Hydrogen Production by Steam Reforming of Ethanol and Dry Reforming of Methane with CO2 on Ni/Vermiculite: Stability Improvement via Acid or Base Treatment of the Support
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
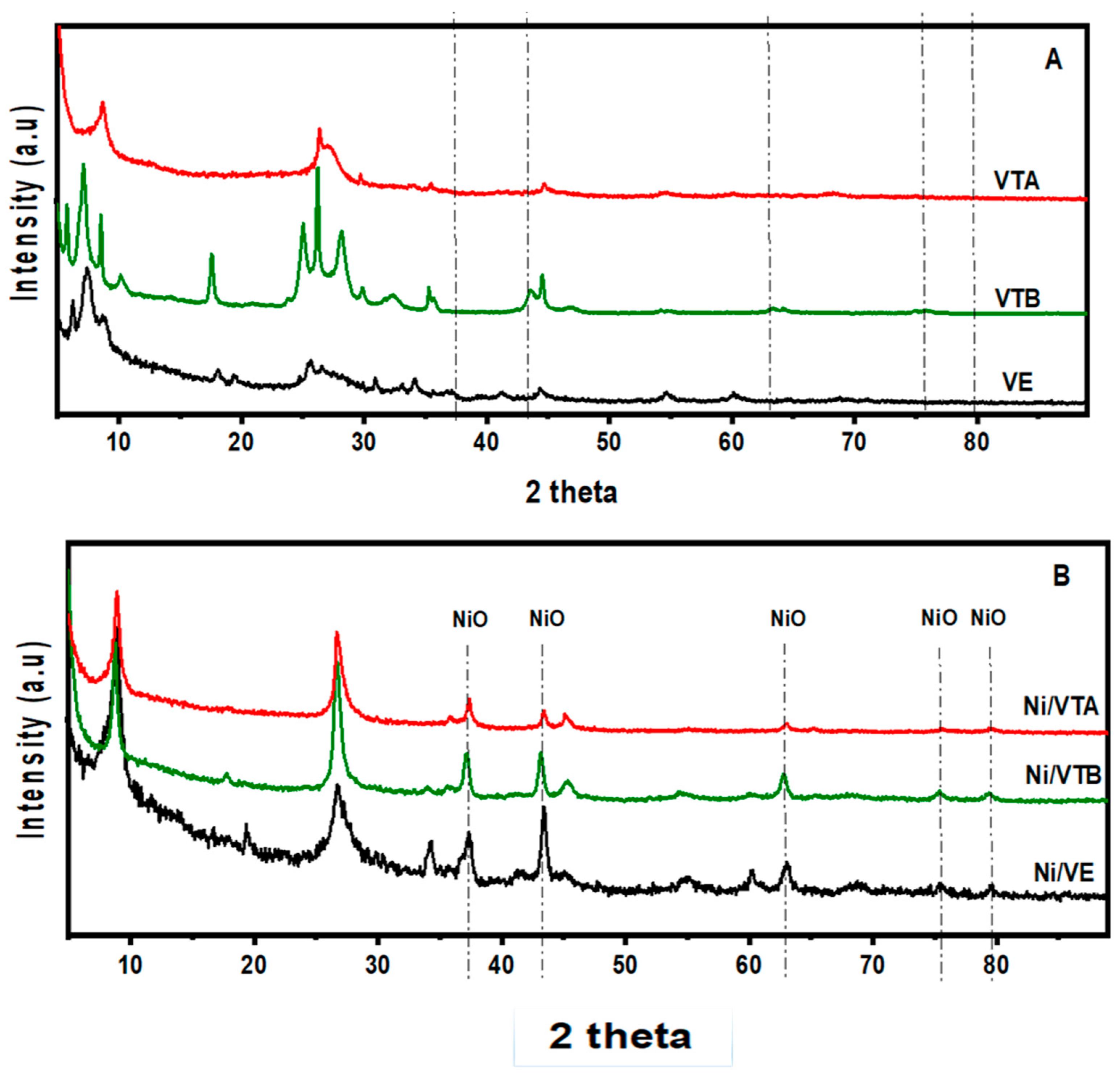
2.1.1. X-ray Diffraction and Surface Area
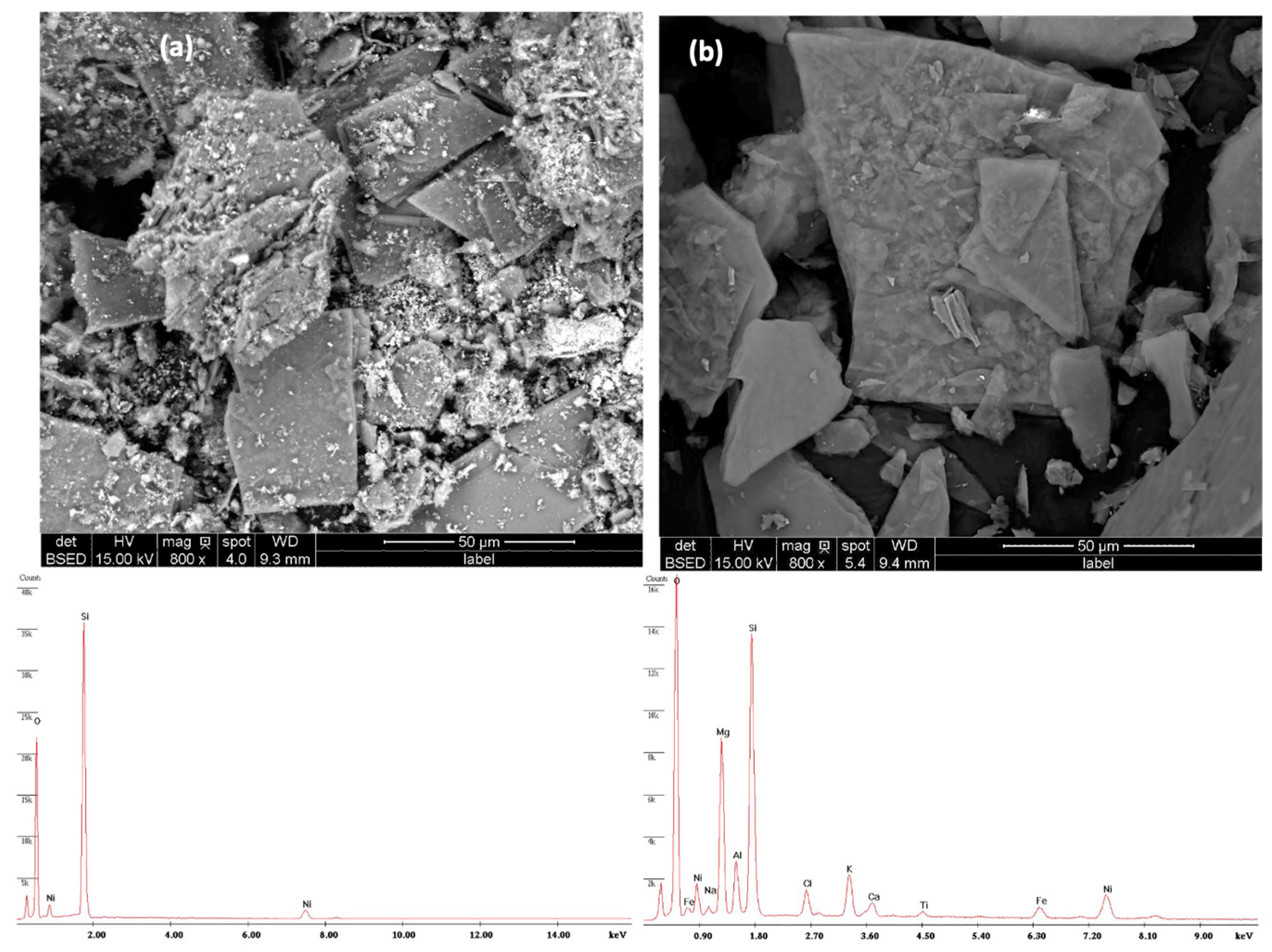
2.1.2. SEM–EDS
2.1.3. FTIR Spectroscopy
2.1.4. TPR Analyses
2.2. Catalytic Activity
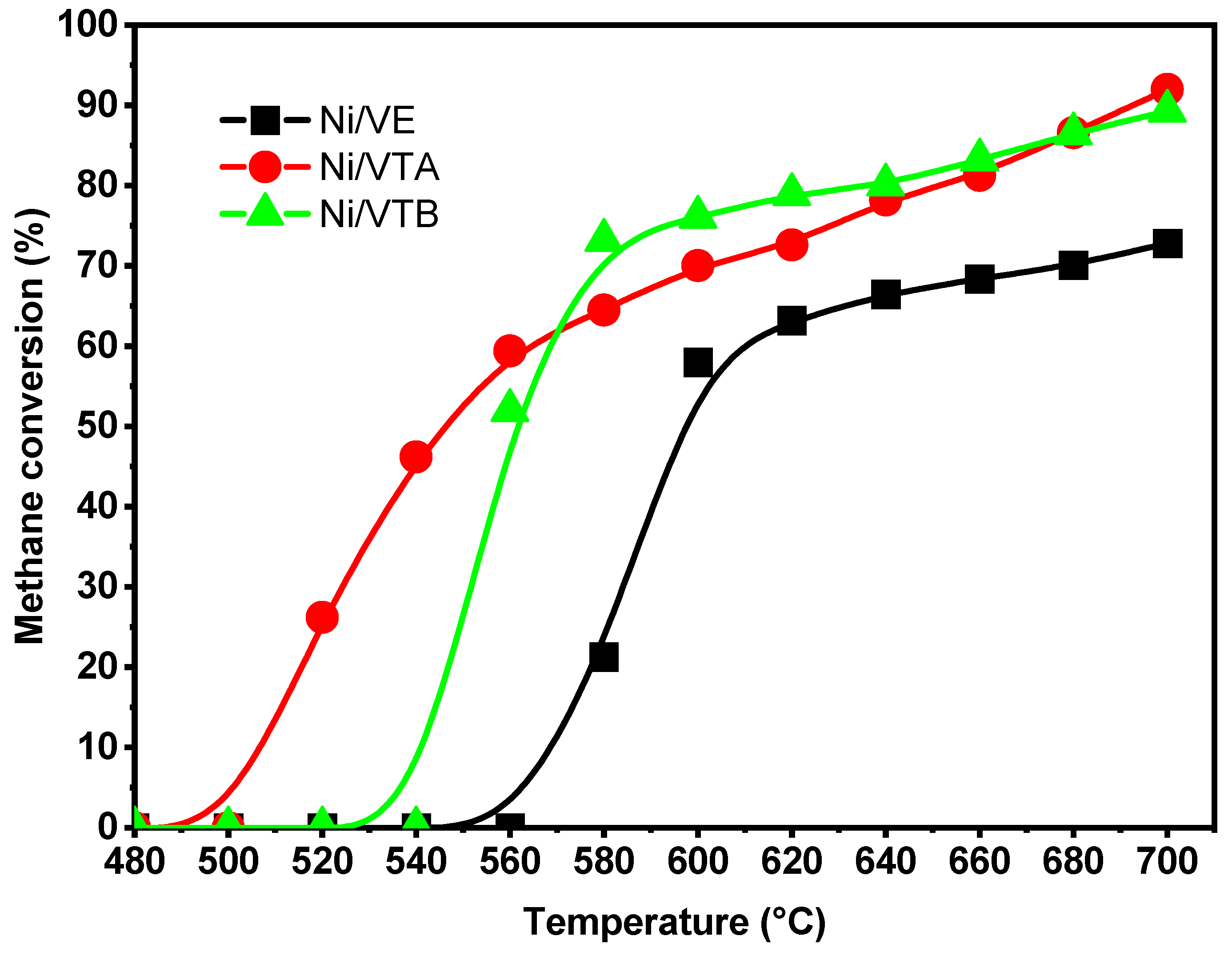
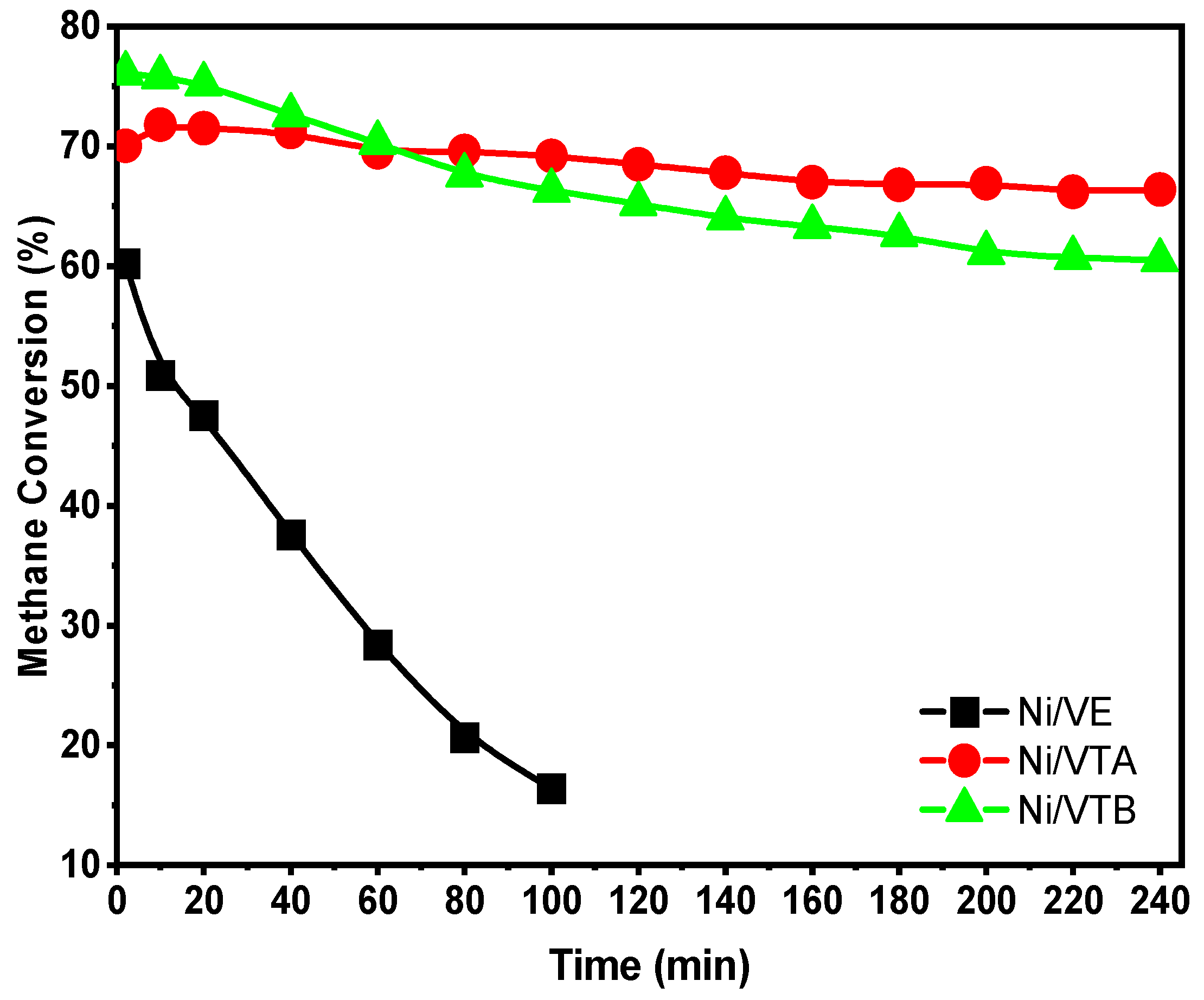
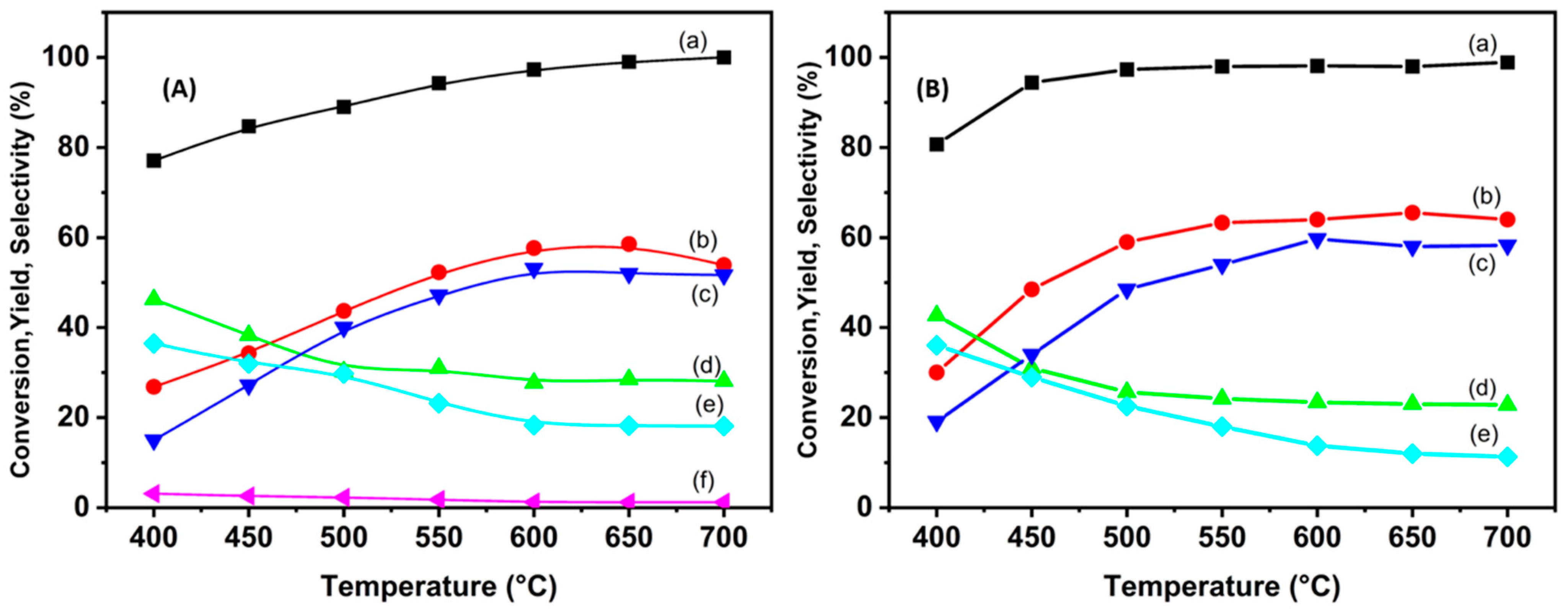
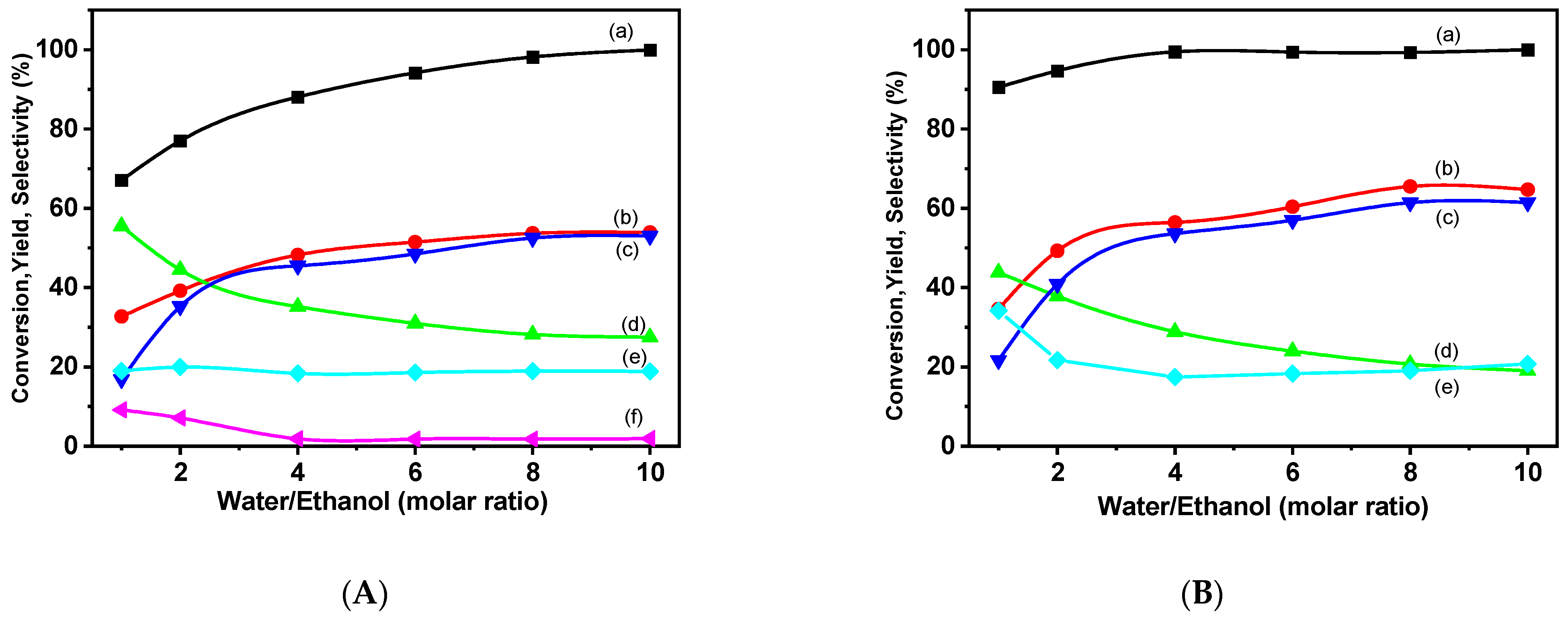
2.2.1. Dry Reforming of Methane
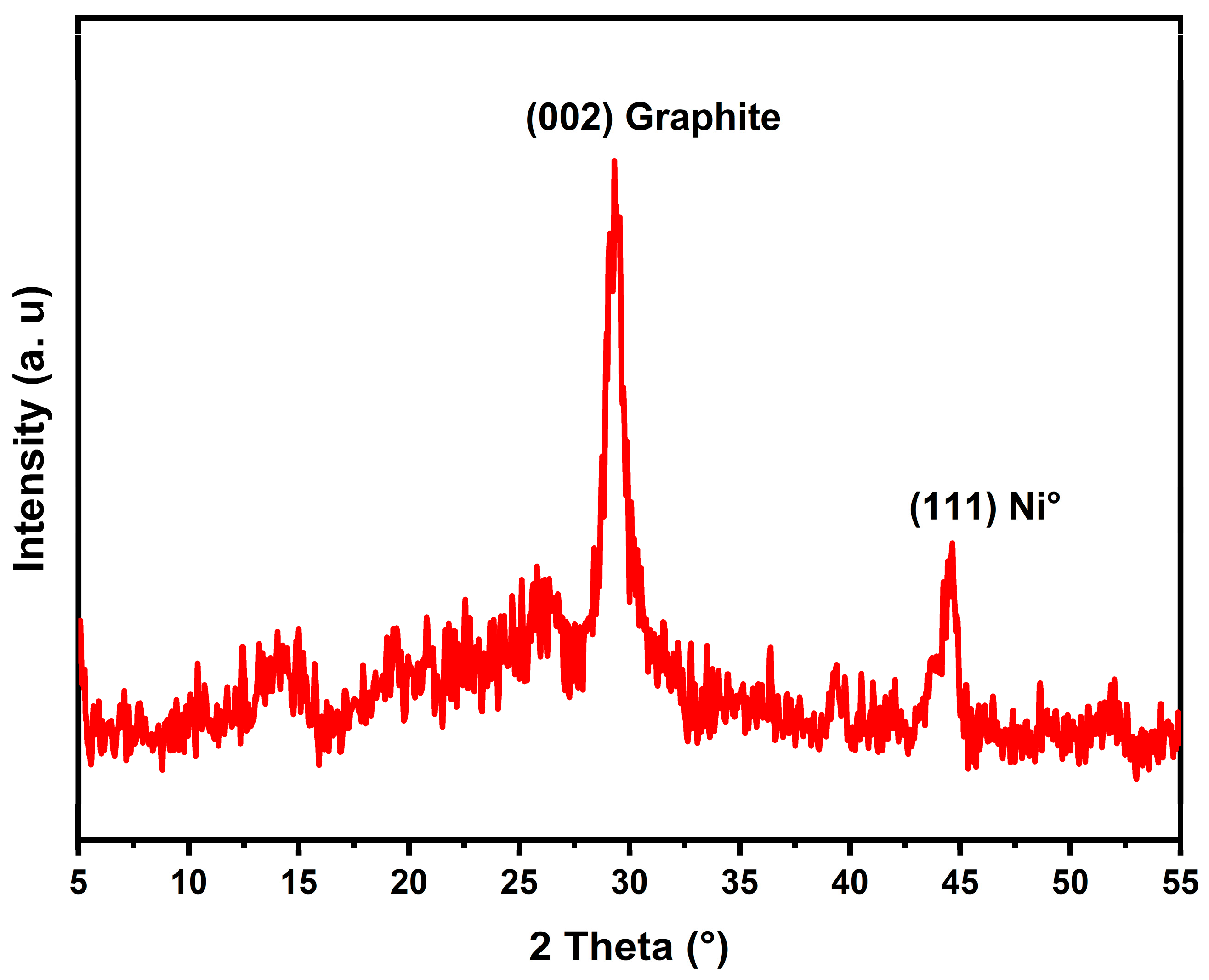
2.2.2. Spent Catalysts Characterization (TGA-TDA and XRD)
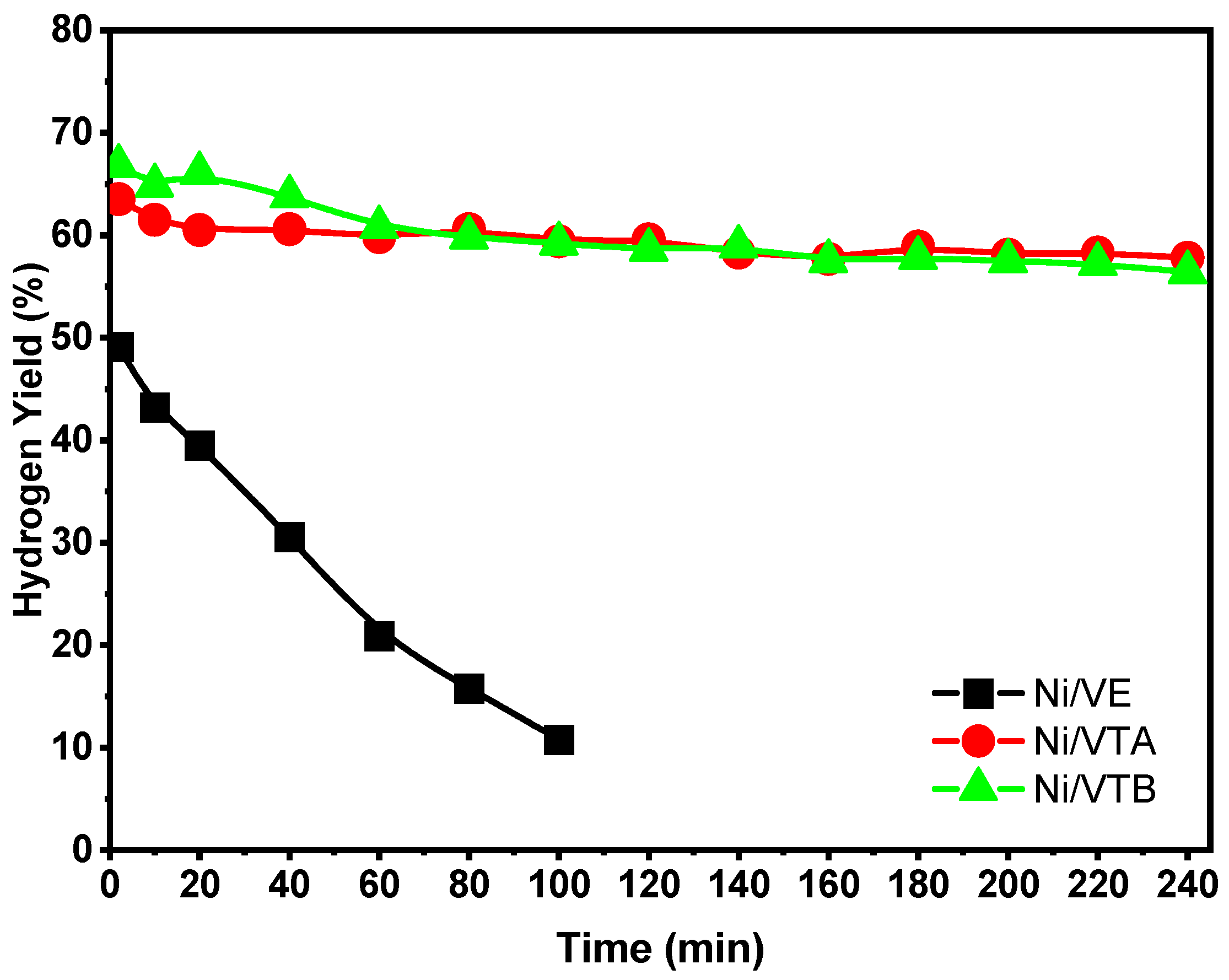
2.2.3. Ethanol Steam Reforming
3. Experimental
3.1. Catalyst Preparation
3.2. Characterization Methods
3.3. Catalytic Tests
3.3.1. Methane Reforming Reaction
3.3.2. Ethanol Steam Reforming (ESR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vermeiren, W.J.M.; Blomsma, E.; Jacobs, P.A. Catalytic and thermodynamic approach of the oxyreforming reaction of methane. Catal. Today 1992, 13, 427–436. [Google Scholar] [CrossRef]
- Corthals, S.; Van Nederkassel, J.; Geboers, J.; De Winne, H.; Van Noyen, J.; Moens, B.; Sels, B.; Jacobs, P. Influence of composition of MgAl2O4 supported NiCeO2ZrO2 catalysts on coke formation and catalyst stability for dry reforming of methane. Catal. Today 2008, 138, 28–32. [Google Scholar] [CrossRef]
- Barroso-Quiroga, M.M.; Castro-Luna, A.E. Catalytic activity and effect of modifiers on Ni-based catalysts for the dry reforming of methane. Int. J. Hydrogen Energy 2010, 35, 6052–6056. [Google Scholar] [CrossRef]
- Asami, K.; Li, X.; Fujimoto, K.; Koyama, Y.; Sakurama, A.; Kometani, N.; Yonezawa, Y. CO2 reforming of CH4 over ceria-supported metal catalysts. Catal. Today 2003, 84, 27–31. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 Reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Rostrupnielsen, J.R.; Hansen, J.H.B. CO2-Reforming of Methane over Transition Metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Selvarajah, K.; Phuc, N.H.H.; Abdullah, B.; Alenazey, F.; Vo, D.-V.N. Syngas production from methane dry reforming over Ni/Al2O3 catalyst. Res. Chem. Intermed. 2016, 42, 269–288. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Román-Martínez, M.C.; Illán-Gómez, M.J. Nickel catalyst activation in the carbon dioxide reforming of methane: Effect of pretreatments. Appl. Catal. A Gen. 2009, 355, 27–32. [Google Scholar] [CrossRef]
- Boukha, Z.; Kacimi, M.; Pereira, M.F.R.; Faria, J.L.; Figueiredo, J.L.; Ziyad, M. Methane dry reforming on Ni loaded hydroxyapatite and fluoroapatite. Appl. Catal. A Gen. 2007, 317, 299–309. [Google Scholar] [CrossRef]
- Mesrar, F.; Kacimi, M.; Liotta, L.F.; Puleo, F.; Ziyad, M. Hydrogen production on Ni loaded apatite-like oxide synthesized by dissolution-precipitation of natural phosphate. Int. J. Hydrogen Energy 2017, 42, 19458–19466. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Chen, R.; Liu, X. Vermiculite as a natural silicate crystal for hydrogen generation from photocatalytic splitting of water under visible light. RSC Adv. 2014, 4, 406–408. [Google Scholar] [CrossRef]
- Gharin Nashtifan, S.; Azadmehr, A.; Maghsoudi, A. Comparative and competitive adsorptive removal of Ni2+ and Cu2+ from aqueous solution using iron oxide-vermiculite composite. Appl. Clay Sci. 2017, 140, 38–49. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Zhu, X.-H. Competitive Adsorption of Ag+, Pb2+, Ni2+, and Cd2+ Ions on Vermiculite. Sep. Sci. Technol. 2010, 45, 277–287. [Google Scholar] [CrossRef]
- Chmielarz, L.; Rutkowska, M.; Jabłońska, M.; Węgrzyn, A.; Kowalczyk, A.; Boroń, P.; Piwowarska, Z.; Matusiewicz, A. Acid-treated vermiculites as effective catalysts of high-temperature N2O decomposition. Appl. Clay Sci. 2014, 101, 237–245. [Google Scholar] [CrossRef]
- Chmielarz, L.; Wojciechowska, M.; Rutkowska, M.; Adamski, A.; Węgrzyn, A.; Kowalczyk, A.; Dudek, B.; Boroń, P.; Michalik, M.; Matusiewicz, A. Acid-activated vermiculites as catalysts of the DeNOx process. Catal. Today 2012, 191, 25–31. [Google Scholar] [CrossRef]
- Xin, H.; Yu, F.; Zhu, M.-Y.; Ouyang, F.-H.; Dai, B.; Dan, J.-M. Hydrochlorination of acetylene using expanded multilayered vermiculite (EML-VMT)-supported catalysts. Chin. Chem. Lett. 2015, 26, 1101–1104. [Google Scholar] [CrossRef]
- Marcos, C.; Arango, Y.C.; Rodriguez, I. X-ray diffraction studies of the thermal behaviour of commercial vermiculites. Appl. Clay Sci. 2009, 42, 368–378. [Google Scholar] [CrossRef]
- Węgrzyn, A.; Chmielarz, L.; Zjeżdżałka, P.; Jabłońska, M.; Kowalczyk, A.; Żelazny, A.; Vázquez Sulleiro, M.; Michalik, M. Vermiculite-based catalysts for oxidation of organic pollutants in water and wastewater. Acta Geodyn. Geomater. 2013, 10, 341–352. [Google Scholar] [CrossRef]
- Maqueda, C.; Perez-Rodriguez, J.L.; Šubrt, J.; Murafa, N. Study of ground and unground leached vermiculite. Appl. Clay Sci. 2009, 44, 178–184. [Google Scholar] [CrossRef]
- Stawiński, W.; Węgrzyn, A.; Freitas, O.; Chmielarz, L.; Mordarski, G.; Figueiredo, S. Simultaneous removal of dyes and metal cations using an acid, acid-base and base modified vermiculite as a sustainable and recyclable adsorbent. Sci. Total Environ. 2017, 576, 398–408. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, C.L.; Shen, S.L.; Zhang, K.; Meng, H.; Guo, K.; Chen, J.F. Effects of silica sources on the properties of magnetic hollow silica. Colloids Surf. A Physicochem. Eng. Asp. 2009, 334, 131–136. [Google Scholar] [CrossRef]
- Kang, K.-M.; Kim, H.-W.; Shim, I.-W.; Kwak, H.-Y. Catalytic test of supported Ni catalysts with core/shell structure for dry reforming of methane. Fuel Process. Technol. 2011, 92, 1236–1243. [Google Scholar] [CrossRef]
- Daza, C.E.; Kiennemann, A.; Moreno, S.; Molina, R. Dry reforming of methane using Ni–Ce catalysts supported on a modified mineral clay. Appl. Catal. A Gen. 2009, 364, 65–74. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Sudarno; Rashid, U.; Zainal, Z. CeO2–SiO2 supported nickel catalysts for dry reforming of methane toward syngas production. Appl. Catal. A Gen. 2013, 468, 359–369. [Google Scholar] [CrossRef]
- Corrente, M.V.; Manfro, R.L.; Souza, M.M.V.M. Hydrogen production by biogas reforming using Ni/MgO-Al2O3 catalysts. Fuel 2024, 368, 131561. [Google Scholar] [CrossRef]
- Alipour, H.; Alavi, S.M.; Rezaei, M.; Akbari, E.; Varbar, M. The role of various preparation techniques and nickel loadings in ethanol steam reforming over mesoporous nanostructured Ni–Al2O3 catalysts. J. Energy Inst. 2024, 112, 101488. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Shi, W. Syngas production from CO2 reforming with methane over core-shell Ni@SiO2 catalysts. J. CO2 Util. 2016, 16, 318–327. [Google Scholar] [CrossRef]
- Koo, K.Y.; Roh, H.-S.; Seo, Y.T.; Seo, D.J.; Yoon, W.L.; Park, S.B. Coke study on MgO-promoted Ni/Al2O3 catalyst in combined H2O and CO2 reforming of methane for gas to liquid (GTL) process. Appl. Catal. A Gen. 2008, 340, 183–190. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, Y.; Li, H.; Zhang, J.; Shi, L.; Zhang, D. Sc promoted and aerogel confined Ni catalysts for coking-resistant dry reforming of methane. RSC Adv. 2017, 7, 4735–4745. [Google Scholar] [CrossRef]
- Li, M.; van Veen, A.C. Coupled reforming of methane to syngas (2H2-CO) over Mg-Al oxide supported Ni catalyst. Appl. Catal. A Gen. 2018, 550, 176–183. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J.L.G.; Sener, C.; Dogu, T. MCM-41 supported PdNi catalysts for dry reforming of methane. Appl. Catal. B Environ. 2009, 92, 250–261. [Google Scholar] [CrossRef]
- Zhang, W.D.; Liu, B.S.; Tian, Y.L. CO2 reforming of methane over Ni/Sm2O3–CaO catalyst prepared by a sol–gel technique. Catal. Commun. 2007, 8, 661–667. [Google Scholar] [CrossRef]
- Baharudin, L.; Rahmat, N.; Othman, N.H.; Shah, N.; Syed-Hassan, S.S.A. Formation, control, and elimination of carbon on Ni-based catalyst during CO2 and CH4 conversion via dry reforming process: A review. J. CO2 Util. 2022, 61, 102050. [Google Scholar] [CrossRef]
- Pinilla, J.L.; de Llobet, S.; Moliner, R.; Suelves, I. Ni-Co bimetallic catalysts for the simultaneous production of carbon nanofibres and syngas through biogas decomposition. Appl. Catal. B Environ. 2017, 200, 255–264. [Google Scholar] [CrossRef]
- Yoshida, H.; Yamaoka, R.; Arai, M. Stable Hydrogen Production from Ethanol through Steam Reforming Reaction over Nickel-Containing Smectite-Derived Catalyst. Int. J. Mol. Sci. 2015, 16, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Trevisanut, C.; Mari, M.; Millet, J.-M.M.; Cavani, F. Chemical-loop reforming of ethanol over metal ferrites: An analysis of structural features affecting reactivity. Int. J. Hydrogen Energy 2015, 40, 5264–5271. [Google Scholar] [CrossRef]
- Carrasco-Ruiz, S.; Zhang, Q.; Gándara-Loe, J.; Pastor-Pérez, L.; Odriozola, J.A.; Reina, T.R.; Bobadilla, L.F. H2-rich syngas production from biogas reforming: Overcoming coking and sintering using bimetallic Ni-based catalysts. Int. J. Hydrogen Energy 2023, 48, 27907–27917. [Google Scholar] [CrossRef]
- Yang, E.; Nam, E.; Jo, Y.; An, K. Coke resistant NiCo/CeO2 catalysts for dry reforming of methane derived from core@shell Ni@Co nanoparticles. Appl. Catal. B Environ. 2023, 339, 123152. [Google Scholar] [CrossRef]
- Wang, M.; Kim, S.Y.; Jamsaz, A.; Pham-Ngoc, N.; Men, Y.; Jeong, D.H.; Shin, E.W. Effect of active sites distributions on temperature dependent–coke formation over Ni/CexZr1−xO2–Al2O3 catalysts for ethanol steam reforming: Coke precursor gasification. Appl. Surf. Sci. 2024, 644, 158746. [Google Scholar] [CrossRef]
- Feng, X.; Zhao, Y.; Zhao, Y.; Wang, H.; Liu, H.; Zhang, Q. A mini review on recent progress of steam reforming of ethanol. RSC Adv. 2023, 13, 23991–24002. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Cortese, M.; Martino, M. Bioalcohol Reforming: An Overview of the Recent Advances for the Enhancement of Catalyst Stability. Catalysts 2020, 10, 665. [Google Scholar] [CrossRef]
- Abdelkader, A.; Daly, H.; Saih, Y.; Morgan, K.; Mohamed, M.A.; Halawy, S.A.; Hardacre, C. Steam reforming of ethanol over Co3O4–Fe2O3 mixed oxides. Int. J. Hydrogen Energy 2013, 38, 8263–8275. [Google Scholar] [CrossRef]
- Braga, A.; dos Santos, J.; Bueno, J.M.C.; Damyanova, S. Hydrogen Production by Ethanol Steam Reforming. Athens Journal of Sciences 2016, 3, 7–16. [Google Scholar] [CrossRef]
- Ob-eye, J.; Praserthdam, P.; Jongsomjit, B. Dehydrogenation of Ethanol to Acetaldehyde over Different Metals Supported on Carbon Catalysts. Catalysts 2019, 9, 66. [Google Scholar] [CrossRef]
- Meng, H.; Zhang, J.; Yang, Y. Recent Status in Catalyst Modification Strategies for Hydrogen Production from Ethanol Steam Reforming. ChemCatChem 2023, 15, e202300733. [Google Scholar] [CrossRef]
- Shtyka, O.; Dimitrova, Z.; Ciesielski, R.; Kedziora, A.; Mitukiewicz, G.; Leyko, J.; Maniukiewicz, W.; Czylkowska, A.; Maniecki, T. Correction to: Steam reforming of ethanol for hydrogen production: Influence of catalyst composition (Ni/Al2O3, Ni/Al2O3–CeO2, Ni/Al2O3–ZnO) and process conditions. React. Kinet. Mech. Catal. 2021, 134, 1089. [Google Scholar] [CrossRef]






| Catalyst | SSA | Pore Volume | Chemical Composition Mass (%) by ICP Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (m2 g−1) | (cm3 g−1) | Ni | SiO2 | MgO | Al2O3 | Fe2O3 | K2O | CaO | Na2O | TiO2 | |
| Ni/VE | 36 | 0.10 | 15.0 | 33.8 | 19.8 | 17.1 | 2.8 | 5.1 | 3.2 | 2.1 | 1.1 |
| Ni/VTB | 38 | 0.11 | 15.0 | 36.4 | 18.6 | 16.6 | 2.6 | 4.9 | 2.9 | 2.0 | 1.0 |
| Ni/VTA | 270 | 0.55 | 15.0 | 78.4 | 2.8 | 2.6 | 0.3 | 0.5 | 0.2 | 0.1 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahir, H.; Benzaouak, A.; Mesrar, F.; El Hamidi, A.; Kacimi, M.; Consentino, L.; Liotta, L.F. Hydrogen Production by Steam Reforming of Ethanol and Dry Reforming of Methane with CO2 on Ni/Vermiculite: Stability Improvement via Acid or Base Treatment of the Support. Molecules 2024, 29, 2575. https://doi.org/10.3390/molecules29112575
Mahir H, Benzaouak A, Mesrar F, El Hamidi A, Kacimi M, Consentino L, Liotta LF. Hydrogen Production by Steam Reforming of Ethanol and Dry Reforming of Methane with CO2 on Ni/Vermiculite: Stability Improvement via Acid or Base Treatment of the Support. Molecules. 2024; 29(11):2575. https://doi.org/10.3390/molecules29112575
Chicago/Turabian StyleMahir, Hanane, Abdellah Benzaouak, Farah Mesrar, Adnane El Hamidi, Mohamed Kacimi, Luca Consentino, and Leonarda Francesca Liotta. 2024. "Hydrogen Production by Steam Reforming of Ethanol and Dry Reforming of Methane with CO2 on Ni/Vermiculite: Stability Improvement via Acid or Base Treatment of the Support" Molecules 29, no. 11: 2575. https://doi.org/10.3390/molecules29112575
APA StyleMahir, H., Benzaouak, A., Mesrar, F., El Hamidi, A., Kacimi, M., Consentino, L., & Liotta, L. F. (2024). Hydrogen Production by Steam Reforming of Ethanol and Dry Reforming of Methane with CO2 on Ni/Vermiculite: Stability Improvement via Acid or Base Treatment of the Support. Molecules, 29(11), 2575. https://doi.org/10.3390/molecules29112575








