Facile Fabrication of Large-Area CuO Flakes for Sodium-Ion Energy Storage Applications
Abstract
:1. Introduction
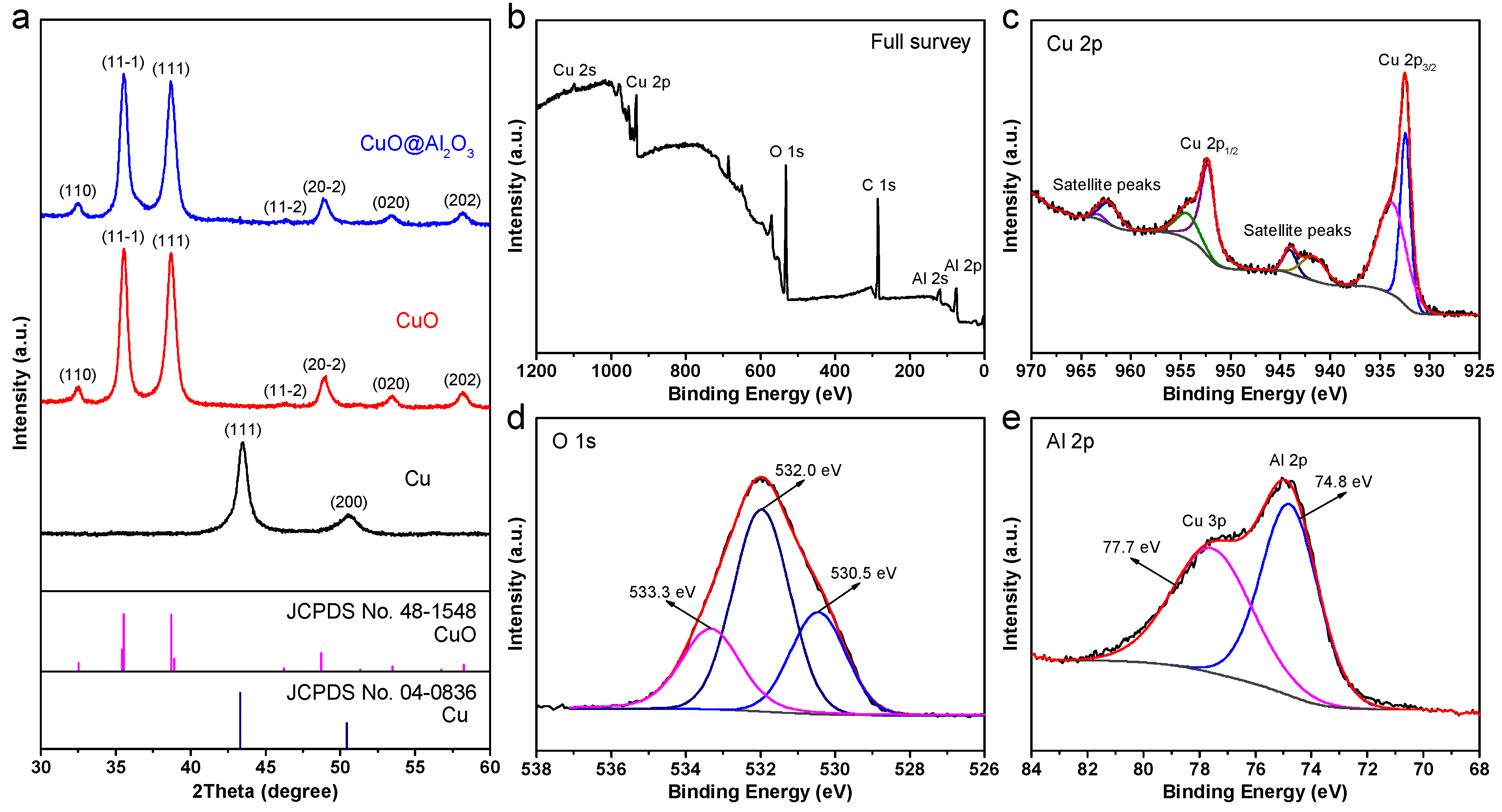
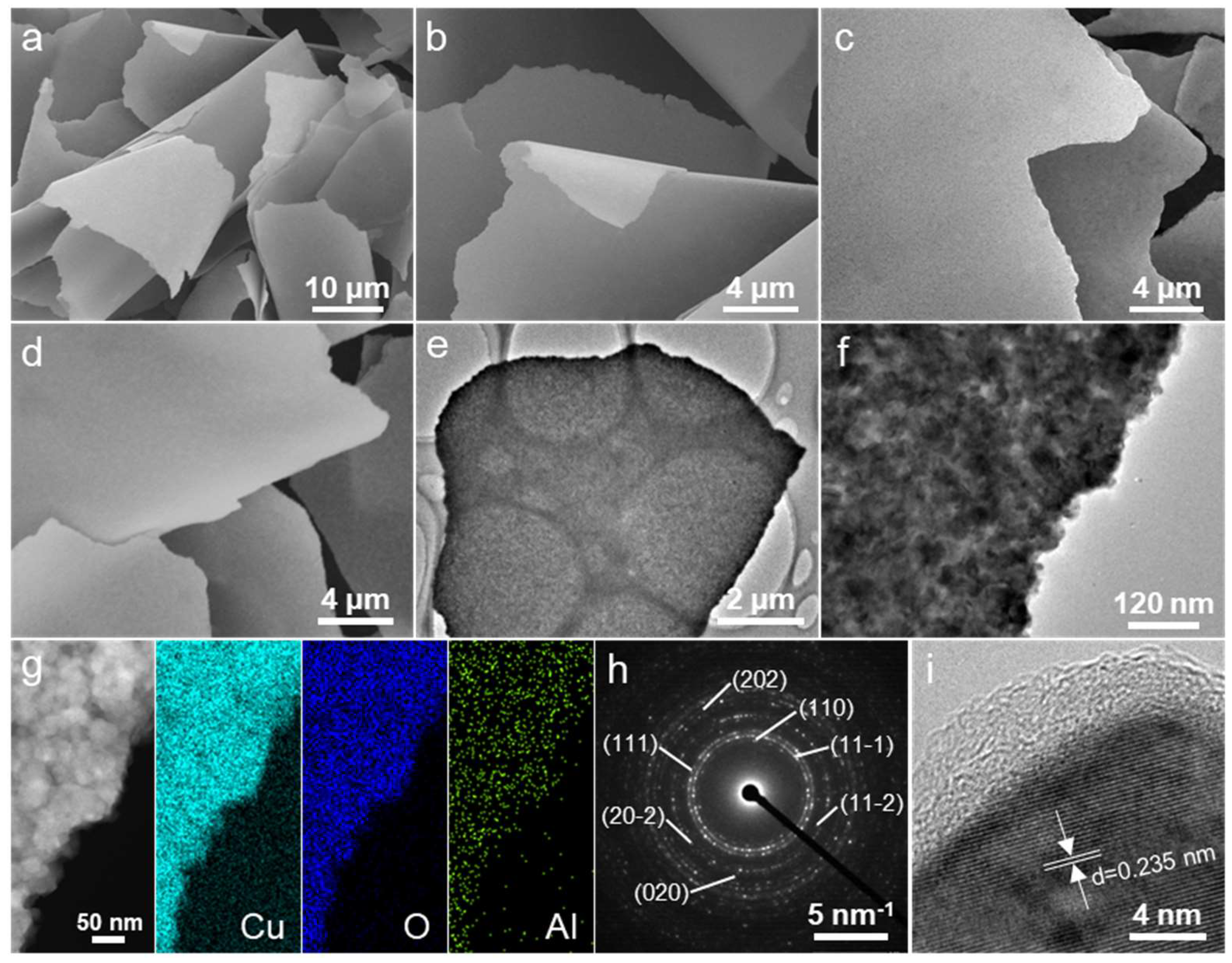
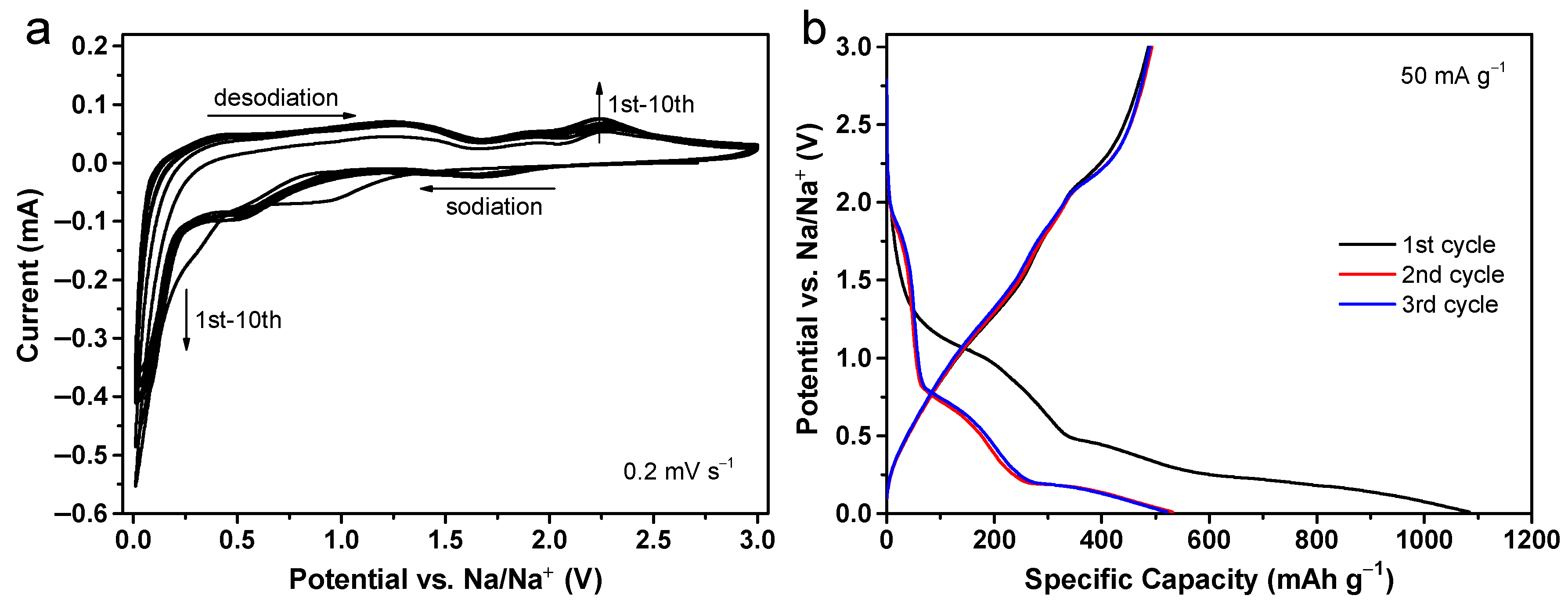
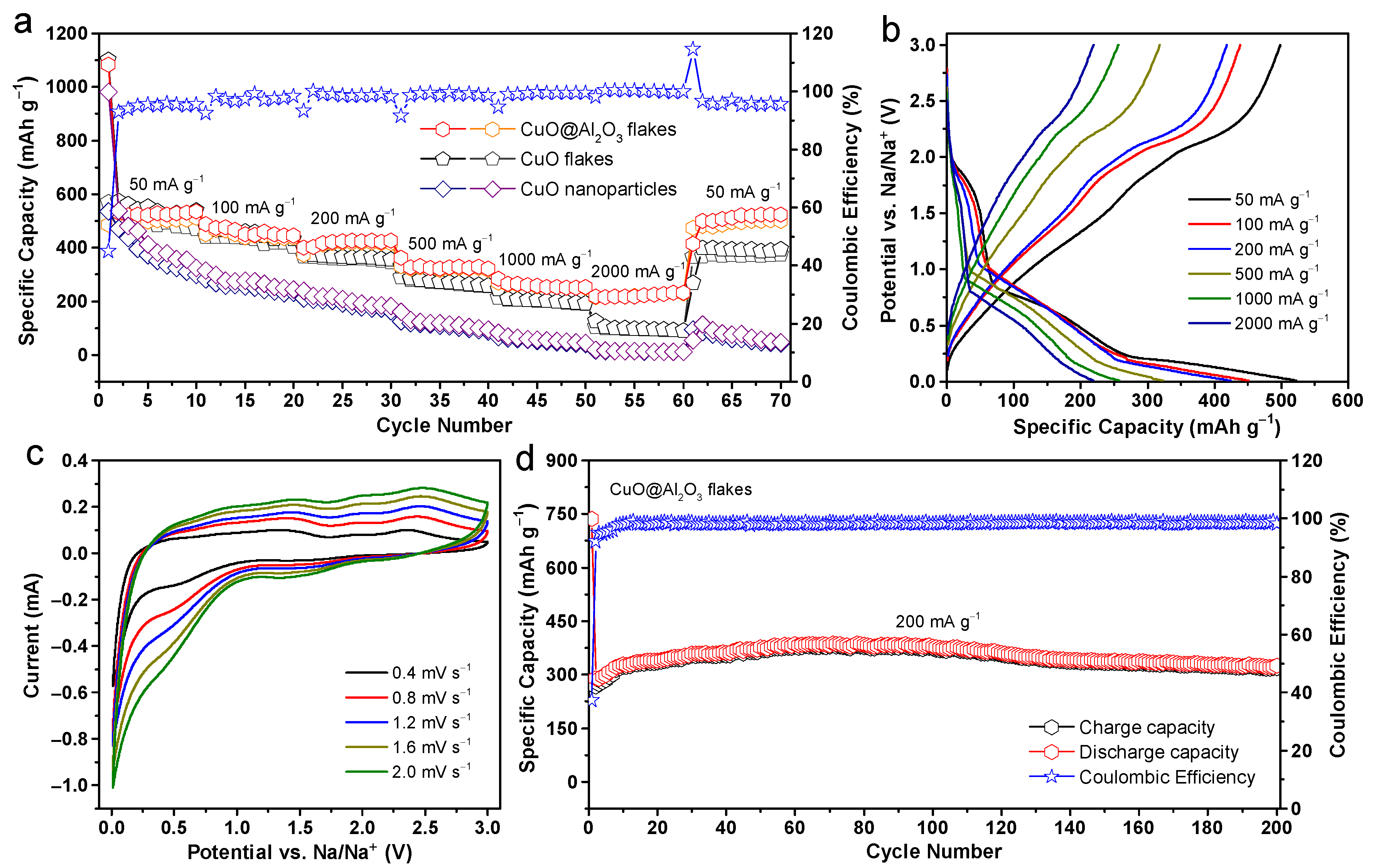
2. Results and Discussion
3. Materials and Methods
3.1. Materials Preparation
3.2. Materials Characterization
3.3. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Liu, C.; Hu, C.; Ma, Y.; Li, X.; Wang, Q.; An, Z.; Liu, S.; Sun, H.; Sun, X. GeO2 Nanoparticles Decorated in Amorphous Carbon Nanofiber Framework as Highly Reversible Lithium Storage Anode. Molecules 2023, 28, 6730. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Y.; Li, Y.; Luo, F. Biomass Alginate Derived Oxygen-Enriched Carbonaceous Materials with Partially Graphitic Nanolayers for High Performance Anodes in Lithium-Ion Batteries. Nanomaterials 2023, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yan, C.; Chen, Y.; Si, W.; Deng, J.; Oswald, S.; Liu, L.; Schmidt, O.G. Three-Dimensionally “Curved” NiO Nanomembranes as Ultrahigh Rate Capability Anodes for Li-Ion Batteries with Long Cycle Lifetimes. Adv. Energy Mater. 2014, 4, 1300912. [Google Scholar] [CrossRef]
- Li, X.; Ye, W.; Xu, P.; Huang, H.; Fan, J.; Yuan, R.; Zheng, M.-S.; Wang, M.-S.; Dong, Q. An Encapsulation-Based Sodium Storage via Zn-Single-Atom Implanted Carbon Nanotubes. Adv. Mater. 2022, 34, 2202898. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hao, G.-P.; Lu, X.; Xi, L.; Liu, B.; Si, W.; Ma, C.; Liu, Q.; Zhang, Q.; Kaskel, S.; et al. High-Defect Hydrophilic Carbon Cuboids Anchored with Co/CoO Nanoparticles as Highly Efficient and Ultra-Stable Lithium-Ion Battery Anodes. J. Mater. Chem. A 2016, 4, 10166–10173. [Google Scholar] [CrossRef]
- Perveen, T.; Siddiq, M.; Shahzad, N.; Ihsan, R.; Ahmad, A.; Shahzad, M.I. Prospects in Anode Materials for Sodium Ion Batteries—A Review. Renew. Sust. Energ. Rev. 2020, 119, 109549. [Google Scholar] [CrossRef]
- Sun, X.; Luo, F. Sodium Storage Properties of Carbonaceous Flowers. Molecules 2023, 28, 4753. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Y.; Lv, W.; Yuan, Y.; Guo, S.; Yan, W. Bismuth-Antimony Alloy Nanoparticles Embedded in 3D Hierarchical Porous Carbon Skeleton Film for Superior Sodium Storage. Molecules 2023, 28, 6464. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, C.; Deng, D.-R.; Weng, J.-C.; Song, J.-X.; Fan, X.-H.; Li, G.-F.; Li, Y.; Wu, Q.-H. Synthesis of Low-Cost and High-Performance Dual-Atom Doped Carbon-Based Materials with a Simple Green Route as Anodes for Sodium-Ion Batteries. Molecules 2023, 28, 7314. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, D.; Luo, Y.; Xu, J.; Guo, K.; Wang, W.; Liu, G.; Wu, N.; Liu, X.; Qin, A. Constructing Abundant Oxygen-Containing Functional Groups in Hard Carbon Derived from Anthracite for High-Performance Sodium-Ion Batteries. Nanomaterials 2023, 13, 3002. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, N.; Zhao, Q.; Liang, J.; Chen, J. Micro-Nanostructured CuO/C Spheres as High-Performance Anode Materials for Na-Ion Batteries. Nanoscale 2015, 7, 2770–2776. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Lu, X.; Huang, S.; Xi, L.; Liu, L.; Liu, B.; Weng, Q.; Zhang, L.; Schmidt, O.G. Reinforcing Germanium Electrode with Polymer Matrix Decoration for Long Cycle Life Rechargeable Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 38556–38566. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Hong, Z.; Ding, H.; Li, J.; Xia, Y.; Zhou, Z.; Yue, G. Facilitating Synthesis of FeP/C@CoP Composites as High-Performance Anode Materials for Sodium-Ion Batteries. Coatings 2023, 13, 2056. [Google Scholar] [CrossRef]
- Chandra Rath, P.; Patra, J.; Saikia, D.; Mishra, M.; Chang, J.-K.; Kao, H.-M. Highly Enhanced Electrochemical Performance of Ultrafine CuO Nanoparticles Confined in Ordered Mesoporous Carbons as Anode Materials for Sodium-Ion Batteries. J. Mater. Chem. A 2016, 4, 14222–14233. [Google Scholar] [CrossRef]
- Fan, M.; Yu, H.; Chen, Y. High-Capacity Sodium Ion Battery Anodes Based on CuO Nanosheets and Carboxymethyl Cellulose Binder. Mater. Technol. 2017, 32, 598–605. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, Y.; Li, X.; Zhang, K.; Pang, Q.; Qin, A. Boosting the Initial Coulomb Efficiency of Sisal Fiber-Derived Carbon Anode for Sodium Ion Batteries by Microstructure Controlling. Nanomaterials 2023, 13, 881. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, J.; Wang, L.; Xie, Y.; Lou, S.; Jiang, Z.; Ren, Y.; Lee, S.; Zuo, P.; Huo, H.; et al. Unraveling the Origins of the “Unreactive Core” in Conversion Electrodes to Trigger High Sodium-Ion Electrochemistry. ACS Energy Lett. 2019, 4, 2007–2012. [Google Scholar] [CrossRef]
- Li, X.; Hector, A.L.; Owen, J.R. Evaluation of Cu3N and CuO as Negative Electrode Materials for Sodium Batteries. J. Phys. Chem. C 2014, 118, 29568–29573. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Qian, J.; Ma, Y.; Li, Y.; Li, W.; Wang, F.; Li, L.; Wu, F.; Chen, R. Freestanding N-Doped Carbon Coated CuO Array Anode for Lithium-Ion and Sodium-Ion Batteries. Energy Technol. 2019, 7, 1900252. [Google Scholar] [CrossRef]
- Li, D.; Yan, D.; Zhang, X.; Li, J.; Lu, T.; Pan, L. Porous CuO/Reduced Graphene Oxide Composites Synthesized from Metal-Organic Frameworks as Anodes for High-Performance Sodium-Ion Batteries. J. Colloid Interface Sci. 2017, 497, 350–358. [Google Scholar] [CrossRef]
- Yuan, S.; Huang, X.-L.; Ma, D.-L.; Wang, H.-G.; Meng, F.-Z.; Zhang, X.-B. Engraving Copper Foil to Give Large-Scale Binder-Free Porous CuO Arrays for a High-Performance Sodium-Ion Battery Anode. Adv. Mater. 2014, 26, 2273–2279. [Google Scholar] [CrossRef]
- Liu, H.; Cao, F.; Zheng, H.; Sheng, H.; Li, L.; Wu, S.; Liu, C.; Wang, J. In Situ Observation of the Sodiation Process in CuO Nanowires. Chem. Commun. 2015, 51, 10443–10446. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.; Pinedo, R.; Berkes, B.B.; Janek, J.; Adelhelm, P. Kinetics and Degradation Processes of CuO as Conversion Electrode for Sodium-Ion Batteries: An Electrochemical Study Combined with Pressure Monitoring and Dems. J. Phys. Chem. C 2017, 121, 8679–8691. [Google Scholar] [CrossRef]
- Hu, S.; Liu, H.; Zheng, H.; Jia, S.; Chen, G.; Jiang, R.; Li, L.; Zhao, L.; Zhao, D.; Wang, J. Coating-Mediated Nanomechanical Behaviors of CuO Electrodes in Li- and Na-Ion Batteries. Adv. Mater. Interfaces 2020, 7, 2001161. [Google Scholar] [CrossRef]
- Chen, C.; Dong, Y.; Li, S.; Jiang, Z.; Wang, Y.; Jiao, L.; Yuan, H. Rapid Synthesis of Three-Dimensional Network Structure CuO as Binder-Free Anode for High-Rate Sodium Ion Battery. J. Power Sources 2016, 320, 20–27. [Google Scholar] [CrossRef]
- Rath, P.C.; Patra, J.; Saikia, D.; Mishra, M.; Tseng, C.-M.; Chang, J.-K.; Kao, H.-M. Comparative Study on the Morphology-Dependent Performance of Various CuO Nanostructures as Anode Materials for Sodium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 10876–10885. [Google Scholar] [CrossRef]
- Majumder, T.; Das, D.; Jena, S.; Mitra, A.; Das, S.; Majumder, S.B. Electrophoretic Deposition of Metal-Organic Framework Derived Porous Copper Oxide Anode for Lithium and Sodium Ion Rechargeable Cells. J. Alloys Compd. 2021, 879, 160462. [Google Scholar] [CrossRef]
- Ma, T.; Gao, L.; Liu, Y.; Zhang, L.; Yang, X. Porous CuO/Cu2O Heterostructured Arrays as Anode for High-Performance Sodium-Ion Batteries. Ionics 2021, 27, 1995–2003. [Google Scholar] [CrossRef]
- Zheng, T.; Li, G.; Li, D.; Meng, X. Based on Cu as Framework Constructed Nanoporous CuO/Cu Composites by a Dealloy Method for Sodium-Ion Battery Anode. J. Nanopart. Res. 2018, 20, 140. [Google Scholar] [CrossRef]
- Wen, J.; Jiang, R.; Huang, J.; Xie, Y.; Ma, L.; Li, X.; Ren, Y.; Liu, Z.; Xiao, B.; Zhou, X. Fabrication of Hollow and Hierarchical CuO Micro–Nano Cubes Wrapped by Reduced Graphene Oxide as a Prospective Anode for SIBs. Langmuir 2024, 40, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-J.; Sun, H.-Y.; Chen, Y.-F.; Zhao, Y.-W.; Zhang, M.-M.; Li, C.-S.; Sun, Y.; Gao, Z.-H.; Li, H.-J.; Jiang, Y. Modulating p-d Orbital Hybridization by CuO/Cu Nanoparticles Enables Carbon Nanofibers High Cycling Stability as Anode for Sodium Storage. Rare Met. 2023, 42, 4039–4047. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, Y.; Zhang, W.; Hu, P.; Chen, C.; Li, Z.; Yuan, L.; Hu, X.; Huang, Y. Facile Fabrication of CuO Nanosheets on Cu Substrate as Anode Materials for Electrochemical Energy Storage. J. Alloys Compd. 2014, 586, 208–215. [Google Scholar] [CrossRef]
- Sun, X.; Xie, W.; Luo, F. Nanoarchitectonics of Multilayered NiO Submicron Flakes for Ultrafast and Stable Lithium Storage. J. Alloys Compd. 2023, 936, 168259. [Google Scholar] [CrossRef]
- Rehman, J.; Lin, S.; Butt, M.K.; Fan, X.; Khattab, T.; Elsayed, K.A.; Shibl, M.F. An Overview of 2D Metal Sulfides and Carbides as Na Host Materials for Na-Ion Batteries. Chem. Eng. J. 2023, 461, 141924. [Google Scholar] [CrossRef]
- Sun, X.; Qiao, L.; Pang, H.; Li, D. Synthesis of Nanosheet-Constructed SnO2 Spheres with Efficient Photocatalytic Activity and High Lithium Storage Capacity. Ionics 2017, 23, 3177–3185. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Y.; Luo, F. Nanoporous Ald-Modified Oxygen-Deficient NiO Flakes as Anodes for Lithium-Ion Batteries. Ceram. Int. 2024, 50, 3480–3490. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Qin, Y.; Li, X.; Qiao, L.; Feng, N.; Hu, D.; He, D. Synthesis of Novel Pompon-Like Porous SnO2 and Its Application in Lithium-Ion Battery. Mater. Lett. 2012, 66, 193–195. [Google Scholar] [CrossRef]
- Liu, H.; Zheng, H.; Li, L.; Jia, S.; Meng, S.; Cao, F.; Lv, Y.; Zhao, D.; Wang, J. Surface-Coating-Mediated Electrochemical Performance in CuO Nanowires During the Sodiation–Desodiation Cycling. Adv. Mater. Interfaces 2018, 5, 1701255. [Google Scholar] [CrossRef]
- Ni, J.; Jiang, Y.; Wu, F.; Maier, J.; Yu, Y.; Li, L. Regulation of Breathing CuO Nanoarray Electrodes for Enhanced Electrochemical Sodium Storage. Adv. Funct. Mater. 2018, 28, 1707179. [Google Scholar] [CrossRef]
- Wen, L.; Wang, X.; Liu, G.Q.; Luo, H.Z.; Liang, J.; Dou, S.X. Novel Surface Coating Strategies for Better Battery Materials. Surf. Innov. 2018, 6, 13–18. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, D.; Yoon, B.S.; Lee, Y.-S.; Il Park, Y.; Ko, C.H. Atomic Layer Deposition-Based Synthesis of TiO2 and Al2O3 Thin-Film Coatings on Nanoparticle Powders for Sodium-Ion Batteries with Enhanced Cyclic Stability. J. Alloys Compd. 2022, 897, 163113. [Google Scholar] [CrossRef]
- Sun, X.; Jing, M.; Dong, H.; Xie, W.; Luo, F. CuO-ZnO Submicroflakes with Nanolayered Al2O3 Coatings as High Performance Anode Materials in Lithium-Ion Batteries. J. Alloys Compd. 2023, 953, 170137. [Google Scholar] [CrossRef]
- Negi, R.S.; Culver, S.P.; Wiche, M.; Ahmed, S.; Volz, K.; Elm, M.T. Optimized Atomic Layer Deposition of Homogeneous, Conductive Al2O3 Coatings for High-Nickel NCM Containing Ready-to-Use Electrodes. Phys. Chem. Chem. Phys. 2021, 23, 6725–6737. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.; Pinedo, R.; Hering, P.; Polity, A.; Janek, J.; Adelhelm, P. Reaction Mechanism and Surface Film Formation of Conversion Materials for Lithium- and Sodium-Ion Batteries: An XPS Case Study on Sputtered Copper Oxide (CuO) Thin Film Model Electrodes. J. Phys. Chem. C 2016, 120, 1400–1414. [Google Scholar] [CrossRef]
- Subramanyan, K.; Akshay, M.; Lee, Y.-S.; Aravindan, V. Fabrication of Na-Ion Full-Cells Using Carbon-Coated Na3V2(PO4)2O2F Cathode with Conversion Type CuO Nanoparticles from Spent Li-Ion Batteries. Small Methods 2022, 6, 2200257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Xie, D.; Tang, Y.; Wu, C.; Cui, L.; Li, Y.; Ning, X.; Shan, Z. In Situ Transmission Electron Microscopy Study of the Electrochemical Sodiation Process for a Single CuO Nanowire Electrode. RSC Adv. 2016, 6, 11441–11445. [Google Scholar] [CrossRef]
- Bai, X.; Wu, N.; Yu, G.; Li, T. Recent Advances in Anode Materials for Sodium-Ion Batteries. Inorganics 2023, 11, 289. [Google Scholar] [CrossRef]
- Sun, X.; Si, W.; Liu, X.; Deng, J.; Xi, L.; Liu, L.; Yan, C.; Schmidt, O.G. Multifunctional Ni/NiO Hybrid Nanomembranes as Anode Materials for High-Rate Li-Ion Batteries. Nano Energy 2014, 9, 168–175. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, P.; Wang, S.; Chen, W.; Wei, B.; Lu, X.; Qian, G.; Kan, W.H.; Chen, H.; Yin, W.; et al. Slight Compositional Variation-Induced Structural Disorder-to-Order Transition Enables Fast Na+ Storage in Layered Transition Metal Oxides. Nat. Commun. 2022, 13, 7888. [Google Scholar] [CrossRef]
- Wang, W.; Qin, J.; Yin, Z.; Cao, M. Achieving Fully Reversible Conversion in MoO3 for Lithium Ion Batteries by Rational Introduction of CoMoO4. ACS Nano 2016, 10, 10106–10116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, Y.; Yue, W.-C.; Zheng, S.-M.; Li, X.; Gao, N.; Zhu, T.; Zhang, Y.-J.; Xia, G.-M.; Wang, B. Facile Fabrication of Hollow CuO Nanocubes for Enhanced Lithium/Sodium Storage Performance. CrystEngComm 2021, 23, 6107–6116. [Google Scholar] [CrossRef]
- Liang, J.; Ning, Y.; Wu, X.; Chen, W.; Wu, W.; Wang, K. Controlled Synthesis of Uniform Porous CuO Microspheres for Enhanced Sodium Storage. Mater. Lett. 2020, 263, 127231. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, J.; Ci, L.; Tian, Y.; Xiong, S. Mental-Organic Framework Derived CuO Hollow Spheres as High Performance Anodes for Sodium Ion Battery. Mater. Technol. 2016, 31, 497–500. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Wang, Y.; Jiao, L. CuO Quantum Dots Embedded in Carbon Nanofibers as Binder-Free Anode for Sodium Ion Batteries with Enhanced Properties. Small 2016, 12, 4865–4872. [Google Scholar] [CrossRef]
- Xu, S.; Lu, L.; Zhang, Q.; Jiang, Q.; Luo, Z.; Wang, S.; Li, G.; Feng, C. A Facile Synthesis of Flower-Like CuO as Anode Materials for Lithium (Sodium) Ion Battery Applications. J. Nanosci. Nanotechnol. 2016, 16, 7655–7661. [Google Scholar] [CrossRef]

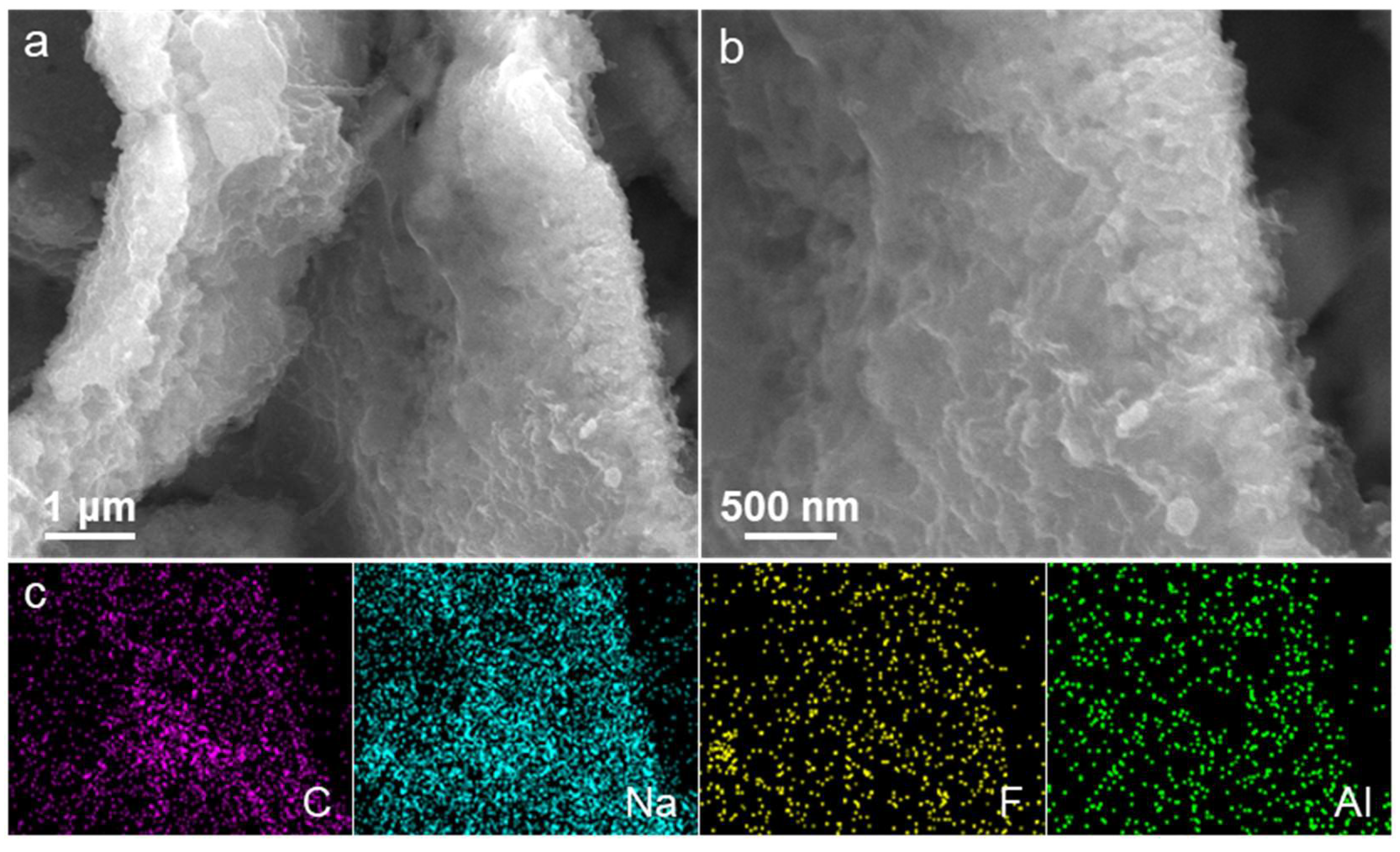
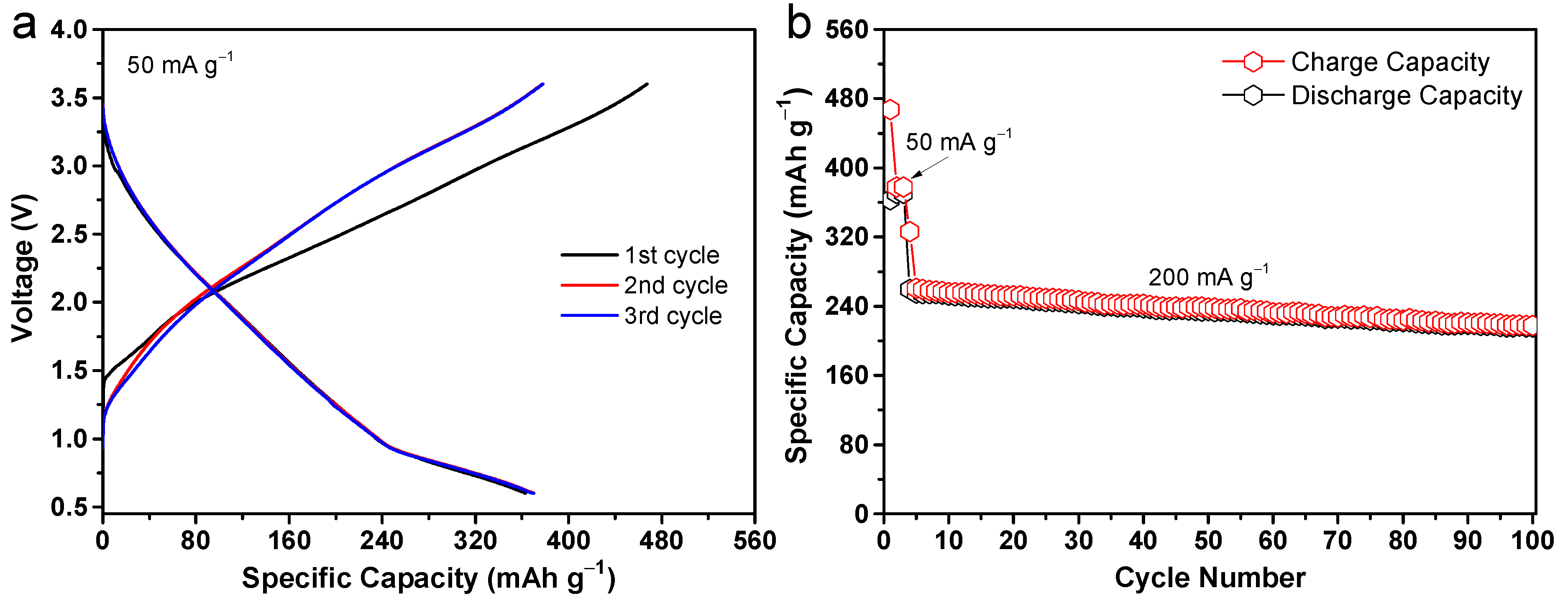
| Samples | BET Surface Area (m2 g−1) | Average Pore Diameter (nm) |
|---|---|---|
| CuO flakes | 15.8 | 16.6 |
| CuO@Al2O3 flakes | 16.1 | 16.7 |
| Materials | Rate Capability | Specific Capacity | Voltage Range | References |
|---|---|---|---|---|
| CuO@Al2O3 flakes | 220 mAh g−1 (2000 mA g−1) | 319 mAh g−1 (200 cycles, 200 mA g−1) | 0.01–3.0 V | This work |
| CuO micro-nano cubes | 218 mAh g−1 (800 mA g−1) | 213 mAh g−1 (100 cycles, 100 mA g−1) | 0.01–3.0 V | [31] Langmuir 2024, 40, 348–361. |
| CuO/Cu/C nanofibers | 120 mAh g−1 (2000 mA g−1) | 300 mAh g−1 (400 cycles, 100 mA g−1) | 0.01–3.0 V | [32] Rare Metals 2023, 42, 4039–4047. |
| CuO nanocubes | 216 mAh g−1 (500 mA g−1) | 170 mAh g−1 (100 cycles, 100 mA g−1) | 0.01–3.0 V | [52] CrystEngComm 2021, 23, 6107–6116. |
| CuO microspheres | 224 mAh g−1 (1000 mA g−1) | 284 mAh g−1 (50 cycles, 100 mA g−1) | 0.01–3.0 V | [53] Mater. Lett. 2020, 263, 127231. |
| CuO nanoellipsoids | 110 mAh g−1 (2000 mA g−1) | 188 mAh g−1 (100 cycles, 100 mA g−1) | 0.01–3.0 V | [27] ACS Sustainable Chem. Eng. 2018, 6, 10876–10885. |
| CuO nanosheets | 230 mAh g−1 (2000 mA g−1) | 394 mAh g−1 (20 cycles, 100 mA g−1) | 0.01–3.0 V | [16] Mater. Technol. 2017, 32, 598–605. |
| CuO nanoparticles | 196 mAh g−1 (1500 mA g−1) | 162 mAh g−1 (100 cycles, 100 mA g−1) | 0.01–3.0 V | [15] J. Mater. Chem. A 2016, 4, 14222–14233. |
| CuO@C nanofibers | 384 mAh g−1 (1000 mA g−1) | 477 mAh g−1 (200 cycles, 100 mA g−1) | 0.01–3.0 V | [55] Small 2016, 12, 4865–4872. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Luo, F. Facile Fabrication of Large-Area CuO Flakes for Sodium-Ion Energy Storage Applications. Molecules 2024, 29, 2528. https://doi.org/10.3390/molecules29112528
Sun X, Luo F. Facile Fabrication of Large-Area CuO Flakes for Sodium-Ion Energy Storage Applications. Molecules. 2024; 29(11):2528. https://doi.org/10.3390/molecules29112528
Chicago/Turabian StyleSun, Xiaolei, and Feng Luo. 2024. "Facile Fabrication of Large-Area CuO Flakes for Sodium-Ion Energy Storage Applications" Molecules 29, no. 11: 2528. https://doi.org/10.3390/molecules29112528






