Fabrication of a Molybdenum Dioxide/Multi-Walled Carbon Nanotubes Nanocomposite as an Anodic Modification Material for High-Performance Microbial Fuel Cells
Abstract
:1. Introduction
2. Results and Discussion
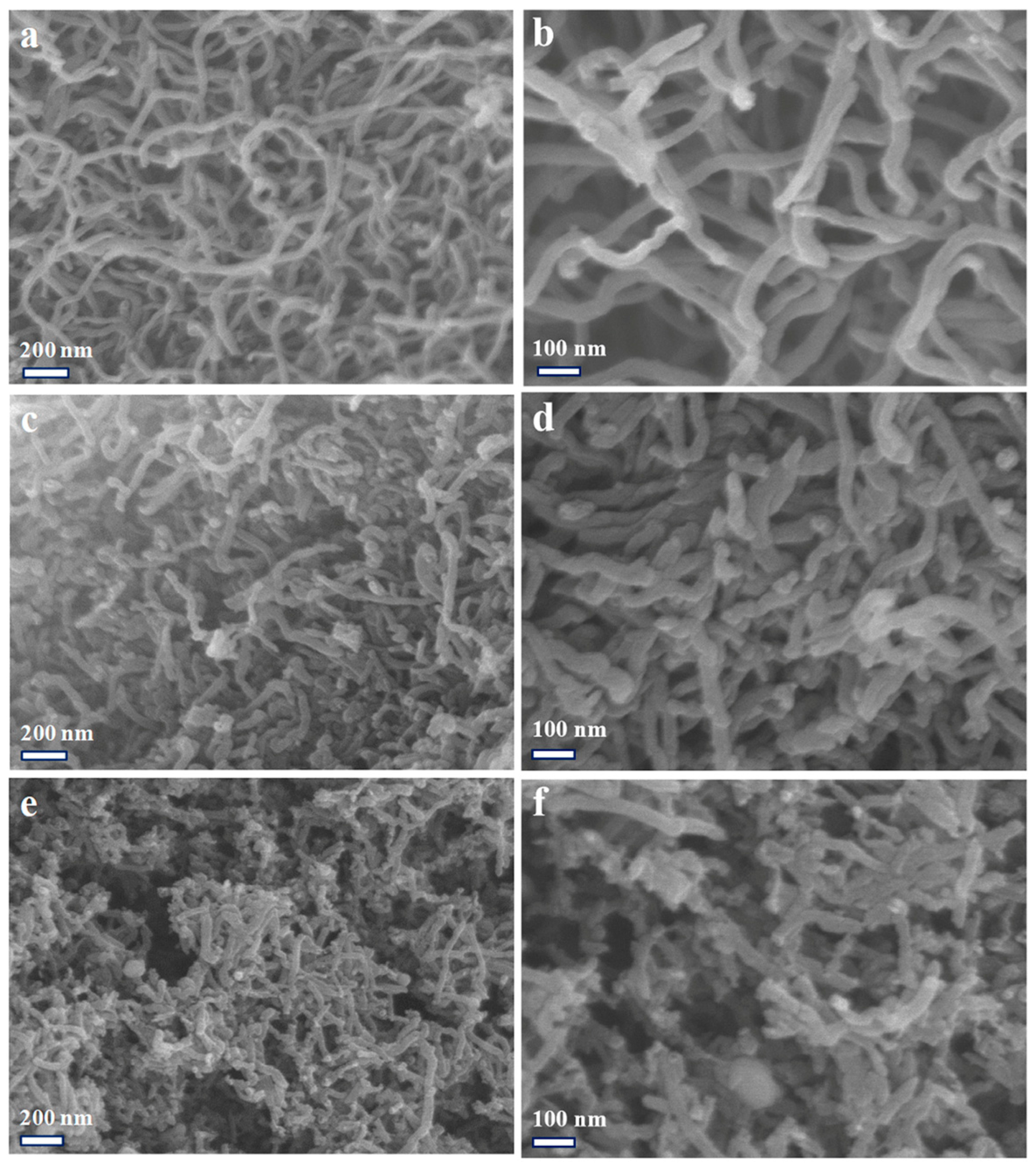
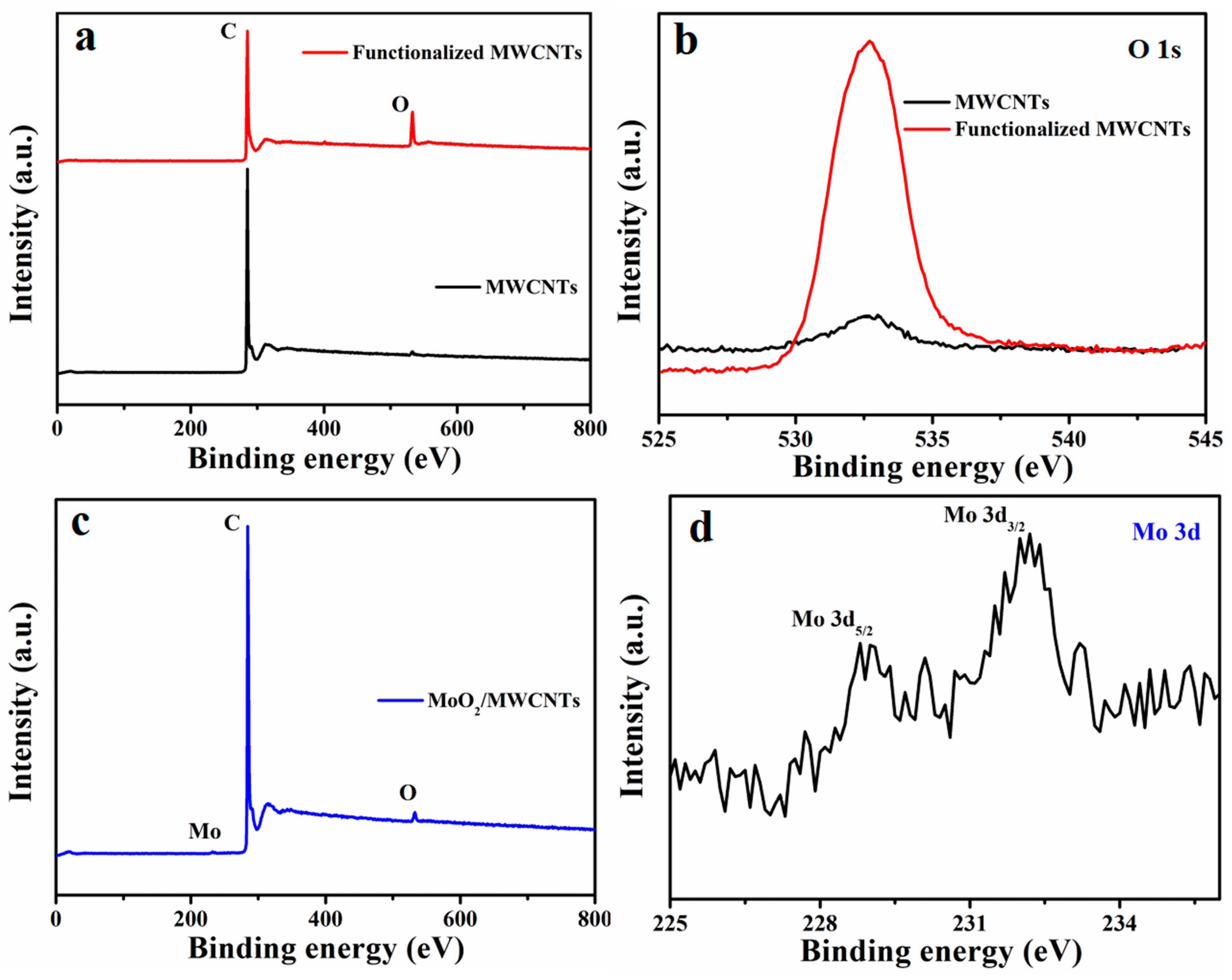
2.1. Physical Characterization of Materials
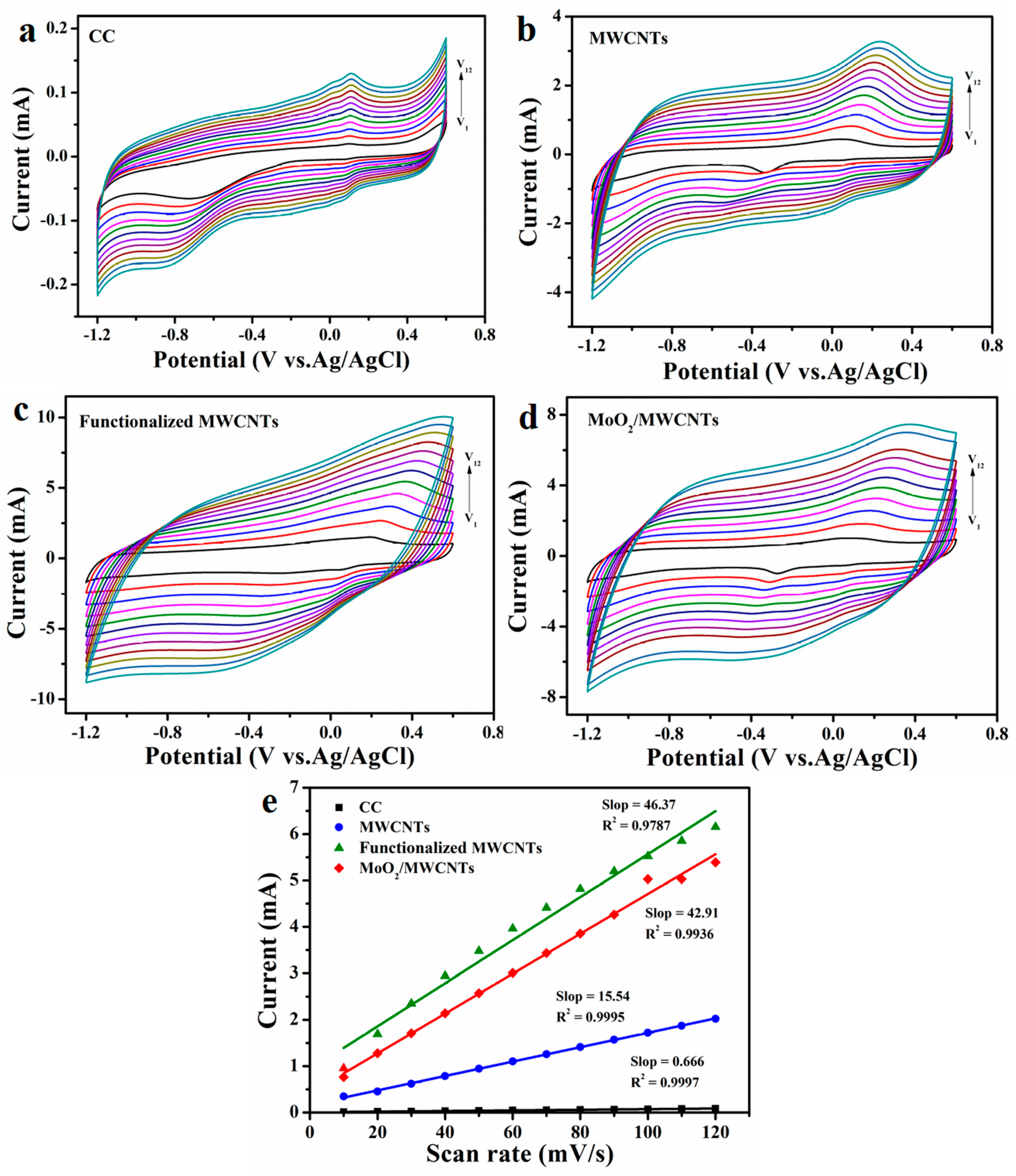
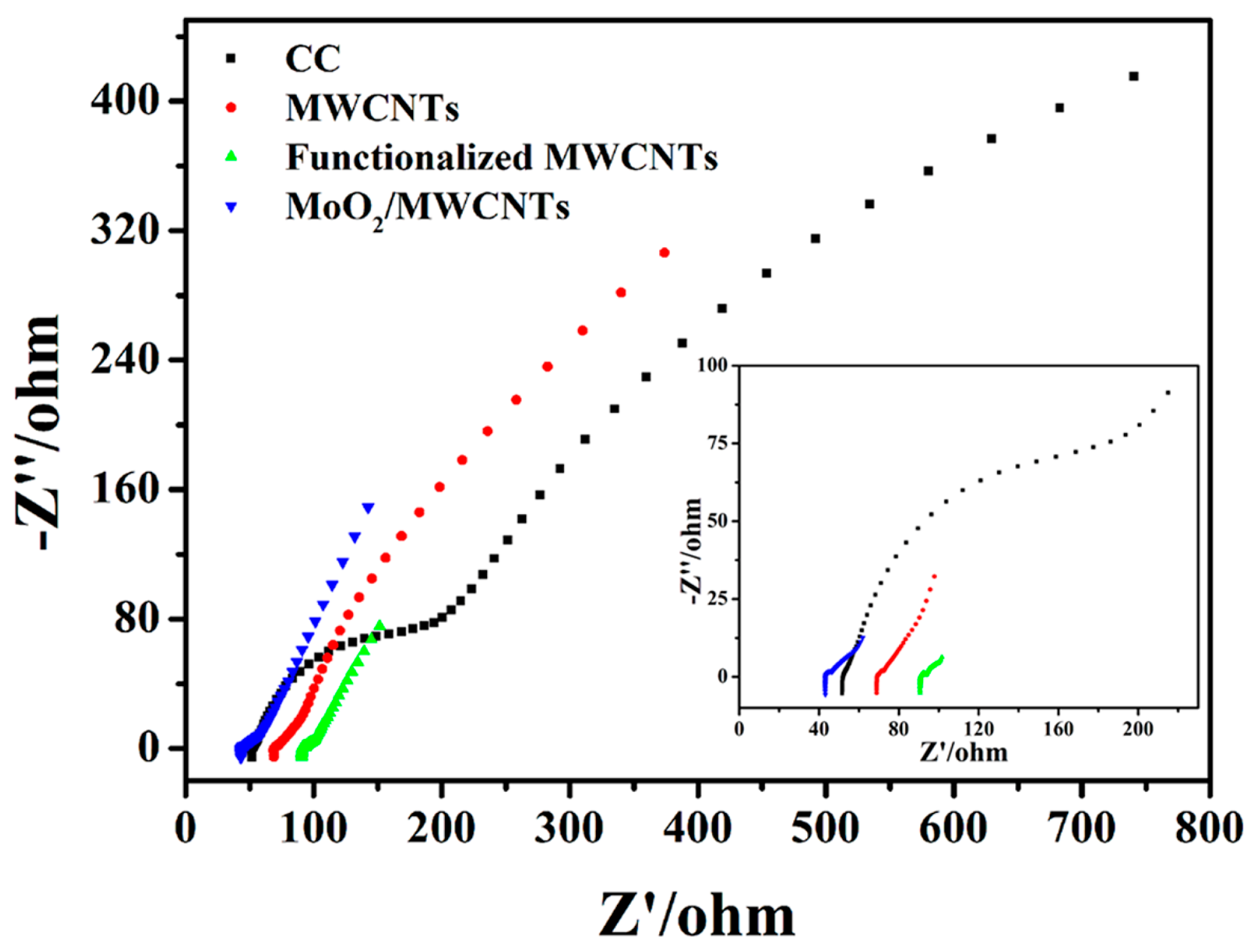
2.2. Electrochemical Behaviors of Electrodes
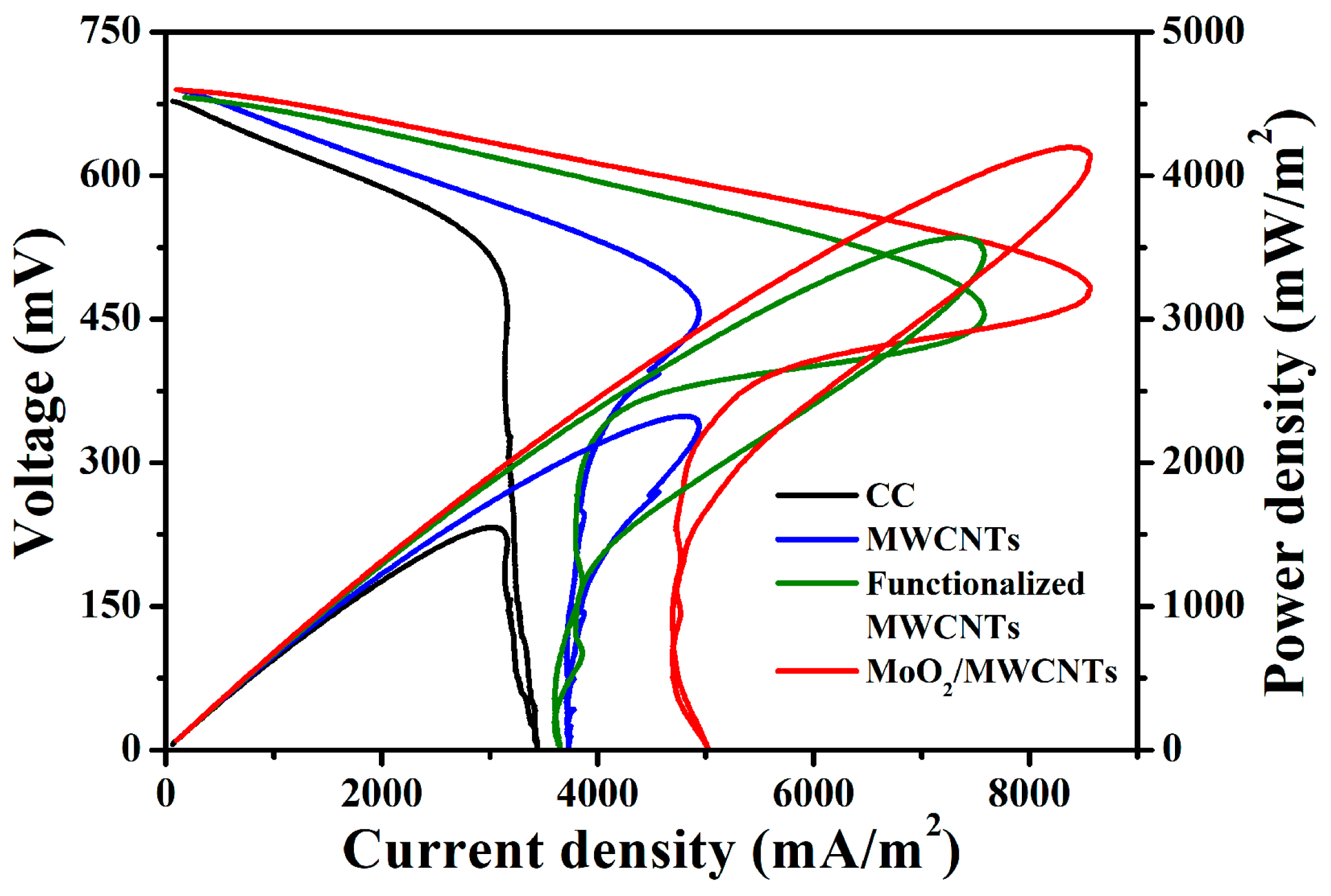
2.3. Investigation of MFC Performance
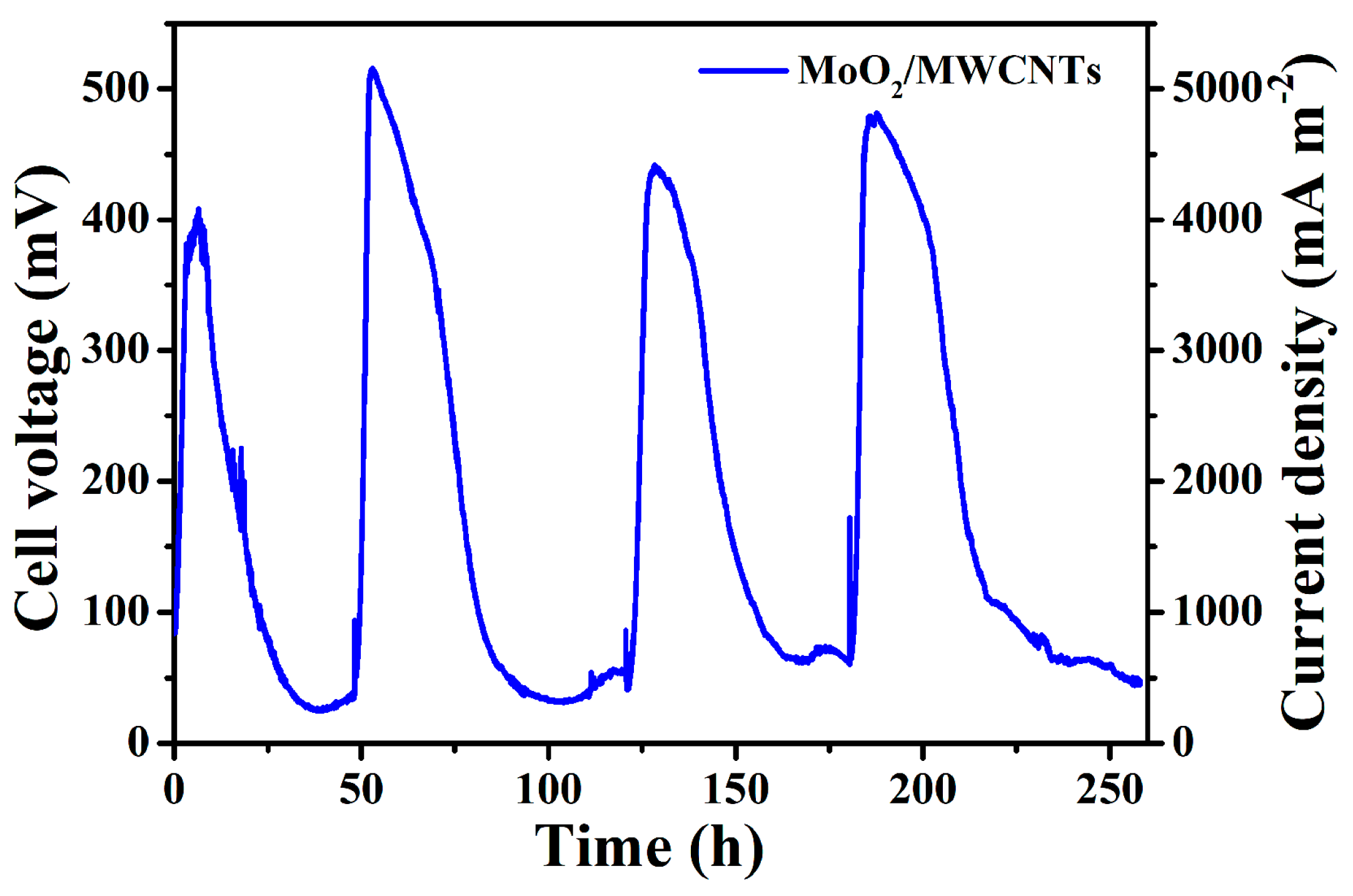
2.4. Discharge Repeatability
3. Experimental Section
3.1. Fabrication and Characterization of Materials
3.2. Electrode Construction
3.3. Establishment, Operation, and Evaluation of E. coli-Inoculated MFCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, M.; Meng, L.; Zhang, Z.; Zheng, Q.; Wang, R.; Yang, C.; Guo, W. Hierarchical modulation of extracellular electron transfer processes in microbial fuel cell anodes for enhanced power output through improved Geobacter adhesion. Electrochim. Acta 2024, 487, 144165. [Google Scholar] [CrossRef]
- Zou, L.; Huang, Y.H.; Wu, X.; Long, Z.E. Synergistically promoting microbial biofilm growth and interfacial bioelectrocatalysis by molybdenum carbide nanoparticles functionalized graphene anode for bioelectricity production. J. Power Sources 2019, 413, 174–181. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Kumar, G.G.; Awan, Z.; Nahm, K.S.; Xavier, J.S. Nanotubular MnO2/graphene oxide composites for the application of open air-breathing cathode microbial fuel cells. Biosens. Bioelectron. 2014, 53, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Chouler, J.; Padgett, G.A.; Cameron, P.J.; Preuss, K.; Titirici, M.-M.; Ieropoulos, I.; Di Lorenzo, M. Towards effective small scale microbial fuel cells for energy generation from urine. Electrochim. Acta 2016, 192, 89–98. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, K.; Li, H.; Dai, Y.; Zhang, H.; Zuo, J.; Yan, J.; Xiao, T.; Liu, X.; Lu, Y.; et al. Bimetallic hybrids modified with carbon nanotubes as cathode catalysts for microbial fuel cell: Effective oxygen reduction catalysis and inhibition of biofilm formation. J. Power Sources 2021, 485, 229273. [Google Scholar] [CrossRef]
- Ma, J.C.; Zhang, J.; Zhang, Y.Z.; Guo, Q.L.; Hu, T.J.; Xiao, H.; Jia, J.F. Progress on anodic modification materials and future development directions in microbial fuel cells. J. Power Sources 2023, 556, 232486. [Google Scholar] [CrossRef]
- Rossi, R.; Cario, B.P.; Santoro, C.; Yang, W.; Saikaly, P.E.; Logan, B.E. Evaluation of electrode and solution area-based resistances enables quantitative comparisons of factors impacting microbial fuel cell performance. Environ. Sci. Technol. 2019, 53, 3977–3986. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, N.; Pannipara, M.; Al-Sehemi, A.G.; Kumar, G.G. PEDOT/NiFe2O4 nanocomposites on biochar as a free-standing anode for high-performance and durable microbial fuel cells. New J. Chem. 2019, 43, 7743–7750. [Google Scholar] [CrossRef]
- Marks, S.; Makinia, J.; Fernandez-Morales, F.J. Performance of microbial fuel cells operated under anoxic conditions. Appl. Energy 2019, 250, 1–6. [Google Scholar] [CrossRef]
- Li, B.; Zhou, J.; Zhou, X.; Wang, X.; Li, B.; Santoro, C.; Grattieri, M.; Babanova, S.; Artyushkova, K.; Atanassov, P.; et al. Surface modification of microbial fuel cells anodes: Approaches to practical design. Electrochim. Acta 2014, 134, 116–126. [Google Scholar] [CrossRef]
- Gnana Kumar, G.; Kirubaharan, C.J.; Udhayakumar, S.; Ramachandran, K.; Karthikeyan, C.; Renganathan, R.; Nahm, K.S. Synthesis, Structural, and Morphological Characterizations of Reduced Graphene Oxide-Supported Polypyrrole Anode Catalysts for Improved Microbial Fuel Cell Performances. ACS Sustain. Chem. Eng. 2014, 2, 2283–2290. [Google Scholar] [CrossRef]
- Babanova, S.; Jones, J.; Phadke, S.; Lu, M.; Angulo, C.; Garcia, J.; Carpenter, K.; Cortese, R.; Chen, S.; Phan, T.T.; et al. Continuous flow, large-scale, microbial fuel cell system for the sustained treatment of swine waste. Water Environ. Res. 2020, 92, 60–72. [Google Scholar] [CrossRef]
- Li, F.; Wang, D.; Liu, Q.; Wang, B.; Zhong, W.; Li, M.; Liu, K.; Lu, Z.; Jiang, H.; Zhao, Q.; et al. The construction of rod-like polypyrrole network on hard magnetic porous textile anodes for microbial fuel cells with ultra-high output power density. J. Power Sources 2019, 412, 514–519. [Google Scholar] [CrossRef]
- Yu, B.; Li, Y.; Feng, L. Enhancing the performance of soil microbial fuel cells by using a bentonite-Fe and Fe3O4 modified anode. J. Hazard. Mater. 2019, 377, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, D.; Yan, M.; Zhang, L.; Chang, W.; Sun, Z.; Liu, S.; Guo, C. Three-dimensional high performance free-standing anode by one-step carbonization of pinecone in microbial fuel cells. Bioresour. Technol. 2019, 292, 121956. [Google Scholar] [CrossRef]
- Gnana Kumar, G.; Kirubaharan, C.J.; Yoo, D.J.; Kim, A.R. Graphene/poly(3,4-ethylenedioxythiophene)/Fe3O4 nanocomposite—An efficient oxygen reduction catalyst for the continuous electricity production from wastewater treatment microbial fuel cells. Int. J. Hydrogen Energy 2016, 41, 13208–13219. [Google Scholar] [CrossRef]
- Catal, T.; Kul, A.; Atalay, V.E.; Bermek, H.; Ozilhan, S.; Tarhan, N. Efficacy of microbial fuel cells for sensing of cocaine metabolites in urine-based wastewater. J. Power Sources 2019, 414, 1–7. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lai, Y.-C.; Lin, C.-W. Enhancement of power generation by microbial fuel cells in treating toluene-contaminated groundwater: Developments of composite anodes with various compositions. Appl. Energy 2019, 233–234, 922–929. [Google Scholar] [CrossRef]
- Ren, P.; Ci, S.; Ding, Y.; Wen, Z. Molten-salt-mediated synthesis of porous Fe-containing N-doped carbon as efficient cathode catalysts for microbial fuel cells. Appl. Surf. Sci. 2019, 481, 1206–1212. [Google Scholar] [CrossRef]
- Kirubaharan, C.J.; Kumar, G.G.; Sha, C.; Zhou, D.; Yang, H.; Nahm, K.S.; Raj, B.S.; Zhang, Y.; Yong, Y.-C. Facile fabrication of Au@polyaniline core-shell nanocomposite as efficient anodic catalyst for microbial fuel cells. Electrochim. Acta 2019, 328, 135136. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, Z.; Li, X.; Zhang, M.; Song, H. Good microbial affinity of phenolic carbon felt as an efficient anode for microbial fuel cells. Bioelectrochemistry 2021, 138, 107700. [Google Scholar] [CrossRef]
- Muthukumar, H.; Mohammed, S.N.; Chandrasekaran, N.; Sekar, A.D.; Pugazhendhi, A.; Matheswaran, M. Effect of iron doped zinc oxide nanoparticles coating in the anode on current generation in microbial electrochemical cells. Int. J. Hydrogen Energy 2019, 44, 2407–2416. [Google Scholar] [CrossRef]
- Mehdinia, A.; Ziaei, E.; Jabbari, A. Multi-walled carbon nanotube/SnO2 nanocomposite: A novel anode material for microbial fuel cells. Electrochim. Acta 2014, 130, 512–518. [Google Scholar] [CrossRef]
- Mohan, Y.; Das, D. Effect of ionic strength, cation exchanger and inoculum age on the performance of microbial fuel cells. Int. J. Hydrogen Energy 2009, 34, 7542–7546. [Google Scholar] [CrossRef]
- Li, H.; Liao, B.; Xiong, J.; Zhou, X.; Zhi, H.; Liu, X.; Li, X.; Li, W. Power output of microbial fuel cell emphasizing interaction of anodic binder with bacteria. J. Power Sources 2018, 379, 115–122. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Wu, C.-C.; Lee, C.-Y.; Shih, E.P. Microbial fuel cell performance of multiwall carbon nanotubes on carbon cloth as electrodes. J. Power Sources 2009, 194, 199–205. [Google Scholar] [CrossRef]
- Sun, J.-J.; Zhao, H.-Z.; Yang, Q.-Z.; Song, J.; Xue, A. A novel layer-by-layer self-assembled carbon nanotube-based anode: Preparation, characterization, and application in microbial fuel cell. Electrochim. Acta 2010, 55, 3041–3047. [Google Scholar] [CrossRef]
- Peng, L.; You, S.-J.; Wang, J.-Y. Carbon nanotubes as electrode modifier promoting direct electron transfer from shewanella oneidensis. Biosens. Bioelectron. 2010, 25, 1248–1251. [Google Scholar] [CrossRef]
- Liang, P.; Wang, H.; Xia, X.; Huang, X.; Mo, Y.; Cao, X.; Fan, M. Carbon nanotube powders as electrode modifier to enhance the activity of anodic biofilm in microbial fuel cells. Biosens. Bioelectron. 2011, 26, 3000–3004. [Google Scholar] [CrossRef]
- Liu, X.-W.; Huang, Y.-X.; Sun, X.-F.; Sheng, G.-P.; Zhao, F.; Wang, S.-G.; Yu, H.-Q. Conductive carbon nanotube hydrogel as a bioanode for enhanced microbial electrocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 8158–8164. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.; Vostrowsky, O. Functionalization of carbon nanotubes. ChemInform 2005, 245, 193–237. [Google Scholar]
- Tasis, D.; Tagmatarchis, N.; Georgakilas, V.; Pantarotto, D.; Vaccari, L.; Bianco, A.; Guldi, D.M.; Prato, M. Organic functionalization of carbon nanotubes. ChemInform 2003, 124, 282–286. [Google Scholar]
- Liu, Y.; Yu, G.; Li, G.D.; Sun, Y.; Asefa, T.; Chen, W.; Zou, X. Coupling Mo2C with nitrogen-rich nanocarbon leads to efficient hydrogen-evolution electrocatalytic sites. Angew. Chem. 2015, 54, 10752–10757. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Gong, C.; Guo, C.X.; Li, C.M. Mo2C/CNTs supported Pd nanoparticles for highly efficient catalyst towards formic acid electrooxidation. J. Mater. Chem. A 2013, 1, 1179–1184. [Google Scholar] [CrossRef]
- Mai, E.F.; Machado, M.A.; Davies, T.E.; Lopez-Sanchez, J.A.; da Silva, V.T. Molybdenum carbide nanoparticles within carbon nanotubes as superior catalysts for γ-valerolactone production via levulinic acid hydrogenation. Green Chem. 2014, 16, 4092–4097. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Watson, G.W.; Payne, D.J.; Atkinson, G.R.; Law, D.S.L. Theoretical and experimental study of the electronic structures of MoO3 and MoO2. J. Phys. Chem. C 2010, 114, 4636–4645. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, B.; Corr, S.A.; Shi, Q.; Hu, Y.-S.; Heier, K.R.; Chen, L.; Seshadri, R.; Stucky, G.D. Ordered mesoporous metallic MoO2 materials with highly reversible lithium storage capacity. Nano Lett. 2009, 9, 4215–4220. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Chen, X.; Li, H.; Xiong, J.; Hu, M.; Li, X.; Li, W. Highly dispersed polydopamine-modified Mo2C/MoO2 nanoparticles as anode electrocatalyst for microbial fuel cells. Electrochim. Acta 2018, 283, 528–537. [Google Scholar] [CrossRef]
- Li, L.X.; Hu, M.; Zeng, L.; Xiong, J.; Tang, B.; Hu, Z.; Xing, L.; Huang, Q.; Li, W. Co-modified MoO2 nanoparticles highly dispersed on N-doped carbon nanorods as anode electrocatalyst of microbial fuel cells. Biosens. Bioelectron. 2019, 145, 111727. [Google Scholar] [CrossRef]
- Hu, F.M.; Qiu, Z.H.; Zhang, Z.Q.; Zheng, J.Y.; He, L.J.; Gao, H.P.; Lin, C.G. In-situ growth of N@MoO2 microflowers on carbon cloth for high-performance anodes in microbial fuel cells. J. Environ. Chem. Eng. 2022, 10, 107869. [Google Scholar] [CrossRef]
- Gao, X.T.; Wang, Y.F.; Fu, L.; Zhang, R.X.; Li, R.M.; Gao, Z.H.; Yan, Z.F.; Liu, Y.M.; Huang, W.; Liu, L.; et al. High-performance Mo2C/MWCNT electrocatalyst for MOR: Comparison with MoO2/MWCNT and MoO3/MWCNT. Int. J. Hydrogen Energy 2023, 48, 32408–32419. [Google Scholar] [CrossRef]
- Guan, J.P.; Zhao, L.P.; Xing, C.Y.; Li, Y.J. Novel flexible MWCNTs@MoO2-C nanocable composites with excellent electrochemical performance for lithium ion battery anodes. Mater. Res. Express 2015, 2, 095502. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, L. A study of the electrical properties of carbon nanotube-NiFe2O4 composites: Effect of the surface treatment of the carbon nanotubes. Carbon 2005, 43, 47–52. [Google Scholar] [CrossRef]
- Xiong, J.; Hu, M.; Li, X.; Li, H.; Li, X.; Liu, X.; Cao, G.; Li, W. Porous graphite: A facile synthesis from ferrous gluconate and excellent performance as anode electrocatalyst of microbial fuel cell. Biosens. Bioelectron. 2018, 109, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, S.; Yuan, Y.; Cai, X.; Zhou, S. In situ formation of graphene layers on graphite surfaces for efficient anodes of microbial fuel cells. Biosens. Bioelectron. 2015, 71, 387–395. [Google Scholar] [CrossRef]
- Mo, C.; Jian, J.; Li, J.; Fang, Z.; Zhao, Z.; Yuan, Z.; Yang, M.; Zhang, Y.; Dai, L.; Yu, D. Boosting water oxidation on metal-free carbon nanotubes via directional interfacial charge-transfer induced by adsorbed polyelectrolyte. Energy Environ. Sci. 2018, 11, 3334–3341. [Google Scholar] [CrossRef]
- Ma, J.; Shi, N.; Zhang, Y.; Zhang, J.; Hu, T.; Xiao, H.; Tang, T.; Jia, J. Facile preparation of polyelectrolyte-functionalized reduced graphene oxide for significantly improving the performance of microbial fuel cells. J. Power Sources 2020, 450, 227628. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef]
- Wang, R.; Yan, M.; Li, H.; Zhang, L.; Peng, B.; Sun, J.; Liu, D.; Liu, S. FeS2 nanoparticles decorated graphene as microbial-fuel-cell anode achieving high power density. Adv. Mater. 2018, 30, 1800618. [Google Scholar] [CrossRef]
- Bian, B.; Shi, D.; Cai, X.; Hu, M.; Guo, Q.; Zhang, C.; Wang, Q.; Sun, A.X.; Yang, J. 3D printed porous carbon anode for enhanced power generation in microbial fuel cell. Nano Energy 2018, 44, 174–180. [Google Scholar] [CrossRef]
- Liu, J.; Rinzler, A.G.; Dai, H.J.; Hafner, J.H.; Bradley, R.K.; Boul, P.J.; Lu, A.; Iverson, T.; Shelimov, K.; Huffman, C.B.; et al. Fullerene pipes. Science 1998, 280, 1253–1255. [Google Scholar] [CrossRef]
- Ma, J.; Shi, N.; Jia, J. Fe3O4 nanospheres decorated reduced graphene oxide as anode to promote extracellular electron transfer efficiency and power density in microbial fuel cells. Electrochim. Acta 2020, 362, 137126. [Google Scholar] [CrossRef]
- Zhang, T.; Cui, C.; Chen, S.; Ai, X.; Yang, H.; Ping, S.; Peng, Z. A novel mediatorless microbial fuel cell based on direct biocatalysis of Escherichia coli. Chem. Commun. 2006, 21, 2257–2259. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Wang, L.; Zhang, Y.; Jia, J. Fabrication of a Molybdenum Dioxide/Multi-Walled Carbon Nanotubes Nanocomposite as an Anodic Modification Material for High-Performance Microbial Fuel Cells. Molecules 2024, 29, 2541. https://doi.org/10.3390/molecules29112541
Ma J, Wang L, Zhang Y, Jia J. Fabrication of a Molybdenum Dioxide/Multi-Walled Carbon Nanotubes Nanocomposite as an Anodic Modification Material for High-Performance Microbial Fuel Cells. Molecules. 2024; 29(11):2541. https://doi.org/10.3390/molecules29112541
Chicago/Turabian StyleMa, Jianchun, Lifang Wang, Yezhen Zhang, and Jianfeng Jia. 2024. "Fabrication of a Molybdenum Dioxide/Multi-Walled Carbon Nanotubes Nanocomposite as an Anodic Modification Material for High-Performance Microbial Fuel Cells" Molecules 29, no. 11: 2541. https://doi.org/10.3390/molecules29112541
APA StyleMa, J., Wang, L., Zhang, Y., & Jia, J. (2024). Fabrication of a Molybdenum Dioxide/Multi-Walled Carbon Nanotubes Nanocomposite as an Anodic Modification Material for High-Performance Microbial Fuel Cells. Molecules, 29(11), 2541. https://doi.org/10.3390/molecules29112541




