Study on Quality Characteristic of Chebulae Fructus and Its Adulterants and Degradation Pathway of Hydrolyzable Tannins
Abstract
:1. Introduction
2. Results and Discussion
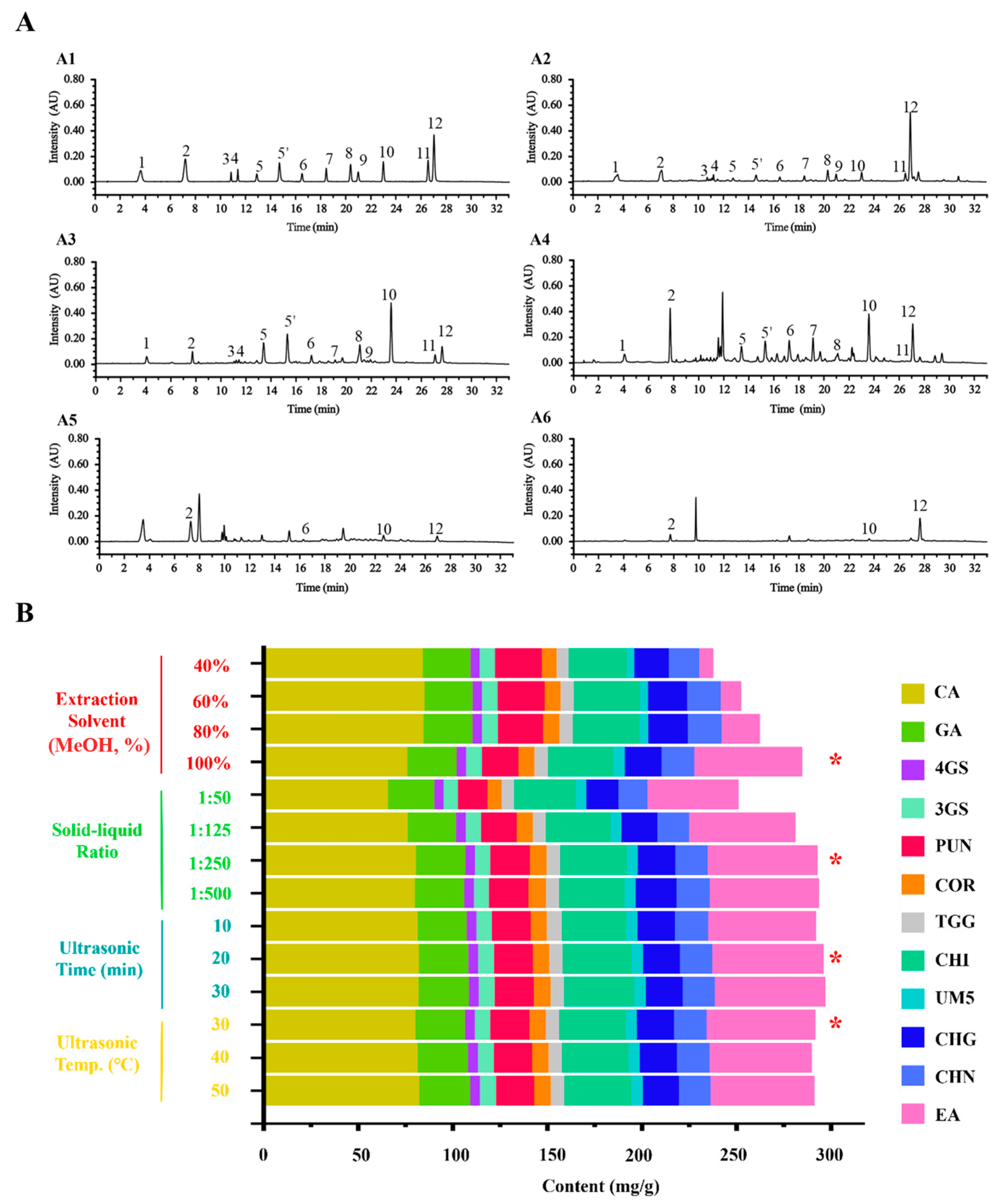
2.1. Optimization and Methodological Validation of the Quantitative Analysis for the Focused Compounds
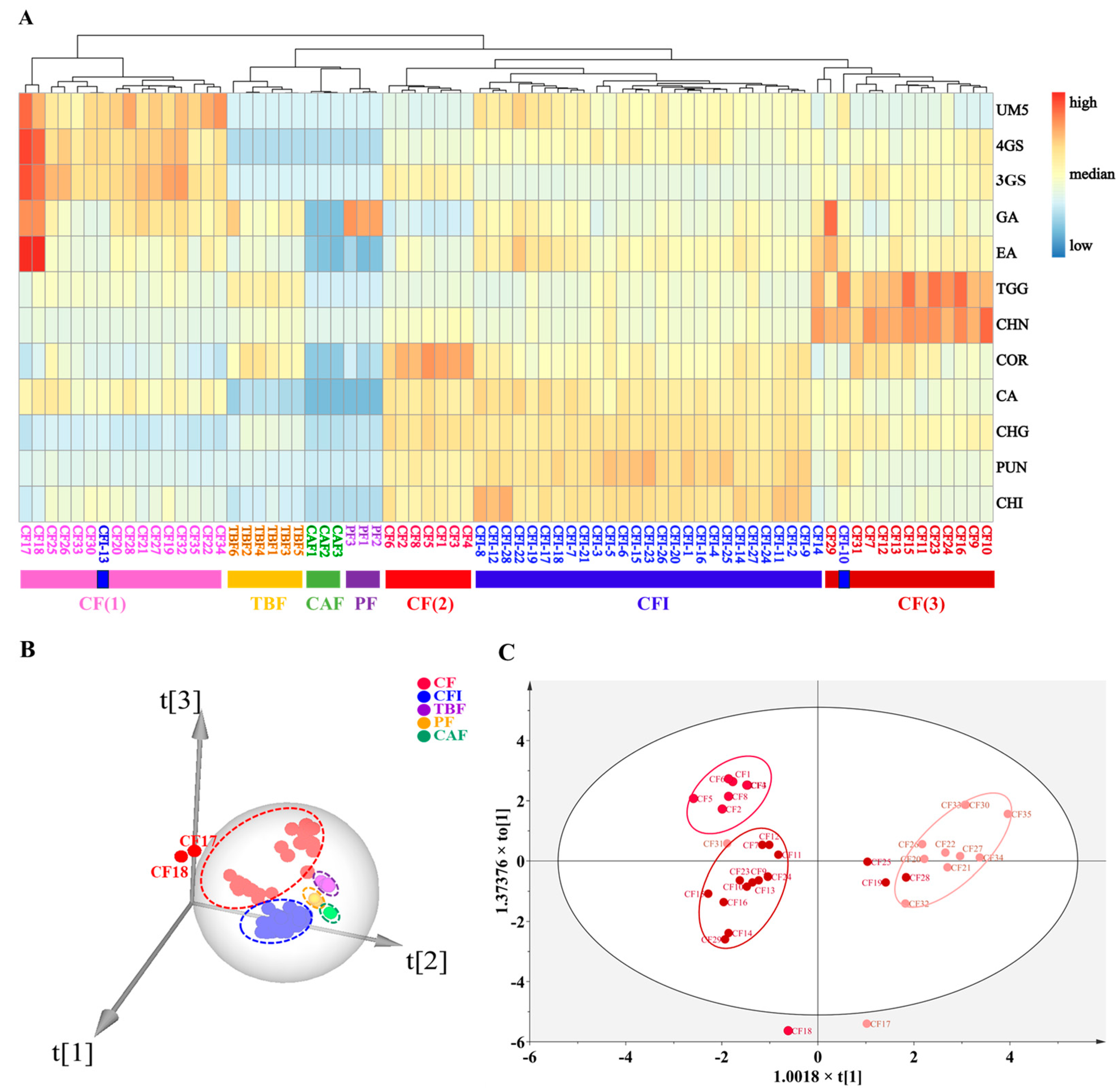
2.2. Quantitative Determination of the Twelve Observed Constituents in CF, CFI, TBF, PF, and CAF
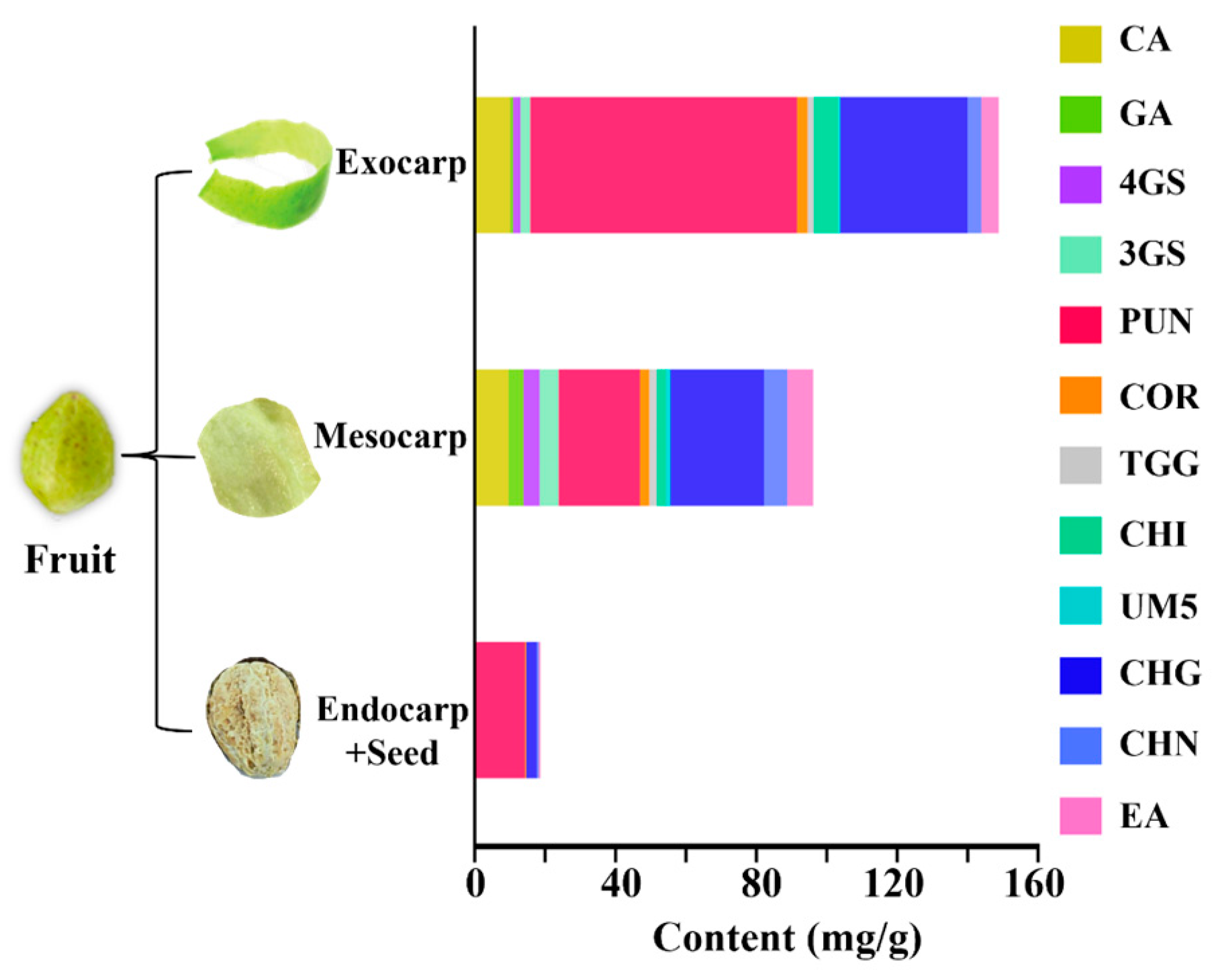
2.3. Distribution of the Observed Constituents in Different Medicinal Parts of CF
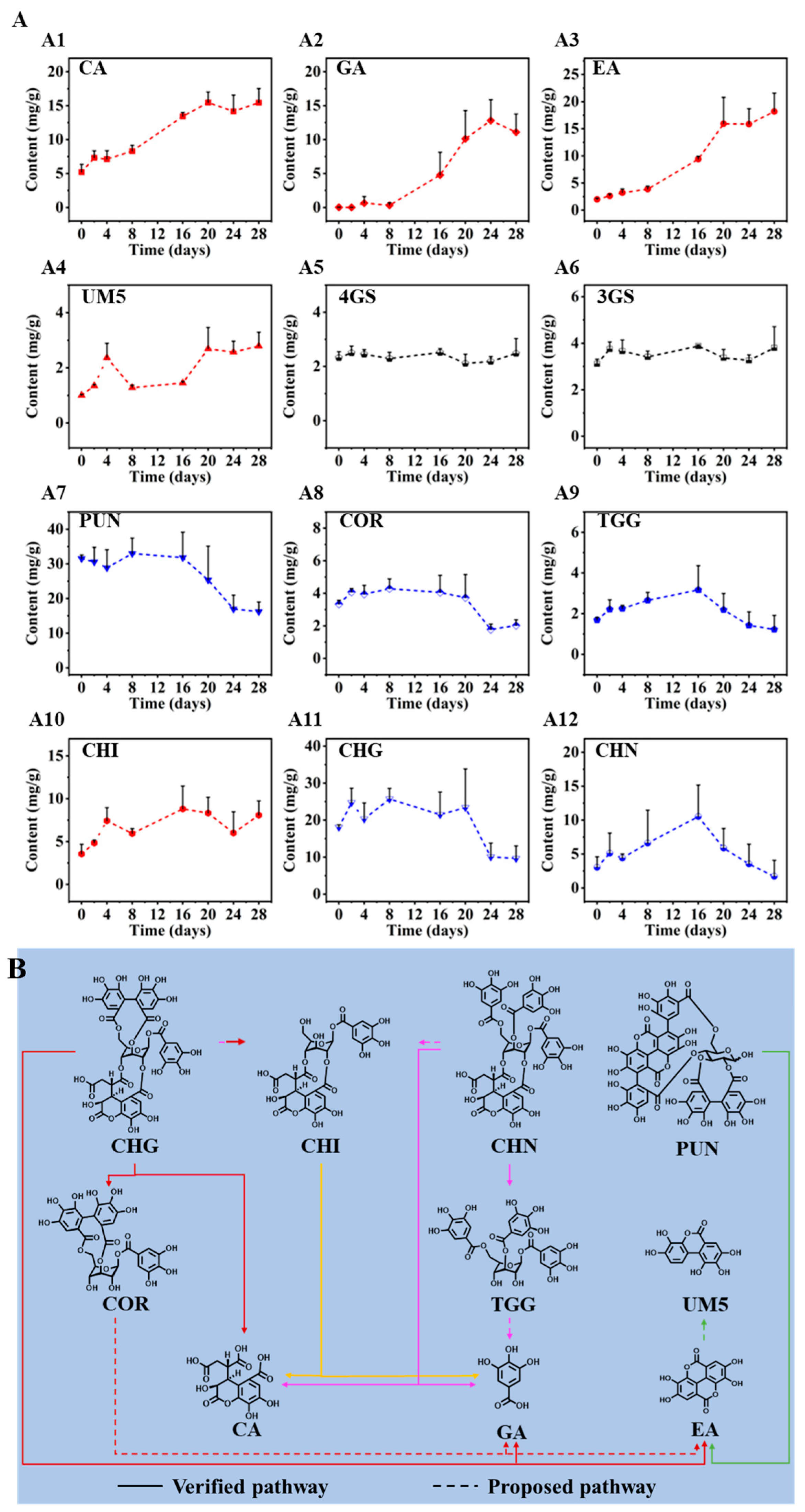
2.4. Discovery on Degradation of the Observed Hydrolyzable Tannins during the Drying Process of CF
2.5. Verification of the Degradation Pathways of Hydrolyzable Tannins from CF at High Temperature
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of Standard and Sample Solution
3.2.1. Preparation of Standard Solution
3.2.2. Sample Preparation
3.3. UPLC-PDA Analysis
3.4. Methodological Validation
3.5. UHPLC-QTOF-MS Analysis
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CA | Chebulic acid |
| CAF | Canarii Fructus |
| CF | Chebulae Fructus |
| CFI | Chebulae Fructus Immaturus |
| CHG | Chebulagic acid |
| CHI | Chebulanin acid |
| CHN | Chebulinic acid |
| COR | Corilagin |
| EA | Ellagic acid |
| GA | Gallic acid |
| HCA | Hierarchical cluster analysis |
| HTs | Hydrolyzable tannins |
| PCA | Principal component analysis |
| PF | Phyllanthi Fructus |
| PUN | Punicalagin |
| PTCs | Phenolcarboxylic and tannic constituents |
| TBF | Terminaliae Belliricae Fructus |
| TGG | 1,3,6-tri-O-galloyl-β-d-glucose |
| UPLC-PDA | Ultra-performance liquid chromatography with a photo-diode array detector |
| UM5 | Urolithin M5 |
| 3GS | 3-O-galloyl-(−)-shikimic acid |
| 4GS | 4-O-galloyl-(−)-shikimic acid |
References
- National Commission of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2020.
- Zhang, X.R.; Qiao, Y.J.; Zhu, H.T.; Kong, Q.H.; Wang, D.; Yang, C.R.; Zhang, Y.J. Multiple in vitro biological effects of phenolic compounds from Terminalia chebula var. tomentella. J. Ethnopharmacol. 2021, 275, 114135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Duan, Z.; Wang, Y.; Wang, M.; Liu, Y.; Wang, X.; Li, H. Protective effect of Terminalia chebula Retz. extract against Aβ aggregation and Aβ-induced toxicity in Caenorhabditis elegans. J. Ethnopharmacol. 2021, 268, 113640. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Bhattacharyya, S.K.; Chattopadhyay, R.R. The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac. J. Trop. Biomed. 2013, 3, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.K.; Lee, D.; Kim, H.P.; Baek, S.H. Structure analysis and antioxidant activities of an amylopectin-type polysaccharide isolated from dried fruits of Terminalia chebula. Carbohydr. Polym. 2019, 211, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Paul Khurana, S.M. Phytochemistry and medicinal value of harad (Terminalia chebula Retz.) the ‘King of Medicinal Plants’. Pharma Chem 2018, 10, 186–195. [Google Scholar]
- Lin, K.; Zhou, M.; Leng, C.; Tao, X.; Zhou, R.; Li, Y.; Sun, B.; Shu, X.; Liu, W. Neuroprotective effect of polyphenol extracts from Terminalia chebula Retz. against cerebral ischemia-reperfusion injury. Molecules 2022, 27, 6449. [Google Scholar] [CrossRef] [PubMed]
- Ankegowda, V.M.; Kollur, S.P.; Prasad, S.K.; Pradeep, S.; Dhramashekara, C.; Jain, A.S.; Prasad, A.; Srinivasa, C.; Sridhara Setty, P.B.; Gopinath, S.M.; et al. Phyto-Mediated synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of its cytotoxic and antimicrobial potential. Molecules 2020, 25, 5042. [Google Scholar] [CrossRef] [PubMed]
- Hassan Bulbul, M.R.; Uddin Chowdhury, M.N.; Naima, T.A.; Sami, S.A.; Imtiaj, M.S.; Huda, N.; Uddin, M.G. A comprehensive review on the diverse pharmacological perspectives of Terminalia chebula Retz. Heliyon 2022, 8, e10220. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Ito, H. Tannins of constant structure in medicinal and food plants—Hydrolyzable tannins and polyphenols related to tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Adhikari-Devkota, A.; Dirar, A.I.; Hassan, M.M.; Adhikari, A.; Belwal, T.; Devkota, H.P. Fruits of Terminalia chebula Retz.: A review on traditional uses, bioactive chemical constituents and pharmacological activities. Phytother. Res. 2020, 34, 2518–2533. [Google Scholar] [CrossRef]
- Peterson, C.T.; Denniston, K.; Chopra, D. Therapeutic uses of triphala in Ayurvedic medicine. J. Altern. Complement. Med. 2017, 23, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kumar, R.; Bhattacharyya, P.; Bishayee, A.; Pandey, A.K. Terminalia bellirica (Gaertn.) roxb. (Bahera) in health and disease: A systematic and comprehensive review. Phytomedicine 2020, 77, 153278. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Sharma, N.; Oladeji, O.S.; Sourirajan, A.; Dev, K.; Zengin, G.; El-Shazly, M.; Kumar, V. Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114570. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Peng, C.; Chen, P.; Zhang, W.; Jiang, C.; Sang, S.; Zhu, W.; Yuan, Y.; Hong, Y.; Yao, M. In-vitro anti-helicobacter pylori activity and preliminary mechanism of action of Canarium album Raeusch. fruit extracts. J. Ethnopharmacol. 2022, 283, 114578. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, B.; Liu, J.; Qiu, F.; Diao, Y.; Lei, Y.; Liu, J.; Zhang, W. Phenolic acids from Fructus Chebulae Immaturus alleviate intestinal ischemia-reperfusion injury in mice through the PPARα/NF-κB pathway. Molecules 2022, 27, 5227. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.P.; Babu, K.B.; Perumal, S.S.; Rajendran, D. Repeated oral dose toxicity study on hydrolysable tannin rich fraction isolated from fruit pericarps of Terminalia chebula Retz in Wistar albino rats. Regul. Toxicol. Pharmacol. 2018, 92, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Juang, L.J.; Sheu, S.J.; Lin, T.C. Determination of hydrolyzable tannins in the fruit of Terminalia chebula Retz. by high-performance liquid chromatography and capillary electrophoresis. J. Sep. Sci. 2004, 27, 718–724. [Google Scholar] [CrossRef]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Harvey, I. Analysis of hydrolysable tannins. Anim. Feed Sci. Technol. 2001, 91, 3–20. [Google Scholar] [CrossRef]
- Bidikar, C.M.; Hurkadale, P.J.; Nandanwadkar, S.M.; Hegde, H.V. A validated spectro densitometric regulatory compliant USP-HP-TLC protocol for quantification of polyphenols and antioxidants from polyherbal formulations containing Terminalia species. J. Chromatography. B 2022, 1207, 123379. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.K.; Zahiruddin, S.; Newton, K.G.; Sawant, L.; Mitra, R.; Kumar Rai, R.; Ahmad, S. Validation and standardization of gallic acid and ellagic acid in Quercus infectoria, Terminalia chebula, and Pistacia integerrima. J. AOAC Int. 2023, 106, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhan, S.; Zhang, P.; Jia, C.; Zhu, Q.; Dai, Q.; Yu, M.; Cheng, L.; Xiong, L.; Sun, F.; et al. Simultaneous quantitative analysis and in vitro anti-arthritic effects of five polyphenols from Terminalia chebula. Front. Physiol. 2023, 14, 1138947. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Miao, X.; Ge, M.; Wu, M.; Bai, Y.; Lv, Z.; Ogaji, O.; Chang, Y.; Ouyang, H.; He, J. Comparative analysis of chemical components in fruits of Chebulae Fructus and its pulp based on chromatographic technology coupled with multivariate chemometric methods. J. Pharm. Biomed. Anal. 2023, 236, 115735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.H.; Li, Y.J.; Guo, Z.H.; Chen, J. Screening of acetylcholinesterase inhibitory and antioxidant active compounds from Terminalia chebula fruits by spectrum-effect relationship and liquid chromatography-mass spectrometry analysis. J. Sep. Sci. 2022, 45, 3412–3421. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jiang, S.; Liu, M.; Tian, S. Simultaneous process optimization of ultrasound-assisted extraction of polyphenols and ellagic acid from pomegranate (Punica granatum L.) flowers and its biological activities. Ultrason. Sonochemistry 2021, 80, 105833. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.F.M.; Aquino-Martins, V.G.Q.; Silva, A.P.D.; Oliveira Rocha, H.A.; Scortecci, K.C. Biological and pharmacological aspects of tannins and potential biotechnological applications. Food Chem. 2023, 414, 135645. [Google Scholar] [CrossRef] [PubMed]
- Rohman, A.; Wijayanti, T.; Windarsih, A.; Riyanto, S. The authentication of java turmeric (Curcuma xanthorrhiza) using thin layer chromatography and 1H-NMR based-metabolite fingerprinting coupled with multivariate analysis. Molecules 2020, 25, 3928. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Tian, Y.; Yi, Y.; Li, K.; Qiao, X.; Ye, M. Comparative bioactivity evaluation and chemical profiling of different parts of the medicinal plant Glycyrrhiza uralensis. J. Pharm. Biomed. Anal. 2022, 215, 114793. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Zhang, G.; Gao, J.; Yan, Y.G.; Wei, Y.; Chen, Y. Dynamic changes of tannins during fruit development in Cornus officinalis. Acta Bot. Boreali-Occident. Sin. 2021, 41, 1834–1842. [Google Scholar] [CrossRef]
- Venusova, E.; Kolesarova, A.; Horky, P.; Slama, P. Physiological and Immune Functions of Punicalagin. Nutrients 2021, 13, 2150. [Google Scholar] [CrossRef] [PubMed]
- Jukov, A.; Ajala, O.S.; Gao, J.; Ma, C.M. Quantification of six bioactive constituents in the three parts of Terminalia Fruit by liquid chromatography-quadropole mass spectrometry. J. Inn. Mong. Univ. 2016, 47, 90–95. [Google Scholar] [CrossRef]
- Li, Q.; Wang, F.T.; Zhang, L. The impact of kernel removal of Terminalia chebula Retz. on the therapeutic efficacy of the pharmacopoeia variety of jiebaiwan. West. J. Tradit. Chin. Med. 2006, 9, 45–46. [Google Scholar]
- Wang, X.; Xu, J.; Zhang, L.H.; Yang, W.; Yu, H.; Zhang, M.; Wang, Y.; Wu, H.H. Global profiling of the antioxidant constituents in Chebulae Fructus based on an integrative strategy of UHPLC/IM-QTOF-MS, MS/MS molecular networking, and spectrum-effect correlation. Antioxidants 2023, 12, 2093. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. In vitro bioaccessibility, human gut microbiota metabolites and hepatoprotective potential of chebulic ellagitannins: A case of padma hepaten® formulation. Nutrients 2015, 7, 8456–8477. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Z.; Wei, X.C.; Lin, J.Z.; Tan, P.; Fan, S.H.; Han, L.; Zhang, D.K. Tannin transformation during the reflux process of Phyllanthus emblica L. and discussion of the content determination method in Chinese Pharmacopoeia. Chin. Pharm. 2019, 54, 581–587. [Google Scholar] [CrossRef]
- Gao, H.; Huang, Y.N.; Xu, P.Y.; Kawabata, J. Inhibitory effect on α-glucosidase by the fruits of Terminalia chebula Retz. Food Chem. 2007, 105, 628–634. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, H.W.; Yang, H.; Sung, S.H. Hydrolyzable tannins from the fruits of Terminalia chebula Retz and their α-glucosidase inhibitory activities. Phytochemistry 2017, 137, 109–116. [Google Scholar] [CrossRef]

| Compounds | Linear Regression | LOD | LOQ | Precision (RSD, %) | Repeatability | Stability | |||
|---|---|---|---|---|---|---|---|---|---|
| Regression Equation | r2 | Linear Range (μg/mL) | (μg/mL) | (μg/mL) | Intra-Day | Inter-Day | (n = 6, RSD, %) | (n = 7, RSD, %) | |
| CA | y = 5584.6x + 6891.1 | 0.9999 | 7.823–500.7 | 0.9779 | 1.956 | 0.80 | 0.80 | 0.7 | 0.9 |
| GA | y = 23,460x + 20342 | 0.9999 | 3.294–210.8 | 0.4118 | 0.8236 | 1.00 | 0.40 | 1.1 | 0.8 |
| 4GS | y = 12,053x + 2204.6 | 0.9999 | 0.7984–51.10 | 0.0998 | 0.3992 | 0.80 | 0.40 | 1.2 | 0.7 |
| 3GS | y = 13,392x + 4111.8 | 0.9999 | 1.039–66.48 | 0.1298 | 0.5194 | 1.00 | 0.40 | 2.9 | 1.0 |
| # PUN | y = 15,351x + 8207.5 | 0.9999 | 3.795–242.9 | 0.4744 | 0.9488 | 0.60 | 0.70 | 1.4 | 1.2 |
| COR | y = 13,855x + 3831.5 | 0.9999 | 1.024–65.52 | 0.1228 | 0.2559 | 1.70 | 0.90 | 1.4 | 1.2 |
| TGG | y = 19,369x + 6044.1 | 0.9998 | 1.068–68.32 | 0.1334 | 0.2669 | 2.70 | 0.60 | 3.0 | 0.6 |
| CHI | y = 9103.7x + 7343.6 | 0.9999 | 3.606–230.8 | 0.4507 | 0.9014 | 0.50 | 0.30 | 1.4 | 0.5 |
| UM5 | y = 32,378x + 3274.4 | 0.9999 | 0.5578–35.70 | 0.1395 | 0.2789 | 1.00 | 1.00 | 1.2 | 0.9 |
| CHG | y = 12,668x + 8627.8 | 0.9999 | 2.869–183.6 | 0.3586 | 0.7172 | 0.50 | 0.60 | 0.7 | 0.2 |
| CHN | y = 13,309x + 10,763 | 0.9999 | 2.913–186.4 | 0.3641 | 0.7281 | 1.70 | 0.70 | 2.7 | 0.3 |
| EA | y = 35,524x + 16,314 | 0.9999 | 2.739–175.3 | 0.3424 | 0.6848 | 2.40 | 0.70 | 0.9 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wang, X.; Yu, H.; Chai, X.; Zhang, M.; Wu, H.-H.; Wang, Y. Study on Quality Characteristic of Chebulae Fructus and Its Adulterants and Degradation Pathway of Hydrolyzable Tannins. Molecules 2024, 29, 2399. https://doi.org/10.3390/molecules29102399
Xu J, Wang X, Yu H, Chai X, Zhang M, Wu H-H, Wang Y. Study on Quality Characteristic of Chebulae Fructus and Its Adulterants and Degradation Pathway of Hydrolyzable Tannins. Molecules. 2024; 29(10):2399. https://doi.org/10.3390/molecules29102399
Chicago/Turabian StyleXu, Jian, Xiangdong Wang, Huijuan Yu, Xin Chai, Min Zhang, Hong-Hua Wu, and Yuefei Wang. 2024. "Study on Quality Characteristic of Chebulae Fructus and Its Adulterants and Degradation Pathway of Hydrolyzable Tannins" Molecules 29, no. 10: 2399. https://doi.org/10.3390/molecules29102399
APA StyleXu, J., Wang, X., Yu, H., Chai, X., Zhang, M., Wu, H.-H., & Wang, Y. (2024). Study on Quality Characteristic of Chebulae Fructus and Its Adulterants and Degradation Pathway of Hydrolyzable Tannins. Molecules, 29(10), 2399. https://doi.org/10.3390/molecules29102399






