Corrosion Inhibition Properties of Corrosion Inhibitors to under-Deposit Corrosion of X65 Steel in CO2 Corrosion Conditions
Abstract
:1. Introduction
2. Experimental Methods and Materials
2.1. Material Preparation
2.2. Synthesis of Corrosion Inhibitors
2.3. Pre-Scaling
2.4. Weight-Loss Experiments
2.5. Electrochemical Measurements
2.6. Characterization of Corrosion Products
3. Results and Discussion
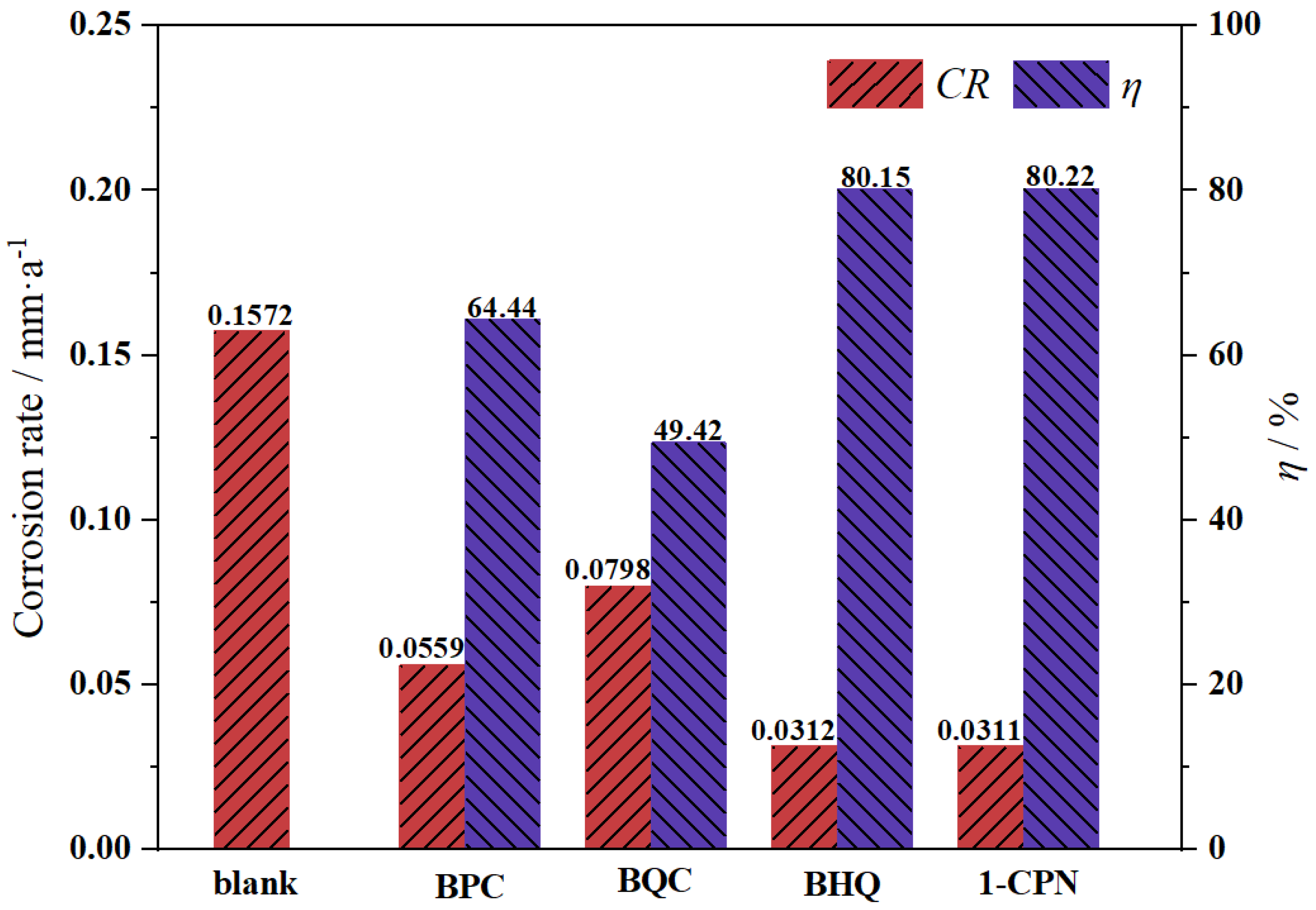
3.1. Corrosion and Inhibition Behavior by Weight-Loss Measurements
3.2. Electrochemical Behavior of Corrosion Inhibitors to Scale-Coated X65 Steel
3.2.1. OCP Variations
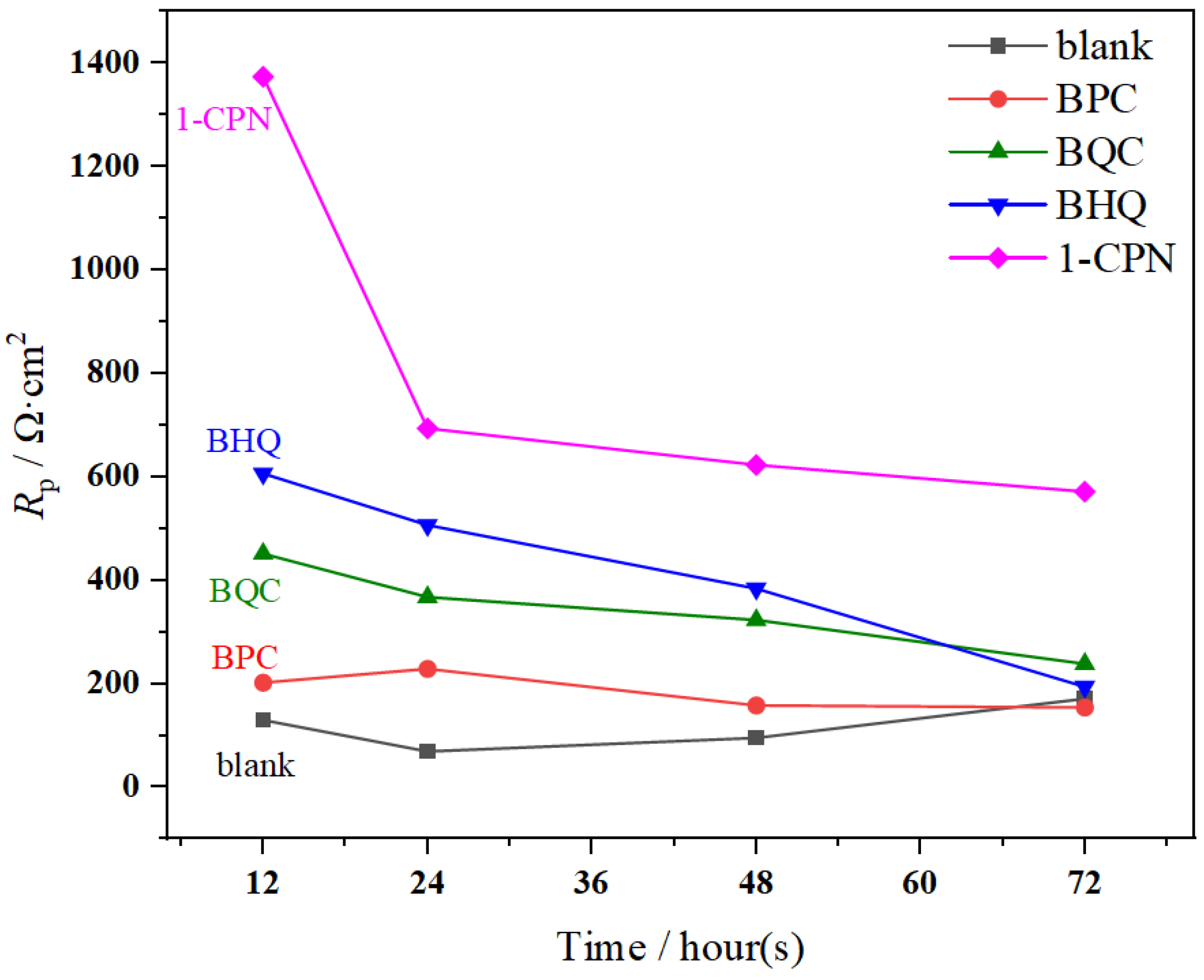
3.2.2. Linear Polarization Resistance
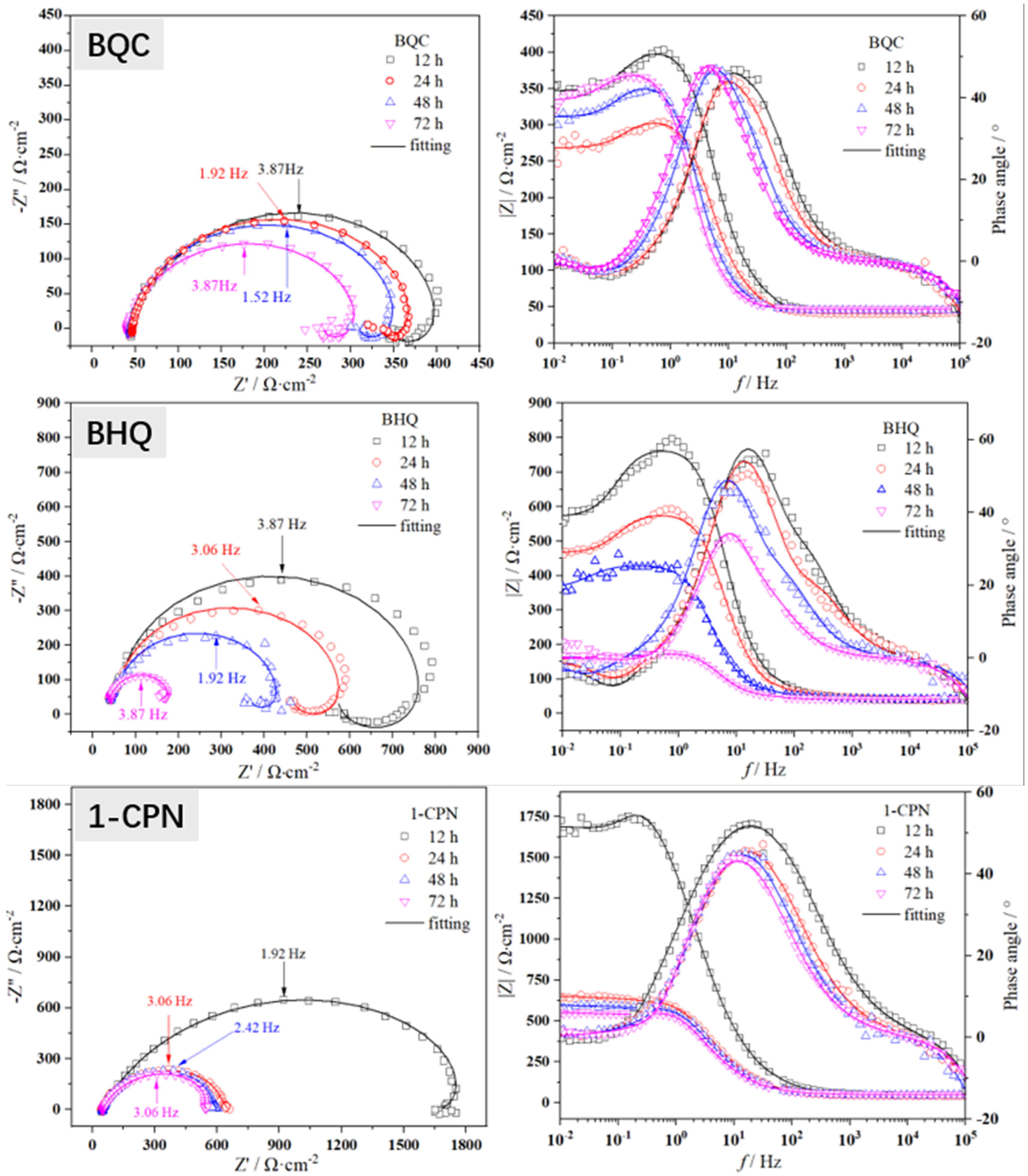
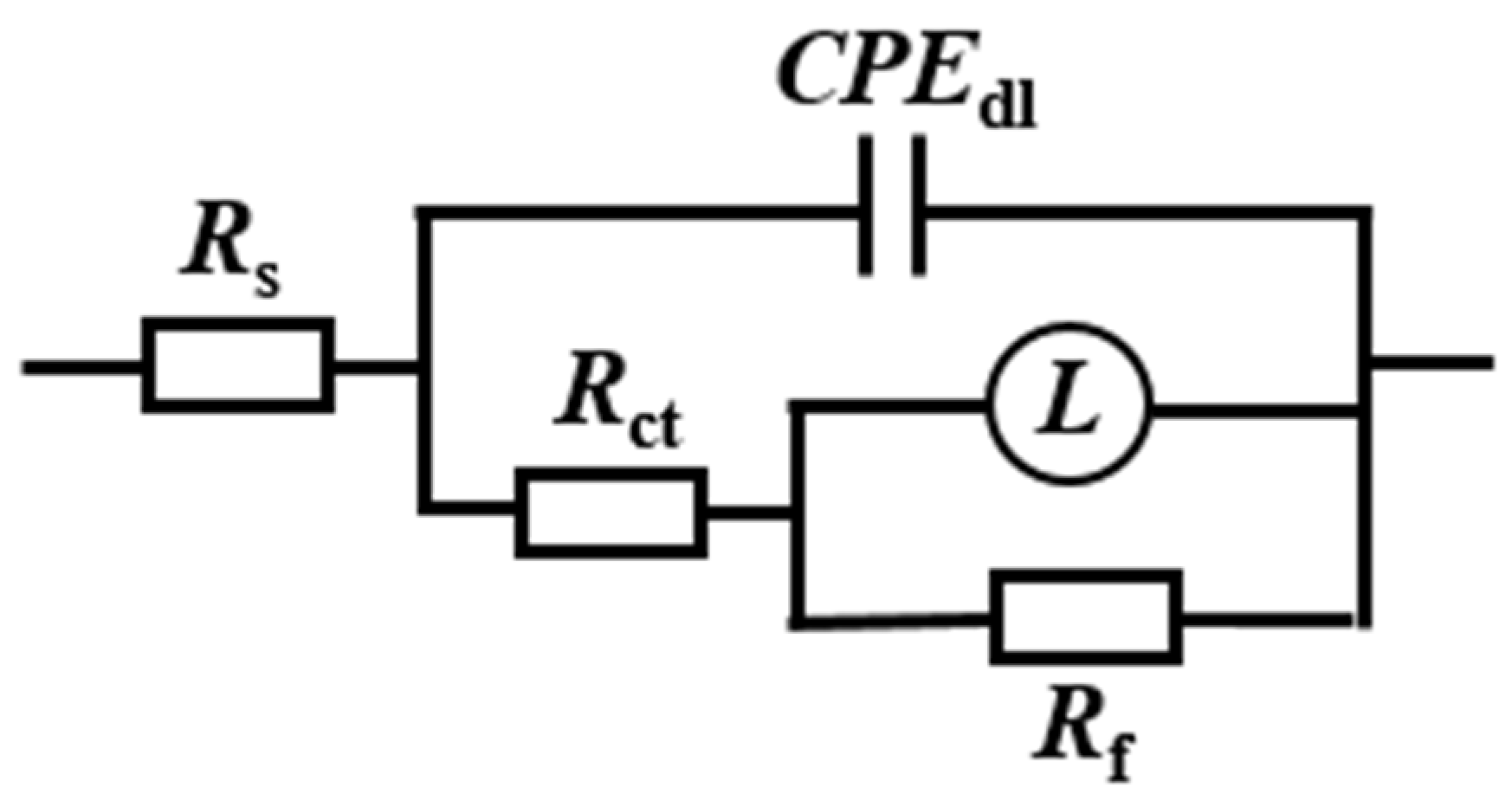
3.2.3. Electrochemical Impedance Spectroscopy
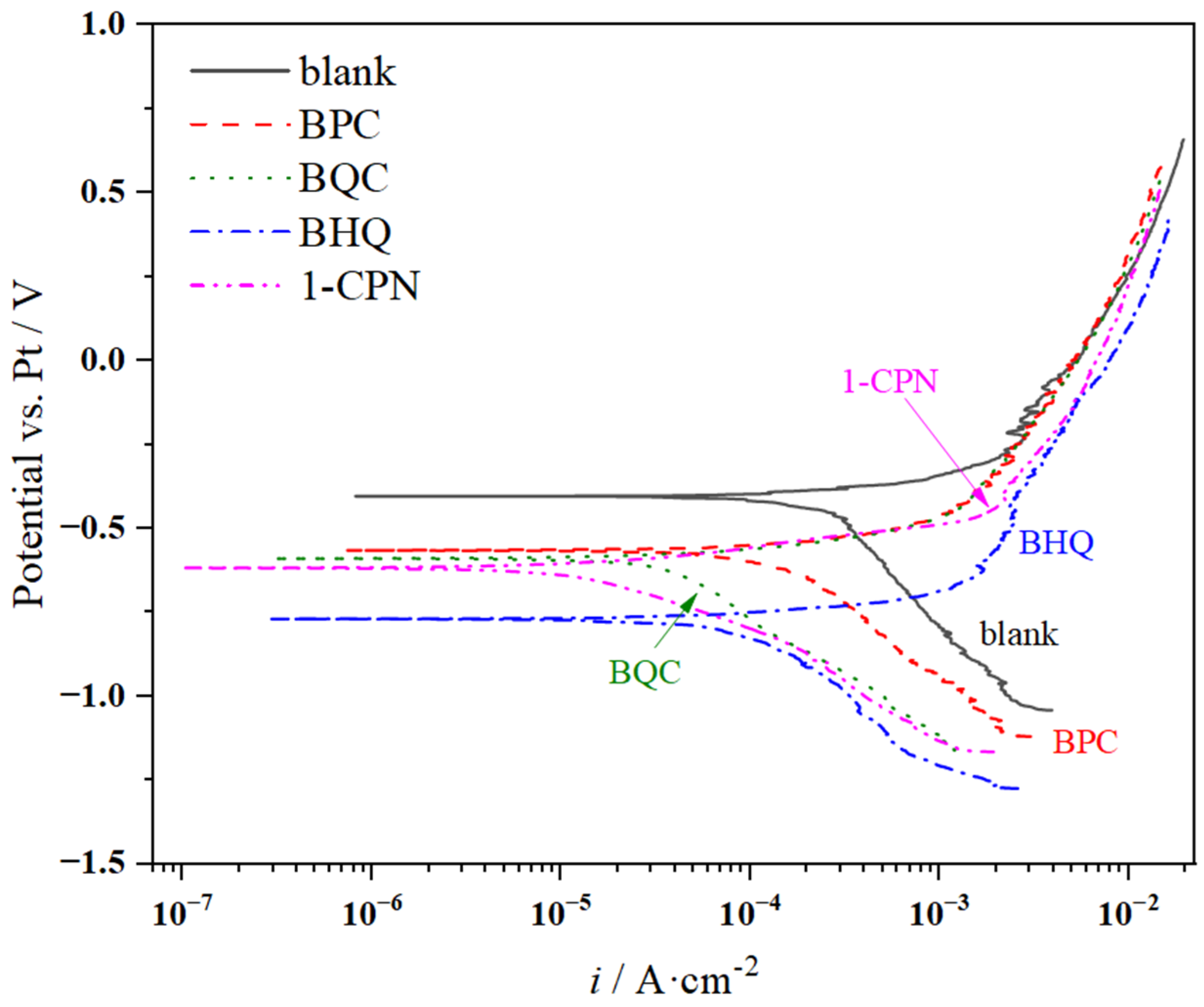
3.2.4. Polarization Curves
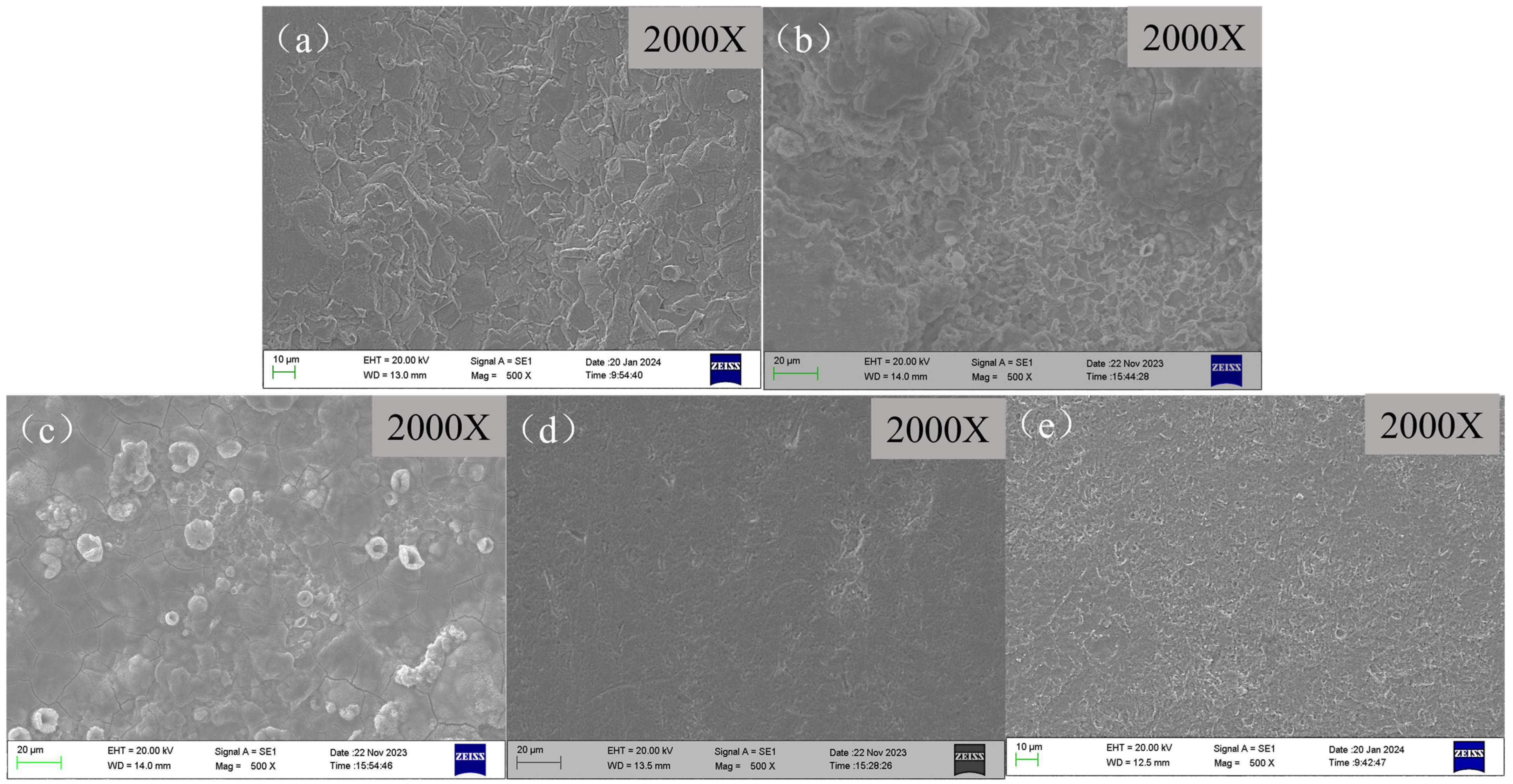
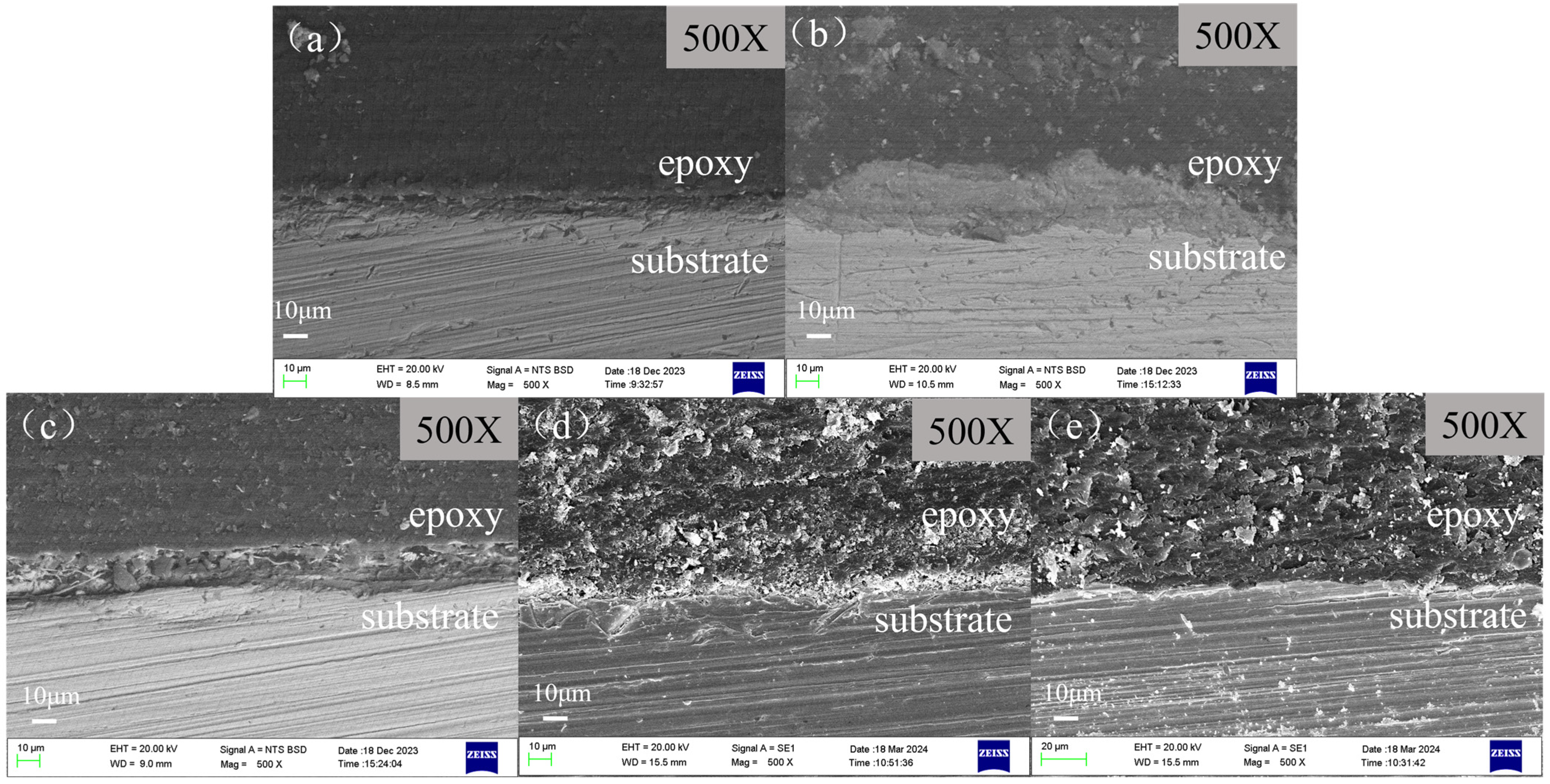
3.3. Surface Morphologies of Samples via SEM
3.3.1. Surface Morphologies of X65 Steel Samples after Corrosion Experiments
3.3.2. Cross-Sectional Morphologies of Scale-Coated Samples after Corrosion Experiments
4. Conclusions
- (1)
- Weight-loss experiments have shown that different corrosion inhibitors can effectively inhibit under-deposit corrosion. But there are different capabilities in inhibiting the under-deposit corrosion of X65 steels coated with CaCO3 layer. The inhibition efficiencies of the corrosion inhibitors from high to low are as follows: 1-CPN < BHQ < BQC < BPC.
- (2)
- The differences in electrochemical behavior under different corrosion inhibitor addition indicate different corrosion inhibition properties to the corrosion under-deposit layer. The pre-scaled CaCO3, which forms before corrosion occurs, provides adequate protection in the early stage. However, as immersion was prolonged, a decrease in Rp and Rct occurred, which inferred the increase in corrosion rate. Both EIS and PC results proved the best inhibition efficiency for 1-CPN.
- (3)
- The surface morphology of the sample in the absence of a corrosion inhibitor exhibits as a loose and porous layer, which can provide less protection to the substrate. However, in the presence of a different corrosion inhibitor, pre-scaled X65 steels exhibit varying degrees of corrosion. Under the action of corrosion inhibitors, the under-deposit corrosion is inhibited to varying degrees, with BHQ and 1-CPN showing the best inhibition effect to under-deposit corrosion. In the cross-sectional morphologies of BPC and BQC, it exhibited as uneven and the corrosion under the deposit was relatively severe. The corrosion product of 1-CPN covers the substrate surface as a thin and uniform layer. A slightly corroded surface can be observed after the product layer is removed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Melchers, R.E. Long-term under-deposit pitting corrosion of carbon steel pipes. Ocean. Eng. 2017, 133, 231–243. [Google Scholar] [CrossRef]
- Huang, J.; Brown, B.; Nesic, S.; Papavinasam, S.; Gould, D. Localized Corrosion of Mild Steel under Silica Deposits in Inhibited Aqueous CO2 Solutions. In Proceedings of the NACE CORROSION 2013, Orlando, FL, USA, 17–21 March 2013. [Google Scholar]
- Kung, S.; Miller, N.; Eiyaakobi, F.; Moloney, J. Mitigation of Elemental Sulfur and Iron Sulfide Under-Deposit Corrosion. In Proceedings of the AMPP Annual Conference + Expo, Denver, CO, USA, 19–23 March 2023. [Google Scholar]
- Kermani, M.B.; Morshed, A. Carbon dioxide corrosion in oil and gas production—A compendium. Corrosion 2003, 59, 659–683. [Google Scholar] [CrossRef]
- Nešić, S. Key issues related to modelling of internal corrosion of oil and gas pipelines—A review. Corros. Sci. 2007, 49, 4308–4338. [Google Scholar] [CrossRef]
- Mansoori, H.; Young, D.; Brown, B.; Singer, M. Influence of calcium and magnesium ions on CO2 corrosion of carbon steel in oil and gas production systems-A review. J. Nat. Gas. Sci. Eng. 2018, 59, 287–296. [Google Scholar] [CrossRef]
- Mansoori, H.; Mirzaee, R.; Esmaeilzadeh, F.; Vojood, A.; Dowrani, A.S. Pitting corrosion failure analysis of a wet gas pipeline. Eng. Fail. Anal. 2017, 82, 16–25. [Google Scholar] [CrossRef]
- Barker, R.; Burkle, D.; Charpentier, T.; Thompson, H.; Neville, A. A review of iron carbonate (FeCO3) formation in the oil and gas industry. Corros. Sci. 2018, 142, 312–341. [Google Scholar] [CrossRef]
- Almeida, T.; Bandeira, M.; Moreira, R.M.; Mattos, O.R. New insights on the role of CO2 in the mechanism of carbon steel corrosion. Corros. Sci. 2017, 120, 239–250. [Google Scholar] [CrossRef]
- Xiedong, R.; Yuan, L.; Qiang, W.; Liusi, Y.; Kaiyuan, Z.; Jiayi, T.; Hu, W.; Juan, X. The influence of Ca2+ on the growth mechanism of corrosion product film on N80 steel in CO2 corrosion environments. Corros. Sci. 2023, 218, 111168. [Google Scholar]
- Almeida, T.C.; Bandeira, M.C.E.; Moreira, R.M.; Mattos, O.R. Discussion on “Electrochemistry of CO2 corrosion of mild steel: Effect of CO2 on iron dissolution reaction” by A. Kahyarian, B. Brown, S. Nesic, [Corros. Sci. 129 (2017) 146–151]. Corros. Sci. 2018, 133, 417–422. [Google Scholar] [CrossRef]
- Ren, X.; Wang, H.; Wei, Q.; Lu, Y.; Xiao, B.; Xie, J. Electrochemical behaviour of N80 steel in CO2 environment at high temperature and pressure conditions. Corros. Sci. 2021, 189, 109619. [Google Scholar] [CrossRef]
- Gao, M.; Pang, X.; Gao, K. The growth mechanism of CO2 corrosion product films. Corros. Sci. 2011, 53, 557–568. [Google Scholar] [CrossRef]
- Du, C.; Wang, X.; Chen, Y.; Lu, X.; Wang, H. Synergistic effect between two quaternary ammonium salts and thiourea as corrosion inhibitors for X70 steels in CO2 saturated 3.5% NaCl solution. Int. J. Electrochem. Sci. 2024, 19, 100443. [Google Scholar] [CrossRef]
- Wu, X.D.; Li, J.B.; Deng, C.Y.; Yang, L.; Lv, J.; Fu, L.P. Novel carbon dots as effective corrosion inhibitor for N80 steel in 1 M HCl and CO2-saturated 3.5 wt% NaCl solutions. J. Mol. Struct. 2022, 1250, 131897. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.; Zhang, G. Efficient inhibition of mild steel corrosion in acidic medium by novel pyrimidine derivatives: Inhibitive effect evaluation and interface adsorption mechanism. J. Mol. Struct. 2023, 1291, 136005. [Google Scholar] [CrossRef]
- Xiaolin, Y.; Qiang, W.; Yuan, L.; Ming, D.; Hu, W.; Juan, X. The Synergistic Inhibition Effect between Imidazoline and 2-Mercaptoethanol on Carbon Steel Corrosion in CO2-saturated 3.5% NaCl solution. Int. J. Electrochem. Sci. 2022, 17, 220556. [Google Scholar]
- El Basiony, N.M.; Elgendy, A.; El-Tabey, A.E.; Al-Sabagh, A.M.; Abd El-Hafez, G.M.; Abd El-raouf, M.; Migahed, M.A. Synthesis, characterization, experimental and theoretical calculations (DFT and MC) of ethoxylated aminothiazole as inhibitor for X65 steel corrosion in highly aggressive acidic media. J. Mol. Liq. 2020, 297, 111940. [Google Scholar] [CrossRef]
- Sharma, S.; Saha, S.K.; Kang, N.; Ganjoo, R.; Thakur, A.; Assad, H.; Kumar, A. Multidimensional analysis for corrosion inhibition by Isoxsuprine on mild steel in acidic environment: Experimental and computational approach. J. Mol. Liq. 2022, 357, 119129. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mehta, R.K.; Kumari, N.; Yadav, M.; Obot, I.B. Study on benzylidine derivatives as corrosion inhibitors for mild steel in 15% HCl medium: Experimental & theoretical investigation. J. Phys. Chem. Solids. 2023, 183, 111632. [Google Scholar]
- Feng, X.J.; Yan, N.; Wang, Y.Q.; Mei, P.; Chen, W.; Lu, L.L.; Lai, L. Corrosion Inhibition Studies of 8-Hydroxyquinoline Derivatives for N80 Steel in a 1.0 M HCl Solution: Experimental, Computational Chemistry, and Quantitative Structure-Activity Relationship Studies. Langmuir 2023, 39, 519–532. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Ali, I.H.; Alanazi, A.K.; Younas, M.; Lin, Y.H. Long chain imidazole derivative as a novel corrosion inhibitor for Q235 steel in 15 % HCl medium under hydrodynamic condition: Experimental and theoretical examinations. Mater. Chem. Phys. 2024, 313, 128798. [Google Scholar] [CrossRef]
- Hegazy, M.A.; Abdallah, M.; Awad, M.K.; Rezk, M. Three novel di-quaternary ammonium salts as corrosion inhibitors for API X65 steel pipeline in acidic solution. Part I: Experimental results. Corros. Sci. 2014, 81, 54–64. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Chauhan, D.S.; Quraishi, M.A.; Lgaz, H.; Chung, I.M. Comprehensive investigation of steel corrosion inhibition at macro/micro level by ecofriendly green corrosion inhibitor in 15% HCl medium. J. Colloid Interface Sci. 2020, 560, 225–236. [Google Scholar] [CrossRef]
- Sharma, D.; Thakur, A.; Sharma, M.K.; Kumar, A.; Jakhar, K.; Kumar, S.; Sihmar, A.; Dahiya, H.; Kumar, A.; Sharma, A.K.; et al. A convenient synthesis, electrochemical profiling, and morphological studies of a pyridine-based 1,3,4-oxadiazole hybrid: A promising study for corrosion mitigation of mild steel in strongly acidic environment. Inorg. Chem. Commun. 2023, 158, 111554. [Google Scholar] [CrossRef]
- Tang, J.; Hu, Y.; Han, Z.; Wang, H.; Zhu, Y.; Wang, Y.; Nie, Z.; Wang, Y. Experimental and Theoretical Study on the Synergistic Inhibition Effect of Pyridine Derivatives and Sulfur-Containing Compounds on the Corrosion of Carbon Steel in CO2-Saturated 3.5 wt.% NaCl Solution. Molecules 2018, 23, 3270. [Google Scholar] [CrossRef]
- Ma, L.R.; Lu, W.Q.; Yang, D.; Shen, J.J.; Gao, Z.S.; Zhang, S.Y.; Liao, Q.Q. Dithiocarbamate modified glucose as a novel eco-friendly corrosion inhibitor for copper in sodium chloride media. Sustain. Chem. Pharm. 2021, 22, 100488. [Google Scholar] [CrossRef]
- Zeng, W.J.; Tan, B.C.; Zheng, X.W.; Chen, X.D.; Chen, J.D.; Li, W.P. Penetration into the inhibition performance of two piperazine derivatives as high-efficiency inhibitors for copper in sulfuric acid environment. J. Mol. Liq. 2022, 356, 119015. [Google Scholar] [CrossRef]
- Abouchane, M.; Dkhireche, N.; Rbaa, M.; Benhiba, F.; Ouakki, M.; Galai, M.; Lakhrissi, B.; Zarrouk, A.; Touhami, M.E. Insight into the corrosion inhibition performance of two quinoline-3-carboxylate derivatives as highly efficient inhibitors for mild steel in acidic medium: Experimental and theoretical evaluations. J. Mol. Liq. 2022, 360, 119470. [Google Scholar] [CrossRef]
- Yahya, S.; Kee, K.E.; Puad MJ, M.; Ismail, M.C. Evaluation on steel corrosion in water-based drilling fluids: Inhibitors and scale involvement. J. Pet. Sci. Eng. 2022, 211, 110127. [Google Scholar] [CrossRef]
- He, J.; Yu, H.; Fu, Z.; Yue, P. Effect of Water-soluble Corrosion Inhibitor on Corrosion Behavior of Q235 Pipeline Steel for Construction. J. Chin. Soc. Corros. Prot. 2023, 43, 1041–1048. [Google Scholar]
- Zhang, D.; Ma, F.; Wu, Y.; Dong, Z. Optimization of Injection Technique of Corrosion Inhibitor in CO2-flooding Oil Recovery. J. Southw. Pet. Univ. (Sci. Tech. Ed.) 2020, 42, 103–109. [Google Scholar]
- Xu, Y.; Huang, Y.; Ying, L.; Wang, X.; Yang, F. Corrosion of X65 Pipeline Steel Under Deposit and Effect of Corrosion Inhibitor. J. Mater. Eng. 2016, 44, 100–108. [Google Scholar]
- Wang, B.; Xu, L.; Li, D.; Lu, M. Corrosion Inhibition Mechanism of Carbon Steel in O2/CO2 Coexisting Environment. J. Mater. Eng. 2017, 45, 38–45. [Google Scholar]
- Xu, Y.; Huang, Y.; Ying, L.; Yang, F.; Li, B.; Wang, X. Corrosion behavior of pipeline steel under deposit corrosion and the inhibition performance of organic phosphine inhibitor. Acta. Metal. Sin. 2016, 52, 320–330. [Google Scholar]
- Ye, Z.; Wang, G.; Zhou, X.; Zhao, L.; Jiang, W.; Yi, R.; Cui, X.; Qiu, Z.; Yang, Z.; Chen, Y.; et al. Co-Evolution Law and Control Technology of Corrosion and Scaling in Water Injection Wells of a Middle East Carbonate Oilfield. In Proceedings of the International Petroleum Technology Conference, Bangkok, Thailand, 1–3 March 2023. [Google Scholar]
- Wang, Q.; Wu, W.; Li, Q.; Zhang, D.; Yu, Y.; Cao, B.; Liu, Z. Under-deposit corrosion of tubing served for injection and production wells of CO2 flooding. Eng. Fail. Anal. 2021, 127, 105540. [Google Scholar] [CrossRef]
- Gomez, J.L.A.; Barquera, S.S.; Perez, R. Effect of Wet Hydrogen Sulfide Environments on the Cracking Susceptibility of Medium Strength Microalloyed Pipeline Steels for Oil and Gas Transport. In Proceedings of the CORROSION 2003, San Diego, CA, USA, 16–20 March 2003. [Google Scholar]
- Sliem, M.H.; Fayyad, E.M.; Abdullah, A.M.; Younan, N.A.; Al-Qahtani, N.; Nabhan, F.F.; Arora, D. Monitoring of under deposit corrosion for the oil and gas industry: A review. J. Pet. Sci. Eng. 2021, 204, 108752. [Google Scholar] [CrossRef]
- Standlee, S.; Efird, K.D.; Spiller, D. Under Deposit Corrosion from IRON sulfide. In Proceedings of the NACE CORROSION, Houston, TX, USA, 13–17 March 2011. [Google Scholar]
- Kvarekvål, J.; Svenningsen, G. Effect of Iron Sulfide Deposits on Sour Corrosion of Carbon Steel. In Proceedings of the NACE CORROSION, Vancouver, BC, Canada, 6–10 March 2016; Volume 2, pp. 1513–1526. [Google Scholar]
- Vera, J.R.; Daniels, D.; Achour, M.H. Under Deposit Corrosion (UDC) in the Oil and Gas Industry: A Review of Mechanisms, Testing and Mitigation. In Proceedings of the Nace Corrosion, Salt Lake City, UT, USA, 11–15 March 2012; Volume 4, pp. 3028–3040. [Google Scholar]
- Chen, X.; Yang, X.; Zeng, M.; Wang, H. Influence of CO2 partial pressure and flow rate on the corrosion behavior of N80 steel in 3.5% NaCl. Int. J. Electrochem. Sci. 2023, 18, 100218. [Google Scholar] [CrossRef]
- Sun, W.; Chokshi, K.; Nesic, S. Iron Carbonate Scale Growth and the Effect of Inhibition in CO2 Corrosion of Mild Steel. In Proceedings of the NACE CORROSION, Houston, TX, USA, 3–7 April 2005. [Google Scholar]
- Wong, J.E.; Park, N. Effect of Corrosion Inhibitor Active Components on the Growth of Iron Carbonate Scale under CO2 Conditions. In Proceedings of the NACE CORROSION, New Orleans, LA, USA, 16–20 March 2008. [Google Scholar]
- Ciolkowski, M.; Neville, A.; Hu, X.; Mavredaki, E.; Sanders, L. Influence of Brine with Different Supersaturation Ratio on Corrosion Processes for Pipeline Material Carbon Steel X65, Scale Deposition and Performance of Combined Inhibitor. In Proceedings of the SPE International Oilfield Corrosion Conference and Exhibition, Aberdeen, UK, 28–29 May 2012. [Google Scholar]
- Tang, J.; Shi, Y.; He, S.; Luo, J.; Liu, Y.; Zhai, K.; Duan, M.; Wang, H.; Xie, J. Study on the corrosion inhibition properties of some quinoline derivatives as acidizing corrosion inhibitors for steel. Int. J. Electrochem. Sci. 2024, 19, 100547. [Google Scholar] [CrossRef]
- Stalker, R.; Graham, G.M.; Simpson, C. The Impact of Inorganic Scale Deposits and Their Removal on General CO2 Corrosion Rates and Corrosion Inhibitor Performance. In Proceedings of the SPE International Symposium on Oilfield Corrosion, Aberdeen, UK, 28 May 2004. [Google Scholar]
- Lu, Y.; Wei, Q.; Ren, X.; Wang, H.; Xie, J. Investigation of Scale Inhibition Mechanism by Electrochemical Quartz Crystal Microbalance. Int. J. Electrochem. Sci. 2021, 16, 21057. [Google Scholar]
- He, S.; Zhao, H.; Tontiwachwuthikul, P.; Qiu, Y.; Luo, S.; Xia, X. Investigation on corrosion behavior and mechanism of carbon steel under CaCO3 deposit in CO2-flooding environment. Int. J. Greenh. Gas Control 2022, 121, 103799. [Google Scholar] [CrossRef]
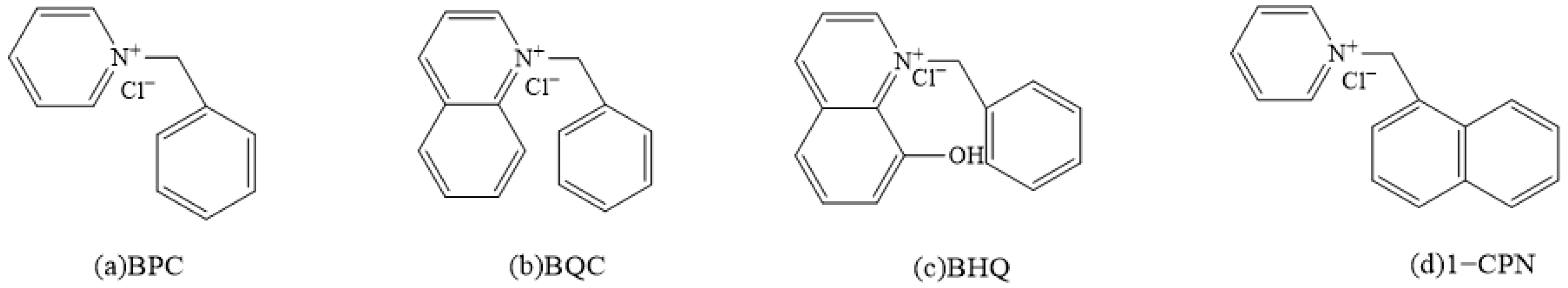



| Element | C | Ti | Mn | P | S | Cr | Mo | Ni | N | V | Al | Nb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 1.60 | 0.30 | 0.02 | 0.004 | 0.35 | 0.30 | 0.25 | 0.06 | 0.30 | 0.025 | 0.009 | 0.06 |
| Inhibitor | Time (h) | LPR Rp (Ω·cm2) | IE (%) |
|---|---|---|---|
| Blank | 12 | 130.32 | - |
| 24 | 69.855 | - | |
| 48 | 96.203 | - | |
| 72 | 171.55 | - | |
| BPC | 12 | 202.73 | 35.72 |
| 24 | 229.7 | 69.59 | |
| 48 | 158.97 | 39.48 | |
| 72 | 154.78 | - | |
| BQC | 12 | 452.11 | 71.18 |
| 24 | 368 | 81.01 | |
| 48 | 323.55 | 70.27 | |
| 72 | 238.89 | 28.19 | |
| BHQ | 12 | 606.74 | 78.52 |
| 24 | 507.2 | 86.22 | |
| 48 | 384.54 | 74.98 | |
| 72 | 194.57 | 11.83 | |
| 1-CPN | 12 | 1373.6 | 90.51 |
| 24 | 694.09 | 89.94 | |
| 48 | 623.65 | 84.57 | |
| 72 | 572.08 | 70.01 |
| Inhibitor | Time (h) | Rs (Ω·cm2) | CPEdl (μF·cm2) | n | Rct (Ω·cm2) | L (H) | Rf (Ω·cm2) | IE (%) |
|---|---|---|---|---|---|---|---|---|
| Blank | 12 | 35.0 | 352.0 | 0.87 | 102.1 | 19.2 | 33.3 | - |
| 24 | 35.3 | 414.2 | 0.94 | 87.9 | 10.1 | 17.3 | - | |
| 48 | 33.2 | 656.2 | 0.95 | 83.1 | 15.2 | 20.8 | - | |
| 72 | 32.7 | 1004.0 | 0.92 | 96.1 | 5.5 | 14.8 | - | |
| BPC | 12 | 41.2 | 190.7 | 0.88 | 163.8 | 57.0 | 67.2 | 33.12 |
| 24 | 40.7 | 298.7 | 0.88 | 174.4 | 3.8 | 23.5 | 42.72 | |
| 48 | 44.0 | 418.4 | 0.92 | 121.0 | 21.8 | 42.6 | 29.52 | |
| 72 | 42.3 | 605.1 | 0.91 | 120.0 | 7.1 | 28.1 | 20.64 | |
| BQC | 12 | 43.7 | 147.0 | 0.89 | 305.0 | 51.3 | 76.4 | 63.06 |
| 24 | 41.0 | 204.5 | 0.91 | 229.9 | 33.1 | 50.5 | 24.32 | |
| 48 | 45.0 | 273.2 | 0.93 | 267.1 | 54.6 | 59.9 | 31.08 | |
| 72 | 47.0 | 343.6 | 0.94 | 291.1 | 71.2 | 49.2 | 51.20 | |
| BHQ | 12 | 41.8 | 86.4 | 0.85 | 554.2 | 212.2 | 268.8 | 77.00 |
| 24 | 43.0 | 134.5 | 0.83 | 442.4 | 108.2 | 192.5 | 74.62 | |
| 48 | 42.6 | 262.7 | 0.84 | 345.6 | 80.8 | 99.2 | 70.04 | |
| 72 | 42.0 | 456.9 | 0.87 | 122.9 | 7.3 | 30.9 | 21.89 | |
| 1-CPN | 12 | 46.1 | 95.0 | 0.74 | 1647.0 | 336.7 | 319.3 | 91.90 |
| 24 | 42.2 | 165.0 | 0.77 | 610.7 | 14.1 | 66.4 | 81.13 | |
| 48 | 47.4 | 175.7 | 0.78 | 552.2 | 17.0 | 80.4 | 80.60 | |
| 72 | 50.4 | 209.6 | 0.78 | 501.4 | 27.6 | 115.0 | 76.66 |
| Inhibitor | ba (mV) | bc (mV) | icorr (mA·cm−2) | Ecorr (V) | H (%) |
|---|---|---|---|---|---|
| blank | 150 | 1070 | 0.531 | −0.404 | - |
| BPC | 211 | 1562 | 0.323 | −0.566 | 39.17 |
| BQC | 125 | 921 | 0.070 | −0.590 | 86.82 |
| BHQ | 95 | 483 | 0.098 | −0.770 | 81.54 |
| 1-CPN | 86 | 269 | 0.018 | −0.618 | 96.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Chen, X.; Luo, Z.; Xu, J.; Lu, P.; Xie, T.; Tang, J.; Wang, H. Corrosion Inhibition Properties of Corrosion Inhibitors to under-Deposit Corrosion of X65 Steel in CO2 Corrosion Conditions. Molecules 2024, 29, 2611. https://doi.org/10.3390/molecules29112611
Lin H, Chen X, Luo Z, Xu J, Lu P, Xie T, Tang J, Wang H. Corrosion Inhibition Properties of Corrosion Inhibitors to under-Deposit Corrosion of X65 Steel in CO2 Corrosion Conditions. Molecules. 2024; 29(11):2611. https://doi.org/10.3390/molecules29112611
Chicago/Turabian StyleLin, Hai, Xiaorong Chen, Zhongming Luo, Jun Xu, Ping Lu, Tianyi Xie, Jiayi Tang, and Hu Wang. 2024. "Corrosion Inhibition Properties of Corrosion Inhibitors to under-Deposit Corrosion of X65 Steel in CO2 Corrosion Conditions" Molecules 29, no. 11: 2611. https://doi.org/10.3390/molecules29112611
APA StyleLin, H., Chen, X., Luo, Z., Xu, J., Lu, P., Xie, T., Tang, J., & Wang, H. (2024). Corrosion Inhibition Properties of Corrosion Inhibitors to under-Deposit Corrosion of X65 Steel in CO2 Corrosion Conditions. Molecules, 29(11), 2611. https://doi.org/10.3390/molecules29112611






