Kaolin-Derived Porous Silico-Aluminate Nanoparticles as Absorbents for Emergency Disposal of Toluene Leakage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Porous Silico-Aluminate Nanoparticles
2.3. Experimental Method for Capturing Toluene in Porous Silico-Aluminate Nanoparticles
2.3.1. Effects of Different Dosages
2.3.2. Effect of the Capture Time
2.3.3. Effect of pH
2.3.4. Effect of Temperature
2.3.5. Kinetic Study Methods
2.4. Determination of Toluene Concentration in Aqueous Solution
2.5. Characterization
2.6. Calculation of the Adsorption Rate of Toluene in Aqueous Solution
2.7. Calculation of the Adsorption Capacity of Toluene in Aqueous Solution
3. Results and Discussion
3.1. Structural Characterization of Silico-Aluminate Nanoparticles
3.2. Effects of Different Dosages of Porous Silico-Aluminate Nanoparticles on the Removal Efficiency of Toluene
3.3. Effect of the Capture Time on the Removal Efficiency of Toluene
3.4. Effect of pH on the Removal Efficiency of Toluene
3.5. Effect of Temperature on the Removal Efficiency of Toluene
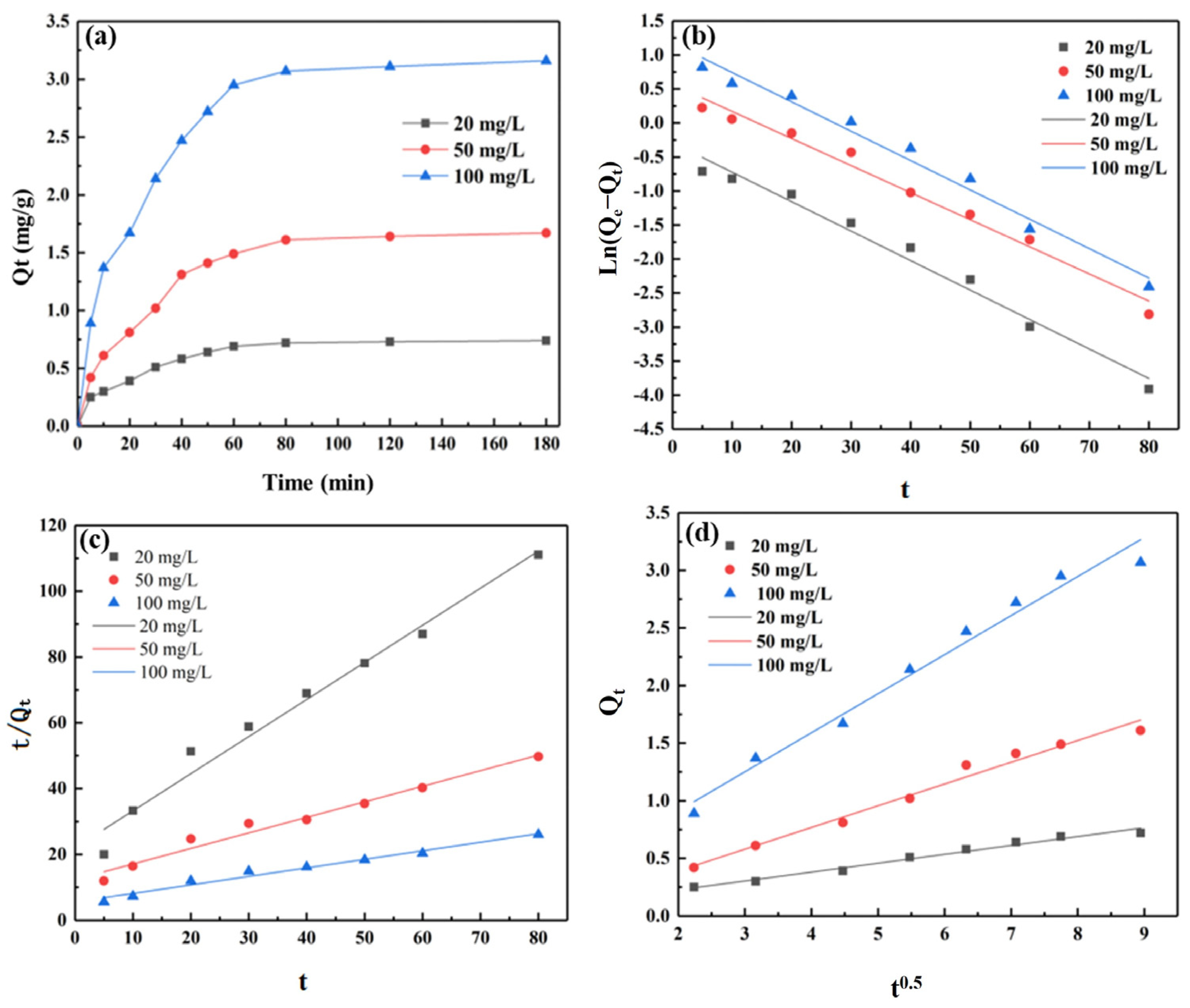
3.6. Adsorption Kinetic Study
3.6.1. The Pseudo-First-Order Kinetics
3.6.2. The Pseudo-Second-Order Kinetics
3.6.3. Weber–Morris Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jähnisch, K.; Baerns, M.; Hessel, V.; Ehrfeld, W.; Haverkamp, V.; Löwe, H.; Wille, C.; Guber, A. Direct fluorination of toluene using elemental fluorine in gas/liquid microreactors. J. Fluorine Chem. 2000, 105, 117–128. [Google Scholar] [CrossRef]
- Barjoee, S.S.; Elmi, M.R.; Varaoon, V.T.; Keykhosravi, S.S.; Karimi, F. Hazards of toluene storage tanks in a petrochemical plant: Modeling effects, consequence analysis, and comparison of two modeling programs. Environ. Sci. Pollut. Res. 2022, 29, 4587–4615. [Google Scholar] [CrossRef]
- Chae, Y.; Kim, L.; Lee, J.; Kim, D.; Cui, R.; An, Y.-J. Estimation of hazardous concentration of toluene in the terrestrial ecosystem through the species sensitivity distribution approach. Environ. Pollut. 2021, 289, 117836. [Google Scholar] [CrossRef]
- Turco, A.; Primiceri, E.; Frigione, M.; Maruccio, G.; Malitesta, C. An innovative, fast and facile soft-template approach for the fabrication of porous PDMS for oil–water separation. J. Mater. Chem. A 2017, 5, 23785–23793. [Google Scholar] [CrossRef]
- Zhao, X.T.; Su, Y.L.; Liu, Y.A.; Lip, Y.F.; Jiang, Z.Y. Free-Standing Graphene Oxide-Palygorskite Nanohybrid Membrane for Oil/Water Separation. ACS Appl. Mater. Interfaces 2016, 8, 8247–8256. [Google Scholar] [CrossRef]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Tu, L.; Duan, W.; Xiao, W.; Fu, C.; Wang, A.; Zheng, Y. Calotropis gigantea fiber derived carbon fiber enables fast and efficient absorption of oils and organic solvents. Sep. Purif. Technol. 2018, 192, 30–35. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, X.; Song, Z.; Lu, K.; Zhu, S.; Yang, Y.; Zhang, Y.; Fang, W.; Jin, J. Microporous polymer adsorptive membranes with high processing capacity for molecular separation. Nat. Commun. 2022, 13, 4169. [Google Scholar] [CrossRef]
- Ohto, K. Review of adsorbents incorporating calixarene derivatives used for metals recovery and hazardous ions removal: The concept of adsorbent design and classification of adsorbents. J. Incl. Phenom. Macrocycl. Chem. 2021, 101, 175–194. [Google Scholar] [CrossRef]
- Rao, G.S.; Nabipour, H.; Zhang, P.; Wang, X.; Xing, W.Y.; Song, L.; Hu, Y. Lightweight, hydrophobic and recyclable carbon foam derived from lignin-resorcinol-glyoxal resin for oil and solvent spill capture. J. Mater. Res. Technol. 2020, 9, 4655–4664. [Google Scholar] [CrossRef]
- Wang, X.; Nie, S.B.; Zhang, P.; Song, L.; Hu, Y. Superhydrophobic and superoleophilic graphene aerogel for ultrafast removal of hazardous organics from water. J. Mater. Res. Technol. 2020, 9, 667–674. [Google Scholar] [CrossRef]
- Inagaki, M.; Konno, H.; Toyoda, M.; Moriya, K.; Kihara, T. Sorption and recovery of heavy oils by using exfoliated graphite Part II: Recovery of heavy oil and recycling of exfoliated graphite. Desalination 2000, 128, 213–218. [Google Scholar] [CrossRef]
- Zhu, H.T.; Qiu, S.S.; Jiang, W.; Wu, D.X.; Zhang, C.Y. Evaluation of Electrospun Polyvinyl Chloride/Polystyrene Fibers As Sorbent Materials for Oil Spill Cleanup. Environ. Sci. Technol. 2011, 45, 4527–4531. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]
- Choi, H.M.; Cloud, R.M. Natural Sorbents in Oil-Spill Cleanup. Environ. Sci. Technol. 1992, 26, 772–776. [Google Scholar] [CrossRef]
- Mysore, D.; Viraraghavan, T.; Jin, Y.C. Treatment of oily waters using vermiculite. Water Res. 2005, 39, 2643–2653. [Google Scholar] [CrossRef]
- Annunciado, T.R.; Sydenstricker, T.H.D.; Amico, S.C. Experimental investigation of various vegetable fibers as sorbent materials for oil spills. Mar. Pollut. Bull. 2005, 50, 1340–1346. [Google Scholar] [CrossRef]
- Bazargan, A.; Tan, J.; Hui, C.W.; McKay, G. Utilization of rice husks for the production of oil sorbent materials. Cellulose 2014, 21, 1679–1688. [Google Scholar] [CrossRef]
- Sathasivam, K.; Haris, M.R.H.M. Adsorption Kinetics and Capacity of Fatty Acid-Modified Banana Trunk Fibers for Oil in Water. Water Air Soil Pollut. 2010, 213, 413–423. [Google Scholar] [CrossRef]
- Said, A.E.A.A.; Ludwick, A.G.; Aglan, H.A. Usefulness of raw bagasse for oil absorption: A comparison of raw and acylated bagasse and their components. Bioresour. Technol. 2009, 100, 2219–2222. [Google Scholar] [CrossRef]
- Sun, X.-F.; Sun, R.-C.; Sun, J.-X. Acetylation of Rice Straw with or without Catalysts and Its Characterization as a Natural Sorbent in Oil Spill Cleanup. J. Agric. Food Chem. 2002, 50, 6428–6433. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.A.; Rahmah, A.U.; Man, Z. Physicochemical and sorption characteristics of Malaysian Ceiba pentandra (L.) Gaertn. as a natural oil sorbent. J. Hazard. Mater. 2010, 177, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Moura, F.C.C.; Lago, R.M. Catalytic growth of carbon nanotubes and nanofibers on vermiculite to produce floatable hydrophobic “nanosponges” for oil spill remediation. Appl. Catal. B Environ. 2009, 90, 436–440. [Google Scholar] [CrossRef]
- Deschamps, G.; Caruel, H.; Borredon, M.E.; Bonnin, C.; Vignoles, C. Oil removal from water by selective sorption on hydrophobic cotton fibers. 1. Study of sorption properties and comparison with other cotton fiber-based sorbents. Environ. Sci. Technol. 2003, 37, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Kenes, K.; Yerdos, O.; Zulkhair, M.; Yerlan, D. Study on the effectiveness of thermally treated rice husks for petroleum adsorption. J. Non-Cryst. Solids 2012, 358, 2964–2969. [Google Scholar] [CrossRef]
- Angelova, D.; Uzunov, I.; Uzunova, S.; Gigova, A.; Minchev, L. Kinetics of oil and oil products adsorption by carbonized rice husks. Chem. Eng. J. 2011, 172, 306–311. [Google Scholar] [CrossRef]
- Chen, K.; Yu, Z.; Mousavi, S.H.; Singh, R.; Gu, Q.; Snurr, R.Q.; Webley, P.A.; Li, G.K. Regulating adsorption performance of zeolites by pre-activation in electric fields. Nat. Commun. 2023, 14, 5479. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host–guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. [Google Scholar] [CrossRef]
- Qu, H.; Ma, Y.; Li, B.; Wang, L. Hierarchical zeolites: Synthesis, structural control, and catalytic applications. Emerg. Mater. 2020, 3, 225–245. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef]
- Yan, Z.; Tang, S.; Zhou, X.; Yang, L.; Xiao, X.; Chen, H.; Qin, Y.; Sun, W. All-silica zeolites screening for capture of toxic gases from molecular simulation. Chin. J. Chem. Eng. 2019, 27, 174–181. [Google Scholar] [CrossRef]
- Mofarahi, M.; Gholipour, F. Gas adsorption separation of CO2/CH4 system using zeolite 5A. Microporous Mesoporous Mater. 2014, 200, 1–10. [Google Scholar] [CrossRef]
- Cha, Y.H.; Mun, S.; Lee, K.B. Development of modified zeolite for adsorption of mixed sulfur compounds in natural gas by combination of ion exchange and impregnation. Appl. Surf. Sci. 2023, 619, 156634. [Google Scholar] [CrossRef]
- Mu, L.; Feng, W.; Zhang, H.; Hu, X.; Cui, Q. Synthesis and catalytic performance of a small crystal NaY zeolite with high SiO2/Al2O3 ratio. RSC Adv. 2019, 9, 20528–20535. [Google Scholar] [CrossRef] [PubMed]
- Dabbawala, A.A.; Ismail, I.; Vaithilingam, B.V.; Polychronopoulou, K.; Singaravel, G.; Morin, S.; Berthod, M.; Al Wahedi, Y. Synthesis of hierarchical porous Zeolite-Y for enhanced CO2 capture. Microporous Mesoporous Mater. 2020, 303, 110261. [Google Scholar] [CrossRef]
- Tahari, N.; de Hoyos-Martinez, P.L.; Abderrabba, M.; Ayadi, S.; Labidi, J. Lignin—Montmorillonite hydrogels as toluene adsorbent. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125108. [Google Scholar] [CrossRef]
- Jafari, D.; Esfandyari, M.; Mojahed, M. Optimization of removal of toluene from industrial wastewater using RSM Box–Behnken experimental design. Sustain. Environ. Res. 2023, 33, 30. [Google Scholar] [CrossRef]
- Shimizu, S.; Matubayasi, N. Temperature Dependence of Sorption. Langmuir 2021, 37, 11008–11017. [Google Scholar] [CrossRef]
- Bandura, L.; Kołodyńska, D.; Franus, W. Adsorption of BTX from aqueous solutions by Na-P1 zeolite obtained from fly ash. Process Saf. Environ. Prot. 2017, 109, 214–223. [Google Scholar] [CrossRef]
- Eydi, E.F.; Shariati, A.; Khosravi-Nikou, M.R. Separation of BTEX compounds (benzene, toluene, ethylbenzene and xylenes) from aqueous solutions using adsorption process. J. Dispers. Sci. Technol. 2019, 40, 453–463. [Google Scholar] [CrossRef]
- Zou, X.Q.; El Fallah, J.; Goupil, J.M.; Zhu, G.S.; Valtchev, V.; Mintova, S. Green removal of aromatic organic pollutants from aqueous solutions with a zeolite-hemp composite. RSC Adv. 2012, 2, 3115–3122. [Google Scholar] [CrossRef]
- Mèçabih, Z. Adsorption-Desorption of BTX (Benzene, Toluene and O-xylene) on Fe, Fe-Al Pillared Clay. J. Encapsul. Adsorpt. Sci. 2017, 7, 40–66. [Google Scholar] [CrossRef]
- Garg, S.; Singh, S.; Khan, N.A.; Samuel, J.; Ramamurthy, P.C.; Singh, J. Equilibrium and kinetic modeling of Cr(VI) removal by novel tolerant bacteria species along with zero-valent iron nanoparticles. Sci. Rep. 2024, 14, 8611. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
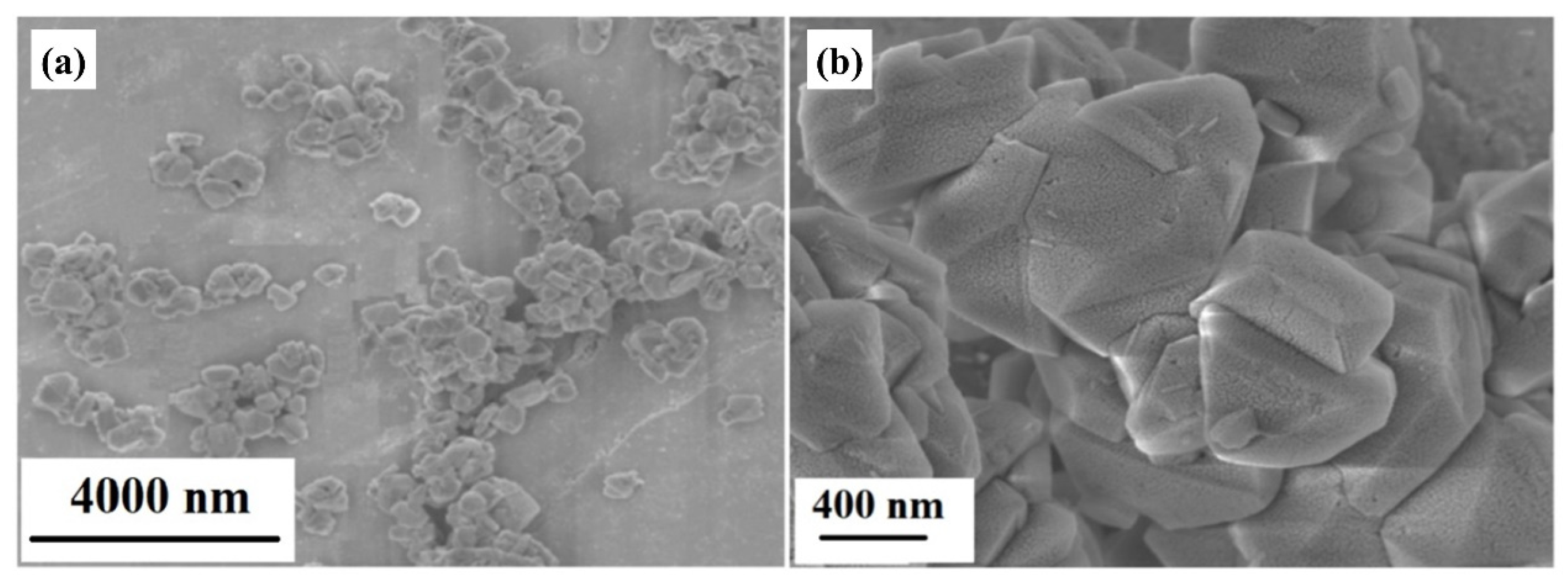
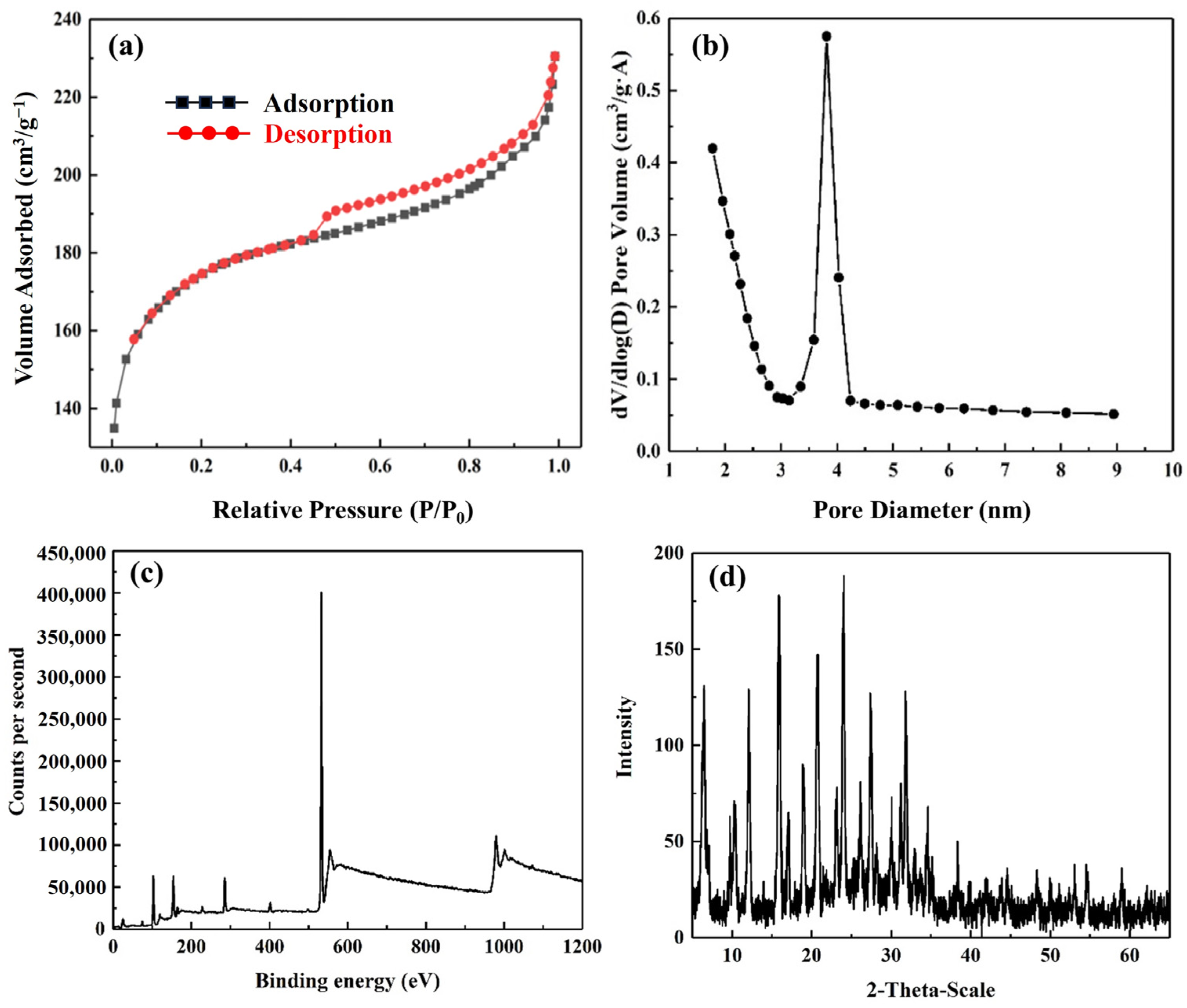

| Atom | O | Si | C | Al | Na |
|---|---|---|---|---|---|
| Atomic conc. (%) | 55.0 | 21.7 | 19.7 | 3.3 | 0.3 |
| Sorbent Name | Temperature (°C) | Dosage (g) | Adsorption Time | Removal Rate (%) | References |
|---|---|---|---|---|---|
| Zeolite Na-P1 | 20 | 0.5 | 24 h | <60 | [39] |
| Zeolite SMZ-100 | 20 | 1.5 | 24 h | <80 | [40] |
| Zeolite–hemp composite | 25 | -- | 300 min | <80 | [41] |
| Fe-Al/B | 80 | 1.0 | 120 min | <70 | [42] |
| SANs | 20 | 0.6 | 80 min | 86 | This Work |
| Initial Concentration C0 (mg·L−1) | Fitting Equation | Correlation Coefficient (R) | R2 | Rate Constant k1 (min−1) | Adsorption Capacity at Equilibrium Qe (mg·g−1) |
|---|---|---|---|---|---|
| 20 | ln(Qe − Qt) = −0.28951 − 0.04333t | 0.99022 | 0.9805 | 0.04333 | 0.7486 |
| 50 | ln(Qe − Qt) = 0.5683 − 0.03981t | 0.99107 | 0.9822 | 0.03981 | 1.7653 |
| 100 | ln(Qe − Qt) = 1.1736 − 0.04315t | 0.99034 | 0.9808 | 0.04315 | 3.2336 |
| Initial Concentration C0 (mg·L−1) | Fitting Equation | Correlation Coefficient (R) | R2 | Rate Constant k2 [g·(mg·min)−1] | Adsorption Capacity at Equilibrium Qe (mg·g−1) |
|---|---|---|---|---|---|
| 20 | t/Qt = 21.9904 + 1.1277t | 0.98962 | 0.97935 | 0.05782 | 0.8868 |
| 50 | t/Qt = 12.3681 + 0.4726t | 0.98760 | 0.97535 | 0.01806 | 2.1161 |
| 100 | t/Qt = 5.52834 + 0.2595t | 0.98894 | 0.97800 | 0.01218 | 3.8530 |
| Initial Concentration C0 (mg·L−1) | Fitting Equation | Correlation Coefficient (R) | R2 | Rate Constant Kpi [g·(mg·min0.5)−1] | Diffusion Constant C |
|---|---|---|---|---|---|
| 20 | Qt = 0.07715t0.5 + 0.07188 | 0.99028 | 0.98065 | 0.07715 | 0.07188 |
| 50 | Qt = 0.18876t0.5 + 0.01303 | 0.99019 | 0.98048 | 0.18876 | 0.01303 |
| 100 | Qt = 0.33916t0.5 + 0.23387 | 0.99002 | 0.98014 | 0.33916 | 0.23387 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Rao, G.; Zhou, F.; Bian, F.; Hu, Y. Kaolin-Derived Porous Silico-Aluminate Nanoparticles as Absorbents for Emergency Disposal of Toluene Leakage. Molecules 2024, 29, 2624. https://doi.org/10.3390/molecules29112624
Wang X, Rao G, Zhou F, Bian F, Hu Y. Kaolin-Derived Porous Silico-Aluminate Nanoparticles as Absorbents for Emergency Disposal of Toluene Leakage. Molecules. 2024; 29(11):2624. https://doi.org/10.3390/molecules29112624
Chicago/Turabian StyleWang, Xin, Guishi Rao, Feng Zhou, Fuli Bian, and Yuan Hu. 2024. "Kaolin-Derived Porous Silico-Aluminate Nanoparticles as Absorbents for Emergency Disposal of Toluene Leakage" Molecules 29, no. 11: 2624. https://doi.org/10.3390/molecules29112624
APA StyleWang, X., Rao, G., Zhou, F., Bian, F., & Hu, Y. (2024). Kaolin-Derived Porous Silico-Aluminate Nanoparticles as Absorbents for Emergency Disposal of Toluene Leakage. Molecules, 29(11), 2624. https://doi.org/10.3390/molecules29112624







