Synthesis of Thionated Perylenediimides: State of the Art and First Investigations of an Alternative to Lawesson’s Reagent †
Abstract
1. Introduction
2. Overview of the Synthesis of Thionated Perylenediimides
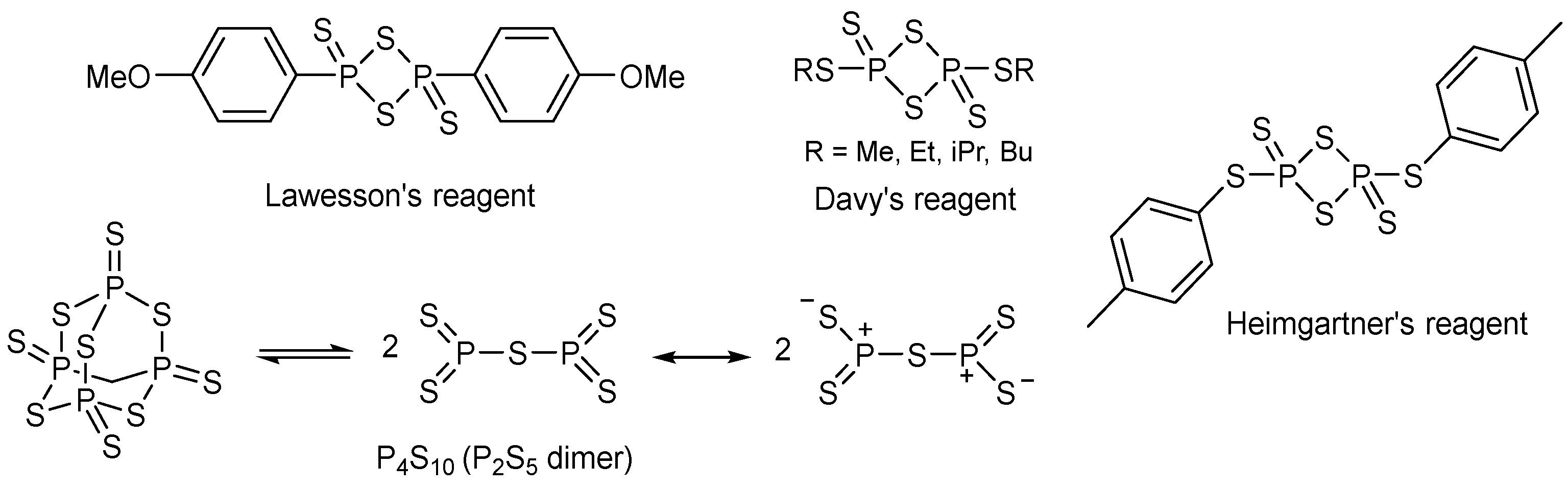
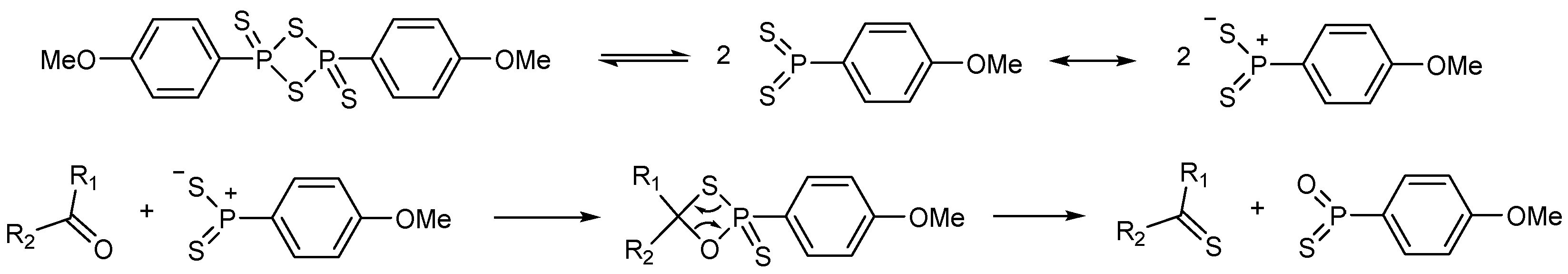
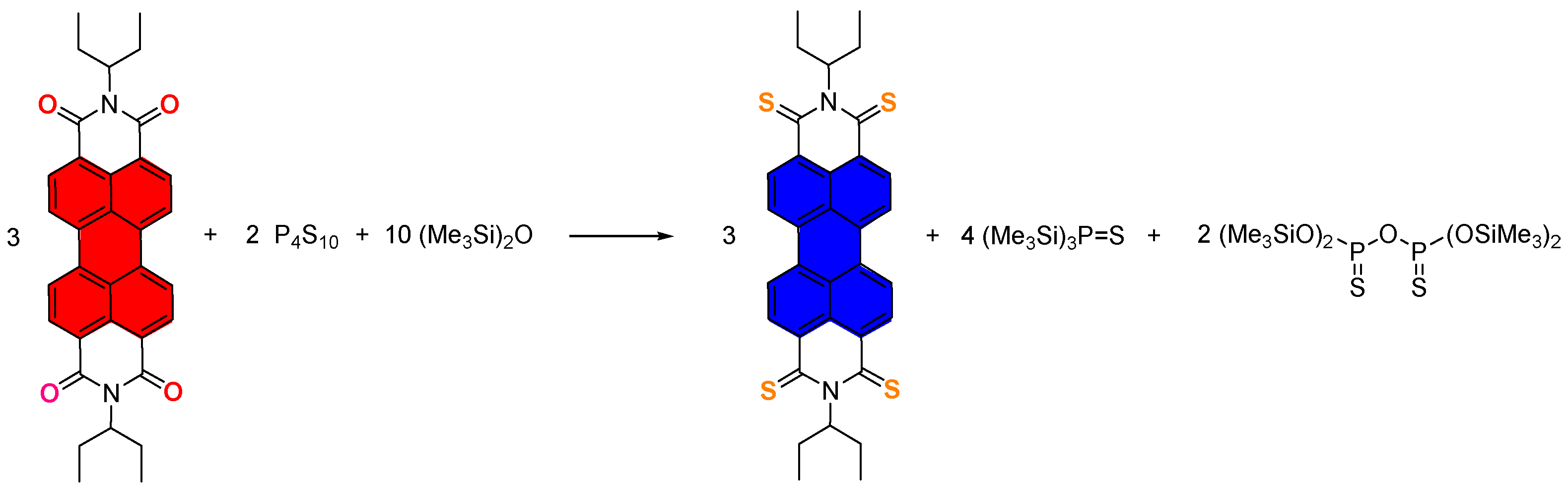
3. Investigations of Novel Reagents in Thionated Perylenediimides Synthesis
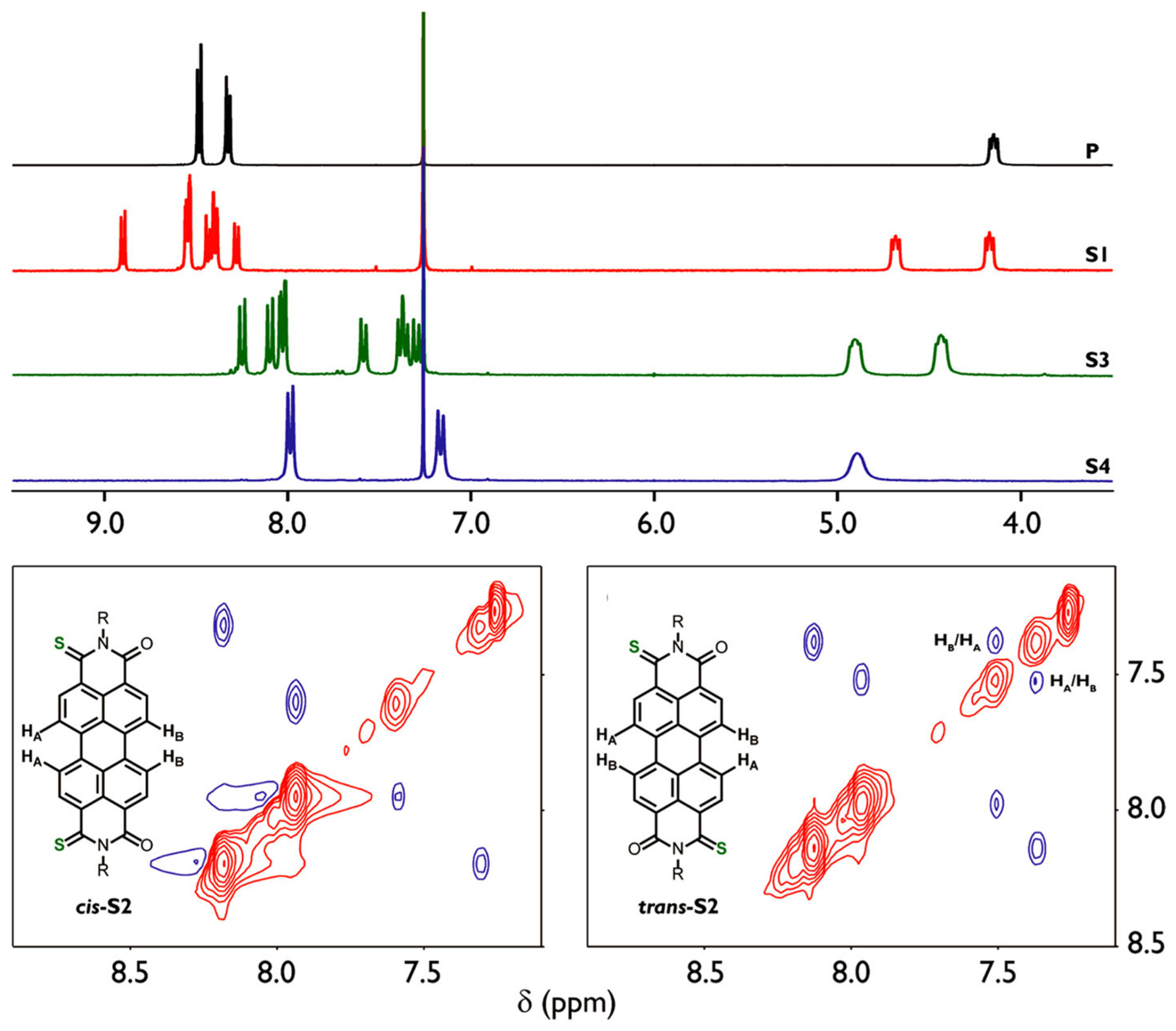
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017, 117, 3479–3716. [Google Scholar] [CrossRef]
- Borissov, A.; Maurya, Y.K.; Moshniaha, L.; Wong, W.-S.; Żyła-Karwowska, M.; Stępień, M. Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds. Chem. Rev. 2022, 122, 565–788. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Che, Y.; Moore, J.S. One-Dimensional Self-Assembly of Planar π-Conjugated Molecules: Adaptable Building Blocks for Organic Nanodevices. Acc. Chem. Res. 2008, 41, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Król, A.; Shoyama, K.; Stolte, M.; Würthner, F. Naphthalene and perylene diimides–better alternatives to fullerenes for organic electronics? Chem. Commun. 2018, 54, 13763–13772. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.T.E.; Zhu, J.; Li, X.; Wang, J.; Li, Y. Recent progress in the development of n-type organic semiconductors for organic field effect transistors. J. Mater. Chem. C 2017, 5, 8654–8681. [Google Scholar] [CrossRef]
- Qin, Y.; Li, G.; Qi, T.; Huang, H. Aromatic imide/amide-based organic small-molecule emitters for organic light-emitting diodes. Mater. Chem. Front. 2020, 4, 1554–1568. [Google Scholar] [CrossRef]
- Zink-Lorre, N.; Font-Sanchis, E.; Sastre-Santos, Á.; Fernández-Lázaro, F. Perylenediimides as more than just non-fullerene acceptors: Versatile components in organic, hybrid and perovskite solar cells. Chem. Commun. 2020, 56, 3824–3838. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Koenig, J.D.B.; Welch, G.C. Perylene diimide based non-fullerene acceptors: Top performers and an emerging class featuring N-annulation. J. Mater. Chem. A 2021, 9, 6775–6789. [Google Scholar] [CrossRef]
- Murugan, P.; Ravindran, E.; Sangeetha, V.; Liu, S.-Y.; Jung, J.W. Perylene-diimide for organic solar cells: Current scenario and prospects in molecular geometric, functionalization, and optoelectronic properties. J. Mater. Chem. A 2023, 11, 26393–26425. [Google Scholar] [CrossRef]
- Akash; Tiwari, J.P. Recent advancements in perylene diimide as an electron acceptor in organic solar cells. J. Mater. Chem. C 2024, 12, 838–853. [Google Scholar] [CrossRef]
- Görl, D.; Zhang, X.; Würthner, F. Molecular Assemblies of Perylene Bisimide Dyes in Water. Angew. Chem. Int. Ed. 2012, 51, 6328–6348. [Google Scholar] [CrossRef]
- Sun, M.; Müllen, K.; Yin, M. Water-soluble perylenediimides: Design concepts and biological applications. Chem. Soc. Rev. 2016, 45, 1513–1528. [Google Scholar] [CrossRef]
- Zhang, X.; Rehm, S.; Safont-Sempere, M.M.; Würthner, F. Vesicular perylene dye nanocapsules as supramolecular fluorescent pH sensor systems. Nat. Chem. 2009, 1, 623–629. [Google Scholar] [CrossRef]
- Krupka, O.; Hudhomme, P. Recent Advances in Applications of Fluorescent Perylenediimide and Perylenemonoimide Dyes in Bioimaging, Photothermal and Photodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 6308. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Q. Recent Advances in Perylene Diimides (PDI)-based Small Molecules Used for Emission and Photothermal Conversion. ChemPhotoChem 2024, 8, e202300213. [Google Scholar] [CrossRef]
- Nowak-Król, A.; Würthner, F. Progress in the synthesis of perylene bisimide dyes. Org. Chem. Front. 2019, 6, 1272–1318. [Google Scholar] [CrossRef]
- Kardos, M. Über einige Aceanthrenchinon- und 1.9-Anthracen-Derivate. Berichte Dtsch. Chem. Ges. 1913, 46, 2086–2091. [Google Scholar] [CrossRef]
- Quinn, J.; Zheng, Y.; Chen, Z.; Usta, H.; Newman, C.; Yan, H.; Facchetti, A. Organic Semiconductors and Devices Incorporating Same. U.S. Patent 8.440,828 B2, 29 December 2010. [Google Scholar]
- Shibahara, F.; Sugiura, R.; Murai, T. Direct Thionation and Selenation of Amides Using Elemental Sulfur and Selenium and Hydrochlorosilanes in the Presence of Amines. Org. Lett. 2009, 11, 3064–3067. [Google Scholar] [CrossRef]
- McGregor, W.M.; Sherrington, D.C. Some recent synthetic routes to thioketones and thioaldehydes. Chem. Soc. Rev. 1993, 22, 199–204. [Google Scholar] [CrossRef]
- Ozturk, T.; Ertas, E.; Mert, O. A Berzelius Reagent, Phosphorus Decasulfide (P4S10), in Organic Syntheses. Chem. Rev. 2010, 110, 3419–3478. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N. Phosphorus Pentasulfide in Heterocycle Synthesis. In Lawesson’s Reagent in Heterocycle Synthesis, Springer: Singapore, 2021; pp. 245–306.
- Cava, M.P.; Levinson, M.I. Thionation reactions of lawesson’s reagents. Tetrahedron 1985, 41, 5061–5087. [Google Scholar] [CrossRef]
- Jesberger, M.; Davis, T.P.; Barner, L. Applications of Lawesson’s Reagent in Organic and Organometallic Syntheses. Synthesis 2003, 2003, 1929–1958. [Google Scholar] [CrossRef]
- Ozturk, T.; Ertas, E.; Mert, O. Use of Lawesson’s Reagent in Organic Syntheses. Chem. Rev. 2007, 107, 5210–5278. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, H.; Abdulmalek, E. A Focused Review of Synthetic Applications of Lawesson’s Reagent in Organic Synthesis. Molecules 2021, 26, 6937. [Google Scholar] [CrossRef] [PubMed]
- Jackson, Y.A.; Rajagopal, D.; Bendolph, J.; Guillory, M.; Lakshmikantham, M.V.; Yang, J.; Cava, M.P. Thiophene Isosteres of 9,10-Dithioanthraquinone. Org. Lett. 2003, 5, 1883–1885. [Google Scholar] [CrossRef] [PubMed]
- Wipf, P.; Jenny, C.; Heimgartner, H. 2,4-Bis(4-methylpheylthio)-1,3,2λ5,4λ5-dithiadiphosphetan-2,4-dithion: Ein neues Reagens zur Schwefelung von N,N-disubstituierten Amiden. Helv. Chim. Acta 1987, 70, 1001–1011. [Google Scholar] [CrossRef]
- Curphey, T.J. A superior procedure for the conversion of 3-oxoesters to 3H-1,2-dithiole-3-thiones. Tetrahedron Lett. 2000, 41, 9963–9966. [Google Scholar] [CrossRef]
- Curphey, T.J. Thionation of esters and lactones with the reagent combination of phosphorus pentasulfide and hexamethyldisiloxane. Tetrahedron Lett. 2002, 43, 371–373. [Google Scholar] [CrossRef]
- Curphey, T.J. Thionation with the Reagent Combination of Phosphorus Pentasulfide and Hexamethyldisiloxane. J. Org. Chem. 2002, 67, 6461–6473. [Google Scholar] [CrossRef]
- Bergman, J.; Pettersson, B.; Hasimbegovic, V.; Svensson, P.H. Thionations using a P4S10-pyridine complex in solvents such as acetonitrile and dimethyl sulfone. J. Org. Chem. 2011, 76, 1546–1553. [Google Scholar] [CrossRef]
- Kingi, N.; Bergman, J. Thionation of Tryptanthrin, Rutaecarpine, and Related Molecules with a Reagent Prepared from P4S10 and Pyridine. J. Org. Chem. 2016, 81, 7711–7716. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Kaushik, M.P. A new, efficient and simple method for the thionation of ketones to thioketones using P4S10/Al2O3. Tetrahedron Lett. 2004, 45, 6255–6257. [Google Scholar] [CrossRef]
- Morel, S.; Chatel, F.; Boyer, G.; Galy, J.-P. Synthesis of New Cyclopenta-acridinone and -phenothiazine Derivatives. J. Chem. Res. (S) 1998, 1, 4–5. [Google Scholar] [CrossRef]
- Degl’Innocenti, A.; Capperucci, A.; Nocentini, T.; Castagnoli, G.; Malesci, I.; Cerreti, A. HMDST as Useful Tool in Organic Synthesis: A Further Step in the Delivery of Sulfur Functionalities. Phosphorus Sulfur Silicon Relat. Elem. 2005, 180, 1247–1251. [Google Scholar] [CrossRef]
- Lecher, H.Z.; Greenwood, R.A.; Whitehouse, K.C.; Chao, T.H. The Phosphonation of Aromatic Compounds with Phosphorus Pentasulfide. J. Am. Chem. Soc. 1956, 78, 5018–5022. [Google Scholar] [CrossRef]
- Perregaard, J.; Scheibye, S.; Meyer, H.J.; Thomsen, I.; Lawesson, S.-O. Studies on Organophosphorus Compounds XVIII. Oxidation of Tertiary Alicyclic Amines with Elemental Sulfur in Hexamethylphosphoric Triamide (HMPA). Oxidative Rearrangements of Hexahydroazepines and Octahydroazocines to Bis(3-Pyrrolyl)Polysulfides. Bull. Soc. Chim. Belg. 1977, 86, 679–691. [Google Scholar] [CrossRef]
- Orzeszko, A.; Maurin, J.K.; Melon-Ksyta, D. Investigation of the Thionation Reaction of Cyclic Imides. Z. Naturforschung B 2001, 56, 1035–1040. [Google Scholar] [CrossRef]
- Tilley, A.J.; Pensack, R.D.; Lee, T.S.; Djukic, B.; Scholes, G.D.; Seferos, D.S. Ultrafast Triplet Formation in Thionated Perylene Diimides. J. Phys. Chem. C 2014, 118, 9996–10004. [Google Scholar] [CrossRef]
- Symons, H.E.; Hagemann, M.J.L.; Harniman, R.L.; Faul, C.F.J. Thionated PDI supramolecular polymers: Controlling aggregation mechanisms, morphology and function. J. Mater. Chem. C 2022, 10, 2828–2837. [Google Scholar] [CrossRef]
- Llewellyn, B.A.; Davies, E.S.; Pfeiffer, C.R.; Cooper, M.; Lewis, W.; Champness, N.R. Thionated perylene diimides with intense absorbance in the near-IR. Chem. Commun. 2016, 52, 2099–2102. [Google Scholar] [CrossRef]
- Mandal, K.; Yadav, D.; Saini, P.; Mukhopadhyay, P. Synthesis, optical and redox attributes of core-/bay-substituted thionated NDIs, PDIs and their diverse radical anions. J. Mater. Chem. C 2023, 11, 12543–12549. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Y.; Jin, X.; Deng, Q.; Yin, Z.; Tong, S.; Qing, W.; Huang, Y. Regioisomer-manipulating thio-perylenediimide nanoagents for photothermal/photodynamic theranostics. J. Mater. Chem. B 2020, 8, 5535–5544. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ji, C.; Fan, Z.; Ma, R.; Yin, M. A facile design of thio-perylenediimides with controllable fluorescent, photodynamic and photothermal effects towards cancer theranostics. Chem. Commun. 2021, 57, 13126–13129. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-L.; Chou, Y.-T.; Su, B.-K.; Wu, C.-c.; Wang, C.-H.; Chang, K.-H.; Ho, J.-a.A.; Chou, P.-T. Comprehensive Thione-Derived Perylene Diimides and Their Bio-Conjugation for Simultaneous Imaging, Tracking, and Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2022, 144, 17249–17260. [Google Scholar] [CrossRef]
- An, F.; Zhao, Y.; Li, H.; Meng, J.; Jiao, L.; Zhang, Z.; Li, X.; Sun, X. Intramolecular charge transfer versus intersystem crossing: The way toward super-high photothermal efficiency by thionation. Dyes Pigment. 2023, 217, 111411. [Google Scholar] [CrossRef]
- Tilley, A.J.; Guo, C.; Miltenburg, M.B.; Schon, T.B.; Yan, H.; Li, Y.; Seferos, D.S. Thionation Enhances the Electron Mobility of Perylene Diimide for High Performance n-Channel Organic Field Effect Transistors. Adv. Funct. Mater. 2015, 25, 3321–3329. [Google Scholar] [CrossRef]
- Kozycz, L.M.; Guo, C.; Manion, J.G.; Tilley, A.J.; Lough, A.J.; Li, Y.; Seferos, D.S. Enhanced electron mobility in crystalline thionated naphthalene diimides. J. Mater. Chem. C 2015, 3, 11505–11515. [Google Scholar] [CrossRef]
- Pahlavanlu, P.; Tilley, A.J.; McAllister, B.T.; Seferos, D.S. Microwave Synthesis of Thionated Naphthalene Diimide-Based Small Molecules and Polymers. J. Org. Chem. 2017, 82, 12337–12345. [Google Scholar] [CrossRef]
- Weitz, R.T.; Amsharov, K.; Zschieschang, U.; Villas, E.B.; Goswami, D.K.; Burghard, M.; Dosch, H.; Jansen, M.; Kern, K.; Klauk, H. Organic n-Channel Transistors Based on Core-Cyanated Perylene Carboxylic Diimide Derivatives. J. Am. Chem. Soc. 2008, 130, 4637–4645. [Google Scholar] [CrossRef]
- Gsänger, M.; Oh, J.H.; Könemann, M.; Höffken, H.W.; Krause, A.-M.; Bao, Z.; Würthner, F. A Crystal-Engineered Hydrogen-Bonded Octachloroperylene Diimide with a Twisted Core: An n-Channel Organic Semiconductor. Angew. Chem. Int. Ed. 2010, 49, 740–743. [Google Scholar] [CrossRef]
- Ortiz-Rodríguez, L.A.; Crespo-Hernández, C.E. Thionated organic compounds as emerging heavy-atom-free photodynamic therapy agents. Chem. Sci. 2020, 11, 11113–11123. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, L.; Loredo, A.; Cole, C.; Xiao, H. Single-atom replacement as a general approach towards visible-light/near-infrared heavy-atom-free photosensitizers for photodynamic therapy. Chem. Sci. 2020, 11, 6701–6708. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-N.; Park, S.J.; Qi, S.; Ha, J.; Heo, S.; Yim, Y.; Baek, G.; Lim, C.S.; Lee, D.J.; Kim, H.M.; et al. Design and synthesis of efficient heavy-atom-free photosensitizers for photodynamic therapy of cancer. Chem. Commun. 2020, 56, 11489–11492. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-N.; Qi, S.; Kim, S.; Kwon, N.; Kim, G.; Yim, Y.; Park, S.; Yoon, J. An Emerging Molecular Design Approach to Heavy-Atom-Free Photosensitizers for Enhanced Photodynamic Therapy under Hypoxia. J. Am. Chem. Soc. 2019, 141, 16243–16248. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rodríguez, L.A.; Hoehn, S.J.; Loredo, A.; Wang, L.; Xiao, H.; Crespo-Hernández, C.E. Electronic Relaxation Pathways in Heavy-Atom-Free Photosensitizers Absorbing Near-Infrared Radiation and Exhibiting High Yields of Singlet Oxygen Generation. J. Am. Chem. Soc. 2021, 143, 2676–2681. [Google Scholar] [CrossRef] [PubMed]
- Deiana, M.; Josse, P.; Dalinot, C.; Osmolovskyi, A.; Marqués, P.S.; Castán, J.M.A.; Abad Galán, L.; Allain, M.; Khrouz, L.; Maury, O.; et al. Site-selected thionated benzothioxanthene chromophores as heavy-atom-free small-molecule photosensitizers for photodynamic therapy. Commun. Chem. 2022, 5, 142. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meng, J.; Sun, X. Substitution effects on NIR-absorbing Perylene Diimide based on DFT calculation. Inorg. Chem. Commun. 2019, 105, 194–198. [Google Scholar] [CrossRef]
- Henry, L. Ueber eine neue Bildungs- und Darstellungsweise der Nitrile. Ann. Chem. Pharm. 1869, 152, 148–152. [Google Scholar] [CrossRef]
- Wislicenus, J. Vorläufige Mittheilungen. Z. Chem. 1869, 12, 324–326. [Google Scholar]
- Hofmann, A.W. Ueber die Daratellung der geachwefelten Amide. Berichte Dtsch. Chem. Ges. 1878, 11, 338–340. [Google Scholar] [CrossRef]
- Sureshbabu, V.V.; Nagendra, G.; Venkataramanarao, R. Ultrasound accelerated conversion of Nα-urethane protected peptide esters to their thiopeptides using P2S5. Ultrason. Sonochem. 2008, 15, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Demmig, S.; Langhals, H. Leichtlösliche, lichtechte Perylen-Fluoreszenzfarbstoffe. Chem. Berichte 1988, 121, 225–230. [Google Scholar] [CrossRef]
- Hendsbee, A.D.; Sun, J.-P.; Law, W.K.; Yan, H.; Hill, I.G.; Spasyuk, D.M.; Welch, G.C. Synthesis, Self-Assembly, and Solar Cell Performance of N-Annulated Perylene Diimide Non-Fullerene Acceptors. Chem. Mater. 2016, 28, 7098–7109. [Google Scholar] [CrossRef]
- Perrin, L.; Hudhomme, P. Synthesis, Electrochemical and Optical Absorption Properties of New Perylene-3,4:9,10-bis(dicarboximide) and Perylene-3,4:9,10-bis(benzimidazole) Derivatives. Eur. J. Org. Chem. 2011, 2011, 5427–5440. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Nivsarkar, M.; Paradashani, D.; Kaushik, M.P. Thionation of carbonyl compounds using phosphorus pentasulfide and hexamethyldisiloxane under microwave irradiations. J. Chem. Res. 2004, 2004, 474–476. [Google Scholar] [CrossRef]
- Krstić, N.M.; Bjelaković, M.S.; Dabović, M.M.; Pavlović, V.D. Thionation of some alpha,beta-unsaturated steroidal ketones. Molecules 2010, 15, 3462–3477. [Google Scholar] [CrossRef] [PubMed]
- Rocard, L.; Goujon, A.; Hudhomme, P. Nitro-Perylenediimide: An Emerging Building Block for the Synthesis of Functional Organic Materials. Molecules 2020, 25, 1402. [Google Scholar] [CrossRef] [PubMed]
- El-Berjawi, R.; Hudhomme, P. Synthesis of a perylenediimide-fullerene C60 dyad: A simple use of a nitro leaving group for a Suzuki-Miyaura coupling reaction. Dyes Pigment. 2018, 159, 551–556. [Google Scholar] [CrossRef]
- Rocard, L.; Hatych, D.; Chartier, T.; Cauchy, T.; Hudhomme, P. Original Suzuki–Miyaura Coupling Using Nitro Derivatives for the Synthesis of Perylenediimide-Based Multimers. Eur. J. Org. Chem. 2019, 2019, 7635–7643. [Google Scholar] [CrossRef]
- Rocard, L.; Hudhomme, P. Recent Developments in the Suzuki–Miyaura Reaction Using Nitroarenes as Electrophilic Coupling Reagents. Catalysts 2019, 9, 213. [Google Scholar] [CrossRef]
- Goujon, A.; Rocard, L.; Cauchy, T.; Hudhomme, P. An Imine Photocyclization as an Alternative to the Pictet–Spengler Reaction for the Synthesis of AzaBenzannulated Perylenediimide Dyes. J. Org. Chem. 2020, 85, 7218–7224. [Google Scholar] [CrossRef] [PubMed]
- El-Berjawi, R.; Rocard, L.; Goujon, A.; Cauchy, T.; Hudhomme, P. Visible-Light-Mediated Synthesis of AzaBenzannulated Perylenediimide-Based Light-Harvesting Dyads. J. Org. Chem. 2020, 85, 12252–12261. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-P.; Hendsbee, A.D.; Dobson, A.J.; Welch, G.C.; Hill, I.G. Perylene diimide based all small-molecule organic solar cells: Impact of branched-alkyl side chains on solubility, photophysics, self-assembly, and photovoltaic parameters. Org. Electron. 2016, 35, 151–157. [Google Scholar] [CrossRef]
- Kudchadker, M.V.; Zingaro, R.A.; Irgolic, K.J. Chemistry of phosphorus pentaselenide. I. Its reaction with alcohols. Can. J. Chem. 2011, 46, 1415–1424. [Google Scholar] [CrossRef]
- Tedy, A.M.; Manna, A.K. Nature and energetics of low-lying excited singlets/triplets and intersystem crossing rates in selone analogs of perylenediimide: A theoretical perspective. J. Chem. Phys. 2024, 160, 114306. [Google Scholar] [CrossRef]
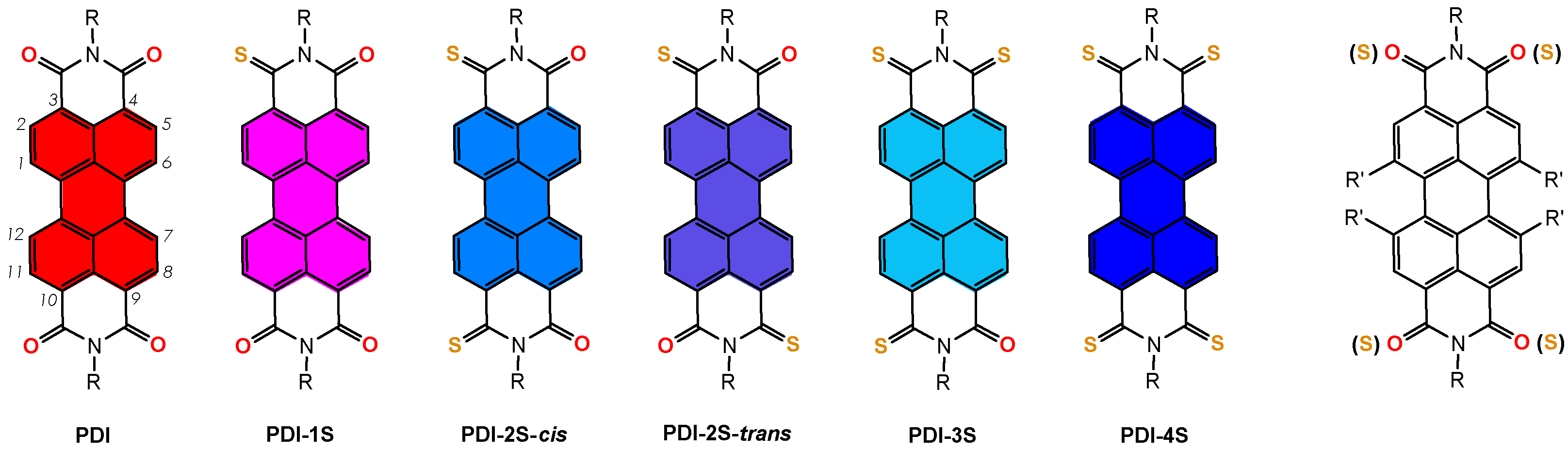






| Entry | R | R’ | Experimental Conditions | Yield PDI 1S to 4S | Ref |
|---|---|---|---|---|---|
| 1 |  | H | LR (2.7 eq.) 1-MeNaphth., 180 °C, 30 min | PDI-1S: < 5% PDI-2S cis: 20–30% PDI-2S trans: 29% PDI-3S: 1% PDI-4S: traces | [18] |
| 2 | -CH(CH2CH3)2 | H | DR (2 eq.) o-C6H4Cl2, 180 °C, 7 min | PDI-2S cis: 26% PDI-2S trans: 27% | [18] |
| 3 |  | CN (1,6 and 1,7 isomers) | DR (2.2 eq.) o-C6H4Cl2, 180 °C, 15 min | PDI-2S trans: 22% | [18] |
| 4 | 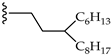 | H | LR (2 eq.) Tol., 110 °C, 18 h | PDI-1S: 10% PDI-2S cis: 13% PDI-2S trans: 17% PDI-3S: 24% PDI-4S: traces | [40] |
| 5 | 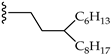 | H | LR (5 eq.) Tol., 110 °C, 50 h | PDI-3S: 13% PDI-4S: 29% | [40] |
| 6 | 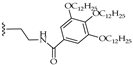 | H | LR (8 eq.) Tol., 110 °C, 48 h | PDI-1S: 21% PDI-2S cis: 10% PDI-2S trans: 9% | [41] |
| 7 | -C4H9 |  (1,6 and 1,7 isomers) | LR (6 eq.) Tol., 110 °C, 48 h | 1,7-PDI-4S: 16% 1,6-PDI-4S: 17% | [42] |
| 8 | -C6H11 | Br 1,7 isomer | LR (4 eq.) Tol., 85 °C, 36 h | PDI-1S: 13% and 9% PDI-2S cis: 11% PDI-2S trans: 15% | [43] |
| 9 | -CH(C6H13)2 | H | LR (10 eq.) Tol., 110 °C, 18 h | PDI-2S cis: 5.4% PDI-2S trans: 10.9% | [44] |
| 10 | -C8H17 | 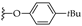 (1,6,7,12 tetra substituted) | LR (4 eq.) Xyl., MW 150 W, 103 °C, 20 min | PDI-1S: 10% PDI-2S trans: 15% PDI-3S: 19% PDI-4S: 24% | [45] |
| 11 | -CH(C2H5)2 | H | LR (6 eq.) Tol., 110 °C, overnight | PDI-1S: 20% PDI-2S cis: 30% PDI-2S trans: 35% | [46] |
| 12 | 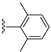 | H | LR (6 eq.) Tol., 110 °C, overnight | PDI-1S: 10% PDI-2S cis: 18% PDI-2S trans: 19% PDI-3S: 15% PDI-4S: 9% | [46] |
| 13 | -C6H11 | -NH-C6H11 1,7 isomer | LR (6 eq.) Tol., 110 °C, 3 days | PDI-1S: 20% PDI-2S-trans: 35% PDI-3S: 12% | [47] |
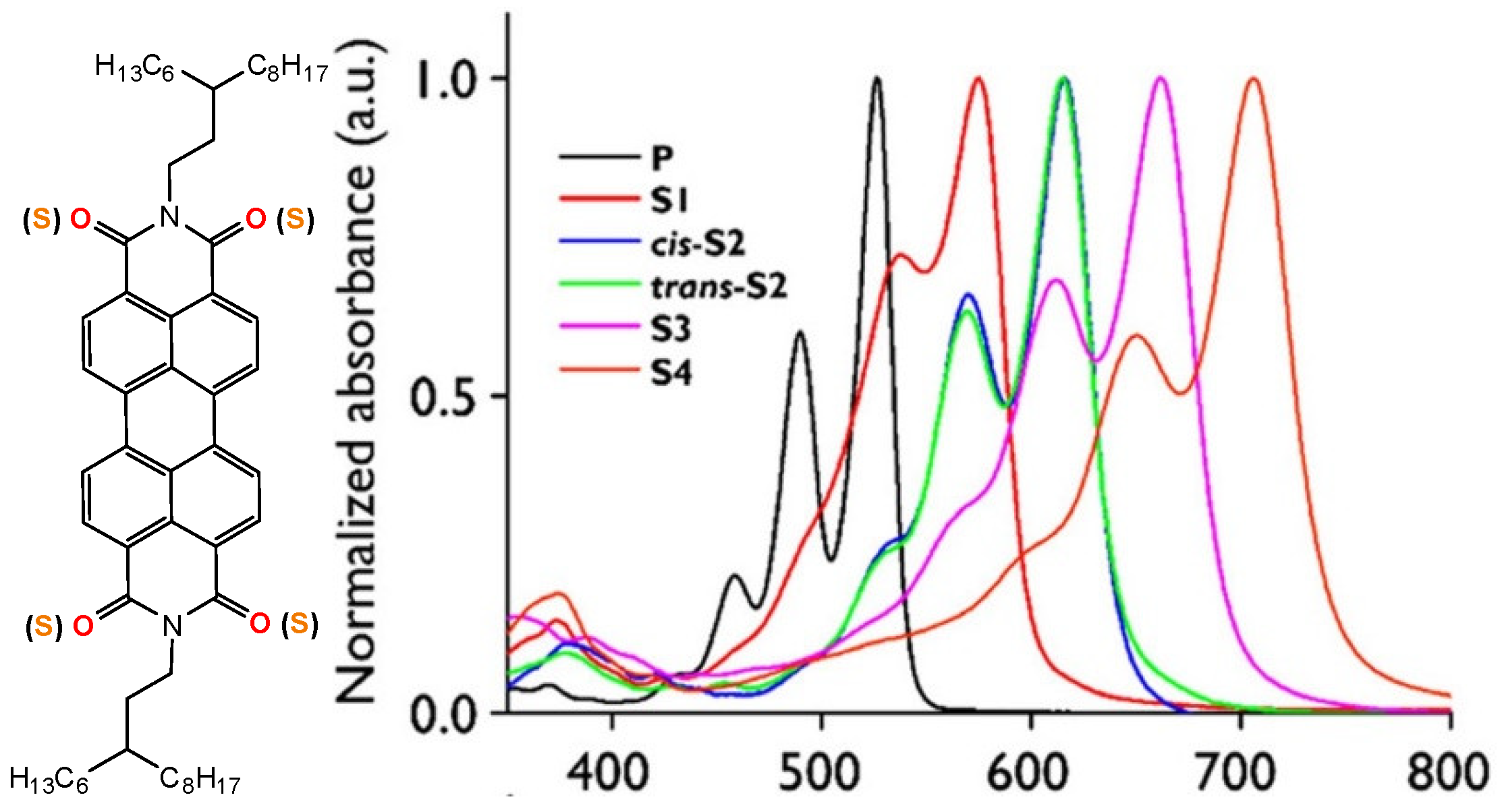
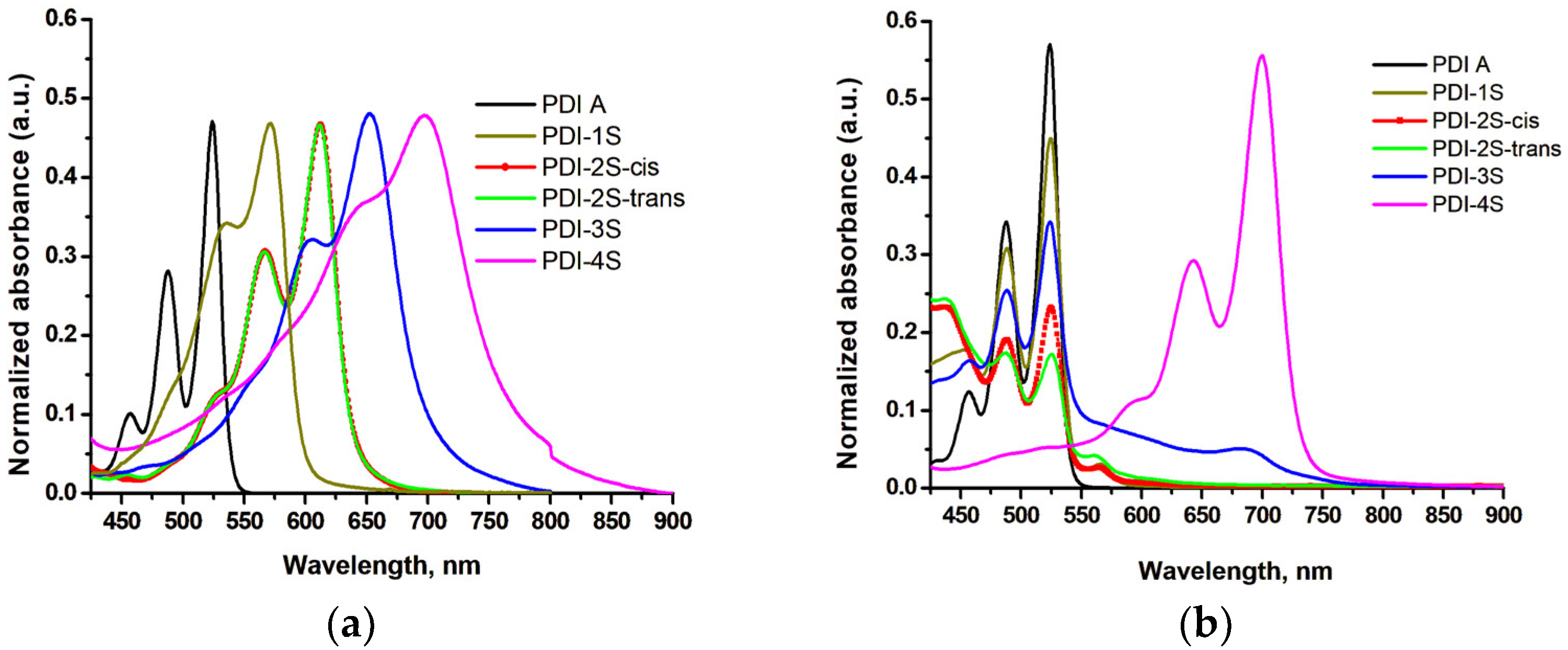
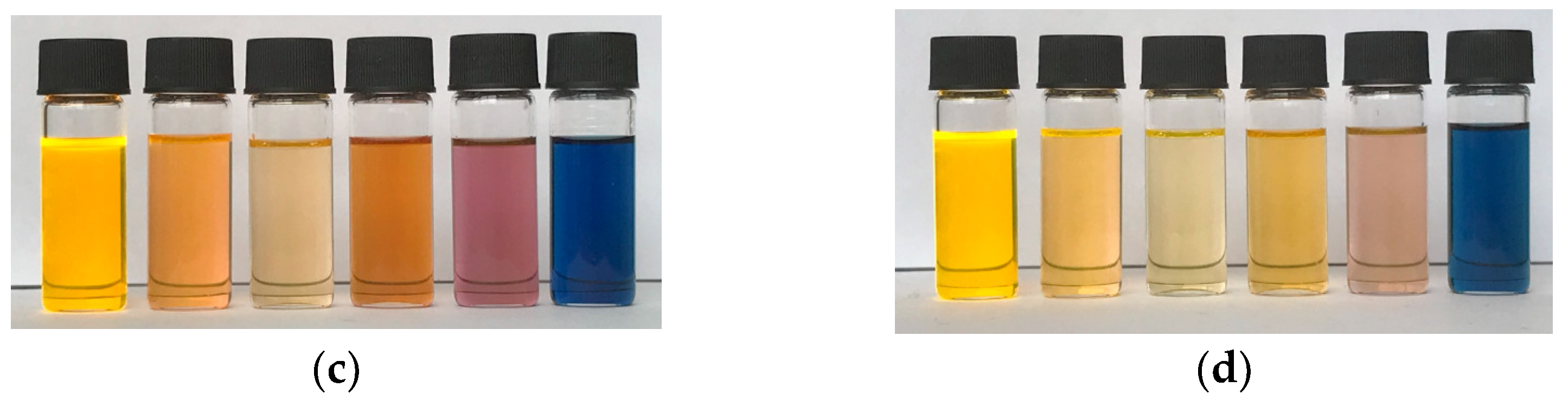
| Compound | λmax (nm) | Optical Band Gap (eV) | E1/2red1 (V) | E1/2red2 (V) |
|---|---|---|---|---|
| PDI (P) | 526 | 2.25 | −0.68 | −0.91 |
| PDI-1S (S1) | 574 | 2.06 | −0.55 | −0.72 |
| PDI-2S-cis (S2-cis) | 616 | 1.91 | −0.48 | −0.57 |
| PDI-2S-trans (S2-trans) | 615 | 1.90 | −0.51 | −0.61 |
| PDI-3S (S3) | 663 | 1.78 | −0.36 | −0.45 |
| PDI-4S (S4) | 706 | 1.64 | −0.23 | −0.33 |
| Compound | HOMO (eV) | LUMO (eV) | ||
|---|---|---|---|---|
| PDI (P) | −5.92 (−6.23) |  | −3.67 (−3.76) | 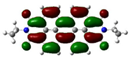 |
| PDI-1S (S1) | −5.85 (−6.15) | 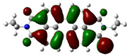 | −3.80 (−3.88) |  |
| PDI-2S-cis (S2-cis) | −5.78 (−6.09) | 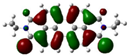 | −3.87 (−3.99) | 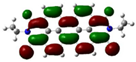 |
| PDI-2S-trans (S2-trans) | −5.74 (−6.08) | 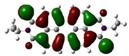 | −3.84 (−3.97) | 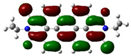 |
| PDI-3S (S3) | −5.77 (−6.04) | 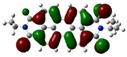 | −3.99 (−4.07) |  |
| PDI-4S (S4) | −5.76 (−5.98) |  | −4.12 (−4.15) | 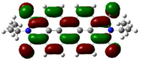 |
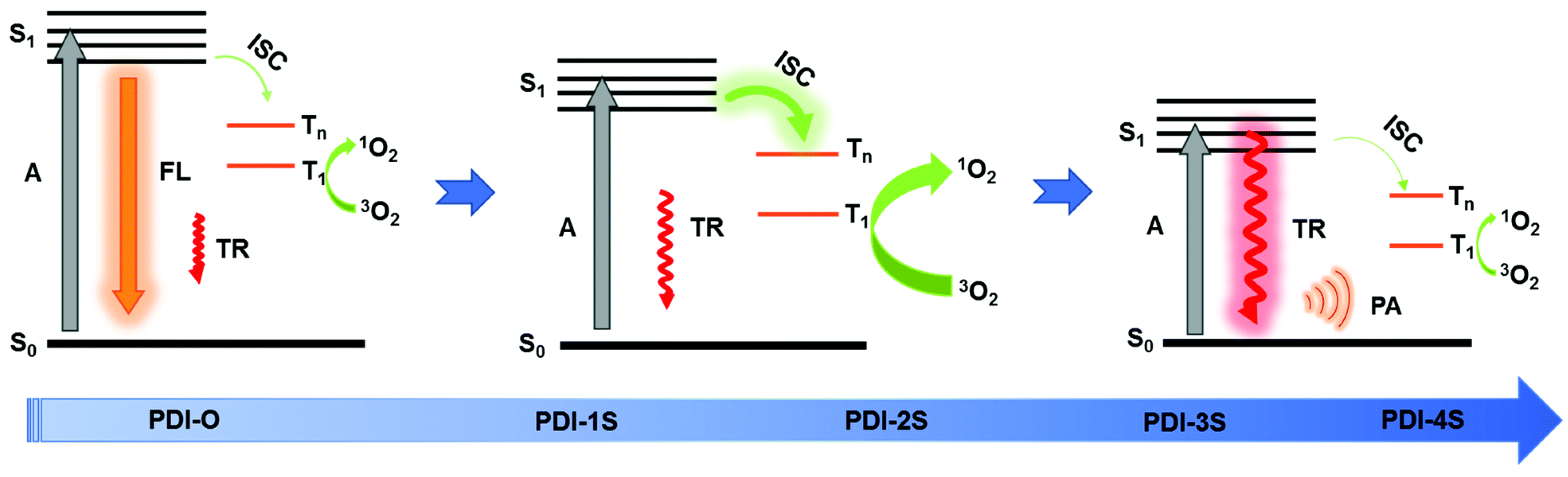
| Compound | λmax (nm) | Optical Band Gap (eV) | ΦPL (%) | ΦΔ (%) |
|---|---|---|---|---|
| PDI | 575 | 2.31 | 0.92 | 1 |
| PDI-1S | 623 | 2.13 | - | 95.6 |
| PDI-2S | 666 | 1.99 | - | 45.8 |
| PDI-3S | 719 | 1.81 | - | 11.1 |
| PDI-4S | 769 | 1.68 | - | 0.5 |
| Entry | PDI | Reagent | Solvent, Time | Yields (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| PDI | PDI-1S | PDI-2S-cis | PDI-2S-trans | PDI-3S | PDI-4S | ||||
| 1 | A | LR (3 mmol) | Δ Tol., 24 h | 21 | 35 | 9 | 8 | nd | nd |
| 2 | A | P4S10 (0.33 mmol) HMDSO (1.66 mmol) | Δ Tol., 24 h | trace | 4 | 40 | 42 | 8 | trace |
| 3 | A | P4S10 (0.33 mmol) HMDSO (1.66 mmol) | Δ Xyl., 24 h | nd | 5 | 33 | 31 | 17 | trace |
| 4 | A | P4S10 (0.75 mmol) HMDSO (3.75 mmol) | Δ Tol., 24 h | nd | trace | 28 | 29 | 20 | trace |
| 5 | A | P4S10 (0.75 mmol) HMDSO (7.5 mmol) | Δ Tol., 24 h | nd | 1 | 24 | 23 | 27 | trace |
| 6 | A | P4S10 (1.5 mmol) HMDSO (15 mmol) | Δ Tol., 24 h | nd | nd | 12 | 15 | 7 | trace |
| 7 | A | P4S10 (1.5 mmol) HMDSO (15 mmol) | Δ Tol., 120 h | nd | nd | 11 | 18 | 9 | trace |
| 8 | A | P4S10 (3 × 0.33 mmol) HMDSO (3 × 1.66 mmol) | Δ Tol., 3 × 24 h | nd | 2 | 27 | 28 | 16 | trace |
| 9 | A | P4S10 (0.75 mmol) HMDST (3.75 mmol)) | Δ Tol., 24 h | 2 | 13 | 18 | 23 | 1 | trace |
| 10 | B | LR (3 mmol) | Δ Tol., 24 h | trace | 13 | 19 | 15 | 8 | 5 |
| 11 | B | P4S10 (0.33 mmol) HMDSO (1.66 mmol) | Δ Tol., 24 h | nd | 11 | 14 | 17 | 20 | 12 |
| 12 | B | P4S10 (0.75 mmol) HMDSO (7.5 mmol) | Δ Tol., 24 h | nd | trace | 2 | 2 | 18 | 32 |
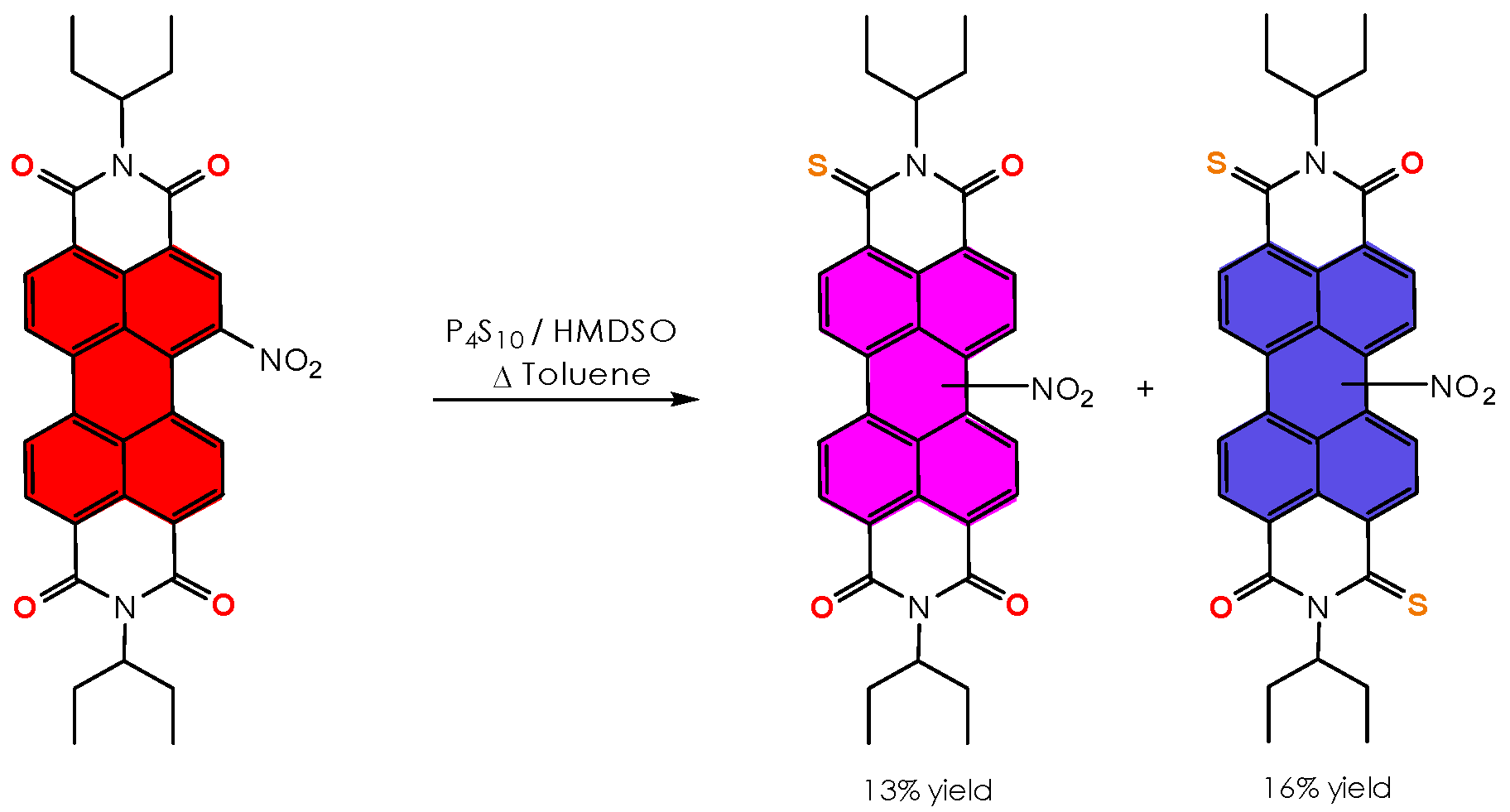
| 13 | C | P4S10 (0.75 mmol) HMDSO (7.5 mmol) | Δ Tol., 24 h | nd | trace | trace | trace | trace | 89 |
| 14 | C | P4S10 (1 mmol) HMDSO (10 mmol) | Δ Tol., 24 h | nd | trace | trace | trace | trace | 84 |
| 15 | D | P4S10 (0.75 mmol) HMDSO (7.5 mmol) | Δ Tol., 24 h | nd | 13 | 16 | trace | nd | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharchenko, O.; Hryniuk, A.; Krupka, O.; Hudhomme, P. Synthesis of Thionated Perylenediimides: State of the Art and First Investigations of an Alternative to Lawesson’s Reagent. Molecules 2024, 29, 2538. https://doi.org/10.3390/molecules29112538
Kharchenko O, Hryniuk A, Krupka O, Hudhomme P. Synthesis of Thionated Perylenediimides: State of the Art and First Investigations of an Alternative to Lawesson’s Reagent. Molecules. 2024; 29(11):2538. https://doi.org/10.3390/molecules29112538
Chicago/Turabian StyleKharchenko, Oksana, Anna Hryniuk, Oksana Krupka, and Piétrick Hudhomme. 2024. "Synthesis of Thionated Perylenediimides: State of the Art and First Investigations of an Alternative to Lawesson’s Reagent" Molecules 29, no. 11: 2538. https://doi.org/10.3390/molecules29112538
APA StyleKharchenko, O., Hryniuk, A., Krupka, O., & Hudhomme, P. (2024). Synthesis of Thionated Perylenediimides: State of the Art and First Investigations of an Alternative to Lawesson’s Reagent. Molecules, 29(11), 2538. https://doi.org/10.3390/molecules29112538






