Application of High-Z Nanoparticles to Enhance Current Radiotherapy Treatment
Abstract
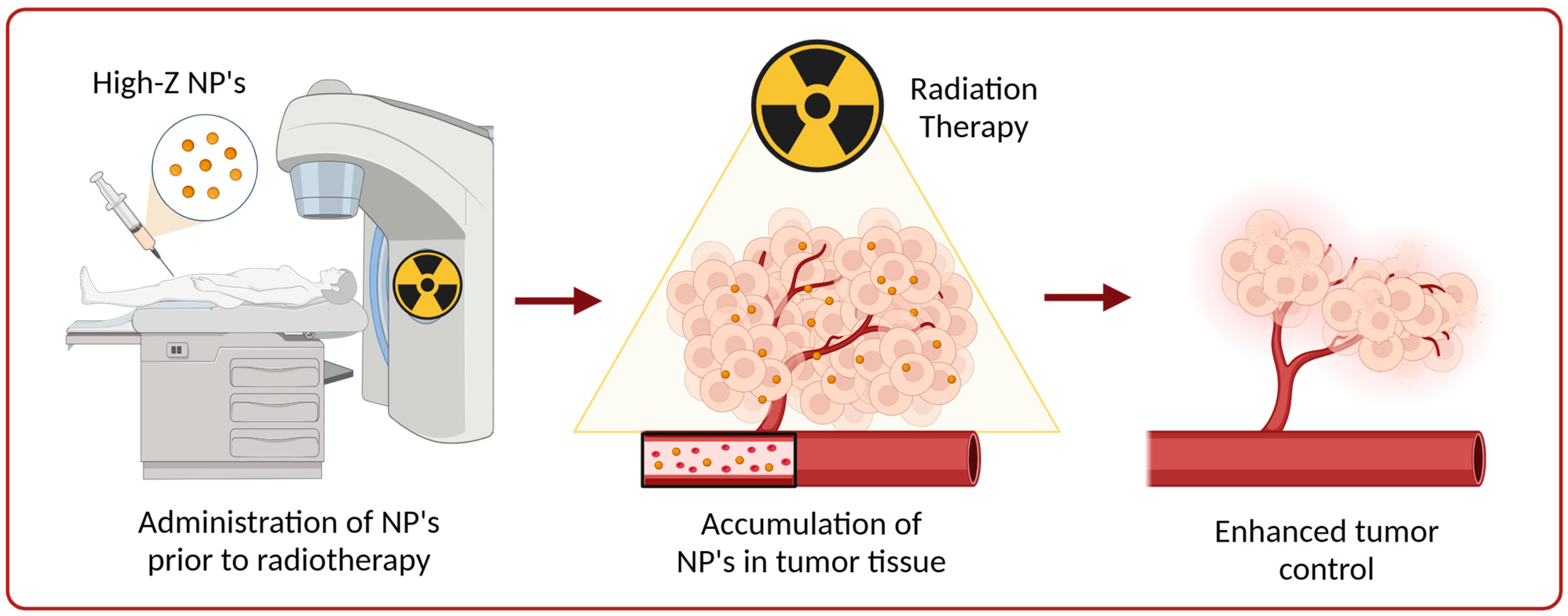
:1. Introduction
2. Tumor-Targeting Capabilities of Nanoparticles
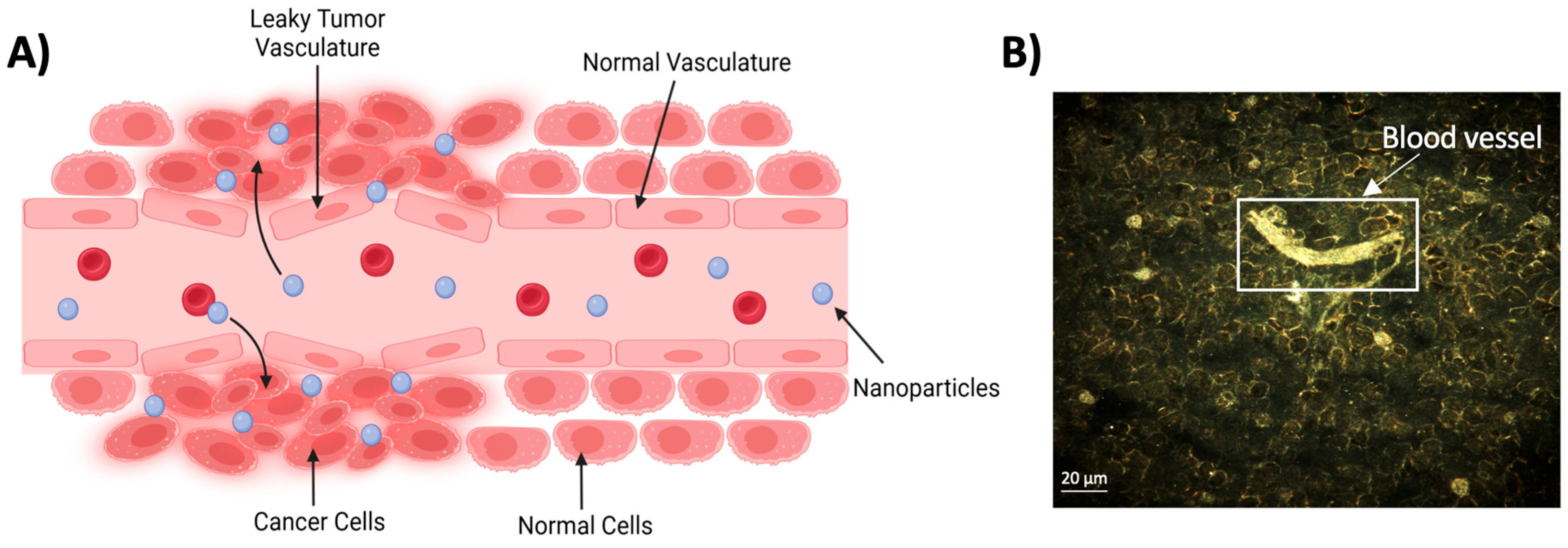
2.1. Passive Targeting
2.2. Active Targeting
2.2.1. Antibody-Coated NPs
2.2.2. Folate-Coated NPs

2.2.3. Peptide-Capped NPs
3. Mechanisms of Radiosensitization
3.1. Physical Mechanism of NP-Based Radiosensitization
3.2. Production of Reactive Oxygen Species (ROS) and Oxidative Stress
3.3. Cell Cycle Effects
4. Application of Nanoparticles as Radiosensitizers
4.1. High-Z Nanoparticles as Radiosensitizers

4.2. Targeted Delivery of Radiosensitizing Chemotherapeutics via High-Z Nanoparticles
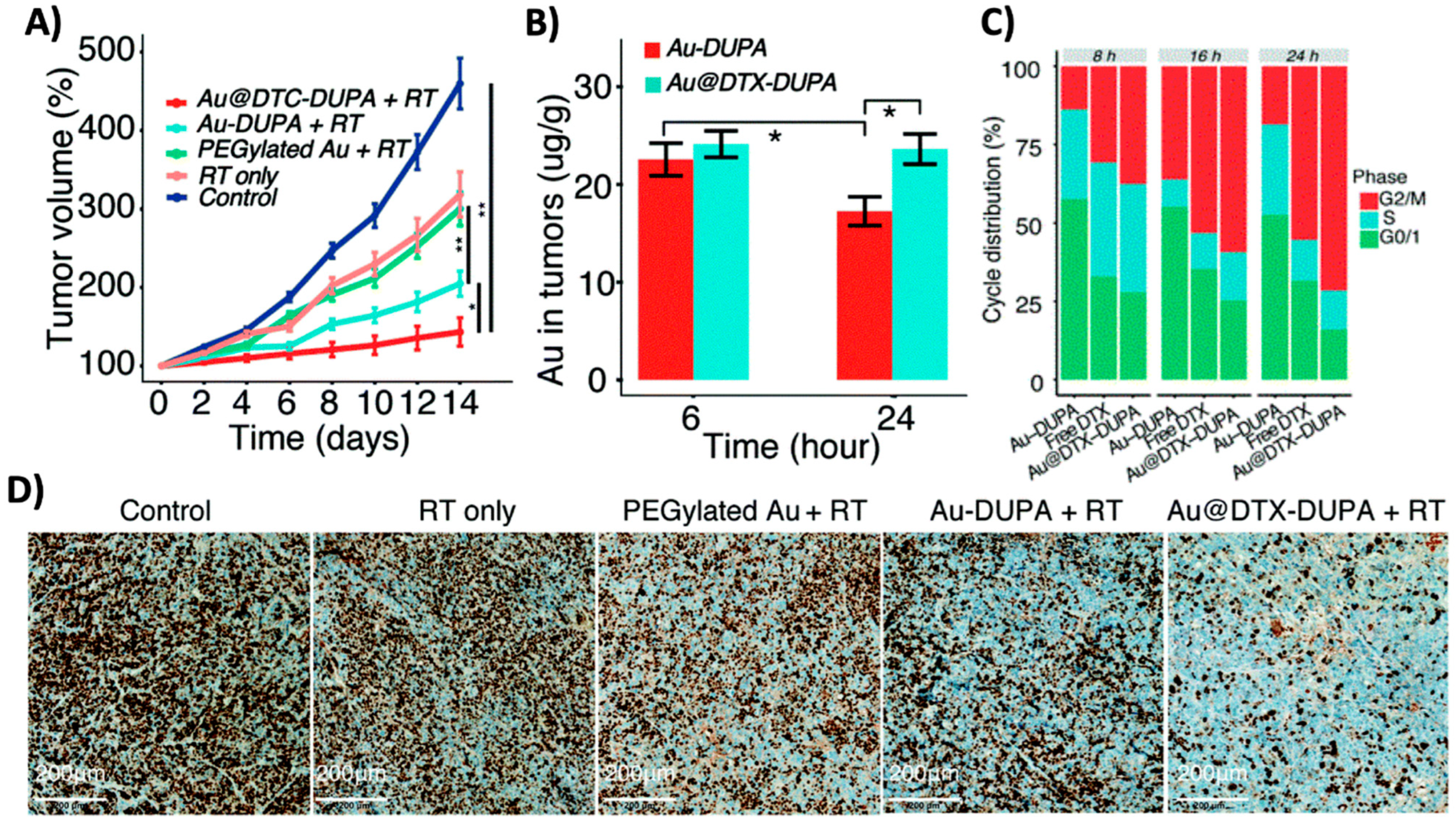
4.3. Challenges and Considerations in the Application of High-Z NPs as Radiosensitizers
4.4. Clinical Translation of High-Z Nanoparticles as Radiosensitizers
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Delaney, G.P.; Barton, M.B. Evidence-based Estimates of the Demand for Radiotherapy. Clin. Oncol. 2015, 27, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Joiner, M.; van der Kogel, A. Basic Clinical Radiobiology, 5th ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Cho, B. Intensity-modulated radiation therapy: A review with a physics perspective. Radiat. Oncol. J. 2018, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Otto, K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med. Phys. 2008, 35, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mu, X.; He, H.; Zhang, X.-D. Cancer Radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Wardman, P. Chemical Radiosensitizers for Use in Radiotherapy. Clin. Oncol. 2007, 19, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Monge-Cadet, J.; Moyal, E.; Supiot, S.; Guimas, V. DNA repair inhibitors and radiotherapy. Cancer Radiothér. 2022, 26, 947–954. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, A.; Manchanda, R.; McGoron, A.J. Theranostic Applications of Nanomaterials in Cancer: Drug Delivery, Image-Guided Therapy, and Multifunctional Platforms. Appl. Biochem. Biotechnol. 2011, 165, 1628–1651. [Google Scholar] [CrossRef]
- Song, X.; Sun, Z.; Li, L.; Zhou, L.; Yuan, S. Application of nanomedicine in radiotherapy sensitization. Front. Oncol. 2023, 13, 1088878. [Google Scholar] [CrossRef]
- Shinde, V.R.; Revi, N.; Murugappan, S.; Singh, S.P.; Rengan, A.K. Enhanced permeability and retention effect: A key facilitator for solid tumor targeting by nanoparticles. Photodiagnosis Photodyn. Ther. 2022, 39, 102915. [Google Scholar] [CrossRef]
- Pearce, A.K.; O’Reilly, R.K. Insights into Active Targeting of Nanoparticles in Drug Delivery: Advances in Clinical Studies and Design Considerations for Cancer Nanomedicine. Bioconjugate Chem. 2019, 30, 2300–2311. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between Defective Endothelial Cells Explain Tumor Vessel Leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Matsumura, Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit. Rev. Ther. Drug Carr. Syst. 1989, 6, 193–210. [Google Scholar]
- Yang, C.; Bromma, K.; Chithrani, D. Peptide Mediated In Vivo Tumor Targeting of Nanoparticles through Optimization in Single and Multilayer In Vitro Cell Models. Cancers 2018, 10, 84. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Chithrani, D.B.; Cruje, C. Polyethylene Glycol Density and Length Affects Nanoparticle Uptake by Cancer Cells. J. Nanomed. Res. 2014, 1. [Google Scholar] [CrossRef]
- Bromma, K.; Cicon, L.; Beckham, W.; Chithrani, D.B. Gold nanoparticle mediated radiation response among key cell components of the tumour microenvironment for the advancement of cancer nanotechnology. Sci. Rep. 2020, 10, 12096. [Google Scholar] [CrossRef]
- Mitri, Z.; Constantine, T.; O’Regan, R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother. Res. Pract. 2012, 2012, 743193. [Google Scholar] [CrossRef]
- Cao, N.; Li, S.; Wang, Z.; Ahmed, K.M.; Degnan, M.E.; Fan, M.; Dynlacht, J.R.; Li, J.J. NF-κB-Mediated HER2 Overexpression in Radiation-Adaptive Resistance. Radiat. Res. 2009, 171, 9–21. [Google Scholar] [CrossRef]
- Bradley, R.; Braybrooke, J.; Gray, R.; Hills, R.; Liu, Z.; Peto, R.; Davies, L.; Dodwell, D.; McGale, P.; Pan, H.; et al. Trastuzumab for early-stage, HER2-positive breast cancer: A meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021, 22, 1139–1150. [Google Scholar] [CrossRef]
- Cai, Z.; Chattopadhyay, N.; Yang, K.; Kwon, Y.L.; Yook, S.; Pignol, J.-P.; Reilly, R.M. 111In-labeled trastuzumab-modified gold nanoparticles are cytotoxic in vitro to HER2-positive breast cancer cells and arrest tumor growth in vivo in athymic mice after intratumoral injection. Nucl. Med. Biol. 2016, 43, 818–826. [Google Scholar] [CrossRef]
- Altunay, B.; Morgenroth, A.; Beheshti, M.; Vogg, A.; Wong, N.C.L.; Ting, H.H.; Biersack, H.-J.; Stickeler, E.; Mottaghy, F.M. HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1371–1389. [Google Scholar] [CrossRef]
- Pourshohod, A.; Jamalan, M.; Zeinali, M.; Ghanemi, M.; Kheirollah, A. Enhancement of X-ray radiotherapy by specific delivery of Z(HER2) affibody-conjugated gold nanoparticles to HER2-positive malignant cells. J. Drug Deliv. Sci. Technol. 2019, 52, 934–941. [Google Scholar] [CrossRef]
- Pourshohod, A.; Zeinali, M.; Ghaffari, M.A.; Kheirollah, A.; Jamalan, M. Improvement of specific aiming of X-ray radiotherapy on HER2-overexpressing cancerous cell lines by targeted delivery of silver nanoparticle. J. Drug Deliv. Sci. Technol. 2022, 76, 103746. [Google Scholar] [CrossRef]
- Bhattarai, S.; Mackeyev, Y.; Venkatesulu, B.P.; Krishnan, S.; Singh, P.K. CXC chemokine receptor 4 (CXCR4) targeted gold nanoparticles potently enhance radiotherapy outcomes in breast cancer. Nanoscale 2021, 13, 19056–19065. [Google Scholar] [CrossRef]
- Martín-Sabroso, C.; Torres-Suárez, A.I.; Alonso-González, M.; Fernández-Carballido, A.; Fraguas-Sánchez, A.I. Active Targeted Nanoformulations via Folate Receptors: State of the Art and Future Perspectives. Pharmaceutics 2021, 14, 14. [Google Scholar] [CrossRef]
- Daniele, R.; Brazzale, C.; Arpac, B.; Tognetti, F.; Pesce, C.; Malfanti, A.; Sayers, E.; Mastrotto, F.; Jones, A.T.; Salmaso, S.; et al. Influence of Folate-Targeted Gold Nanoparticles on Subcellular Localization and Distribution into Lysosomes. Pharmaceutics 2023, 15, 864. [Google Scholar] [CrossRef] [PubMed]
- Kefayat, A.; Ghahremani, F.; Motaghi, H.; Amouheidari, A. Ultra-small but ultra-effective: Folic acid-targeted gold nanoclusters for enhancement of intracranial glioma tumors’ radiation therapy efficacy. Nanomedicine 2019, 16, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Cruje, C.; Chithrani, B.D. Integration of Peptides for Enhanced Uptake of PEGylayed Gold Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 2125–2131. [Google Scholar] [CrossRef]
- Schuemann, J.P.; Berbeco, R.P.; Chithrani, D.B.P.; Cho, S.H.P.; Kumar, R.P.; McMahon, S.J.P.; Sridhar, S.P.; Krishnan, S.M.D. Roadmap to Clinical Use of Gold Nanoparticles for Radiation Sensitization. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 189–205. [Google Scholar] [CrossRef]
- Paro, A.D.; Hossain, M.; Webster, T.J.; Su, M. Monte Carlo and analytic simulations in nanoparticle-enhanced radiation therapy. Int. J. Nanomed. 2016, 11, 4735–4741. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: A preliminary Monte Carlo study. Phys. Med. Biol. 2005, 50, N163–N173. [Google Scholar] [CrossRef]
- Gray, T.; Bassiri, N.; David, S.; Patel, D.Y.; Stathakis, S.; Kirby, N.; Mayer, K.M. A detailed experimental and Monte Carlo analysis of gold nanoparticle dose enhancement using 6 MV and 18 MV external beam energies in a macroscopic scale. Appl. Radiat. Isot. 2021, 171, 109638. [Google Scholar] [CrossRef]
- Butterworth, K.T.; McMahon, S.J.; Currell, F.J.; Prise, K.M. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale 2012, 4, 4830–4838. [Google Scholar] [CrossRef]
- Chithrani, D.B.; Jelveh, S.; Jalali, F.; van Prooijen, M.; Allen, C.; Bristow, R.G.; Hill, R.P.; Jaffray, D.A. Gold Nanoparticles as Radiation Sensitizers in Cancer Therapy. Radiat. Res. 2010, 173, 719–728. [Google Scholar] [CrossRef]
- Stewart, C.; Konstantinov, K.; McKinnon, S.; Guatelli, S.; Lerch, M.; Rosenfeld, A.; Tehei, M.; Corde, S. First proof of bismuth oxide nanoparticles as efficient radiosensitisers on highly radioresistant cancer cells. Phys. Medica 2016, 32, 1444–1452. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Charmi, J.; Yaray, K.; Ghaffarlou, M.; Balcioglu, E.; Ertas, Y.N. Enhanced In Vivo Radiotherapy of Breast Cancer Using Gadolinium Oxide and Gold Hybrid Nanoparticles. ACS Appl. Bio Mater. 2023, 6, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, P.; Ma, J.; Li, D.; Yang, H.; Chen, W.; Jiang, Y. Enhancement of Radiosensitization by Silver Nanoparticles Functionalized with Polyethylene Glycol and Aptamer As1411 for Glioma Irradiation Therapy. Int. J. Nanomed. 2019, 14, 9483–9496. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, S.; Liao, Y.; Yang, H.; Chen, Z.; Hu, Y.; Fu, S.; Wu, J. Albumin-Modified Gold Nanoparticles as Novel Radiosensitizers for Enhancing Lung Cancer Radiotherapy. Int. J. Nanomed. 2023, 18, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Misawa, M.P.; Takahashi, J.P. Generation of reactive oxygen species induced by gold nanoparticles under X-ray and UV Irradiations. Nanomedicine 2011, 7, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.J.; Jung, K.O.; Graves, E.E.; Pratx, G. A gold nanoparticle system for the enhancement of radiotherapy and simultaneous monitoring of reactive-oxygen-species formation. Nanotechnology 2018, 29, 504001. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, Z.; Jin, X.; Liu, Y.; Li, P.; Shen, Z.; Wu, A.; Zheng, X.; Chen, W.; Li, Q. Radiosensitizing Effect of Gadolinium Oxide Nanocrystals in NSCLC Cells Under Carbon Ion Irradiation. Nanoscale Res. Lett. 2019, 14, 328. [Google Scholar] [CrossRef]
- Wu, H.; Lin, J.; Liu, P.; Huang, Z.; Zhao, P.; Jin, H.; Ma, J.; Wen, L.; Gu, N. Reactive oxygen species acts as executor in radiation enhancement and autophagy inducing by AgNPs. Biomaterials 2016, 101, 1–9. [Google Scholar] [CrossRef]
- Nakayama, M.; Sasaki, R.; Ogino, C.; Tanaka, T.; Morita, K.; Umetsu, M.; Ohara, S.; Tan, Z.; Nishimura, Y.; Akasaka, H.; et al. Titanium peroxide nanoparticles enhanced cytotoxic effects of X-ray irradiation against pancreatic cancer model through reactive oxygen species generation in vitro and in vivo. Radiat. Oncol. 2016, 11, 91. [Google Scholar] [CrossRef]
- Tabatabaie, F.; Franich, R.; Feltis, B.; Geso, M. Oxidative Damage to Mitochondria Enhanced by Ionising Radiation and Gold Nanoparticles in Cancer Cells. Int. J. Mol. Sci. 2022, 23, 6887. [Google Scholar] [CrossRef]
- Bemidinezhad, A.; Mirzavi, F.; Gholamhosseinian, H.; Gheybi, F.; Soukhtanloo, M. Gold-containing liposomes and glucose-coated gold nanoparticles enhances the radiosensitivity of B16F0 melanoma cells via increasing apoptosis and ROS production. Life Sci. 2023, 318, 121495. [Google Scholar] [CrossRef] [PubMed]
- Mateo, D.; Morales, P.; Ávalos, A.; Haza, A.I. Oxidative stress contributes to gold nanoparticle-induced cytotoxicity in human tumor cells. Toxicol. Mech. Methods 2014, 24, 161–172. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, Y.; Huang, L.; Lv, G.; Lei, C.; Fan, X.; Lin, F.; Zhang, Y.; Wu, L.; Yang, Y. In vitro cytotoxicity of gold nanorods in A549 cells. Environ. Toxicol. Pharmacol. 2015, 39, 871–878. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.; Alrokayan, S. Toxicity Mechanism of Gadolinium Oxide Nanoparticles and Gadolinium Ions in Human Breast Cancer Cells. Curr. Drug Metab. 2019, 20, 907–917. [Google Scholar] [CrossRef]
- Hvolbæk, B.; Janssens, T.V.W.; Clausen, B.S.; Falsig, H.; Christensen, C.H.; Nørskov, J.K. Catalytic activity of Au nanoparticles. Nano Today 2007, 2, 14–18. [Google Scholar] [CrossRef]
- Shcherbakov, V.; Denisov, S.A.; Mostafavi, M. A mechanistic study of gold nanoparticles catalysis of O2 reduction by ascorbate and hydroethidine, investigating reactive oxygen species reactivity. RSC Adv. 2023, 13, 8557–8563. [Google Scholar] [CrossRef]
- Pan, Y.; Leifert, A.; Ruau, D.; Neuss, S.; Bornemann, J.; Schmid, G.; Brandau, W.; Simon, U.; Jahnen-Dechent, W. Gold Nanoparticles of Diameter 1.4 nm Trigger Necrosis by Oxidative Stress and Mitochondrial Damage. Small 2009, 5, 2067–2076. [Google Scholar] [CrossRef]
- Jawaid, P.; Rehman, M.U.; Zhao, Q.-L.; Misawa, M.; Ishikawa, K.; Hori, M.; Shimizu, T.; Saitoh, J.-i.; Noguchi, K.; Kondo, T. Small size gold nanoparticles enhance apoptosis-induced by cold atmospheric plasma via depletion of intracellular GSH and modification of oxidative stress. Cell Death Discov. 2020, 6, 83. [Google Scholar] [CrossRef]
- Abudayyak, M.; Öztaş, E.; Arici, M.; Özhan, G. Investigation of the toxicity of bismuth oxide nanoparticles in various cell lines. Chemosphere 2017, 169, 117–123. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Yuan, Q.; An, D.; Li, J.; Gao, X. The Au clusters induce tumor cell apoptosis via specifically targeting thioredoxin reductase 1 (TrxR1) and suppressing its activity. Chem. Commun. 2014, 50, 10687–10690. [Google Scholar] [CrossRef]
- Roa, W.; Zhang, X.; Guo, L.; Shaw, A.; Hu, X.; Xiong, Y.; Gulavita, S.; Patel, S.; Sun, X.; Chen, J.; et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology 2009, 20, 375101. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, Y.; Li, X.; Hu, L. Thioglucose-bound gold nanoparticles increase the radiosensitivity of a triple-negative breast cancer cell line (MDA-MB-231). Breast Cancer 2015, 22, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Tse, K.; Zahedi, P.; Harding, S.M.; Zafarana, G.; Jaffray, D.A.; Bristow, R.G.; Allen, C. Hypoxia and Cellular Localization Influence the Radiosensitizing Effect of Gold Nanoparticles (AuNPs) in Breast Cancer Cells. Radiat. Res. 2014, 182, 475–488. [Google Scholar] [CrossRef]
- Butterworth, K.T.; Coulter, J.A.; Jain, S.; Forker, J.; McMahon, S.J.; Schettino, G.; Prise, K.M.; Currell, F.J.; Hirst, D.G. Evaluation of cytotoxicity and radiation enhancement using 1.9 nm gold particles: Potential application for cancer therapy. Nanotechnology 2010, 21, 295101. [Google Scholar] [CrossRef] [PubMed]
- Hanžić, N.; Horvat, A.; Bibić, J.; Unfried, K.; Jurkin, T.; Dražić, G.; Marijanović, I.; Slade, N.; Gotić, M. Syntheses of gold nanoparticles and their impact on the cell cycle in breast cancer cells subjected to megavoltage X-ray irradiation. Mater. Sci. Eng. C 2018, 91, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Holmila, R.J.; Vance, S.A.; King, S.B.; Tsang, A.W.; Singh, R.; Furdui, C.M. Silver Nanoparticles Induce Mitochondrial Protein Oxidation in Lung Cells Impacting Cell Cycle and Proliferation. Antioxidants 2019, 8, 552. [Google Scholar] [CrossRef]
- Li, Q.; Huang, C.; Liu, L.; Hu, R.; Qu, J. Effect of surface coating of gold nanoparticles on cytotoxicity and cell cycle progression. Nanomaterials 2018, 8, 1063. [Google Scholar] [CrossRef]
- Matsudaira, H.; Ueno, A.M.; Furuno, I. Iodine Contrast Medium Sensitizes Cultured Mammalian Cells to X Rays but Not to γ Rays. Radiat. Res. 1980, 84, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Santos Mello, R.; Callisen, H.; Winter, J.; Kagan, A.R.; Norman, A. Radiation dose enhancement in tumors with iodine. Med. Phys. 1983, 10, 75–78. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Smilowitz, H.M.; O’Connor, M.J.; Dilmanian, F.A.; Slatkin, D.N. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine 2013, 8, 1601–1609. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Zhong, Z.; Slatkin, D.N.; Kalef-Ezra, J.A.; Smilowitz, H.M. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 2010, 55, 3045–3059. [Google Scholar] [CrossRef]
- Liu, S.; Piao, J.; Liu, Y.; Tang, J.; Liu, P.; Yang, D.; Zhang, L.; Ge, N.; Jin, Z.; Jiang, Q.; et al. Radiosensitizing effects of different size bovine serum albumin-templated gold nanoparticles on H22 hepatoma-bearing mice. Nanomedicine 2018, 13, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Mehrnia, S.S.; Hashemi, B.; Mowla, S.J.; Arbabi, A. Enhancing the effect of 4MeV electron beam using gold nanoparticles in breast cancer cells. Phys. Medica 2017, 35, 18–24. [Google Scholar] [CrossRef]
- Cunningham, C.; de Kock, M.; Engelbrecht, M.; Miles, X.; Slabbert, J.; Vandevoorde, C. Radiosensitization Effect of Gold Nanoparticles in Proton Therapy. Front. Public Health 2021, 9, 699822. [Google Scholar] [CrossRef]
- Liu, P.; Jin, H.; Guo, Z.; Ma, J.; Zhao, J.; Li, D.; Wu, H.; Gu, N. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int. J. Nanomed. 2016, 11, 5003–5014. [Google Scholar] [CrossRef]
- Wu, C.; Cai, R.; Zhao, T.; Wu, L.; Zhang, L.; Jin, J.; Xu, L.; Li, P.; Li, T.; Zhang, M.; et al. Hyaluronic Acid-Functionalized Gadolinium Oxide Nanoparticles for Magnetic Resonance Imaging-Guided Radiotherapy of Tumors. Nanoscale Res. Lett. 2020, 15, 94. [Google Scholar] [CrossRef]
- Liu, P.; Huang, Z.; Chen, Z.; Xu, R.; Wu, H.; Zang, F.; Wang, C.; Gu, N. Silver nanoparticles: A novel radiation sensitizer for glioma? Nanoscale 2013, 5, 11829–11836. [Google Scholar] [CrossRef]
- Deng, J.; Xu, S.; Hu, W.; Xun, X.; Zheng, L.; Su, M. Tumor targeted, stealthy and degradable bismuth nanoparticles for enhanced X-ray radiation therapy of breast cancer. Biomaterials 2018, 154, 24–33. [Google Scholar] [CrossRef]
- Blanchard, P.M.D.; Lee, A.P.; Marguet, S.M.; Leclercq, J.M.; Ng, W.T.M.D.; Ma, J.P.; Chan, A.T.C.P.; Huang, P.-Y.M.D.; Benhamou, E.M.D.; Zhu, G.M.D.; et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An update of the MAC-NPC meta-analysis. Lancet Oncol. 2015, 16, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Kirwan, J.; Tierney, J.; Vale, C.; Symonds, P.; Fresco, L.; Williams, C.; Collingwood, M. Concomitant chemotherapy and radiation therapy for cancer of the uterine cervix. Cochrane Database Syst. Rev. 2005, CD002225. [Google Scholar] [CrossRef]
- Grabenbauer, G.G. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer. Results of a phase III randomized trial. Strahlenther. Onkol. 1998, 174, 108–109. [Google Scholar] [CrossRef]
- Pignon, J.-P.; Maître, A.l.; Maillard, E.; Bourhis, J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Gaitanis, A.; Staal, S. Liposomal doxorubicin and nab-paclitaxel: Nanoparticle cancer chemotherapy in current clinical use. Methods Mol. Biol. 2010, 624, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Mason, K.A.; Hunter, N.R.; Milas, M.; Abbruzzese, J.L.; Milas, L. Docetaxel enhances tumor radioresponse in vivo. Clin. Cancer Res. 1997, 3, 2431–2438. [Google Scholar] [PubMed]
- Hennequin, C.; Giocanti, N.; Favaudon, V. Interaction of ionizing radiation with paclitaxel (Taxol) and docetaxel (Taxotere) in HeLa and SQ20B cells. Cancer Res. 1996, 56, 1842–1850. [Google Scholar] [PubMed]
- Alhussan, A.; Bromma, K.; Perez, M.M.; Beckham, W.; Alexander, A.S.; Howard, P.L.; Chithrani, D.B. Docetaxel-Mediated Uptake and Retention of Gold Nanoparticles in Tumor Cells and in Cancer-Associated Fibroblasts. Cancers 2021, 13, 3157. [Google Scholar] [CrossRef] [PubMed]
- Alhussan, A.; Jackson, N.; Eaton, S.; Santos, N.D.; Barta, I.; Zaifman, J.; Chen, S.; Tam, Y.Y.C.; Krishnan, S.; Chithrani, D.B. Lipid-Nanoparticle-Mediated Delivery of Docetaxel Prodrug for Exploiting Full Potential of Gold Nanoparticles in the Treatment of Pancreatic Cancer. Cancers 2022, 14, 6137. [Google Scholar] [CrossRef] [PubMed]
- Bromma, K.; Dos Santos, N.; Barta, I.; Alexander, A.; Beckham, W.; Krishnan, S.; Chithrani, D.B. Enhancing nanoparticle accumulation in two dimensional, three dimensional, and xenograft mouse cancer cell models in the presence of docetaxel. Sci. Rep. 2022, 12, 13508. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.; Hill, I.; Alhussan, A.; Bromma, K.; Morgan, J.; Abousaida, B.; Zahra, Y.; Mackeyev, Y.; Beckham, W.; Herchko, S.; et al. Dual enhancement in the radiosensitivity of prostate cancer through nanoparticles and chemotherapeutics. Cancer Nanotechnol. 2023, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-x.; Guo, Z.-h.; Lu, J.-s.; Xie, W.-s.; Zhong, Q.-z.; Sun, X.-d.; Wang, X.-m.; Wang, J.-y.; Liu, M.; Zhao, L.-y. All-purpose nanostrategy based on dose deposition enhancement, cell cycle arrest, DNA damage, and ROS production as prostate cancer radiosensitizer for potential clinical translation. Nanoscale 2021, 13, 14525–14537. [Google Scholar] [CrossRef]
- Chen, J.L.-Y.; Yang, S.-J.; Pan, C.-K.; Lin, L.-C.; Tsai, C.-Y.; Wang, C.-H.; Huang, Y.-S.; Lin, Y.-L.; Kuo, S.-H.; Shieh, M.-J. Cisplatin and Albumin-Based Gold–Cisplatin Nanoparticles Enhance Ablative Radiation Therapy–Induced Antitumor Immunity in Local and Distant Tumor Microenvironment. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 1135–1149. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Arnida; Malugin, A.; Ghandehari, H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: A comparative study of rods and spheres. J. Appl. Toxicol. 2010, 30, 212–217. [Google Scholar] [CrossRef]
- Yohan, D.; Cruje, C.; Lu, X.; Chithrani, D.B. Size-Dependent Gold Nanoparticle Interaction at Nano–Micro Interface Using Both Monolayer and Multilayer(Tissue-Like) Cell Models. Nano-Micro Lett. 2016, 8, 44–53. [Google Scholar] [CrossRef]
- Bromma, K.; Alhussan, A.; Perez, M.M.; Howard, P.; Beckham, W.; Chithrani, D.B. Three-Dimensional Tumor Spheroids as a Tool for Reliable Investigation of Combined Gold Nanoparticle and Docetaxel Treatment. Cancers 2021, 13, 1465. [Google Scholar] [CrossRef]
- Schmid, G. The relevance of shape and size of Au55 clusters. Chem. Soc. Rev. 2008, 37, 1909–1930. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Cheng, T.-M.; Chu, H.-Y.; Huang, H.-M.; Li, Z.-L.; Chen, C.-Y.; Shih, Y.-J.; Whang-Peng, J.; Cheng, R.H.; Mo, J.-K.; Lin, H.-Y.; et al. Toxicologic Concerns with Current Medical Nanoparticles. Int. J. Mol. Sci. 2022, 23, 7597. [Google Scholar] [CrossRef]
- Bonvalot, S.P.; Rutkowski, P.L.P.; Thariat, J.P.; Carrère, S.M.D.; Ducassou, A.M.D.; Sunyach, M.-P.M.D.; Agoston, P.M.D.; Hong, A.M.; Mervoyer, A.M.D.; Rastrelli, M.M.D.; et al. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): A multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019, 20, 1148–1159. [Google Scholar] [CrossRef]
- Hu, Y.; Paris, S.; Barsoumian, H.; Abana, C.O.; He, K.; Sezen, D.; Wasley, M.; Masrorpour, F.; Chen, D.; Yang, L.; et al. A radioenhancing nanoparticle mediated immunoradiation improves survival and generates long-term antitumor immune memory in an anti-PD1-resistant murine lung cancer model. J. Nanobiotechnol. 2021, 19, 416. [Google Scholar] [CrossRef]
- Hu, Y.; Paris, S.; Barsoumian, H.; Abana, C.O.; He, K.; Wasley, M.; Younes, A.I.; Masrorpour, F.; Chen, D.; Yang, L.; et al. Radiation Therapy Enhanced by NBTXR3 Nanoparticles Overcomes Anti-PD1 Resistance and Evokes Abscopal Effects. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 647–657. [Google Scholar] [CrossRef]
- Hu, Y.; Paris, S.; Bertolet, G.; Barsoumian, H.B.; Wang, Q.; Da Silva, J.; Patel, N.B.; Nguyen, N.; Doss, D.J.; Huang, A.; et al. NBTXR3 improves the efficacy of immunoradiotherapy combining nonfucosylated anti-CTLA4 in an anti-PD1 resistant lung cancer model. Front. Immunol. 2022, 13, 1022011. [Google Scholar] [CrossRef]
- Lux, F.; Vu Long, T.; Thomas, E.; Dufort, S.; Rossetti, F.; Martini, M.; Truillet, C.; Doussineau, T.; Bort, G.; Denat, F.; et al. AGuIX (R) from bench to bedside-Transfer of an ultrasmall theranostic gadolinium-based nanoparticle to clinical medicine. Br. J. Radiol. 2019, 92, 20180365. [Google Scholar] [CrossRef]
- Quatre, R.; Jacquet, T.; Atallah, I.; Tillement, O.; Lux, F.; Coll, J.L.; Dufort, S.; Righini, C.A. Evaluation of the theranostic properties of gadolinium-based nanoparticles for head and neck cancer. Head Neck 2019, 41, 403–410. [Google Scholar] [CrossRef]
- Sun, H.; Cai, H.; Xu, C.; Zhai, H.; Lux, F.; Xie, Y.; Feng, L.; Du, L.; Liu, Y.; Sun, X.; et al. AGuIX nanoparticles enhance ionizing radiation-induced ferroptosis on tumor cells by targeting the NRF2-GPX4 signaling pathway. J. Nanobiotechnol. 2022, 20, 449. [Google Scholar] [CrossRef]
- Du, Y.; Sun, H.; Lux, F.; Xie, Y.; Du, L.; Xu, C.; Zhang, H.; He, N.; Wang, J.; Liu, Y.; et al. Radiosensitization Effect of AGuIX, a Gadolinium-Based Nanoparticle, in Nonsmall Cell Lung Cancer. ACS Appl. Mater. Interfaces 2020, 12, 56874–56885. [Google Scholar] [CrossRef]
- Verry, C.; Dufort, S.; Villa, J.; Gavard, M.; Iriart, C.; Grand, S.; Charles, J.; Chovelon, B.; Cracowski, J.-L.; Quesada, J.-L.; et al. Theranostic AGuIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). Radiother. Oncol. 2021, 160, 159–165. [Google Scholar] [CrossRef]


| Nanoparticle | Condition | Phase | Status | Identifier |
|---|---|---|---|---|
| NBTXR3 | Adult soft-tissue sarcoma | Phase I | Completed | NCT01433068 |
| Pancreatic ductal adenocarcinoma | Phase I | Recruiting | NCT04484909 | |
| Lung non-small-cell carcinoma | Phase I | Recruiting | NCT04505267 | |
| Metastatic malignant solid neoplasm | Phase I/II | Recruiting | NCT05039632 | |
| Esophageal adenocarcinoma | Phase I | Recruiting | NCT04615013 | |
| Head and neck squamous cell carcinoma | Phase II | Recruiting | NCT04862455 | |
| Advanced cancers | Phase I | Recruiting | NCT03589339 | |
| Head and neck squamous | Phase I | Active | NCT01946867 | |
| Adult soft-tissue sarcoma | Phase II/III | Completed | NCT02379845 | |
| Head and neck squamous cell carcinoma | Phase III | Recruiting | NCT04892173 | |
| AGuIX | Glioblastoma | Phase I/II | Recruiting | NCT04881032 |
| Brain metastases | Phase I | Completed | NCT02820454 | |
| Brain metastases | Phase II | Recruiting | NCT03818386 | |
| Gynecological cancers | Phase I | Recruiting | NCT03308604 | |
| Brain metastases | Phase II | Recruiting | NCT04899908 | |
| Lung tumors and pancreatic cancer | Phase I/II | Recruiting | NCT04789486 | |
| Recurrent cancer | Phase I | Not yet recruiting | NCT04784221 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, N.; Cecchi, D.; Beckham, W.; Chithrani, D.B. Application of High-Z Nanoparticles to Enhance Current Radiotherapy Treatment. Molecules 2024, 29, 2438. https://doi.org/10.3390/molecules29112438
Jackson N, Cecchi D, Beckham W, Chithrani DB. Application of High-Z Nanoparticles to Enhance Current Radiotherapy Treatment. Molecules. 2024; 29(11):2438. https://doi.org/10.3390/molecules29112438
Chicago/Turabian StyleJackson, Nolan, Daniel Cecchi, Wayne Beckham, and Devika B. Chithrani. 2024. "Application of High-Z Nanoparticles to Enhance Current Radiotherapy Treatment" Molecules 29, no. 11: 2438. https://doi.org/10.3390/molecules29112438






