Highly Efficient and Selective Hydrogenation of Biomass-Derived Furfural Using Interface-Active Rice Husk-Based Porous Carbon-Supported NiCu Alloy Catalysts
Abstract
:1. Introduction
2. Results and Discussion
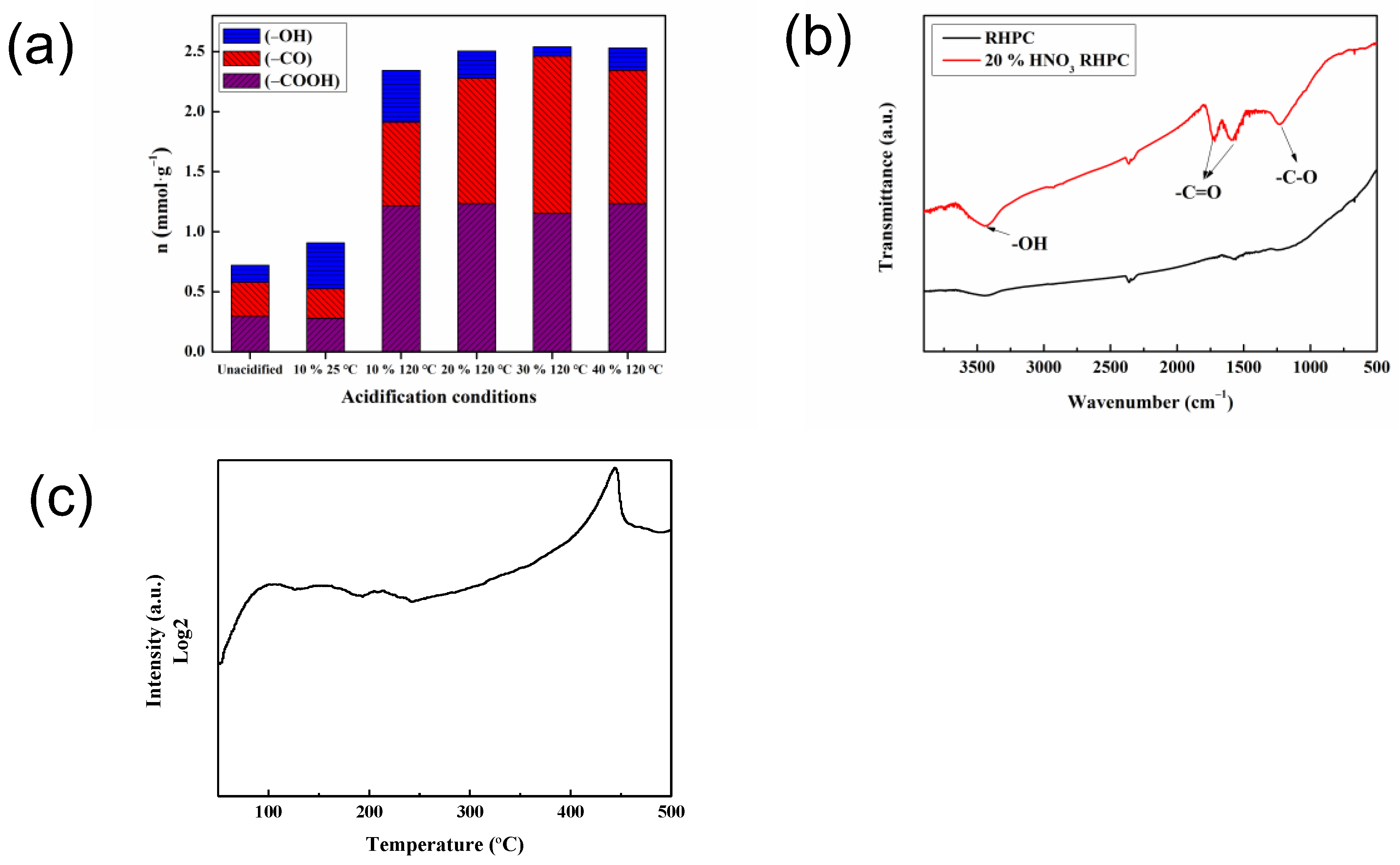
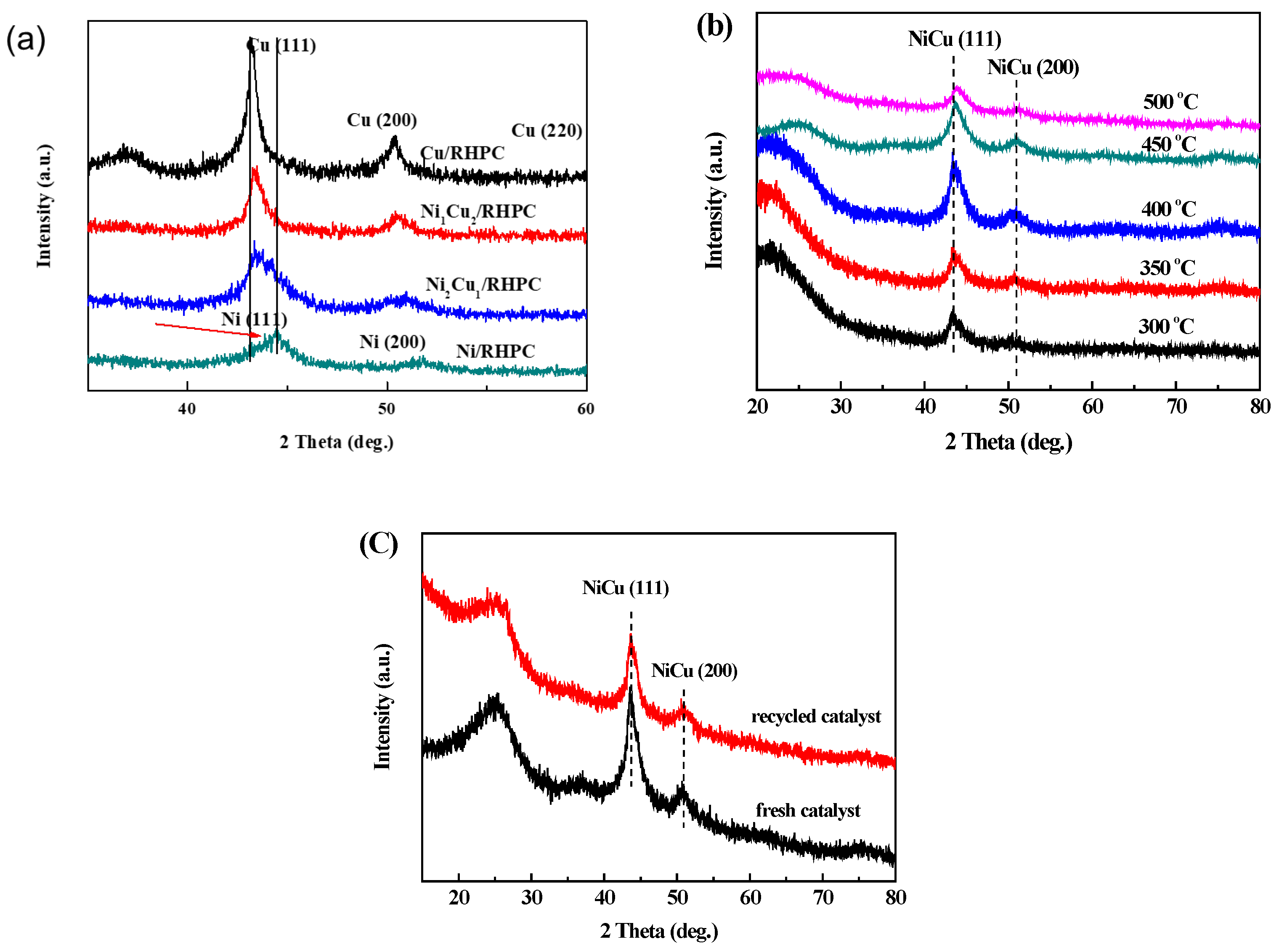
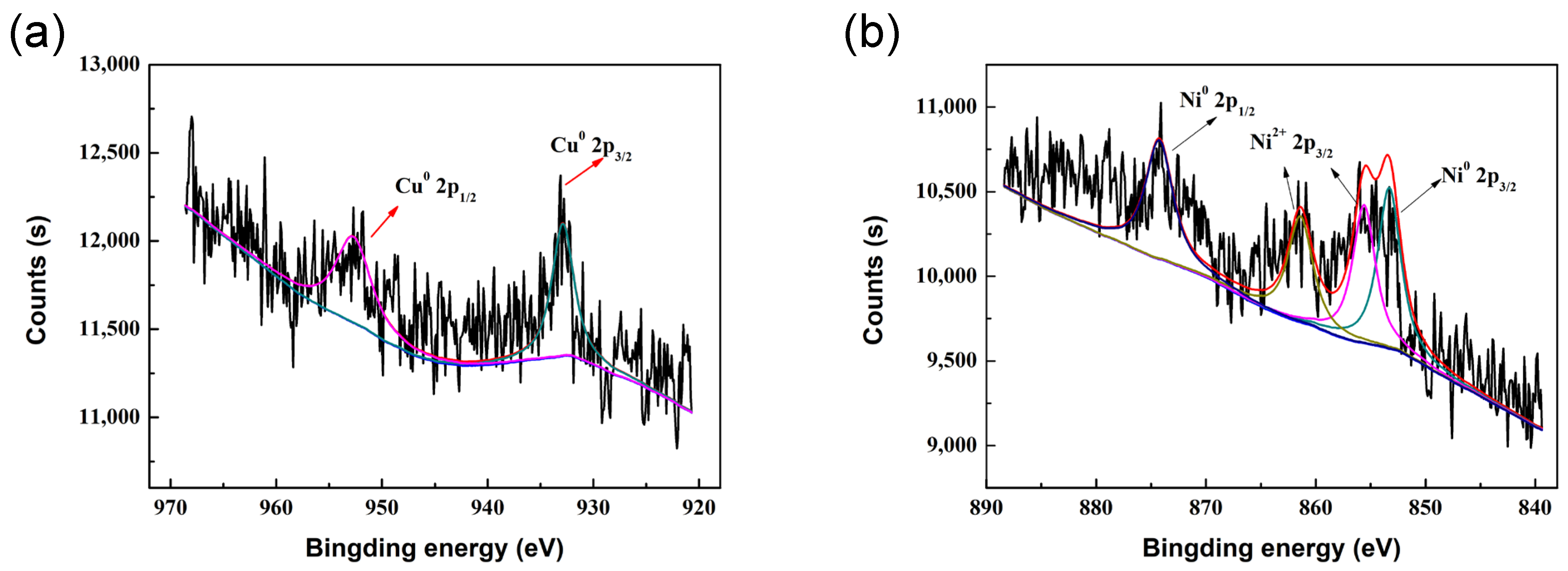
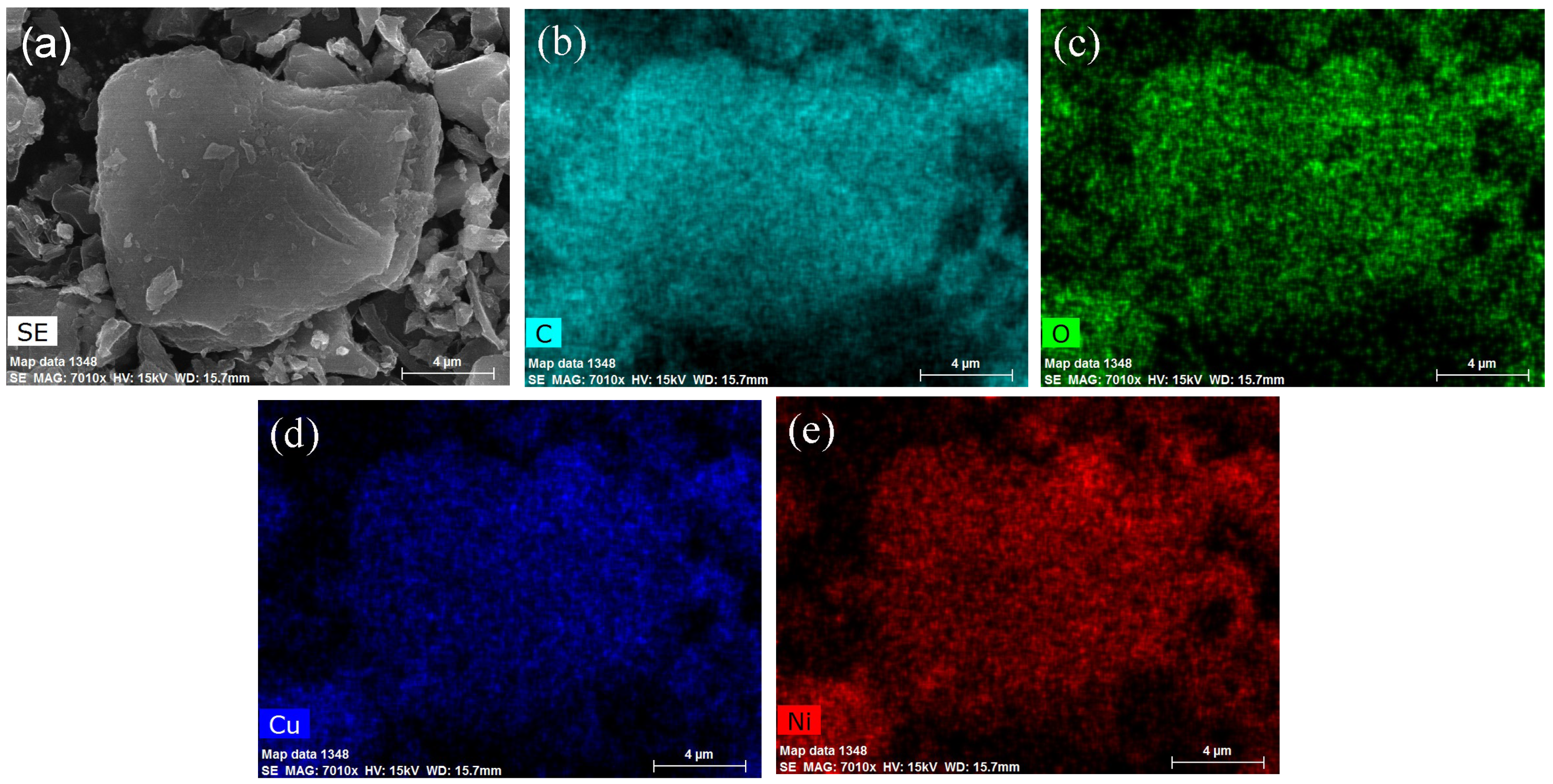
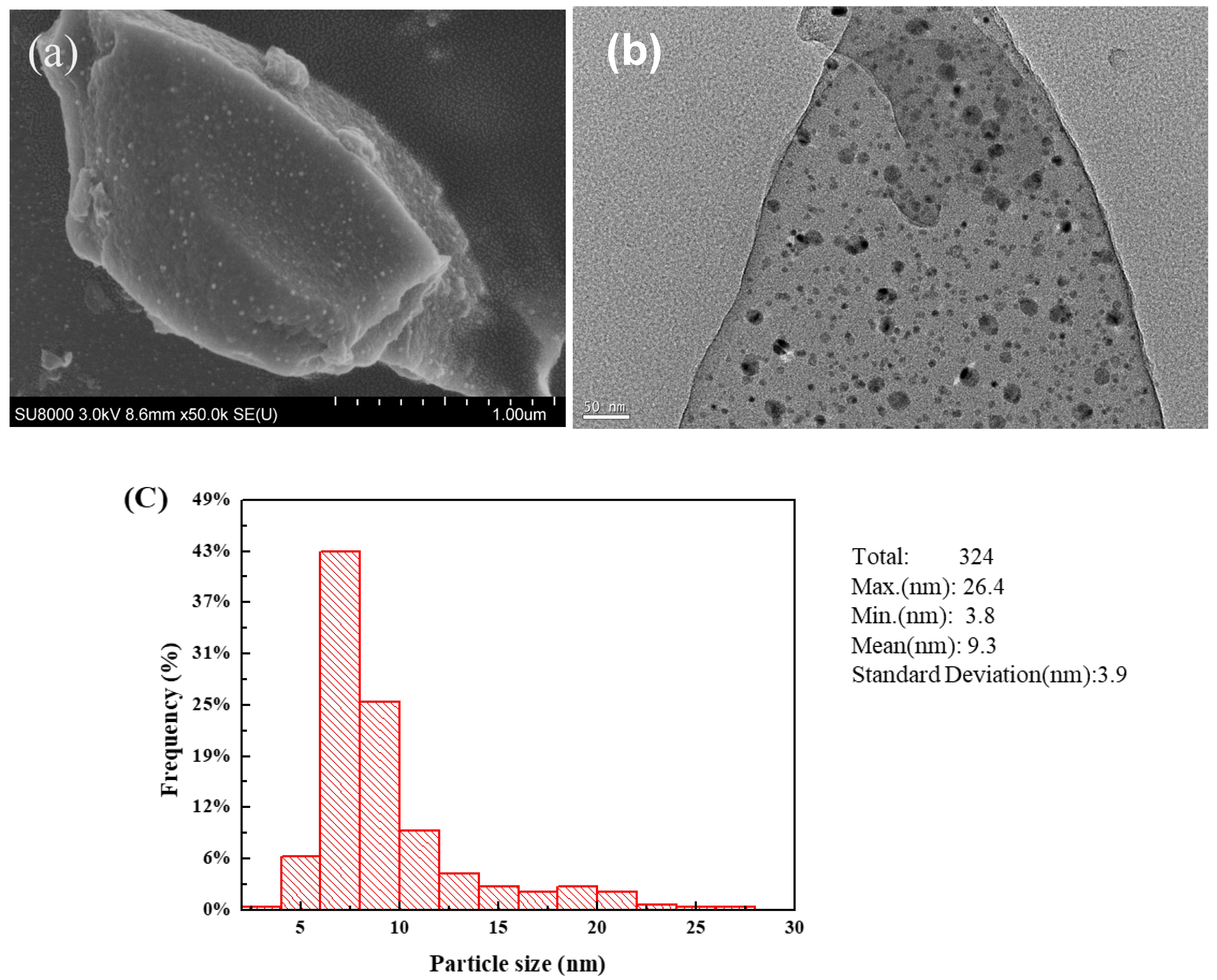
2.1. Characterization of Catalysts
2.2. Catalytic Performance of NixCuy/RHPC Catalysts
3. Experimental Section
3.1. Materials and Reagents
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Furfural Hydrogenation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Sun, W.; Xiao, N.; Yan, Y.; Liu, S. Experimental study for liquid phase selective hydrogenation of furfuryl alcohol to tetrahydrofurfuryl alcohol on supported Ni catalysts. Chem. Eng. J. 2007, 126, 5–11. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakazawa, H.; Watanabe, H.; Tomishige, K. Total Hydrogenation of Furfural over a Silica-Supported Nickel Catalyst Prepared by the Reduction of a Nickel Nitrate Precursor. ChemCatChem 2012, 4, 1791–1797. [Google Scholar] [CrossRef]
- Banerjee, A.; Mushrif, S.H. Investigating Reaction Mechanisms for Furfural Hydrodeoxygenation on Ni and the Effect of Boron Doping on the Activity and Selectivity of the Catalyst. J. Phys. Chem. C 2018, 122, 18383–18394. [Google Scholar] [CrossRef]
- Nandi, S.; Saha, A.; Patel, P.; Khan, N.H.; Kureshy, R.I.; Panda, A.B. Hydrogenation of Furfural with Nickel Nanoparticles Stabilized on Nitrogen-Rich Carbon Core-Shell and Its Transformations for the Synthesis of gamma-Valerolactone in Aqueous Conditions. ACS Appl. Mater. Interfaces 2018, 10, 24480–24490. [Google Scholar] [CrossRef] [PubMed]
- Manfro, R.L.; Pires, T.P.M.D.; Ribeiro, N.F.P.; Souza, M.M.V.M. Aqueous-phase reforming of glycerol using Ni–Cu catalysts prepared from hydrotalcite-like precursors. Catal. Sci. Technol. 2013, 3, 1278–1287. [Google Scholar] [CrossRef]
- Li, D.; Lu, M.; Aragaki, K.; Koike, M.; Nakagawa, Y.; Tomishige, K. Characterization and catalytic performance of hydrotalcite-derived Ni-Cu alloy nanoparticles catalysts for steam reforming of 1-methylnaphthalene. Appl. Catal. B Environ. 2016, 192, 171–181. [Google Scholar] [CrossRef]
- Thongratkaew, S.; Kiatphuengporn, S.; Junkaew, A.; Kuboon, S.; Chanlek, N.; Seubsai, A.; Rungtaweevoranit, B.; Faungnawakij, K. Solvent effects in integrated reaction-separation process of liquid-phase hydrogenation of furfural to furfuryl alcohol over CuAl2O4 catalysts. Catal. Commun. 2022, 169, 106468. [Google Scholar] [CrossRef]
- Seemala, B.; Cai, C.M.; Wyman, C.E.; Christopher, P. Support Induced Control of Surface Composition in Cu–Ni/TiO2 Catalysts Enables High Yield Co-Conversion of HMF and Furfural to Methylated Furans. ACS Catal. 2017, 7, 4070–4082. [Google Scholar] [CrossRef]
- Wang, Y.; Sang, S.Y.; Zhu, W.; Gao, L.J.; Xiao, G.M. CuNi@C catalysts with high activity derived from metal-organic frameworks precursor for conversion of furfural to cyclopentanone. Chem. Eng. J. 2016, 299, 104–111. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Kang, H.; Xia, C.; Chen, J. Selective hydrogenolysis of biomass-derived furfuryl alcohol into 1,2- and 1,5-pentanediol over highly dispersed Cu-Al2O3 catalysts. Chin. J. Catal. 2016, 37, 700–710. [Google Scholar] [CrossRef]
- Wu, J.; Gao, G.; Li, J.L.; Sun, P.; Long, X.D.; Li, F.W. Efficient and versatile CuNi alloy nanocatalysts for the highly selective hydrogenation of furfural. Appl Catal B Env. 2017, 203, 227–236. [Google Scholar] [CrossRef]
- Shu, R.Y.; Lin, Y.K.; Zhou, L.X.; Luo, B.W.; Yang, S.; Tian, Z.P.; Wang, C.; Shi, Z.J.; Nayak, R.R.; Gupta, N.K. Enhanced lignin hydrogenolysis through synergy-induced bimetallic NiCu catalyst for chemocatalytic production of aromatic monomers. Chem. Eng. Sci. 2024, 286. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Gulyaeva, T.I.; Nizovskii, A.I.; Kalinkin, A.V.; Bukhtiyarov, V.I.; Lavrenov, A.V.; Likholobov, V.A. Effect of the nature of carbon support on the formation of active sites in Pd/C and Ru/C catalysts for hydrogenation of furfural. Catal. Today 2015, 249, 145–152. [Google Scholar] [CrossRef]
- Kim, G.; Kim, Y.E.; Jae, J.; Lee, M.S. Effect of physicochemical properties of carbons on the performance of bead-type Pd/C catalysts for furfural hydrogenation. Mol. Catal. 2024, 558, 114025. [Google Scholar] [CrossRef]
- Liu, L.-J.; Guo, H.-M.; Xue, B.; Lou, H.; Chen, M. Hydrogenation in supercritical conditions catalyzed by palladium supported on modified activated carbon. Rsc Adv. 2015, 5, 66704–66710. [Google Scholar] [CrossRef]
- Liu, L.; Lou, H.; Chen, M. Selective hydrogenation of furfural over Pt based and Pd based bimetallic catalysts supported on modified multiwalled carbon nanotubes (MWNT). Appl. Catal. A Gen. 2018, 550, 1–10. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Tu, R.; Sun, Y.; Jiang, E. Hydrogen from Rice Husk Pyrolysis Volatiles via Non-Noble Ni–Fe Catalysts Supported on Five Differently Treated Rice Husk Pyrolysis Carbon Supports. ACS Sustain. Chem. Eng. 2018, 6, 8325–8339. [Google Scholar] [CrossRef]
- Singh, P.; Kulkarni, M.V.; Gokhale, S.P.; Chikkali, S.H.; Kulkarni, C.V. Enhancing the hydrogen storage capacity of Pd-functionalized multi-walled carbon nanotubes. Appl. Surf. Sci. 2012, 258, 3405–3409. [Google Scholar] [CrossRef]
- Chen, Z.M.; Wang, X.F.; Xue, B.C.; Li, W.; Ding, Z.Y.; Yang, X.M.; Qiu, J.S.; Wang, Z.C. Rice husk-based hierarchical porous carbon for high performance supercapacitors: The structure-performance relationship. Carbon 2020, 161, 432–444. [Google Scholar] [CrossRef]
- Naghash, A.R.; Etsell, T.H.; Xu, S. XRD and XPS study of Cu-Ni interactions on reduced copper-nickel-aluminum oxide solid solution catalysts. Chem. Mat. 2006, 18, 2480–2488. [Google Scholar] [CrossRef]
- Wu, Q.X.; Duchstein, L.D.L.; Chiarello, G.L.; Christensen, J.M.; Damsgaard, C.D.; Elkjaer, C.F.; Wagner, J.B.; Temel, B.; Grunwaldt, J.D.; Jensen, A.D. In Situ Observation of Cu-Ni Alloy Nanoparticle Formation by X-Ray Diffraction, X-Ray Absorption Spectroscopy, and Transmission Electron Microscopy: Influence of Cu/Ni Ratio. Chemcatchem 2014, 6, 301–310. [Google Scholar] [CrossRef]
- Mansour, A.N. Nickel monochromated Al Kalpha XPS spectra from the Physical Electronics Model 5400 spectrometer. Surf. Sci. Spectra 1994, 3, 221–230. [Google Scholar] [CrossRef]
- Mansour, A.N. Nickel Mg Kalpha XPS spectra from the Physical Electronics Model 5400 spectrometer. Surf. Sci. Spectra 1994, 3, 211–220. [Google Scholar] [CrossRef]
- Conner, G.R. Combination analysis of metal-oxides using esca, aes, and sims. J. Vac. Sci. Technol. 1978, 15, 343–347. [Google Scholar] [CrossRef]
- Nemoshkalenko, V.V.; Didyk, V.V.; Krivitskii, V.P.; Senkevich, A.I. Study of the charge state of atoms in iron, cobalt and nickel phosphides. Zhurnal Neorg. Khimii 1983, 28, 2182–2186. [Google Scholar]
- Dube, C.E.; Workie, B.; Kounaves, S.P.; Robbat, A.; Aksu, M.L.; Davies, G. Electrodeposition of metal alloy and mixed-oxide films using a single-precursor tetranuclear copper-nickel complex. J. Electrochem. Soc. 1995, 142, 3357–3365. [Google Scholar] [CrossRef]
- Xia, J.; He, G.; Zhang, L.; Sun, X.; Wang, X. Hydrogenation of nitrophenols catalyzed by carbon black-supported nickel nanoparticles under mild conditions. Appl. Catal. B Environ. 2016, 180, 408–415. [Google Scholar] [CrossRef]
- Lin, W.; Cheng, H.; Ming, J.; Yu, Y.; Zhao, F. Deactivation of Ni/TiO2 catalyst in the hydrogenation of nitrobenzene in water and improvement in its stability by coating a layer of hydrophobic carbon. J. Catal. 2012, 291, 149–154. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Zhang, Y.; Zhou, H.; Wang, H.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. Efficient Synthesis of Furfuryl Alcohol from H2-Hydrogenation/Transfer Hydrogenation of Furfural Using Sulfonate Group Modified Cu Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2172–2180. [Google Scholar] [CrossRef]
- Du, H.; Ma, X.; Yan, P.; Jiang, M.; Zhao, Z.; Zhang, Z.C. Catalytic furfural hydrogenation to furfuryl alcohol over Cu/SiO2 catalysts: A comparative study of the preparation methods. Fuel Process. Technol. 2019, 193, 221–231. [Google Scholar] [CrossRef]

| Run | Catalyst | wt % | |
|---|---|---|---|
| Ni | Cu | ||
| 1 | Cu/RHPC | 0 | 21.4 |
| 2 | Ni1Cu2/RHPC | 7.0 | 15.7 |
| 3 | Ni2Cu1/RHPC | 15.8 | 8.3 |
| 4 | Ni/RHPC | 22.0 | 0 |
| 5 | Ni2Cu1/RHAC-Recycled | 9.4 | 5.0 |
| Samples | SBET m2/g | Vtotal cm3/g | Vmicro cm3/g | Vmeso cm3/g | Vmicro/Vtotal % | Vmeso/Vtotal % | Dave. nm |
|---|---|---|---|---|---|---|---|
| RHPC | 1847 | 1.06 | 0.65 | 0.41 | 61 | 39 | 2.3 |
| RHPC-HNO3 a | 974 | 0.60 | 0.26 | 0.34 | 43 | 57 | 1.8 |
| Ni2Cu1/RHPC | 591 | 0.37 | 0.12 | 0.25 | 32 | 68 | 2.5 |
| Run | Acidification Conditions | Con./% | Sel./% | |
|---|---|---|---|---|
 | Others | |||
| 1 | — | >99.9 | 93 | 7 |
| 2 | 10% HNO3 25 °C | >99.9 | 98 | 2 |
| 3 | 10% HNO3 120 °C | >99.9 | 98 | 2 |
| 4 | 20% HNO3 120 °C | >99.9 | >99 | 0 |
| 5 | 30% HNO3 120 °C | >99.9 | 97 | 3 |
| 6 | 40% HNO3 120 °C | >99.9 | 97 | 3 |
| Run | T/°C | Con./% | Sel./% | |
|---|---|---|---|---|
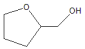 | Others | |||
| 1 | 300 | >99.9 | 94 | 6 |
| 2 | 350 | >99.9 | 96 | 4 |
| 3 | 400 | >99.9 | >99 | 0 |
| 4 | 450 | >99.9 | 98 | 2 |
| 5 | 500 | >99.9 | 96 | 4 |
| Run | Catalyst | Con./% | Sel./% | ||
|---|---|---|---|---|---|
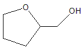 | 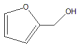 | Others | |||
| 1 a | Cu/RHPC | 63 | 0 | 100 | 0 |
| 2 a | Ni1Cu2/RHPC | >99.9 | 74 | 21 | 5 |
| 3 a | Ni2Cu1/RHPC | >99.9 | >99 | 0 | 0 |
| 4 a | Ni/RHPC | 98 | 26 | 67 | 7 |
| 5 b | Ni/RHPC (20 mg)+ Cu/RHPC (10 mg) | >99.9 | 86 | 9 | 5 |
| Run | Solvent | Con./% | Sel./% | |
|---|---|---|---|---|
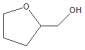 | 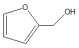 | |||
| 1 | Water | >99.9 | >99 | 0 |
| 2 | Ethanol | 99 | 31 | 69 |
| 3 | Decalin | 86 | 29 | 71 |
| Run | T/°C | Con./% | Sel./% | ||
|---|---|---|---|---|---|
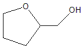 | 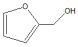 | Others | |||
| 1 | 30 | 92 | 13 | 87 | 0 |
| 2 | 40 | >99.9 | 54 | 41 | 5 |
| 3 | 50 | >99.9 | >99 | 0 | 0 |
| Run | nfurfural/mmol | Con./% | Sel./% | ||
|---|---|---|---|---|---|
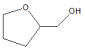 | 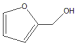 | Others | |||
| 1 | 0.36 | >99.9 | >99 | 0 | 0 |
| 2 | 0.60 | >99.9 | >99 | 0 | 0 |
| 3 | 0.90 | >99.9 | 97 | 0 | 3 |
| 4 | 1.20 | >99.9 | 85 | 7 | 8 |
| Run | Cycle | Con./% | Sel./% | |
|---|---|---|---|---|
 | Others | |||
| 1 | — | >99.9 | >99 | 0 |
| 2 | 1st | >99.9 | 99 | 1 |
| 3 | 2nd | >99.9 | 97 | 3 |
| 4 | 3rd | >99.9 | 97 | 3 |
| 5 | 4th | >99.9 | 97 | 3 |
| 6 | 5th | >99.9 | 97 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Gao, Y.; Hu, L.; Yang, X. Highly Efficient and Selective Hydrogenation of Biomass-Derived Furfural Using Interface-Active Rice Husk-Based Porous Carbon-Supported NiCu Alloy Catalysts. Molecules 2024, 29, 2638. https://doi.org/10.3390/molecules29112638
Ding Z, Gao Y, Hu L, Yang X. Highly Efficient and Selective Hydrogenation of Biomass-Derived Furfural Using Interface-Active Rice Husk-Based Porous Carbon-Supported NiCu Alloy Catalysts. Molecules. 2024; 29(11):2638. https://doi.org/10.3390/molecules29112638
Chicago/Turabian StyleDing, Zhiyao, Yujun Gao, Lianghai Hu, and Xiaomin Yang. 2024. "Highly Efficient and Selective Hydrogenation of Biomass-Derived Furfural Using Interface-Active Rice Husk-Based Porous Carbon-Supported NiCu Alloy Catalysts" Molecules 29, no. 11: 2638. https://doi.org/10.3390/molecules29112638




