Abstract
In this paper, an interesting γ′-carbon 1,6-conjugate addition for phosphine-catalyzed α-succinimide substituted allenoates has been disclosed. A wide array of substrates was found to participate in the reaction, resulting in the production of diverse 4-diarylmethylated 3,4-disubstituted maleimides with satisfactory to outstanding yields. Furthermore, a plausible mechanism for the reaction was proposed by the investigators.
1. Introduction
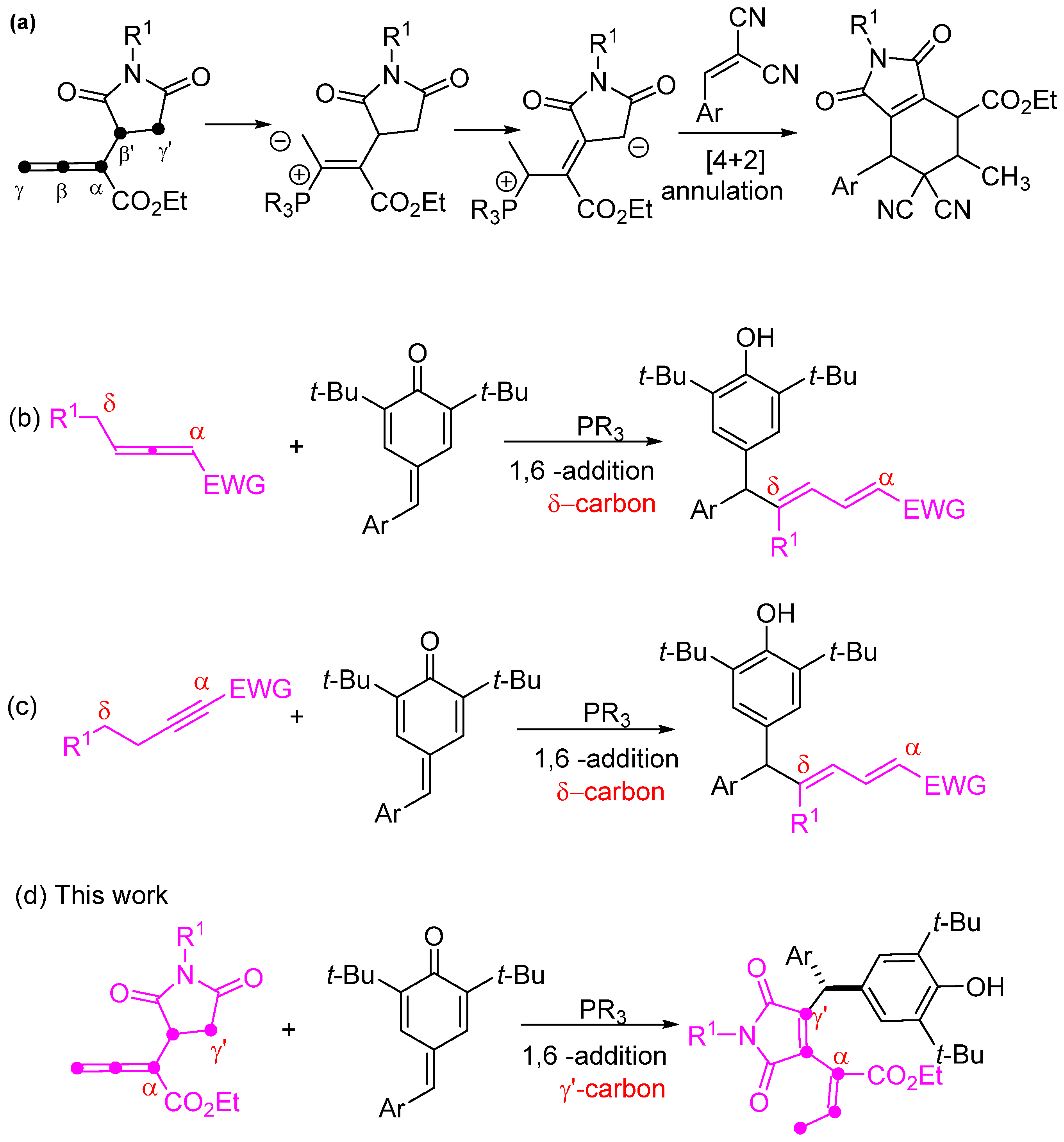
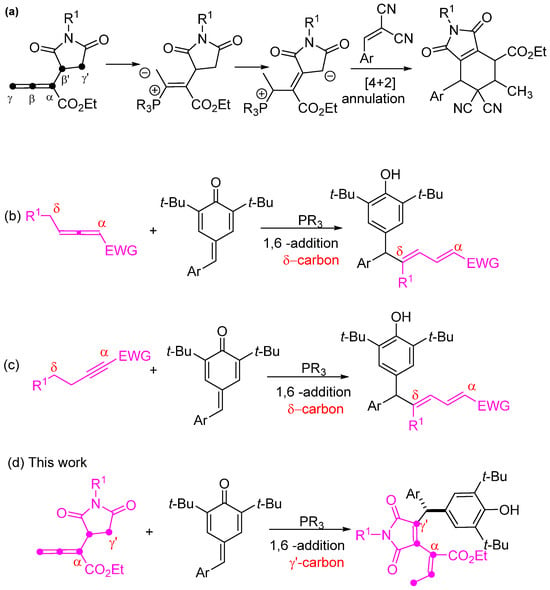
Over the past two decades, there has been a notable growth in phosphine catalysis within organic synthesis. This expansion is attributed to the versatile nature of phosphine, attracting attention from numerous research groups [1,2,3,4,5,6,7,8,9,10,11,12]. Since the pioneering work of Kwon’s group in 2003, where they introduced α-substituted allenoates for phosphine-catalyzed [4 + 2] annulation [13], the potential of these allenoates in phosphine catalysis has been extensively explored. While various electrophilic partners have been employed in phosphine-catalyzed nucleophilic additions or annulations with α-substituted allenoates [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33], there remains value in investigating novel α-substituted allenoates with diverse structures. Such research endeavors hold promise for revealing new reaction pathways and enriching synthetic routes for a wide array of functional molecules. Very recently, our group was the first to report a series of new α-succinimide substituted allenoates, which can undergo a [4 + 2] annulation reaction with 1,1-dicyanoalkenes under phosphine catalysis, involving the γ′-carbon of the allenoates (Scheme 1a) [34]. But there have been very few works related to the γ′-carbon addition of allenoates under phosphine catalysis [35,36]. Therefore, the potential of α-succinimide substituted allenoates in nucleophilic phosphine catalysis remains underexplored. Therefore, continuous exploration of the addition reactions involving this allenoate is highly recommended.
Scheme 1.
Reaction mode of α-succinimide substituted allenoates and overview of 1, 6-conjugated addition of phosphine catalysis. (a) Phosphine-catalyzed α-succinimide substituted allenoate [4 + 2] annulation reaction with 1,1-dicyanoalkenes; (b) Phosphine-catalyzed allenoate isomerization/1,6-conjugate addition reaction with para-quinone methides; (c) Phosphine-catalyzed 1,6-conjugate addition of an alkynoate with para-quinone methides; (d) Phosphine-catalyzed γ′-carbon 1,6-addition of α-succinimide substituted allenoates to para-quinone methides.
In recent years, para-quinone methides have emerged as versatile building blocks for 1,6-conjugate addition reactions, facilitating the synthesis of functionalized diarylmethane derivatives [37,38,39,40,41,42,43,44,45,46,47]. Catalytic reactions play an important role in many transformations of para-quinone methides, and some have been established through the use of catalysts such as Lewis acids, Brønsted acids, bases, transition metals, and N-heterocyclic carbenes [38,48]. In a recent investigation, these 1,6-addition reactions to para-quinone methides taking place under phosphine catalysis were already reported, achieving the phosphine-catalyzed direct dienylation with para-quinone methides. Xu et al. documented a chiral phosphine-catalyzed allenoate isomerization/1,6-conjugate addition cascade reaction with para-quinone methides. The reaction exclusively proceeds via the δ-carbon under phosphine catalysis (Scheme 1b) [49]. Another study by Wang et al. in 2021 revealed a phosphine-catalyzed protocol for the 1,6-conjugate addition of an alkynoate via the δ-carbon with para-quinone methides (Scheme 1c) [50]. To the best of our knowledge, γ′-carbon 1,6-conjugate addition with para-quinone methides catalyzed by phosphine has never been reported before.
Heterocyclic diarylmethane molecules have exhibited a wide range of biological applications [51,52,53,54,55,56,57,58,59]. As part of our ongoing efforts to develop new methodologies for synthesizing functionalized organic compounds under phosphine catalysis, we introduce an innovative and highly regioselective synthesis of 4-diarylmethylated 3,4-disubstituted maleimides. Herein, we report the first example of a phosphine catalyzed γ′-carbon 1,6-addition of α-succinimide substituted allenoates to para-quinone methides (Scheme 1d).
2. Results and Discussion
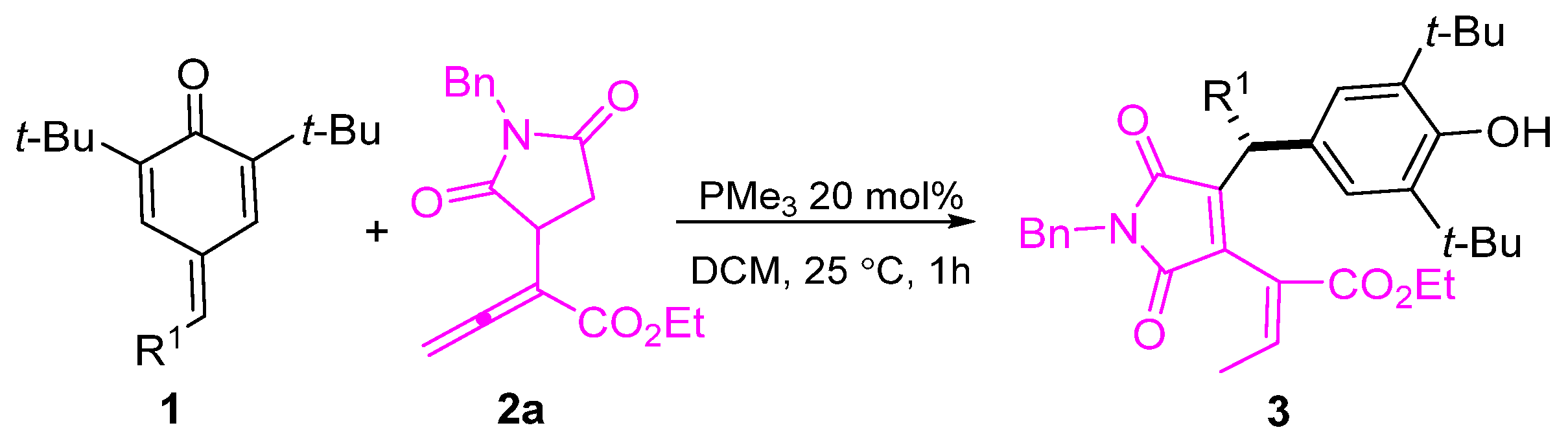
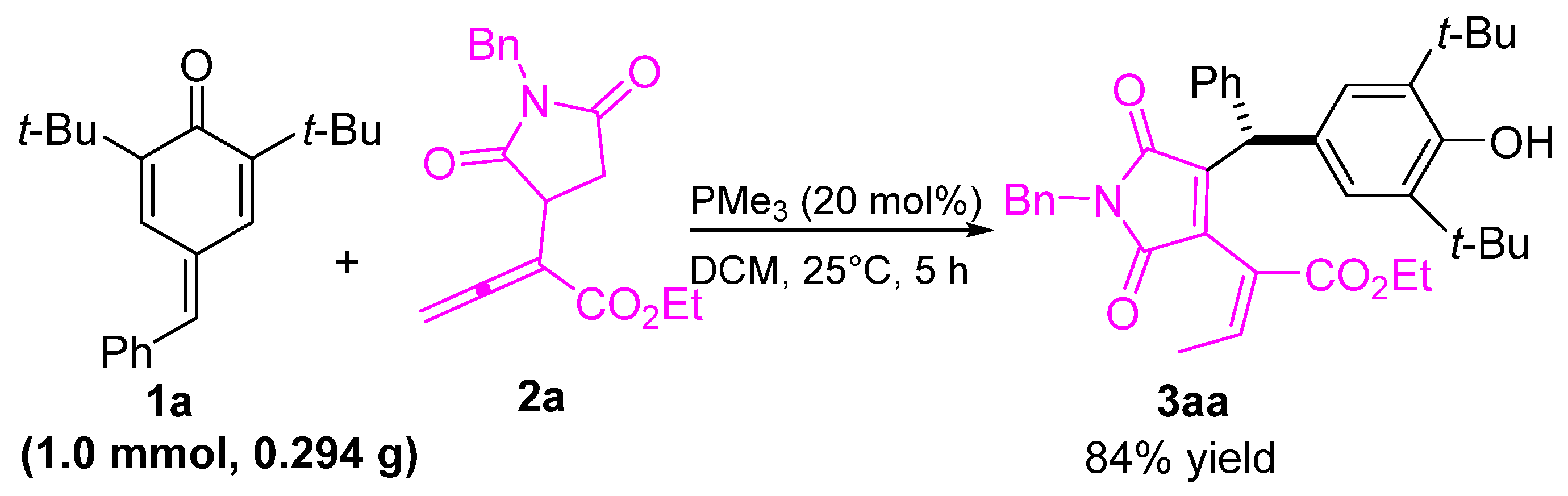
This investigation commenced by examining the reaction between α-succinimide allenoate 2a and para-quinone methide (p-QM) 1a. A catalyst, MePPh2, was employed at 20 mol % with DCM as the solvent. The desired product 3aa was obtained with a yield of 34% (Table 1, entry 1). Various phosphines were also tested for the cascade remote 1, 6-addition reactions. However, no enhancement in reactivity was observed with the phosphines EtPPh2 and PrPPh2 (Table 1, entries 2, 3). Fortunately, when the reaction was attempted with Me2PPh and PMe3, smooth conversion was achieved, yielding the 1,6-conjugate addition product 3aa with yields of 67% and 92%, respectively (Table 1, entries 4, 5). It is noteworthy that the presence of PCy3 or PBu3, with their bulky substitution groups, resulted in decreased yields (Table 1, entries 6, 7). Considering the optimal catalyst, PMe3, we further optimized the reactions, using different solvents. Solvent screening revealed that DCE yielded similar results to DCM (Table 1, entry 8), while toluene, THF, and EtOAc exhibited low reactivity as solvents (Table 1, entries 9–11). Conversely, CH3CN and CH3OH resulted in inferior outcomes (Table 1, entries 12, 13). By reducing the loading of PMe3 to 10 mol % and 5 mol %, the yields of 3aa decreased to 85% and 75%, respectively (Table 1, entries 14, 15). Therefore, the optimal reaction conditions were determined to be DCM as the solvent, room temperature, and 20 mol% of PMe3 as the catalyst.

Table 1.
Reaction yields under different reaction conditions.
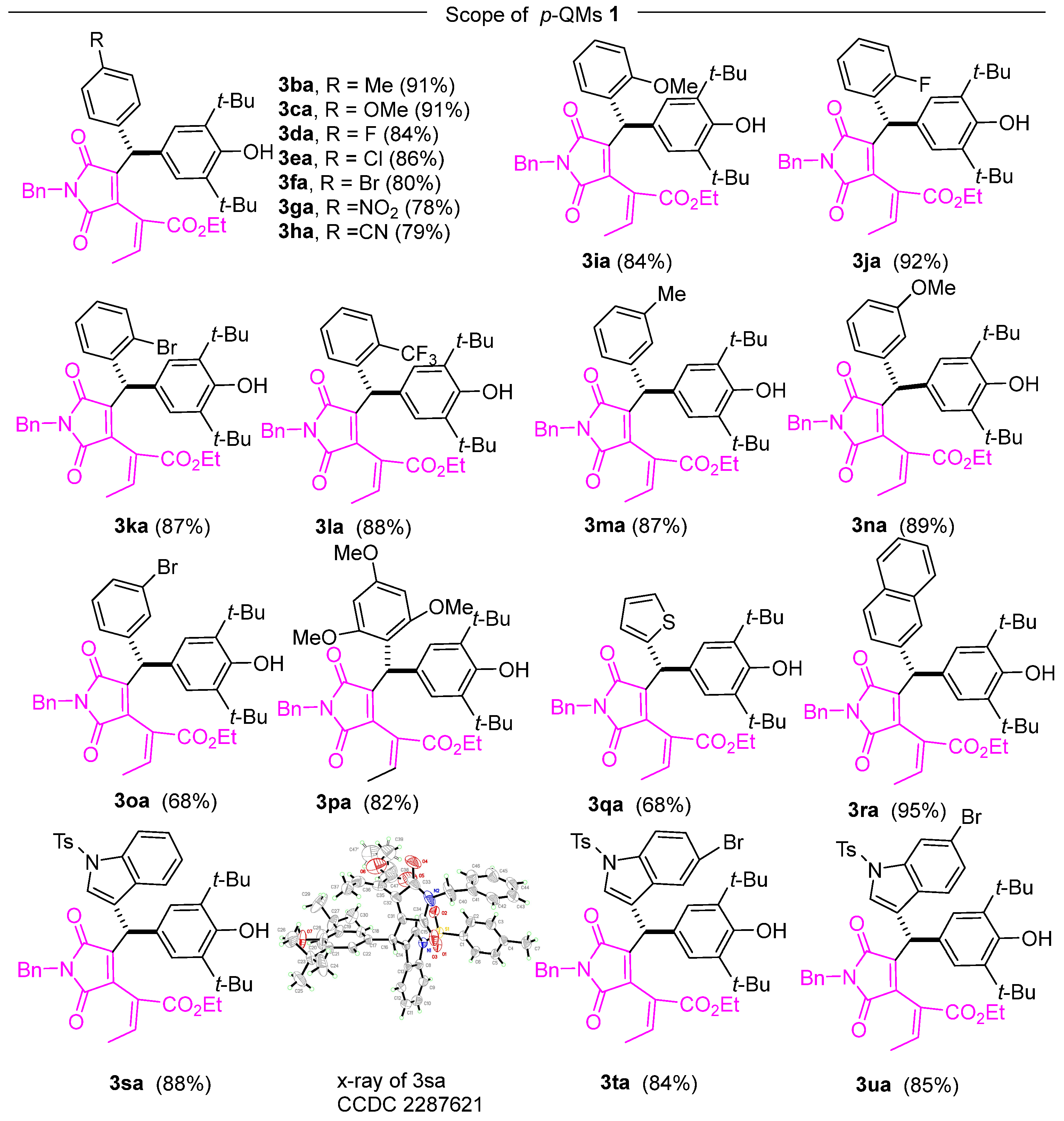
Following the determination of optimal conditions for 1,6-conjugate addition, we investigated the substrate scope of this reaction. In Scheme 1, we explored the influence of various functional groups on the benzene ring of p-QMs. Under the optimized conditions, it was observed that the p-QMs carrying various electronically diverse functional groups at the para-position of the phenyl ring facilitated the formation of conjugate adducts with yields ranging from good to excellent. Smooth conjugate addition was achieved with electron-rich methyl- and methoxy-substituted aryl quinone methides, resulting in excellent yields of the adducts (Scheme 2, 3ba, 3ca). Similarly, the reactions effectively yielded the corresponding products in good yields (Scheme 2, 3da–3ha) when employing arenes containing electron-deficient functional groups such as halogens, nitro groups, and cyano groups. Additionally, satisfactory performance was demonstrated in the reaction with p-QMs containing an ortho-substituted aryl moiety (Scheme 2, 3ia–3la). The applicability of the reaction was expanded to include meta-substituted aryl quinone methides (Scheme 2, 3ma–3oa). Furthermore, trisubstituted aryl quinone methides were investigated, and the conjugate addition yielded the desired in good yields (Scheme 2, 3pa). Moreover, the reaction was employed with p-QMs derived from β-naphthaldehyde, resulting in enhanced product yields (Scheme 2, 3ra). The reaction also showed success with the p-QM substrates generated from thiophene-2-carboxaldehyde and indole-3-carboxaldehyde (Scheme 2, 3qa, 3sa–3ua). The structure of 3sa was confirmed via X-ray analysis (CCDC 2287621) [60]. These data are accessible free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. 9 August 2023. (DOI: 10.5517/ccdc.csd.cc2gsg7z). For further details regarding the crystal structure of 3sa, please refer to the Supplementary Materials.
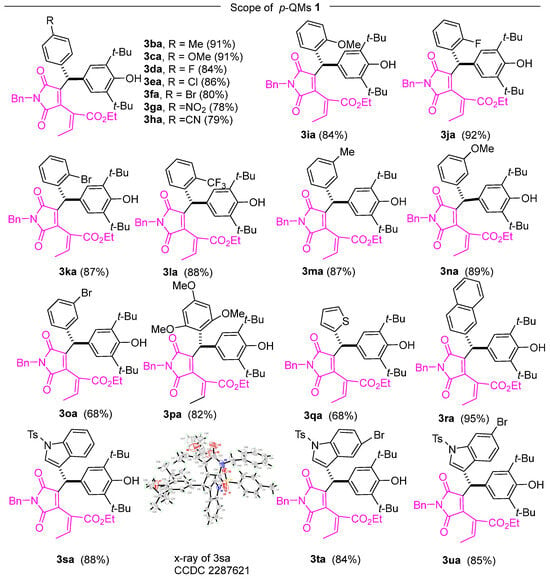
Scheme 2.
Substrate scope of the p-QMs.
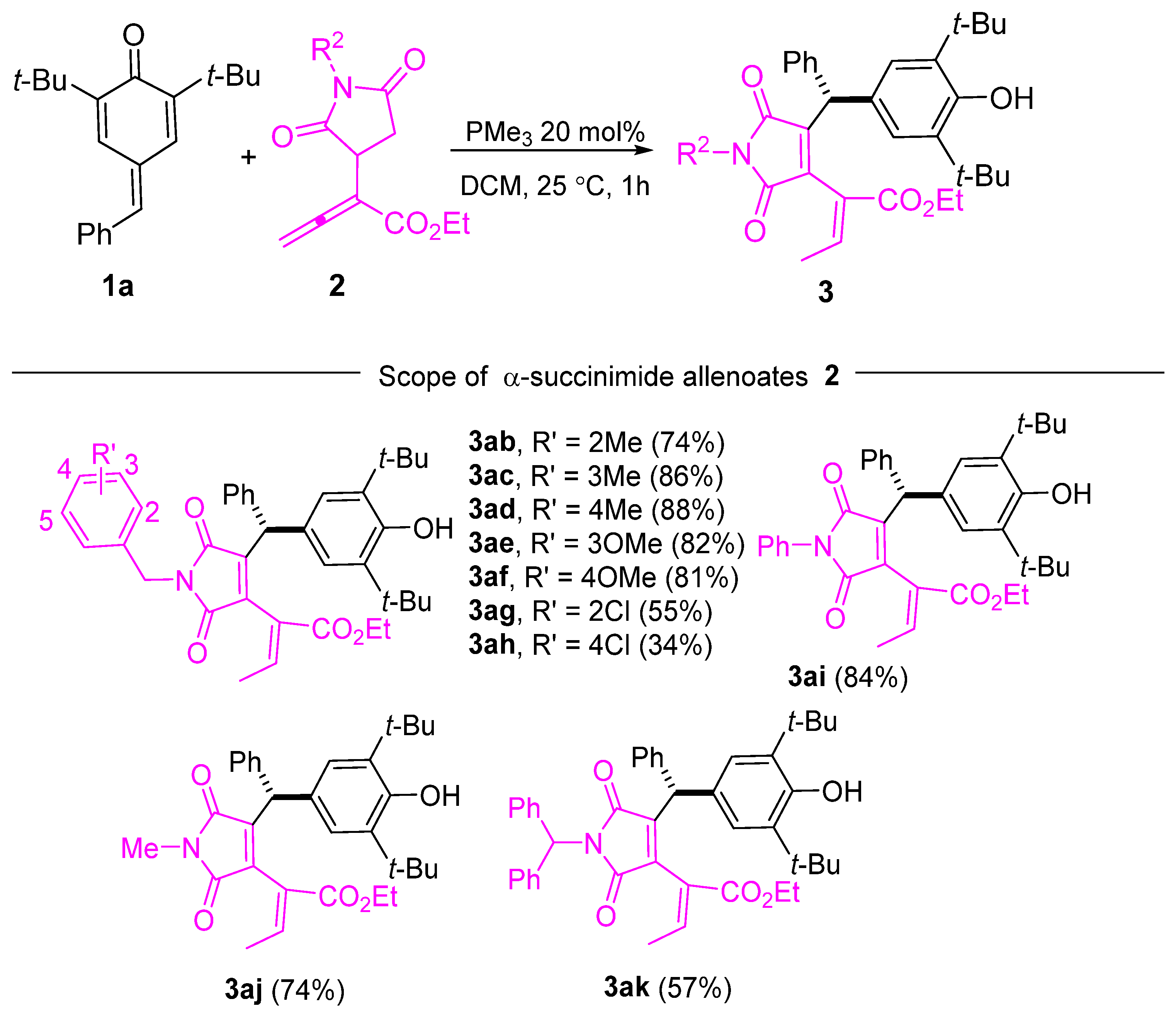
In addition to p-QMs, we explored the substrate scope of α-succinimide allenoates with various R2 groups using 1a as the coupling partner (Scheme 3). We were pleased to observe that the reactivity of the substrates remained unaffected when electron-donating substituents were introduced into the benzyl phenyl ring, resulting in yields of 74–81% (Scheme 3, 3ab–3af). However, the yields slightly decreased to 55% and 34% for 3ag and 3ah, respectively, when R’ groups with electron-withdrawing properties were employed. Encouragingly, the reaction displayed good tolerance toward the phenyl group, as evidenced by the successful formation of the corresponding products in 84% yield (Scheme 3, 3ai). Additionally, when R2 was a methyl group or a diphenyl-substituted methyl group, the products were obtained with respective yields of 74% (Scheme 2, 3aj) and 57% (Scheme 3, 3ak).
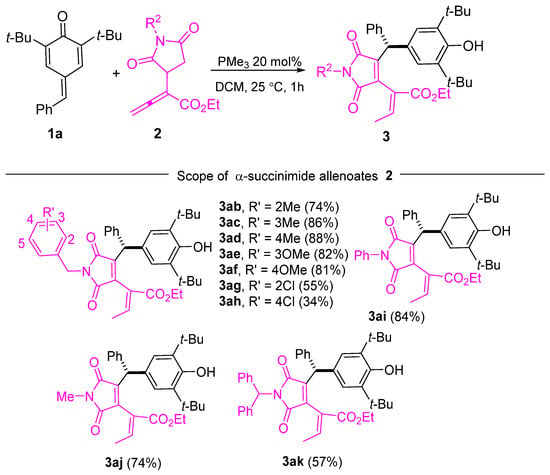
Scheme 3.
Substrate scope of the α-succinimide allenoates.
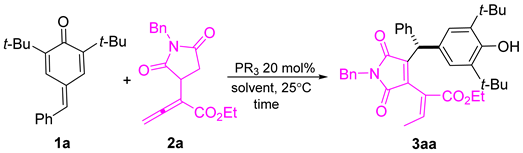
To demonstrate the applicability of our method, we conducted a scale-up reaction. As depicted in Scheme 4, the reaction between 1a and allenoate 2a proceeded smoothly under optimal conditions, yielding desired product 3aa at the gram scale without any noticeable loss in yield.
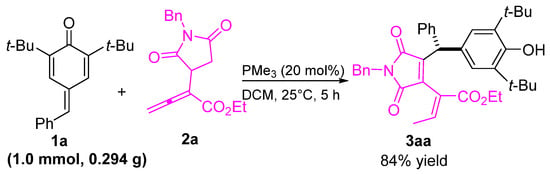
Scheme 4.
The scale-up reaction.
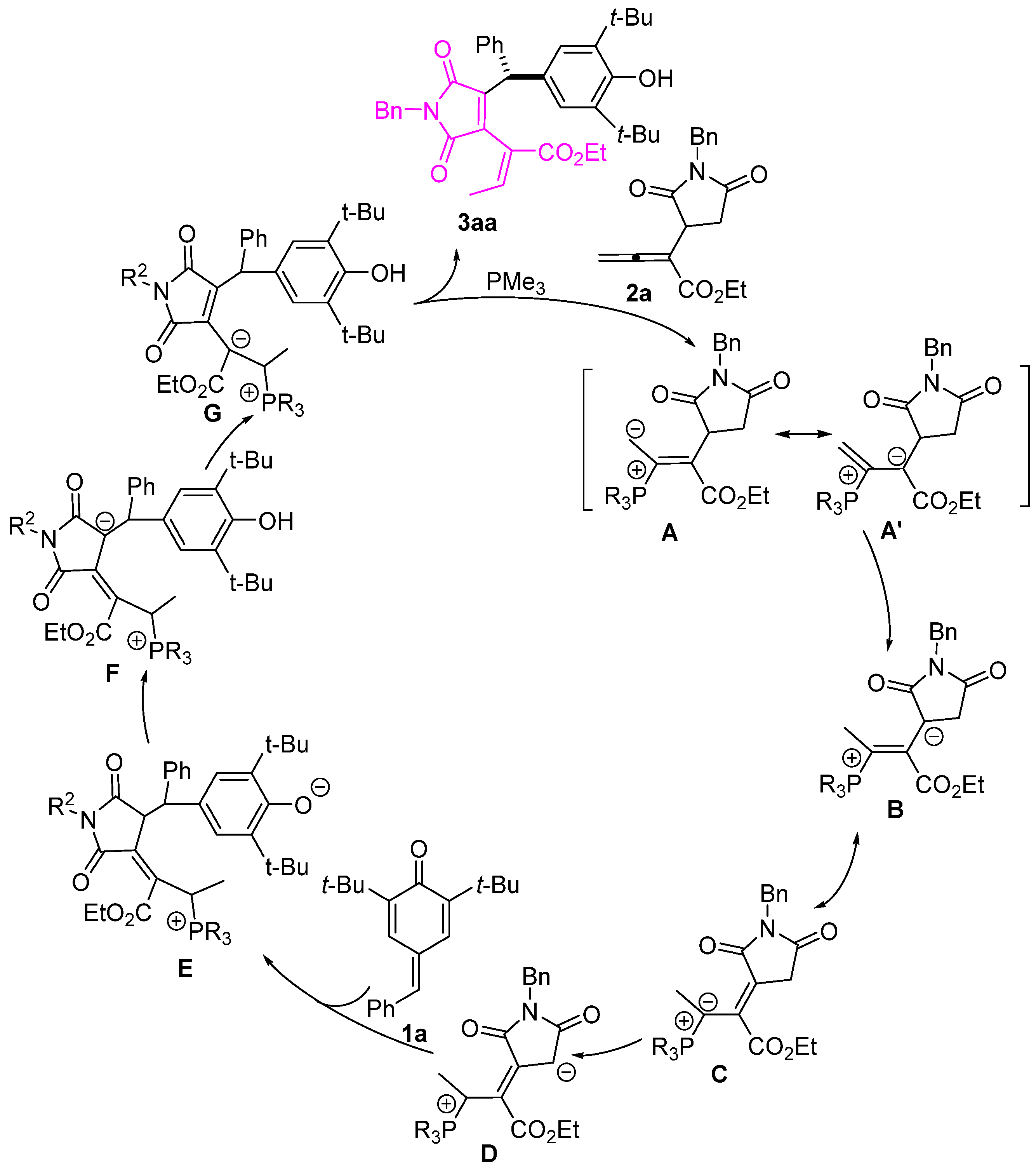
A plausible reaction mechanism is proposed in Scheme 5. This addition reaction begins with the nucleophilic addition of a phosphine to the allenoates 2a, forming zwitterionic intermediates (A⟷A′). Subsequent proton transfer leads to intermediate B, followed by the formation of intermediate D through isomerization and proton transfer. Intermediate D then attacks p-QM 1a, forming intermediate E. Subsequently, intermediate E undergoes a series of H-transfers to form intermediate G. The elimination of PR3 from G produces product 3aa, which regenerates PR3 to complete the catalytic cycle.
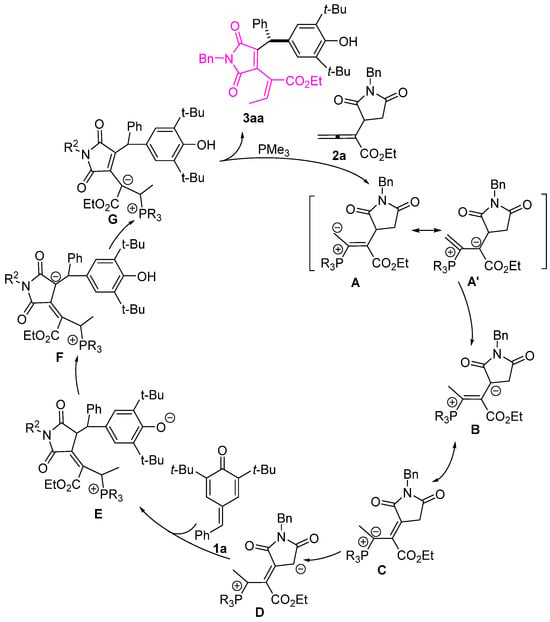
Scheme 5.
Proposed mechanism. The letter numbers A-G indicate possible intermediate structures in the plausible reaction mechanism.
3. Materials and Methods
All chemical reactions were carried out under an argon atmosphere using oven-dried glassware. This setup included magnetic stirring to ensure proper mixing of reagents. Unless otherwise stated, all chemicals were purchased from commercial suppliers and used as received without any additional purification. However, all solvents used in the reactions were purified and dried prior to use, according to standard laboratory procedures. The progress of the chemical reactions was monitored by thin-layer chromatography (TLC) on glass plates precoated with silica gel, and fluorescence quenching with UV light at a wavelength of 254 nm was used to visualize the chromatograms. Any necessary purification of the products was carried out by flash column chromatography using the Qingdao Haiyang flash silica gel (Qingdao, China) with a particle size range of 100–200 mesh. Nuclear magnetic resonance (NMR) spectra, both proton (1H) and carbon (13C), were recorded in deuterated CDCl3 or DMSO-d6 using the 500 MHz NMR spectrometer. The melting points of the compounds were determined using an X-4 digital micro-melting point apparatus from Shanghai Jingke (Shanghai, China) to ensure accuracy. An Agilent instrument using electrospray ionization mass spectrometry (ESI-MS) (Campus Drive Stanford, CA, USA) was used to obtain accurate mass measurements. In addition, X-ray crystallographic data were collected using a Bruker D8 VENTURE instrument (Billerica, Germany) to provide detailed structural information on the synthesized compounds. Characterization data of compounds, NMR spectra of compounds and crystallographic data for product 3sa, See Supplementary Materials.
3.1. General Procedure for the Synthesis of Para-Quinone Methides 1
Aldehyde (10 mmol) was added to a solution of the phenol (10 mmol) in toluene (40 mL). The reaction mixture was heated in a Dean–Stark apparatus to reflux. Piperidine (20 mmol) was added dropwise over 1 h, and heating continued until all the starting material had been consumed. After the mixture had cooled just below the boiling point of toluene (100 °C), acetic anhydride (20 mmol) was added. The solution was stirred for 15 min. The residue was extracted three times with dichloromethane. The combined organic layers were sequentially washed with water and brine, dried over magnesium sulfate, filtered, and concentrated. The crude product was purified by flash column chromatography on silica gel to afford the corresponding product 1.
3.2. General Procedure for the Synthesis of α-Succinimide Substituted Allenoates 2
Maleimide (0.15 mmol), allene (0.30 mmol), DABCO (0.030 mmol), and 1,4-dioxane (1.0 mL) were added to a Schlenk tube. The reaction mixture was stirred at room temperature for 3 h, the solvent was then removed under reduced pressure, and the residue was purified by flash column chromatography (PE/EA = 4/1~2/1) to afford products 2.
3.3. General Procedure for the Phosphine-Catalyzed Direct 1,6-Conjugate Addition
Under argon atmosphere, 1 mL of DCM was added to a mixture of para-quinone methide 1 (0.10 mmol), α-succinimide substituted allenoate 2 (0.12 mmol) and catalyst PMe3 (20 mol%, 0.02 mmol) in a Schlenk tube at room temperature. The resulting mixture was stirred until the starting material was completely consumed (monitored by TLC) and then concentrated to dryness. The residue was purified through flash column chromatography (PE/EtOAc = 8:1) to afford corresponding cycloaddition products 3.
4. Conclusions
In conclusion, we have introduced a novel method for synthesizing functionalized 4-diarylmethylated 3,4-disubstituted maleimides in satisfactory yields. This method involves a phosphine-catalyzed 1,6-conjugated addition reaction between α-succinimide substituted allenoates and p-QMs. Considering the extensive research on the biological activity of natural products and industrially useful compounds containing the 3,4-disubstituted maleimide moiety, our methodology presents a new and efficient protocol for their synthesis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29112593/s1, Full experimental procedures, characterization data, NMR spectra for all new compounds, as well as crystallographic data for product 3sa are available [61,62].
Author Contributions
Z.G. and X.W. were responsible for conceptualization and data validation, L.L. were responsible for writing, review, and editing; X.Z. and D.L. were responsible for the catalysis experiments; B.N. and X.C. were responsible for the spectroscopic and analytical analysis. J.W. and H.L. were responsible for the data curation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Shandong Province (ZR2021MB110), the National Natural Science Foundation of China (No. 22101002), the Support Plan on Science and Technology for Youth Innovation of Universities in Shandong Province (2022KJ111)”, Innovation and Entrepreneurship Training Program Liaocheng University (CXCY2023464), and the Special Construction Project Fund for Shandong Province Taishan Scholars.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, Z.; Xu, X.; Kwon, O. Phosphine Catalysis of Allenes with Electrophiles. Chem. Soc. Rev. 2014, 43, 2927–2940. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Sun, Z.; Guo, H.; Kwon, O. Chiral Phosphines in Nucleophilic Organocatalysis. Beilstein J. Org. Chem. 2014, 10, 2089–2121. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, J. Recent Developments in the Synthesis and Utilization of Chiral β-Aminophosphine Derivatives as Catalysts or Ligands. Chem. Soc. Rev. 2016, 45, 1657–1677. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Guo, H.; Kwon, O. Nucleophilic Chiral Phosphines: Powerful and Versatile Catalysts for Asymmetric Annulations. Aldrichim. Acta 2016, 49, 3–41. [Google Scholar]
- Wang, T.; Han, X.; Zhong, F.; Yao, W.; Lu, Y. Amino Acid Derived Bifunctional Phosphines for Enantioselective Transformations. Acc. Chem. Res. 2016, 49, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Guo, H. Acidic Hydrogen-Tethered Electron-Deficient Acceptors for Phosphine-Catalyzed Annulations. Synlett 2024, 35. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, M. Lu’s [3 + 2] Cycloaddition of Allenes with Electrophiles: Discovery, Development and Synthetic Application. Org. Chem. Front. 2017, 4, 1876–1890. [Google Scholar] [CrossRef]
- Ni, H.; Chan, W.L.; Lu, Y. Phosphine-Catalyzed Asymmetric Organic Reactions. Chem. Rev. 2018, 118, 9344–9411. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Fan, Y.C.; Sun, Z.; Wu, Y.; Kwon, O. Phosphine Organocatalysis. Chem. Rev. 2018, 118, 10049–10293. [Google Scholar] [CrossRef]
- Li, E.Q.; Huang, Y. Recent Advances in Phosphine catalysis Involving γ-Substituted Allenoates. Chem. Commun. 2020, 56, 680–694. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liao, J.; Wang, W.; Liu, H.; Guo, H. Synthesis of Heterocyclic Compounds through Nucleophilic Phosphinecatalysis. Chem. Commun. 2020, 56, 15235–15281. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Smaligo, A.J.; Song, X.-R.; Kwon, O. Phosphorus-Based Catalysis. ACS Cent. Sci. 2021, 7, 536–558. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Lan, J.; Kwon, O. An Expedient Phosphine-Catalyzed [4 + 2] Annulation: Synthesis of Highly Functionalized Tetrahydropyridines. J. Am. Chem. Soc. 2003, 125, 4716–4717. [Google Scholar] [CrossRef] [PubMed]
- Wurz, R.P.; Fu, G.C. Catalytic Asymmetric Synthesis of Piperidine Derivatives through the [4 + 2] Annulation of Imines with Allenes. J. Am. Chem. Soc. 2005, 127, 12234–12235. [Google Scholar] [CrossRef] [PubMed]
- Tran, Y.S.; Kwon, O. Phosphine-Catalyzed [4 + 2] Annulation: Synthesis of Cyclohexenes. J. Am. Chem. Soc. 2007, 129, 12632–12633. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xu, Q.; Kwon, O. PhosphinePromoted [3 + 3] Annulations of Aziridines with Allenoates: Facile Entry into Highly Functionalized Tetrahydropyridines. J. Am. Chem. Soc. 2009, 131, 6318–6319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, L.; Tong, X. 2-(Acetoxymethyl)buta-2,3-dienoate, a Versatile 1,4-Biselectrophile for Phosphine-Catalyzed [4+n] Annulations with 1,n-Bisnucleophiles (n =1, 2). J. Am. Chem. Soc. 2010, 132, 2550–2551. [Google Scholar] [CrossRef] [PubMed]
- Na, R.; Jing, C.; Xu, Q.; Jiang, H.; Wu, X.; Shi, J.; Zhong, J.; Wang, M.; Benitez, D.; Tkatchouk, E.; et al. Phosphine Catalyzed Annulations of Azomethine Imines: Allene-Dependent [3 + 2], [3 + 3], [4 + 3], and [3 + 2 + 3] Pathways. J. Am. Chem. Soc. 2011, 133, 13337–13348. [Google Scholar] [CrossRef] [PubMed]
- Szeto, J.; Sriramurthy, V.; Kwon, O. Phosphine-Initiated General Base Catalysis: Facile Access to Benzannulated 1,3-Diheteroatom Five-Membered Rings via Double-Michael Reactions of Allenes. Org. Lett. 2011, 13, 5420–5423. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Qiu, S.; Zhao, C.; Zhang, L.; Liu, H.; Zhou, L.; Xiao, Y.; Guo, H. Phosphine-Catalyzed [4 + 2] Cycloaddition of Unsaturated Pyrazolones with Allenoates: A Concise Approach toward Spiropyrazolones. RSC Adv. 2015, 5, 62343–62347. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Yuan, C.; Wang, G.-P.; Zhu, S.-F.; Wu, Y.; Wang, B.; Sun, Z.; Xiao, Y.; Zhou, Q.-L.; et al. Enantioselective Synthesis of Spirobarbiturate Cyclohexenes through Phosphine-Catalyzed Asymmetric [4 + 2] Annulation of Barbiturate-Derived Alkenes with Allenoates. Org. Lett. 2016, 18, 1302–1305. [Google Scholar] [CrossRef]
- Yuan, C.; Zhou, L.; Xia, M.; Sun, Z.; Wang, D.; Guo, H. Phosphine-Catalyzed Enantioselective [4 + 3] Annulation of Allenoates with C, N-Cyclic Azomethine Imines: Synthesis of Quinazoline-Based Tricyclic Heterocycles. Org. Lett. 2016, 18, 5644–5647. [Google Scholar] [CrossRef]
- Ni, H.; Yao, W.; Waheed, A.; Ullah, N.; Lu, Y. Enantioselective [4 + 2]-Annulation of Oxadienes and Allenones catalyzed by an Amino Acid Derived Phosphine: Synthesis of Functionalized Dihydropyrans. Org. Lett. 2016, 18, 2138–2141. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Z.; Zhou, L.; Yuan, C.; Sun, Z.; Xiao, Y.; Guo, H. Phosphine-Catalyzed [2 + 4] Annulation of Allenoates with Thiazolone-Derived Alkenes: Synthesis of Functionalized 6,7- Dihydro-5H-pyrano [2,3-d]thiazoles. Org. Lett. 2016, 18, 3418–3421. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, H.; Su, Q.; Hu, P.; Shao, P.L.; He, Y.; Lu, Y. Enantioselective Synthesis of Tetrahydropyridines/piperidines via Stepwise [4 + 2]/[2 + 2] Cyclizations. Org. Lett. 2017, 19, 3111–3114. [Google Scholar] [CrossRef]
- Wang, C.; Jia, H.; Zhang, C.; Gao, Z.; Zhou, L.; Yuan, C.; Xiao, Y.; Guo, H. Phosphine-Catalyzed Enantioselective [2 + 4] Cycloaddition to Synthesize Pyrrolidin-2-one Fused Dihydropyrans Using α-Substituted Allenoates as C2 Synthons. J. Org. Chem. 2017, 82, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, W.; Zhang, J. Ferrocene Derived Bifunctional Phosphine-Catalyzed Asymmetric Oxa-[4 + 2] Cycloaddition of αSubstituted Allenones with Enones. Chem.-Eur. J. 2017, 23, 13587–13590. [Google Scholar] [CrossRef]
- Ni, H.; Tang, X.; Zheng, W.; Yao, W.; Ullah, N.; Lu, Y. Enantioselective Phosphine-Catalyzed Formal [4 + 4] Annulation of α,β-Unsaturated Imines and Allene Ketones: Construction of Eight-membered Rings. Angew. Chem. Int. Ed. 2017, 56, 14222–14226. [Google Scholar] [CrossRef]
- Zielke, K.; Waser, M. Formal [4 + 1]-Addition of Allenoates to o-QuinoneMethides. Org. Lett. 2018, 20, 768–771. [Google Scholar] [CrossRef]
- Yao, C.; Bao, Y.; Lu, T.; Zhou, Q. Stereoselective Synthesis of Functionalized Benzooxazepino[5,4-a]isoindolone Derivatives via Cesium Carbonate Catalyzed Formal [5 + 2] Annulation of 2-(2-Hydroxy-phenyl)-isoindoline-1,3-dione with Allenoates. Org. Lett. 2018, 20, 2152–2155. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, C.; Zhou, L.; Yuan, C.; Xiao, Y.; Guo, H. Phosphine-Catalyzed [8 + 2]-Annulation of Heptafulvenes with Allenoates and Its Asymmetric Variant: Construction of Bicyclo[5.3.0]decane Scaffold. Org. Lett. 2018, 20, 4302–4305. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, D.; Huang, Y. Phosphine Sequentially Catalyzed Domino 1,6-Addition/Annulation: Access to Functionalized Chromans and Tetrahydroquinolines with an Ethynyl-Substituted All Carbon Quaternary Center. Org. Lett. 2019, 21, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jin, H.; Feng, J.; Zhu, Y.; Jia, P.; Wu, C.; Huang, Y. Sequential Phosphine-Catalyzed [4 + 2] Annulation of β′-Acetoxy Allenoates: Enantioselective Synthesis of 3-Ethynyl-Substituted Tetrahydroquinolines. Org. Lett. 2019, 21, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhou, X.; Xie, L.; Wang, X.; Wang, S.; Liu, H.; Guo, H. Phosphine-Catalyzed [4 + 2] Annulation of Allenoates Bearing Acidic Hydrogen with 1,1-Dicyanoalkenes. J. Org. Chem. 2024, 89, 7169–7174. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Shi, W.; Liao, J.; Liu, H.; Zhang, C.; Guo, H. Phosphine-Catalyzed [4 + 2] Annulation of Allenoate with SulfamateDerived Cyclic Imines: A Reaction Mode Involving γ′-Carbon of α-Substituted Allenoate. Org. Lett. 2017, 19, 6340–6343. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Ren, Y.; Zhao, H.; Tang, Y.; Piao, S.; Mao, B.; Wang, W.; Wu, Y.; Wang, B.; Guo, H. Phosphine-Catalyzed (4 + 2) Annulation of Allenoates with Benzofuran-Derived Azadienes and Subsequent Thio-Michael Addition. Org. Lett. 2022, 24, 3747–3752. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, X.; Zhang, P.; Li, P. Recent Advances in the Catalytic Enantioselective Reactions of para-Quinone Methides. Chem. Asian J. 2018, 13, 2350–2359. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, W.; Tu, S.; Jiang, B. Recent Developments in 1,6-Addition Reactions of para-Quinone Methides (p-QMs). Org. Chem. Front. 2020, 13, 1743–1778. [Google Scholar] [CrossRef]
- Lima, C.; Pauli, F.; Costa, D.; Souza, A.; Forezi, L.; Ferreira, V.; Silva, F. para-Quinone Methides as Acceptors in 1,6-Nucleophilic Conjugate Addition Reactions for the Synthesis of Structurally Diverse Molecules. Eur. J. Org. Chem. 2020, 18, 2650–2692. [Google Scholar] [CrossRef]
- More, S.; Rupanawar, B.; Suryavanshi, G. Metal-Free, Acid-Catalyzed 1, 6-Conjugate Addition of NH-Sulfoximines to para-Quinone Methides: Accessing to Diarylmethine Imino Sulfanone. J. Org. Chem. 2021, 86, 10129–10139. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Ge, X.; Fan, Y.; Liu, X.; Yang, X.; Yang, Y.; Zhao, X.; An, X.; Fan, C. Iron(iii)-Catalyzed Tandem Annulation of Indolyl-Substituted p-Quinone Methides with Ynamides for the Synthesis of Cyclopenta[b]indoles. Chem. Commun. 2022, 58, 8710–8713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, W.; Tu, S.; Jiang, B. Catalytic Asymmetric 1,6-Conjugate Addition of para-Quinone Methides: Formation of All-Carbon Quaternary Stereocenters. Angew. Chem. Int. Ed. 2015, 54, 13711–13714. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, B.; Shi, Z.; Tan, C.; Fan, R.; Li, Z.; Tan, J. Hf(OTf)4-Catalyzed 1, 6-Conjugate Addition of 2-Alkyl-azaarenes to para -Quinone Methides. J. Org. Chem. 2021, 86, 3615–3624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Cao, W.; Liu, X.; Feng, X. Diastereo- and Enantioselective 1, 6-Conjugate Addition of 2-Azaarylacetamides to para-Quinone Methides. Org. Lett. 2019, 21, 6063–6067. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Liu, X.; Feng, X. Recent Advances in Metal-Catalyzed Asymmetric 1, 4-Conjugate Addition (ACA) of Nonorganometallic Nucleophiles. Chem. Rev. 2018, 118, 7586–7656. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Huang, Y.; Zhao, Y. Stereoselective 1,6-Conjugate Addition/Annulation of para-Quinone Methides with Vinyl Epoxides/Cyclopropanes. ACS Catal. 2016, 6, 6408–6412. [Google Scholar] [CrossRef]
- Sharma, B.; Shinde, D.; Jain, R.; Begari, E.; Satbhaiya, S.; Gonnade, R.; Kumar, P. Unravelling the Nucleophilicity of Butenolides for 1,6-Conjugate Addition to p-Quinone Methides: A Direct Access to Diversely Substituted Butenolide-Derived Diarylmethanes. Org. Lett. 2018, 20, 2787–2791. [Google Scholar] [CrossRef] [PubMed]
- Ranga, P.; Ahmad, F.; Nager, P.; Rana, P.; Anand, R. Bis(amino)cyclopropenium Ion as a Hydrogen-Bond Donor Catalyst for 1,6-Conjugate Addition Reactions. J. Org. Chem. 2021, 86, 4994–5010. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Song, Z.; Wang, W.; Xu, T. Highly Regio- and Enantioselective Dienylation of p-Quinone Methides Enabled by an Organocatalyzed Isomerization/Addition Cascade of Allenoates. Org. Lett. 2019, 21, 3963–3967. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wang, W.; Liu, Z.; Lu, Y.; Wang, D. Phosphine-Catalyzed Intermolecular Dienylation of Alkynoate with para-Quinone Methides. J. Org. Chem. 2021, 86, 8590–8599. [Google Scholar] [CrossRef]
- Wood, P.; Woo, L.; Labrosse, J.; Trusselle, M.; Abbate, S.; Longhi, G.; Castiglioni, E.; Lebon, F.; Purohit, A.; Reed, M.; et al. Chiral Aromatase and Dual Aromatase-Steroid Sulfatase Inhibitors from the Letrozole Template: Synthesis, Absolute Configuration, and In Vitro Activity. J. Med. Chem. 2008, 51, 4226–4238. [Google Scholar] [CrossRef] [PubMed]
- Shiri, M.; Zolfigol, M.; Kruger, H.; Tanbakouchian, Z. Bis- and Trisindolylmethanes (BIMs and TIMs). Chem. Rev. 2010, 110, 2250–2293. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Sun, F.; Zheng, X.; You, S. Brønsted Acid Catalyzed Synthesis of Unsymmetrical Arylbis(3-indolyl)-methanes. Synlett 2009, 7, 1111–1114. [Google Scholar]
- Mondal, S.; Panda, G. Synthetic Methodologies of Achiral Diarylmethanols, Diaryl and Triarylmethanes (TRAMs) and Medicinal Properties of Diaryl and Triarylmethanes-an overview. RSC Adv. 2014, 4, 28317–28358. [Google Scholar] [CrossRef]
- Cho, S.; Yoon, K.; Chintharlapalli, S.; Abdelrahim, M.; Lei, P. Nur77 Agonists Induce Proapoptotic Genes and Responses in Colon Cancer Cells through Nuclear Receptor–Dependent and Nuclear Receptor–Independent Pathways. Cancer Res. 2007, 67, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Antus, S.; Schindlbeck, E.; Ahmad, S.; Seligmann, O.; Chari, V.; Wagner, H. Synthesis of Melanervin from Melaleuca Quinquenervia, the First Naturally Occurring Compound with a Triphenylmethane Structure. Tetrahedron 1982, 38, 133–137. [Google Scholar] [CrossRef]
- Duxbury, D.F. The Photochemistry and Photophysics of Triphenylmethane Dyes in Solid and Liquid Media. Chem. Rev. 1993, 93, 381–433. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Wei, W.; Lin, A.; Yao, H. Organocatalyzed Asymmetric 1, 6-Conjugate Addition of para-Quinone Methides with Dicyanoolefins. Org. Lett. 2016, 18, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Arde, P.; Anand, R. Expedient Access to Unsymmetrical Triarylmethanes through N-Heterocyclic Carbene Catalysed 1,6-Conjugate Addition of 2-Naphthols to para-Quinone Methides. RSC Adv. 2016, 6, 77111–77115. [Google Scholar] [CrossRef]
- Crystallographic data for 3sa has been deposited with the Cambridge Crystallograohic Data Centre as deposition number CCDC 2287621. 2023. [CrossRef]
- Goswami, P.; Anand, R.V. Bi (OTf) 3 Catalyzed Solvent Free Approach to Unsymmetrical Diaryl (2-indolyl) methanes through 1, 6-Conjugate Addition of 3-Substituted Indoles to para-Quinone Methides. ChemistrySelect 2016, 1, 2556–2559. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Pei, C.K.; Guan, X.Y.; Shi, M. Enantioselective Intermolecular Rauhut–Currier Reaction of Electron-Deficient Allenes with Maleimides. Adv. Synth. Catal. 2011, 353, 1973–1979. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).