A Convenient In Situ Preparation of Cu2ZnSnS4–Anatase Hybrid Nanocomposite for Photocatalysis/Photoelectrochemical Water-Splitting Hydrogen Production
Abstract
:1. Introduction
2. Results and Discussion
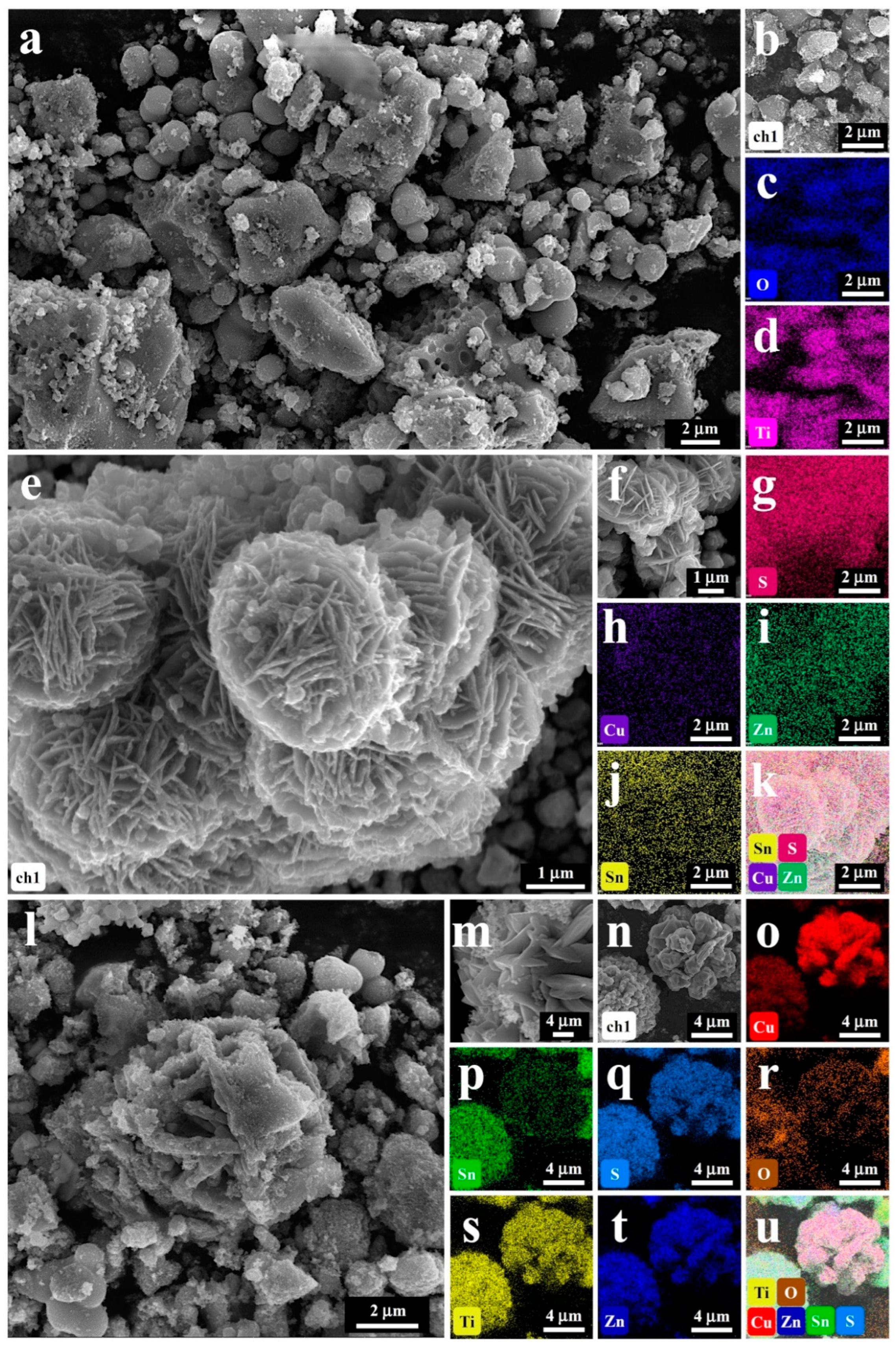
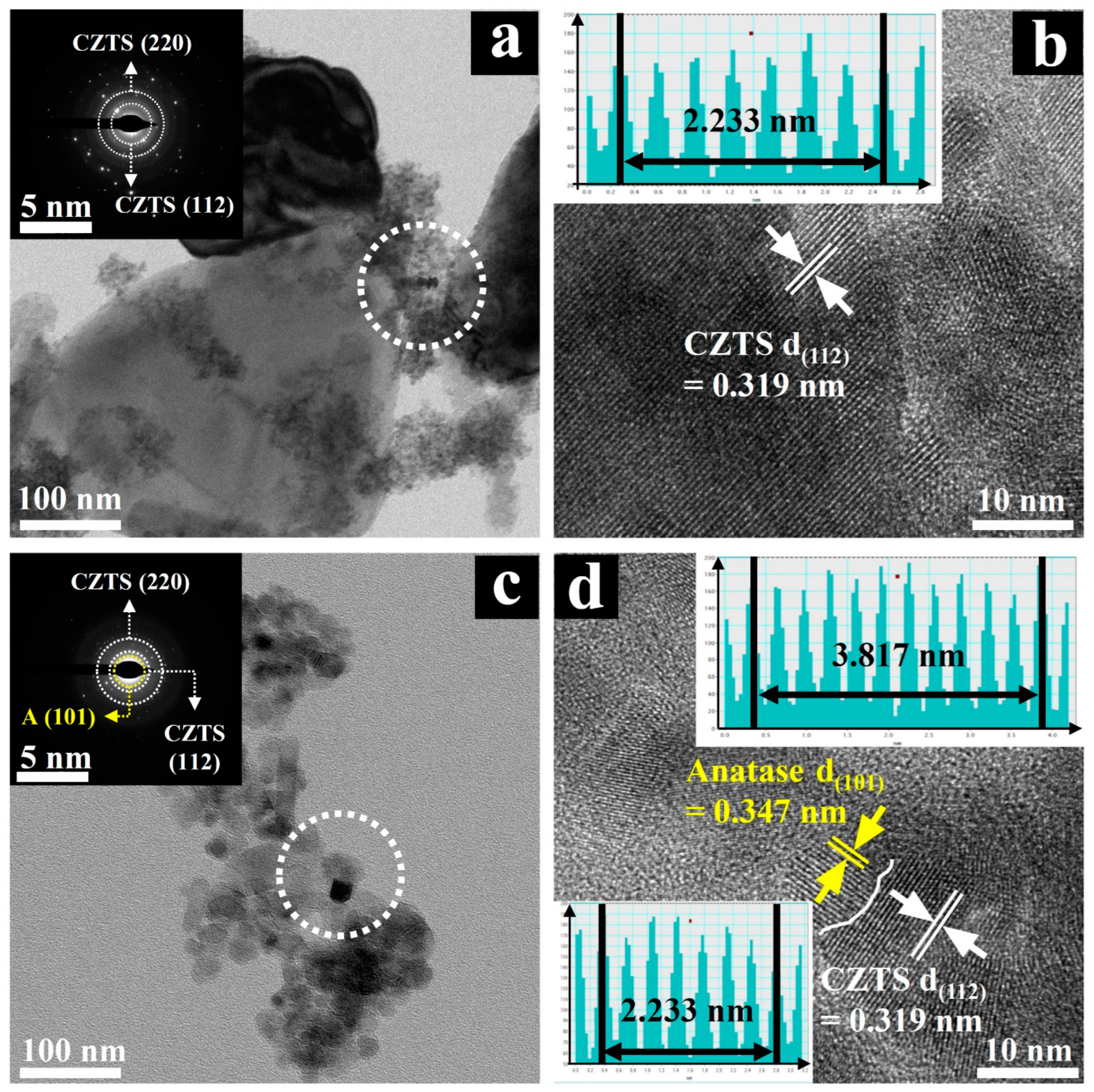
2.1. Structure and Morphology
2.2. XPS Characterization
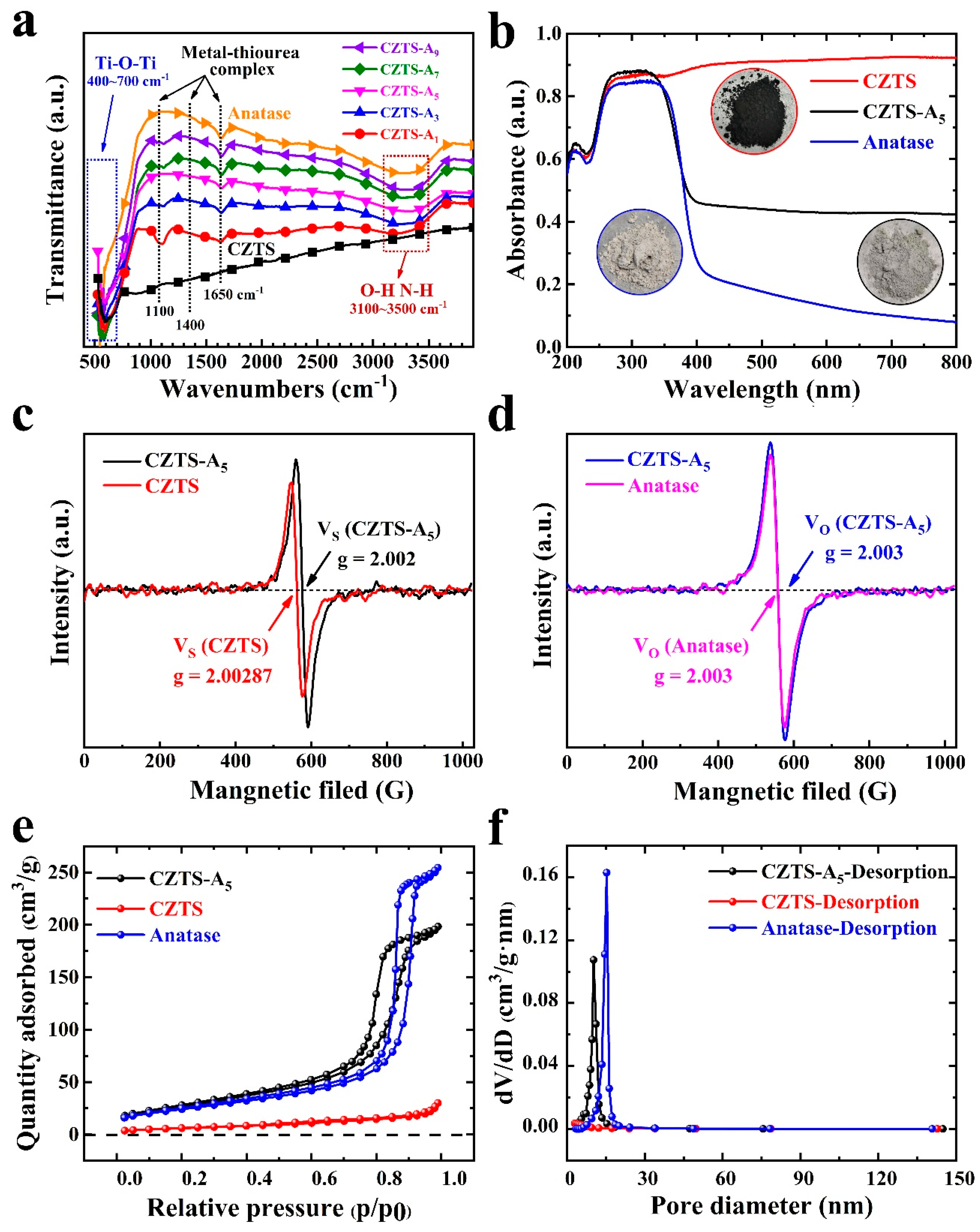
2.3. FTIR Characterization and Optical Characterization
2.4. EPR Characterization
2.5. BET Characterization
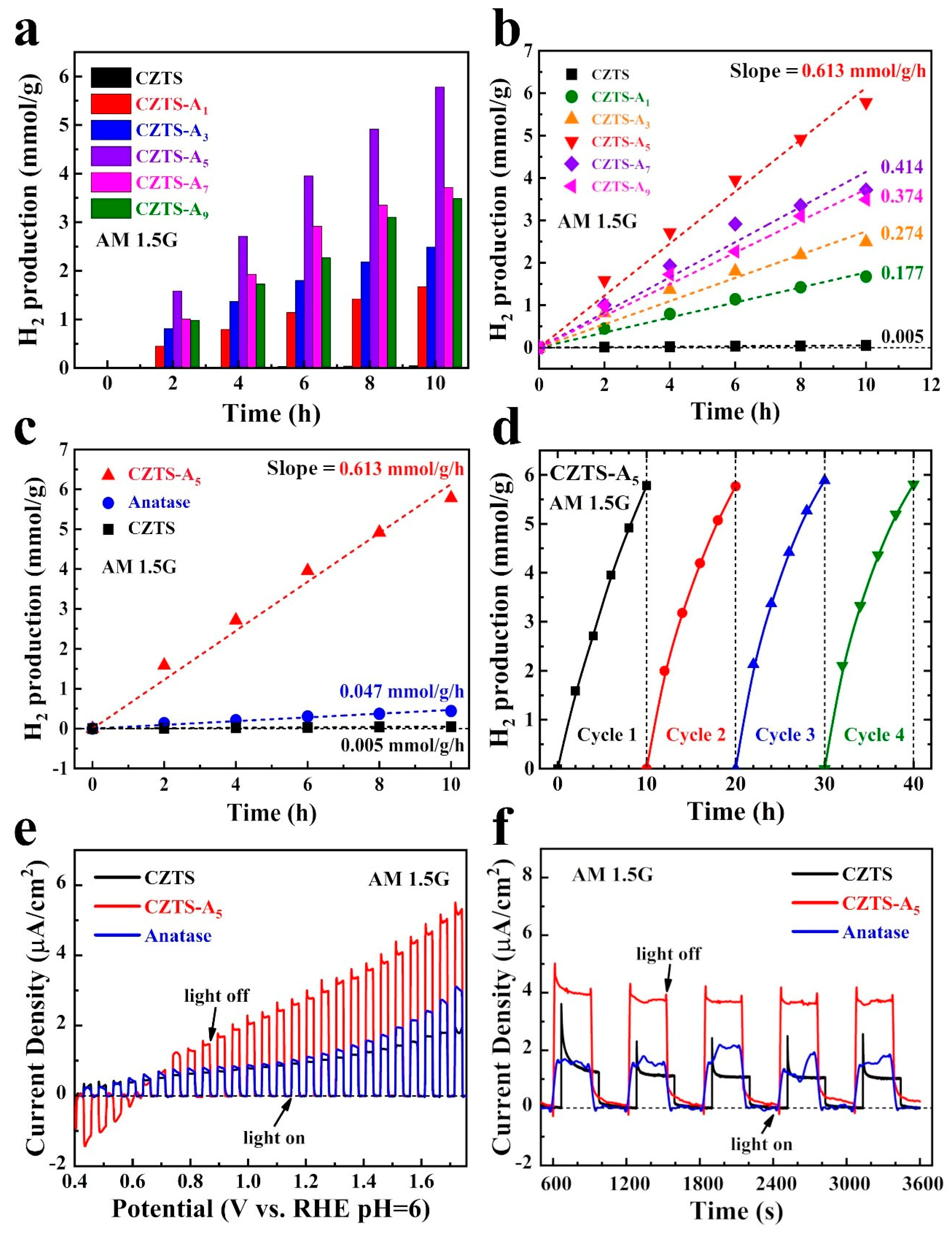
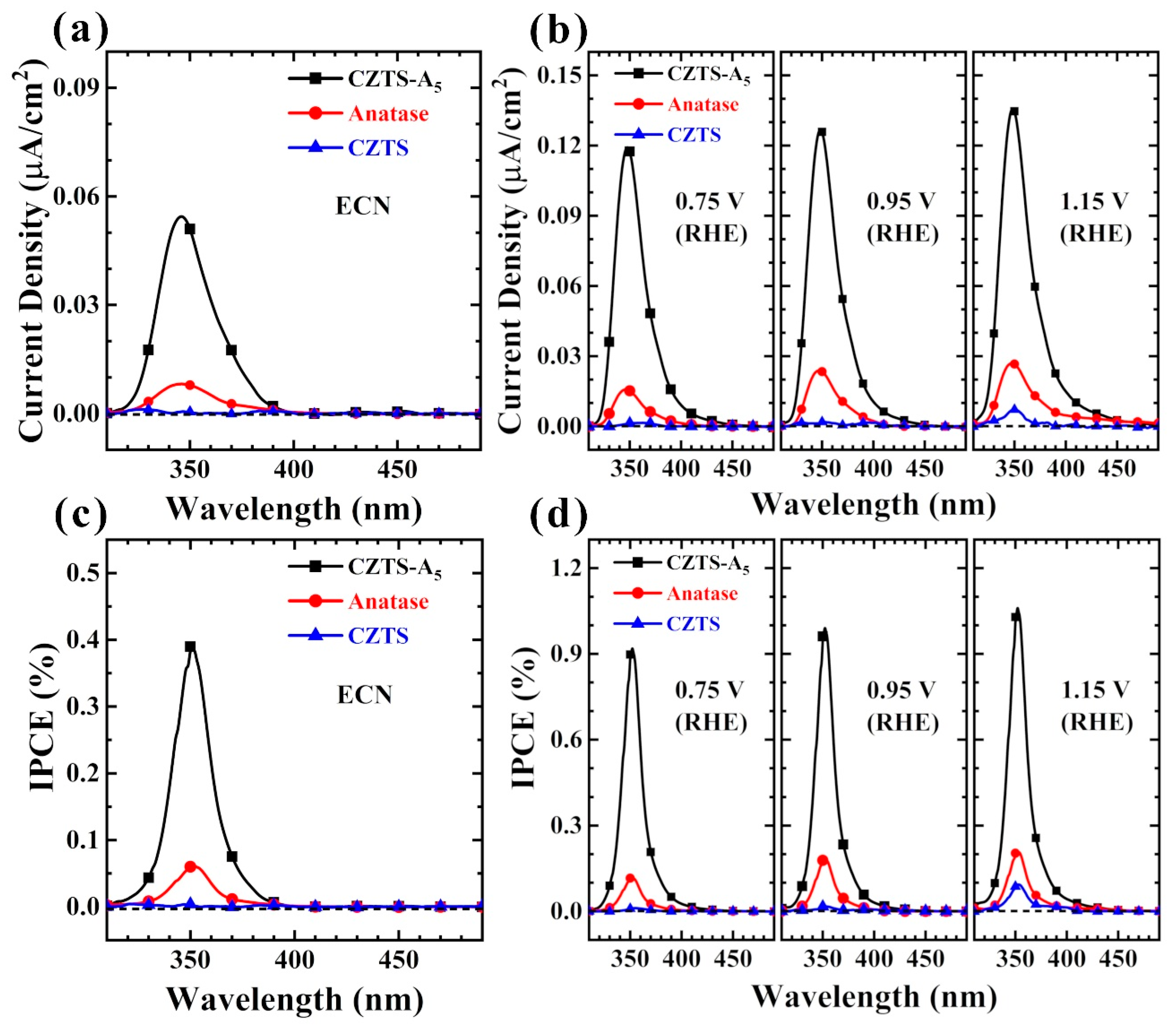
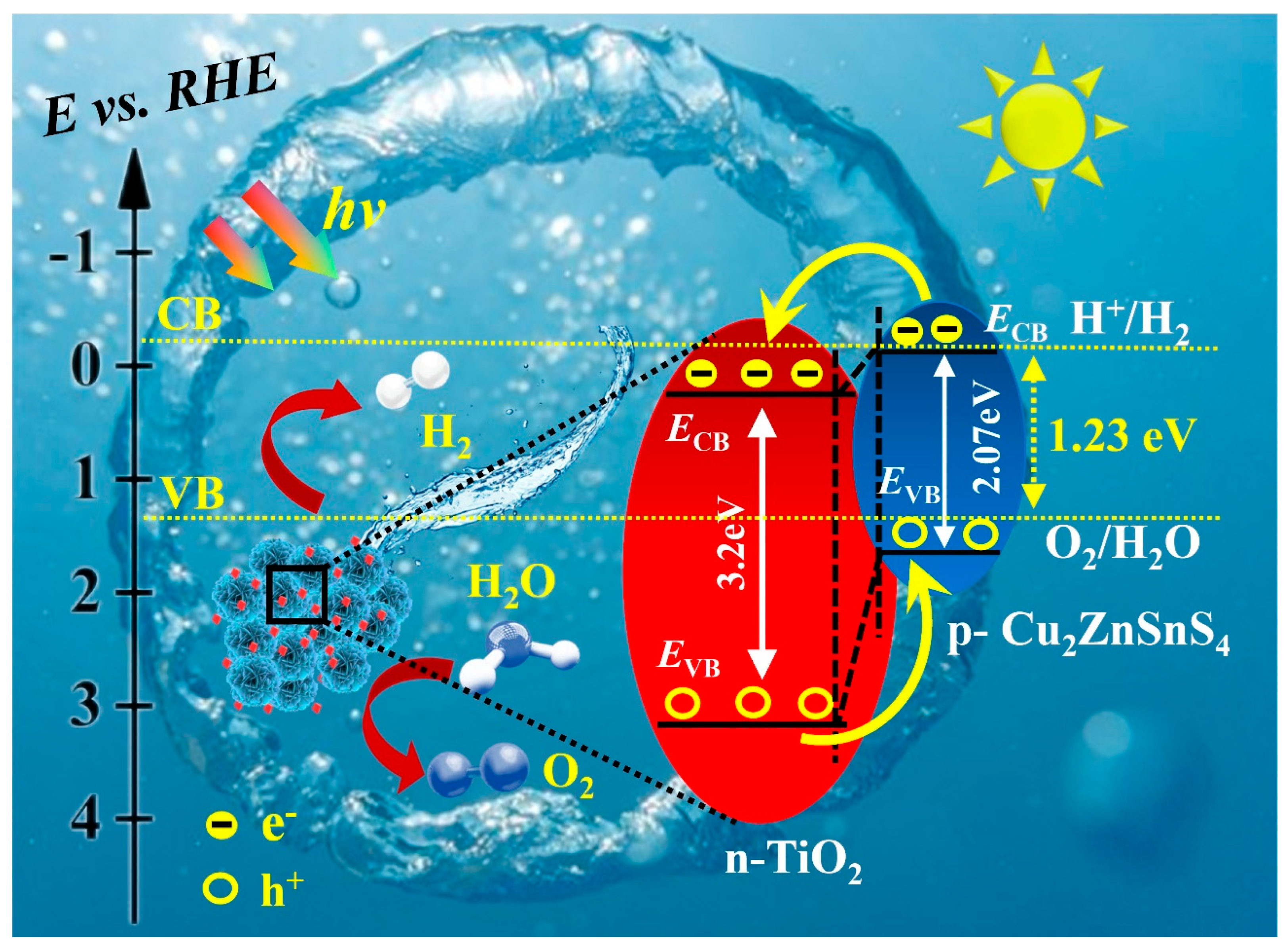
2.6. Electrochemical (EC), Photoelectrochemical (PEC) and Photocatalytic (PC) Performance
3. Materials and Methods
3.1. Materials
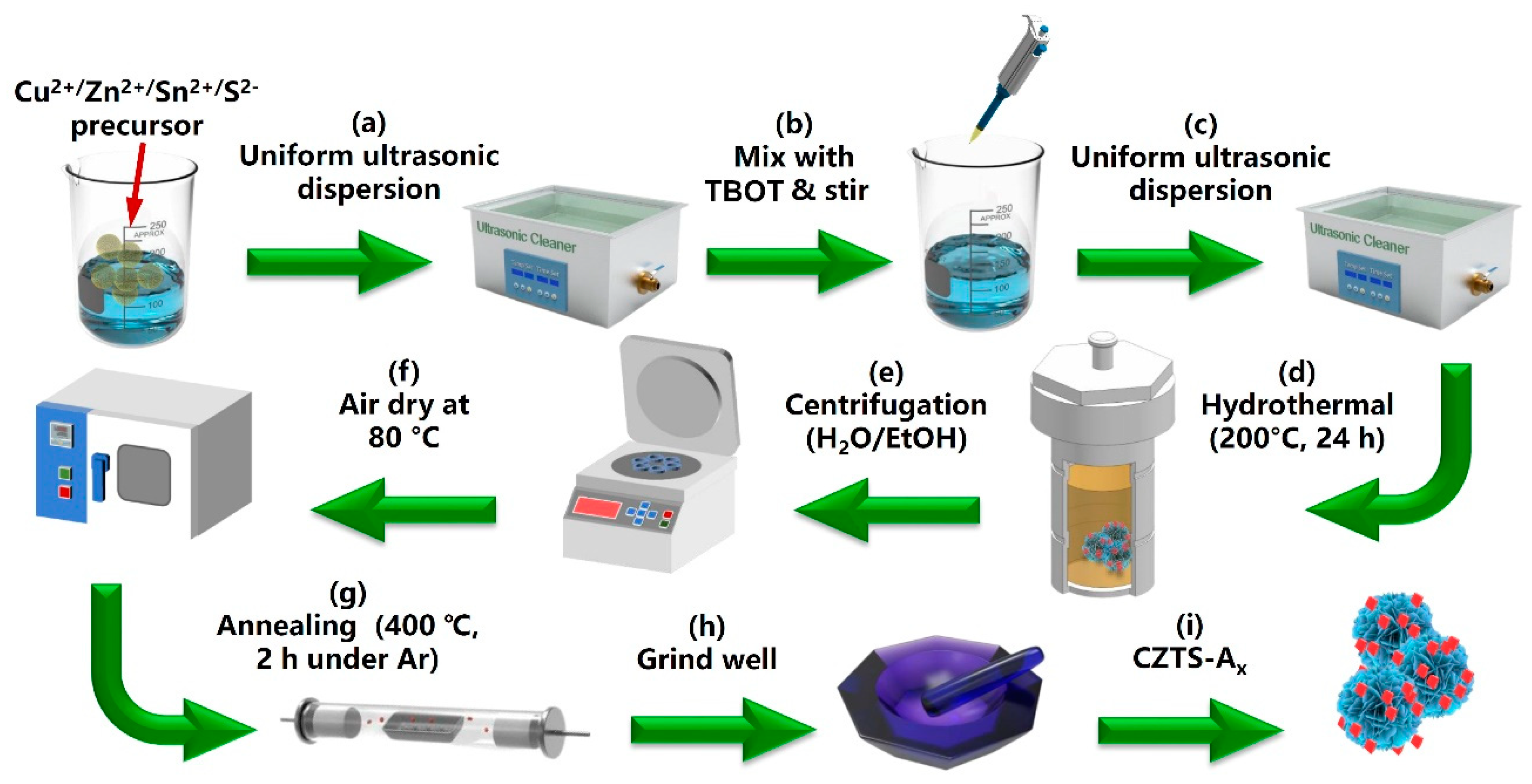
3.2. Synthesis Method
3.3. Characterization
3.4. Photoelectrochemical (PEC) Measurements
3.5. Photocatalytic (PC) Hydrogen Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic and Electrocatalytic Hydrogen Evolution Reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef]
- Yokoyama, D.; Minegishi, T.; Jimbo, K.; Hisatomi, T.; Ma, G.; Katayama, M.; Kubota, J.; Katagiri, H.; Domen, K. H2 Evolution from Water on Modified Cu2ZnSnS4 Photoelectrode under Solar Light. Appl. Phys. Express 2010, 3, 101202. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting-materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, T.; Minegishi, T.; Domen, K. Kinetic Assessment and Numerical Modeling of Photocatalytic Water Splitting toward Efficient Solar Hydrogen Production. Bull. Chem. Soc. Jpn. 2012, 85, 647–655. [Google Scholar] [CrossRef]
- Tong, M.-H.; Wang, T.-M.; Lin, S.-W.; Chen, R.; Jiang, X.; Chen, Y.-X.; Lu, C.-Z. Ultra-thin carbon doped TiO2 nanotube arrays for enhanced visible-light photoelectrochemical water splitting. Appl. Surf. Sci. 2023, 623, 156980. [Google Scholar] [CrossRef]
- Wang, J.; Ong, W.L.; Gao, M.; Zhu, L.; Ho, G.W. TiO2-Based Heterogeneous Catalysis for Photocatalytic Hydrogen Generation and Photodegradation. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 1–29. [Google Scholar] [CrossRef]
- Efthimiopoulos, I.; Küllmey, T.; Speziale, S.; Pakhomova, A.S.; Quennet, M.; Paulus, B.; Ritscher, A.; Lerch, M.; Koch-Müller, M. Pressure-induced structural and electronic transitions in kesterite-type Cu2ZnSnS4. J. Appl. Phys. 2018, 124, 085905. [Google Scholar] [CrossRef]
- Liao, M.; Wang, T.; Zuo, T.; Meng, L.; Yang, M.; Chen, Y.X.; Hu, T.; Xie, Y. Design and Solvothermal Synthesis of Polyoxometalate-Based Cu(II)-Pyrazolate Photocatalytic Compounds for Solar-Light-Driven Hydrogen Evolution. Inorg. Chem. 2021, 60, 13136–13149. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Y.; Jiang, X.; Ji, M.; Shi, H.; Wang, H.; Chen, Y.; Lu, C. Scheme Z Pd-NPs Embedded in 3DOM-LaCoO3-Dispersed pCN Heterojunction for Stimulating Visible Light-Catalyzed Solar H2 Production. ACS Appl. Energy Mater. 2023, 6, 11288–11298. [Google Scholar] [CrossRef]
- Lin, S.; Tong, M.; Chen, Y.; Chen, R.; Zhao, H.; Jiang, X.; Yang, K.; Lu, C. CeO2/TiO2 Heterojunction Nanotube Arrays for Highly Efficient Visible Light Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2023, 6, 1093–1102. [Google Scholar] [CrossRef]
- Yu, Y.; Dong, X.; Chen, P.; Geng, Q.; Wang, H.; Li, J.; Zhou, Y.; Dong, F. Synergistic Effect of Cu Single Atoms and Au-Cu Alloy Nanoparticles on TiO2 for Efficient CO2 Photoreduction. ACS Nano 2021, 15, 14453–14464. [Google Scholar] [CrossRef]
- Sakar, M.; Mithun Prakash, R.; Do, T.O. Insights into the TiO2-Based Photocatalytic Systems and Their Mechanisms. Catalysts 2019, 9, 680. [Google Scholar] [CrossRef]
- Raza, A.; Shen, H.; Haidry, A.A.; Shahzad, M.K.; Sun, L. Facile in-situ fabrication of TiO2-Cu2ZnSnS4 hybrid nanocomposites and their photoreduction of CO2 to CO/CH4 generation. Appl. Surf. Sci. 2020, 529, 147005. [Google Scholar] [CrossRef]
- Shi, W.; Cao, L.; Shi, Y.; Zhong, W.; Chen, Z.; Wei, Y.; Guo, F.; Chen, L.; Du, X. Boosted built-in electric field and active sites based on Ni-doped heptazine/triazine crystalline carbon nitride for achieving high-efficient photocatalytic H2 evolution. J. Mol. Struct. 2023, 1280, 135076. [Google Scholar] [CrossRef]
- Zhang, X.; Han, M.; Zeng, Z.; Lin, H.Q. The instability of S vacancies in Cu2ZnSnS4. RSC Adv. 2016, 6, 15424–15429. [Google Scholar] [CrossRef]
- Levcenko, S.; Hajdeu-Chicarosh, E.; Garcia-Llamas, E.; Caballero, R.; Serna, R.; Bodnar, I.V.; Victorov, I.A.; Guc, M.; Merino, J.M.; Pérez-Rodriguez, A.; et al. Spectroscopic ellipsometry study of Cu2ZnSnS4 bulk poly-crystals. Appl. Phys. Lett. 2018, 112, 161901. [Google Scholar] [CrossRef]
- Zhang, R.; Wen, X.; Xu, F.; Zhang, Q.; Sun, L. A Density Functional Theory Study of the Cu2ZnSnS4 Monolayer as a Photo-electrointegrated Catalyst for Water Splitting and Hydrogen Evolution. J. Phys. Chem. C 2020, 124, 11922–11929. [Google Scholar] [CrossRef]
- Kageshima, Y.; Shiga, S.; Ode, T.; Takagi, F.; Shiiba, H.; Htay, M.T.; Hashimoto, Y.; Teshima, K.; Domen, K.; Nishikiori, H. Photocatalytic and Photoelectrochemical Hydrogen Evolution from Water over Cu2SnxGe1−xS3 Particles. J. Am. Chem. Soc. 2021, 143, 5698–5708. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Isotta, E.; Fanciulli, C.; Ataollahi, N.; Scardi, P. Topological Anderson Insulator in Cation-Disordered Cu2ZnSnS4. Nanomaterials 2021, 11, 2595. [Google Scholar] [CrossRef]
- Zhu, X.; Xiong, J.; Wang, Z.; Chen, R.; Cheng, G.; Wu, Y. Metallic Copper Containing Composite Photocatalysts: Fundamental Materials Design and Photoredox Applications. Small Methods 2022, 6, 2101001. [Google Scholar] [CrossRef]
- Chen, G.L.; Wang, W.H.; Lin, P.Y.; Cai, H.L.; Chen, B.W.; Huang, X.J.; Zhang, J.M.; Chen, S.Y.; Huang, Z.G. The effect of binary sulfides precursors with different value states on CZTS thin films. Ceram. Int. 2018, 44, 18408–18412. [Google Scholar] [CrossRef]
- Semalti, P.; Sharma, V.; Sharma, S.N. A novel method of water remediation of organic pollutants and industrial wastes by solution-route processed CZTS nanocrystals. J. Mater. 2021, 7, 904–919. [Google Scholar] [CrossRef]
- Chen, S.; Liu, T.; Zheng, Z.; Ishaq, M.; Liang, G.; Fan, P.; Chen, T.; Tang, J. Recent progress and perspectives on Sb2Se3-based photocathodes for solar hydrogen production via photoelectrochemical water splitting. J. Energy Chem. 2022, 67, 508–523. [Google Scholar] [CrossRef]
- Chen, S.; Liu, T.; Chen, M.; Ishaq, M.; Tang, R.; Zheng, Z.; Su, Z.; Li, X.; Qiao, X.; Fan, P.; et al. Crystal growth promotion and interface optimization enable highly efficient Sb2Se3 photocathodes for solar hydrogen evolution. Nano Energy 2022, 99, 107417. [Google Scholar] [CrossRef]
- Dursun, S.; Sarıipek, F.B.; Kılıç, S.; Gezgin, S.Y.; Gündoğdu, Y.; Kılıç, H.Ş. Investigation of photocatalytic activity (under visible light) of ultrathin CZTS films produced in different thicknesses by PLD method. Opt. Quantum Electron. 2023, 55, 166. [Google Scholar] [CrossRef]
- Liang, G.; Li, Z.; Ishaq, M.; Zheng, Z.; Su, Z.; Ma, H.; Zhang, X.; Fan, P.; Chen, S. Charge Separation Enhancement Enables Record Photocurrent Density in Cu2ZnSn(S,Se)4 Photocathodes for Efficient Solar Hydrogen Production. Adv. Energy Mater. 2023, 13, 2300215. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Singh, S.; Khare, N. Visible light active CZTS sensitized CdS/TiO2 tandem photoanode for highly efficient photoelectrochemical hydrogen generation. Sol. Energy 2019, 181, 37–42. [Google Scholar] [CrossRef]
- Zhu, L.; Tao, J.; Tao, H.; Chen, S.; Shen, Y.; Xu, A.; Jiang, J.; Pan, L. In-situ growth of Cu2ZnSnS4 nanosheets on TiO2 nanowires for enhanced photoelectrochemical performance. J. Alloys Compd. 2015, 649, 704–711. [Google Scholar] [CrossRef]
- Efthimiopoulos, I.; Küllmey, T.; Speziale, S.; Pakhomova, A.S.; Quennet, M.; Paulus, B.; Ritscher, A.; Lerch, M. High-pressure behavior of disordered kesterite-type Cu2ZnSnS4. Appl. Phys. A-Mater. Sci. Process. 2021, 127, 616. [Google Scholar] [CrossRef]
- Al-Ani, A.A.-S.; Tokay, B.; Zhu, W.; Chen, G.Z. Enhancement of photoconversion efficiency and light harvesting ability of TiO2 nanotube-arrays with Cu2ZnSnS4. Int. J. Hydrogen Energy 2022, 47, 31003–31013. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, Z.; Pan, X.; Ma, Z.; Zhang, X. Ultrafine Cu2ZnSnS4 quantum dots functionalized TiO2 nanotube arrays for potential optoelectronic applications. Ceram. Int. 2020, 46, 2940–2948. [Google Scholar] [CrossRef]
- Suryawanshi, M.P.; Ghorpade, U.V.; Shin, S.W.; Gang, M.G.; Wang, X.; Park, H.; Kang, S.H.; Kim, J.H. Enhanced Solar Water Oxidation Performance of TiO2 via Band Edge Engineering: A Tale of Sulfur Doping and Earth-Abundant CZTS Nanoparticles Sensitization. ACS Catal. 2017, 7, 8077–8089. [Google Scholar] [CrossRef]
- Sawant, J.P.; Kale, R.B. CZTS counter electrode in dye-sensitized solar cell: Enhancement in photo conversion efficiency with morphology of TiO2 nanostructured thin films. J. Solid State Electrochem. 2019, 24, 461–472. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, Y.; Zhang, J.; Liang, N.; Long, L.; Liu, J. Insight into the Growth Mechanism of Mixed Phase CZTS and the Photocatalytic Performance. Nanomaterials 2022, 12, 1439. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, M.; Xu, Y.; Li, X.; Zhou, X.; Hong, L.; Jiang, L.; Lin, W.-F. S vacancy modulated ZnxCd1−xS/CoP quantum dots for efficient H2 evolution from water splitting under visible light. J. Energy Chem. 2021, 61, 210–218. [Google Scholar] [CrossRef]
- Xin, X.; He, M.; Han, W.; Jung, J.; Lin, Z. Low-cost copper zinc tin sulfide counter electrodes for high-efficiency dye-sensitized solar cells. Angew. Chem. Int. Ed. 2011, 50, 11739–11742. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, L.; Su, Y.; Shao, D.; Zeng, S.; Wang, W. Photoreduction of carbon dioxide of atmospheric concentration to methane with water over CoAl-layered double hydroxide nanosheets. J. Mater. Chem. A 2018, 6, 8366–8373. [Google Scholar] [CrossRef]
- Lin, S.J.; Ting, J.M.; Hsu, K.C.; Fu, Y.S. A Composite Photocatalyst Based on Hydrothermally-Synthesized Cu2ZnSnS4 Powders. Materials 2018, 11, 158. [Google Scholar] [CrossRef]
- Wu, F.; Yang, R.; Lu, S.; Du, W.; Zhang, B.; Shi, Y. Unveiling Partial Transformation and Activity Origin of Sulfur Vacancies for Hydrogen Evolution. ACS Energy Lett. 2022, 7, 4198–4203. [Google Scholar] [CrossRef]
- Yang, M.; Liu, J.; Xu, H.; Pei, Y.; Jiang, C.; He, D.; Xiao, X. Intrinsic defects of nonprecious metal electrocatalysts for energy conversion: Synthesis advanced characterization and fundamentals. ChemPhysMater 2022, 1, 155–182. [Google Scholar] [CrossRef]
- Jin, J.; Wang, X.; Hu, Y.; Zhang, Z.; Liu, H.; Yin, J.; Xi, P. Precisely Control Relationship between Sulfur Vacancy and H Absorption for Boosting Hydrogen Evolution Reaction. Nanomicro Lett. 2024, 16, 63. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, Y.X.; Zhou, J.W.; Lin, S.W.; Lu, C.Z. Pollen Carbon Based Rare Earth Composite Material for Highly Efficient Photocatalytic Hydrogen Production from Ethanol Water Mixtures. ACS Omega 2022, 7, 30495–30503. [Google Scholar] [CrossRef]
- Jian, J.; Kang, H.; Yu, D.; Qiao, X.; Liu, Y.; Li, Y.; Qin, W.; Wu, X. Bi-Functional Co/Al Modified 1T-MoS2/rGO Catalyst for Enhanced Uranium Extraction and Hydrogen Evolution Reaction in Seawater. Small 2023, 19, 2207378. [Google Scholar] [CrossRef]
- Li, H.; Xie, C.; Liao, Y.; Liu, Y.; Zou, Z.; Wu, J. Characterization of Incidental Photon-to-electron Conversion Efficiency (IPCE) of porous TiO2/SnO2 composite film. J. Alloys Compd. 2013, 569, 88–94. [Google Scholar] [CrossRef]
- Wang, S.; Qiao, Y.; Liu, X.; Zhu, S.; Zheng, Y.; Jiang, H.; Zhang, Y.; Shen, J.; Li, Z.; Liang, Y.; et al. Reduced Graphene Oxides Modified Bi2Te3 Nanosheets for Rapid Photo-Thermoelectric Catalytic Therapy of Bacteria-Infected Wounds. Adv. Funct. Mater. 2022, 33, 2210098. [Google Scholar] [CrossRef]
- Zhou, Y.; Ye, X.; Lin, D. Enhance photocatalytic hydrogen evolution by using alkaline pretreated corn stover as a sacrificial agent. Int. J. Energy Res. 2020, 44, 4616–4628. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.R.; Sahoo, L.; Dutta, S.; Gautam, U.K. Light-Induced Hypoxia in Carbon Quantum Dots and Ultrahigh Photocatalytic Efficiency. J. Am. Chem. Soc. 2022, 144, 2580–2589. [Google Scholar] [CrossRef]



| Samples | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Size (nm) | Most Frequent Pore Diameter (nm) |
|---|---|---|---|---|
| anatase | 87.223 | 0.372 | 1.805 | – |
| CZTS | 22.022 | 0.042 | 8.488 | – |
| CZTS–A3 | 132.280 | 0.301 | 9.101 | 8.743 |
| CZTS–A5 | 98.954 | 0.312 | 1.239 | 10.300 |
| CZTS–A9 | 126.612 | 0.306 | 9.562 | 8.739 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.-X.; Li, C.-H.; Shi, H.-Y.; Chen, R.; She, A.-S.; Yang, Y.; Jiang, X.; Chen, Y.-X.; Lu, C.-Z. A Convenient In Situ Preparation of Cu2ZnSnS4–Anatase Hybrid Nanocomposite for Photocatalysis/Photoelectrochemical Water-Splitting Hydrogen Production. Molecules 2024, 29, 2514. https://doi.org/10.3390/molecules29112514
Li K-X, Li C-H, Shi H-Y, Chen R, She A-S, Yang Y, Jiang X, Chen Y-X, Lu C-Z. A Convenient In Situ Preparation of Cu2ZnSnS4–Anatase Hybrid Nanocomposite for Photocatalysis/Photoelectrochemical Water-Splitting Hydrogen Production. Molecules. 2024; 29(11):2514. https://doi.org/10.3390/molecules29112514
Chicago/Turabian StyleLi, Ke-Xian, Cai-Hong Li, Hao-Yan Shi, Rui Chen, Ao-Sheng She, Yang Yang, Xia Jiang, Yan-Xin Chen, and Can-Zhong Lu. 2024. "A Convenient In Situ Preparation of Cu2ZnSnS4–Anatase Hybrid Nanocomposite for Photocatalysis/Photoelectrochemical Water-Splitting Hydrogen Production" Molecules 29, no. 11: 2514. https://doi.org/10.3390/molecules29112514






