Accumulation of Bisphenol A® by Pleurotus spp.—Flow Injection Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Scheme of the Experiment
2.2. Mushroom Materials
2.2.1. Initial Mycelial Cultures (Step 1)
2.2.2. Experimental Mycelial Cultures (Step 2)
2.3. Mushroom Material Lyophilization Analysis (Step 3)
2.3.1. Sorption and Desorption
2.3.2. Extraction of Bisphenol A® into Artificial Digestive Juices (Step 3)
Preparation of Artificial Digestive Juices
Extraction Process
2.4. Reagents
2.5. Analytical Tools Applied
2.5.1. Surface Characteristics
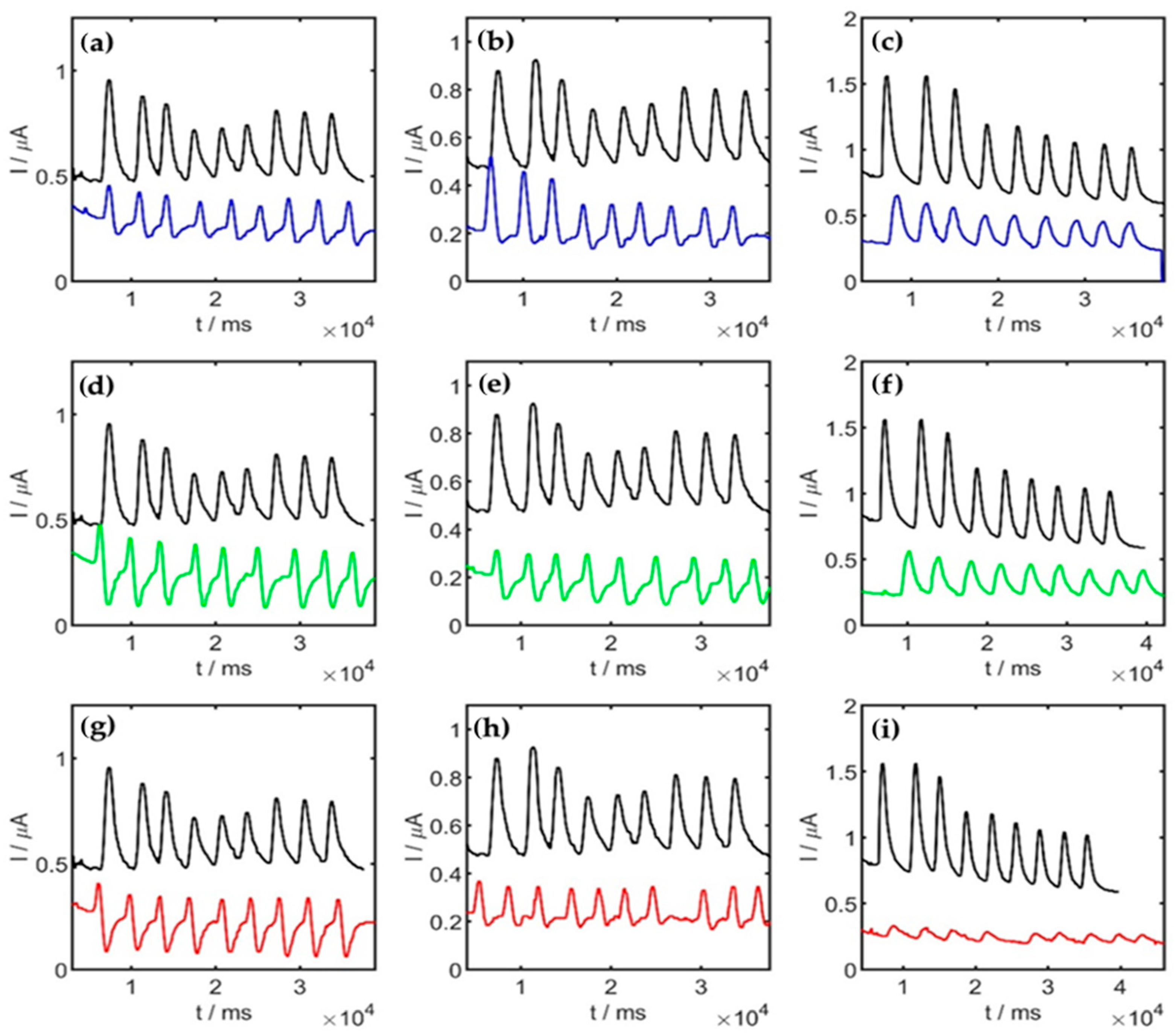
2.5.2. Bisphenol A® Content Analysis (FIA Detection) (Step 4)
3. Results and Discussion
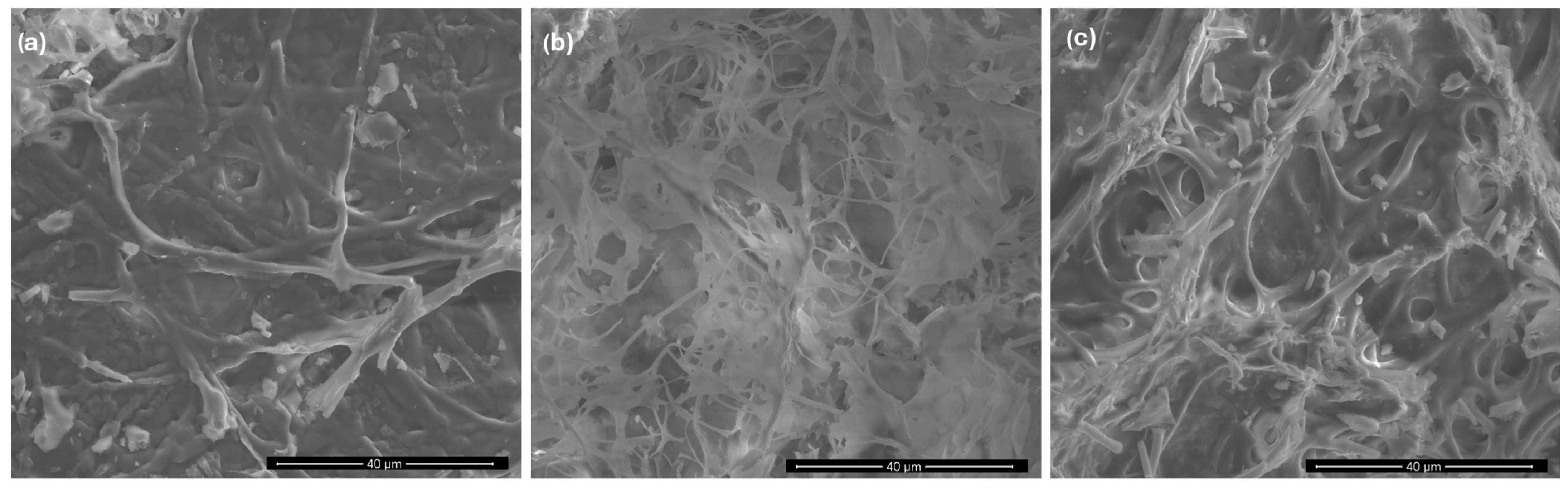
3.1. Material Characteristics
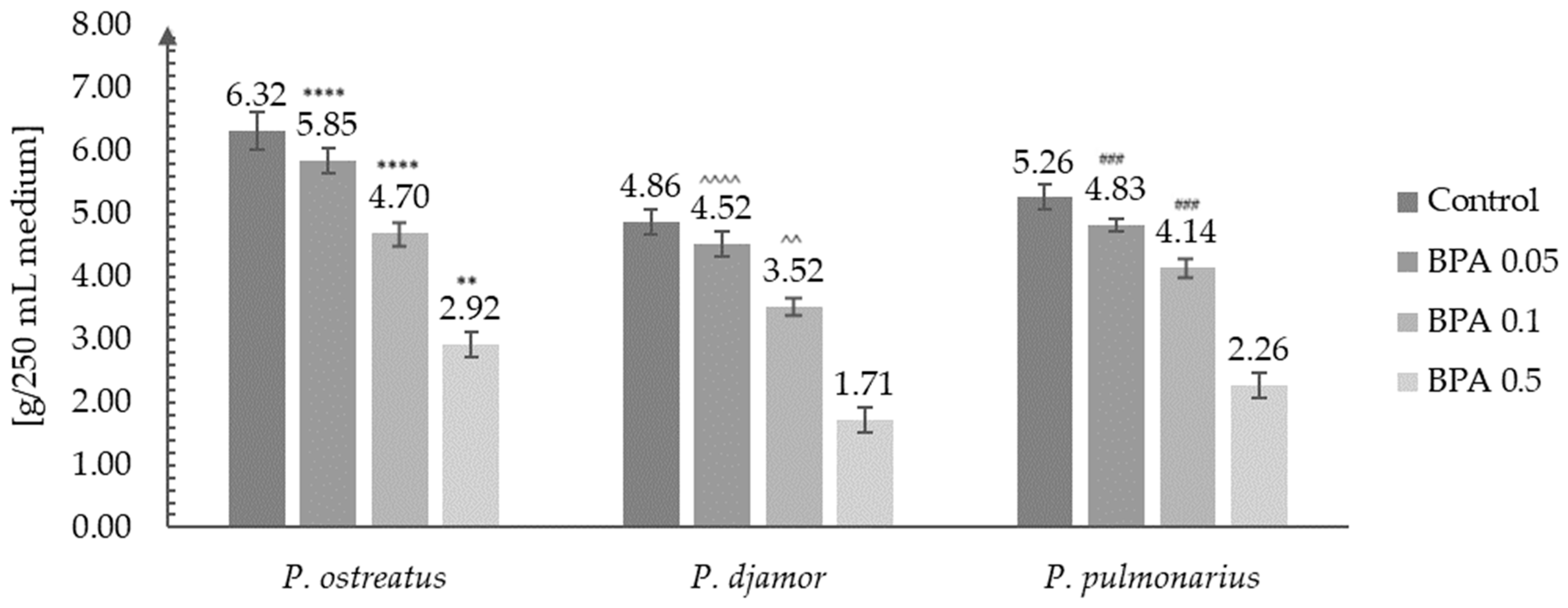
3.2. Biomass Growth Analysis
3.3. Sorption Analysis
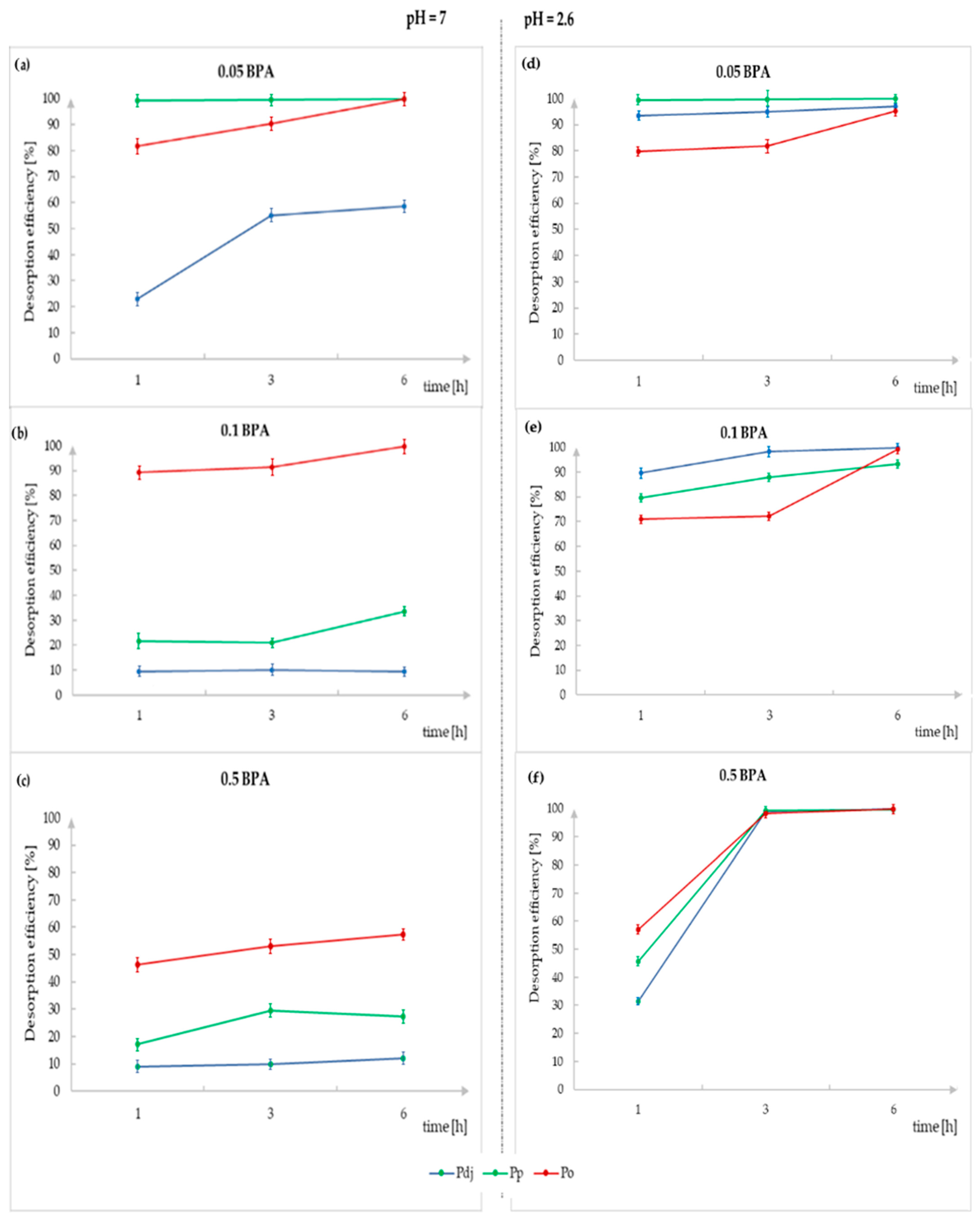
3.4. Desorption Analysis
3.4.1. Desorption in Water
3.4.2. Desorption in Acidic Media
3.5. Extraction into Artificial Digestive Juices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boamponsem, G.A.; Obeng, A.K.; Osei-Kwateng, M.; Badu, A.O. Accumulation of heavy metals by Pleurotus ostreatus from soils of metal scrap sites. Int. J. Cur. Res. Rev. 2013, 5, 1–9. [Google Scholar]
- Ronda, O.; Grządka, E.; Ostolska, I.; Orzeł, J.; Cieślik, B.M. Accumulation of radioisotopes and heavy metals in selected species of mushrooms. Food Chem. 2022, 367, 130670. [Google Scholar] [CrossRef] [PubMed]
- Brzostowski, A.; Falandysz, J.; Jarzyńska, G.; Zhang, D. Bioconcentration potential of metallic elements by Poison Pax (Paxillus involutus) mushroom. J. Environ. Health 2011, 46, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Kojta, A.K.; Jarzyńska, G.; Drewnowska, A.; Dryżałowska, A.; Wydmańska, D.; Szefer, P. Mercury in Bay Bolete Xerocomus badius: Bioconcentration by fungus and assessment of element intake by humans eating fruiting bodies. Food Addit. Contam. 2012, 29, 951–961. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Stuper-Szablewska, K.; Rissmann, I.; Sobieralski, K.; Goliński, P. Accumulation of elements by edible mushroom species. Problem of trace element toxicity in mushrooms. J. Environ. Sci. Health 2013, 28, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A.; Rukhsana Bajwa, R.; Shafique, U.; Anwar, J. Removal of heavy metals by adsorption on Pleurotus ostreatus. Biomass Bioenergy 2011, 35, 1675–1682. [Google Scholar] [CrossRef]
- Byrne, A.R.; Ravnik, V.; Kosta, L. Trace element concentrations in higher fungi. Sci. Total Environ. 1976, 6, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Stijve, T.; Besson, R. Mercury, cadmium, lead, and selenium content of mushroom species belonging to the genus Agaricus. Chemosphere 1976, 5, 151–158. [Google Scholar] [CrossRef]
- Muszyńska, B.; Kała, K.; Włodarczyk, A.; Krakowska, A.; Ostachowicz, B.; Gdula-Argasińska, J.; Suchocki, P. Lentinula edodes as a source of bioelements released into artificial digestive juices and potential anti-inflammatory material. Biol. Trace Elem. Res. 2020, 194, 603–613. [Google Scholar] [CrossRef]
- Zięba, P.; Kała, K.; Włodarczyk, A.; Szewczyk, A.; Kunicki, E.; Sękara, A.; Muszyńska, B. Selenium and zinc biofortification of Pleurotus eryngii mycelium and fruiting bodies as a tool for controlling their biological activity. Molecules 2020, 25, 889. [Google Scholar] [CrossRef]
- Krakowska, A.; Zięba, P.; Włodarczyk, A.; Kała, K.; Sułkowska-Ziaja, K.; Bernaś, E.; Sękara, A.; Ostachowicz, B.; Muszyńska, B. Selected edible medicinal mushrooms from Pleurotus genus as an answer for human civilization diseases. Food Chem. 2020, 327, 127084. [Google Scholar] [CrossRef]
- Reczyński, W.; Muszyńska, B.; Opoka, W.; Smalec, A.; Sułkowska-Ziaja, K.; Malec, M. Comparative study of metals accumulation in cultured in vitro mycelium and naturally grown fruiting bodies of Boletus badius and Contharellus cibarius. Biol. Trace Elem. Res. 2013, 155, 355–362. [Google Scholar] [CrossRef]
- Krakowska, A.; Reczyński, W.; Muszyńska, B. Optimization of the liquid culture medium composition to obtain the mycelium of Agaricus bisporus rich in essential minerals. Biol. Trace Elem. Res. 2016, 173, 231–240. [Google Scholar] [CrossRef]
- Yamanaka, K. Mushroom cultivation in Japan. Mushroom Prod. Bull. 2011, 10, 455–459. [Google Scholar]
- Gapiński, M.; Woźniak, W.; Ziobra, M. Boczniak Technologia Uprawy i Przetwarzania. Państwowe Wydaw. Rol. I Leśne 2001, 23, 12–15. [Google Scholar]
- Wondratschek, I.; Röder, U. Monitoring of heavy metals in soils by higher fungi. In Plants as Biomonitors, Indicators for Heavy Metals in the Terrestrial Environment; Markert, B., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 1993; pp. 345–363. [Google Scholar]
- Reider, S.R.; Brunner, I.; Horvat, M.; Jacobs, A.; Frey, B. Accumulation of mercury and methylmercury by mushrooms and earthworms from forest soils. Environ. Pol. 2011, 159, 2861–2869. [Google Scholar] [CrossRef]
- Svoboda, L.; Chrastný, V. Levels of eight trace elements in edible mushrooms from a rural area. Food Additiv. Contam. 2008, 25, 51–58. [Google Scholar] [CrossRef]
- Campos, J.A.; Tejera, N.A.; Sánchéz, C.J. Substrate role in the accumulation of heavy metals in sporocarps of wild fungi. Biometals 2009, 22, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, L.; Zimmermannová, K.; Kalač, P. Concentrations of mercury, cadmium, lead and copper in fruiting bodies of edible mushrooms in an emission area of a copper smelter and a mercury smelter. Sci. Total Environ. 2000, 246, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Burridge, E. Bisphenol A: Product profile. Eur Chem. News. 2003, 17, 14. [Google Scholar]
- Konieczna, A.; Rutkowska, A.; Rachoń, D. Health risk of exposure to bisphenol A (BPA). Rocz. Państwowego Zakładu Hig. 2015, 66, 5–11. [Google Scholar]
- Wetherill, Y.; Akingbemi, J.; Kanno, I.; Mclachlan, J.; Nadal, A.; Sonneschein, C.; Belcher, S. In vitro molecular mechanisms of bisphenol A action. Reprod. Toxicol. 2007, 24, 178. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and Human Health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Geens, T. A review of dietary and non-dietary exposure to bisphenol A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of Urinary Bisphenol A Concentration With Medical Disorders and Laboratory Abnormalities in Adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.; Williams, P.; Missmer, A.; Flaws, A.; Ye, X.; Calafat, A.; Hauser, R. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum. Reprod. 2012, 27, 3583. [Google Scholar] [CrossRef] [PubMed]
- Bloom, M.; Vom Saal, F.; Kim, D.; Taylor, J.; Lamb, J.; Fujimoto, V. Serum unconjugated bisphenol A concentrations in men may influence embryo quality indicators during in vitro fertilization. Environ. Toxicol. Pharmacol. 2011, 32, 319. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.; Williams, P.; Missmer, A.; Flaws, A.; Berry, K.; Calafat, A.; Hauser, R. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ. Health. Perspect. 2012, 120, 978. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.; Calafat, A.; Hauser, R. Bisphenol A and thyroid hormones. Environ. Sci. Technol. 2010, 44, 1458. [Google Scholar] [CrossRef]
- Wang, F.; Hua, J.; Chen, M.; Xia, Y.; Zhang, Q.; Zhao, R. Occupational exposure to bisphenol A (BPA) in a plastic injection molding factory in Malaysia. Occupenviron. Med. 2012, 69, 679. [Google Scholar]
- Zhang, Y.; Chen, X.; Xie, L. Pleurotus pulmonarius Strain: Arsenic(III)/Cadmium(II) Accumulation, Tolerance, and Simulation Application in Environmental Remediation. Int. J. Environ. Res. Public Health 2023, 20, 5056. [Google Scholar] [CrossRef] [PubMed]
- Matkovits, A.; Fodor, M.; Jókai, Z. Analysis of Polyphenol Patterns of Pleurotus ostreatus Cultivars by UHPLC-ESI-MS/MS; Application of FT-NIR and Chemometric Methods, Classification Options. Chemosensors 2024, 12, 19. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Govindan, S.; Ramani, P. Investigation of chemical and biological properties of an acidic polysaccharide fraction from Pleurotus eous (Berk.) Sacc. Food Biosci. 2021, 42, 101209. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Krakowska, A.; Sułkowska-Ziaja, K.; Suchanek, M.; Zięba, P.; Opoka, W.; Muszyńska, B. Pleurotus spp. Mycelia Enriched in Magnesium and Zinc Salts as a Potential Functional Food. Molecules 2021, 26, 162. [Google Scholar]
- Arvidson, K.; Johasson, E.G. Galvanic current between dental alloys in vitro. Scand. J. Dent. Res. 1985, 93, 467–473. [Google Scholar] [CrossRef]
- Neumann, M.; Goderska, K.; Grajek, K.; Grajek, W. Modele przewodu pokarmowego in vitro do badań nad biodostępnością składników odżywczych. Żywność Nauka Technol. Jakość 2006, 1, 30–45. [Google Scholar]
- Polish Pharmakopeia, Wydanie X; PTFarm: Warszawa, Poland, 2014.
- Opoka, W.; Muszyńska, B.; Rojowski, J.; Rumian, J. Gastroel–2014. Poland Patent Application P 417238, 18 May 2016. [Google Scholar]
- Confortin, F.G.; Marchetto, R.; Bettin, F.; Camassola, M.; Salvador, M.; Dillon, A.J.P. Production of Pleurotus sajor-caju strain PS-2001 biomass in submerged culture. J. Ind. Microbiol. Biotechnol. 2018, 35, 1149. [Google Scholar] [CrossRef] [PubMed]
- Rosado, F.R.; Germano, S.; Carbonero, E.R.; Costa, S.M.; Iacomini, M.; Kemmelmeier, C. Biomass and exopolysaccharide production in submerged cultures of Pleurotus ostreatoroseus Sing. and Pleurotus ostreatus “Xorida” (Jack.: Fr.) Kummer. J. Basic Microbiol. 2003, 43, 230–237. [Google Scholar] [CrossRef]
- Poursaeid, N.; Azadbakht, A.; Balali, G.R. Improvement of zinc bioaccumulation and biomass yield in the mycelia and fruiting bodies of Pleurotus florida cultured on liquid media. Biotechnol. Appl. Biochem. 2015, 175, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, M.; Sandeep, T.; Sucharitha, K.; Godi, S. Biodegradation of plastic polymers by fungi: A brief review. Bioresour. Bioprocess. 2022, 9, 42. [Google Scholar] [CrossRef]
- Kała, K.; Krakowska, A.; Sułkowska-Ziaja, K.; Szewczyk, A.; Reczyński, W.; Opoka, W.; Muszyńska, B. Kinetics of extracted bioactive components from mushrooms in artificial digestive juices. Int. J. Food Prop. 2017, 20, 1796–1817. [Google Scholar] [CrossRef]



| Chemical Compounds | Weight (g)/L | Direction of extraction | |
| Saliva (pH = 6.7) | CaCl2 | 0.20 | |
| C6H8O7 | 0.03 | ||
| KHCO3 | 1.50 | ||
| KH2PO4 | 0.35 | ||
| MgCl2 | 0.01 | ||
| Na2HPO4 | 0.35 | ||
| Gastric juice (pH = 2) | HCl | 0.10 | |
| NaCl | 2.00 | ||
| Pepsin | 3.20 | ||
| Intestinal juice (pH = 8) | Bile salt | 0.15 | |
| NaHCO3 | 8.50 | ||
| Pancreatic extract | 0.02 |
| Sorption of 0.05 g of BPA/250 mL of medium [%] | |
| Pdj | 92.8 ± 6.4 |
| Pp | 95.9 ± 3.1 |
| Po | 99.1 ± 0.8 |
| Sorption of 0.1 g of BPA/250 mL of medium [%] | |
| Pdj | 91.3 ± 1.5 |
| Pp | 93.3 ± 1.2 |
| Po | 99.0 ± 0.6 |
| Sorption of 0.5 g of BPA/250 mL of medium [%] | |
| Pdj | 65.3 ± 7.3 |
| Pp | 66.9 ± 4.6 |
| Po | 97.5 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krakowska, A.; Suchanek, M.; Piech, R.; Paczosa-Bator, B.; Skalski, T.; Muszyńska, B. Accumulation of Bisphenol A® by Pleurotus spp.—Flow Injection Analysis. Molecules 2024, 29, 2520. https://doi.org/10.3390/molecules29112520
Krakowska A, Suchanek M, Piech R, Paczosa-Bator B, Skalski T, Muszyńska B. Accumulation of Bisphenol A® by Pleurotus spp.—Flow Injection Analysis. Molecules. 2024; 29(11):2520. https://doi.org/10.3390/molecules29112520
Chicago/Turabian StyleKrakowska, Agata, Małgorzata Suchanek, Robert Piech, Beata Paczosa-Bator, Tomasz Skalski, and Bożena Muszyńska. 2024. "Accumulation of Bisphenol A® by Pleurotus spp.—Flow Injection Analysis" Molecules 29, no. 11: 2520. https://doi.org/10.3390/molecules29112520








