independent of apoptosis induction. J. Neuro-Oncol. 2018, 140, 497–507. [Google Scholar] [CrossRef]
- Dilruba, S.; Kalayda, G.V. Platinum-based drugs: Past, present and future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, A.; Murthy, H.C.A.; Enyew, A.Z.; Revathi, R.; Perumal, R.V. Emerging Trends in Metal-based Anticancer Agents: Drug Design to Clinical Trials and their Mechanism of Action. Chem. Sel. 2023, 8, e202302113. [Google Scholar] [CrossRef]
- Zajac, J.; Novohradsky, V.; Markova, L.; Brabec, V.; Kasparkova, J. Platinum (IV) derivatives with cinnamate axial ligands as potent agents against both differentiated and tumorigenic cancer stem rhabdomyosarcoma cells. Angew. Chem. Int. Ed. 2020, 59, 3329–3335. [Google Scholar] [CrossRef]
- Ravera, M.; Gabano, E.; McGlinchey, M.J.; Osella, D. A view on multi-action Pt(IV) antitumor prodrugs. Inorg. Chim. Acta 2019, 492, 32–47. [Google Scholar] [CrossRef]
- Aher, S.; Zhu, J.; Bhagat, P.; Borse, L.; Liu, X. Pt(IV) Complexes in the Search for Novel Platinum Prodrugs with Promising Activity. Top. Curr. Chem. 2024, 382, 6. [Google Scholar] [CrossRef] [PubMed]
- Štarha, P.; Křikavová, R. Platinum(IV) and platinum(II) anticancer complexes with biologically active releasable ligands. Coord. Chem. Rev. 2024, 501, 215578. [Google Scholar] [CrossRef]
- Gibson, D. Multi-action Pt(IV) anticancer agents; do we understand how they work? J. Inorg. Biochem. 2019, 191, 77–84. [Google Scholar] [CrossRef]
- Gibson, D. Platinum(IV) anticancer agents; are we en route to the holy grail or to a dead end? J. Inorg. Biochem. 2021, 217, 111353. [Google Scholar] [CrossRef] [PubMed]
- Kostrhunova, H.; Zajac, J.; Markova, L.; Brabec, V.; Kasparkova, J. A multi-action PtIV conjugate with oleate and cinnamate ligands targets human epithelial growth factor receptor HER2 in aggressive breast cancer cells. Angew. Chem. Int. Ed. 2020, 59, 21157–21162. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Zhang, S.; Gan, Z.; Wang, X.; Zhao, X.; Zhu, Y.; Cao, M.; Wang, X.; Li, W. ctc-[Pt(NH3)2(cinnamate)(valproate)Cl2] is a highly potent and low-toxic triple action anticancer prodrug. Dalton Trans. 2021, 50, 11180. [Google Scholar] [CrossRef]
- Tan, M.-X.; Wang, Z.-F.; Qin, Q.-P.; Zou, B.-Q.; Liang, H. Oxoplatin complexes with rhein and ferulic acid ligands as platinum (IV) prodrugs with high anti-tumor activity. Dalton Trans. 2020, 49, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Pellerito, C.; Emanuele, S.; Ferrante, F.; Celesia, A.; Giuliano, M.; Fiore, T. Tributyltin(IV) ferulate, a novel synthetic ferulic acid derivative, induces autophagic cell death in colon cancer cells: From chemical synthesis to biochemical effects. J. Inorg. Biochem. 2020, 205, 110999. [Google Scholar] [CrossRef]
- Puszyńska-Tuszkanow, M.; Zierkiewicz, W.; Grabowski, T.; Daszkiewicz, M.; Maciejewska, G.; Adach, A.; Kucharska-Ziembicka, K.; Wietrzyk, J.; Filip-Psurska, B.; Cieślak-Golonka, M. Magnesium cinnamate complex, [Mg(cinn)2(H2O)2]n; structural, spectroscopic, thermal, biological and pharmacokinetical characteristics. J. Mol. Struct. 2017, 1134, 199–207. [Google Scholar] [CrossRef]
- Aragón-Muriel, A.; Camprubí-Robles, M.; González-Rey, E.; Salinas-Castillo, A.; Rodríguez-Diéguez, A.; Gómez-Ruiz, S.; Polo-Cerón, D. Dual investigation of lanthanide complexes with cinnamate and phenylacetate ligands: Study of the cytotoxic properties and the catalytic oxidation of styrene. Polyhedron 2014, 80, 117–128. [Google Scholar] [CrossRef]
- Sarwar, S.; Iftikhar, T.; Rauf, M.K.; Badshah, A.; Waseem, D.; Tahir, M.N.; Khan, K.M.; Khan, G.M. Synthesis of heteroleptic pentavalent antimonials bearing heterocyclic cinnamate moieties and their biological studies. Inorg. Chim. Acta 2018, 476, 12–19. [Google Scholar] [CrossRef]
- Gogoi, A.; Das, A.; Frontera, A.; Verma, A.; Bhattacharyya, M. Energetically significant unconventional π−π contacts involving fumarate in a novel coordination polymer of Zn(II): In-vitro anticancer evaluation and theoretical studies. Inorg. Chim. Acta 2019, 493, 1–13. [Google Scholar] [CrossRef]
- Hou, H.; Tang, D.; Zhang, L.; Zhao, D.; Xiao, H.; Li, B. NIR light triggered intracellular polymerization via nanoparticles containing acrylates prodrugs and azo-polymers for inhibiting cisplatin efflux for combined chemotherapy and immunotherapy. Nano Today 2023, 50, 101858. [Google Scholar] [CrossRef]
- Shahlaei, M.; Asl, S.M.; Derakhshani, A.; Kurek, L.; Karges, J.; Macgregor, R.; Saeidifar, M.; Kostova, I.; Saboury, A.A. Platinum-based drugs in cancer treatment: Expanding horizons and overcoming resistance. J. Mol. Struct. 2024, 1301, 137366. [Google Scholar] [CrossRef]
- Patil, V.M.; Gupta, S.P.; Masand, N.; Balasubramanian, K. Experimental and computational models to understand protein-ligand, metal-ligand and metal-DNA interactions pertinent to targeted cancer and other therapies. Eur. J. Med. Chem. Rep. 2024, 10, 100133. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, P.; Wang, C.; Sun, Y.; Gao, C. Recent updates in nanoscale delivery systems of platinum(IV) antitumor prodrugs. Coord. Chem. Rev. 2024, 508, 215774. [Google Scholar] [CrossRef]
- Maciel-Flores, C.E.; Lozano-Alvarez, J.A.; Bivián-Castro, E.Y. Recently Reported Biological Activities and Action Targets of Pt(II)- and Cu(II)-Based Complexes. Molecules 2024, 29, 1066. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Nag, S. Homo and heterometallic ruthenium and platinum complexes with multiple targets for therapeutic applications: A review. Rev. Inorg. Chem. 2024, 44, 1–23. [Google Scholar] [CrossRef]
- Casini, A.; Pöthig, A. Metals in Cancer Research: Beyond Platinum Metallodrugs. ACS Cent. Sci. 2024, 10, 242–250. [Google Scholar] [CrossRef]
- Abdolmaleki, S.; Aliabadi, A.; Khaksar, S. Riding the metal wave: A review of the latest developments in metal-based anticancer agents. Coord. Chem. Rev. 2024, 501, 215579. [Google Scholar] [CrossRef]
- Kumar, S.; Kumari, S.; Karan, R.; Kumar, A.; Rawal, R.K.; Gupta, P.K. Anticancer perspectives of vanadium complexes. Inorg. Chem. Commun. 2024, 161, 112014. [Google Scholar] [CrossRef]
- Wu, H.; Wang, H.; Wang, X.; Pan, G.; Shi, F.; Zhang, Y.; Bai, Y.; Kong, J. V-shaper ligand bis(2-benzimidazolylmethyl)amine containing three copper (II) ternary complexes: Synthesis, structure, DNA-binding properties and antioxidant activity. N. J. Chem. 2014, 38, 1052. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Kaur, P.; Kaur, P.; Singh, H.; El-Saber Batiha, G.; Verma, I. Exploring multifunctional antioxidants as potential agents for management of neurological disorders. Environ. Sci. Pollut. Res. 2022, 29, 24458–24477. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, H.; Xu, Y.; Aderinto, S.O.; Fan, X. Mono-, bi- and multinuclear silver complexes constructed from bis(benzimidazole)-2-oxapropane ligands and methacrylate: Synthesis, crystal structures, DNA-binding properties and antioxidant activities. RSC Adv. 2016, 6, 83697–83708. [Google Scholar] [CrossRef]
- Wu, H.; Wang, F.; Shi, F.; Yang, Z.; Zhang, H.; Peng, H. Three- and four-coordinate Ag(I) complexes of crotonate and bis(benzimidazole)-2-oxapropane ligands: Syntheses, crystal structures, DNA-binding studies and antioxidant activities. Trans. Met. Chem. 2015, 40, 555–564. [Google Scholar] [CrossRef]
- Wu, H.; Yang, Z.; Chen, C.; Zhang, J.; Zhang, H.; Peng, H.; Wang, F. Synthesis, crystal structures, antioxidant activities and DNA-binding studies of two silver (I) complexes with 1,3-bis(1-ethylbenzimidazol-2-yl)-2-thiapropane and α,β-unsaturated carboxylates. J. Coord. Chem. 2016, 69, 1076–1087. [Google Scholar] [CrossRef]
- Mao, S.; Shen, K.; Shi, X.; Xu, Y.; Wu, H. Two silver (I) complexes with bis(benzimidazole)-2-oxopropane ligands: Syntheses, crystal structures and DNA binding studies. Appl. Organomet. Chem. 2017, 31, e3747. [Google Scholar] [CrossRef]
- Tang, X.; Yang, Z.; Zhang, J.; Chen, C.; Wu, H. Synthesis, structure, antioxidation and DNA-binding studies of a zinc (II) complex with and cinnamate. Res. Chem. Intermed. 2015, 41, 4349–4360. [Google Scholar] [CrossRef]
- Hafeez, S.T.; Ali, S.; Tahir, M.N.; Iqbal, M.; Munawar, K.S. One-pot synthesis, structural elucidation, DNA binding and alkaline phosphatase inhibition studies on zinc (II) complexes with 4-nitrocinnamic acid and ethylene diamine. J. Coord. Chem. 2014, 67, 2479–2495. [Google Scholar] [CrossRef]
- Semenov, S.A.; Drobot, D.V.; Musatova, V.Y.; Pronin, A.S.; Pomogailo, A.D.; Dzhardimalieva, G.I.; Popenko, V.I. Synthesis and thermal conversions of unsaturated cobalt(II) dicarboxylates as precursors of metallopolymer nanocomposites. Russ. J. Inorg. Chem. 2015, 60, 897–905. [Google Scholar] [CrossRef]
- Shershnev, V.A.; Dzhardimalieva, G.I.; Kiryukhin, D.P.; Zhorin, V.A.; Pomogailo, A.D. Synthesis and reactivity of metal-containing monomers 72. Monomeric and polymeric metal acetylenecarboxylates and their nanocomposite products: Synthesis, structures, and properties. Russ. Chem. Bull. 2013, 62, 1649–1658. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasile Scaeteanu, G.; Badea, M.; Olar, R. Coordinative Compounds Based on Unsaturated Carboxylate with Versatile Biological Applications. Molecules 2024, 29, 2321. https://doi.org/10.3390/molecules29102321
Vasile Scaeteanu G, Badea M, Olar R. Coordinative Compounds Based on Unsaturated Carboxylate with Versatile Biological Applications. Molecules. 2024; 29(10):2321. https://doi.org/10.3390/molecules29102321
Chicago/Turabian StyleVasile Scaeteanu, Gina, Mihaela Badea, and Rodica Olar. 2024. "Coordinative Compounds Based on Unsaturated Carboxylate with Versatile Biological Applications" Molecules 29, no. 10: 2321. https://doi.org/10.3390/molecules29102321
APA StyleVasile Scaeteanu, G., Badea, M., & Olar, R. (2024). Coordinative Compounds Based on Unsaturated Carboxylate with Versatile Biological Applications. Molecules, 29(10), 2321. https://doi.org/10.3390/molecules29102321








