Exploring Sustainable Agriculture with Nitrogen-Fixing Cyanobacteria and Nanotechnology
Abstract
:1. Introduction
2. Nanoparticle Uptake and Distribution in Plants
2.1. Mechanisms of Nanoparticle Entry into Plant Cells
2.2. Cyanobacterial Intercellular Transportation
| Type | Moss Species and Relationship with Cyanobacteria | Cyanobacteria | Reference |
|---|---|---|---|
| Hornwort-cyanobacteria symbiont | Anthoceros sp. Dendroceros sp. Notothylas sp. Phaeoceros sp. | Nostoc sp. | [99,100,101,102,103] |
| Liverwort-cyanobacteria symbiont | Anastrophyllum involutifolium Blasia sp. Cavicularia sp Chiloscyphusleptanthus Marchantia sp. Porella sp. | Nostoc sp. Calothrix sp. Stigonema sp. Chlorogloeopsis sp. | [99,100,101,102,104] |
| Moss-cyanobacteria symbiont | Acroschisma wilsonii Andreaea alpine Andreaea laxifolia Aulacomnium palustre Blepharidophyllum densifolium Bryum sp. Calliergon richardsonii Ceratodon purpureus Clasmatocolea humilis Cryptochila grandiflora Dendroligotrichum squamosum Dicranoloma chilense Ditrichum cylindricarpum Drepanocladus sp Drepanocladus exannulatus Dupontia sp. Grimmia sp. Heteroscyphus magellanicus Hylocomium splendens Paludella squarrosa Pleurozium schreberi Ptilium sp. Racomitrium Subcrispipilum Racomitriumlaevigatum Racomitrium lanuginosum Racomitrium didymium Sanionia uncinate Sphagnum lindebergii Sphagnum riparium Tomentypnum nitens Weisia controversa | Nostoc sp. Nostoc muscorum Calothrix sp Stigonema sp Scytonema sp. Anobena sp. Oscillatoria sp. Lyngbya sp. | [99,100,101,102,105,106,107,108,109] |
2.3. Root-Shoot Translocation
2.4. Systemic Distribution and Long-Distance Signaling
2.5. Cyanobacterial Symbiosis
3. Molecular Insights into Nanoparticle Toxicity
3.1. Plants and Cyanobacteria Cellular Reactions to Exposure to Metal Nanoparticles
3.2. Oxidative Stress and Its Implications for Plant-Cyanobacteria Health
3.3. Signaling Pathways Triggered by Nanoparticle-Induced Stress in Mutualistic Systems
4. Enhancement of Nutrient Uptake and Availability
4.1. MNPs as Carriers of Essential Nutrients: Root Interactions and Cyanobacterial Associations
4.2. Nutrient-Use Efficiency and Crop Yield Enhancement through Plant-Cyanobacteria Synergy
4.3. Ecological and Agricultural Implications
5. Stress Tolerance and Disease Resistance
5.1. Nanoparticle-Mediated Stress Responses
5.2. Reinforcement of Pathogen Defense Mechanisms
5.3. Mitigation of Abiotic Stress Factors
6. Impact on Key Plant and Cyanobacterial Processes
6.1. Impact on Photosynthesis and Carbon Fixation
6.2. Effects of Nanoparticles on Photosynthesis, Energy Conversion, and Nitrogen Fixation by Cyanobacteria
6.3. Effects on Root Architecture, Mycorrhizal Associations, and Cyanobacterial Aggregates
7. Phytoremediation Potential
7.1. Mechanisms of Phytoremediation Assisted by Metal Nanoparticles
7.2. Synergistic Effects on Plant-Microbe Interactions
7.3. Ecological Considerations, Cyanobacterial Contributions, and Long-Term Effectiveness
8. Cutting-Edge Developments in Agricultural Nanotechnology
8.1. Integrating Cyanobacterial Solutions
8.2. Cyanobacterial Biofertilizers and Soil Fertility
8.3. Role of Cyanobacteria in Nanoparticle Applications
8.4. Ethical Considerations and Regulatory Implications
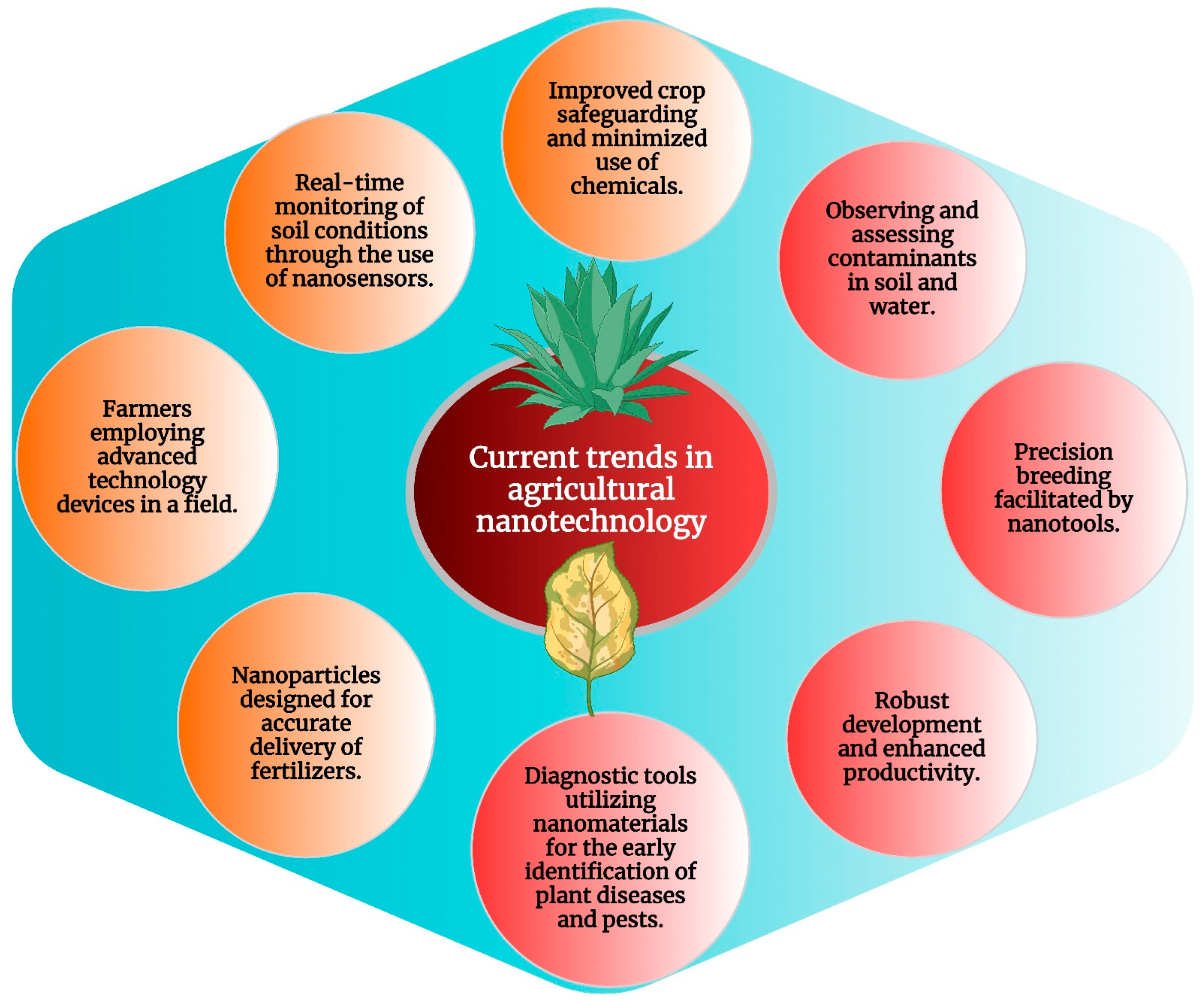
9. Current Trends in Agricultural Nanotechnology
9.1. Integrating Metal Nanoparticles into Modern Agricultural Practices
9.2. Integrating Cyanobacterial Solutions
9.3. Synergies with Precision Farming, Sustainable Agriculture, and Cyanobacterial Biofertilizers
9.4. Balancing Benefits, Ethical Concerns, and the Pivotal Role of Cyanobacteria in Nanoparticle Applications
10. Future Prospects and Challenges
10.1. Unexplored Areas of Research in Plant-Cyanobacteria-Nanoparticle Interactions
10.2. Regulatory Aspects, Ethical Considerations, and the Potential of Cyanobacterial Bioinoculants
10.3. Steps toward Responsible and Safe Utilization of Nanoparticles in a Cyanobacteria-Augmented Environment
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gu, J.; Yang, J. Nitrogen (N) transformation in paddy rice field: Its effect on N uptake and relation to improved N management. Crop Environ. 2022, 1, 7–14. [Google Scholar] [CrossRef]
- Jiao, X.; Takishita, Y.; Zhou, G.; Smith, D.L. Plant associated rhizobacteria for biocontrol and plant growth enhancement. Front. Plant Sci. 2021, 12, 634796. [Google Scholar] [CrossRef] [PubMed]
- Bellenger, J.P.; Darnajoux, R.; Zhang, X.; Kraepiel, A.M.L. Biological nitrogen fixation by alternative nitrogenases in terrestrial ecosystems: A review. Biogeochemistry 2020, 149, 53–73. [Google Scholar] [CrossRef]
- Smercina, D.N.; Evans, S.E.; Friesen, M.L.; Tiemann, L.K. To fix or not to fix: Controls on free-living nitrogen fixation in the rhizosphere. Appl. Environ. Microbiol. 2019, 85, e02546-18. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.; Bosch, J.; Coclet, C.; Johnson, J.; Lebre, P.; Salawu-Rotimi, A.; Vikram, S.; Makhalanyane, T.; Cowan, D. Microbial nitrogen cycling in Antarctic soils. Microorganisms 2020, 8, 1442. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sangwan, S.; Kaur, H.; Patra, A.; Anamika; Mehta, S. Diversity and evolution of nitrogen fixing bacteria. In Sustainable Agriculture Reviews 60: Microbial Processes in Agriculture; Springer: Berlin/Heidelberg, Germany, 2023; Volume 60, pp. 95–120. [Google Scholar]
- Nawaz, T.; Gu, L.; Fahad, S.; Saud, S.; Harrison, M.T.; Zhou, R. Sustainable protein production through genetic engineering of cyanobacteria and use of atmospheric N2 gas. Food Energy Secur. 2024, 13, e536. [Google Scholar] [CrossRef]
- Hu, C.; Rzymski, P. Non-photosynthetic Melainabacteria (Cyanobacteria) in human gut: Characteristics and association with health. Life 2022, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Runge, E.A.; Mansor, M.; Kappler, A.; Duda, J.P. Microbial biosignatures in ancient deep-sea hydrothermal sulfides. Geobiology 2023, 21, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, T.; Gu, L.; Fahad, S.; Saud, S.; Hassan, S.; Harrison, M.T.; Liu, K.; Zhou, R. Unveiling the antioxidant capacity of fermented foods and food microorganisms: A focus on cyanobacteria. J. Umm Al-Qura Univ. Appl. Sci. 2024, 10, 232–243. [Google Scholar] [CrossRef]
- Callieri, C.; Cabello-Yeves, P.J.; Bertoni, F. The “dark side” of picocyanobacteria: Life as we do not know it (yet). Microorganisms 2022, 10, 546. [Google Scholar] [CrossRef]
- Mallén-Ponce, M.J.; Huertas, M.J.; Florencio, F.J. Exploring the diversity of the thioredoxin systems in cyanobacteria. Antioxidants 2022, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Garlapati, D.; Chandrasekaran, M.; Devanesan, A.; Mathimani, T.; Pugazhendhi, A. Role of cyanobacteria in agricultural and industrial sectors: An outlook on economically important byproducts. Appl. Microbiol. Biotechnol. 2019, 103, 4709–4721. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.P.; Nguyen, L.N.; Zdarta, J.; Nga, T.T.; Nghiem, L.D. Blue-green algae in surface water: Problems and opportunities. Curr. Pollut. Rep. 2020, 6, 105–122. [Google Scholar] [CrossRef]
- Righini, H.; Francioso, O.; Martel Quintana, A.; Roberti, R. Cyanobacteria: A natural source for controlling agricultural plant diseases caused by fungi and oomycetes and improving plant growth. Horticulturae 2022, 8, 58. [Google Scholar] [CrossRef]
- Margesin, R.; Collins, T. Microbial ecology of the cryosphere (glacial and permafrost habitats): Current knowledge. Appl. Microbiol. Biotechnol. 2019, 103, 2537–2549. [Google Scholar] [CrossRef] [PubMed]
- Kollmen, J.; Strieth, D. The beneficial effects of cyanobacterial co-culture on plant growth. Life 2022, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Pernil, R.; Schleiff, E. Metalloproteins in the Biology of Heterocysts. Life 2019, 9, 32. [Google Scholar] [CrossRef]
- Alleman, A.B.; Peters, J.W. Mechanisms for Generating Low Potential Electrons across the Metabolic Diversity of Nitrogen-Fixing Bacteria. Appl. Environ. Microbiol. 2023, 89, e00378-23. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting biological nitrogen fixation: A route towards a sustainable agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Carvalho, S.; Kürten, B.; Krokos, G.; Hoteit, I.; Ellis, J. The red sea. In World Seas: An Environmental Evaluation; Academic Press: Cambridge, MA, USA, 2019; pp. 49–74. [Google Scholar]
- Williamson, C.E.; Neale, P.J.; Hylander, S.; Rose, K.C.; Figueroa, F.L.; Robinson, S.A.; Häder, D.P.; Wängberg, S.Å.; Worrest, R.C. The interactive effects of stratospheric ozone depletion, UV radiation, and climate change on aquatic ecosystems. Photochem. Photobiol. Sci. 2019, 18, 717–746. [Google Scholar] [CrossRef]
- McGregor, G.B.; Sendall, B.C. Iningainema pulvinus gen nov., sp nov. (Cyanobacteria, Scytonemataceae) a new nodularin producer from Edgbaston Reserve, north-eastern Australia. Harmful Algae 2017, 62, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The nitrogen cascade. Bioscience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Fadeel, B.; Garcia-Bennett, A.E. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv. Drug Deliv. Rev. 2010, 62, 362–374. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Zaman, M.A.; Abbas, R.Z.; Qamar, W.; Qamar, M.F.; Mehreen, U.; Shahid, Z.; Kamran, M. Role of secondary metabolites of medicinal plants against Ascaridia galli. Worlds Poult. Sci. J. 2020, 76, 639–655. [Google Scholar] [CrossRef]
- Huq, M.E.; Fahad, S.; Shao, Z.; Sarven, M.S.; Khan, I.A.; Alam, M.; Saeed, M.; Ullah, H.; Adnan, M.; Saud, S.; et al. Arsenic in a groundwater environment in Bangladesh: Occurrence and mobilization. J. Environ. Manag. 2020, 262, 110318. [Google Scholar] [CrossRef]
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; Iqbal, S.; Kamran, M.; El-Sabrout, A.M.; Elansary, H.O.; et al. Role of promising secondary metabolites to confer resistance against environmental stresses in crop plants: Current scenario and future perspectives. Front. Plant Sci. 2022, 13, 881032. [Google Scholar] [CrossRef]
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A review on silver nanoparticles: Classification, various methods of synthesis, and their potential roles in biomedical applications and water treatment. Water 2021, 13, 2216. [Google Scholar] [CrossRef]
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of cadmium: Physiological, biochemical, and molecular mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef]
- Hussain, M.; Zahra, N.; Lang, T.; Zain, M.; Raza, M.; Shakoor, N.; Adeel, M.; Zhou, H. Integrating nanotechnology with plant microbiome for next-generation crop health. Plant Physiol. Biochem. 2023, 196, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Medina-Velo, I.A.; Cota-Ruiz, K.; Moreno-Olivas, F.; Gardea-Torresdey, J.L. Can abiotic stresses in plants be alleviated by manganese nanoparticles or compounds? Ecotoxicol. Environ. Saf. 2019, 184, 109671. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Z.; Habib, Z.; Hyun, H.; Shahzad, H.M.A. Overview on recent developments in the design, application, and impacts of nanofertilizers in agriculture. Sustainability 2022, 14, 9397. [Google Scholar] [CrossRef]
- do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef]
- Kumar, B.; Singhal, R.K.; Chand, S.; Chauhan, J.; Kumar, V.; Mishra, U.N.; Hidangmayum, A.; Singh, A.; Bose, B. Nanopriming in sustainable agriculture: Recent advances, emerging challenges and future prospective. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 339–365. [Google Scholar] [CrossRef]
- Garg, R.; Maheshwari, S. Seed Priming and Fortification of Seeds Using Nanotechnology: A review. EPH—Int. J. Environ. Agric. Res. 2023, 9, 11–17. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Adhikari, P.; Djebaili, R.; Thathola, P.; Joshi, K.; Pellegrini, M.; Adeyemi, N.O.; Khoshru, B.; Kaur, K.; Priyadarshini, A.; et al. Biosynthesis and characterization of nanoparticles, its advantages, various aspects and risk assessment to maintain the sustainable agriculture: Emerging technology in modern era science. Plant Physiol. Biochem. 2023, 196, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Elmer, W.; White, J.C. The future of nanotechnology in plant pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef]
- Guleria, A.; Sachdeva, H.; Saini, K.; Gupta, K.; Mathur, J. Recent trends and advancements in synthesis and applications of plant-based green metal nanoparticles: A critical review. Appl. Organomet. Chem. 2022, 36, e6778. [Google Scholar] [CrossRef]
- Jain, A.; Ranjan, S.; Dasgupta, N.; Ramalingam, C. Nanomaterials in food and agriculture: An overview on their safety concerns and regulatory issues. Crit. Rev. Food Sci. Nutr. 2018, 58, 297–317. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Marei, I.; Crovella, S.; Abou-Saleh, H. Recent developments in nanomaterials-based drug delivery and upgrading treatment of cardiovascular diseases. Int. J. Mol. Sci. 2022, 23, 1404. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Jinnouchi, R.; Asahi, R. Predicting catalytic activity of nanoparticles by a DFT-aided machine-learning algorithm. J. Phys. Chem. Lett. 2017, 8, 4279–4283. [Google Scholar] [CrossRef]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Villamor, E.; Begines, B.; Alcudia, A. Environmental impact of nanoparticles’ application as an emerging technology: A review. Materials 2020, 14, 166. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Tran, C.L. (Eds.) Nanotoxicology: Progress toward Nanomedicine; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Schloemer, T.; Narayanan, P.; Zhou, Q.; Belliveau, E.; Seitz, M.; Congreve, D.N. Nanoengineering Triplet–Triplet Annihilation Upconversion: From Materials to Real-World Applications. ACS Nano 2023, 17, 3259–3288. [Google Scholar] [CrossRef]
- Heilgeist, S.; Sekine, R.; Sahin, O.; Stewart, R.A. Finding nano: Challenges involved in monitoring the presence and fate of engineered titanium dioxide nanoparticles in aquatic environments. Water 2021, 13, 734. [Google Scholar] [CrossRef]
- Renn, O.; Roco, M.C. Nanotechnology and the need for risk governance. In Emerging Technologies; Routledge: London, UK, 2020; pp. 321–359. [Google Scholar] [CrossRef]
- Rozaki, Z. Food security challenges and opportunities in Indonesia post COVID-19. Adv. Food Secur. Sustain. 2021, 6, 119–168. [Google Scholar] [CrossRef]
- Majumdar, S.; Keller, A.A. Omics to address the opportunities and challenges of nanotechnology in agriculture. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2595–2636. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Boddu, V.M.; Chadha, N.K.; Chakraborty, P.; Kumar, J.; Krishna, G.; Pathak, H. Metallic and non-metallic nanoparticles from plant, animal, and fisheries wastes: Potential and valorization for application in agriculture. Environ. Sci. Pollut. Res. 2022, 29, 81130–81165. [Google Scholar] [CrossRef]
- El Semary, N.A. Nitrogen-fixing Cyanothece sp. as a mixotroph and silver nanoparticle synthesizer: A multitasking exceptional cyanobacterium. Braz. J. Biol. 2022, 82, e265135. [Google Scholar] [CrossRef]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S.Z. Synthesis, characterization, and applications of metal nanoparticles. In Biomaterials and Bionanotechnology; Academic Press: Cambridge, MA, USA, 2019; pp. 527–612. [Google Scholar] [CrossRef]
- Brandelli, A.; Ritter, A.C.; Veras, F.F. Antimicrobial activities of metal nanoparticles. In Metal Nanoparticles in Pharma; Springer: Berlin/Heidelberg, Germany, 2017; pp. 337–363. [Google Scholar] [CrossRef]
- Nawaz, T.; Saud, S.; Gu, L.; Khan, I.; Fahad, S.; Zhou, R. Cyanobacteria: Harnessing the Power of Microorganisms for Plant Growth Promotion, Stress Alleviation, and Phytoremediation in the Era of Sustainable Agriculture. Plant Stress 2024, 11, 100399. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef] [PubMed]
- Burketová, L.; Martinec, J.; Siegel, J.; Macůrková, A.; Maryška, L.; Valentová, O. Noble metal nanoparticles in agriculture: Impacts on plants, associated microorganisms, and biotechnological practices. Biotechnol. Adv. 2022, 58, 107929. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Rizvi, A.; Ali, K.; Lee, J.; Zaidi, A.; Khan, M.S.; Musarrat, J. Nanoparticles in the soil–plant system: A review. Environ. Chem. Lett. 2021, 19, 1545–1609. [Google Scholar] [CrossRef]
- Hussain, M.; Shakoor, N.; Adeel, M.; Ahmad, M.A.; Zhou, H.; Zhang, Z.; Xu, M.; Rui, Y.; White, J.C. Nano-enabled plant microbiome engineering for disease resistance. Nano Today 2023, 48, 101752. [Google Scholar] [CrossRef]
- Li, X.; He, F.; Wang, Z.; Xing, B. Roadmap of environmental health research on emerging contaminants: Inspiration from the studies on engineered nanomaterials. Eco-Environ. Health 2022, 1, 181–197. [Google Scholar] [CrossRef] [PubMed]
- De la Torre Roche, R.; Servin, A.; Hawthorne, J.; Xing, B.; Newman, L.A.; Ma, X.; Chen, G.; White, J.C. Terrestrial Trophic Transfer of Bulk and Nanoparticle La2O3 Does Not Depend on Particle Size. Environ. Sci. Technol. 2015, 49, 11866–11874. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Gu, Z.; Pan, Y.; Chen, J.; Xie, Q.; Xu, S.; Gao, M.; Cao, X.; Liu, S.; Wang, W.; et al. Biotransformation of rare earth oxide nanoparticles eliciting microbiota imbalance. Part. Fibre Toxicol. 2021, 18, 17. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Shang, E.; Xia, X.; Niu, J.; Crittenden, J. Effects of Chloride Ions on Dissolution, ROS Generation, and Toxicity of Silver Nanoparticles under UV Irradiation. Environ. Sci. Technol. 2018, 52, 4842–4849. [Google Scholar] [CrossRef] [PubMed]
- Shang, E.; Li, Y.; Niu, J.; Zhou, Y.; Wang, T.; Crittenden, J.C. Relative importance of humic and fulvic acid on ROS generation, dissolution, and toxicity of sulfide nanoparticles. Water Res. 2017, 124, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, B.; Hasnain, A.; Wang, J.; Zheng, H.; Naqvi, S.A.H.; Prasad, R.; Rehman Au Sohail, M.A.; Hassan, M.Z.; Farhan, M.; Khan, M.A. Current Progress and Open Challenges for Combined Toxic Effects of Manufactured Nano-Sized Objects (MNO’s) on Soil Biota and Microbial Community. Coatings 2023, 13, 212. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of physico-chemical properties of nanoparticles on their intracellular uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jin, L.; Wang, X. Cadmium absorption and transportation pathways in plants. Int. J. Phytoremediat. 2017, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Jin, M.; Yao, L.; He, B.; Ahmed, S.; Safdar, W.; Ahmad, I.; Cheng, D.B.; Lei, Z.; Sun, T. Physicochemical properties, pharmacokinetics, toxicology and application of nanocarriers. J. Mater. Chem. B 2023, 11, 716–733. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Root system architecture, copper uptake and tissue distribution in soybean (Glycine max L.) Merr.) grown in copper oxide nanoparticle (CuONP)-amended soil and implications for human nutrition. Plants 2020, 9, 1326. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kumar, A. Understanding the effect of the interaction of nanoparticles with roots on the uptake in plants. J. Environ. Nanotechnol. 2020, 3, 277–304. [Google Scholar] [CrossRef]
- Apodaca, S.A.; Cota-Ruiz, K.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Arbuscular mycorrhizal fungi alleviate phytotoxic effects of copper-based nanoparticles/compounds in spearmint (Mentha spicata). ACS Agric. Sci. Technol. 2022, 2, 661–670. [Google Scholar] [CrossRef]
- Arruda, S.C.C.; Silva, A.L.D.; Galazzi, R.M.; Azevedo, R.A.; Arruda, M.A.Z. Nanoparticles applied to plant science: A review. Talanta 2015, 131, 693–705. [Google Scholar] [CrossRef]
- Columbus, S.; Ramachandran, K.; Shameer, M.; Daoudi, K.; Gaidi, M. Antimicrobial nanosystems for environmental remediation applications. In Antimicrobial Nanosystems; Elsevier: Amsterdam, The Netherlands, 2023; pp. 417–435. [Google Scholar] [CrossRef]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. J. Nanomater. 2021, 2021, 6677616. [Google Scholar] [CrossRef]
- Chou, L.Y.; Ming, K.; Chan, W.C. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011, 40, 233–245. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef] [PubMed]
- Zangi, R.; Filella, M. Transport routes of metalloids into and out of the cell: A review of the current knowledge. Chem. Biol. Interact. 2012, 197, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.R.; Zia-ur-Rehman, M.; Sohail, M.I.; Haq, M.A.; Khalid, H.; Ayub, M.A.; Ishaq, G. Effects of rare earth oxide nanoparticles on plants. In Nanomaterials in Plants, Algae, and Microorganisms; Academic Press: Cambridge, MA, USA, 2018; pp. 239–275. [Google Scholar] [CrossRef]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H.; Singh, S.C. Plant-nanoparticle interaction: An approach to improve agricultural practices and plant productivity. Int. J. Pharm. Sci. Invent. 2015, 4, 25–40. [Google Scholar]
- Park, J.H.; Gu, L.; Von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liao, C.; Liu, S.; Xia, T.; Jiang, G. Nanotechnology: New opportunities for the development of patch-clamps. J. Nanobiotechnol. 2021, 19, 97. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Chandler, M.; Johnson, M.B.; Panigaj, M.; Afonin, K.A. Innate immune responses triggered by nucleic acids inspire the design of immunomodulatory nucleic acid nanoparticles (NANPs). In Therapeutic RNA Nanotechnology; Taylor and Francis: Abingdon, UK, 2021; pp. 1045–1060. [Google Scholar] [CrossRef]
- da Silva Santos, E.; Nogueira, K.A.B.; Fernandes, L.C.C.; Martins, J.R.P.; Reis, A.V.F.; Neto, J.D.B.V.; da Silva Júnior, I.J.; Pessoa, C.; Petrilli, R.; Eloy, J.O. EGFR targeting for cancer therapy: Pharmacology and immunoconjugates with drugs and nanoparticles. Int. J. Pharm. 2021, 592, 120082. [Google Scholar] [CrossRef]
- Gu, L.; Nawaz, T.; Qiu, Y.; Wu, Y.; Zhou, R. Photosynthetic conversion of CO2 and H2O to long-chain terpene alcohol by genetically engineered N2-fixing cyanobacteria. In Photosynthesis; Academic Press: Cambridge, MA, USA, 2023; pp. 451–461. [Google Scholar] [CrossRef]
- Flores, E.; Herrero, A. Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat. Rev. Microbiol. 2010, 8, 39–50. [Google Scholar] [CrossRef]
- Kieninger, A.K.; Maldener, I. Cell–cell communication through septal junctions in filamentous cyanobacteria. Curr. Opin. Microbiol. 2021, 61, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M.; Brown, A.C. Bacterial outer membrane vesicles as antibiotic delivery vehicles. Front. Immunol. 2021, 12, 733064. [Google Scholar] [CrossRef] [PubMed]
- Biller, S.J.; McDaniel, L.D.; Breitbart, M.; Rogers, E.; Paul, J.H.; Chisholm, S.W. Membrane vesicles in sea water: Heterogeneous DNA content and implications for viral abundance estimates. ISME J. 2017, 11, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Naz, T.; Iqbal, M.M.; Fahad, S.; Akhtar, J.; Saqib, M.; Alamri, S.; Siddiqui, M.H.; Saud, S.; Khattak, J.Z.K.; Ali, S.; et al. Bio-fortification of Two Wheat Cultivars with Iron and Zinc Through Their Soil and Foliar Application in Salt-Factored Soil: Growth, Ionic, Physiological, and Biochemical Modifications. J. Plant Growth Regul. 2023, 42, 5727–5745. [Google Scholar] [CrossRef]
- Lea-Smith, D.J.; Ross, N.; Zori, M.; Bendall, D.S.; Dennis, J.S.; Scott, S.A.; Smith, A.G.; Howe, C.J. Thylakoid terminal oxidases are essential for the cyanobacterium Synechocystis sp. PCC 6803 to survive rapidly changing light intensities. Plant Physiol. 2013, 162, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Patterson-Fortin, L.M. LexA-Mediated Repression of the Cyanobacterial RNA Helicase, crhR; University of Alberta: Edmonton, AL, Canada, 2008. [Google Scholar] [CrossRef]
- Rippka, R.; Waterbury, J.B.; Stanier, R.Y. Provisional generic assignments for cyanobacteria in pure culture. In The Prokaryotes: A Handbook on Habitats, Isolation, and Identification of Bacteria; Springer: Berlin/Heidelberg, Germany, 1981; pp. 247–256. [Google Scholar] [CrossRef]
- Houle, D.; Bilodeau Gauthier, S.; Paquet, S.; Planas, D.; Warren, A. Identification of two genera of N2-fixing cyanobacteria growing on three feather moss species in boreal forests of Quebec, Canada. Botany 2006, 84, 1025–1029. [Google Scholar] [CrossRef]
- Meeks, J.C. Physiological adaptations in nitrogen-fixing Nostoc–plant symbiotic associations. In Prokaryotic Symbionts in Plants; Springer: Berlin/Heidelberg, Germany, 2007; pp. 181–205. [Google Scholar] [CrossRef]
- Arróniz-Crespo, M.; Pérez-Ortega, S.; De Los Ríos, A.; Green, T.G.; Ochoa-Hueso, R.; Casermeiro, M.Á.; de la Cruz, M.T.; Pintado, A.; Palacios, D.; Rozzi, R.; et al. Bryophyte-cyanobacteria associations during primary succession in recently Deglaciated areas of Tierra del Fuego (Chile). PLoS ONE 2014, 9, e96081. [Google Scholar] [CrossRef]
- Warshan, D.; Liaimer, A.; Pederson, E.; Kim, S.Y.; Shapiro, N.; Woyke, T.; Altermark, B.; Pawlowski, K.; Weyman, P.D.; Dupont, C.L.; et al. Genomic changes associated with the evolutionary transitions of Nostoc to a plant symbiont. Mol. Biol. Evol. 2018, 35, 1160–1175. [Google Scholar] [CrossRef]
- West, N.J.; Adams, D.G. Phenotypic and genotypic comparison of symbiotic and free-living cyanobacteria from a single field site. Appl. Environ. Microbiol. 1997, 63, 4479–4484. [Google Scholar] [CrossRef]
- Gentili, F.; Nilsson, M.C.; Zackrisson, O.; DeLuca, T.H.; Sellstedt, A. Physiological and molecular diversity of feather moss associative N2-fixing cyanobacteria. J. Exp. Bot. 2005, 56, 3121–3127. [Google Scholar] [CrossRef]
- Shakoor, N.; Hussain, M.; Adeel, M.; Azeem, I.; Ahmad, M.A.; Zain, M.; Zhang, P.; Li, Y.; Quanlong, W.; Horton, R.; et al. Lithium-induced alterations in soybean nodulation and nitrogen fixation through multifunctional mechanisms. Sci. Total Environ. 2023, 904, 166438. [Google Scholar] [CrossRef] [PubMed]
- Rousk, K.; Jones, D.L.; Deluca, T.H. Moss-cyanobacteria associations as biogenic sources of nitrogen in boreal forest ecosystems. Front. Microbiol. 2013, 4, 150. [Google Scholar] [CrossRef] [PubMed]
- van den Elzen, E.; Bengtsson, F.; Fritz, C.; Rydin, H.; Lamers, L.P. Variation in symbiotic N2 fixation rates among Sphagnum mosses. PLoS ONE 2020, 15, e0228383. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, M.L.; Nusinow, D.A. Time will tell: Intercellular communication in the plant clock. Trends Plant Sci. 2021, 26, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Regot, S.; Macia, J.; Conde, N.; Furukawa, K.; Kjellén, J.; Peeters, T.; Hohmann, S.; De Nadal, E.; Posas, F.; Solé, R. Distributed biological computation with multicellular engineered networks. Nature 2011, 469, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Habibullah, G.; Viktorova, J.; Ruml, T. Current strategies for noble metal nanoparticle synthesis. Nanoscale Res. Lett. 2021, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.; Prakash, R.; Rai, M.P. Latest advances and status analysis of nanomaterials for microalgae photosystem, lipids and biodiesel: A state of art. J. Environ. Chem. Eng. 2022, 11, 109111. [Google Scholar] [CrossRef]
- Scharwies, J.D.; Dinneny, J.R. Water transport, perception, and response in plants. J. Plant Res. 2019, 132, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Leyser, O. Auxin, self-organisation, and the colonial nature of plants. Curr. Biol. 2011, 21, R331–R337. [Google Scholar] [CrossRef]
- Singh, S.; Bhoi, T.K.; Vyas, V. Interceding Microbial Biofertilizers in Agroforestry System for Enhancing Productivity. In Plant Growth Promoting Microorganisms of Arid Region; Springer: Singapore, 2023; pp. 161–183. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.K.; Zhou, Y.; Wang, J.; Jiang, Y.; Shen, N.; Wang, Y.; Yang, L.; Jiang, M. Unlocking the strength of plant growth promoting Pseudomonas in improving crop productivity in normal and challenging environments: A review. J. Plant Interact. 2022, 17, 220–238. [Google Scholar] [CrossRef]
- Abdellatif, A.A.; Khan, R.A.; Alhowail, A.H.; Alqasoumi, A.; Sajid, S.M.; Mohammed, A.M.; Alsharidah, M.; Al Rugaie, O.; Mousa, A.M. Octreotide-conjugated silver nanoparticles for active targeting of somatostatin receptors and their application in a nebulized rat model. Nanotechnol. Rev. 2021, 11, 266–283. [Google Scholar] [CrossRef]
- Bouallegui, Y.; Ben Younes, R.; Oueslati, R.; Sheehan, D. Redox proteomic insights into involvement of clathrin-mediated endocytosis in silver nanoparticles toxicity to Mytilus galloprovincialis. PLoS ONE 2018, 13, e0205765. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M.; et al. Nanotoxicology and Nanosafety: Safety-By-Design and Testing at a Glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In Vitro Methods for Assessing Nanoparticle Toxicity. Methods Mol. Biol. 2019, 1894, 1–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drasler, B.; Sayre, P.; Steinhäuser, K.G.; Petri-Fink, A.; Rothen-Rutishauser, B. In vitro approaches to assess the hazard of nanomaterials. NanoImpact 2017, 8, 99–116. [Google Scholar] [CrossRef]
- Li, J.; Hu, C.; Liu, B.; Liu, Z. Dual pathway reduction of Mo4+ and photogenerated electrons restore catalytic sites to enhance heterogeneous peroxymonosulfate activation system. Chem. Eng. J. 2023, 452, 139246, ISSN 1385-8947. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Liang, C.; Duan, L. Novel technology for synergistic SO2 reduction during the carbonation process via a CaO-Char mixed system. Chem. Eng. J. 2024, 488, 150678, ISSN 1385-8947. [Google Scholar] [CrossRef]
- Chen, X.; Lu, R.; Liu, P.; Li, X. Effects of nano-TiO2 on Chlamydomonas reinhardtii cell surface under UV, natural light conditions. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 217–222. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, G.; Sabella, S.; Sorce, B.; Brunetti, V.; Malvindi, M.A.; Cingolani, R.; Pompa, P.P. Effects of cell culture media on the dynamic formation of protein-nanoparticle complexes and influence on the cellular response. ACS Nano 2010, 47, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chang, Y.; Chen, Y. Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere 2010, 78, 209–215. [Google Scholar] [CrossRef]
- Wang, H.; Wick, R.L.; Xing, B. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ. Pollut. 2009, 157, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Long, T.C.; Saleh, N.; Tilton, R.D.; Lowry, G.V.; Veronesi, B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): Implications for nanoparticle neurotoxicity. Environ. Sci. Technol. 2006, 40, 4346–4352. [Google Scholar] [CrossRef]
- Lynch, I.; Cedervall, T.; Lundqvist, M.; Cabaleiro-Lago, C.; Linse, S.; Dawson, K.A. The nanoparticle–protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv. Colloid Interface Sci. 2007, 134, 167–174. [Google Scholar] [CrossRef]
- Hillegass, J.M.; Shukla, A.; Lathrop, S.A.; MacPherson, M.B.; Fukagawa, N.K.; Mossman, B.T. Assessing nanotoxicity in cells in vitro. Wiley Interdisciplinary Reviews: Nanomed. Nanobiotechnology 2010, 2, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Simon-Deckers, A.; Loo, S.; Mayne-L’hermite, M.; Herlin-Boime, N.; Menguy, N.; Reynaud, C.; Gouget, B.; Carrière, M. Size, composition and shape dependent toxicological impact of metal oxide nanoparticles and carbon nanotubes toward bacteria. Environ. Sci. Technol. 2009, 43, 8423–8429. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Azameti, M.K.; Imoro, A.W. Nanotechnology: A promising field in enhancing abiotic stress tolerance in plants. Crop Des. 2023, 28, 100037. [Google Scholar] [CrossRef]
- Allouzi, M.M.; Tang, D.Y.; Chew, K.W.; Rinklebe, J.; Bolan, N.; Allouzi, S.M.; Show, P.L. Micro (nano) plastic pollution: The ecological influence on soil-plant system and human health. Sci. Total Environ. 2021, 20, 147815. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Wang, L.; Rojanasakul, Y. The effects of carbon nanotubes on lung and dermal cellular behaviors. Nanomedicine 2014, 9, 895–912. [Google Scholar] [CrossRef] [PubMed]
- Simon-Deckers, A.; Gouget, B.; Mayne-L’Hermite, M.; Herlin-Boime, N.; Reynaud, C.; Carriere, M. In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology 2008, 253, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Vinson, D.; Abt, D.; Hurt, R.H.; Rand, D.M. Differential toxicity of carbon nanomaterials in Drosophila: Larval dietary uptake is benign, but adult exposure causes locomotor impairment and mortality. Environ. Sci. Technol. 2009, 43, 6357–6363. [Google Scholar] [CrossRef]
- Petersen, E.J.; Henry, T.B.; Zhao, J.; MacCuspie, R.I.; Kirschling, T.L.; Dobrovolskaia, M.A.; Hackley, V.; Xing, B.; White, J.C. Identification and avoidance of potential artifacts and misinterpretations in nanomaterial ecotoxicity measurements. Environ. Sci. Technol. 2014, 48, 4226–4246. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Brunet, L.; Hinkal, G.W.; Wiesner, M.R.; Alvarez, P.J. Antibacterial activity of fullerene water suspensions (nC60) is not due to ROS-mediated damage. Nano Lett. 2008, 8, 1539–1543. [Google Scholar] [CrossRef]
- Liu, J.; Aruguete, D.M.; Murayama, M.; Hochella, M.F., Jr. Influence of size and aggregation on the reactivity of an environmentally and industrially relevant nanomaterial (PbS). Environ. Sci. Technol. 2009, 43, 8178–8183. [Google Scholar] [CrossRef]
- Carvalho, L.Â.; Oya-Silva, L.F.; Perussolo, M.C.; Guaita, G.O.; Brito, J.C.; Evans, A.A.; Prodocimo, M.M.; Cestari, M.M.; Braga, T.T.; de Assis, H.C. Experimentally exposed toxic effects of long-term exposure to environmentally relevant concentrations of CIP in males and females of the silver catfish Rhamdia quelen. Chemosphere 2023, 336, 139216. [Google Scholar] [CrossRef]
- Kent, R.D.; Vikesland, P.J. Dissolution and persistence of copper-based nanomaterials in undersaturated solutions with respect to cupric solid phases. Environ. Sci. Technol. 2016, 50, 6772–6781. [Google Scholar] [CrossRef]
- Schmidt, S.N.; Mayer, P. Linking algal growth inhibition to chemical activity: Baseline toxicity required 1% of saturation. Chemosphere 2015, 120, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 213902. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.S.; Koh, D.C.; Kong, I.C. Toxicity evaluation of individual and mixtures of nanoparticles based on algal chlorophyll content and cell count. Materials 2018, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Madler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Blinova, I.; Ivask, A.; Heinlaan, M.; Mortimer, M.; Kahru, A. Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ. Pollut. 2010, 158, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Thill, A.; Zeyons, O.; Spalla, O.; Chauvat, F.; Rose, J.; Auffan, M.; Flank, A.M. Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Sci. Total Environ. 2006, 40, 6151–6156. [Google Scholar] [CrossRef] [PubMed]
- Keenan, C.R.; Goth-Goldstein, R.; Lucas, D.; Sedlak, D.L. Oxidative stress induced by zero-valent iron nanoparticles and Fe (II) in human bronchial epithelial cells. Environ. Sci. Technol. 2009, 43, 4555–4560. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano-and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In Vitro 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Kawata, K.; Osawa, M.; Okabe, S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environ. Sci. Technol. 2009, 43, 6046–6051. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Greden, K.; Alvarez, P.J.; Gregory, K.B.; Lowry, G.V. Adsorbed polymer and NOM limits adhesion and toxicity of nano scale zerovalent iron to E. coli. Environ. Sci. Technol. 2010, 44, 3462–3467. [Google Scholar] [CrossRef]
- Stebounova, L.V.; Adamcakova-Dodd, A.; Kim, J.S.; Park, H.; O’Shaughnessy, P.T.; Grassian, V.H.; Thorne, P.S. Nanosilver induces minimal lung toxicity or inflammation in a subacute murine inhalation model. Part. Fibre Toxicol. 2011, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.Y.; Byeon, J.H.; Park, J.H.; Hwang, J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 2007, 373, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Fujioka, K.; Oku, T.; Suga, M.; Sasaki, Y.F.; Ohta, T.; Yasuhara, M.; Suzuki, K.; Yamamoto, K. Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett. 2004, 4, 2163–2169. [Google Scholar] [CrossRef]
- Hussain, S.M.; Javorina, A.K.; Schrand, A.M.; Duhart, H.M.; Ali, S.F.; Schlager, J.J. The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol. Sci. 2006, 92, 456–463. [Google Scholar] [CrossRef]
- Jiang, W.; Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Braydich-Stolle, L.K.; Speshock, J.L.; Castle, A.; Smith, M.; Murdock, R.C.; Hussain, S.M. Nanosized aluminum altered immune function. ACS Nano 2010, 4, 3661–3670. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- De La Torre-Roche, R.; Hawthorne, J.; Deng, Y.; Xing, B.; Cai, W.; Newman, L.A.; Wang, Q.; Ma, X.; Hamdi, H.; White, J.C. Multiwalled carbon nanotubes and C60 fullerenes differentially impact the accumulation of weathered pesticides in four agricultural plants. Environ. Sci. Technol. 2013, 47, 12539–12547. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers pathways and processes for uptake, translocation and accumulation of nanomaterials in plants–Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef]
- Sanati, M.; Afshari, A.R.; Kesharwani, P.; Sukhorukov, V.N.; Sahebkar, A. Recent trends in the application of nanoparticles in cancer therapy: The involvement of oxidative stress. J. Control. Release 2022, 348, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants and animals. Comp. Biochem. Physiol. Part C Toxicol. Appl. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Krumova, K.; Cosa, G. Overview of Reactive Oxygen Species; The Royal Society of Chemistry: London, UK, 2016; pp. 1–21. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Soares, C.; Sousa, B.; Martins, M.; Kumar, V.; Shahzad, B.; Sidhu, G.P.; Bali, A.S.; Asgher, M.; Bhardwaj, R.; et al. Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: A review on molecular and biochemical aspects. Physiol. Plant. 2020, 168, 318–344. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Clague, L.A. Comparative Genomics and Transcriptomics of Steinernema carpocapsae and Caenorhabditis elegans; University of California: Irvine, CA, USA, 2020. [Google Scholar]
- Nawaz, T.; Gu, L.; Fahad, S.; Saud, S.; Jiang, Z.; Hassan, S.; Harrison, M.Y.; Liu, K.; Khan, M.A.; Liu, K.; et al. A comprehensive review of the therapeutic potential of cyanobacterial marine bioactives: Unveiling the hidden treasures of the sea. Food Energy Secur. 2023, 12, e495. [Google Scholar] [CrossRef]
- Vurro, M.; Miguel-Rojas, C.; Pérez-de-Luque, A. Safe nanotechnologies for increasing the effectiveness of environmentally friendly natural agrochemicals. Pest Manag. Sci. 2019, 75, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Mani, P.K.; Mondal, S. Agri-nanotechniques for plant availability of nutrients. In Plant Nanotechnology: Principles and Practices; Springer: Berlin/Heidelberg, Germany, 2016; pp. 263–303. [Google Scholar] [CrossRef]
- Busby, P.E.; Soman, C.; Wagner, M.R.; Friesen, M.L.; Kremer, J.; Bennett, A.; Morsy, M.; Eisen, J.A.; Leach, J.E.; Dangl, J.L. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017, 15, e2001793. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, A.; Hoshino, A.; Hanaki, K.I.; Suzuki, K.; Yamamoto, K. On the cyto-toxicity caused by quantum dots. Microbiol. Immunol. 2004, 48, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Bao, M.; Wang, B.; Wu, S.; Luo, L.; Li, B.; Lin, J. Inhibitory effects of Cu2O/SiO2 on the growth of Microcystis aeruginosa and its mechanism. Nanomaterials 2019, 9, 1669. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, I.R.; Olsson, A.L.; Tufenkji, N. Deposition kinetics of quantum dots and polystyrene latex nanoparticles onto alumina: Role of water chemistry and particle coating. Environ. Sci. Technol. 2013, 47, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Frey-Klett, P.; Burlinson, P.; Deveau, A.; Barret, M.; Tarkka, M.; Sarniguet, A. Bacterial-fungal interactions: Hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol. Mol. Biol. 2011, 75, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Basir, A.; Adnan, M.; Fahad, S.; Ali, J.; Mussart, M.; Mian, I.A.; Ahmad, M.; Saleem, M.H.; Naseem, W.; et al. Biochar to Improve Crops Yield and Quality Under a Changing Climate. In Sustainable Agriculture Reviews 61: Biochar to Improve Crop Production and Decrease Plant Stress under a Changing Climate; Springer International Publishing: Cham, Switzerland, 2023; pp. 57–73. [Google Scholar] [CrossRef]
- Bityutsky, V.S.; Tsekhmistrenko, S.I.; Tsekhmistrenko, O.S.; Tymoshok, N.O.; Spivak, M.Y. Regulation of redox processes in biological systems with the participation of the Keap1/Nrf2/ARE signaling pathway, biogenic selenium nanoparticles as Nrf2 activators. Regul. Mech. Biosyst. 2020, 11, 483–493. [Google Scholar] [CrossRef]
- Abideen, Z.; Hanif, M.; Munir, N.; Nielsen, B.L. Impact of nanomaterials on the regulation of gene expression and metabolomics of plants under salt stress. Plants 2022, 11, 691. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Wu, F.; Tang, M. Comparative photochemistry activity and antioxidant responses in male and female Populus cathayana cuttings inoculated with arbuscular mycorrhizal fungi under salt. Sci. Rep. 2016, 6, 37663. [Google Scholar] [CrossRef]
- Atkinson, J.T. Controlling Bioenergetic Systems Using Protein Design and Synthetic Biology. Ph.D. Thesis, Rice University, Houston, TX, USA, 2019. [Google Scholar]
- Shah, S.; Chen, C.; Sun, Y.; Wang, D.; Nawaz, T.; El-Kahtany, K.; Fahad, S. Mechanisms of nitric oxide involvement in plant-microbe interaction and its enhancement of stress resistance. Plant Stress 2023, 2, 100191. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Szufa, S.; Grzesik, M.; Piotrowski, K.; Janas, R. The promotive effect of cyanobacteria and Chlorella sp. foliar biofertilization on growth and metabolic activities of willow (Salix viminalis L.) plants as feedstock production, solid biofuel and biochar as C carrier for fertilizers via torrefaction process. Energies 2021, 14, 5262. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Los, D.A.; Schmitt, F.J.; Zharmukhamedov, S.K.; Kuznetsov, V.V.; Allakhverdiev, S.I. The impact of the phytochromes on photosynthetic processes. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 400–408. [Google Scholar] [CrossRef]
- Lin, W.R.; Tan, S.I.; Hsiang, C.C.; Sung, P.K.; Ng, I.S. Challenges and opportunity of recent genome editing and multi-omics in cyanobacteria and microalgae for biorefinery. Bioresour. Technol. 2019, 291, 121932. [Google Scholar] [CrossRef]
- Santini, G.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Plant biostimulants from cyanobacteria: An emerging strategy to improve yields and sustainability in agriculture. Plants 2021, 10, 643. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Lu, X. Engineering cyanobacteria to improve photosynthetic production of alka (e) nes. Biotechnol. Biofuels 2013, 6, 69. [Google Scholar] [CrossRef]
- Bombar, D.; Heller, P.; Sanchez-Baracaldo, P.; Carter, B.J.; Zehr, J.P. Comparative genomics reveals surprising divergence of two closely related strains of uncultivated UCYN-A cyanobacteria. ISME J. 2014, 8, 2530–2542. [Google Scholar] [CrossRef]
- Rogers, E.D.; Benfey, P.N. Regulation of plant root system architecture: Implications for crop advancement. Curr. Opin. Biotechnol. 2015, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant Root Growth, Architecture and Function; Springer: Berlin/Heidelberg, Germany, 2009; pp. 153–187. [Google Scholar] [CrossRef]
- Derakhshan Nejad, Z.; Jung, M.C.; Kim, K.H. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ. Geochem. Health 2018, 40, 927–953. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Chen, H.; Sarsaiya, S.; Qin, S.; Liu, H.; Awasthi, M.K.; Kumar, S.; Singh, L.; Zhang, Z.; Bolan, N.S.; et al. Current research trends on micro-and nano-plastics as an emerging threat to global environment: A review. Hazard. Mater. 2021, 409, 124967. [Google Scholar] [CrossRef]
- Chouhan, G.K.; Verma, J.P.; Jaiswal, D.K.; Mukherjee, A.; Singh, S.; de Araujo Pereira, A.P.; Liu, H.; Abd_Allah, E.F.; Singh, B.K. Phytomicrobiome for promoting sustainable agriculture and food security: Opportunities, challenges, and solutions. Microbiol. Res. 2021, 248, 126763. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. New roots for agriculture: Exploiting the root phenome. Philos. Trans. R. Soc. B 2012, 367, 1598–1604. [Google Scholar] [CrossRef] [PubMed]
- Shenton, M.; Iwamoto, C.; Kurata, N.; Ikeo, K. Effect of wild and cultivated rice genotypes on rhizosphere bacterial community composition. Rice 2016, 9, 42. [Google Scholar] [CrossRef]
- Szoboszlay, M.; Lambers, J.; Chappell, J.; Kupper, J.V.; Moe, L.A.; McNear, D.H., Jr. Comparison of root system architecture and rhizosphere microbial communities of Balsas teosinte and domesticated corn cultivars. Soil Biol. Biochem. 2015, 80, 34–44. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Mazza Rodrigues, J.L.; Brisson, V.L.; Kent, A.; Gaudin, A.C.M. Impacts of directed evolution and soil management legacy on the maize rhizobiome. Soil Biol. Biochem. 2020, 145, 107794. [Google Scholar] [CrossRef]
- Saleem, M.; Law, A.D.; Sahib, M.R.; Pervaiz, Z.H.; Zhang, Q. Impact of root system architecture on rhizosphere and root microbiome. Rhizosphere 2018, 6, 47–51. [Google Scholar] [CrossRef]
- Corneo, P.E.; Suenaga, H.; Kertesz, M.A.; Dijkstra, F.A. Effect of twenty-four wheat genotypes on soil biochemical and microbial properties. Plant Soil 2016, 404, 141–155. [Google Scholar] [CrossRef]
- Robertson-Albertyn, S.; Alegria Terrazas, R.; Balbirnie, K.; Blank, M.; Janiak, A.; Szarejko, I.; Chmielewska, B.; Karcz, J.; Morris, J.; Hedley, P.E.; et al. Root hair mutations displace the barley rhizosphere microbiota. Front. Plant Sci. 2017, 8, 1094. [Google Scholar] [CrossRef]
- Pérez-Jaramillo, J.E.; Carrión, V.J.; Bosse, M.; Ferrão, L.F.V.; de Hollander, M.; Garcia, A.A.F.; Ramírez, C.A.; Mendes, R.; Raaijmakers, J.M. Linking rhizosphere microbiome composition of wild and domesticated Phaseolus vulgaris to genotypic and root phenotypic traits. ISME J. 2017, 11, 2244–2257. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.; Kumari, A.; Shende, S.S.; Ranjan, A.; Faizan, M.; Barakvov, A.; Gromovik, A.; Gorbunova, N.; Rajput, P.; et al. A review on nanobioremediation approaches for restoration of contaminated soil. Eurasian J. Soil Sci. 2022, 11, 43–60. [Google Scholar] [CrossRef]
- Sharma, N.; Kumari, R.M.; Gupta, N.; Syed, A.; Bahkali, A.H.; Nimesh, S. Poly-(lactic-co-glycolic) acid nanoparticles for synergistic delivery of epirubicin and paclitaxel to human lung cancer cells. Molecules 2020, 25, 4243. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, Z.; Liu, Y.; Cao, T.; Zhang, H.; Hao, M.; Chen, R.; Dzakpasu, M.; Wang, X.C. Phytoremediation mechanisms and plant eco-physiological response to microorganic contaminants in integrated vertical-flow constructed wetlands. J. Hazard. Mater. 2022, 424, 127611. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Bhuyan, S.; Chowdhury, R.; Sarma, R.; Roy, S.; Neog, P.R. Nanoremediation strategies to address environmental problems. Sci. Total Environ. 2023, 10, 163998. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Afridi, M.S.; Javed, M.A.; Saleem, A.; Hafeez, A.; Khan, A.R.; Zeeshan, M.; Ali, B.; Azhar, W.; Sumaira; et al. Nano-priming against abiotic stress: A way forward towards sustainable agriculture. Sustainability 2022, 14, 14880. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A. Multifunctional hybrid nanomaterials for sustainable agri-food and ecosystems: A note from the editor. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Li, M.; Sun, X.; Yin, M.; Shen, J.; Yan, S. Recent advances in nanoparticle-mediated co-delivery system: A promising strategy in medical and Agricultural Field. Int. J. Mol. Sci. 2023, 24, 5121. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Golińska, P. Myconanotechnology Emerging Trends and Applications; Routledge: London, UK, 2023. [Google Scholar]
- Agarwal, P.; Soni, R.; Kaur, P.; Madan, A.; Mishra, R.; Pandey, J.; Singh, S.; Singh, G. Cyanobacteria as a promising alternative for sustainable environment: Synthesis of biofuel and biodegradable plastics. Front. Microbiol. 2022, 13, 939347. [Google Scholar] [CrossRef] [PubMed]
- Kothari, R.; Ahmad, S.; Pathak, V.V.; Pandey, A.; Kumar, A.; Shankarayan, R.; Black, P.N.; Tyagi, V.V. Algal-based biofuel generation through flue gas and wastewater utilization: A sustainable prospective approach. Biomass Convers. Biorefin. 2021, 11, 1419–1442. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Singh, M. Cyanobacteria: A sustainable and commercial bio-resource in production of bio-fertilizer and bio-fuel from waste waters. Environ. Sustain. 2019, 3, 100008. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef]
- Cao, T.N.; Mukhtar, H.; Le, L.T.; Tran, D.P.; Ngo, M.T.; Nguyen, T.B.; Bui, X.T. Roles of microalgae-based biofertilizer in sustainability of green agriculture and food-water-energy security nexus. Sci. Total Environ. 2023, 870, 161927. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Rajakumar, G.; Chung, I.M. Nanotechnology: Current uses and future applications in the food industry. 3 Biotech 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Litter, M.I.; Ahmad, A. (Eds.) Industrial Applications of Nanoparticles; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Hamida, R.S.; Ali, M.A.; Redhwan, A.; Bin-Meferij, M.M. Cyanobacteria—A promising platform in green nanotechnology: A review on nanoparticles fabrication and their prospective applications. Int. J. Nanomed. 2020, 13, 6033–6066. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Wang, Y.; Qin, Y.X. Promoting neuroregeneration by applying dynamic magnetic fields to a novel nanomedicine: Superparamagnetic iron oxide (SPIO)-gold nanoparticles bounded with nerve growth factor (NGF). Nanomed. Nanotechnol. Biol. 2018, 14, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Costantini, M.; Pal, S.; Kumar, A. Oxygenation therapies for improved wound healing: Current trends and technologies. J. Mater. Chem. B. 2022, 10, 7905–7923. [Google Scholar] [CrossRef] [PubMed]
- Smith, O.M.; Cohen, A.L.; Reganold, J.P.; Jones, M.S.; Orpet, R.J.; Taylor, J.M.; Thurman, J.H.; Cornell, K.A.; Olsson, R.L.; Ge, Y.; et al. Landscape context affects the sustainability of organic farming systems. Proc. Natl. Acad. Sci. USA 2020, 117, 2870–2878. [Google Scholar] [CrossRef]
- Rosenhauer, A.M.; Beach, L.Q.; Jeffress, E.C.; Thompson, B.M.; McCann, K.E.; Partrick, K.A.; Diaz, B.; Norvelle, A.; Choi, D.C.; Huhman, K.L. Brain-derived neurotrophic factor signaling mitigates the impact of acute social stress. Neuropharmacology 2019, 148, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour Adeh, E.; Selker, J.S.; Higgins, C.W. Remarkable agrivoltaic influence on soil moisture, micrometeorology and water-use efficiency. PLoS ONE 2018, 13, e0203256. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Brestic, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Raliya, R.; Biswas, P.; Tarafdar, J.C. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol. Rep. 2015, 5, 22–26. [Google Scholar] [CrossRef]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Vyas, P.; Dhaliwal, H.S.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. Biocatal. Agric. Biotechnol. 2020, 23, 101487. [Google Scholar] [CrossRef]
- Raimi, A.; Adeleke, R.; Roopnarain, A. Soil fertility challenges and Biofertiliser as a viable alternative for increasing smallholder farmer crop productivity in sub-Saharan Africa. Cogent Food Agric. 2017, 3, 1400933. [Google Scholar] [CrossRef]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical fertilizers and their impact on soil health. Microbiota and Biofertilizers, Volume 2: Ecofriendly Tools for Reclamation of Degraded Soil Environs; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–20. [Google Scholar] [CrossRef]
- Rajput, V.D.; Kumari, A.; Upadhyay, S.K.; Minkina, T.; Mandzhieva, S.; Ranjan, A.; Sushkova, S.; Burachevskaya, M.; Rajput, P.; Konstantinova, E.; et al. Can nanomaterials improve the soil microbiome and crop productivity? Agriculture 2023, 13, 231. [Google Scholar] [CrossRef]
- Gebbers, R.; Adamchuk, V.I. Precision agriculture and food security. Science 2010, 327, 828–831. [Google Scholar] [CrossRef] [PubMed]

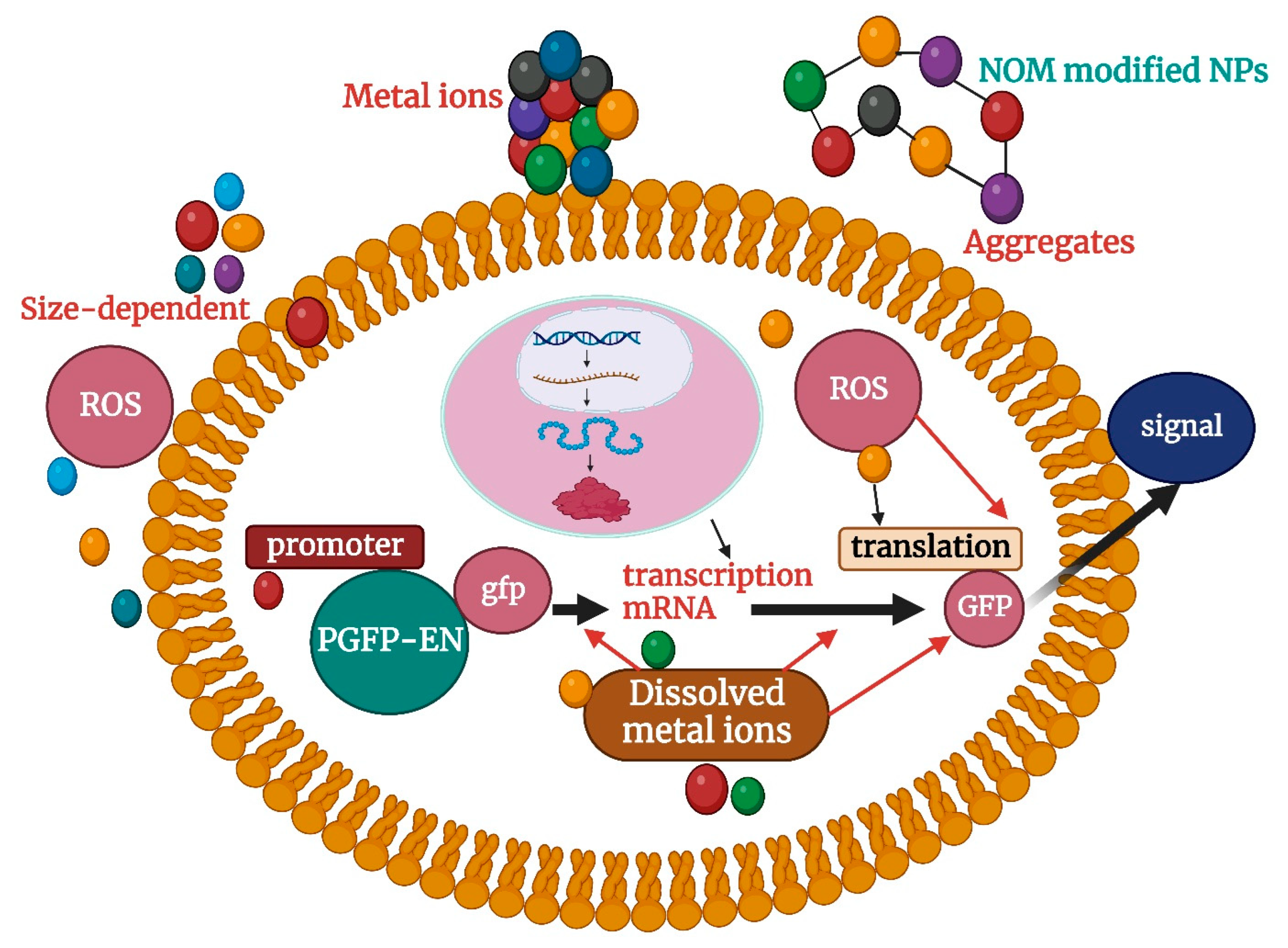

| Kinds of NPs | Tested Objects | Toxicological Effects | References |
|---|---|---|---|
| nanoparticle containing carbon | human lung epithelial cells A549 | inhibition 22% (40 g/cm2 exposure 18 h); 15% DNA damage (40 µg/cm2 exposure 4 h) | [134] |
| alveolar macrophage | (single-walled) inhibition 20% (1.41 µg/cm2′ exposure 6 h), (multi-walled) inhibition 14% (22 µg/cm2 exposure 6 h) | [135] | |
| human epidermal keratinocytes (HEKs) | (multi-walled) altered the expression of 36 proteins (exposure 24 h): altered 106 (exposure 48 h) | [136] | |
| Cupriavidus metallidurans CH34 | CNT are internalized (10 µg/mL exposure 24 h) | [137] | |
| Escherichia coli MG1655 | CNT are internalized (10 µg/mL exposure 24 h) | [137] | |
| Drosophila melanogaster | strongly adhering CB significantly reduced survivorship | [138] | |
| adult oysters | 40% cell damage (100 µg/L, exposure 4 days) | [139] | |
| Crassostrea virginica | embryonic development and lysosomal destabilization (10 µg/L exposure 24 h) | [140] | |
| Daphnia: fathead minnow | THF-nCgo-exposed fish 100% mortality (6 and 18 h), water-46 stirred-nC60-exposed fish no effects (48 h). However significantly increased expression of CYP2 family isozymes in liver | [125] | |
| Escherichia coli | attributed to photocatalytically generated ROS. Exerts ROS-independent oxidative stress (10 mg/L exposure 15 min) | [140] | |
| metal oxide | Drosophila melanogaster | less adhering was indistinguishable from unexposed control | [141] |
| human lung epithelial cells A549 | DNA damaged 19% (40 µg/cm2 exposure 4 h); | [134] | |
| Daphnia magna | 19/25 acute toxicity EC50 > 100 mg/L (exposure 48 h) | [142] | |
| Chlamydomonas reinhardtii | transient up-regulation of genes as low as 1.0 mg/L: Sodl.gpx, cat and ptox2 (exposure 6 h); | [137] | |
| Caenorhabditis elegans | LC50 80 mg/L (exposure 24 h) | [138] | |
| Cupriavidus metallidurans CH34 | cell internalized and induced significant ROS production at 500 mg/L; | [137] | |
| Escherichia coli MG1655 | cell internalized and induced significant ROS production at 500 mg/L; | [137] | |
| brain microglia (BV2) | produced ROS; neurotoxicity (2.5–120 mg/L exposure 1, 6, 18 h) | [127] | |
| Bacillus subtilis: Escherichia coli | low toxicity on the three tested bacteria (20 mg/L exposure 24 h | [140] | |
| Pseudomonas fluorescens | inhibition ratio 38% (40 g/cm2 exposure 18 h), DNA damaged 12% (exposure 4 h) | [134] | |
| human lung epithelial cells A549 | LC50 (2.3 mg/L exposure 4 h) | [143] | |
| Caenorhabditis elegans RAW 264.7 and BEAS-2B | generated ROS, inflammation, cell death (25 pg/mL exposure1–16 h) | [144] | |
| Daphnia magna | acute toxicity; EC50 = lmg/L (exposure 48 h) | [144] | |
| Caenorhabditis elegans | LC50 2.3 mg/L (exposure 24 h) | [145] | |
| Escherichia coli; Staphylococcus aureus | inhibition concentrations > 3.4 mM | [146] | |
| Bacillus subtilis: Escherichia coli: Pseudomonas fluorescens | inhibited concentration > lmM | [147] | |
| Staphylococcus aureus | causing 100% mortality to the three tested bacteria (20 mg/L exposure 24 h). | [146] | |
| Daphniamagna; Thamnocephalusplatyurus; Tetrahymena thermophila | L (E) C50 values 1.1–16 gm/L | [147] | |
| RAW 264.7 and BEAS-2B | inhibit ROS generation, resist oxidative stress, no inflammation and cell death (25 µg/mL exposure 1–16 h) | [147] | |
| Escherichia coli | no survival above 230 mg/L; 90% survival rate (exposure 330.9 mg/L) | [148] | |
| human lung epithelial cells A549 | 40 pg/cm′ exposure 18 h inhibition 96%, after exposure 4 h DNA damaged 41% | [134] | |
| Daphnia magna; Thamnocephalus platyurus: Tetrahymena thermophila | L(E)C50 values 90–224 µg | [149] | |
| human lung epithelial cells A549 | catalytic effects with ROS generating (100–200 µM exposure 60 min) | [150] | |
| Caenorhabditis elegans | LC50 at 82 mg/L (exposure 24 h) | [145] | |
| Cupriavidus metallidurans CH34 | cell internalized and induced a drastic increase in ROS level (2 h); | [145] | |
| Escherichia coli MG1655 | cell internalized and induced a drastic increase in ROS level (2 h); | [145] | |
| Bacillus subtilis; Escherichia coli; Pseudomonas fluorescens | inhibited 57% B. subtilis, 36% E. coli, 70% P. fluorescens (20 mg/L exposure 24 h) | [151] | |
| pure metal | PC-12 cells | produce cell shrinkage, irregular membrane (5–50 g/mL exposure 24 h) | [151] |
| rat liver cells BRL3A | mitochondrial perturbation (5–50 g/µL exposure 24 h) | [151] | |
| Escherichia coli | 40 nm susceptibility 0.0236 mL/µg | [152] | |
| Bacillus subtilis | 40 nm susceptibility 0.0622 mL/µg | [153] | |
| Escherichia coli ATCC 10536 | shape dependent toxicity (1–100 g exposure 24 h) | [154] | |
| Chlamydomonas reinhardtii | time-dependent toxicity; NP Ag appeared to be higher than AgNO ECg3 300: 572 nM (1 h); 1 049 ± 396 nM (2 h) | [155] | |
| HepG2 human hepatoma cells | accelerated cell proliferation at low dose (0.5 mg/L exposure 24 h | [156] | |
| Zebrafish Embryos | almost 100% mortality (250 µM exposure 120 h) | [157] | |
| Termed HeLa: SK-Mel-28: 1929: J774A1 | size-dependent toxicity (Hela cell; IC50 is 250 µM, 140 µM) | [158] | |
| Zebrafish Embryos Escherichia coli 33876 | less than 3% mortality (250 µM exposure 120 h) | [159] | |
| human bronchiiall epithelial cells | oxidative stress (100–200 µM exposure 60 min) | [150] | |
| Escherichia coli | 100 nm susceptibility 0.04 mL/µg | [160] | |
| Bacillus subtilis | 100 nm susceptibility 0.04 mL/µg | [160] | |
| quantum dots | WTK1 cell line | DNA damaged (2 µM exposure 2 h) | [161] |
| Hela cell line, human primary, hepatocyte | cytotoxicity (0.1 mg/mL exposure 24 h) | [161] | |
| Cupriavidus metallidurans CH 34 | cellular ROS level increasing and about 2.5-fold increase of 84 the cells with damaged and leaky membranes (20 nM exposure 30 min | [162] | |
| Pseudomonas aeruginosa | cell membrane damaged; intracellular ROS; a concentration threshold of 50 mg/L | [163] | |
| Chlamydomonas reinhardtii | (HS-CH-CO0-) Sod1, gpx, cat and ptox2 (exposure 6 h); Transient up-regulation of genes as low as 0.1 mg/L | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, T.; Gu, L.; Fahad, S.; Saud, S.; Bleakley, B.; Zhou, R. Exploring Sustainable Agriculture with Nitrogen-Fixing Cyanobacteria and Nanotechnology. Molecules 2024, 29, 2534. https://doi.org/10.3390/molecules29112534
Nawaz T, Gu L, Fahad S, Saud S, Bleakley B, Zhou R. Exploring Sustainable Agriculture with Nitrogen-Fixing Cyanobacteria and Nanotechnology. Molecules. 2024; 29(11):2534. https://doi.org/10.3390/molecules29112534
Chicago/Turabian StyleNawaz, Taufiq, Liping Gu, Shah Fahad, Shah Saud, Bruce Bleakley, and Ruanbao Zhou. 2024. "Exploring Sustainable Agriculture with Nitrogen-Fixing Cyanobacteria and Nanotechnology" Molecules 29, no. 11: 2534. https://doi.org/10.3390/molecules29112534
APA StyleNawaz, T., Gu, L., Fahad, S., Saud, S., Bleakley, B., & Zhou, R. (2024). Exploring Sustainable Agriculture with Nitrogen-Fixing Cyanobacteria and Nanotechnology. Molecules, 29(11), 2534. https://doi.org/10.3390/molecules29112534










