Homo- and Heterogeneous Benzyl Alcohol Catalytic Oxidation Promoted by Mononuclear Copper(II) Complexes: The Influence of the Ligand upon Product Conversion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
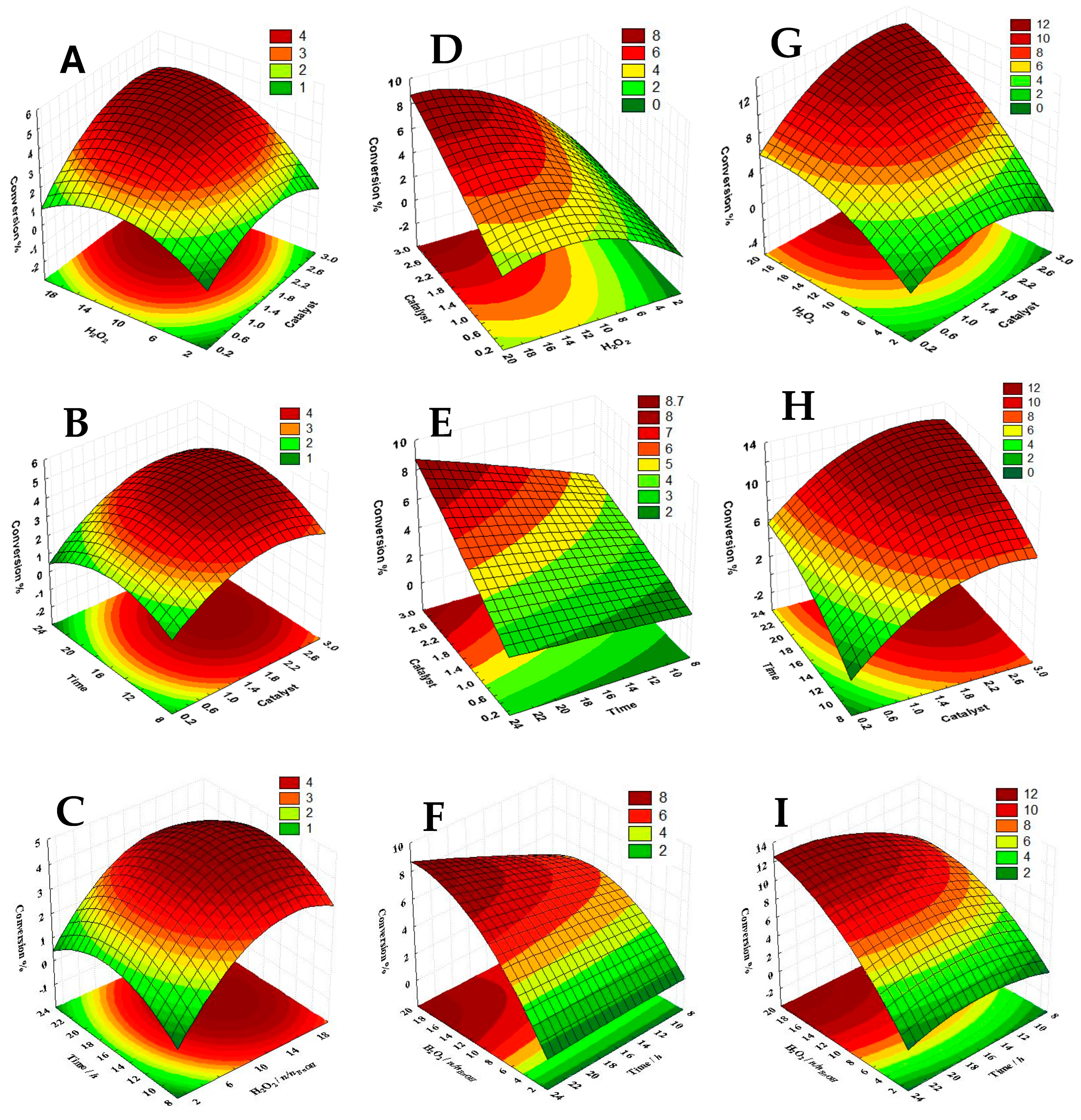
2.2. Homogeneous Catalytic Assays
2.3. Heterogeneous Catalytic Assays
2.4. Theoretical Insights
3. Experimental Procedures
3.1. Materials and Instruments
3.2. DFT Methodology
3.3. Preparation of the Copper Complexes
3.3.1. [Cu(en)2](ClO4)2 (1)
3.3.2. [Cu(amp)2](ClO4)2 (2)
3.3.3. [Cu(bpy)2](ClO4)2 (3)
3.4. Preparation of Composite Catalyst 3-Fe3O4@SiO2
3.5. Homogeneous Catalytic Assays
3.6. Separation and Quantification of the Substrate and Products
3.7. Multivariate Statistical Analysis
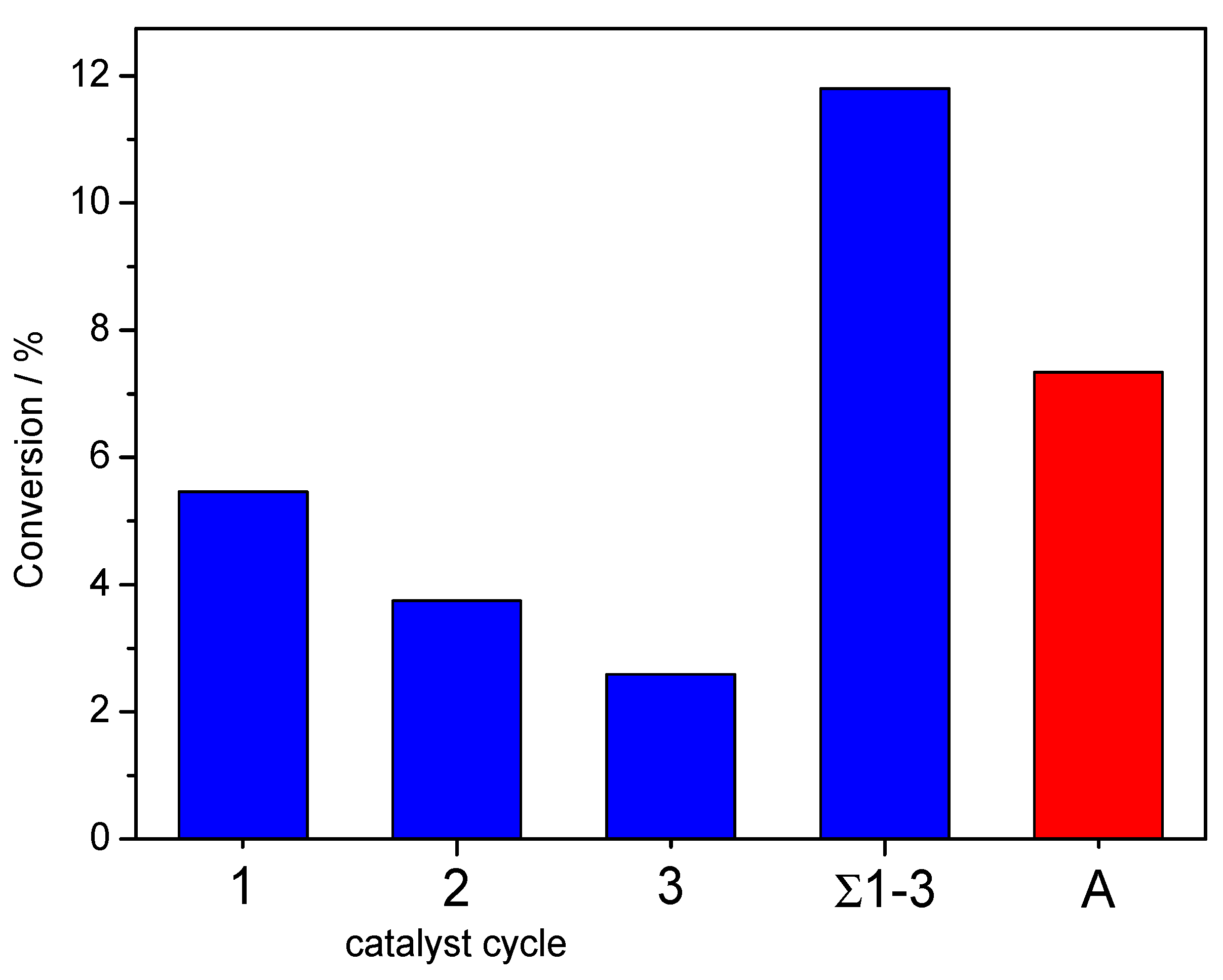
3.8. Heterogenous Catalytic Assays and Catalyst Recycling Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenzweig, A.C.; Sazinsky, M.H. Structural Insights into Dioxygen-Activating Copper Enzymes. Curr. Opin. Struct. Biol. 2006, 16, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Mydy, L.S.; Chigumba, D.N.; Kersten, R.D. Plant Copper Metalloenzymes As Prospects for New Metabolism Involving Aromatic Compounds. Front. Plant Sci. 2021, 12, 692108. [Google Scholar] [CrossRef]
- Belle, C.; Rammal, W.; Pierre, J.L. Sulfur Ligation in Copper Enzymes and Models. J. Inorg. Biochem. 2005, 99, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Urresti, S.; Lafond, M.; Johnston, E.M.; Derikvand, F.; Ciano, L.; Berrin, J.-G.; Henrissat, B.; Walton, P.H.; Davies, G.J.; et al. Structure–Function Characterization Reveals New Catalytic Diversity in the Galactose Oxidase and Glyoxal Oxidase Family. Nat. Commun. 2015, 6, 10197. [Google Scholar] [CrossRef] [PubMed]
- Parikka, K.; Master, E.; Tenkanen, M. Oxidation with Galactose Oxidase: Multifunctional Enzymatic Catalysis. J. Mol. Catal. B Enzym. 2015, 120, 47–59. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, H.B.; Ji, L.N.; Mao, Z.W. Insights into Metalloenzyme Microenvironments: Biomimetic Metal Complexes with a Functional Second Coordination Sphere. Chem. Soc. Rev. 2013, 42, 8360–8375. [Google Scholar] [CrossRef] [PubMed]
- Quist, D.A.; Diaz, D.E.; Liu, J.J.; Karlin, K.D. Activation of Dioxygen by Copper Metalloproteins and Insights from Model Complexes. J. Biol. Inorg. Chem. 2017, 22, 253–288. [Google Scholar] [CrossRef] [PubMed]
- Marais, L.; Swarts, A.J. Biomimetic Cu/Nitroxyl Catalyst Systems for Selective Alcohol Oxidation. Catalysts 2019, 9, 395. [Google Scholar] [CrossRef]
- Oshita, H.; Shimazaki, Y. Recent Advances in One-Electron-Oxidized CuII–Diphenoxide Complexes as Models of Galactose Oxidase: Importance of the Structural Flexibility in the Active Site. Chem.-Eur. J. 2020, 26, 8324–8340. [Google Scholar] [CrossRef]
- Orio, M.; Jarjayes, O.; Kanso, H.; Philouze, C.; Neese, F.; Thomas, F. X-Ray Structures of Copper(II) and Nickel(II) Radical Salen Complexes: The Preference of Galactose Oxidase for Copper(II). Angew. Chem. Int. Ed. 2010, 49, 4989–4992. [Google Scholar] [CrossRef]
- Kundu, B.K.; Ranjan, R.; Mukherjee, A.; Mobin, S.M.; Mukhopadhyay, S. Mannich Base Cu(II) Complexes as Biomimetic Oxidative Catalyst. J. Inorg. Biochem. 2019, 195, 164–173. [Google Scholar] [CrossRef]
- Gao, J.; Ren, Z.G.; Lang, J.P. Oxidation of Benzyl Alcohols to Benzaldehydes in Water Catalyzed by a Cu(II) Complex with a Zwitterionic Calix[4]Arene Ligand. J. Organomet. Chem. 2015, 792, 88–92. [Google Scholar] [CrossRef]
- Santiago, P.H.O.; Aiube, C.M.; de Macedo, J.L.; Gatto, C.C. Hydrazone-Derived Copper(II) Coordination Polymer as a Selective Liquid-Phase Catalyst: Synthesis, Crystal Structure and Performance towards Benzyl Alcohol Oxidation. Mol. Cat. 2020, 496, 111177. [Google Scholar] [CrossRef]
- Borgaonkar, H.V.; Raverkar, S.R.; Chandalla, S.B. Liquid Phase Oxidation of Toluene to Benzaldehyde by Air. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 455–458. [Google Scholar] [CrossRef]
- Godhani, D.R.; Nakum, H.D.; Parmar, D.K.; Mehta, J.P.; Desai, N.C. Zeolite-Y Immobilized Metallo-Ligand Complexes: A Novel Heterogenous Catalysts for Selective Oxidation. Inorg. Chem. Commun. 2016, 72, 105–116. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Savara, A.; Villa, A. Selective Benzyl Alcohol Oxidation over Pd Catalysts. Catalysts 2018, 8, 8100431. [Google Scholar] [CrossRef]
- Göksu, H.; Burhan, H.; Mustafov, S.D.; Şen, F. Oxidation of Benzyl Alcohol Compounds in the Presence of Carbon Hybrid Supported Platinum Nanoparticles (Pt@CHs) in Oxygen Atmosphere. Sci. Rep. 2020, 10, 5439. [Google Scholar] [CrossRef]
- Kodchasee, J.; Chanloi, C.; Khemthong, P.; Uapipatanakul, B.; Ehara, M.; Bobuatong, K. Catalytic Oxidation of Benzyl Alcohol to Benzaldehyde on Au8 and Au6pd2 Clusters: A Dft Study on the Reaction Mechanism. Catalysts 2021, 11, 720. [Google Scholar] [CrossRef]
- Torbina, V.V.; Vodyankin, A.A.; Ten, S.; Mamontov, G.V.; Salaev, M.A.; Sobolev, V.I.; Vodyankina, O.V. Ag-Based Catalysts in Heterogeneous Selective Oxidation of Alcohols: A Review. Catalysts 2018, 8, 447. [Google Scholar] [CrossRef]
- Trong, D.T.; Van Long, D.; Khanh, P.Q.; Anh Tuan, V.; Nhat, P.H.; Tien Thao, N. Efficient Oxidation of Benzyl Alcohol on Copper Phyllosilicate Catalysts. J. Sci. Adv. Mater. Devices 2023, 8, 100564. [Google Scholar] [CrossRef]
- Yamamoto, R.; Sawayama, Y.S.; Shibahara, H.; Ichihashi, Y.; Nishiyama, S.; Tsuruya, S. Promoted Partial Oxidation Activity of Supported Ag Catalysts in the Gas-Phase Catalytic Oxidation of Benzyl Alcohol. J. Catal. 2005, 234, 308–317. [Google Scholar] [CrossRef]
- Mao, J.; Deng, M.; Xue, Q.; Chen, L.; Lu, Y. Thin-Sheet Ag/Ni-Fiber Catalyst for Gas-Phase Selective Oxidation of Benzyl Alcohol with Molecular Oxygen. Catal. Commun. 2009, 10, 1376–1379. [Google Scholar] [CrossRef]
- Feng, J.B.; Wu, X.F. Transition Metal-Catalyzed Oxidative Transformations of Methylarenes. Appl. Organomet. Chem. 2015, 29, 63–86. [Google Scholar] [CrossRef]
- Adam, M.S.S.; Khalil, A.; Taha, A.; Mostafa, M.M.; Makhlouf, M.M.; Mahmoud, H.A. Facile Synthetic Route of TiO2–ZnO Heteronanostructure Coated by Oxovanadium (IV) Bis-Schiff Base Complex as a Potential Effective Homogeneous/Heterogeneous Catalysts for Alcohols Redox Systems. Surf. Interfaces 2023, 39, 102914. [Google Scholar] [CrossRef]
- Adam, M.S.S.; Taha, A.; Hereba, A.T.; Makhlouf, M.M.; Mahmoud, H.A. Immobilized Transition Metal (II) Bis–Imine Chelate on ZnO@TiO2 Nanoparticles as Sufficient Catalysts for Alcohol Oxidation and for α,β-Cinnamic Acid Decarboxylative Bromination. Appl. Organomet. Chem. 2024, e7438. [Google Scholar] [CrossRef]
- Li, Y.S.; Church, J.S.; Woodhead, A.L.; Moussa, F. Preparation and Characterization of Silica Coated Iron Oxide Magnetic Nano-Particles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 76, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Sarkheil, M.; Lashanizadegan, M. Copper(II) Schiff Base Complex Immobilized on Superparamagnetic Fe3O4@SiO2 as a Magnetically Separable Nanocatalyst for Oxidation of Alkenes and Alcohols. Appl. Organomet. Chem. 2017, 31, e3726. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Sun, X.C.; Guo, Z.M.; Su, L.; Zhao, J.Q. Synthesis of Pyridinecarboxaldimine Grafted to Magnetic Nanoparticles (Fe3O4@SiO2) and Its Application in the Aerobic Oxidation of Primary Alcohols Catalyzed by CuBr2/TEMPO. Russ. J. Gen. Chem. 2016, 86, 944–952. [Google Scholar] [CrossRef]
- Vailati, A.F.; Huelsmann, R.D.; Martendal, E.; Bortoluzzi, A.J.; Xavier, F.R.; Peralta, R.A. Multivariate Analysis Applied to Oxidation of Cyclohexane and Benzyl Alcohol Promoted by Mononuclear Iron and Copper Complexes. New J. Chem. 2020, 44, 2514–2526. [Google Scholar] [CrossRef]
- Munshi, S.; Sinha, A.; Yiga, S.; Banerjee, S.; Singh, R.; Hossain, M.K.; Haukka, M.; Valiati, A.F.; Huelsmann, R.D.; Martendal, E.; et al. Hydrogen-Atom and Oxygen-Atom Transfer Reactivities of Iron. Dalton Trans. 2022, 51, 870–884. [Google Scholar] [CrossRef] [PubMed]
- Chimilouski, L.; Morgan, F.; Ekanayake, D.; El-Harakeh, N.; Peralta, R.A.; Martendal, E.; Verani, C.N.; Xavier, F.R. Bioinspired Oxidation of Benzyl Alcohol: The Role of Environment and Nuclearity of the Catalyst Evaluated by Multivariate Analysis. J. Inorg. Biochem. 2023, 240, 112095. [Google Scholar] [CrossRef] [PubMed]
- Percy, G.C.; Tlfornton, D.A. Structural Information on Copper(II) and Copper(I) Complexes of 2,2′-Bipyridine and 1,10-Phenanthroline from Their Infrared Spectra. J. Mol. Struct. 1972, 14, 313–319. [Google Scholar] [CrossRef]
- Niven, M.L.; Percy, G.C.; Thornton, D.A. The Infrared Spectra of 2-Aminomethylpyridine Complexes of Metal(II) Ions. J. Mol. Struct. 1980, 68, 73–80. [Google Scholar] [CrossRef]
- Mcwhinnie, W.R. Infra-Red Spectra of Some Sulphato (and Perchlorato) Complexes of Copper(II)-II. J. Inorg. Nucl. Chem. 1964, 26, 21–24. [Google Scholar] [CrossRef]
- Swanson, H.E.; McMurdie, H.F.; Morris, M.C.; Evans, E.H. Standard X-ray Diffraction Powder Patterns; U.S. Government Printing Office: Washington, DC, USA, 1967. [Google Scholar]
- Singh, N.; Botcha, N.K.; Jones, T.M.; Ertem, M.Z.; Niklas, J.; Farquhar, E.R.; Poluektov, O.G.; Mukherjee, A. Reactivity of Bio-Inspired Cu(II) (N2/Py2) Complexes with Peroxide at Room Temperature. J. Inorg. Biochem. 2019, 197, 110674. [Google Scholar] [CrossRef] [PubMed]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen-Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-Bis(N-Methylbenzimidazol-2′-Yl)-2,6-Dithiaheptane]Copper(II) Perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Lide, D.R.; Haynes, W.M.M.; Baysinger, G.; Berger, L.I.; Roth, D.L.; Zwillinger, D.; Frenkel, M.; Goldberg, R.N. CRC Handbook of Chemistry and Physics, 90th ed.; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Gagne, R.R.; Koval, C.A.; Lisensky, G.C. Ferrocene as an Internal Standard for Electrochemical Measurements. Inorg. Chem. 1980, 19, 2854–2855. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colic-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully Optimized Contracted Gaussian Basis Sets for Atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully Optimized Contracted Gaussian Basis Sets of Triple Zeta Valence Quality for Atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.G.L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Izsák, R.; Neese, F. An Overlap Fitted Chain of Spheres Exchange Method. J. Chem. Phys. 2011, 135, 144105. [Google Scholar] [CrossRef]
- Izsák, R.; Neese, F.; Klopper, W. Robust Fitting Techniques in the Chain of Spheres Approximation to the Fock Exchange: The Role of the Complementary Space. J. Chem. Phys. 2013, 139, 094111. [Google Scholar] [CrossRef] [PubMed]
- Helmich-Paris, B.; de Souza, B.; Neese, F.; Izsák, R. An Improved Chain of Spheres for Exchange Algorithm. J. Chem. Phys. 2021, 155, 104109. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, T.; Kossmann, S.; Neese, F. Efficient Time-Dependent Density Functional Theory Approximations for Hybrid Density Functionals: Analytical Gradients and Parallelization. J. Chem. Phys. 2011, 134, 054116. [Google Scholar] [CrossRef]
- Cammi, R.; Mennucci, B.; Tomasi, J. Fast Evaluation of Geometries and Properties of Excited Molecules in Solution: A Tamm-Dancoff Model with Application to 4-Dimethylaminobenzonitrile. J. Phys. Chem. A 2000, 104, 5631–5637. [Google Scholar] [CrossRef]
- Neese, F. Software Update: The ORCA Program System, Version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, 4–9. [Google Scholar] [CrossRef]
- Zhurko, G.A.; Zhurko, D.A. Chemcraft—Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 1 March 2024).
- Chae, H.S.; Kim, S.D.; Piao, S.H.; Choi, H.J. Core-Shell Structured Fe3O4@SiO2 Nanoparticles Fabricated by Sol–Gel Method and Their Magnetorheology. Colloid Polym. Sci. 2016, 294, 647–655. [Google Scholar] [CrossRef]



| Entry | Catalyst/mol% | H2O2 a | Time/h | Conversion/% | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||||
| BA | BzA | BA | BzA | BA | BzA | ||||
| 1 | 0.10 | 10.5 | 8 | 0.79 | - | 5.19 | - | 1.75 | - |
| 2 | 3.0 | 10.5 | 8 | 2.56 | - | 4.74 | 1.26 | 5.00 | - |
| 3 | 1.55 | 1.0 | 8 | 0.27 | - | 1.18 | - | 1.27 | - |
| 4 | 1.55 | 20 | 8 | 2.71 | 1.33 | 2.20 | - | 6.83 | - |
| 5 | 3.0 | 10.5 | 24 | 3.21 | - | 5.63 | 1.05 | 7.37 | 5.60 |
| 6 | 1.55 | 1.0 | 24 | 0.35 | - | 0.56 | - | 1.47 | - |
| 7 | 0.1 | 10.5 | 24 | 0.37 | - | 2.68 | - | 5.05 | - |
| 8 | 1.55 | 20 | 24 | 2.52 | 1.34 | 6.05 | 0.62 | 12.07 | 5.25 |
| 9 | 0.1 | 1.0 | 16 | 0.48 | - | 0.37 | n.d | 0.73 | - |
| 10 | 3.0 | 1.0 | 16 | 0.38 | - | 0.76 | n.d | 1.07 | - |
| 11 | 0.1 | 20 | 16 | 1.08 | - | 1.30 | n.d | 3.31 | - |
| 12 | 3.0 | 20 | 16 | 3.66 | 5.93 | 7.20 | 1.40 | 10.85 | 2.65 |
| 13 | 1.55 | 10.5 | 16 | 4.28 | 1.05 | 4.96 | 1.10 | 8.02 | - |
| 14 | 1.55 | 10.5 | 16 | 3.87 | 1.26 | 4.64 | 1.05 | 9.15 | - |
| 15 | 1.55 | 10.5 | 16 | 4.85 | 1.67 | 4.81 | 1.08 | 9.68 | - |
| Entry | Complex | Catalyst/mol% | H2O2/n nsubstrate−1 | Time/h | Conversion/% (±sd.) | Selectivity/% | TON e (×103) | TOF f/h−1 (×103) | |
|---|---|---|---|---|---|---|---|---|---|
| BA | BzA | ||||||||
| 1 | 1 | 2.20 | 14.5 | 16.3 | 5.94 ± 0.27 [4.76 ± 0.51] | 3.72 ± 0.33 | 62 | 48.0 | 3.00 |
| 2 | 2 | 3.00 | 20.0 | 24.0 | 7.75 ± 0.28 [8.68 ± 0.40] | 5.24 ± 0.14 | 60 | 45.8 | 1.91 |
| 3 | 3 | 2.50 | 20.0 | 24.0 | 11.20 ± 0.59 [12.60 ± 0.46] | 3.28 ± 0.10 | 78 | 79.2 | 3.30 |
| 4 | CS a | 2.20 | 14.5 | 16.3 | 4.22 ± 0.04 | - | 100 | ||
| 5 | CS a | 3.00 | 20.0 | 24.0 | 7.42 ± 0.16 | - | 100 | ||
| 6 | CS a | 2.50 | 20.0 | 24.0 | 7.69 ± 0.77 | - | 100 | ||
| 7 | - | - | 14.5 | 16.3 | 0.54 ± 0.36 | - | 100 | ||
| 8 | - | - | 20.0 | 24.0 | 0.57 ± 0.33 | - | 100 | ||
| 9 | 1 b | 2.20 | 14.5 | 16.3 | - | 93.6 ± 6.8 | 100 | ||
| 10 | 2 b | 3.00 | 20.0 | 24.0 | - | 71.6 ± 7.4 | 100 | ||
| 11 | 3 b | 2.50 | 20.0 | 24.0 | - | 56.2 ± 1.8 | 100 | ||
| 12 | - b | - | 14.5 | 16.3 | - | - | 100 | ||
| 13 | - b | - | 20.0 | 24.0 | - | - | - | ||
| 14 | 1 c | 2.20 | 14.5 | 16.3 | 2.26 ± 0.12 | - | 100 | ||
| 15 | 2 c | 3.00 | 20.0 | 24.0 | 3.44 ± 0.29 | - | 100 | ||
| 16 | 3 c | 2.50 | 20.0 | 24.0 | 5.12 ± 0.15 | - | 100 | ||
| 17 | 1 d | 2.20 | 14.5 | 16.3 | - | 42.2 ± 7.9 | 100 | ||
| 18 | 2 d | 3.00 | 20.0 | 24.0 | - | 59.9 ± 6.0 | 100 | ||
| 19 | 3 d | 2.50 | 20.0 | 24.0 | - | 61.9 ± 3.0 | 100 | ||
| Entry | Catalyst/mol% | H2O2/n nsubstrate−1 | Time/h | RV a/mL | Conversion/% (±sd.) | Selectivity/% | TON d (×103) | TOF e/h−1 (×103) |
|---|---|---|---|---|---|---|---|---|
| BA | ||||||||
| 1 | 1.00 | 20.0 | 24.0 | 3.0 | 5.46 ± 0.31 | 100 | 97.4 | 4.06 |
| 2 | 1.00 | 20.0 | 24.0 | 4.5 | 2.89 ± 0.30 | 100 | - | - |
| 3 | 1.00 | 20.0 | 24.0 | 9.0 | 1.21 ± 0.26 | 100 | - | - |
| 4 | 1.00 | 20.0 | 24.0 | 3.0 | 7.34 ± 0.33 b | 100 | 130.2 | 5.42 |
| 5 | - | 20.0 | 24.0 | 3.0 | - | - | - | - |
| 6 | 1.00 | 20.0 | 24.0 | 3.0 | 5.48 ± 0.31 c | 100 | - | - |
| 7 | 1.00 | 20.0 | 24.0 | 3.0 | 3.75 ± 0.50 | 100 | - | - |
| 8 | 1.00 | 20.0 | 24.0 | 3.0 | 2.59 ± 0.07 | 100 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chimilouski, L.; Slominski, W.H.; Tillmann, A.I.; Will, D.; dos Santos, A.M.; Farias, G.; Martendal, E.; Naidek, K.P.; Xavier, F.R. Homo- and Heterogeneous Benzyl Alcohol Catalytic Oxidation Promoted by Mononuclear Copper(II) Complexes: The Influence of the Ligand upon Product Conversion. Molecules 2024, 29, 2634. https://doi.org/10.3390/molecules29112634
Chimilouski L, Slominski WH, Tillmann AI, Will D, dos Santos AM, Farias G, Martendal E, Naidek KP, Xavier FR. Homo- and Heterogeneous Benzyl Alcohol Catalytic Oxidation Promoted by Mononuclear Copper(II) Complexes: The Influence of the Ligand upon Product Conversion. Molecules. 2024; 29(11):2634. https://doi.org/10.3390/molecules29112634
Chicago/Turabian StyleChimilouski, Larissa, William H. Slominski, Ana I. Tillmann, Daniella Will, Aaron M. dos Santos, Giliandro Farias, Edmar Martendal, Karine P. Naidek, and Fernando R. Xavier. 2024. "Homo- and Heterogeneous Benzyl Alcohol Catalytic Oxidation Promoted by Mononuclear Copper(II) Complexes: The Influence of the Ligand upon Product Conversion" Molecules 29, no. 11: 2634. https://doi.org/10.3390/molecules29112634







