Improving Water-Based Drilling Mud Performance Using Biopolymer Gum: Integrating Experimental and Machine Learning Techniques
Abstract
:1. Introduction
2. Results and Discussion
2.1. Material Characterization
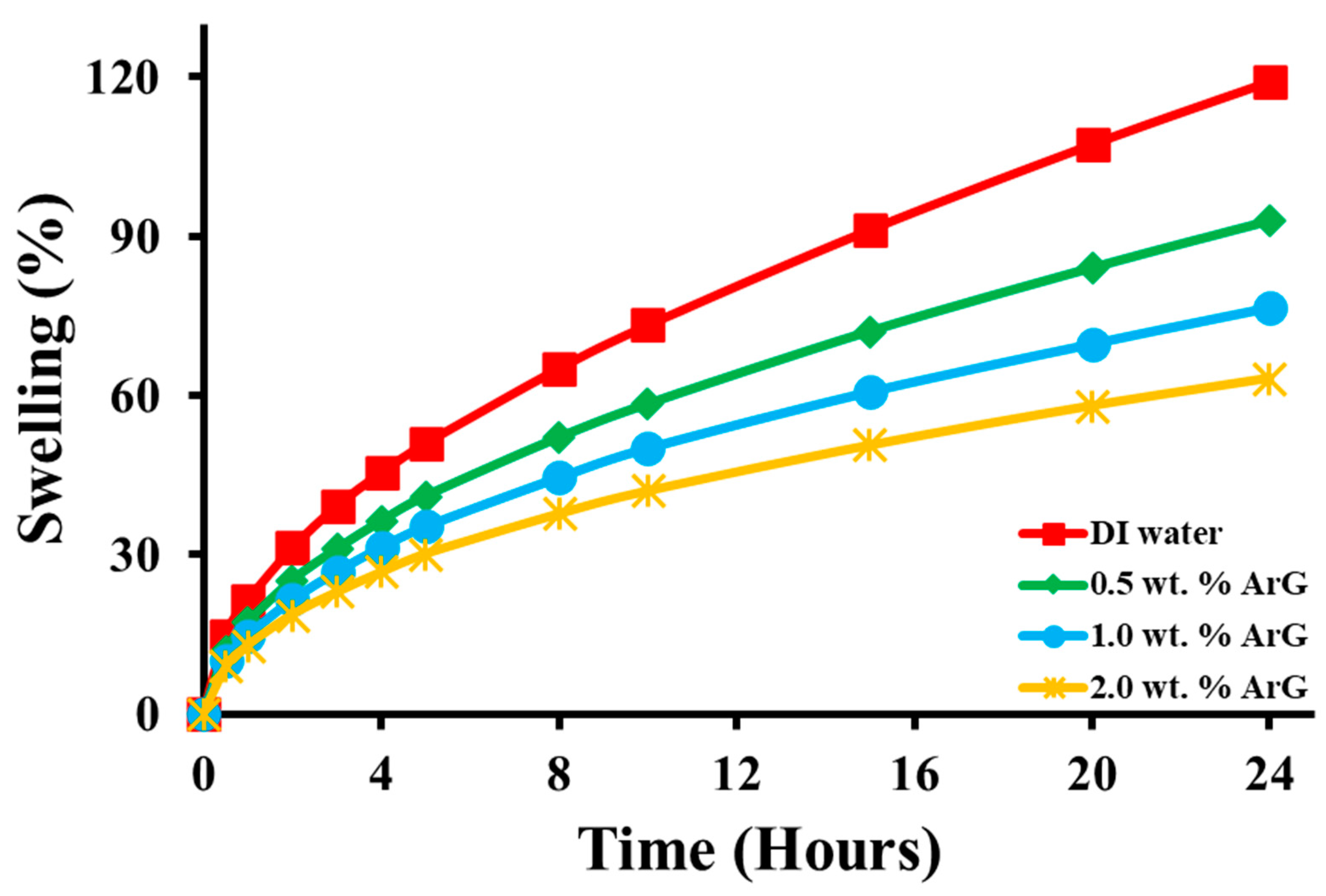
2.2. Swelling Inhibition
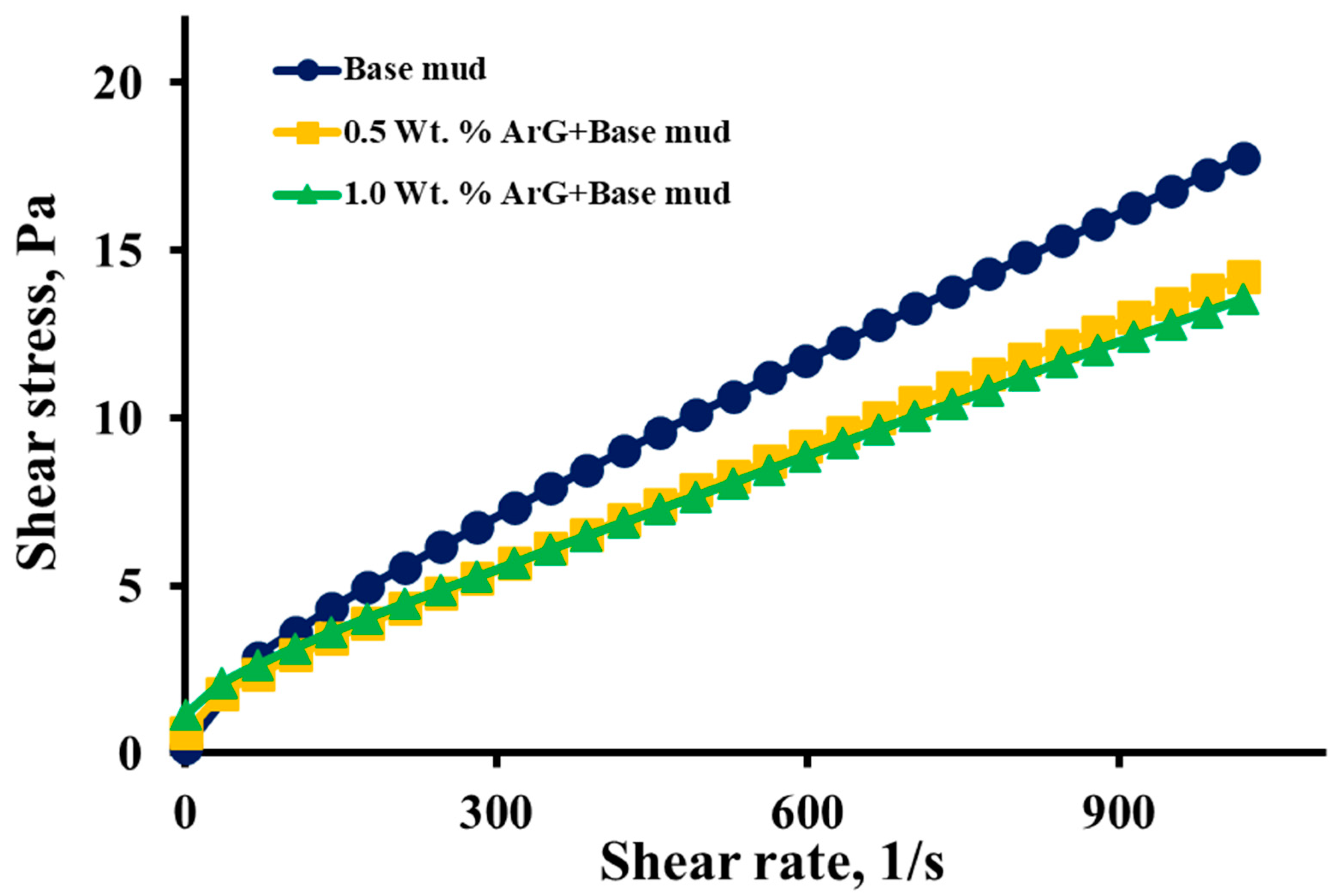
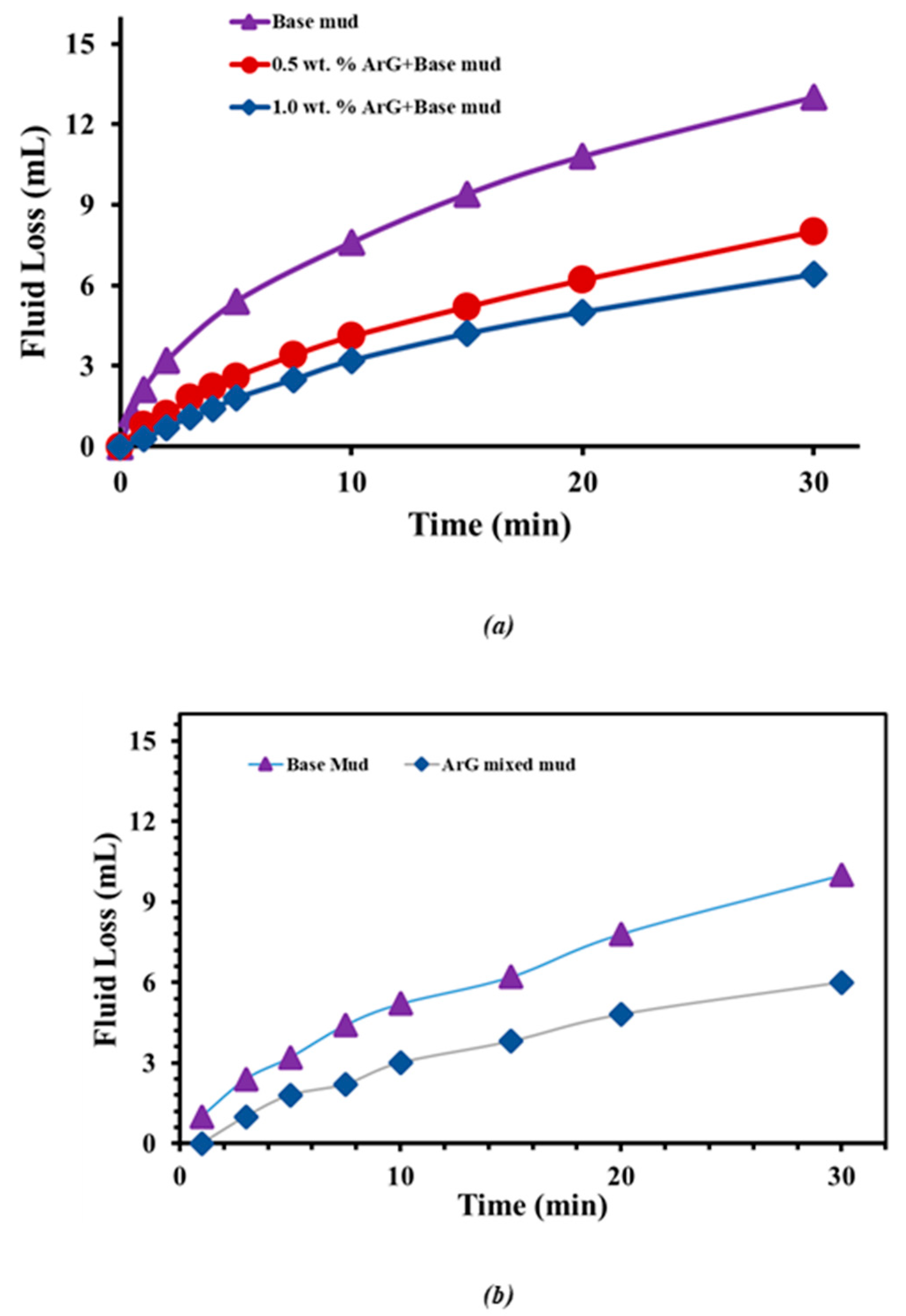
2.3. Rheology and Fluid Loss Tests
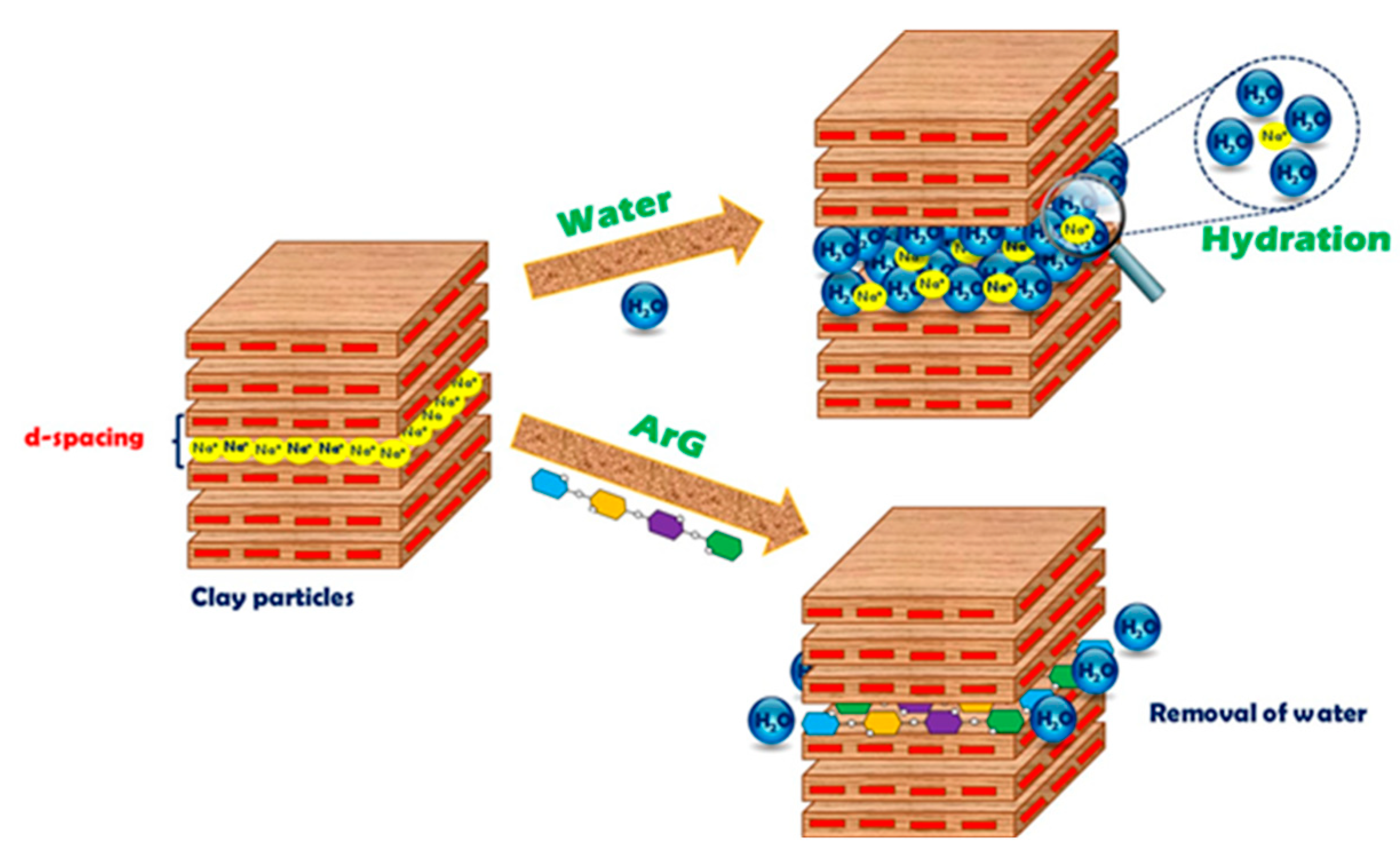
2.4. Inhibition Mechanism
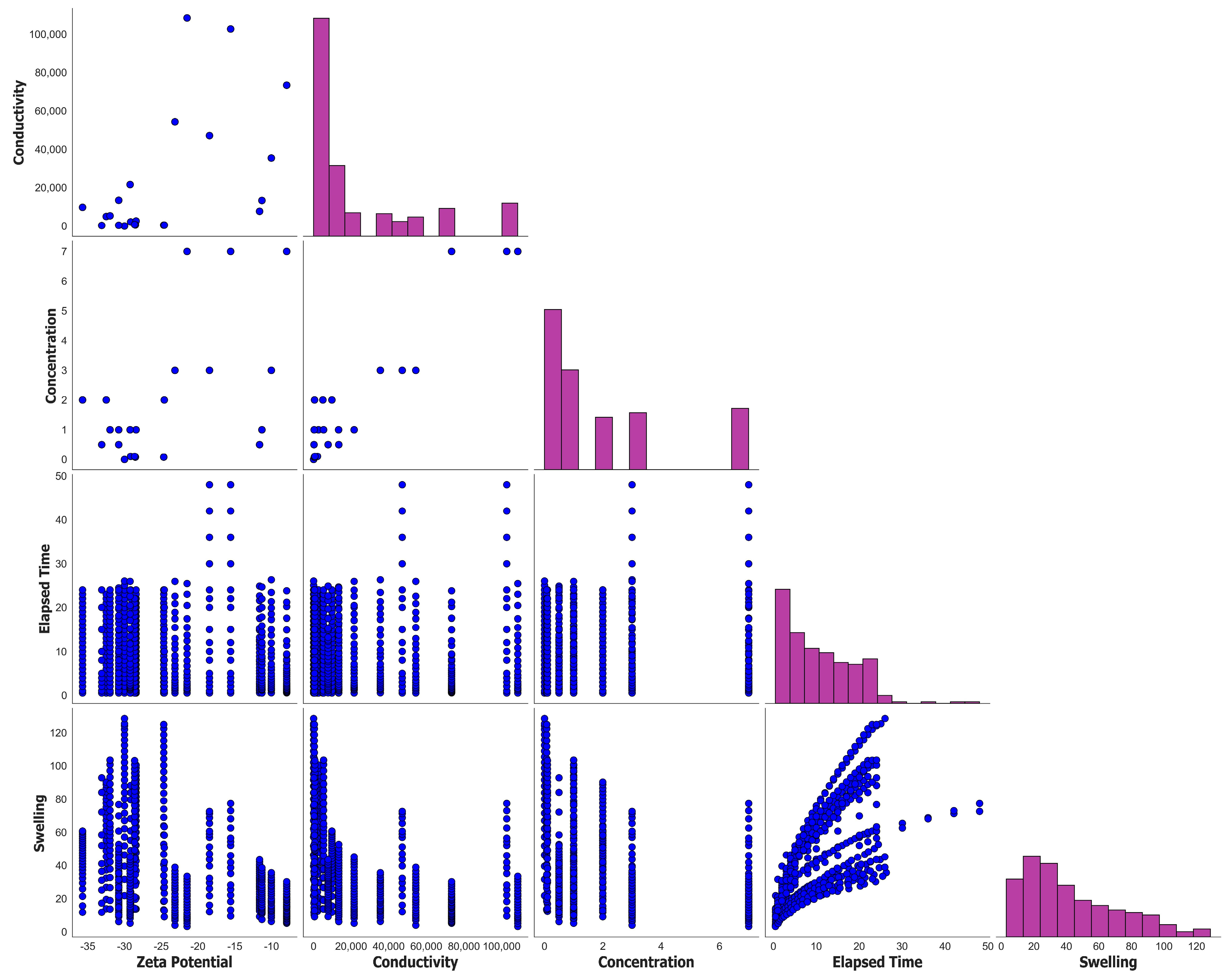
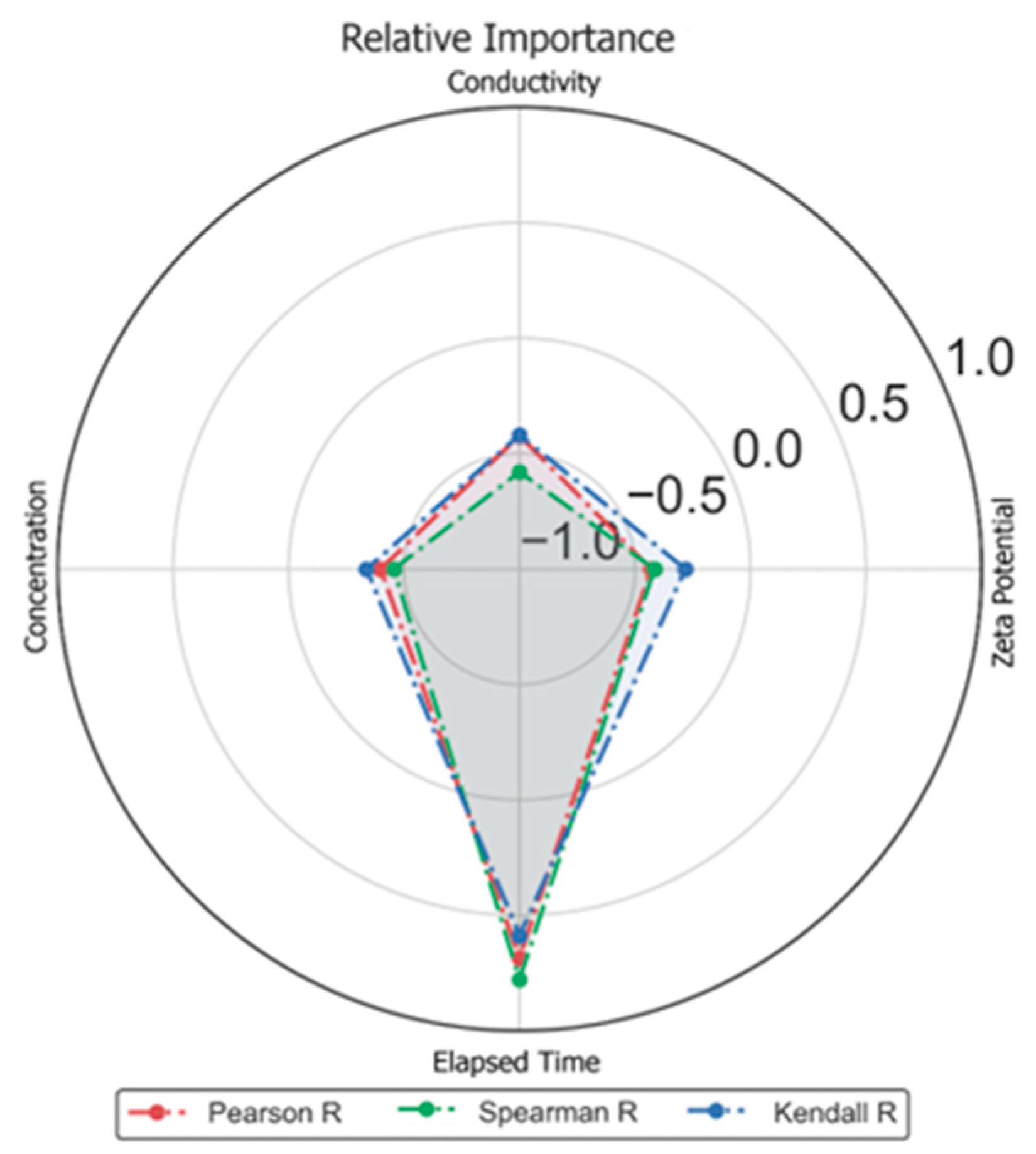
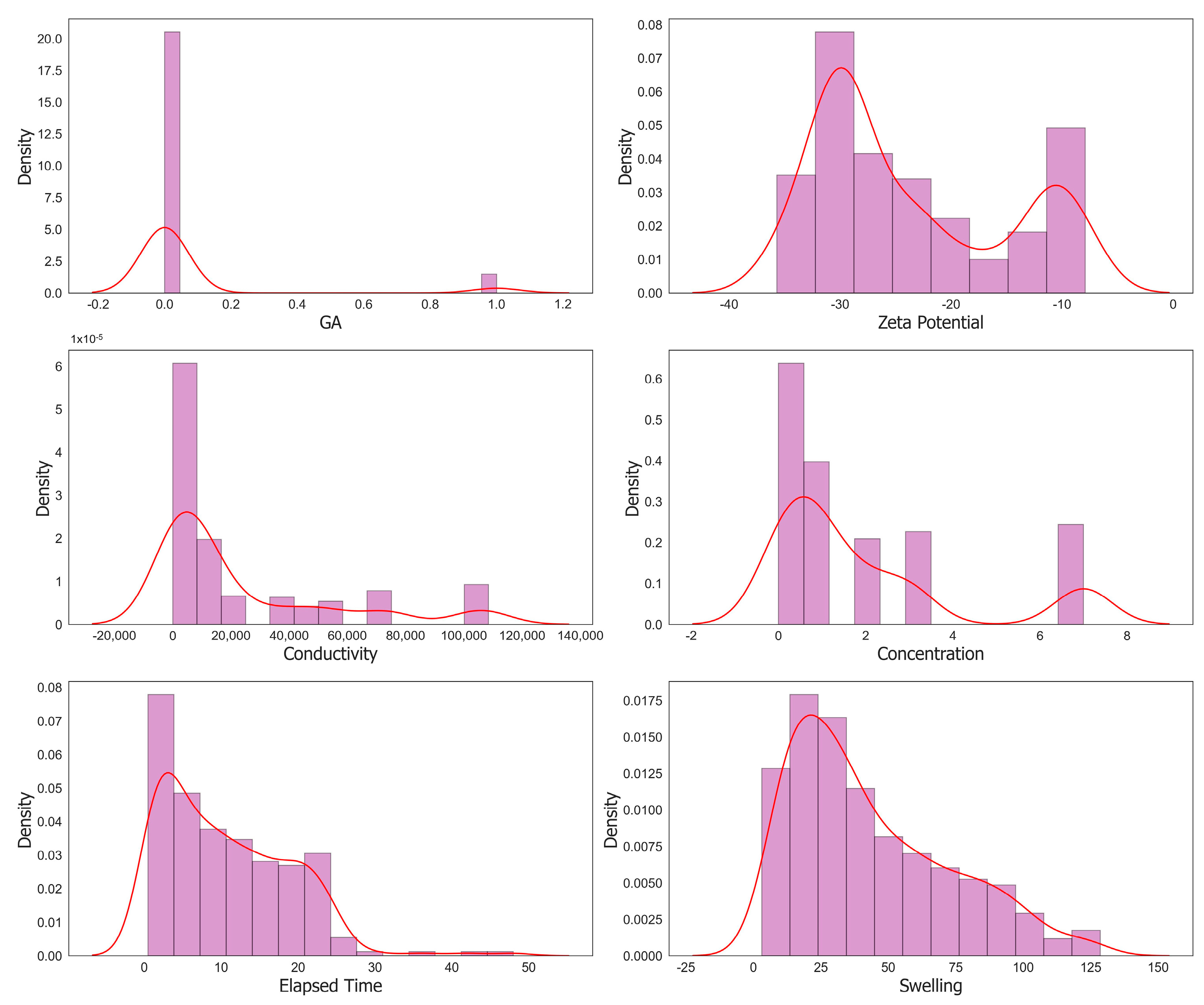
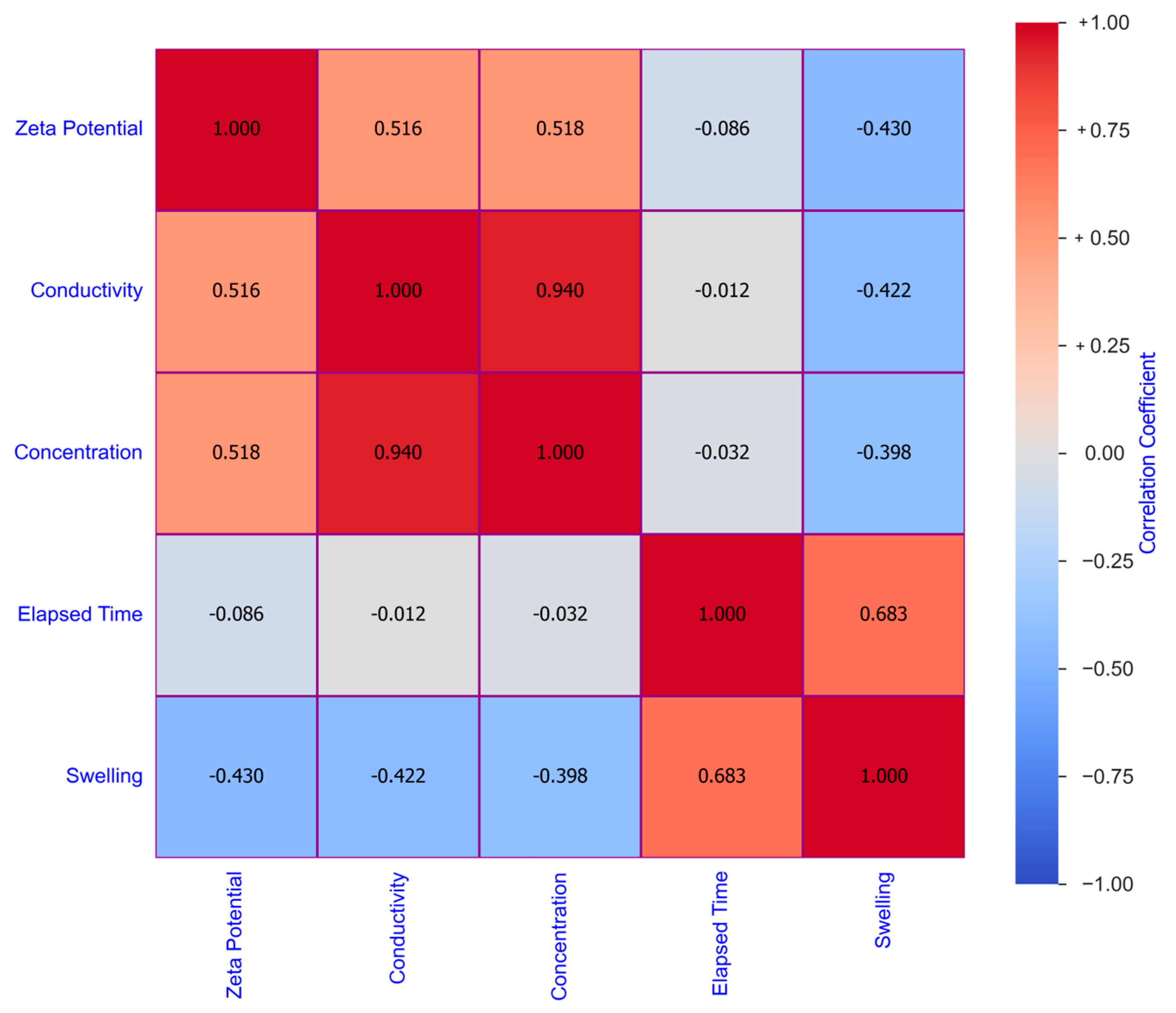
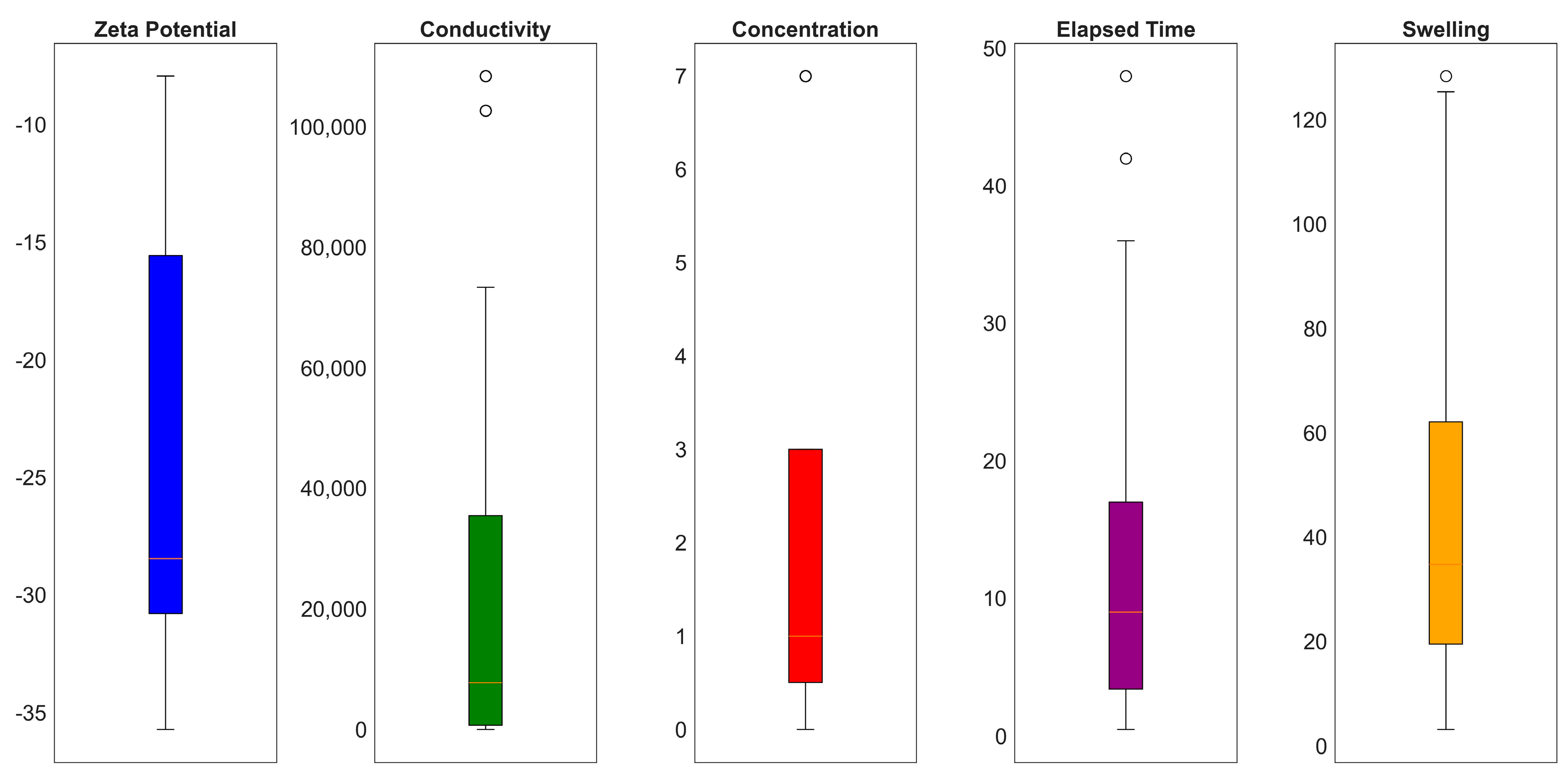
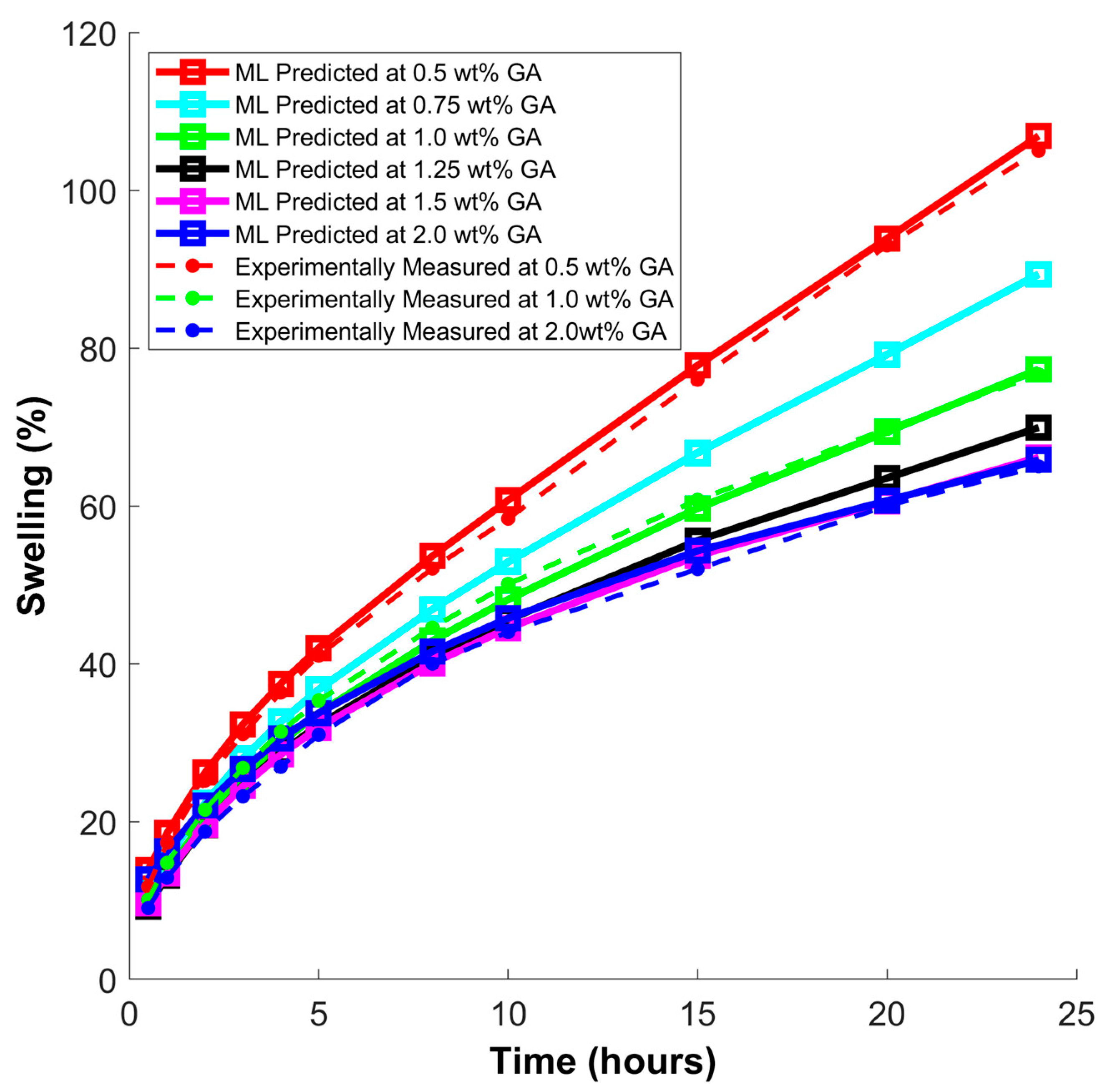
3. Machine Learning Modeling
4. Materials and Procedures
4.1. Materials
4.2. Preparation of Drilling Mud
4.3. Material Characterization Techniques
4.3.1. Swelling Inhibition Tests
4.3.2. Rheology and Fluid Loss Tests
5. Conclusions
- The linear swelling test results revealed that the effectiveness of ArG in inhibiting swelling depended on its concentration.
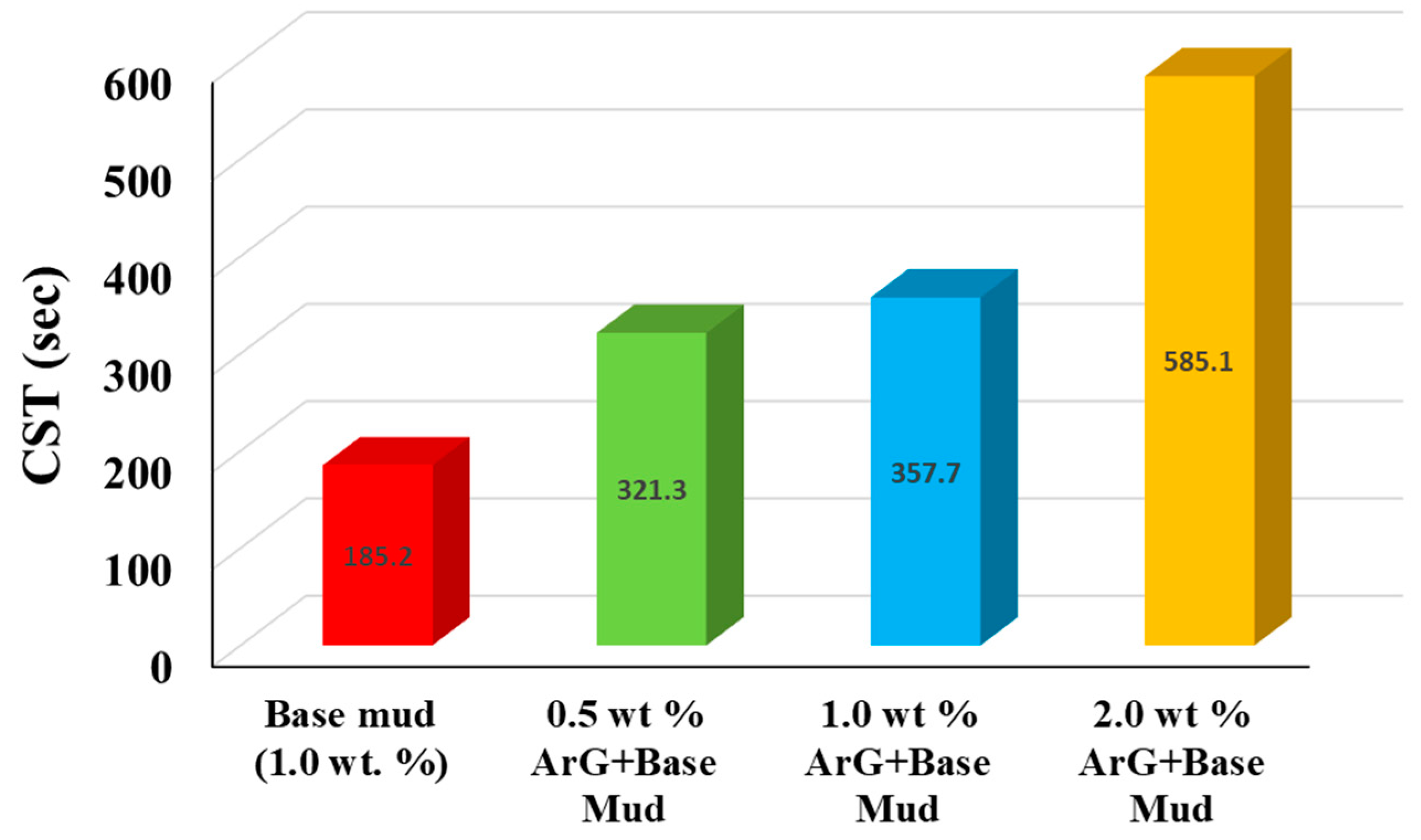
- The CST results showed that ArG prevented fluid loss in a highly concentration-dependent manner.
- The zeta potential results showed that intercalation and adsorption of the ArG on the clay changed the average surface charge of the clay.
- There was no significant alteration in the rheological behavior of the base mud upon the addition of ArG. This indicated that ArG integration did not adversely affect the mud’s flow and viscosity properties.
- When ArG was introduced into the base mud, a significant improvement in fluid loss control was observed. The addition of 0.5 wt. % ArG reduced the fluid loss from 13.0 mL to 8.0 mL, and 1.0 wt. % ArG further decreased it to 6.4 mL.
- ArG is an environmentally friendly material which can be added to drilling muds to control shale swelling and fluid loss to enhance the biodegradability of the drilling fluids.
- A developed FNN (feedforward neural network) model exhibited exceptional accuracy in predicting the swelling behavior of shales treated with ArG. This finding suggests that the FNN model can be a valuable tool for accurately forecasting the impact of ArG on clay swelling, aiding in the formulation of mud systems for various industrial applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vryzas, Z.; Kelessidis, V.C. Nano-Based Drilling Fluids: A Review. Energies 2017, 10, 540. [Google Scholar] [CrossRef]
- Bourgoyne, A.T., Jr.; Millheim, K.K.; Chenevert, M.E.; Young, F.S., Jr. Applied Drilling Engineering; Society of Petroleum Engineers, Inc.: Richardson, USA, 1991; ISBN 1-55563-001-4. [Google Scholar]
- Deville, J.P. Drilling Fluids. In Fluid Chemistry, Drilling and Completion; Elsevier: Amsterdam, The Netherlands, 2022; pp. 115–185. [Google Scholar]
- Hossain, M.E.; Al-Majed, A.A. Fundamentals of Sustainable Drilling Engineering; Wiley-Scrivener: Austin, TX, USA, 2015. [Google Scholar]
- Gautam, R.; Sahai, M.; Kumar, S. Recent Advances in Application of Nanomaterials as Additives for Drilling Fluids. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–24. [Google Scholar] [CrossRef]
- Murtaza, M.; Ahmed Khan, R.; Kamal, M.S.; Hussain, S.M.S.; Mahmoud, M. Poly(Oxyethylene)-Amidoamine Based Gemini Cationic Surfactants with Hydrophilic Spacers as Clay Stabilizers. Energy Fuels 2020, 34, 10619–10630. [Google Scholar] [CrossRef]
- Rana, A.; Murtaza, M.; Saleh, T.A.; Shahzad Kamal, M.; Mahmoud, M. Green Nanocomposite Synthesis and Application: Electrochemically Exfoliated Graphene-Modified Biopolymer as an Effective Clay Swelling Inhibitor for Water-Based Drilling Muds. Geoenergy Sci. Eng. 2023, 231, 212394. [Google Scholar] [CrossRef]
- Hassan, A.; Murtaza, M.; Alade, O.; Tariq, Z.; Kamal, M.S.; Mahmoud, M. Chapter 3—Interactions of Drilling and Completion Fluids during Drilling and Completion Operations. In Developments in Petroleum Science; Hussein, I.A., Mahmoud, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 78, pp. 41–74. ISBN 0376-7361. [Google Scholar]
- Wang, Z.; Liu, J.; Lv, K.; Shao, Z.; Zhang, X.; Xu, Z.; Zhang, F.; Dong, X.; Sun, J. Hydrophobically Modified Low Molecular Weight Polymers as High Temperature Resistant Shale Inhibitor. J. Mol. Liq. 2023, 382, 121856. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, F.; Lv, K.; Chang, X. A Novel Film-Forming Silicone Polymer as Shale Inhibitor for Water-Based Drilling Fluids. e-Polymers 2019, 19, 574–578. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, K.; Gong, J.; Wang, Z.; Huang, X.; Sun, J.; Yao, X.; Liu, K.; Rong, K.; Li, M. Synthesis of a Hydrophobic Quaternary Ammonium Salt as a Shale Inhibitor for Water-Based Drilling Fluids and Determination of the Inhibition Mechanism. J. Mol. Liq. 2022, 362, 119474. [Google Scholar] [CrossRef]
- Yang, L.; Kong, D.; Chang, X.; Jiang, G.; Ao, T.; Xie, C.; Kinkeyi Moukoko, A.D.; Ma, J. Counterion-Specific Shale Hydration Inhibiting Performance of Vinylimdazolium Ionic Liquids. J. Mol. Liq. 2021, 335, 116544. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, C.; Li, X.; Zhang, Z.; Wang, X.; Dai, X.; Zhou, M.; Liu, Q. Investigation of the Inhibition Mechanism of Polymer/Nano-Silica Composite as Shale Inhibitor in Water-Based Drilling Fluids. Colloids Surf. A Physicochem. Eng. Asp. 2022, 636, 128099. [Google Scholar] [CrossRef]
- López, D.; Chamat, N.M.; Galeano-Caro, D.; Páramo, L.; Ramirez, D.; Jaramillo, D.; Cortés, F.B.; Franco, C.A. Use of Nanoparticles in Completion Fluids as Dual Effect Treatments for Well Stimulation and Clay Swelling Damage Inhibition: An Assessment of the Effect of Nanoparticle Chemical Nature. Nanomaterials 2023, 13, 388. [Google Scholar] [CrossRef]
- Baba Hamed, S.; Belhadri, M. Rheological Properties of Biopolymers Drilling Fluids. J. Pet. Sci. Eng. 2009, 67, 84–90. [Google Scholar] [CrossRef]
- Zhu, W.; Zheng, X. Effective Modified Xanthan Gum Fluid Loss Agent for High-Temperature Water-Based Drilling Fluid and the Filtration Control Mechanism. ACS Omega 2021, 6, 23788–23801. [Google Scholar] [CrossRef] [PubMed]
- William, J.K.M.; Ponmani, S.; Samuel, R.; Nagarajan, R.; Sangwai, J.S. Effect of CuO and ZnO Nanofluids in Xanthan Gum on Thermal, Electrical and High Pressure Rheology of Water-Based Drilling Fluids. J. Pet. Sci. Eng. 2014, 117, 15–27. [Google Scholar] [CrossRef]
- Phillips, G.O.; Ogasawara, T.; Ushida, K. The Regulatory and Scientific Approach to Defining Gum Arabic (Acacia Senegal and Acacia Seyal) as a Dietary Fibre. Food Hydrocoll. 2008, 22, 24–35. [Google Scholar] [CrossRef]
- Annu; Ahmed, S. Advanced Green Materials: An Overview. In Advanced Green Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–13. [Google Scholar]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Preparation of Chitosan/Gum Arabic Nanoparticles and Their Use as Novel Stabilizers in Oil/Water Pickering Emulsions. Carbohydr. Polym. 2019, 224, 115190. [Google Scholar] [CrossRef] [PubMed]
- Chanamai, R.; McClements, D.J. Comparison of Gum Arabic, Modified Starch, and Whey Protein Isolate as Emulsifiers: Influence of PH, CaCl2 and Temperature. J. Food Sci. 2002, 67, 120–125. [Google Scholar] [CrossRef]
- Atgié, M.; Chennevière, A.; Masbernat, O.; Roger, K. Emulsions Stabilized by Gum Arabic: How Diversity and Interfacial Networking Lead to Metastability. Langmuir 2019, 35, 14553–14565. [Google Scholar] [CrossRef] [PubMed]
- Da Luz, R.C.S.; Fagundes, F.P.; Balaban, R. de C. Water-Based Drilling Fluids: The Contribution of Xanthan Gum and Carboxymethylcellulose on Filtration Control. Chem. Pap. 2017, 71, 2365–2373. [Google Scholar] [CrossRef]
- Kroll, C.; Warchold, A.; Pradhan, P. Sustainable Development Goals (SDGs): Are We Successful in Turning Trade-Offs into Synergies? Palgrave Commun. 2019, 5, 140. [Google Scholar] [CrossRef]
- Axon, S.; James, D. The UN Sustainable Development Goals: How Can Sustainable Chemistry Contribute? A View from the Chemical Industry. Curr. Opin. Green Sustain. Chem. 2018, 13, 140–145. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nakajima, L. Sustainable Development Goals for Advanced Materials Provided by Industrial Wastes and Biomass Sources. Curr. Opin. Green Sustain. Chem. 2021, 28, 100439. [Google Scholar] [CrossRef]
- Daoub, R.M.A.; Elmubarak, A.H.; Misran, M.; Hassan, E.A.; Osman, M.E. Characterization and Functional Properties of Some Natural Acacia Gums. J. Saudi Soc. Agric. Sci. 2018, 17, 241–249. [Google Scholar] [CrossRef]
- Gils, P.S.; Ray, D.; Mohanta, G.P.; Manavalan, R.; Sahoo, P.K. Designing of New Acrylic Based Macropoprous Superabsorbent Polymer Hydrogel and Its Suitability for Drug Delivery. Int. J. Pharm. Pharm. Sci. 2009, 1, 43–54. [Google Scholar]
- Jamaludin, J.; Adam, F.; Rasid, R.A.; Hassan, Z. Thermal Studies on Arabic Gum—Carrageenan Polysaccharides Film. Chem. Eng. Res. Bull. 2017, 19, 80. [Google Scholar] [CrossRef]
- Zohuriaan, M.J.; Shokrolahi, F. Thermal Studies on Natural and Modified Gums. Polym. Test 2004, 23, 575–579. [Google Scholar] [CrossRef]
- Koubaa, M.; Ktata, A.; Barba, F.J.; Grimi, N.; Mhemdi, H.; Bouaziz, F.; Driss, D.; Chaabouni, S.E. Water-Soluble Polysaccharides from Opuntia Stricta Haw. Fruit Peels: Recovery, Identification and Evaluation of Their Antioxidant Activities. Int. Agrophys. 2015, 29, 299–306. [Google Scholar] [CrossRef]
- Hua, D.; Zhang, D.; Huang, B.; Yi, P.; Yan, C. Structural Characterization and DPPH· Radical Scavenging Activity of a Polysaccharide from Guara Fruits. Carbohydr. Polym. 2014, 103, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, F.; Koubaa, M.; Ben Jeddou, K.; Kallel, F.; Boisset Helbert, C.; Khelfa, A.; Ellouz Ghorbel, R.; Ellouz Chaabouni, S. Water-Soluble Polysaccharides and Hemicelluloses from Almond Gum: Functional and Prebiotic Properties. Int. J. Biol. Macromol. 2016, 93, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, O.; de Pinto, G.L.; Rincón, F.; Picton, L.; Cozic, C.; Le Cerf, D.; Muller, G. Acacia Macracantha Gum as a Possible Source of Arabinogalactan–Protein. Carbohydr. Polym. 2008, 72, 88–94. [Google Scholar] [CrossRef]
- Bashir, M.; Haripriya, S. Assessment of Physical and Structural Characteristics of Almond Gum. Int. J. Biol. Macromol. 2016, 93, 476–482. [Google Scholar] [CrossRef]
- Louati, S.; Baklouti, S.; Samet, B. Geopolymers Based on Phosphoric Acid and Illito-Kaolinitic Clay. Adv. Mater. Sci. Eng. 2016, 2016, 2359759. [Google Scholar] [CrossRef]
- Wang, L.; Schiraldi, D.A.; Sánchez-Soto, M. Foamlike Xanthan Gum/Clay Aerogel Composites and Tailoring Properties by Blending with Agar. Ind. Eng. Chem. Res. 2014, 53, 7680–7687. [Google Scholar] [CrossRef]
- De Bussetti, S.G.; Ferreiro, E.A. Adsorption of Poly(Vinyl Alcohol) on Montmorillonite. Clays Clay Miner. 2004, 52, 334–340. [Google Scholar] [CrossRef]
- Dias, F.T.G.; Souza, R.R.; Lucas, E.F. Influence of Modified Starches Composition on Their Performance as Fluid Loss Additives in Invert-Emulsion Drilling Fluids. Fuel 2015, 140, 711–716. [Google Scholar] [CrossRef]
- Avci, E.; Mert, B.A. The Rheology and Performance of Geothermal Spring Water-Based Drilling Fluids. Geofluids 2019, 2019, 3786293. [Google Scholar] [CrossRef]
- Liao, K.; An, J.; Fu, L.; Zhang, H.; Wei, M.; Bai, J.; He, Y. Adsorption of Welan Gum on Montmorillonite and Its Influencing Factors. Polymers 2022, 14, 2599. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A.; Rana, A. Surface-Modified Biopolymer as an Environment-Friendly Shale Inhibitor and Swelling Control Agent. J. Mol. Liq. 2021, 342, 117275. [Google Scholar] [CrossRef]
- Li, X.; Jiang, G.; Shen, X.; Li, G. Poly-L-Arginine as a High-Performance and Biodegradable Shale Inhibitor in Water-Based Drilling Fluids for Stabilizing Wellbore. ACS Sustain. Chem. Eng. 2020, 8, 1899–1907. [Google Scholar] [CrossRef]
- Li, X.; Jiang, G.; Yang, L.; Peng, S. Study of Gelatin as Biodegradable Shale Hydration Inhibitor. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 539, 192–200. [Google Scholar] [CrossRef]
- Tang, Z.; Qiu, Z.; Zhong, H.; Kang, Y.; Guo, B. Wellbore Stability through Novel Catechol-Chitosan Biopolymer Encapsulator-Based Drilling Mud. Gels 2022, 8, 307. [Google Scholar] [CrossRef]
- Alizadeh, S.M.; Alruyemi, I.; Daneshfar, R.; Mohammadi-Khanaposhtani, M.; Naseri, M. An Insight into the Estimation of Drilling Fluid Density at HPHT Condition Using PSO-, ICA-, and GA-LSSVM Strategies. Sci. Rep. 2021, 11, 7033. [Google Scholar] [CrossRef] [PubMed]
- Bahaloo, S.; Mehrizadeh, M.; Najafi-Marghmaleki, A. Review of Application of Artificial Intelligence Techniques in Petroleum Operations. Petroleum Res. 2023, 8, 167–182. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Gum Arabic. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2021; pp. 627–652. [Google Scholar]
- Rana, A.; Murtaza, M.; Saleh, T.A.; Kamal, M.S.; Mahmoud, M. An Efficient, Cost-Effective, and Green Natural Extract in Water-Based Drilling Muds for Clay Swelling Inhibition. J. Pet. Sci. Eng. 2022, 214, 110332. [Google Scholar] [CrossRef]
- American Petroleum Institute API RP 13B-1 Recommended Practice for Field Testing of Water Based Drilling Fluids; American Petroleum Institute: Washington, DC, USA, 2019.
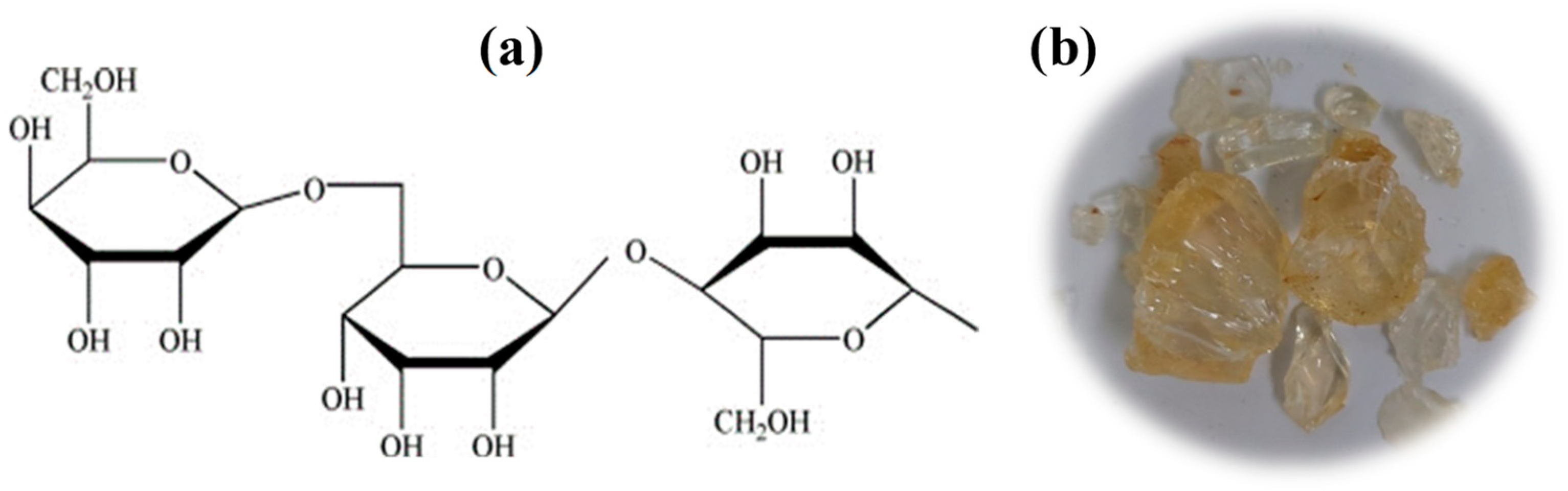
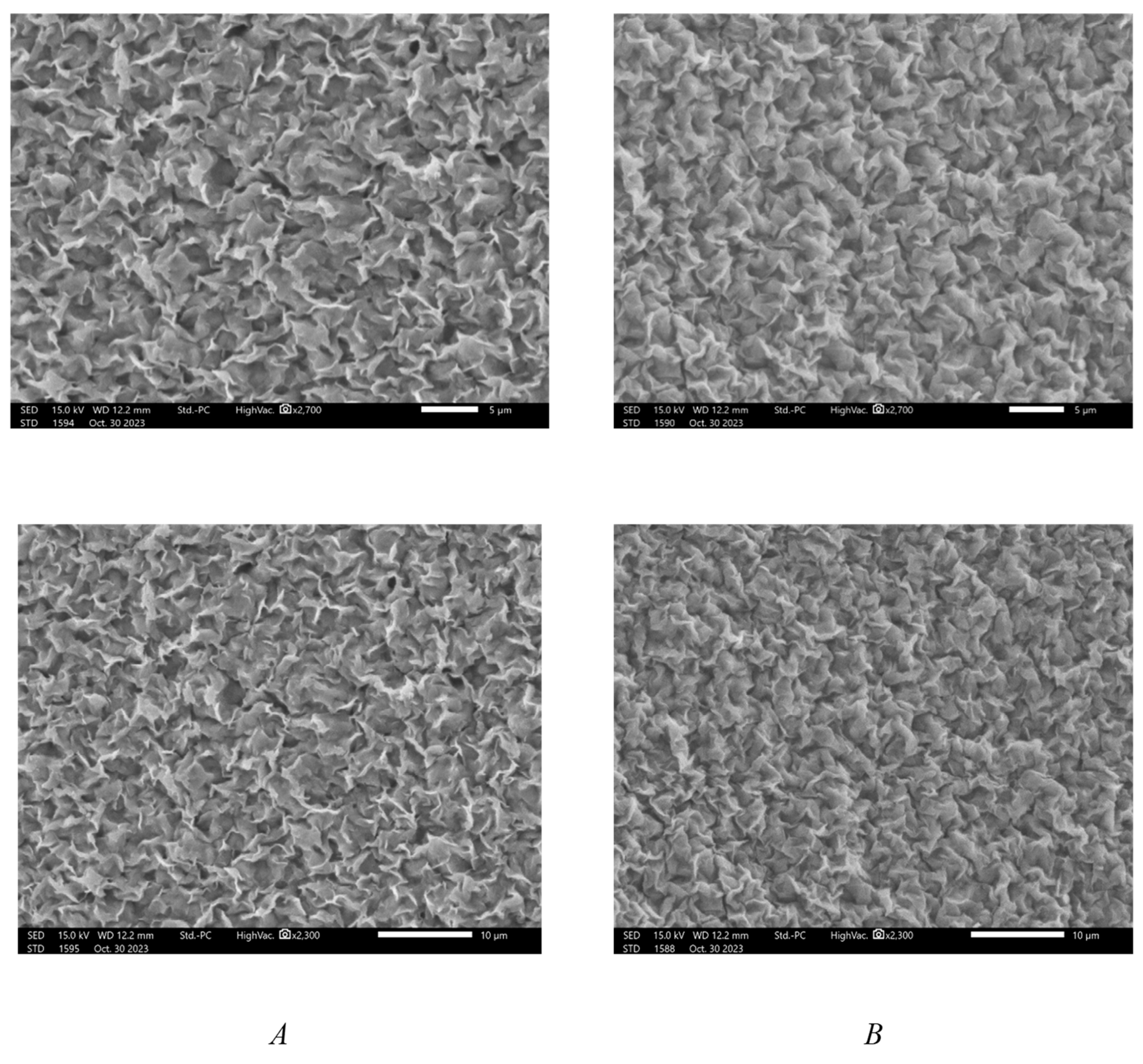
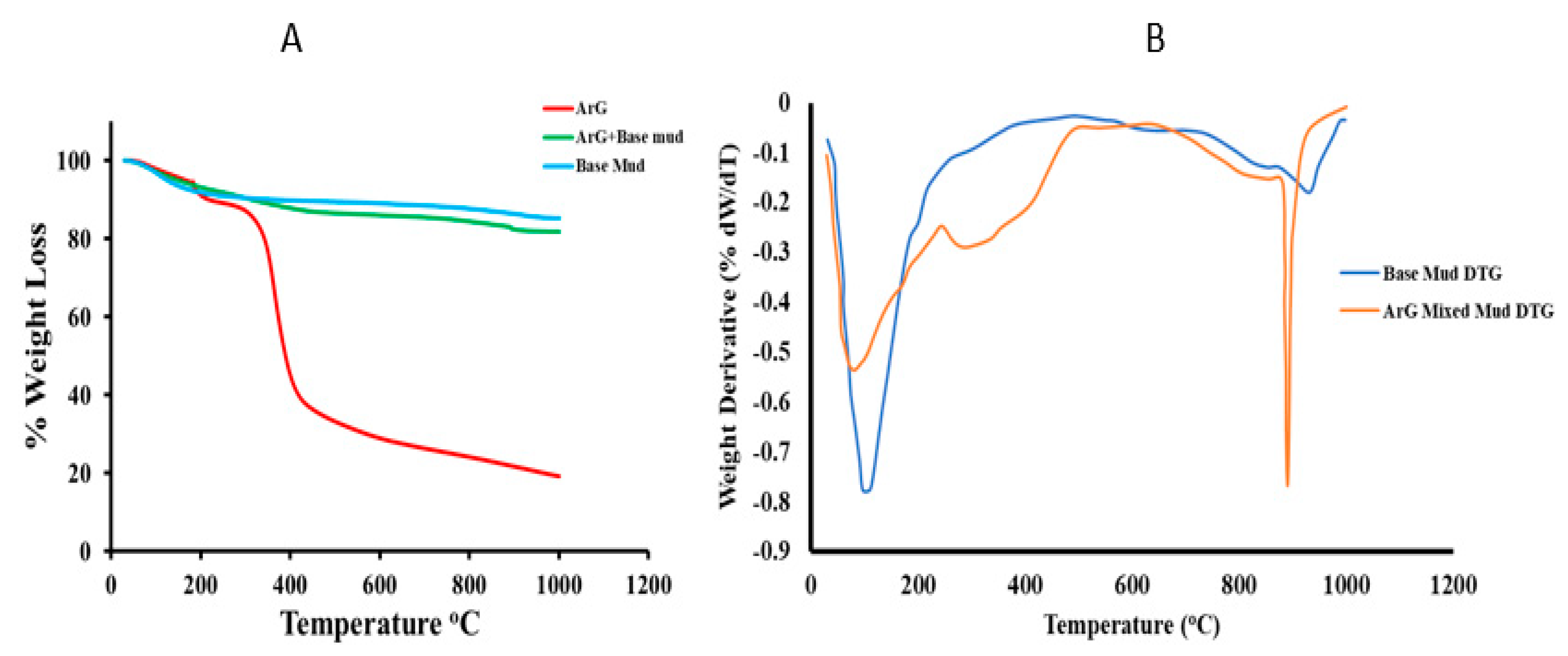
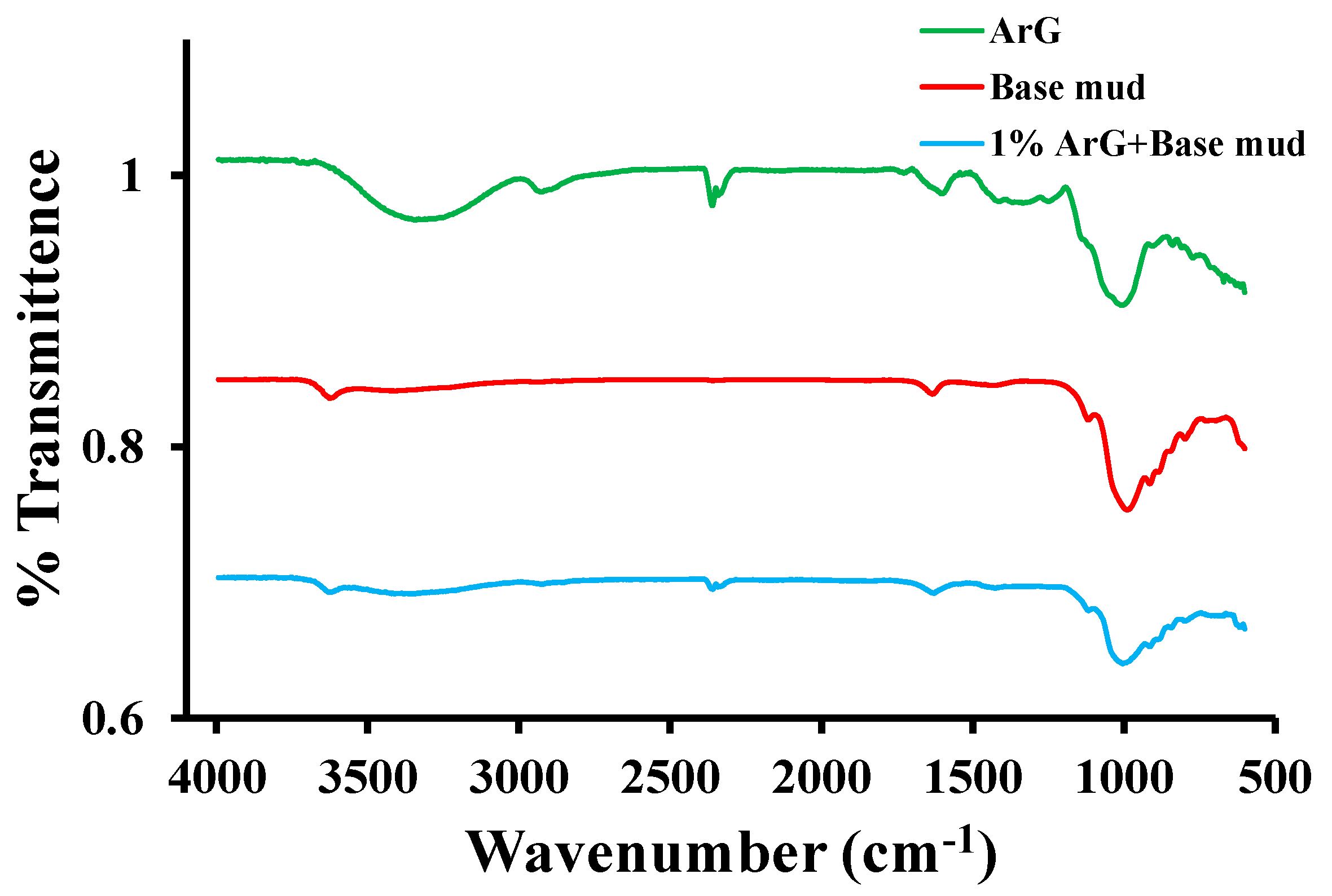


| Formulations | Zeta Potential (mV) |
|---|---|
| Base mud (0.5 wt. %) | −35.42 |
| 0.5 wt. % ArG | −30.74 |
| 1.0 wt. % ArG | −27.29 |
| 2.0 wt. % ArG | −22.72 |
| Mud Formulation | PV (cp) | YP (lb/ft2) | YP/PV (Pa/mPA.s) | Gel10s (lb/ft2) | Gel10min (lb/ft2) | Fluid Loss (mL) |
|---|---|---|---|---|---|---|
| Unmodified Base Mud | 8.4 | 7.5 | 0.9 | 2.6 | 3.7 | 10.0 |
| ArG-Modified Base Mud | 7.7 | 6.9 | 0.9 | 2.1 | 3.2 | 6.0 |
| Compounds | Swelling Rate Test | Rheological Features | Fluid Loss (mL) | Refs | |||
|---|---|---|---|---|---|---|---|
| PV (cp) | YP (lb/ft2) | Gel10s (lb/ft2) | Gel10min (lb/ft2) | ||||
| ArG | 63% reduction in swelling | 7.7 | 6.9 | 2.1 | 3.2 | 6.0 | This work |
| Styrene-modified cellulose | 56% reduction in swelling | 10.3 | 7.0 | 5.2 | 7.6 | - | [42] |
| Poly-L-arginine | 53% reduction in swelling | 36 | 12 | 2.5 | 4.0 | 4.2 | [43] |
| Gelatin | 69% reduction in swelling | - | - | - | - | - | [44] |
| Catechol-Chitosan Biopolymer | 32% reduction in swelling | 10 | - | - | - | 80 | [45] |
| Statistical Parameters | Mean | Variance | Kurtosis | Skewness | Range | Minimum | Maximum | |
|---|---|---|---|---|---|---|---|---|
| DW | 0.0549 | 0.2280 | 0.0520 | 13.4285 | 3.9210 | 1.0000 | 0.0000 | 1.0000 |
| GA | 0.0671 | 0.2504 | 0.0627 | 10.0955 | 3.4720 | 1.0000 | 0.0000 | 1.0000 |
| Zeta Potential | −23.7771 | 8.7073 | 75.8178 | −1.0824 | 0.6115 | 27.7819 | −35.7088 | −7.9269 |
| Conductivity | 2.34 × 104 | 3.17 × 104 | 1.00 × 109 | 1.17 × 100 | 1.53 × 100 | 1.08 × 105 | 1.20 × 100 | 1.08 × 105 |
| Concentration | 1.9557 | 2.2591 | 5.1036 | 0.7208 | 1.4164 | 7.0000 | 0.0000 | 7.0000 |
| Elapsed Time | 10.6557 | 8.2912 | 68.7443 | 1.1906 | 0.9229 | 47.5297 | 0.4703 | 48.0000 |
| Swelling | 43.3333 | 29.3014 | 858.5740 | −0.1684 | 0.8392 | 125.3740 | 3.1160 | 128.4900 |
| Hyperparameter | Typical Range | Optimum Value (Approximate) |
|---|---|---|
| Number of Hidden Layers | 1 to 3 | 1 |
| Number of Neurons per Layer | 30 | 25 |
| Activation Function | ReLU, Leaky ReLU, or PReLU | ReLU |
| Learning Rate | 0.001 to 0.01 | 0.001 |
| Epochs | 100 to 1000 | 100 |
| Optimizer | Adam, RMSprop, etc. | Adam |
| Dropout Rate | 0.0 to 0.3 | Requires tuning |
| Learning Rate Schedule | Learning rate decay, etc. | Learning rate schedule |
| Loss Function | Mean Squared Error (MSE) | MSE |
| Contents and Properties | Acacia Senegal |
|---|---|
| % Galactose | 38.9 |
| % Arabinose | 25.7 |
| % Rhamnose | 9.5 |
| % Glucuronic acid | 21.5 |
| 4-O-methyl glucuronic acid | 1.5 |
| % Nitrogen | 0.36 |
| Average molecular mass | 640,000 |
| Density at 1.0 wt. % in g/cm3 | 1.0016 |
| Viscosity at 1.0 wt. % in mpa.S | 2.084 |
| pH at 1.0 wt. % | 5.52 |
| Formulation (g) | Mixing Time, min | Base Field Drilling Mud | Modified Field Drilling Mud |
|---|---|---|---|
| Water (mL) | - | 350 mL | 350 mL |
| Bentonite | 20 | 10 g | 10 g |
| Sodium hydroxide | 5 | 0.2 g | 0.2 g |
| XC polymer | 20 | 0.5 g | 0.5 g |
| Starch | 10 | 0.4 g | 0.4 g |
| Barite | 10 | 50 g | 50 g |
| ArG | 10 | - | 3.5 g |
| Property | Description | Units |
|---|---|---|
| Plastic viscosity (PV) | θ600–θ300 | cP |
| Initial gel (IG) | θ3 after 10 s | lb/100 ft2 |
| Final gel (FG) | θ3 after 10 min | lb/100 ft2 |
| Yield point (YP) | θ300–PV | lb/100 ft2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murtaza, M.; Tariq, Z.; Kamal, M.S.; Rana, A.; Saleh, T.A.; Mahmoud, M.; Alarifi, S.A.; Syed, N.A. Improving Water-Based Drilling Mud Performance Using Biopolymer Gum: Integrating Experimental and Machine Learning Techniques. Molecules 2024, 29, 2512. https://doi.org/10.3390/molecules29112512
Murtaza M, Tariq Z, Kamal MS, Rana A, Saleh TA, Mahmoud M, Alarifi SA, Syed NA. Improving Water-Based Drilling Mud Performance Using Biopolymer Gum: Integrating Experimental and Machine Learning Techniques. Molecules. 2024; 29(11):2512. https://doi.org/10.3390/molecules29112512
Chicago/Turabian StyleMurtaza, Mobeen, Zeeshan Tariq, Muhammad Shahzad Kamal, Azeem Rana, Tawfik A. Saleh, Mohamed Mahmoud, Sulaiman A. Alarifi, and Nadeem Ahmed Syed. 2024. "Improving Water-Based Drilling Mud Performance Using Biopolymer Gum: Integrating Experimental and Machine Learning Techniques" Molecules 29, no. 11: 2512. https://doi.org/10.3390/molecules29112512






