The Interaction between Iron Cyanide and Lead Ions in Pyrite Flotation
Abstract
1. Introduction
2. Experimental Setup
2.1. Materials
2.2. Methods
2.2.1. Grinding and Flotation
2.2.2. Cryo-XPS Analysis
2.2.3. Zeta Potential Analysis
2.2.4. EIS Analysis
3. Results and Discussion
3.1. Flotation
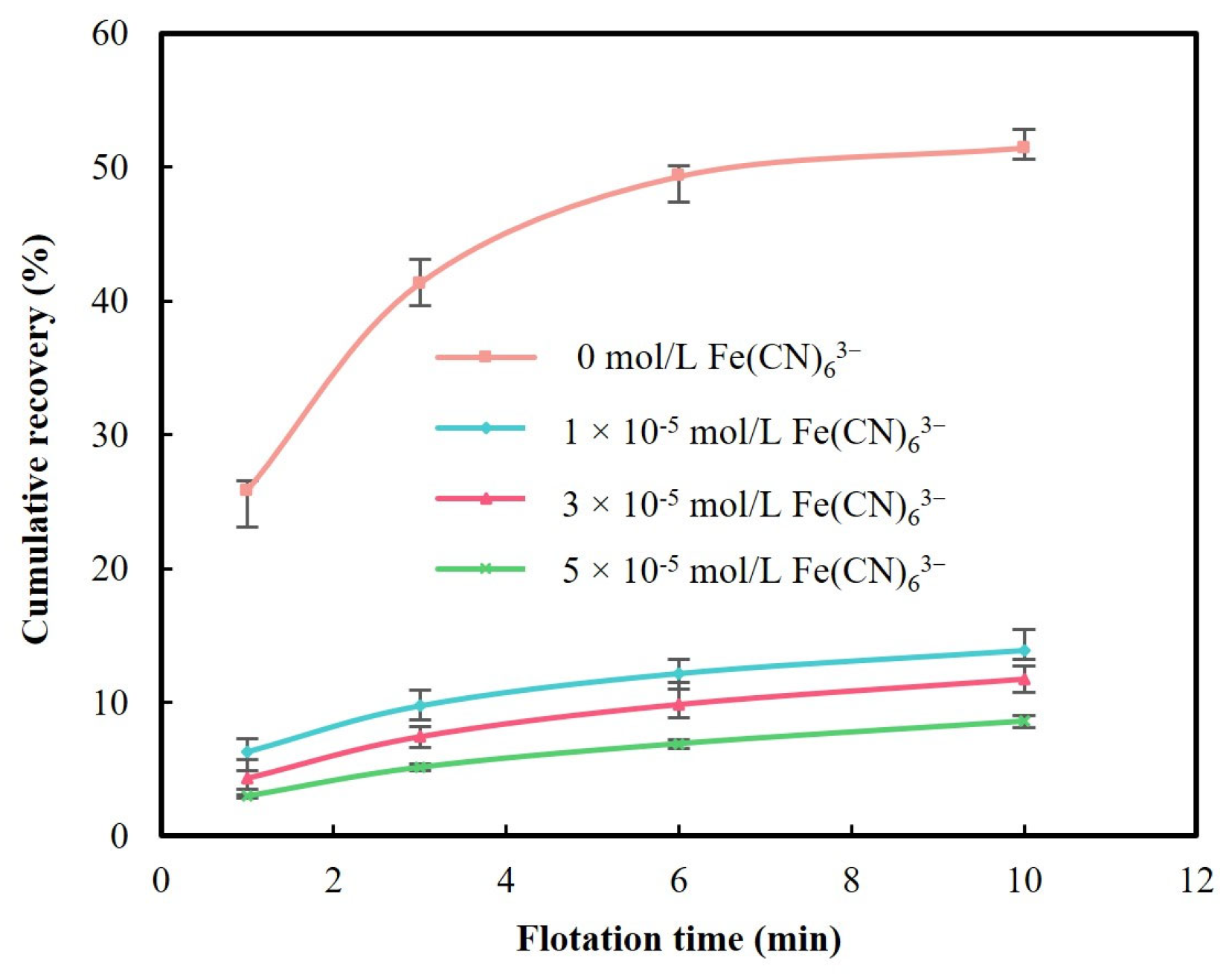
3.1.1. Effect of Iron Cyanide on Pyrite Flotation
3.1.2. Effect of Lead Ions on Pyrite Flotation in the Presence of Iron Cyanide
3.2. Surface Species on Pyrite
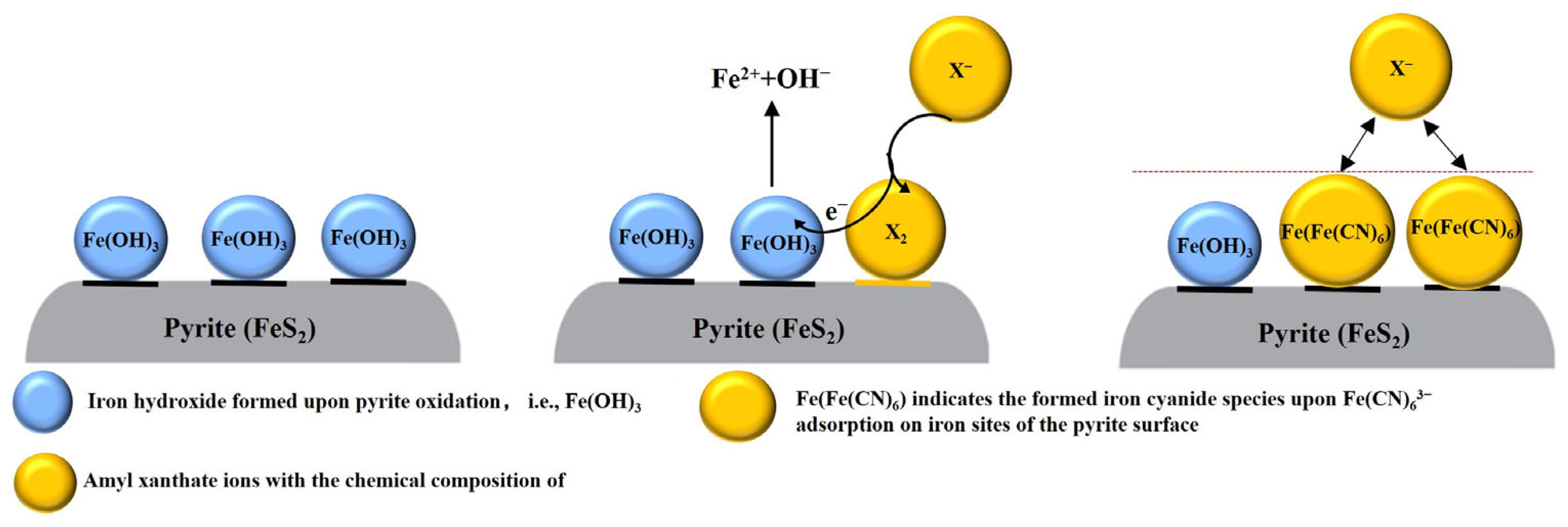
3.2.1. Surface Species Underpinning Pyrite Depression by Fe(CN)63−
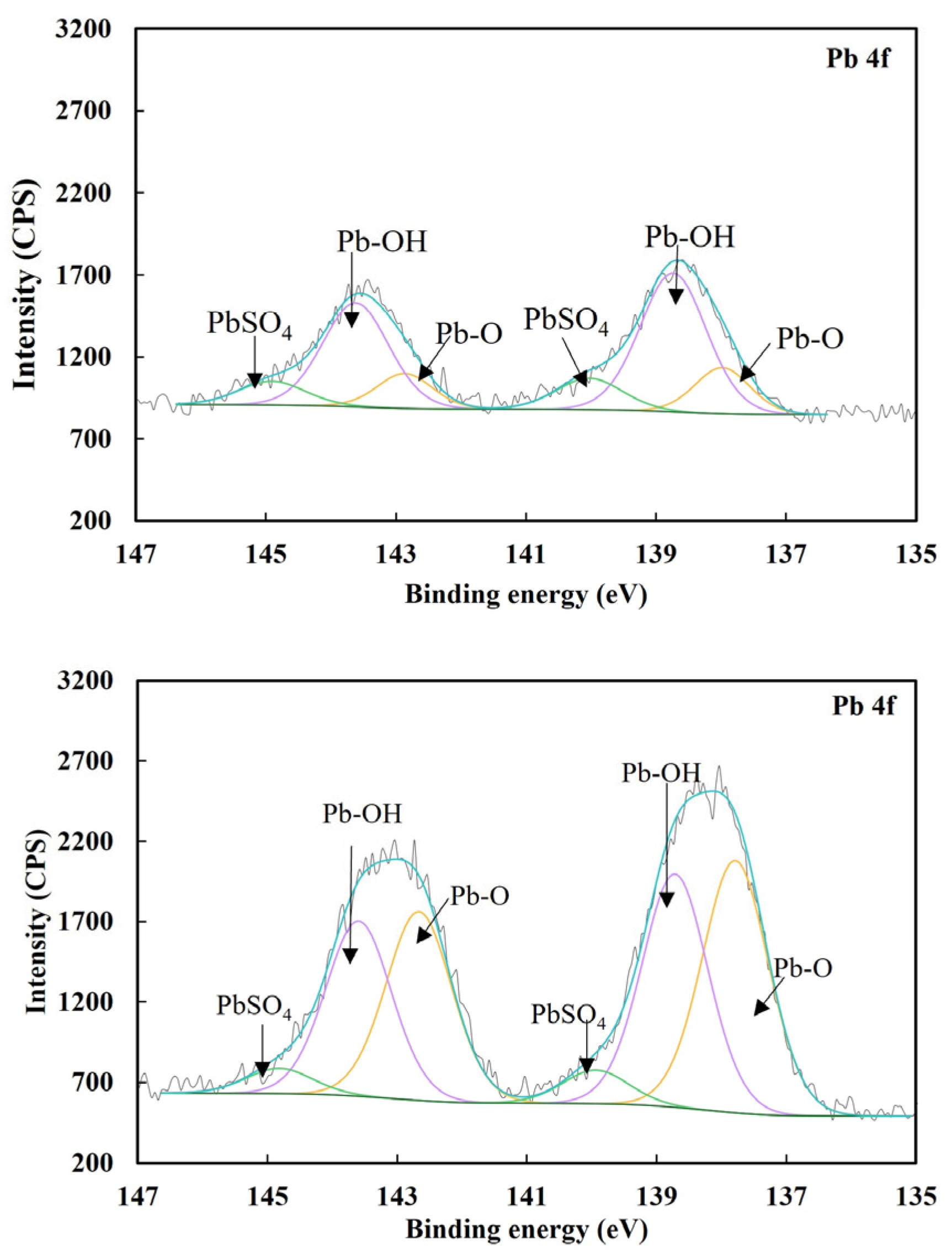
3.2.2. Surface Species Underpinning Pyrite Activation by Lead Ions
3.2.3. Zeta Potential of Pyrite Surfaces upon Addition of Lead Ions and/or Fe(CN)63−
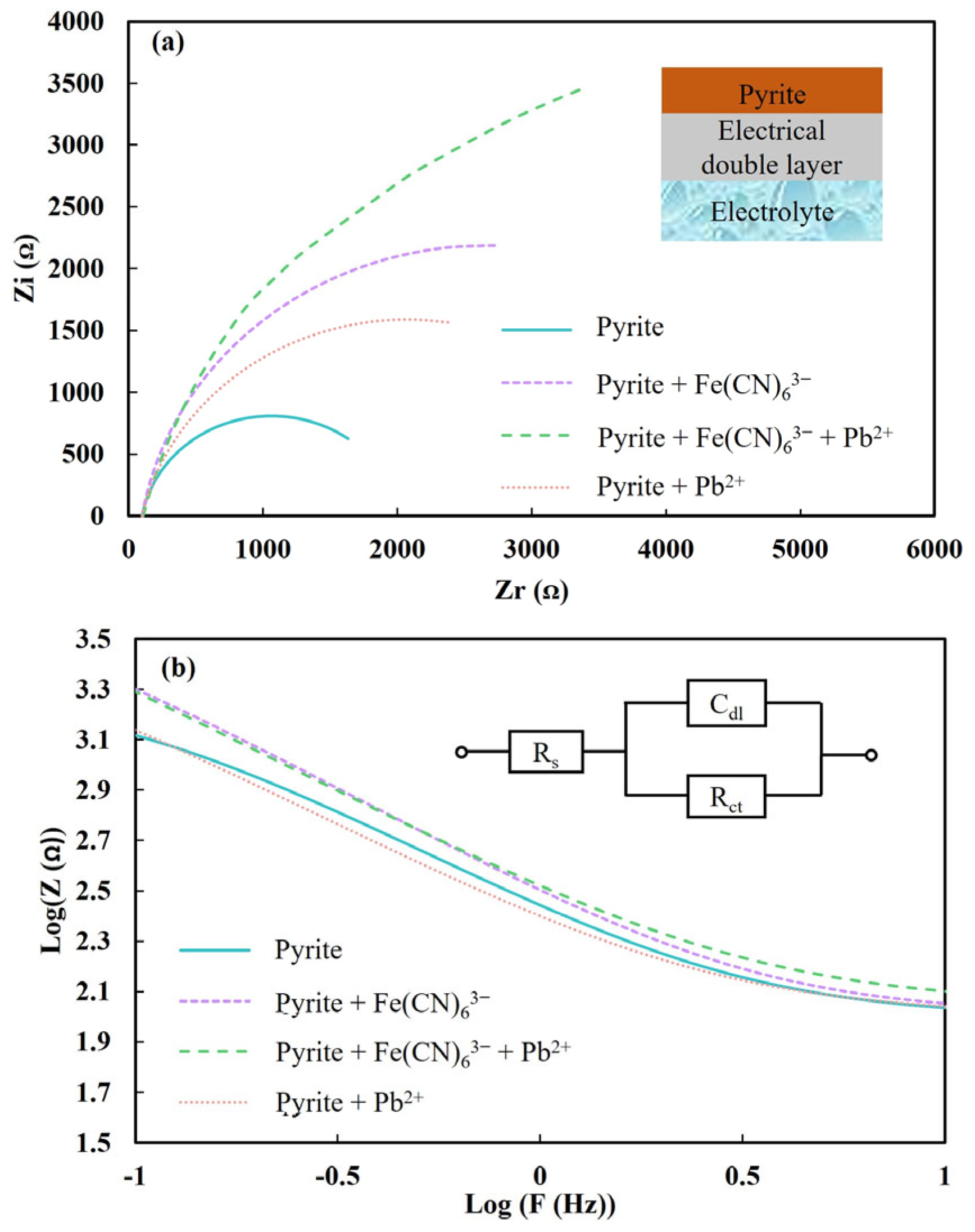
3.3. EIS of Pyrite Surfaces
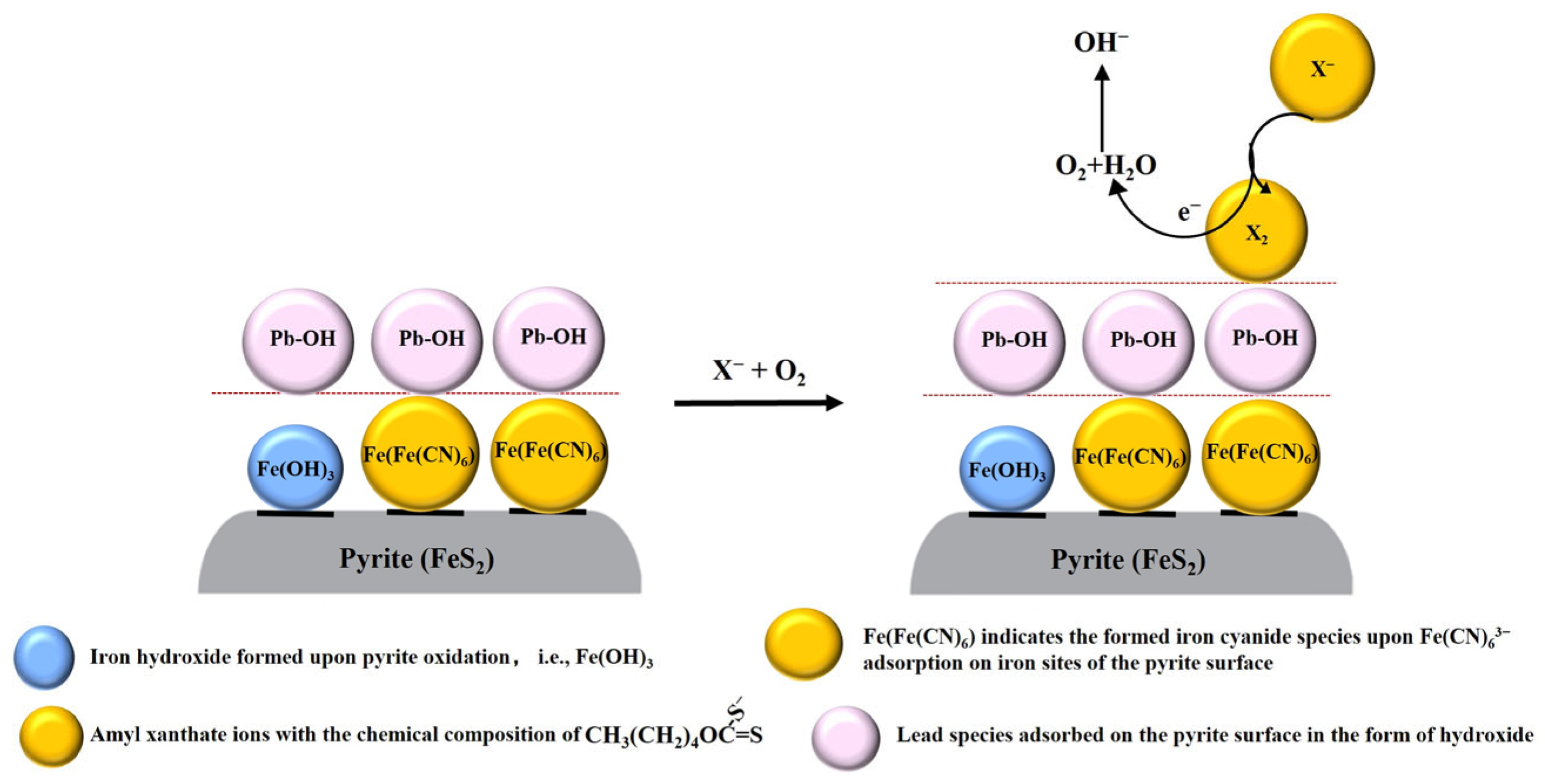
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neisiani, A.A.; Saneie, R.; Mohammadzadeh, A.; Wonyen, D.G.; Chelgani, S.C. Polysaccharides-based pyrite depressants for green flotation separation: An overview. Int. J. Min. Sci. Technol. 2023, 33, 1229–1241. [Google Scholar] [CrossRef]
- Faraz, S.; Hossna, D.; Rezgar, B.; Piroz, Z. Improved recovery of a low-grade refractory gold ore using flotation–preoxidation–cyanidation methods. Int. J. Min. Sci. Technol. 2014, 24, 537–542. [Google Scholar] [CrossRef]
- Adams, M.D. Impact of recycling cyanide and its reaction products on upstream unit operations. Miner. Eng. 2013, 53, 241–255. [Google Scholar] [CrossRef]
- Wang, X.H.; Forssberg, K.S.E. The solution electrochemistry of sulfide-xanthate-cyanide systems in sulfide mineral flotation. Miner. Eng. 1996, 9, 527–546. [Google Scholar] [CrossRef]
- De Wet, J.R.; Pistorius, P.C.; Sandenbergh, R.F. The influence of cyanide on pyrite flotation from gold leach residues with sodium isobutyl xanthate. Int. J. Miner. Process. 1997, 49, 149–169. [Google Scholar] [CrossRef]
- Prestidge, C.A.; Ralston, J.; Smart, R.S.C. The competitive adsorption of cyanide and ethyl xanthate on pyrite and pyrrhotite surfaces. Int. J. Miner. Process. 1993, 38, 205–233. [Google Scholar] [CrossRef]
- Guo, B.; Peng, Y.; Espinosa-Gomez, R. Cyanide chemistry and its effect on mineral flotation. Miner. Eng. Miner. Eng. 2014, 66–68, 25–32. [Google Scholar] [CrossRef]
- Wang, X. Interfacial Electrochemistry of Pyrite Oxidation and Flotation: II. FTIR Studies of Xanthate Adsorption on Pyrite Surfaces in Neutral pH Solutions. J. Colloid Interface Sci. 1995, 171, 413–428. [Google Scholar] [CrossRef]
- Sutherland, K.L.; Wark, I.W. Principles of Flotation; Australasian Institute of Mining and Metallurgy: Carlton, Australia, 1955. [Google Scholar]
- Zhao, W.; Yang, B.; Yi, Y.; Feng, Q.; Liu, D. Synergistic activation of smithsonite with copper-ammonium species for enhancing surface reactivity and xanthate adsorption. Int. J. Min. Sci. Technol. 2023, 33, 519–527. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef]
- Chandra, A.P.; Puskar, L.; Simpson, D.J.; Gerson, A.R. Copper and xanthate adsorption onto pyrite surfaces: Implications for mineral separation through flotation. Int. J. Miner. Process. 2012, 114–117, 16–26. [Google Scholar] [CrossRef]
- Guo, B.; Peng, Y. The interaction between copper species and pyrite surfaces in copper cyanide solutions. Int. J. Miner. Process. 2017, 158, 85–92. [Google Scholar] [CrossRef]
- Zheng, J.; Ritchie, I.; La Brooy, S.; Singh, P. Study of gold leaching in oxygenated solutions containing cyanide-copper-ammonia using a rotating quartz crystal microbalance. Hydrometallurgy 1995, 39, 277–292. [Google Scholar] [CrossRef]
- Yang, X.; Mu, Y.; Peng, Y. Comparing lead and copper activation on pyrite with different degrees of surface oxidation. Miner. Eng. 2021, 168, 106926. [Google Scholar] [CrossRef]
- Yang, X.; Mu, Y.; Peng, Y. The roles of lead ions in restoring the floatability of pyrite depressed by free cyanide. Miner. Eng. 2022, 175, 107289. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y. The role of sodium metabisulphite in depressing pyrite in chalcopyrite flotation using saline water. Miner. Eng. 2019, 142, 105921. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, J.; Wang, Y.; Li, S.; Zhao, H.; Liu, C.; Ma, Z.; Sun, Z. Adsorption characteristics of Pb(II) ions on sulfidized hemimorphite surface under ammonium sulfate system. Int. J. Min. Sci. Technol. 2023, 33, 511–518. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, B.; Gerson, A. The effect of electrochemical potential on the activation of pyrite by copper and lead ions during grinding. Int. J. Miner. Process. 2012, 102–103, 141–149. [Google Scholar] [CrossRef]
- Hongxin, Q.; Xiaohao, S.; Bozeng, W.; Xinqian, S.; Mingzhen, H. Mechanism of arsenic release process from arsenopyrite chemical oxidation. Sci. Total. Environ. 2024, 915, 169969. [Google Scholar] [CrossRef]
- Mu, Y.; Li, L.; Peng, Y. Surface properties of fractured and polished pyrite in relation to flotation. Miner. Eng. 2017, 101, 10–19. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. The depression of copper-activated pyrite in flotation by biopolymers with different compositions. Miner. Eng. 2016, 96–97, 113–122. [Google Scholar] [CrossRef]
- Valdivieso, A.L.; López, A.S.; Song, S. On the cathodic reaction coupled with the oxidation of xanthates at the pyrite/aqueous solution interface. Int. J. Miner. Process. 2005, 77, 154–164. [Google Scholar] [CrossRef]
- Guo, B.; Peng, Y.; Parker, G. Electrochemical and spectroscopic studies of pyrite–cyanide interactions in relation to the depression of pyrite flotation. Miner. Eng. 2016, 92, 78–85. [Google Scholar] [CrossRef]
- Sui, C.; Brienne, S.; Rao, S.R.; Xu, Z.; Finch, J.A. Metal ion production and transfer between sulphide minerals. Miner. Eng. 1995, 8, 1523–1539. [Google Scholar] [CrossRef]
- Laajalehto, K.; Leppinen, J.; Kartio, I.; Laiho, T. XPS and FTIR study of the influence of electrode potential on activation of pyrite by copper or lead. Colloids Surfaces A Physicochem. Eng. Asp. 1999, 154, 193–199. [Google Scholar] [CrossRef]
- Pecina, E.; Uribe, A.; Nava, F.; Finch, J.A. The role of copper and lead in the activation of pyrite in xanthate and non-xanthate systems. Miner. Eng. 2006, 19, 172–179. [Google Scholar] [CrossRef]
- Peng, Y.; Grano, S. Effect of grinding media on the activation of pyrite flotation. Miner. Eng. 2010, 23, 600–605. [Google Scholar] [CrossRef]
- Graf, N.; Yegen, E.; Gross, T.; Lippitz, A.; Weigel, W.; Krakert, S.; Terfort, A.; Unger, W.E.S. XPS and NEXAFS studies of aliphatic and aromatic amine species on functionalized surfaces. Surf. Sci. 2009, 603, 2849–2860. [Google Scholar] [CrossRef]
- Yan, X.; Xu, T.; Chen, G.; Yang, S.; Liu, H.; Xue, Q. Preparation and characterization of electrochemically deposited carbon nitride films on silicon substrate. J. Phys. D Appl. Phys. 2004, 37, 907–913. [Google Scholar] [CrossRef]
- Lisowska-Oleksiak, A.; Nowak, A.; Wilamowska, M.; Sikora, M.; Szczerba, W.; Kapusta, C. Ex situ XANES, XPS and Raman studies of poly(3,4-ethylenedioxythiophene) modified by iron hexacyanoferrate. Synth. Met. 2010, 160, 1234–1240. [Google Scholar] [CrossRef]
- Cano, A.; Lartundo-Rojas, L.; Shchukarev, A.; Reguera, E. Contribution to the coordination chemistry of transition metal nitroprussides: A cryo-XPS study. New J. Chem. 2019, 43, 4835–4848. [Google Scholar] [CrossRef]
- Bi, J.; Huang, X.; Wang, J.; Wang, T.; Wu, H.; Yang, J.; Lu, H.; Hao, H. Oil-phase cyclic magnetic adsorption to synthesize Fe3O4@C@TiO2-nanotube composites for simultaneous removal of Pb(II) and Rhodamine B. Chem. Eng. J. 2019, 366, 50–61. [Google Scholar] [CrossRef]
- Buckley, A.N.; Kravets, I.M.; Shchukarev, A.V.; Woods, R. Interaction of galena with hydrosulphide ions under controlled potentials. J. Appl. Electrochem. 1994, 24, 513–520. [Google Scholar] [CrossRef]
- Lai, H.; Deng, J.; Wen, S.; Liu, Q. Elucidation of lead ions adsorption mechanism on marmatite surface by PCA-assisted ToF-SIMS, XPS and zeta potential. Miner. Eng. 2019, 144, 106035. [Google Scholar] [CrossRef]
- Xu, D.; Tan, X.; Chen, C.; Wang, X. Removal of Pb(II) from aqueous solution by oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2008, 154, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Zingg, D.S.; Hercules, D.M. Electron spectroscopy for chemical analysis studies of lead sulfide oxidation. J. Phys. Chem. 1978, 82, 1992–1995. [Google Scholar] [CrossRef]
- Pecina, E.; Uribe, A.; Finch, J.A.; Nava, F. Mechanism of di-isobutyl dithiophosphinate adsorption onto galena and pyrite. Miner. Eng. 2006, 19, 904–911. [Google Scholar] [CrossRef]
- Elgillani, D.A.; Fuerstenau, M.C. Mechanisms involved in cyanide depression of pyrite. Trans. AIME (Soc. Min. Eng.) 1968, 241, 437–445. [Google Scholar]
- Pajor-Świerzy, A.; Kruk, T.; Warszyński, P. Enhancement of the Electrocatalytic Properties of Prussian Blue Containing Multilayer Films Formed by Reduced Graphene Oxide. Colloid Interface Sci. Commun. 2014, 1, 6–9. [Google Scholar] [CrossRef]
- Zampardi, G.; Sokolov, S.; Batchelor-Mcauley, C.; Compton, R. Potassium (De-)insertion Processes in Prussian Blue Particles: Ensemble versus Single Nanoparticle Behaviour. Chem. A Eur. J. 2017, 23, 14338–14344. [Google Scholar] [CrossRef]
- Yao, C.; Dai, Y.; Chang, S.; Zhang, H. Removal of cesium and strontium for radioactive wastewater by Prussian blue nanorods. Environ. Sci. Pollut. Res. 2023, 30, 36807–36823. [Google Scholar] [CrossRef]
- Huai, Y.; Plackowski, C.; Peng, Y. The effect of gold coupling on the surface properties of pyrite in the presence of ferric ions. Appl. Surf. Sci. 2019, 488, 277–283. [Google Scholar] [CrossRef]




| Category | Species | LogKn |
|---|---|---|
| Free cyanide | CN− | - |
| HCN | 9.2 | |
| WAD | Cd(CN)42− | 17.9 |
| Zn(CN)43− | 19.6 | |
| Cu(CN)2− | 16.3 | |
| Cu(CN)32− | 21.6 | |
| Cu(CN)43− | 23.1 | |
| Ni(CN)42− | 30.2 | |
| Ag(CN)2− | 20.5 | |
| SAD | Fe(CN)64− | 35.4 |
| Fe(CN)63− | 43.6 | |
| Co(CN)63− | 64.0 | |
| Hg(CN)42− | 39.0 | |
| Au(CN)2− | 38.3 |
| Sample | Elements Identified (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | S | Cu | Bi | Pb | Al2O3 | SiO2 | Ti | Zn | |
| Pyrite (0.6–3.2 mm) | 45.4 | 50.5 | 0.1 | 0.02 | 0.13 | 0.03 | 0.35 | 0.04 | 0.05 |
| Samples | Species | N 1s B.E. (eV) | Concentration (at.%) |
|---|---|---|---|
| Pyrite | NH2 | 399.1 | 1.29 |
| O=C-NH | 400.7 | 2.39 | |
| NO− | 401.9 | 2.51 | |
| Pyrite + Fe(CN)63− | Fe-CN species | 397.4 | 1.57 |
| NH2 | 399.1 | 0.68 | |
| C=N | 399.7 | 2.76 | |
| ---NH2/NH3+ | 401.0 | 0.57 | |
| NO− | 402.0 | 0.54 |
| Sample | Species | Pb 4f7/2 B.E. (eV) | Concentration (at.%) |
|---|---|---|---|
| Pyrite + Pb2+ | Pb-O | 137.9 | 0.29 |
| Pb-OH | 138.7 | 1.05 | |
| PbSO4 | 139.8 | 0.24 | |
| Pyrite + Fe(CN)63− + Pb2+ | Pb-O | 137.8 | 1.64 |
| Pb-OH | 138.7 | 1.52 | |
| PbSO4 | 139.9 | 0.21 |
| Sample | Pyrite | Pyrite + Fe(CN)63− | Pyrite + Pb + Fe(CN)63− | Pyrite + Fe(CN)63− + Pb | Pyrite + Pb |
|---|---|---|---|---|---|
| Zeta potential value (mV) | −10.37 | −37.36 | −3.40 | −4.67 | 31.02 |
| Sample | Rs (ꭥ) | Rct (ꭥ) | CPE(μF) | n |
|---|---|---|---|---|
| Pyrite | 98.5 | 1928 | 818 | 0.89 |
| Pyrite + Fe(CN)63− | 101.4 | 5149 | 676 | 0.90 |
| Pyrite + Pb2+ | 99.95 | 3952 | 986 | 0.86 |
| Pyrite + Fe(CN)63− + Pb2+ | 110.4 | 9438 | 729 | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Mu, Y.; Liu, S. The Interaction between Iron Cyanide and Lead Ions in Pyrite Flotation. Molecules 2024, 29, 2517. https://doi.org/10.3390/molecules29112517
Yang X, Mu Y, Liu S. The Interaction between Iron Cyanide and Lead Ions in Pyrite Flotation. Molecules. 2024; 29(11):2517. https://doi.org/10.3390/molecules29112517
Chicago/Turabian StyleYang, Xiaoxia, Yufan Mu, and Shiqi Liu. 2024. "The Interaction between Iron Cyanide and Lead Ions in Pyrite Flotation" Molecules 29, no. 11: 2517. https://doi.org/10.3390/molecules29112517
APA StyleYang, X., Mu, Y., & Liu, S. (2024). The Interaction between Iron Cyanide and Lead Ions in Pyrite Flotation. Molecules, 29(11), 2517. https://doi.org/10.3390/molecules29112517









