Photocatalytic Deposition of Au Nanoparticles on Ti3C2Tx MXene Substrates for Surface-Enhanced Raman Scattering
Abstract
1. Introduction
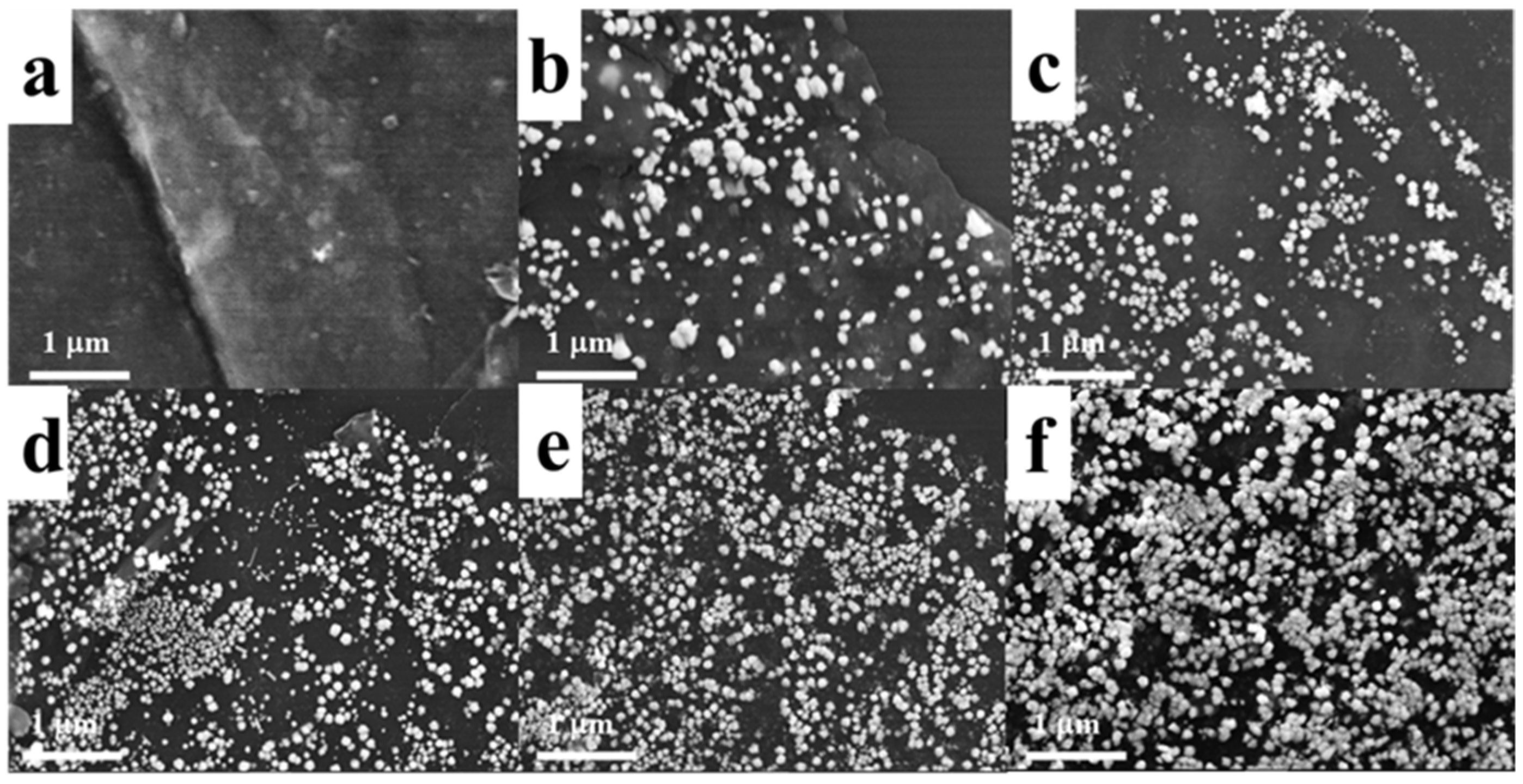
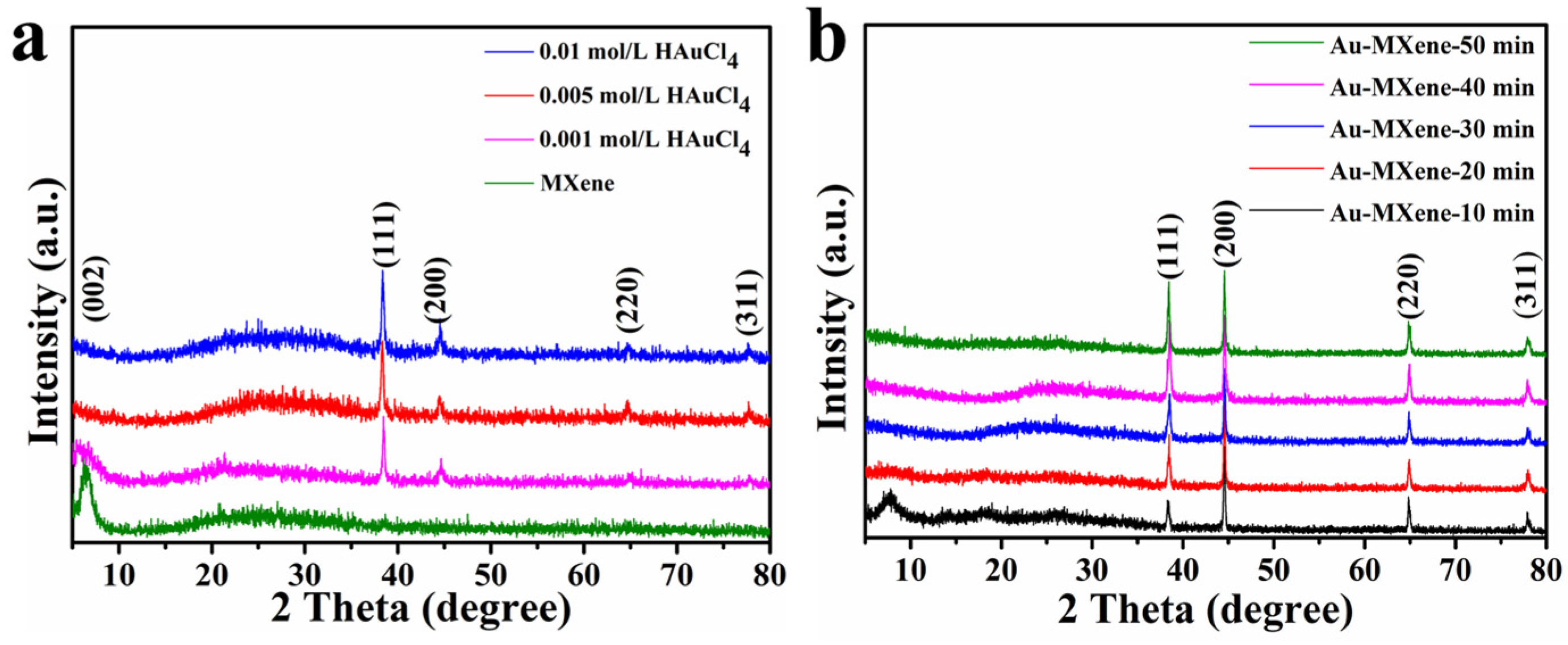
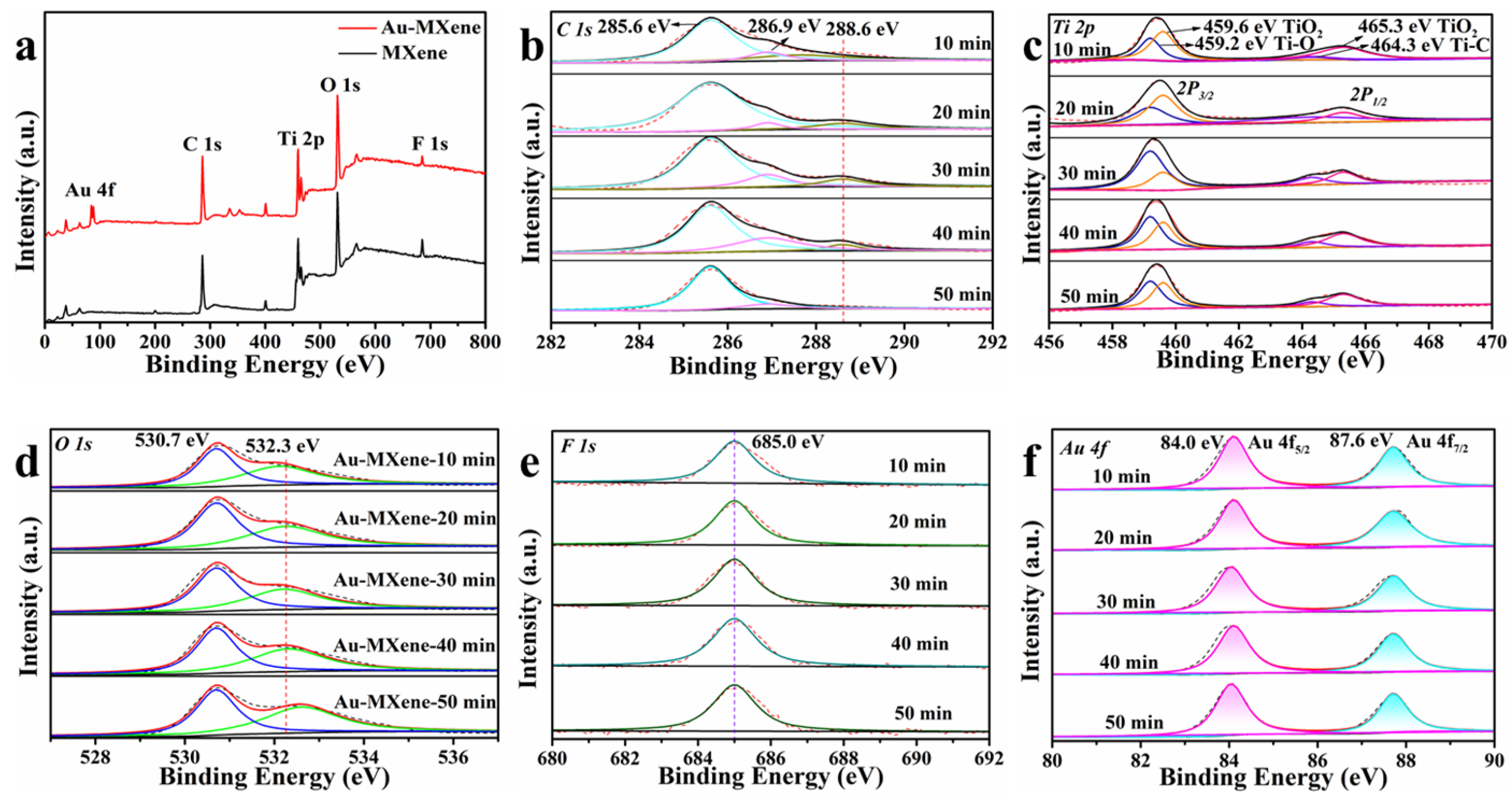
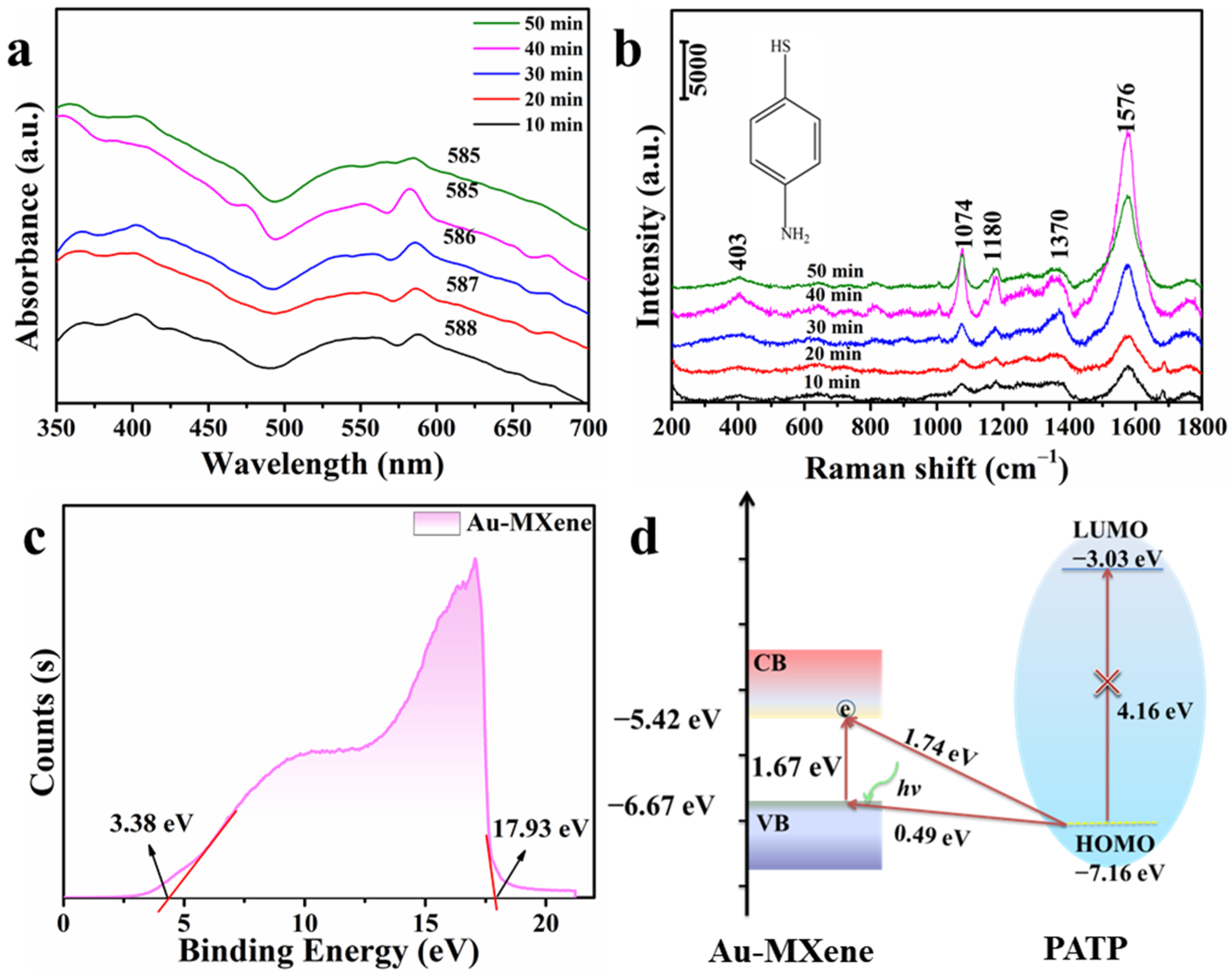
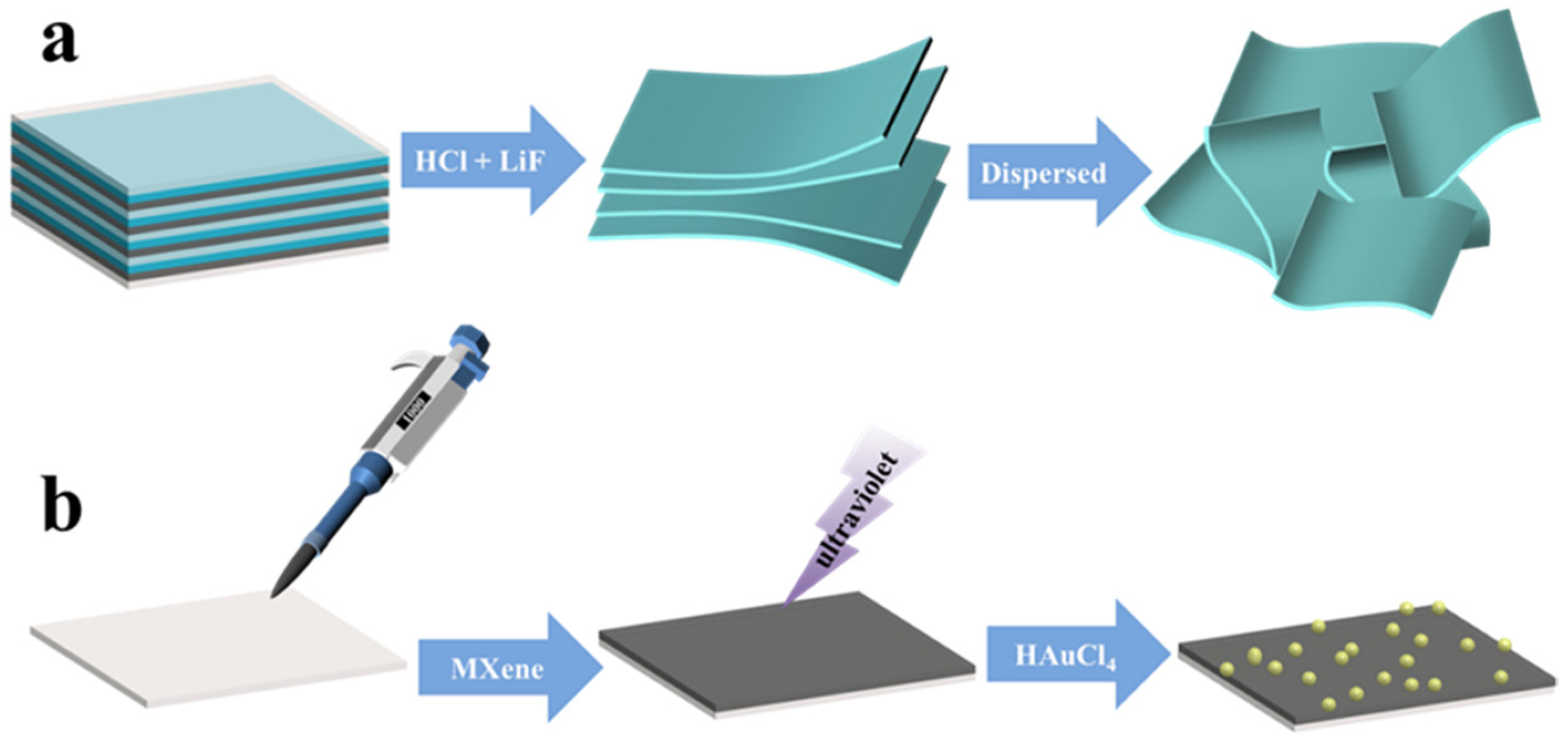
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of MXene (Ti3C2Tx) Nanosheets and Au–MXene Substrate
3.2. SERS Measurement
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.R.; Alvarez Puebla, A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Chio, W.I.; Xie, K.; Zhang, H.; Lan, Y.; Lee, Y. SERS biosensors based on cucurbituril-mediated nanoaggregates for waste water-based epidemiology. TrAC-Trend Anal. Chem. 2022, 146, 116485. [Google Scholar] [CrossRef]
- Ding, S.Y.; You, E.M.; Tian, Z.Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, Q.; Li, J.; Sun, L.; Xie, Y.; Ozaki, Y.; Ji, W. Macroscale TiO2 Microspherical Arrays with Multiple synergistic effect for highly sensitive surface-enhanced Raman scattering. Adv. Funct. Mater. 2024, 34, 2400523. [Google Scholar] [CrossRef]
- Ueno, K.; Misawa, H. Surface plasmon-enhanced photochemical reactions. J. Photochem. Photobiol. C 2013, 15, 31–52. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Peng, Y.; Cai, P.; Yang, L.; Liu, Y.; Zhu, L.; Zhang, Q.; Liu, J.; Huang, Z.; Yang, Y. Theoretical and experimental studies of Ti3C2 MXene for surface-enhanced Raman spectroscopy-based sensing. ACS Omega 2020, 5, 26486–26496. [Google Scholar] [CrossRef]
- Shahzad, F.; Alhabeb, M.; Hatter, C.B.; Anasori, B.; Hong, S.M.; Koo, C.M.; Gogotsi, Y. Electromagnetic interference shielding with 2D transition metal carbides (MXenes). Science 2016, 353, 1137–1140. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, M.; Zhang, W.; Zhen, X.; Pei, Z.; Xue, Q.; Zhi, P.; Shi, C. Ultrathin MXene micropattern-based field-effect transistor for probing neural activity. Adv. Mater. 2016, 28, 3333–3339. [Google Scholar] [CrossRef]
- Vahidmohammadi, A.; Rosen, Y.; Gogotsi, J. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, 6547. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, X.; Imazato, S.; Weir, M.D.; Reynolds, M.A.; Xu, H.H. A proteinrepellent and antibacterial nanocomposite for class-V restorations to inhibit periodontitis-related pathogens. Mater. Sci. Eng. C 2016, 67, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Feng, A.; Yu, Y.; Wang, Y.; Jiang, F.; Yu, Y.; Mi, L.; Song, L. Two-dimensional MXene Ti3C2 produced by exfoliation of Ti3AlC2. Mater. Des. 2017, 114, 161–166. [Google Scholar] [CrossRef]
- Zhu, J.; Ha, E.; Zhao, G.; Zhou, Y.; Huang, D.; Yue, G.; Hu, L.; Sun, N.; Wang, Y.; Lee, L.Y.S.; et al. Recent advance in MXenes: A promising 2D material for catalysis, sensor and chemical adsorption. Coord. Chem. Rev. 2017, 352, 306–327. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsim, Y.; Barsoum, W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Fu, X.; Yang, L.; Xing, S.; Wang, X.F. 2D titanium carbide nanosheets based fluorescent aptasensor for sensitive detection of thrombin. Talanta 2021, 228, 122219. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Gao, F.; Zou, J.; Liu, S.; Li, M.; Gao, Y.; Yu, Y.; Wang, X.; Lu, L. MXene@Ag based ratiometric electrochemical sensing strategy for effective detection of carbendazim in vegetable samples. Food Chem. 2021, 360, 130006. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.F.; Li, Y.X.; Dong, L.M.; Zheng, L.L.; Li, J.Z.; Shen, Y.; Xia, F. Photothermal induced partial leidenfrost superhydrophobic surface as ultrasensitive surface enhanced Raman scattering platform for the detection of neonicotinoid insecticides. Sens. Actuators B 2021, 348, 130728. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.; Duan, M.; Zhu, Y.; Tang, J. TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances. Biosens. Bioelectron. 2015, 74, 1022–1028. [Google Scholar] [CrossRef]
- Yu, M.; Liu, S.; Su, D.; Jiang, S.; Zhang, G.; Qina, Y.; Li, M.Y. Controllable MXene nano-sheet/Au nanostructure architetures for the ultra-sensitive molecule Raman detection. Nanoscale 2019, 11, 22230. [Google Scholar] [CrossRef]
- Li, C.; Wu, C.; Zhang, K.; Chen, M.; Wang, Y.; Shia, J.; Tanga, Z. The charge transfer effect on SERS in a gold decorated surface defect anatase nanosheet/methylene blue (MB) system. New J. Chem. 2021, 45, 19775. [Google Scholar] [CrossRef]
- Pourreza, N.; Rastegarzadeh, S.; Larki, A. Determination of fungicide carbendazim in water and soil samples using dispersive liquid-liquid microextraction and microvolume UV–vis spectrophotometry. Talanta 2015, 134, 24–29. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Zhang, C.; Zhao, Y.; Zhu, Y.; Zhang, J.; Bai, J.; Xiao, X. The effects of carbendazim on acute toxicity, development, and reproduction in caenorhabditis elegans. J. Food Qual. 2020, 2020, 8853537. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, S.; Wu, C.; An, X.; Cai, L.; Zhao, X. Embryonic exposure to carbendazim induces the transcription of genes related to apoptosis, immunotoxicity and endocrine disruption in zebrafish (daniorerio). Fish. Shellfish Immunol. 2014, 41, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Xuan, T.; Wu, Y.; Guo, X.; Ying, Y.; Wen, Y.; Yang, H. Metal-organic-frameworks-enforced surface enhanced Raman scattering chip for elevating detection sensitivity of carbendazim in seawater. Sensor Actuators B Chem. 2021, 326, 128852. [Google Scholar] [CrossRef]
- Ma, C.-H.; Zhang, J.; Hong, Y.-C.; Wang, Y.-R.; Chen, X. Determination of carbendazim in tea using surface enhanced Raman spectroscopy. Chin. Chem. Lett. 2015, 26, 1455–1459. [Google Scholar] [CrossRef]
- Luong, H.N.; Nguyen, N.M.; Nguyen, L.N.T.; Tran, C.K.; Nguyen, T.T.; Duy, L.T.; Nguyen, N.P.; Huynh, T.M.H.; Tran, T.T.; Phan, B.T.; et al. Detection of carbendazim by utilizing multi-shaped Ag NPs decorated ZnO NRs on patterned stretchable substrate through surface-enhanced Raman scattering effect. Sens. Actuators A Phys. 2022, 346, 113816. [Google Scholar] [CrossRef]
- Zhi, S.; Shi, J.; Liang, A.; Jiang, Z. MXene nanosheet decorated gold nanocluster catalytic amplification–aptamer SERS quantitative assay platform for isocarbophos. Talanta 2023, 251, 123771. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jiao, T.; Xing, R. Fabrication of hierarchical MXene-based Au NPs-containing core-shell nanocomposites for high efficient catalysts. Green Energy Environ. 2018, 3, 147–155. [Google Scholar] [CrossRef]
- Li, K.; Jiao, T.; Xing, R.; Zou, G.; Zhou, J.; Zhang, L.; Peng, Q. Fabrication of tunable hierarchical MXene@AuNPs nanocompo-sites constructed by self-reduction reactions with enhanced catalytic performances. Sci. China Mater. 2018, 61, 728–736. [Google Scholar] [CrossRef]
- Tang, H.; Guo, R.; Jiang, M.; Zhang, Y.; Lai, X.; Cui, C.; Xiao, H.; Jiang, S.; Qin, E.; Ren, Q. Construction of Ti3C2 MXene@C@SnS with layered rock stratum structure for high-performance lithium storage. J. Power Source 2020, 462, 228152. [Google Scholar] [CrossRef]
- Klaas, J.; Schulz-Ekloff, G.; Jaeger, N.I. UV-Visible diffuse reflectance spectroscopy of zeolite-hosted mononuclear titanium oxide species. J. Phys. Chem. B 1997, 101, 1305–1311. [Google Scholar] [CrossRef]
- Ji, R.; Sun, W.D.; Chu, Y. One-step hydrothermal synthesis of Ag/Cu2O heterogeneous nanostructures over Cu foil and their SERS applications. RSC Adv. 2014, 4, 6055–6059. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Duyne, R.P.V. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef]
- Liu, X.J.; Cao, L.Y.; Song, W.; Ai, K.L.; Lu, L.H. Functionalizing metal nanostructured film with graphene oxide for ultrasensitive detection of aromatic molecules by surface-enhanced Raman spectroscopy. ACS Appl. Mater. Interfaces 2011, 3, 2944–2952. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, W.N.; Lei, H.X.; Li, B.J. Ag nanowire/nanoparticle-decorated MoS2 monolayers for surface-enhanced Raman scattering applications. Nano Res. 2018, 11, 2181–2189. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, W.; Xing, J.; Ganeev, R.A.; Xin, W.; Cheng, J.; Guo, C. Charge transfer effects on resonance-enhanced Raman scattering for molecules adsorbed on single crystalline perovskite. ACS Photonics 2018, 5, 1619–1627. [Google Scholar] [CrossRef]
- Guo, L.; Mao, Z.; Ma, C.; Wu, J.; Zhu, L.; Zhao, B.; Jung, Y.M. Charge transfer in 4-mercaptobenzoic acid-stabilized Au nanorod@Cu2O nanostructures: Implications for photocatalysis and photoelectric devices. ACS Appl. Nano Mater. 2021, 4, 381–388. [Google Scholar] [CrossRef]
- Sun, H.; Yao, M.; Song, Y.; Zhu, L.; Dong, J.; Liu, R.; Li, P.; Zhao, B.; Liu, B. Pressure-induced SERS enhancement in a MoS2/Au/R6G system by a two-step charge transfer process. Nanoscale 2019, 11, 21493–21501. [Google Scholar] [CrossRef]
- Guo, S.; Jin, S.; Park, E.; Chen, L.; Mao, Z.; Jung, Y.M. Photo-induced charge transfer enhancement for SERS in a SiO2−Ag−reduced graphene oxide system. ACS Appl. Mater. Interfaces 2021, 13, 5699–5705. [Google Scholar] [CrossRef]
- Sun, Z.H.; Wang, C.X.; Yang, J.X.; Zhao, B.; Lombardi, J.R. Nanoparticle metal semiconductor charge transfer in ZnO/PATP/Ag assemblies by surface-enhanced Raman spectroscopy. J. Phys. Chem. C 2008, 112, 6093–6098. [Google Scholar] [CrossRef]
- Furini, L.N.; Sanchez-Cortes, S.; Lopez-Tocon, I.; Otero, J.C.; Aroca, R.F.; Constantino, C.J.L. Detection and quantitative analysis of carbendazim herbicide on Ag nanoparticles via surface-enhanced Raman scattering. J. Raman Spectrosc. 2015, 46, 1095–1101. [Google Scholar] [CrossRef]
- Wang, K.; Sun, D.W.; Pu, H. Stable, fexible, and high performance SERS chip enabled by a ternary flm-packaged plasmonic nanoparticle array. ACS Appl. Mater. Interfaces 2019, 11, 29177–29186. [Google Scholar] [CrossRef] [PubMed]
- Riswana, N.; Wang, T.; Chang, Y. Photochemical synthesis of Ag/Au/AgCl heterostructure from Ag nanowires as a reusable SERS substrate for ultrasensitive detection of analgesics and antibiotics. Chem. Eng. J. 2021, 423, 130191. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Yang, L.; Liu, Y.; Chen, L. Photocatalytic Deposition of Au Nanoparticles on Ti3C2Tx MXene Substrates for Surface-Enhanced Raman Scattering. Molecules 2024, 29, 2383. https://doi.org/10.3390/molecules29102383
Yang Z, Yang L, Liu Y, Chen L. Photocatalytic Deposition of Au Nanoparticles on Ti3C2Tx MXene Substrates for Surface-Enhanced Raman Scattering. Molecules. 2024; 29(10):2383. https://doi.org/10.3390/molecules29102383
Chicago/Turabian StyleYang, Zhi, Lu Yang, Yucun Liu, and Lei Chen. 2024. "Photocatalytic Deposition of Au Nanoparticles on Ti3C2Tx MXene Substrates for Surface-Enhanced Raman Scattering" Molecules 29, no. 10: 2383. https://doi.org/10.3390/molecules29102383
APA StyleYang, Z., Yang, L., Liu, Y., & Chen, L. (2024). Photocatalytic Deposition of Au Nanoparticles on Ti3C2Tx MXene Substrates for Surface-Enhanced Raman Scattering. Molecules, 29(10), 2383. https://doi.org/10.3390/molecules29102383





