Volatilomics of Fruit Wines
Abstract
1. Introduction
2. Volatile Compounds in Wines—Origin and Aromas
2.1. Higher Alcohols
| Compound (IUPAC Name) | Chemical Structure | Aroma Descriptor | Threshold [mg/L] | References |
|---|---|---|---|---|
| Isobutanol (2-methylpropan-1-ol) | 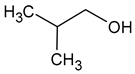 | Solvent, chemical alcoholic, malt notes, wineosity notes | 40 | [14,20] |
| Isoamyl alcohol (3-methylbutan-1-ol) |  | Alcohol notes, nail varnish, solvent amilic notes, malt, whiskey | 30 | [14,21] |
| Isohexanol (4-methylpentan-1-ol) | 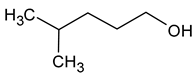 | Almond | 50 | [22] |
| Tert-amyl alcohol (2-methylbutan-2-ol) | 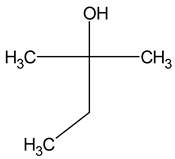 | Nail polish, solvent malt | 30 | [23] |
| Benzyl alcohol (phenylmethanol) | 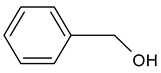 | Sweet, floral | 200 | [24,25] |
| 2-Phenylethanol |  | Floral, rose, honey notes, peach notes | 10–14 | [26] |
| Hexanol (hexan-1-ol) | 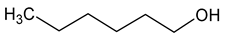 | Grass, green | 8 | [22] |
| Methionol (3-methylsulfanylpropan-1-ol) | 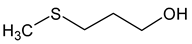 | Crushed potatoes | 1 | [13,14] |
2.2. Esters
2.3. Volatile Fatty Acids
2.4. Terpenes
| Compound (IUPAC Name) | Chemical Structure | Aroma Descriptor | Threshold [μg/L] | References |
|---|---|---|---|---|
| Linalool (3,7-dimethylocta-1,6-dien-3-ol) | 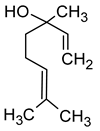 | Flowery, fruity | 0.006–25 | [5,22] |
| Geraniol ((2E)-3,7-dimethylocta-2,6-dien-1-ol) | 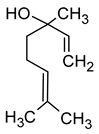 | Rose | 0.03–32 | [5,63] |
| Citronellol (3,7-dimethyloct-6-en-1-ol) | 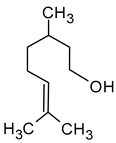 | Green lemon | 0.008–100 | [5,22] |
| Nerol ((2Z)-3,7-dimethylocta-2,6-dien-1-ol) | 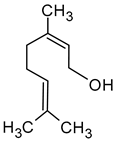 | Floral | 0.3 | [5,64] |
| β-Damascenone ((E)-1-(2,6,6-trimethylcyclohexa-1,3-dien-1-yl)but-2-en-1-one) | 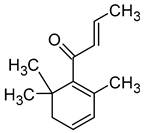 | Honey | 0.05 | [63] |
| (E)-Hotrienol ((5E)-3,7-dimethylocta-1,5,7-trien-3-ol) | 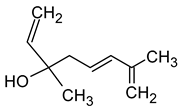 | Faint flowery, older flowers | 0.11 | [5,65] |
| ((−)-cis-Rose oxide ((2S,4R)-4-methyl-2-(2-methylprop-1-enyl)oxane) |  | Geranium oil | 0.0005 | [5,66] |
2.5. Pyrazines
2.6. Thiols
2.7. Ageing Aromas
3. Volatile Compounds of Apple Wines and Ciders
4. Volatile Compounds of Other Fruit Wines
| Compounds | W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 |
|---|---|---|---|---|---|---|---|---|---|
| Ethyl acetate | 1.60 | 1.40 | 5.86 | 0.13 | 8.22 | 12.88 | 6.25 | 33.07 | 1.68 |
| Ethyl 3-methylbutanoate | 0.01 | 0.04 | nd | nd | na | 0.03 | nd | na | na |
| Isopentyl acetate | 0.60 | na | na | na | na | na | na | na | 0.04 |
| Ethyl pentanoate | 0.02 | na | na | nd | na | na | na | na | na |
| Ethyl hexanoate | 0.09 | 0.09 | 0.14 | 0.46 | 0.52 | 0.17 | 0.29 | 0.24 | 0.38 |
| Hexyl acetate | 0.17 | 0.11 | na | na | 0.22 | 0.02 | na | 0.01 | nd |
| Ethyl 3-hexenoate | 0.08 | 0.47 | na | na | nd | na | na | na | na |
| Ethyl lactate | 5.08 | 5.08 | 2.72 | 7.97 | 0.60 | 1.25 | 7.44 | 0.34 | 0.17 |
| Ethyl octanoate | 0.76 | 0.75 | 0.66 | na | 0.46 | 0.37 | 0.51 | 0.82 | na |
| Ethyl decanoate | 0.18 | 0.06 | na | na | 0.49 | 0.19 | na | 0.10 | 0.79 |
| Ethyl benzoate | 0.47 | 0.13 | na | nd | 0.11 | 0.39 | na | 0.32 | 0.01 |
| Diethyl succinate | 0.08 | 0.08 | nd | nd | 0.79 | 0.78 | na | 0.07 | 0.45 |
| Ethyl 2-phenylacetate | 0.01 | 0.01 | na | na | 0.10 | 0.02 | na | na | 0.02 |
| 2-Phenylethyl acetate | 0.03 | 0.22 | 8.13 | na | na | na | 0.78 | 0.01 | nd |
| 1-Propanol | 29.01 | 22.96 | 2.74 | na | 0.86 | 22.18 | 1.41 | 8.53 | 0.72 |
| 2-Methylpropanol | 17.96 | 12.95 | 12.66 | nd | nd | 1.89 | 0.62 | na | na |
| 1-Butanol | 0.75 | 0.55 | 0.74 | 0.33 | 0.31 | na | 0.68 | na | 0.52 |
| 3-Methylbutanol | 49.46 | 12.71 | 9.98 | 102.32 | 1.86 | 13.87 | 10.84 | 14.64 | na |
| 3-Methyl-3-buten-1-ol | 1.53 | na | na | na | na | na | na | na | na |
| 1-Pentanol | 0.01 | 0.06 | na | na | na | na | na | na | na |
| 1-Hexanol | 1.76 | 0.66 | na | 5.89 | 0.309 | 0.46 | 0.66 | 0.50 | 0.80 |
| 2,3-Butanediol | 0.26 | 0.05 | na | na | na | na | na | na | 0.05 |
| 1-Octanol | 0.03 | 0.03 | na | na | na | na | na | na | 0.11 |
| Benzyl alcohol | 17.08 | 5.09 | 3.45 | 3.65 | 1.09 | 18.51 | 2.05 | 17.82 | 2.12 |
| 2-Phenylethyl alcohol | 16.35 | 6.76 | 4.96 | 1.48 | 0.58 | 8.38 | 2.42 | 9.18 | 0.69 |
| Acetic acid | 35.18 | 25.15 | na | 9.81 | 5.28 | 6.62 | 5.35 | 3.73 | 2.10 |
| 2-Methylpropanoic acid | 0.85 | 1.42 | 2.28 | na | na | na | na | na | na |
| Butanoic acid | 0.28 | 0.11 | nd | 0.06 | nd | na | 0.15 | na | 0.20 |
| 3-Methylbutanoic acid | 0.40 | 0.42 | nd | na | na | na | na | na | na |
| Hexanoic acid | 18.17 | 4.92 | 3.69 | 0.29 | nd | 0.61 | 1.83 | 1.17 | 1.15 |
| Octanoic acid | 5.36 | 3.59 | 3.25 | 0.39 | na | 0.98 | 0.39 | 0.11 | 0.51 |
| Decanoic acid | 3.97 | 2.22 | na | na | na | na | na | na | 2.22 |
| Benzaldehyde | 0.12 | 0.58 | 2.25 | 7.37 | 93.78 | 7.50 | 25.41 | 2.31 | 3.75 |
| Linalool | 0.02 | 0.15 | 0.03 | na | na | na | na | 0.15 | 0.37 |
| α-Terpineol | 0.02 | 0.02 | 0.09 | na | na | 0.26 | na | 0.22 | 0.02 |
| β-Citronellol | 0.37 | na | na | 16.80 | na | 0.05 | na | 0.05 | 0.10 |
| β-Damascenone | 0.05 | 1.20 | 0.07 | na | na | na | na | na | na |
| Compounds | W23 | W24 | W25 | W26 | W27 | W28 | W29 | W30 | W31 |
|---|---|---|---|---|---|---|---|---|---|
| Ethyl acetate | 22.50 | 0.05 | na | na | na | na | na | 40.83 | 0.67 |
| Isopentyl acetate | 101.73 | na | na | na | na | na | na | 9.24 | na |
| Ethyl butyrate | na | na | 0.02 | 0.13 | 0.12 | 0.01 | 0.01 | na | 0.04 |
| Ethyl hexanoate | 103.96 | na | na | na | na | na | na | 1.62 | 0.16 |
| Hexyl acetate | 28.81 | na | na | na | na | na | na | 0.11 | na |
| Ethyl 3-hexenoate | na | na | <0.01 | 0.01 | 0.01 | 0.01 | <0.01 | 0.04 | na |
| Ethyl lactate | na | na | 0.20 | 0.26 | 0.01 | 0.41 | 0.01 | na | na |
| 2-Methyl-1-butyl acetate | na | na | na | na | na | na | na | na | 0.19 |
| Ethyl octanoate | 455.74 | 0.13 | <0.01 | 0.01 | 0.13 | <0.01 | 0.00 | na | 1.27 |
| Ethyl decanoate | 519.80 | na | na | na | na | na | na | 0.26 | 0.60 |
| Ethyl caprylate | na | na | na | na | na | na | na | 4.67 | na |
| Diethyl succinate | na | 1.94 | 1.74 | 0.55 | 0.37 | 2.19 | 0.17 | 0.16 | na |
| Ethyl palmitate | na | na | na | na | na | na | na | na | 0.38 |
| Ethyl 2-phenylacetate | 2.00 | na | na | na | na | na | na | na | na |
| 2-Phenylethyl acetate | na | 0.04 | 0.06 | 0.06 | 0.01 | 0.04 | 0.03 | 0.47 | 0.07 |
| 1-Propanol | na | na | na | na | na | na | na | na | na |
| 2-Methylpropanol | na | na | na | na | na | na | na | 88.92 | 0.11 |
| 1-Butanol | na | 0.04 | <0.01 | 0.10 | <0.01 | 0.02 | <0.01 | na | na |
| 3-Methylbutanol | 150.00 | 24.17 | na | na | na | na | na | 389.94 | 1.43 |
| 3-Methyl-3-buten-1-ol | na | na | 0.02 | 0.13 | nd | nd | 0.01 | na | na |
| 1-Pentanol | na | 0.08 | 0.17 | nd | nd | <0.01 | nd | na | na |
| 1-Hexanol | 8.00 | na | <0.01 | 0.03 | 0.04 | 0.01 | <0.01 | na | 0.07 |
| 1-Octanol | na | na | <0.01 | 0.01 | <0.01 | 0.01 | <0.01 | 0.12 | 0.05 |
| Benzyl alcohol | na | na | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.77 | na |
| 2-Phenylethyl alcohol | 50.00 | 17.67 | nd | nd | 0.02 | nd | nd | 2.52 | 0.33 |
| Acetic acid | 3.00 | na | na | na | na | na | na | na | 0.70 |
| 3-Methylbutanoic acid | na | na | 0.14 | 0.33 | 0.11 | 0.02 | 0.03 | na | na |
| Hexanoic acid | 3.00 | 0.38 | 0.54 | 0.63 | 0.24 | 0.15 | 0.39 | 13.97 | na |
| Octanoic acid | 20.50 | 0.81 | 0.01 | 0.43 | 0.43 | 0.45 | 0.51 | 54.41 | 0.12 |
| Decanoic acid | 15.00 | 0.14 | nd | 0.03 | 0.03 | 0.01 | 0.01 | 118.78 | na |
| Benzaldehyde | na | na | na | na | na | na | na | 1.26 | na |
| Linalool | na | na | nd | <0.01 | 0.19 | 0.02 | 0.01 | 4.66 | na |
| α-Terpineol | na | na | 0.01 | 0.21 | 0.01 | 0.04 | 0.28 | 1.86 | na |
| β-Citronellol | na | na | nd | <0.01 | 0.01 | nd | nd | 0.53 | 0.04 |
5. Methods of Analysis—Volatilomics
5.1. Sample Preparation
5.2. Analytical Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saranraj, P.; Sivasakthivelan, P.; Naveen, M. Fermentation of fruit wine and its quality analysis: A review. Aust. J. Sci. Technol. 2017, 1, 85–97. [Google Scholar]
- Matel, F. Technical Guide for Fruit Wine Production. In Science and Technology of Fruit Wine Production; Kosseva, M.R., Joshi, V.K., Panesar, P.S., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 663–703. [Google Scholar]
- Anesi, A.; Stocchero, M.; Dal Santo, S.; Commisso, M.; Zenoni, S.; Ceoldo, S.; Tornielli, G.B.; Siebert, T.E.; Herderich, M.; Pezzotti, M.; et al. Towards a scientific interpretation of the terroir concept: Plasticity of the grape berry metabolome. BMC Plant Biol. 2015, 15, 191. [Google Scholar] [CrossRef] [PubMed]
- Duana, W.-P.; Zhub, B.-Q.; Songa, R.-R.; Zhanga, B.; Lanc, Y.-B.; Zhua, X.; Duanc, C.-Q.; Hana, S.-Y. Volatile composition and aromatic attributes of wine made with Vitisvinifera L. cv Cabernet Sauvignon grapes in the Xinjiang region of China: Effect of different commercial yeasts. Int. J. Food Prop. 2018, 21, 1423–1441. [Google Scholar] [CrossRef]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biot. 2019, 103, 7425–7450. [Google Scholar] [CrossRef] [PubMed]
- Giannoukos, S.; Agapiou, A.; Brkić, B.; Taylor, S. Volatolomics: A broad area of experimentation. J. Chromatogr. B 2019, 1105, 136–147. [Google Scholar] [CrossRef]
- Lytou, A.E.; Panagou, E.Z.; Nychas, G.-J.E. Volatilomics for food quality and authentication. Curr. Opin. Food Sci. 2019, 28, 88–95. [Google Scholar] [CrossRef]
- Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds. Molecules 2021, 26, 644. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research progress of wine aroma components: A critical review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology: The Chemistry of Wine Stabilization and Treatments Volume 2; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Prusova, B.; Humaj, J.; Sochor, J.; Baron, M. Formation, Losses, Preservation and Recovery of Aroma Compounds in the Winemaking Process. Fermentation 2022, 8, 93. [Google Scholar] [CrossRef]
- Nykanen, L. Formation and occurrence of flavor compounds in wine and distilled alcoholic beverages. Am. J. Enol. Vitic. 1986, 37, 84–96. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, L.; Xing, G.; Xu, X.; Tu, C.; Dong, M. Effect of Co-Fermentation with Lactic Acid Bacteria and K. marxianus on Physicochemical and Sensory Properties of Goat Milk. Foods 2020, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and Its Importance to Wine Aroma—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Bagheri, B.; Bauer, F.F.; Cardinali, G.; Setati, M.E. Ecological interactions are a primary driver of population dynamics in wine yeast microbiota during fermentation. Sci. Rep. 2020, 10, 4911. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef]
- Ganigu’e, R.; Sanchez-Paredes, P.; Baneras, L.; Colprim, J. Low Fermentation pH Is a Trigger to Alcohol Production, but a Killer to Chain Elongation. Front. Microbiol. 2016, 7, 702. [Google Scholar] [CrossRef]
- Lai, Y.T.; Hsieh, C.W.; Lo, Y.C.; Liou, B.K.; Lin, H.W.; Hou, C.Y.; Cheng, K.C. Isolation and identification of aroma-producing non-Saccharomyces yeast strains and the enological characteristic comparison in wine making. LWT 2022, 154, 112653. [Google Scholar] [CrossRef]
- Arslan, E.; Çelik, Z.D.; Cabaroğlu, T. Effects of Pure and Mixed Autochthonous Torulaspora delbrueckii and Saccharomyces cerevisiae on Fermentation and Volatile Compounds of Narince Wines. Foods 2018, 7, 147. [Google Scholar] [CrossRef]
- Suklje, K.; Zhang, X.; Antalick, G.; Clark, A.C.; Deloire, A.; Schmidtke, L.M. Berry shriveling significantly alters Shiraz (Vitis vinifera L.) grape and wine chemical composition. J. Agric. Food Chem. 2016, 64, 870–880. [Google Scholar] [CrossRef]
- Roland, A.; Schneider, R.; Razungles, A.; Cavelier, F. Varietal thiols in wine: Discovery, analysis and applications. Chem. Rev. 2011, 111, 7355–7376. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.; Lopez, R.; Cacho, J.F.; Ferreira, V. Prediction of aged red aroma properties from aroma chemical composition. Partial least-squares regression models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-Navajas, M.P.; Campo, E.; Fernández-Zurbano, P.; Valentin, D.; Ferreira, V. An assessment of the effects of wine volatiles on the perception of taste and astringency in wine. Food Chem. 2010, 121, 1139–1149. [Google Scholar] [CrossRef]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Effect of Co-Inoculation of Candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the Industrial Production of Negroamaro Wine in Apulia (Southern Italy). Microorganisms 2020, 8, 726. [Google Scholar] [CrossRef] [PubMed]
- Pubchem Database. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 5 April 2024).
- Fujii, T.; Nagasawa, N.; Iwamatsu, A.; Bogaki, T.; Tamai, Y.; Hamachi, M. Molecular cloning, sequence analysis, and expression of the yeast alcohol acetyltransferase gene. Appl. Environ. Microbiol. 1994, 60, 2786–2792. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef]
- Lee, S.; Villa, K.; Patino, H. Yeast Strain Development for Enhanced Production of Desirable Alcohols/Esters in Beer. J. Am. Soc. Brew. Chem. 1995, 53, 153–156. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Fukushige, T.; Yonezawa, T.; Sone, H. Genetic and physiological analysis of branched-chain alcohols and isoamyl acetate production in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2002, 59, 501–508. [Google Scholar]
- De Revel, G.; Martin, N.; Pripis-Nicolau, L.; Lonvaud-Funel, A.; Bertrand, A. Contribution to the knowledge of malolactic fermentation influence on wine aroma. J. Agric. Food Chem. 1999, 47, 4003–4008. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.A.; Alegría, E.G.; Polo, M.C.; Tenorio, C.; Martín Álvarez, P.J.; Calvo de la Banda, M.T.; Ruiz-Larrea, F.; Moreno-Arribas, M.V. Wine volatile and amino acid composition after malolactic fermentation: Effect of Oenococcus oeni and Lactobacillus plantarum starter cultures. J. Agric. Food Chem. 2005, 53, 8729–8735. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of Grape and Wine Aroma. Part 1. Chemical Components and Viticultural Impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Pérez-Coello, M.S.; González-Viñas, M.A.; García-Romero, E.; Díaz-Maroto, M.C.; Cabezudo, M.D. Influence of storage temperature on the volatile compounds of young white wines. Food Control. 2003, 14, 301–306. [Google Scholar] [CrossRef]
- Ramey, D.D.; Ough, C.S. Volatile ester hydrolysis or formation during storage of model solutions and wines. J. Agric. Food Chem. 1980, 28, 928–934. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Guth, H. Quantification and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Etiévant, P.X. Wine. In Volatile Compounds in Food; Maarse, H., Ed.; Dekker: New York, NY, USA, 1991; pp. 483–544. [Google Scholar]
- Eglinton, J.M.; Henschke, P.A. Yeast starter cultures: I. Physiological basis for fermentative activity. Aus. NZ. Wine Ind. J. 1991, 61, 43–47. [Google Scholar]
- Erasmus, D.J.; Cliff, M.; van Vuuren, H.J. Impact of yeast strain on the production of acetic acid, glycerol, and the sensory attributes of icewine. Am. J. Enol. Vitic. 2004, 55, 371–378. [Google Scholar] [CrossRef]
- Ravaglia, S.; Delfini, C. Production of medium chain fatty acids and their ethyl esters by yeast strains isolated from musts and wines. Ital. J. Food Sci. 1993, 5, 21–36. [Google Scholar]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Silva, R.D.; Chaves, S.R.; Sousa, M.J.; Corte-Real, M. The impact of acetate metabolism on yeast fermentative performance and wine quality: Reduction of volatile acidity of grape musts and wines. Appl. Microbiol. Biotechnol. 2011, 89, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Strauss, C.R.; Wilson, B.; Gooley, P.R.; Williams, P.J. Role of monoterpenes in grape and wine flavor. ACS Symp. Ser. 1986, 317, 222–242. [Google Scholar]
- Maicas, S.; Mateo, J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Ferreira, A.; Clímaco, M.C.; Mendes Faia, A. The role of non-Saccharomyces species in releasing glycosidic bound fraction of grape aroma components—A preliminary study. J. Appl. Microbiol. 2001, 91, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Spagna, G. β-Glucosidase in cellular and acellular form for winemaking application. Enzym. Microb. Technol. 2007, 40, 382–389. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Alastruey-Izquierdo, A.; Navascués, E.; Marquina, D.; Santos, A. Unraveling the enzymatic basis of wine “flavorome”: A phylo-functional study of wine related yeast species. Front. Microbiol. 2016, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Spano, G.; Rinaldi, A.; Ugliano, M.; Moio, L.; Beneduce, L.; Massa, S. A β-glucosidase gene isolated from wine Lactobacillus plantarum is regulated by abiotic stresses. J. Appl. Microbiol. 2005, 98, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Rosi, I.; Vinnella, M.; Domizio, P. Characterization of β-glucosidase activity in yeast of enological origin. J. App. Bacteriol. 1994, 77, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Lerm, E.; Engelbrecht, L.; du Toit, M. Selection and Characterisation of Oenococcus oeni and Lactobacillus plantarum South African Wine Isolates for Use as Malolactic Fermentation Starter Cultures. S. Afr. J. Enol. Vitic. 2011, 32, 280–295. [Google Scholar] [CrossRef]
- Saguir, F.; Campos, I.E.L.; Maturano, C.; de Nadra, M.C.M. Identification of dominant lactic acid bacteria isolated from grape juices. Assessment of its biochemical activities relevant to flavor development in wine. Int. J. Wine Res. 2009, 1, 175–185. [Google Scholar]
- Michlmayr, H.; Nauer, S.; Brandes, W.; Schümann, C.; Kulbe, K.D.; del Hierro, A.M.; Eder, R. Release of wine monoterpenes from natural precursors by glycosidases from Oenococcus oeni. Food Chem. 2012, 135, 80–87. [Google Scholar] [CrossRef]
- Michlmayr, H.; Schümanna, C.; Barreira Braz da Silva, N.M.; Kulbe, K.D.; del Hierro, A.M. Isolation and basic characterization of a β-glucosidase from a strain of Lactobacillus brevis isolated from a malolactic starter culture. J. Appl. Microbiol. 2010, 108, 550–559. [Google Scholar] [CrossRef]
- Ugliano, M.; Bartowsky, E.J.; McCarthy, J.; Moio, L.; Henschke, P.A. Hydrolysis and transformation of grape glycosidically bound volatile compounds during fermentationwith three Saccharomyces yeast strains. J. Agric. Food Chem. 2006, 54, 6322–6331. [Google Scholar] [CrossRef]
- Gil, J.V.; Manzanares, P.; Genoves, S.; Valles, S.; Gonzalez-Candelas, L. Over-production of the major exoglucanase of Saccharomyces cerevisiae leads to an increase in the aroma of wine. Int. J. Food Microbiol. 2005, 103, 57–68. [Google Scholar] [CrossRef]
- Gunata, Z.; Bitteur, S.; Brillouet, J.-M.; Bayonove, C.; Cordonnier, R. Sequential enzymic hydrolysis of potentially aromatic glycosides from grape. Carbohydr. Res. 1988, 184, 139–149. [Google Scholar] [CrossRef]
- Sarry, J.-E.; Gunata, Z. Plant and microbial glycoside hydrolases: Volatile release from glycosidic aroma precursors. Food Chem. 2004, 87, 509–521. [Google Scholar] [CrossRef]
- Ugliano, M.; Moio, L. The influence of malolactic fermentation and Oenococcus oeni strain on glycosidic aroma precursors and related volatile compounds of red wine. J. Sci. Food Agric. 2006, 86, 2468–2476. [Google Scholar] [CrossRef]
- Cosme, F.; Inês, A.; Vilela, A. Microbial and Commercial Enzymes Applied in the Beverage Production Process. Fermentation 2023, 9, 385. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z.W. A Preliminary Study of Aroma Composition and Impact Odorants of Cabernet Franc Wines under Different Terrain Conditions of the Loess Plateau Region (China). Molecules 2018, 23, 1096. [Google Scholar] [CrossRef]
- Zhao, P.; Qian, Y.; He, F.; Li, H.; Qian, M. Comparative characterization of aroma compounds in merlot wine by lichrolut-en-based aroma extract dilution analysis and odor activity value. Chemosens. Percept. 2017, 10, 149–160. [Google Scholar] [CrossRef]
- Simpson, R.F. Some important aroma components of white wine. Food Technol. 1979, 31, 516–522. [Google Scholar]
- Yamamoto, T.; Matsuda, H.; Utsumi, Y.; Hagiwara, T.; Kanisawa, T. Synthesis and odor of optically active rose oxide. Tetrahedron Lett. 2002, 43, 9077–9080. [Google Scholar] [CrossRef]
- Li, Y.; H’eloir, M.C.; Zhang, X.; Geissler, M.; Trouvelot, S.; Jacquens, L.; Henkel, M.; Su, X.; Fang, X.; Wang, Q.; et al. Surfactin and fengycin contribute to the protection of a Bacillus subtilis strain against grape downy mildew by both direct effect and defence stimulation. Mol. Plant Pathol. 2019, 20, 1037–1050. [Google Scholar] [CrossRef]
- Zhao, X.; Ju, Y.; Wei, X.; Dong, S.; Sun, X.; Fang, Y. Significance and Transformation of 3-Alkyl-2-Methoxypyrazines through Grapes to Wine: Olfactory Properties, Metabolism, Biochemical Regulation, and the HP-MP Cycle. Molecules 2019, 24, 4598. [Google Scholar] [CrossRef]
- Rauhut, D. Usage and Formation of Sulphur Compounds. In Biology of Microorganisms on Grapes, in Must and in Wine, 1st ed.; König, H., Unden, G., Fröhlich, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Diaz-Maroto, M.; Schneider, R.; Baumes, R. Formation pathways of ethyl esters of branched short-chain fatty acids during wine aging. J. Agric. Food Chem. 2005, 53, 3503–3509. [Google Scholar] [CrossRef]
- Mazauric, J.-P.; Salmon, J.-P. Interactions between yeast lees and wine polyphenols during simulation of wine aging: I. Analysis of remnant polyphenolic compounds in the resulting wines. J. Agric. Food Chem. 2005, 53, 5647–5653. [Google Scholar] [CrossRef]
- Perez-Seradilla, J.; Luque de Castro, M. Role of lees in wine production: A review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef]
- Martińez Gil, A.; Alamo-Sanza, M.; Barrio-Galán, R.; Nevares, I. Alternative Woods in Oenology: Volatile Compounds Characterisation of Woods with Respect to Traditional Oak and Effect on Aroma in Wine, a Review. Appl. Sci. 2022, 12, 2101. [Google Scholar] [CrossRef]
- Naranjo, A.; Martínez-Lapuente, L.; Ayestarán, B.; Guadalupe, Z.; Pérez, I.; Canals, C.; Adell, E. Aromatic and sensory characterization of Maturana Blanca wines made with different technologies. Beverages 2021, 7, 10. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, X.; Mao, Y.; Zhao, D.; Li, Y. Influence of Saccharomyces cerevisiae autochthonous MQ3 strain on terpenes during the alcoholic fermentation of Chardonnay dry white wine. Aust. J. Grape Wine Res. 2021, 28, 41–49. [Google Scholar] [CrossRef]
- Vaquero, C.; Izquierdo-Cañas, P.M.; Mena-Morales, A.; Marchante-Cuevas, L.; Heras, J.M.; Morata, A. Use of Lachancea thermotolerans for biological vs chemical acidification at pilot-scale in white wines from warm areas. Fermentation 2012, 7, 193. [Google Scholar] [CrossRef]
- Zhao, L.; Ruan, S.; Yang, X.; Chen, Q.; Shi, K.; Lu, K.; He, L.; Liu, S.; Song, Y. Characterization of volatile aroma compounds in litchi (Heiye) wine and distilled spirit. Food Sci. Nutr. 2021, 9, 5914–5927. [Google Scholar] [CrossRef]
- Cravero, M.C. Musty and moldy taint in wines: A review. Beverages 2020, 6, 41. [Google Scholar] [CrossRef]
- Vilela, A.; Ferreira, R.; Nunes, F.; Correia, E. Creation and acceptability of a fragrance with a characteristic Tawny Port winelike aroma. Foods 2020, 9, 1244. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Morata, A.; Loira, I.; Escott, C.; Suarez Lepe, J.A. Evolution of the phenolic fraction and aromatic profile of red wines aged in oak barrels. ACS Omega 2020, 5, 7235–7243. [Google Scholar] [CrossRef]
- Lopez, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- AICV. The European Cider & Fruit Wine Association, European Cider Trends. 2023. Available online: https://aicv.org/files/attachments/.576/AICV_Cider_Trends_2023.pdf (accessed on 12 March 2023).
- Ruppert, V.; Innerhofer, G.; Voit, J.; Hiden, P.; Siegmund, B. The Impact of the Fermentation Strategy on the Flavour Formation of Ilzer Rose (Malus domestica Borkh.) Apple Wine. Foods 2021, 10, 2348. [Google Scholar] [CrossRef]
- Ezemba, C.C.; Anakwenze, V.N.; Ezemba, A.S. Wine Production from Apple (Malus pumila) Using Yeast Isolated from Palmwine. Curr. J. Appl. Sci. Technol. 2022, 41, 1–6. [Google Scholar] [CrossRef]
- Sukhvir, S.; Kocher, G.S. Development of apple wine from Golden Delicious cultivar using a local yeast isolate. J. Food Sci. Technol. 2019, 56, 2959–2969. [Google Scholar] [CrossRef]
- Peng, B.; Li, F.; Cui, L.; Guo, Y. Effects of fermentation temperature on key aroma compounds and sensory properties of apple wine. J. Food Sci. 2015, 80, S2937–S2943. [Google Scholar] [CrossRef]
- Satora, P.; Semik-Szczurak, D.; Tarko, T.; Bułdys, A. Influence of Selected Saccharomyces and Schizosaccharomyces Strains and Their Mixed Cultures on Chemical Composition of Apple Wines. J. Food Sci. 2018, 83, 424–431. [Google Scholar] [CrossRef]
- Anton, M.J.; Valles, B.S.; Hevia, A.G.; Lobo, A.P. Aromatic Profile of Ciders by Chemical Quantitative, Gas Chromatography-Olfactometry, and Sensory Analysis. J. Food Sci. 2014, 79, S92–S99. [Google Scholar] [CrossRef]
- Tangüler, H.; Erten, H. The influence of two yeast strains on fermentation and flavor composition of cider. Flavour Fragr. J. 2022, 37, 144–153. [Google Scholar] [CrossRef]
- Mao, D.; Liu, H.; Li, Z.; Niu, Y.; Xiao, Z.; Zhang, F.; Zhu, J. Impact of sensory interactions among volatile compounds of juice of Red Delicious apples. Hortic. Environ. Biotechnol. 2020, 61, 197–206. [Google Scholar] [CrossRef]
- Satora, P.; Tarko, T.; Duda-Chodak, A.; Sroka, P.; Tuszyński, T.; Czepielik, M. Influence of prefermentative treatments and fermentation on the antioxidant and volatile profiles of apple wines. J. Agric. Food Chem. 2009, 57, 11209–11217. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar] [PubMed]
- Pino, J.A.; Queris, O. Analysis of volatile compounds of mango wine. Food Chem. 2011, 125, 1141–1146. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, T.H.; Um, B.H.; Sohn, E.H.; Han, W.C.; Ji, S.H.; Jang, K.H. Evaluation of physicochemical properties and fermenting qualities of apple wines added with medicinal herbs. Food Sci. Biotechnol. 2013, 22, 1039–1046. [Google Scholar] [CrossRef]
- Villiere, A.; Arvisenet, G.; Bauduin, R.; Le Quere, J.M.; Serot, T. Influence of cider-making process parameters on the odourant volatile composition of hard ciders. J. Inst. Brew. 2015, 121, 95–105. [Google Scholar] [CrossRef]
- Malherbe, S.; Fromion, V.; Hilgert, N.; Sablayrolles, J.M. Modeling the effects of assimilable nitrogen and temperature on fermentation kinetics in enological conditions. Biotechnol. Bioeng. 2004, 86, 261–272. [Google Scholar] [CrossRef]
- Yilmaztekin, M.; Cabaroglu, T.; Erten, H. Effects of fermentation temperature and aeration on production of natural isoamyl acetate by Williopsis saturnus var. saturnus. BioMed Res. Int. 2013, 2013, 870802. [Google Scholar]
- Beltran, G.; Novo, M.; Guillamon, J.M.; Mas, A.; Roses, N. Effect of fermentation temperature and culture media on the yeast lipid composition and wine volatile compounds. Int. J. Food Microbiol. 2008, 121, 169–177. [Google Scholar] [CrossRef]
- Rühmer, T.; Voit, J. Most und Saft—Die veredelte Form von Äpfeln. Haidegger Perspekt. 2020, 3, 6–9. [Google Scholar]
- Herrero, M.; Garcia, L.A.; Diaz, M. The effect of SO2 on the production of ethanol, acetaldehyde, organic acids, and flavor volatiles during industrial cider fermentation. J. Agric. Food Chem. 2003, 51, 3455–3459. [Google Scholar] [CrossRef]
- Kelkar, S.; Dolan, K. Modeling the effects of initial nitrogen content and temperature on fermentation kinetics of hard cider. J. Food Eng. 2012, 109, 588–596. [Google Scholar] [CrossRef]
- Dos Santos, C.M.E.; Alberti, A.; Pietrowski, G.A.M.; Zielinski, A.A.F.; Wosiacki, G.; Nogueira, A.; Jorge, R.M.M. Supplementation of amino acids in apple must for the standardization of volatile compounds in ciders. J. Inst. Brew. 2016, 122, 334–341. [Google Scholar] [CrossRef]
- Lobo, A.P.; Bedriñana, R.P.; Madrera, R.R.; Valles, B.S. Aromatic, olfactometric and consumer description of sweet ciders obtained by cryo-extraction. Food Chem. 2021, 338, 127829. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.; Zhao, G.; Li, J. Rapid Analysis of Flavor Volatiles in Apple Wine Using Headspace Solid-Phase Microextraction. J. Inst. Brew. 2004, 110, 57–65. [Google Scholar] [CrossRef]
- Chen, X.; Lin, M.; Hu, L.; Xu, T.; Xiong, D.; Li, L.; Zhao, Z. Research on the Effect of Simultaneous and Sequential Fermentation with Saccharomyces cerevisiae and Lactobacillus plantarum on Antioxidant Activity and Flavor of Apple Cider. Fermentation 2023, 9, 102. [Google Scholar] [CrossRef]
- Cesnik, U.; Martelanc, M.; Øvsthus, I.; Radovanovic Vukajlovic, T.; Hosseini, A.; Mozetic Vodopivec, B.; Butinar, L. Functional Characterization of Saccharomyces Yeasts from Cider Produced in Hardanger. Fermentation 2023, 9, 824. [Google Scholar] [CrossRef]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Li, S.; Nie, Y.; Ding, Y.; Zhao, J.; Tang, X. Effects of pure and mixed koji cultures with Saccharomyces cerevisiae on apple homogenate cider fermentation. J. Food Proces. Preserv. 2015, 39, 2421–2430. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, F.; Dziugan, P.; Yao, Y.; Zhang, J.; Lv, Z.; Zhang, B. Development of Organic Acids and Volatile Compounds in Cider during Malolactic Fermentation. Czech J. Food Sci. 2014, 32, 69–76. [Google Scholar] [CrossRef]
- Sun, S.Y.; Che, C.Y.; Sun, T.F.; Lv, Z.Z.; He, S.X.; Gu, H.N.; Shen, W.J.; Chi, D.C.; Gao, Y. Evaluation of sequential inoculation of Saccharomyces cerevisiae and Oenococcus oeni strains on the chemical and aromatic profiles of cherry wines. Food Chem. 2013, 138, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhang, X.; Xiao, Z.; Song, S.; Eric, K.; Jia, C.; Yub, H.; Zhub, J. Characterization of odor-active compounds of various cherry wines by gas chromatography–mass spectrometry, gas chromatography–olfactometry and their correlation with sensory attributes. J. Chromatogr. B 2011, 879, 2287–2293. [Google Scholar] [CrossRef]
- Sun, S.Y.; Jiang, W.G.; Zhao, Y.P. Evaluation of different Saccharomyces cerevisiae strains on the profile of volatile compounds and polyphenols in cherry wines. Food Chem. 2011, 27, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liu, S.; Gu, Y.; Xu, N.; Shang, Y.; Zhu, J. Discrimination of Cherry Wines Based on Their Sensory Properties and Aromatic Fingerprinting using HS-SPME-GC-MS and Multivariate Analysis. J. Food Sci. 2014, 79, C284–C294. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Gong, H.S.; Jiang, X.M.; Zhao, Y.P. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae on alcoholic fermentation behaviour and wine aroma of cherry wines. Food Microbiol. 2014, 44, 15–23. [Google Scholar] [CrossRef]
- Li, H.M.; Jiang, D.Q.; Dai, Z.G.; Zhang, Y.S.; Zhang, Y.; Sun, S.Y.; Zhao, Y.P. Aromatic property of cherry wine produced by malolactic fermentation of controlled and spontaneous on the bacterial evolution. Int. J. Food Prop. 2019, 22, 1270–1282. [Google Scholar] [CrossRef]
- Xiao, Z.-B.; Zhang, N.; Niu, Y.-W.; Feng, T.; Tian, H.-X.; Zhu, J.-C.; Yu, H.-Y. Multivariate Classification of Cherry Wines Based on Headspace Solid Phase Microextraction and Gas Chromatography-Mass Spectrometry of Volatile Compounds. Int. J. Food Prop. 2015, 18, 1272–1287. [Google Scholar] [CrossRef]
- Sun, S.Y.; Gong, H.S.; Liu, W.L.; Jin, C.W. Application and validation of autochthonous Lactobacillus plantarum starter cultures for controlled malolactic fermentation and its influence on the aromatic profile of cherry wines. Food Microbiol. 2016, 55, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhou, X.; Niu, Y.; Yu, D.; Zhu, J.; Zhu, G. Optimization and application of headspace-solid-phasemicro-extraction coupled with gas chromatography–massspectrometry for the determination of volatile compounds in cherrywines. J. Chromatogr. B 2015, 978–979, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Zhang, Q.F.; Liu, W.L.; Li, H.M.; Liu, Y.L.; Jiang, X.M.; Zhao, Y.P. Influence of maceration techniques on the chemical, aromatic, sensory and biogenic amine profiles of cherry wine. J. Inst. Brew. 2018, 124, 477–484. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhou, X.; Xiao, Z.; Niu, Y. Characterization of the differences in the aroma of cherry wines from different price segments using gas chromatography–mass spectrometry, odor activity values, sensory analysis, and aroma reconstitution. Food Sci. Biotechnol. 2017, 26, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Chu, Q.; Li, C.; Ma, J.; Zhu, Y.; Sun, S.; Wang, P. Early transcriptional response of Saccharomyces cerevisiae in mixed culture with Torulaspora delbrueckii in cherry wine. LWT 2023, 181, 114749. [Google Scholar] [CrossRef]
- Sun, S.Y.; Gong, H.S.; Zhao, Y.P.; Liua, W.L.; Jin, C.W. Sequential culture with Torulaspora delbrueckii and Saccharomyces cerevisiae and management of fermentation temperature to improve cherry wine quality. Sci. Food Agric. 2016, 96, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, X.; Li, X.; Sheng, X.; Li, T.; Tang, W.; Yu, Z.; Wang, Y. Effect of Hanseniaspora uvarum–Saccharomyces cerevisiae Mixed Fermentation on Aroma Characteristics of Rosa roxburghii Tratt, Blueberry, and Plum Wines. Molecules 2022, 27, 8097. [Google Scholar] [CrossRef]
- Choi, K.-T.; Lee, S.-B.; Choi, J.-S.; Park, H.-D. Influence of Different Pretreatments and Chaptalization Types on the Physiological Characteristics and Antioxidant Activity of Apricot (Prunus armeniaca L.) Wine. Ital. J. Food Sci. 2020, 32, 912–927. [Google Scholar]
- Liang, H.; Gao, D.; Wang, C.; Gao, H.; Guo, Y.; Zhao, Z.; Shi, H. Effect of Fermentation Strategy on the Quality and Aroma Characteristics of Yellow Peach Wines. Fermentation 2022, 8, 604. [Google Scholar] [CrossRef]
- Liu, G.; Wei, P.; Tang, Y.; Pang, Y.; Sunm, J.; Li, J.; Rao, C.; Wu, C.; He, X.; Li, L.; et al. Evaluation of Bioactive Compounds and Bioactivities in Plum (Prunus salicina Lindl.) Wine. Front. Nutr. 2021, 8, 766415. [Google Scholar] [CrossRef]
- Niyomvong, N.; Trakunjae, C.; Boondaeng, A. Fermentation Characteristics and Aromatic Profiles of Plum Wines Produced with Hanseniaspora thailandica Zal1 and Common Wine Yeasts. Molecules 2023, 28, 3009. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, M.; Ouyang, Y.; Zhao, X.; Ju, Y.; Fang, Y. Comparative study of aromatic compounds in fruit wines from raspberry, strawberry, and mulberry in central Shaanxi area. Food Nutr. Res. 2015, 59, 29290. [Google Scholar] [CrossRef] [PubMed]
- Butkhup, L.; Jeenphakdee, M.; Jorjong, S.; Samappito, S.; Samappito, W.; Chowtivannakul, S. HS-SPME-GC-MS Analysis of Volatile Aromatic Compounds in Alcohol Related Beverages Made with Mulberry Fruits. Food Sci. Biotechnol. 2011, 20, 1021–1032. [Google Scholar] [CrossRef]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Vilanova, M.; Teixeira, J.A.; Almeida e Silva, J.B.; Schwan, R.F. Raspberry (Rubus idaeus L.) wine: Yeast selection, sensory evaluation and instrumental analysis of volatile and other compounds. Food Res. Int. 2010, 43, 2303–2314. [Google Scholar] [CrossRef]
- Li, X.; Xia, X.; Wang, Z.; Wang, Y.; Dai, Y.; Yin, L.; Xu, Z.; Zhou, J. Cloning and expression of Lactobacillus brevis β-glucosidase and its effect on the aroma of strawberry wine. J. Food Process. Preserv. 2022, 46, e16368. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Zhao, N.; Xu, J.; Qi, Y.; Wei, X.; Fan, M. Performance of a novel β-glucosidase BGL0224 for aroma enhancement of Cabernet Sauvignon wines. LWT 2021, 144, 111244. [Google Scholar] [CrossRef]
- Hu, L.; Liu, R.; Wang, X.; Zhang, X. The Sensory Quality Improvement of Citrus Wine through Co-Fermentations with Selected Non-Saccharomyces Yeast Strains and Saccharomyces cerevisiae. Microorganisms 2020, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Dellacassa, E.; Trenchs, O.; Fariña, L.; Debernardis, F.; Perez, G.; Boido, E.; Carrau, F. Pineapple (Ananas comosus L. Merr.) wine production in Angola: Characterisation of volatile aroma compounds and yeast native flora. Int. J. Food Microbiol. 2017, 241, 161–167. [Google Scholar]
- Selli, S.; Cabaroglu, T.; Canbas, A. Flavour components of orange wine made from a Turkish cv. Kozan. Int. J. Food Sci. Technol. 2003, 38, 587–593. [Google Scholar] [CrossRef]
- Hu, L.; Wang, J.; Ji, X.; Liu, R.; Chen, F.; Zhang, X. Selection of non-Saccharomyces yeasts for orange wine fermentation based on their enological traits and volatile compounds formation. J. Food Sci. Technol. 2018, 55, 4001–4012. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Q.; Hu, X.; Wu, W.; Zhang, Y.; Liu, S.; Li, C. Evaluation of different Saccharomyces cerevisiae strains on the profile of volatile compounds in pineapple wine. J. Food Sci. Technol. 2018, 55, 4119–4130. [Google Scholar] [CrossRef] [PubMed]
- Selli, S.; Canbas, A.; Varlet, V.; Kelebek, H.; Prost, C.; Serot, T. Characterization of the Most Odor-Active Volatiles of Orange Wine Made from a Turkish cv. Kozan (Citrus sinensis L. Osbeck). J. Agric. Food Chem. 2008, 56, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Teixeira, J.A.; de Almeida e Silva, J.B.; Schwan, R.F. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jaboticaba and umbu. LWT 2010, 43, 1564–1572. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Zhu, Y.; Kortesniemi, M.; Zhu, B.; Li, H. Aromatic Characteristics of Passion Fruit Wines Measured by E-Nose, GC-Quadrupole MS, GC-Orbitrap-MS and Sensory Evaluation. Foods 2022, 11, 3789. [Google Scholar] [CrossRef] [PubMed]
- Salas-Millán, J.Á.; Aznar, A.; Conesa, E.; Conesa-Bueno, A.; Aguayo, E. Fruit Wine Obtained from Melon by-Products: Physico-Chemical and Sensory Analysis, and Characterization of Key Aromas by GC-MS. Foods 2022, 11, 3619. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.A.; de Oliveira Lima, L.C.; Dias, D.R.; Nunes, C.A.; Schwan, R.F. Effects of spontaneous and inoculated fermentation on the volatile profile of lychee (Litchi chinensis Sonn) fermented beverages. Int. J. Food Sci. Technol. 2010, 45, 2358–2365. [Google Scholar] [CrossRef]
- Cardinale, M.; Trinchera, R.; Natrella, G.; Difonzo, G.; De Benedittis, C.; D’amato, I.; Mascellani, M.; Paradiso, V.M.; Rustioni, L. Dynamics of the Fermentation Process and Chemical Profiling of Pomegranate (Punica granatum L.) Wines Obtained by Different CultivarYeast Combinations. Foods 2021, 10, 1913. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.E.S.; Pantoja, L.; Duarte, W.F.; Collela, C.F.; Valarelli, L.T.; Schwan, R.F.; Dias, D.R. Fruit wine produced from cagaita (Eugenia dysenterica DC) by both free and immobilised yeast cell fermentation. Food Res. Int. 2011, 44, 2391–2400. [Google Scholar] [CrossRef]
- Lai, Y.T.; Chen, C.H.; Lo, Y.C.; Hsieh, C.W.; Hsu, F.C.; Cheng, K.C. Application of aroma-producing yeasts and ageing technology in Kyoho-fortified wine. Eur. Food Res. Technol. 2023, 249, 2849–2860. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Li, Y.; Liu, S.; Tu, Q.; Yuan, C. Characterization of wine volatile compounds from different regions and varieties by HS-SPME/GC-MS coupled with chemometrics. Curr. Res. Food Sci. 2023, 6, 100418. [Google Scholar] [CrossRef]
- Tao, Y.; Li, H.; Wang, H.; Zhang, L. Volatile compounds of young Cabernet Sauvignon red wine from Changli County (China). J. Food Comp. Anal. 2008, 21, 689–694. [Google Scholar] [CrossRef]
- Han, S.; Yang, J.; Choi, K.; Kim, J.; Adhikari, K.; Lee, J. Chemical Analysis of Commercial White Wines and Its Relationship with Consumer Acceptability. Foods 2022, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Amores-Arrocha, A.; Sancho-Galán, P.; Jiménez-Cantizano, A.; Palacios, V. A Comparative Study on Volatile Compounds and Sensory Profile of White and Red Wines Elaborated Using Bee Pollen versus Commercial Activators. Foods 2021, 10, 1082. [Google Scholar] [CrossRef] [PubMed]
- Sabon, I.; De Revel, G.; Kotseridis, Y.; Bertrand, A. Determination of Volatile Compounds in Grenache Wines in Relation with Different Terroirs in the Rhone Valley. J. Agric. Food Chem. 2002, 50, 6341–6345. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Liu, M.; Li, J.; Li, Y.; Li, H.; Xu, G. Headspace Solid-Phase Micro-extraction for Determination of Volatile Organic Compounds in Apple Using Gas Chromatography–Mass Spectrometry. Food Anal. Methods 2022, 15, 2734–2743. [Google Scholar] [CrossRef]
- Dias, D.R.; Duarte, W.F.; Schwan, R. Methods of Evaluation of Fruit Wines. In Science and Technology of Fruit Wine Production; Kosseva, M.R., Joshi, V.K., Panesar, P.S., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 227–252. [Google Scholar]
- Sousa, F.M.; Ferreira, R.J.R.; De Sá, S.V.M.; Cunha, S.C.S.; De Oliveira Fernandes, J. Novel analytical approach to assess the profile of volatile phenols in Portuguese red wines. Aust. J. Grape Wine Res. 2020, 26, 90–100. [Google Scholar] [CrossRef]
- Sroka, P.; Tarko, T.; Duda, A. The Impact of Furfural on the Quality of Meads. Molecules 2024, 29, 29. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Pati, S.; Palombi, L.; Grieco, F.; Losito, I. Use of Multivariate Statistics in the Processing of Data on Wine Volatile Compounds Obtained by HS-SPME-GC-MS. Foods 2022, 11, 910. [Google Scholar] [CrossRef] [PubMed]
- Beckner Whitener, M.E.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME–GC×GC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12, 53. [Google Scholar] [CrossRef]
- Özen, M.; Özdemir, N.; Filiz, B.E.; Budak, N.H.; Kök-Taş, T. Sour cherry (Prunus cerasus L.) vinegars produced from fresh fruit or juice concentrate: Bioactive compounds, volatile aroma compounds and antioxidant capacities. Food Chem. 2020, 309, 125664. [Google Scholar] [CrossRef]
- Kim, B.H.; Park, S.K. Volatile aroma and sensory analysis of black raspberry wines fermented by different yeast strains. J. Inst. Brew. 2015, 121, 87–94. [Google Scholar] [CrossRef]
- Kafkas, E.; Cabaroglu, T.; Selli, S.; Bozdoğan, A.; Kürkçüoğlu, M.; Paydaş, S.; Başer, K.H.C. Identification of volatile aroma compounds of strawberry wine using solid-phase microextraction techniques coupled with gas chromatography–mass spectrometry. Flavour Frag. J. 2006, 21, 68–71. [Google Scholar] [CrossRef]
- Arcari, S.G.; Caliari, W.; Sganzerla, M.; Godoy, H.T. Volatile composition of Merlot red wine and its contribution to the aroma: Optimization and validation of analytical method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef] [PubMed]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zin, C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Gupta, S.; Variyar, P.S.; Sharma, A. Application of mass spectrometry based electronic nose and chemometrics for fingerprinting radiation treatment. Radiat. Phys. Chem. 2015, 106, 348–354. [Google Scholar] [CrossRef]
- Piergiovanni, M.; Carlin, S.; Lotti, C.; Vrhovsek, U.; Mattivi, F. Development of a Fully Automated Method HS-SPME-GC-MS/MS for the Determination of Odor-Active Carbonyls in Wines: A “Green” Approach to Improve Robustness and Productivity in the Oenological Analytical Chemistry. J. Agric. Food Chem. 2024, 72, 1995–2007. [Google Scholar] [CrossRef] [PubMed]
- Šorgic, S.; Sredovic Ignjatovic, I.; Antic, M.; Šacirovic, S.; Pezo, L.; Cejic, V.; Ðurovic, S. Monitoring of the Wines’ Quality by Gas Chromatography: HSS-GC/FID Method Development, Validation, Verification, for Analysis of Volatile Compounds. Fermentation 2022, 8, 38. [Google Scholar] [CrossRef]
- Liu, J.; Li, N.; Li, X.; Qian, W.; Liu, J.; Su, Q.; Chen, Y.; Zhang, B.; Zhu, B.; Cheng, X. A high-resolution Orbitrap Mass spectral library for trace volatile compounds in fruit wines. Sci. Data 2022, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Barbara, J.A.; Nicolli, K.P.; Souza-Silva, E.A.; Biasoto, A.C.T.; Welke, J.E.; Zini, C.A. Volatile profile and aroma potential of tropical Syrah wines elaborated in different maturation and maceration times using comprehensive two-dimensional gas chromatography and olfactometry. Food Chem. 2020, 308, 125552. [Google Scholar] [CrossRef]
- Nicolli, K.P.; Biasoto, A.C.T.; Guerra, C.; dos Santos, H.; Correa, L.; Welke, J.E.; Zini, C.A. Effects of soil and vineyard characteristics on volatile, phenolic composition and sensory profile of cabernet sauvignon wines of Campanha Gaúcha. J. Brazil. Chem. Soc. 2020, 31, 1110–1124. [Google Scholar] [CrossRef]
- Welke, J.E.; Nicolli, K.P.; Hernandes, K.C.; Biasoto, A.C.T.; Zini, C.A. Adaptation of an olfactometric system in a GC-FID in combination with GC×GC/MS to evaluate odor-active compounds of wine. Food Chem. 2022, 370, 131004. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.-B.; Xiang, X.-F.; Qian, X.; Wang, J.-M.; Ling, M.-Q.; Zhu, B.-Q.; Liu, T.; Sun, L.-B.; Shi, Y.; Reynolds, A.G.; et al. Characterization and differentiation of key odor-active compounds of ‘Beibinghong’ icewine and dry wine by gas chromatography-olfactometry and aroma reconstitution. Food Chem. 2019, 287, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Tian, X.; Ma, Y.; Sun, Y.; Qi, X.; Miu, C.; Xu, Y. Aroma characteristics of Cabernet Sauvignon wines from Loess Plateau in China by QDA®, Napping® and GC–O analysis. Eur. Food Res. Technol. 2020, 246, 821–832. [Google Scholar] [CrossRef]
- Satora, P.; Pater, A. The Influence of Different Non-Conventional Yeasts on the Odour-Active Compounds of Produced Beers. Appl. Sci. 2023, 13, 2872. [Google Scholar] [CrossRef]
- Pater, A.; Satora, P.; Januszek, M. Effect of Coriander Seed Addition at Different Stages of Brewing on Selected Parameters of Low-Alcohol Wheat Beers. Molecules 2024, 29, 844. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.; Bauer, F.F.; Panzeri, V.; Buica, A. Investigation of olfactory interactions of low levels of five off-flavour causing compounds in a red wine matrix. Food Res. Int. 2020, 128, 108878. [Google Scholar] [CrossRef]
- Tomasino, E.; Song, M.; Fuentes, C. Odor perception interactions between free monoterpene isomers and wine composition of Pinot Gris wines. J. Agric. Food Chem. 2020, 68, 3220–3227. [Google Scholar] [CrossRef]
| Compound (IUPAC Name) | Amino Acid |
|---|---|
| Isobutanol (2-methylpropan-1-ol) | Valine |
| Isoamyl alcohol (3-methylbutan-1-ol) | Leucine |
| Tert-amyl alcohol (2-methylbutan-2-ol) | Isoleucine |
| Phenylethanol (2-phenylethanol) | Phenylalanine |
| Methionol (3-methylsulfanylpropan-1-ol) | Methionine |
| Compound (IUPAC Name) | Chemical Structure | Aroma Descriptor | Threshold [μg/L] | References |
|---|---|---|---|---|
| Ethyl acetate |  | Apple/fruity | 7500 | [20] |
| Hexyl acetate | 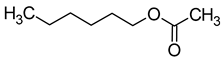 | Apple/fruity | 1500 | [20] |
| Isoamyl acetate (3-methylbutyl acetate) | 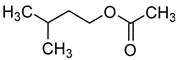 | Banana | 30 | [39] |
| Isobutyl acetate (2-methylpropyl acetate) |  | Solvent | 1600 | [24] |
| Phenylethyl acetate (2-phenylethyl acetate) | 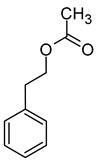 | Roses | 250 | [39] |
| Ethyl cinnamate (ethyl (E)-3-phenylprop-2-enoate) | 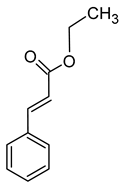 | Cinnamate/sweet | 1,1 | [40] |
| Ethyl isobutyrate (ethyl 2-methylpropanoate) | 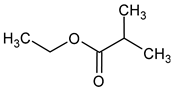 | Fruity/strawberry | 15 | [39] |
| Ethyl lactate (ethyl 2-hydroxypropanoate) | 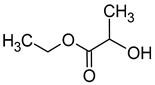 | Fruity | 154 | [41] |
| Ethyl butyrate (ethyl butanoate) | 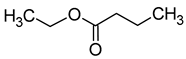 | Fruity | 20 | [39] |
| Ethyl 2-methylbutyrate (ethyl 2-methylbutanoate) | 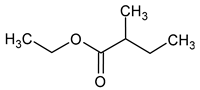 | Fruity/green apple | 1 | [39] |
| Ethyl isovalerate (ethyl 3-methylbutanoate) | 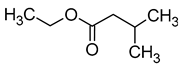 | Fruity/anise | 3 | [40] |
| Ethyl 3-hydroxybutyrate (ethyl 3-hydroxybutanoate) | 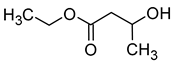 | Apple/tropical fruity | 21 | [20,24] |
| Ethyl caproate (ethyl hexanoate) | 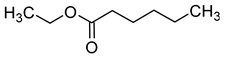 | Fruity/anise | 5 | [39] |
| Ethyl caprylate (ethyl octanoate) | 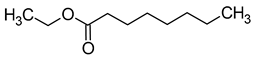 | Fruity/fresh | 2 | [39] |
| Ethyl caprate (ethyl decanoate) |  | Fruity | 200 | [40] |
| Ethyl laurate (ethyl dodecanoate) | 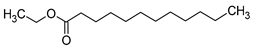 | Floral/waxy | 1500 | [20] |
| Diethyl succinate (diethyl butanedioate) | 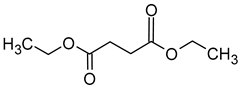 | Fruity | 200 | [41] |
| Compound (IUPAC Name) | Chemical Structure | Aroma Descriptor | Threshold [µg/L] | References |
|---|---|---|---|---|
| Butyric acid (butanoic acid) | 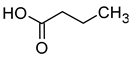 | Cheese, butter | 173 | [20] |
| Caproic acid (hexanoic acid) | 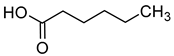 | Cheese, fatty, baked potato | 420 | [20] |
| Isobutyric acid (2-methylpropanoic acid) | 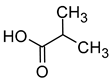 | Cheese | 2300 | [40] |
| 2-Methylbutyric acid (2-methylbutanoic acid) | 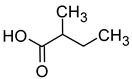 | Cheese | 33 | [40] |
| Isovaleric acid (3-methylbutanoic acid) | 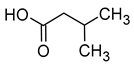 | Cheese | 33 | [40] |
| Caprylic acid (octanoic acid) | 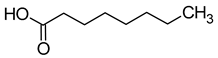 | Fatty/unpleasant | 500 | [40] |
| Capric acid (decanoic acid) | 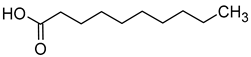 | Cheese | 1000 | [40] |
| Compound (IUPAC Name) | Chemical Structure | Aroma Descriptor | Threshold [µg/L] | References |
|---|---|---|---|---|
| 3-isopropyl-2-methoxypyrazine (2-methoxy-3-propan-2-ylpyrazine) | 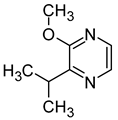 | Asparagus, green beans | 0.002 | [64] |
| 3-isobutyl-2-methoxypyrazine (2-methoxy-3-(2-methylpropyl)pyrazine) | 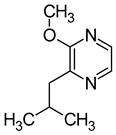 | Green pepper | 0.01–0.016 | [64] |
| 3-sec-butyl-2-methoxypyrazine (2-butan-2-yl-3-methoxypyrazine) | 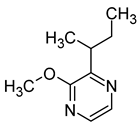 | Pea | 0.001–0.002 | [64] |
| Compound (IUPAC Name) | Chemical Structure | Aroma Descriptor | Threshold [µg/L] | References |
|---|---|---|---|---|
| 3-Mercapto-1-hexanol (3-sulfanylhexan-1-ol) | 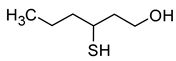 | Grapefruit, citrus peel, passion fruit | 0.06 | [23] |
| 3-Mercaptohexyl acetate (3-sulfanylhexyl acetate) |  | Passion fruit, box tree, boxwood | 0.004 | [23] |
| 4-Mercapto-4-methyl-2-pentanone (4-methyl-4-sulfanylpentan-2-one) | 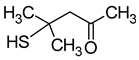 | Blackcurrant, box tree, passion fruit | 0.0008 | [23] |
| Compound (IUPAC Name) | Chemical Structure | Aroma Descriptor | Threshold [μg/L] | References |
|---|---|---|---|---|
| Furfural (2-Furancarboxaldehyde) | 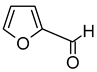 | Sweet, woody, almond, bread | 760–1500 | [73,74] |
| 5-Methylfurfural (5-Methyl-2-furancarboxaldehyde) | 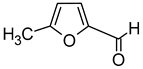 | Caramel, breads, coffee, almond, burnt, sugar | 1.1–1600 | [73,75] |
| 5-Hydroxymethylfurfural (5-Hydroxymethyl-2-furaldehyde) | 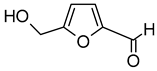 | Vanilla, candy, caramel | 10–10,000 | [73,76] |
| cis-Whiskey lactone ((4R,5R)-5-butyl-4-methyloxolan-2-one) |  | Woody, coconut, nutty, vanilla | 6–46 | [73,77] |
| trans-Whiskey lactone ((4S,5R)-5-butyl-4-methyloxolan-2-one) |  | Spicy, coconut, clove, woody, vanilla | 67–370 | [73,74] |
| Guaiacol (2-Methoxyphenol) | 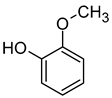 | Clove, smoke, spice, sweet, medicine | 9.5–20 | [73,78] |
| Eugenol (2-Methoxy-4-(prop-2-enyl) Phenol) | 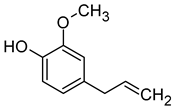 | Clove, cinnamon, honey, spicy, | 6 | [73,74] |
| Isoeugenol (1-Methoxy-4-(prop-2-enyl) phenol) | 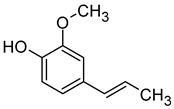 | Spicy, floral, clove, woody | 6 | [24,73] |
| Vanillin (4-Hydroxy-3- Methoxybenzaldehyde) | 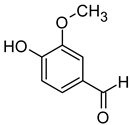 | Vanilla, sweet | 200–1000 | [73,79] |
| Syringaldehyde (4-Hydroxy-3,5-dimethoxybenzaldehyde) | 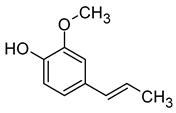 | Woody, sweet, vanilla | 50 | [73,80] |
| o-Cresol (2-benzyl-4-chlorophenol) | 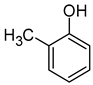 | Leather/spicy | 31 | [81] |
| Compounds | Gloster (W) | Champion (W) | Idared (W) | Fuji (W) | Ilzer Rose (W) | Gala (C) | G. Delicious (C) | Mix (C) | D. de Tresali (C) |
|---|---|---|---|---|---|---|---|---|---|
| Acetaldehyde | 1.4 | 6.9 | 6.4 | na | na | na | 21.47 | na | na |
| Acetone | 1.3 | 0.6 | 0.0 | na | na | na | 0.68 | na | na |
| Ethyl acetate | 5.8 | 27.0 | 30.1 | 54.8 | 0.32 | na | 6.79 | 17 | na |
| Isoamyl acetale | 0.7 | na | na | 16.66 | 1.02 | 0.14 | 0.11 | 0.39 | 1.74 |
| Ethyl hexanoate | 1.3 | na | na | 0.72 | 0.96 | 0.80 | 0.07 | 0.20 | 0.07 |
| Methanol | 18.8 | 121.0 | 20.0 | na | na | na | na | 35 | na |
| Propanol | 94.8 | 20.6 | 23.2 | 8.11 | na | na | 5.71 | 12 | na |
| Isobutanol | 237.8 | 95.0 | 110.0 | 184.85 | 0.44 | 0.05 | 7.34 | 32 | na |
| Amyl alcohols | 260.8 | 322.6 | 329.0 | 232.0 | 3.53 | 3.80 | 73.58 | 168 | na |
| Butanol | na | 20.2 | 27.1 | 8.46 | na | na | na | na | na |
| Pentanol | na | 1.3 | 1.4 | 0.06 | 0.04 | na | na | 0.04 | na |
| Hexanol | na | 5.2 | 6.2 | 2.18 | 0.43 | na | na | 0.04 | 0.59 |
| Phenylethanol | na | 38.4 | 44.2 | 43.5 | 0.63 | 0.02 | na | 17 | na |
| Acetic acid | na | 76.0 | 92.0 | 282.93 | na | na | na | na | na |
| Ethyl butyrate | na | na | na | 2.19 | 0.03 | na | 0.003 | 0.007 | 0.015 |
| 1-Butyl acetate | na | na | na | 0.32 | 0.03 | na | 0.008 | na | na |
| 1-Hexyl acetate | na | na | na | 0.32 | 1.07 | 0.05 | na | na | na |
| Ethyl heptanoate | na | na | na | 0.03 | na | na | na | na | na |
| Ethyl lactate | na | na | na | 4.63 | na | na | na | na | na |
| Ethyl octanoate | na | na | na | 1.09 | 2.08 | 0.53 | 0.12 | 0.18 | 0.95 |
| Heptanol | na | na | na | 0.07 | 0.01 | na | na | na | na |
| 2,3-Butanediol | na | na | na | 0.06 | 0.05 | 0.17 | 0.08 | na | na |
| 1-Octanol | na | na | na | 0.04 | 0.04 | na | na | na | na |
| Methyl decanoate | na | na | na | 0.01 | 0.04 | na | na | na | na |
| Diethyl succinate | na | na | na | 0.24 | na | na | na | na | na |
| Ethyl decanoate | na | na | na | 1.5 | 2.17 | na | na | na | na |
| Methyl dec-4-enoate | na | na | na | 0.01 | 0.01 | na | na | na | na |
| 3-Methylbutyl octanoate | na | na | na | 0.01 | 0.10 | na | na | na | na |
| Ethyl benzoate | na | na | na | 0.99 | 0.00 | na | na | na | na |
| Methyl laurate | na | na | na | 1.27 | na | na | na | na | na |
| Phenylethyl acetate | na | na | na | 7.7 | 0.22 | na | 0.04 | na | na |
| Ethyl laurate | na | na | na | 112.29 | na | na | na | na | na |
| Hexanoic acid | na | na | na | 4.75 | 0.40 | na | 0.04 | na | 2.89 |
| Ethyl myristate | na | na | na | 2.71 | na | na | na | na | na |
| Octanoic acid | na | na | na | 6.11 | 0.72 | na | 0.06 | na | 2.62 |
| Ethyl palmitate | na | na | na | 15.1 | na | na | na | na | na |
| Ethyl 9-hexadecenoate | na | na | na | 16.37 | na | na | na | na | na |
| Decanoic acid | na | na | na | 2.41 | na | na | 0.01 | na | 1.93 |
| Compound | W10 (Plum) | W11 (Apricot) | W12 (Peach) |
|---|---|---|---|
| Ethyl acetate | 52.50 | 729.35 | 333.65 |
| Isobutyl acetate | 2.28 | 42.19 | na |
| Ethyl butanoate | 8.86 | 43.24 | na |
| Butyl acetate | nd | 4.88 | na |
| Ethyl hexanoate | 80.78 | 749.92 | 382.72 |
| Hexyl acetate | 1.34 | 60.09 | 93.01 |
| Methyl octanoate | 1.40 | 34.89 | 2.56 |
| Ethyl octanoate | 0.73 | 2552.69 | 1491.2 |
| Isopentyl hexanoate | 1.60 | na | 3.84 |
| Ethyl nonanoate | 5.46 | 44.32 | 8.11 |
| Ethyl decanoate | 1.11 | 1840.95 | 866.56 |
| Isopentyl octanoate | 10.64 | na | 29.44 |
| Ethyl dodecanoate | 192.00 | 175.20 | na |
| Ethyl tetradecanoate | 8.00 | na | na |
| Ethyl hexadecanoate | 7.50 | na | na |
| Ethyl propionate | na | 18.65 | na |
| Ethyl isobutyrate | na | 11.51 | na |
| Propyl acetate | na | 24.83 | na |
| Isoamyl acetate | na | 2472.35 | 223.16 |
| Ethyl pentanoate | na | 4.24 | na |
| Ethyl heptanoate | na | 9.26 | na |
| Geranyl acetate | na | 96.95 | na |
| Ethyl benzoate | 1.91 | 315.95 | 34.13 |
| 2-Phenylethyl acetate | na | 42.38 | na |
| 1-Propanol | na | 85.48 | na |
| Isobutanol | na | 159.27 | 301.65 |
| Isoamyl alcohol | 300.00 | 2605.68 | 1043.60 |
| 1-Hexanol | na | 20.10 | 145.07 |
| 2,3-Butanediol | nd | 14.70 | na |
| 1-Octanol | nd | 13.62 | na |
| 1-Decanol | na | 5.13 | 1.28 |
| Benzyl alcohol | nd | 20.07 | 10.67 |
| Octanoic acid | 10.50 | na | 57.17 |
| Decanoic acid | 15.00 | na | na |
| Benzaldehyde | nd | na | 5.97 |
| 2-Phenylethanol | 28.56 | 201.16 | 67.41 |
| Hexanoic acid | na | na | 20.05 |
| Eugenol | 3.46 | na | na |
| Linalool | na | 731.90 | 23.47 |
| α-Terpineol | na | 135.46 | 1.28 |
| Citronellol | na | 18.48 | 3.41 |
| Geraniol | na | 40.89 | na |
| Compounds | W13 (Blueberry) | W14 (Raspberry) | W15 (Strawberry) | W16 (Mulberry) |
|---|---|---|---|---|
| Ethyl acetate | 90.00 | 65.83 | 37.84 | 31.41 |
| Ethyl butyrate | 1.34 | na | na | na |
| Isoamyl acetate | 17.60 | na | na | na |
| Ethyl isovalerate | 0.198 | na | na | na |
| Ethyl hexanoate | 47.33 | nd | 5.32 | 11.71 |
| Hexyl acetate | 1.34 | 19.31 | 37.62 | 48.18 |
| Ethyl caprylate | 211.70 | na | na | na |
| Ethyl caprate | 335.20 | na | na | na |
| Methyl salicylate | 0.60 | na | na | na |
| Ethyl phenylacetate | 5.62 | na | na | na |
| Ethyl laurate | 165.00 | na | na | na |
| Ethyl myristate | 4.00 | na | na | na |
| Ethyl octanoate | na | 5.20 | 42.82 | 23.76 |
| Ethyl decanoate | na | 1.25 | 2.11 | 1.16 |
| Diethyl succinate | na | 4.06 | 1.20 | 1.46 |
| 2-Phenylethyl acetate | na | 9.82 | 7.12 | 5.80 |
| Methanol | 2.40 | na | na | na |
| 1-Propanol | na | nd | 3.20 | nd |
| 2-Methylpropanol | na | 152.30 | 778.60 | 480.45 |
| 1-Butanol | na | nd | 19.40 | nd |
| 3-Methylbutanol | na | 3745.00 | 20,568.83 | 7885.75 |
| 1-Pentanol | na | 20.00 | nd | nd |
| 1-Hexanol | 8.00 | 5.03 | 25.21 | 51.73 |
| 1-Octanol | na | 286.53 | 116.60 | 122.49 |
| 2-Phenylethyl alcohol | 84.00 | 25.57 | 19.53 | 16.46 |
| Acetic acid | na | 163.63 | 67.08 | 69.68 |
| 2-Methylpropanoic acid | na | nd | 8.79 | 12.21 |
| Hexanoic acid | 1.26 | nd | nd | 7.97 |
| Octanoic acid | 6.50 | 1.55 | 2.70 | 9.08 |
| Decanoic acid | 8.00 | na | na | na |
| Lauric acid | 1.00 | na | na | na |
| Benzaldehyde | na | nd | 1.22 | 19.39 |
| Eugenol | 3.72 | na | na | na |
| Linalool | 17.82 | na | na | na |
| α-Terpineol | 3.50 | na | na | na |
| Compounds | W17 (Orange) | W18 (Orange) | W19 (Orange) | W20 (Ponkan Mandarin) | W21 (Pineapple) | W22 (Pineapple) |
|---|---|---|---|---|---|---|
| Ethyl acetate | 25.28 | na | na | 4.36 | na | 0.31 |
| Isoamyl acetate | 101.83 | 0.50 | 0.60 | 18.51 | 1.67 | 1.97 |
| Ethyl hexanoate | 22.99 | 0.17 | na | na | na | na |
| Hexyl acetate | na | na | 0.01 | na | na | na |
| Ethyl caprylate | 0.58 | na | na | na | na | na |
| Ethyl caprate | 19.03 | na | na | 25.38 | na | na |
| Ethyl 3-hexenoate | na | na | 0.23 | na | 0.68 | 0.13 |
| Ethyl lactate | na | 1.77 | 0.09 | na | 0.93 | na |
| Ethyl octanoate | na | 0.30 | 0.11 | 31.26 | 1.03 | 0.33 |
| Ethyl decanoate | 5.40 | 0.29 | 0.02 | na | 2.89 | 0.06 |
| Diethyl succinate | na | 0.29 | 0.24 | na | 2.47 | <0.01 |
| 2-Phenylethyl acetate | 40.89 | na | 0.17 | 6.94 | 0.91 | 0.12 |
| 1-Propanol | na | 1.16 | na | na | na | na |
| 2-Methylpropanol | na | 6.20 | 1.37 | na | 2.56 | 0.21 |
| 1-Butanol | na | 0.02 | 0.03 | na | 0.03 | na |
| 3-Methylbutanol | na | 79.04 | 81.33 | na | 46.47 | 1.51 |
| 3-Methyl-3-buten-1-ol | na | 0.03 | 0.01 | na | na | na |
| 1-Pentanol | 0.91 | 0.04 | 0.03 | 53.95 | 0.80 | na |
| 1-Hexanol | na | 0.25 | 0.51 | na | 0.06 | na |
| 2,3-Butanediol | na | 1.80 | na | na | na | 0.02 |
| 1-Octanol | na | 0.17 | 0.64 | na | na | 0.09 |
| Benzyl alcohol | na | 0.05 | 0.05 | na | 0.08 | na |
| 2-Phenylethyl alcohol | 67.63 | 27.26 | 35.46 | 33.21 | 12.20 | 2.39 |
| Acetic acid | na | na | na | na | na | 0.21 |
| Hexanoic acid | 7.21 | 0.56 | 0.81 | 0.13 | 2.60 | 0.09 |
| Capric acid | 1.45 | na | na | na | na | na |
| Octanoic acid | 33.33 | 1.10 | 0.70 | 2.23 | 5.48 | 0.12 |
| Decanoic acid | na | na | na | na | 2.08 | <0.1 |
| Benzaldehyde | na | 0.01 | 0.01 | na | 0.08 | <0.01 |
| Linalool | 1.92 | 1.64 | 3.71 | 0.02 | na | na |
| α-Terpineol | nd | 0.84 | 1.62 | na | na | na |
| β-Citronellol | 1.01 | 0.14 | 0.15 | na | na | na |
| Limonene | na | 0.43 | 0.41 | nd | na | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarko, T.; Duda, A. Volatilomics of Fruit Wines. Molecules 2024, 29, 2457. https://doi.org/10.3390/molecules29112457
Tarko T, Duda A. Volatilomics of Fruit Wines. Molecules. 2024; 29(11):2457. https://doi.org/10.3390/molecules29112457
Chicago/Turabian StyleTarko, Tomasz, and Aleksandra Duda. 2024. "Volatilomics of Fruit Wines" Molecules 29, no. 11: 2457. https://doi.org/10.3390/molecules29112457
APA StyleTarko, T., & Duda, A. (2024). Volatilomics of Fruit Wines. Molecules, 29(11), 2457. https://doi.org/10.3390/molecules29112457







