Dual Optoelectronic Organic Field-Effect Device: Combination of Electroluminescence and Photosensitivity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Frontier Orbitals and Optical Properties from DFT Calculations
2.2. Crystal Growth and Analysis
2.3. Charge-Carrier Mobility Calculations
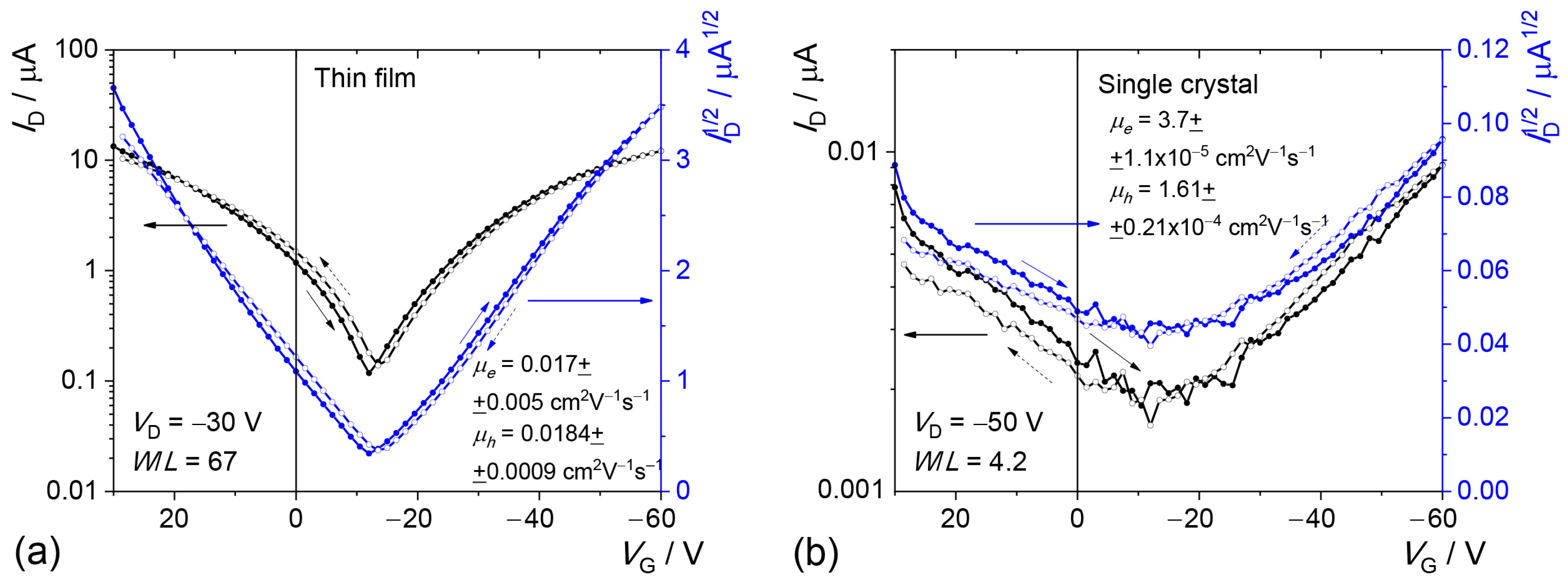
2.4. Electrical Measurements
2.5. Electroluminescence
2.6. Photoelectric Effect
3. Materials and Methods
3.1. Quantum Chemical Calculations
3.2. Experimental Methods
3.2.1. Fabrication of Thin-Film OFETs
3.2.2. Fabrication of Single-Crystal OFETs
3.2.3. OFET Characterization
3.2.4. Electroluminescence
3.2.5. Photoelectric Effect
3.2.6. Optical and Atomic Force Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, C.; Yan, F. Organic Semiconductors in Organic Thin-Film Transistor-Based Chemical and Biological Sensors. Polym. Rev. 2013, 53, 352–406. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, S.; Huang, J. OFET chemical sensors: Chemical sensors based on ultrathin organic field-effect transistors. Polym. Int. 2021, 70, 414–425. [Google Scholar] [CrossRef]
- Muccini, M. A bright future for organic field-effect transistors. Nat. Mater. 2006, 5, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.U.; Muhieddine, K.; Wawrzinek, R.; Sobus, J.; Tandy, K.; Lo, S.-C.; Namdas, E.B. Organic Light-Emitting Transistors: Advances and Perspectives. Adv. Funct. Mater. 2020, 30, 1905282. [Google Scholar] [CrossRef]
- Baeg, K.-J.; Binda, M.; Natali, D.; Caironi, M.; Noh, Y.-Y. Organic Light Detectors: Photodiodes and Phototransistors. Adv. Mater. 2013, 25, 4267–4295. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ji, D.; Fuchs, H.; Hu, W.; Li, T. Recent Progress in Organic Phototransistors: Semiconductor Materials, Device Structures and Optoelectronic Applications. ChemPhotoChem 2020, 4, 9–38. [Google Scholar] [CrossRef]
- Chiba, T.; Kumagai, D.; Udagawa, K.; Watanabe, Y.; Kido, J. Dual mode OPV-OLED device with photovoltaic and light-emitting functionalities. Sci. Rep. 2018, 8, 11472. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Lee, S.-H.; Kim, T.J.; Park, S.H.; Kim, J.; Choi, D.H.; Joo, J. Dual functions of light-emission and light-harvesting using organic donor and acceptor co-deposition. Org. Electron. 2023, 121, 106876. [Google Scholar] [CrossRef]
- Ullbrich, S.; Benduhn, J.; Jia, X.; Nikolis, V.C.; Tvingstedt, K.; Piersimoni, F.; Roland, S.; Liu, Y.; Wu, J.; Fischer, A.; et al. Emissive and charge-generating donor–acceptor interfaces for organic optoelectronics with low voltage losses. Nat. Mater. 2019, 18, 459–464. [Google Scholar] [CrossRef]
- Brütting, W. Organic LEDs and solar cells united. Nat. Mater. 2019, 18, 432–433. [Google Scholar] [CrossRef]
- Yomogida, Y.; Takenobu, T.; Shimotani, H.; Sawabe, K.; Bisri, S.Z.; Yamao, T.; Hotta, S.; Iwasa, Y. Green light emission from the edges of organic single-crystal transistors. Appl. Phys. Lett. 2010, 97, 173301. [Google Scholar] [CrossRef]
- Wang, Y.; Kumashiro, R.; Li, Z.; Nouchi, R.; Tanigaki, K. Light emitting ambipolar field-effect transistors of 2,5-bis(4-biphenyl)bithiophene single crystals with anisotropic carrier mobilities. Appl. Phys. Lett. 2009, 95, 103306. [Google Scholar] [CrossRef]
- Bisri, S.Z.; Takenobu, T.; Yomogida, Y.; Shimotani, H.; Yamao, T.; Hotta, S.; Iwasa, Y. High Mobility and Luminescent Efficiency in Organic Single-Crystal Light-Emitting Transistors. Adv. Funct. Mater. 2009, 19, 1728–1735. [Google Scholar] [CrossRef]
- Noh, Y.-Y.; Kim, D.-Y.; Yoshida, Y.; Yase, K.; Jung, B.-J.; Lim, E.; Shim, H.-K. High-photosensitivity p-channel organic phototransistors based on a biphenyl end-capped fused bithiophene oligomer. Appl. Phys. Lett. 2005, 86, 043501. [Google Scholar] [CrossRef]
- Noh, Y.-Y.; Ghim, J.; Kang, S.-J.; Baeg, K.-J.; Kim, D.-Y.; Yase, K. Effect of light irradiation on the characteristics of organic field-effect transistors. J. Appl. Phys. 2006, 100, 094501. [Google Scholar] [CrossRef]
- Postnikov, V.A.; Odarchenko, Y.I.; Iovlev, A.V.; Bruevich, V.V.; Pereverzev, A.Y.; Kudryashova, L.G.; Sobornov, V.V.; Vidal, L.; Chernyshov, D.; Luponosov, Y.N.; et al. Molecularly Smooth Single-Crystalline Films of Thiophene–Phenylene Co-Oligomers Grown at the Gas–Liquid Interface. Cryst. Growth Des. 2014, 14, 1726–1737. [Google Scholar] [CrossRef]
- Katagiri, T.; Shimizu, Y.; Terasaki, K.; Yamao, T.; Hotta, S. Light-emitting field-effect transistors made of single crystals of an ambipolar thiophene/phenylene co-oligomer. Org. Electron. 2011, 12, 8–14. [Google Scholar] [CrossRef]
- Inada, Y.; Yamao, T.; Inada, M.; Itami, T.; Hotta, S. Giant organic single-crystals of a thiophene/phenylene co-oligomer toward device applications. Synth. Met. 2011, 161, 1869–1877. [Google Scholar] [CrossRef]
- Paasch, G.; Lindner, T.; Rost-Bietsch, C.; Karg, S.; Riess, W.; Scheinert, S. Operation and properties of ambipolar organic field-effect transistors. J. Appl. Phys. 2005, 98, 084505. [Google Scholar] [CrossRef]
- Labram, J.G.; Wöbkenberg, P.H.; Bradley, D.D.C.; Anthopoulos, T.D. Low-voltage ambipolar phototransistors based on a pentacene/PC61BM heterostructure and a self-assembled nano-dielectric. Org. Electron. 2010, 11, 1250–1254. [Google Scholar] [CrossRef]
- Coropceanu, V.; Cornil, J.; da Silva Filho, D.A.; Olivier, Y.; Silbey, R.; Brédas, J.-L. Charge Transport in Organic Semiconductors. Chem. Rev. 2007, 107, 926–952. [Google Scholar] [CrossRef]
- Waldrip, M.; Jurchescu, O.D.; Gundlach, D.J.; Bittle, E.G. Contact Resistance in Organic Field-Effect Transistors: Conquering the Barrier. Adv. Funct. Mater. 2020, 30, 1904576. [Google Scholar] [CrossRef]
- Dominskiy, D.I.; Kharlanov, O.G.; Trukhanov, V.A.; Sosorev, A.Y.; Sorokina, N.I.; Kazantsev, M.S.; Lazneva, E.F.; Gerasimova, N.B.; Sobolev, V.S.; Komolov, A.S.; et al. Polarity Switching in Organic Electronic Devices via Terminal Substitution of Active-Layer Molecules. ACS Appl. Electron. Mater. 2022, 4, 6345–6356. [Google Scholar] [CrossRef]
- Trukhanov, V.A.; Kuevda, A.V.; Dominskiy, D.I.; Mannanov, A.L.; Rybalova, T.V.; Tafeenko, V.A.; Sosorev, A.Y.; Konstantinov, V.G.; Kazantsev, M.S.; Borshchev, O.V.; et al. Strongly polarized surface electroluminescence from an organic light-emitting transistor. Mater. Chem. Front. 2023, 7, 238–248. [Google Scholar] [CrossRef]
- Maslennikov, D.R.; Dominskiy, D.I.; Sosorev, A.Y.; Trukhanov, V.A.; Konstantinov, V.G.; Sorokina, N.I.; Borshchev, O.V.; Skorotetcky, M.S.; Ponomarenko, S.A.; Paraschuk, D.Y. Tuning molecular packing, charge transport, and luminescence in thiophene-phenylene co-oligomer crystals via terminal substituents. J. Phys. Chem. C 2024, 128. in press. [Google Scholar]
- Sosorev, A.Y.; Nuraliev, M.K.; Feldman, E.V.; Maslennikov, D.R.; Borshchev, O.V.; Skorotetcky, M.S.; Surin, N.M.; Kazantsev, M.S.; Ponomarenko, S.A.; Paraschuk, D.Y. Impact of terminal substituents on the electronic, vibrational and optical properties of thiophene–phenylene co-oligomers. Phys. Chem. Chem. Phys. 2019, 21, 11578–11588. [Google Scholar] [CrossRef]
- Parashchuk, O.D.; Mannanov, A.A.; Konstantinov, V.G.; Dominskiy, D.I.; Surin, N.M.; Borshchev, O.V.; Ponomarenko, S.A.; Pshenichnikov, M.S.; Paraschuk, D.Y. Molecular Self-Doping Controls Luminescence of Pure Organic Single Crystals. Adv. Funct. Mater. 2018, 28, 1800116. [Google Scholar] [CrossRef]
- Sosorev, A.Y.; Trukhanov, V.A.; Maslennikov, D.R.; Borshchev, O.V.; Polyakov, R.A.; Skorotetcky, M.S.; Surin, N.M.; Kazantsev, M.S.; Dominskiy, D.I.; Tafeenko, V.A.; et al. Fluorinated Thiophene-Phenylene Co-Oligomers for Optoelectronic Devices. ACS Appl. Mater. Interfaces 2020, 12, 9507–9519. [Google Scholar] [CrossRef] [PubMed]
- Kudryashova, L.G.; Kazantsev, M.S.; Postnikov, V.A.; Bruevich, V.V.; Luponosov, Y.N.; Surin, N.M.; Borshchev, O.V.; Ponomarenko, S.A.; Pshenichnikov, M.S.; Paraschuk, D.Y. Highly Luminescent Solution-Grown Thiophene-Phenylene Co-Oligomer Single Crystals. ACS Appl. Mater. Interfaces 2016, 8, 10088–10092. [Google Scholar] [CrossRef]
- Chen, M.; Yan, L.; Zhao, Y.; Murtaza, I.; Meng, H.; Huang, W. Anthracene-based semiconductors for organic field-effect transistors. J. Mater. Chem. C 2018, 6, 7416–7444. [Google Scholar] [CrossRef]
- Gryn’ova, G.; Lin, K.-H.; Corminboeuf, C. Read between the Molecules: Computational Insights into Organic Semiconductors. J. Am. Chem. Soc. 2018, 140, 16370–16386. [Google Scholar] [CrossRef] [PubMed]
- Skabara, P.J.; Arlin, J.-B.; Geerts, Y.H. Close Encounters of the 3D Kind—Exploiting High Dimensionality in Molecular Semiconductors. Adv. Mater. 2013, 25, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- de Boer, R.W.I.; Gershenson, M.E.; Morpurgo, A.F.; Podzorov, V. Organic single-crystal field-effect transistors. Phys. Status Solidi (A) 2004, 201, 1302–1331. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, S.; Zhang, B.; Shin, E.-Y.; Noh, Y.-Y.; Xu, Y.; Shi, Y.; Li, Y. Role of Schottky Barrier and Access Resistance in Organic Field-Effect Transistors. J. Phys. Chem. Lett. 2020, 11, 1466–1472. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.D.; Yan, Y.; Tsukagoshi, K. Understanding contact behavior in organic thin film transistors. Appl. Phys. Lett. 2010, 97, 063307. [Google Scholar] [CrossRef]
- Zaumseil, J.; Friend, R.H.; Sirringhaus, H. Spatial control of the recombination zone in an ambipolar light-emitting organic transistor. Nat. Mater. 2006, 5, 69–74. [Google Scholar] [CrossRef]
- Yuan, D.; Sharapov, V.; Liu, X.; Yu, L. Design of High-Performance Organic Light-Emitting Transistors. ACS Omega 2020, 5, 68–74. [Google Scholar] [CrossRef]
- Liu, C.-F.; Liu, X.; Lai, W.-Y.; Huang, W. Organic Light-Emitting Field-Effect Transistors: Device Geometries and Fabrication Techniques. Adv. Mater. 2018, 30, 1802466. [Google Scholar] [CrossRef]
- Oyamada, T.; Chang, C.-H.; Chao, T.-C.; Fang, F.-C.; Wu, C.-C.; Wong, K.-T.; Sasabe, H.; Adachi, C. Optical Properties of Oligo(9,9-diarylfluorene) Derivatives in Thin Films and Their Application for Organic Light-Emitting Field-Effect Transistors. J. Phys. Chem. C 2007, 111, 108–115. [Google Scholar] [CrossRef]
- Sakanoue, T.; Yahiro, M.; Adachi, C.; Uchiuzou, H.; Takahashi, T.; Toshimitsu, A. Ambipolar light-emitting organic field-effect transistors using a wide-band-gap blue-emitting small molecule. Appl. Phys. Lett. 2007, 90, 171118. [Google Scholar] [CrossRef]
- Gwinner, M.C.; Kabra, D.; Roberts, M.; Brenner, T.J.K.; Wallikewitz, B.H.; McNeill, C.R.; Friend, R.H.; Sirringhaus, H. Highly Efficient Single-Layer Polymer Ambipolar Light-Emitting Field-Effect Transistors. Adv. Mater. 2012, 24, 2728–2734. [Google Scholar] [CrossRef]
- Hotta, S.; Yamao, T. The thiophene/phenylene co-oligomers: Exotic molecular semiconductors integrating high-performance electronic and optical functionalities. J. Mater. Chem. 2011, 21, 1295–1304. [Google Scholar] [CrossRef]
- Sosorev, A.Y.; Godovsky, D.Y.; Paraschuk, D.Y. Hot kinetic model as a guide to improve organic photovoltaic materials. Phys. Chem. Chem. Phys. 2018, 20, 3658–3671. [Google Scholar] [CrossRef]
- Dimitrov, S.D.; Bakulin, A.A.; Nielsen, C.B.; Schroeder, B.C.; Du, J.; Bronstein, H.; McCulloch, I.; Friend, R.H.; Durrant, J.R. On the Energetic Dependence of Charge Separation in Low-Band-Gap Polymer/Fullerene Blends. J. Am. Chem. Soc. 2012, 134, 18189–18192. [Google Scholar] [CrossRef] [PubMed]
- Bakulin, A.A.; Rao, A.; Pavelyev, V.G.; van Loosdrecht, P.H.M.; Pshenichnikov, M.S.; Niedzialek, D.; Cornil, J.; Beljonne, D.; Friend, R.H. The Role of Driving Energy and Delocalized States for Charge Separation in Organic Semiconductors. Science 2012, 335, 1340–1344. [Google Scholar] [CrossRef]
- Yuan, S.H.; Pei, Z.; Lai, H.C.; Li, P.W.; Chan, Y.J. Pentacene phototransistor with gate voltage independent responsivity and sensitivity by small silver nanoparticles decoration. Org. Electron. 2015, 27, 7–11. [Google Scholar] [CrossRef]
- Kim, H.; Kim, G.; Song, I.; Lee, J.; Abdullah, H.; Yang, C.; Oh, J.H. Ambipolar organic phototransistors based on 6,6′-dibromoindigo. RSC Adv. 2018, 8, 14747–14752. [Google Scholar] [CrossRef]
- Lucas, B.; El Amrani, A.; Chakaroun, M.; Ratier, B.; Antony, R.; Moliton, A. Ultraviolet light effect on electrical properties of a flexible organic thin film transistor. Thin Solid Film. 2009, 517, 6280–6282. [Google Scholar] [CrossRef]
- Kang, H.-S.; Choi, C.-S.; Choi, W.-Y.; Kim, D.-H.; Seo, K.-S. Characterization of phototransistor internal gain in metamorphic high-electron-mobility transistors. Appl. Phys. Lett. 2004, 84, 3780–3782. [Google Scholar] [CrossRef]
- Marjanović, N.; Singh, T.B.; Dennler, G.; Günes, S.; Neugebauer, H.; Sariciftci, N.S.; Schwödiauer, R.; Bauer, S. Photoresponse of organic field-effect transistors based on conjugated polymer/fullerene blends. Org. Electron. 2006, 7, 188–194. [Google Scholar] [CrossRef]
- Narayan, K.S.; Kumar, N. Light responsive polymer field-effect transistor. Appl. Phys. Lett. 2001, 79, 1891–1893. [Google Scholar] [CrossRef]
- Hamilton, M.C.; Martin, S.; Kanicki, J. Thin-film organic polymer phototransistors. IEEE Trans. Electron Devices 2004, 51, 877–885. [Google Scholar] [CrossRef]
- Saragi, T.P.I.; Pudzich, R.; Fuhrmann, T.; Salbeck, J. Organic phototransistor based on intramolecular charge transfer in a bifunctional spiro compound. Appl. Phys. Lett. 2004, 84, 2334–2336. [Google Scholar] [CrossRef]
- Xu, Y.; Berger, P.R.; Wilson, J.N.; Bunz, U.H.F. Photoresponsivity of polymer thin-film transistors based on polyphenyleneethynylene derivative with improved hole injection. Appl. Phys. Lett. 2004, 85, 4219–4221. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Gordon, M.S.; Schmidt, M.W. Chapter 41—Advances in electronic structure theory: GAMESS a decade later. In Theory and Applications of Computational Chemistry; Dykstra, C.E., Frenking, G., Kim, K.S., Scuseria, G.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1167–1189. [Google Scholar]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta (BBA)—Rev. Bioenerg. 1985, 811, 265–322. [Google Scholar] [CrossRef]
- Kirkpatrick, J. An approximate method for calculating transfer integrals based on the ZINDO Hamiltonian. Int. J. Quantum Chem. 2008, 108, 51–56. [Google Scholar] [CrossRef]
- Deng, W.-Q.; Goddard, W.A. Predictions of Hole Mobilities in Oligoacene Organic Semiconductors from Quantum Mechanical Calculations. J. Phys. Chem. B 2004, 108, 8614–8621. [Google Scholar] [CrossRef]
- Materials Studio Package; BIOVIA: Dassault Systèmes: San Diego, CA, USA, 2017.
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.C.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model. 2016, 22, 47. [Google Scholar] [CrossRef]
- Spackman, M.A.; Byrom, P.G. A novel definition of a molecule in a crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallographica Section B 2004, 60, 627–668. [Google Scholar] [CrossRef] [PubMed]
- Trukhanov, V.A.; Dominskiy, D.I.; Parashchuk, O.D.; Feldman, E.V.; Surin, N.M.; Svidchenko, E.A.; Skorotetcky, M.S.; Borshchev, O.V.; Paraschuk, D.Y.; Sosorev, A.Y. Impact of N-substitution on structural, electronic, optical, and vibrational properties of a thiophene–phenylene co-oligomer. RSC Adv. 2020, 10, 28128–28138. [Google Scholar] [CrossRef] [PubMed]
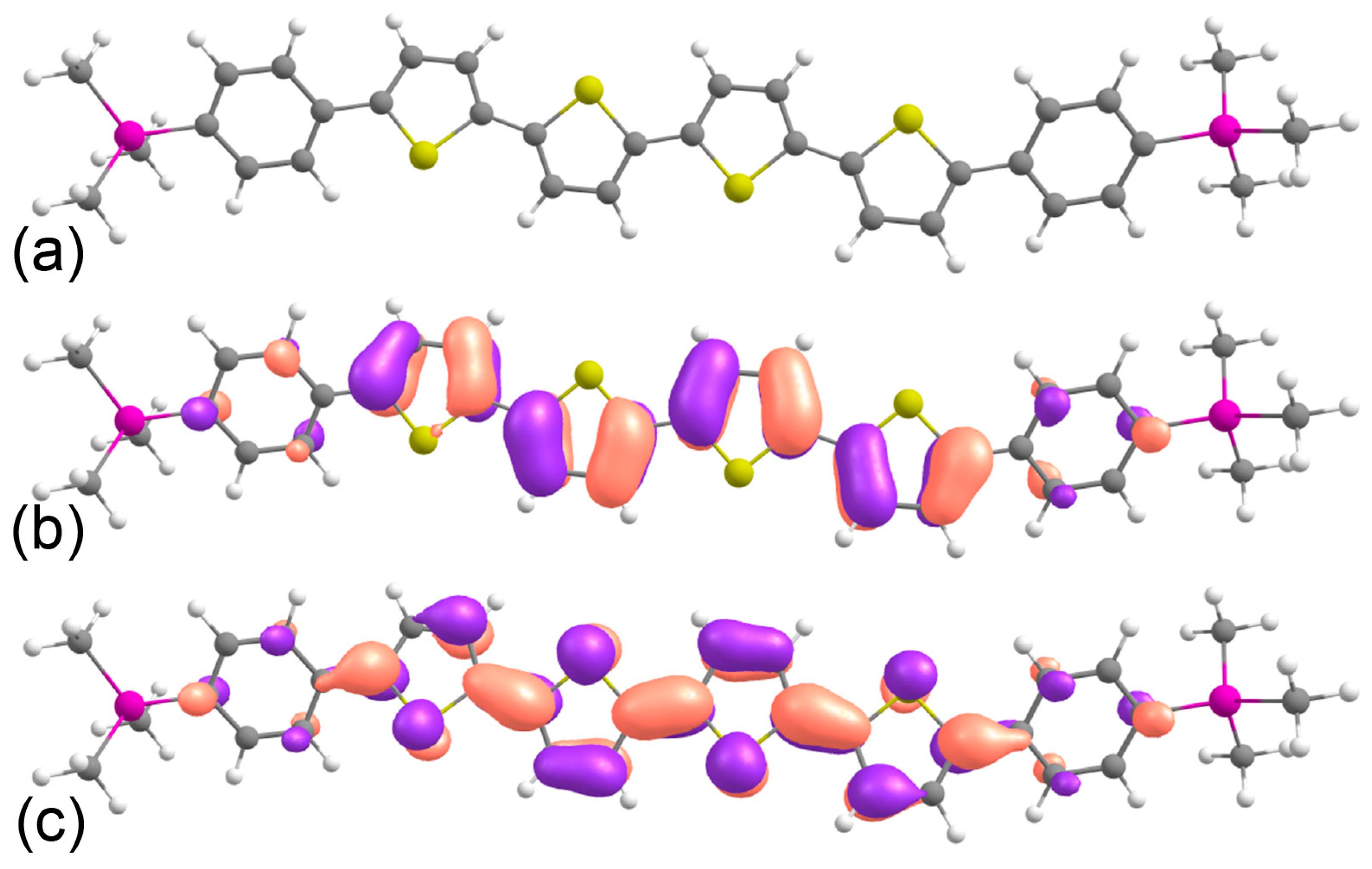

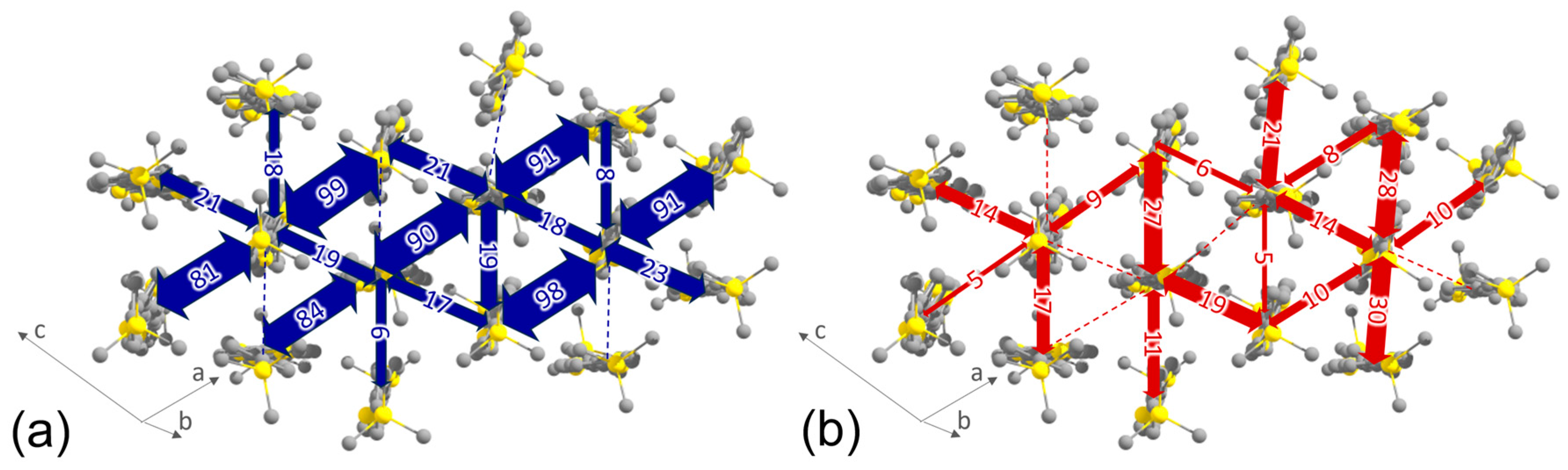


| a, Å | b, Å | c, Å | α, deg | β, deg | γ, deg | Z | Space Group |
|---|---|---|---|---|---|---|---|
| 43.4110(10) | 14.8981(5) | 45.184(2) | 90 | 115.795(3) | 90 | 32 | C 2/c |
| Active Layer | Charge Carriers | Max. Mobility, cm2 V–1 s–1 | Aver. Mobility, cm2 V–1 s–1 | Aver. Threshold Voltage, V |
|---|---|---|---|---|
| Thin film | electrons | 0.0237 | 0.0092 ± 0.0010 | 19 ± 6 |
| holes | 0.0193 | 0.0108 ± 0.0009 | –10 ± 4 | |
| Single crystal | electrons | 1.0·10–4 | (5.0 ± 1.7)·10–5 | –0.3 ± 16 |
| holes | 1.61·10–4 | (1.06 ± 0.22)·10–4 | 5 ± 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trukhanov, V.A.; Sosorev, A.Y.; Dominskiy, D.I.; Fedorenko, R.S.; Tafeenko, V.A.; Borshchev, O.V.; Ponomarenko, S.A.; Paraschuk, D.Y. Dual Optoelectronic Organic Field-Effect Device: Combination of Electroluminescence and Photosensitivity. Molecules 2024, 29, 2533. https://doi.org/10.3390/molecules29112533
Trukhanov VA, Sosorev AY, Dominskiy DI, Fedorenko RS, Tafeenko VA, Borshchev OV, Ponomarenko SA, Paraschuk DY. Dual Optoelectronic Organic Field-Effect Device: Combination of Electroluminescence and Photosensitivity. Molecules. 2024; 29(11):2533. https://doi.org/10.3390/molecules29112533
Chicago/Turabian StyleTrukhanov, Vasiliy A., Andrey Y. Sosorev, Dmitry I. Dominskiy, Roman S. Fedorenko, Victor A. Tafeenko, Oleg V. Borshchev, Sergey A. Ponomarenko, and Dmitry Y. Paraschuk. 2024. "Dual Optoelectronic Organic Field-Effect Device: Combination of Electroluminescence and Photosensitivity" Molecules 29, no. 11: 2533. https://doi.org/10.3390/molecules29112533






