Assessing the Potential of Aconitum Laeve Extract for Biogenic Silver and Gold Nanoparticle Synthesis and Their Biological and Catalytic Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Ag and Au NPs
2.1.1. UV–Visible Spectroscopic Examination
2.1.2. XRD Analysis of the Prepared NPs@AL
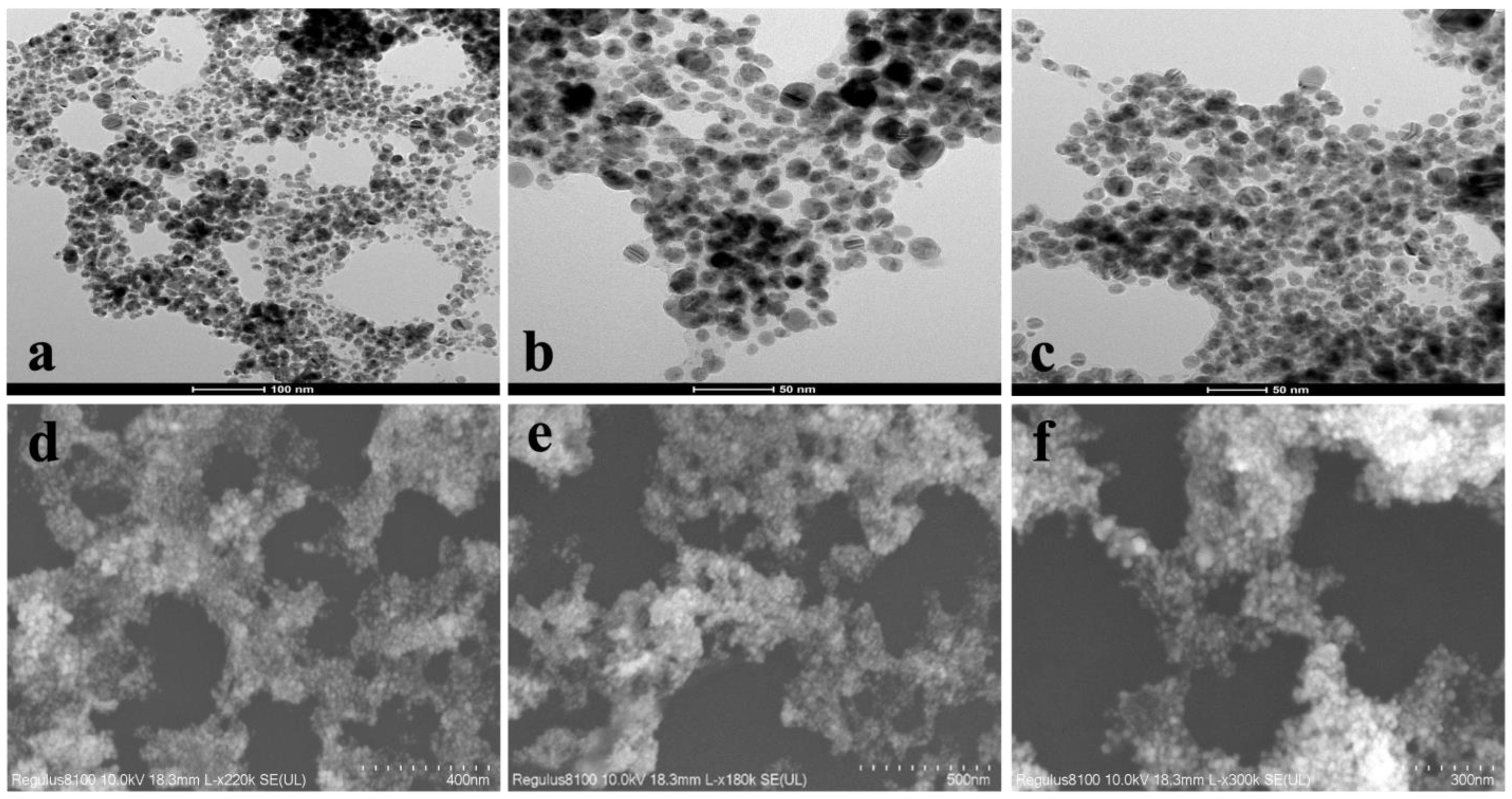
2.1.3. Morphological Analysis
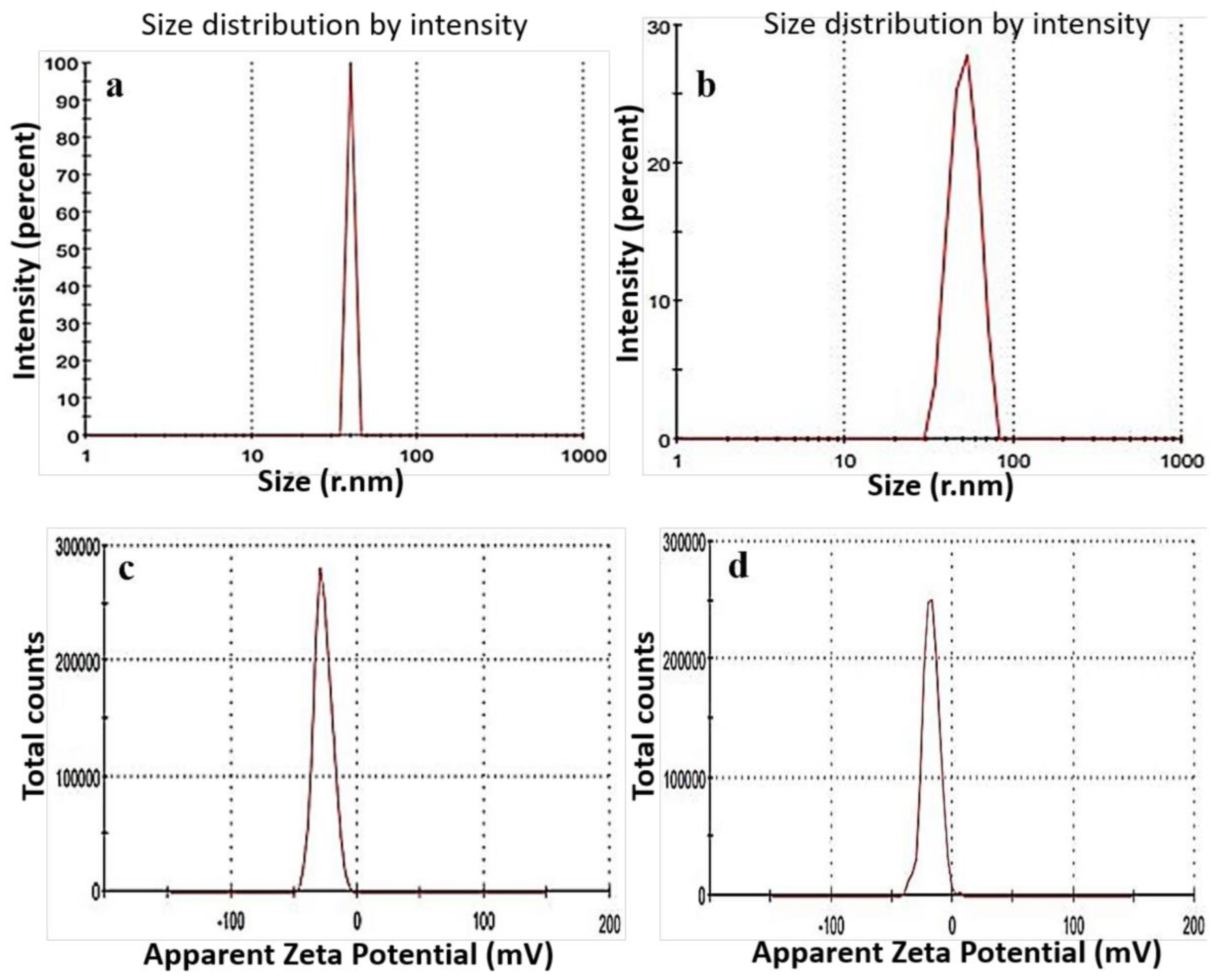
2.1.4. Dynamic Light Scattering Study
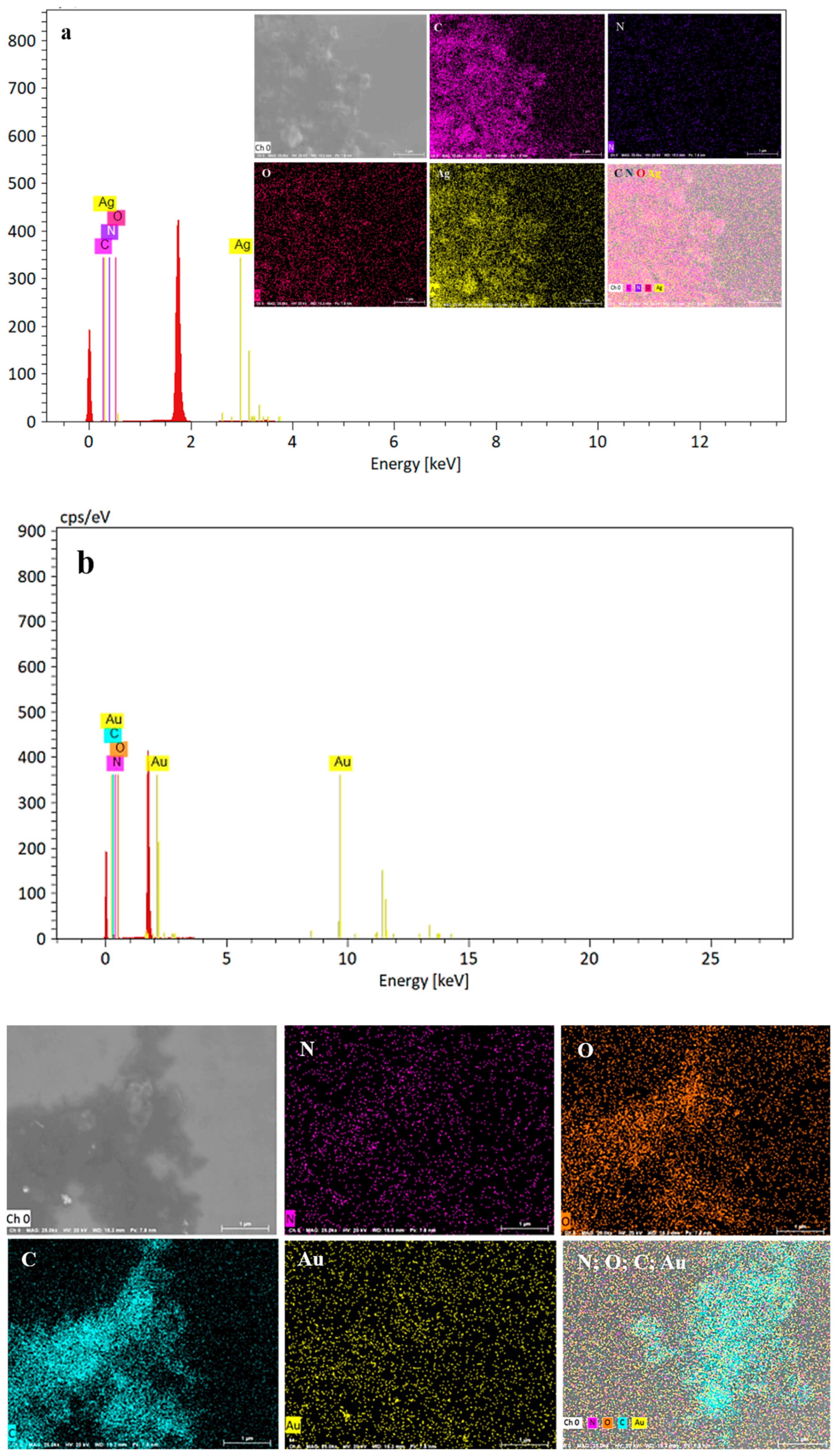
2.1.5. EDX Analysis of the Prepared Ag and AuNPs
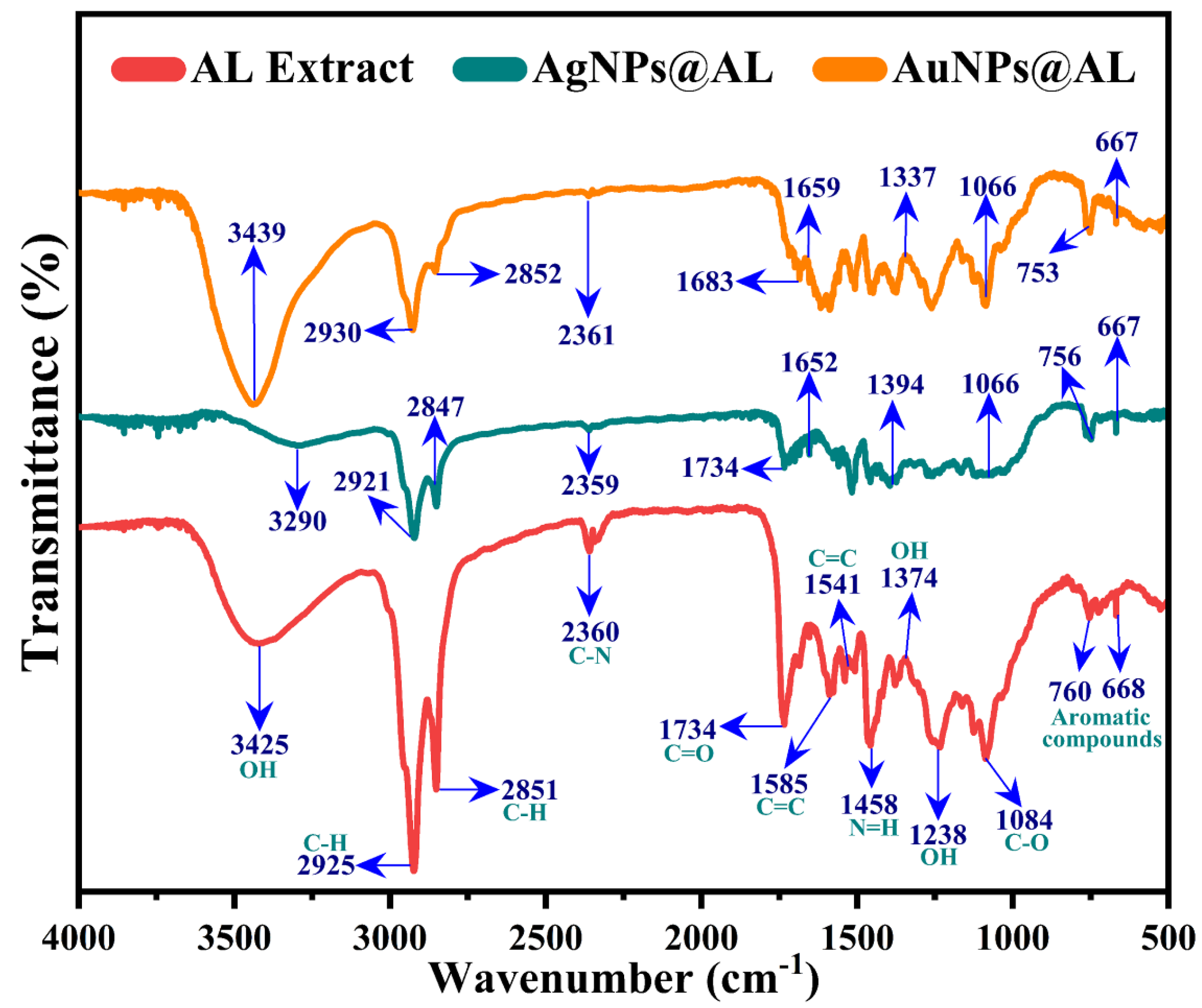
2.1.6. FTIR Investigation of the Plant Extract and NPs@AL
2.1.7. XPS Spectral Study
2.2. Biological Applications of Ag and AuNP@AL
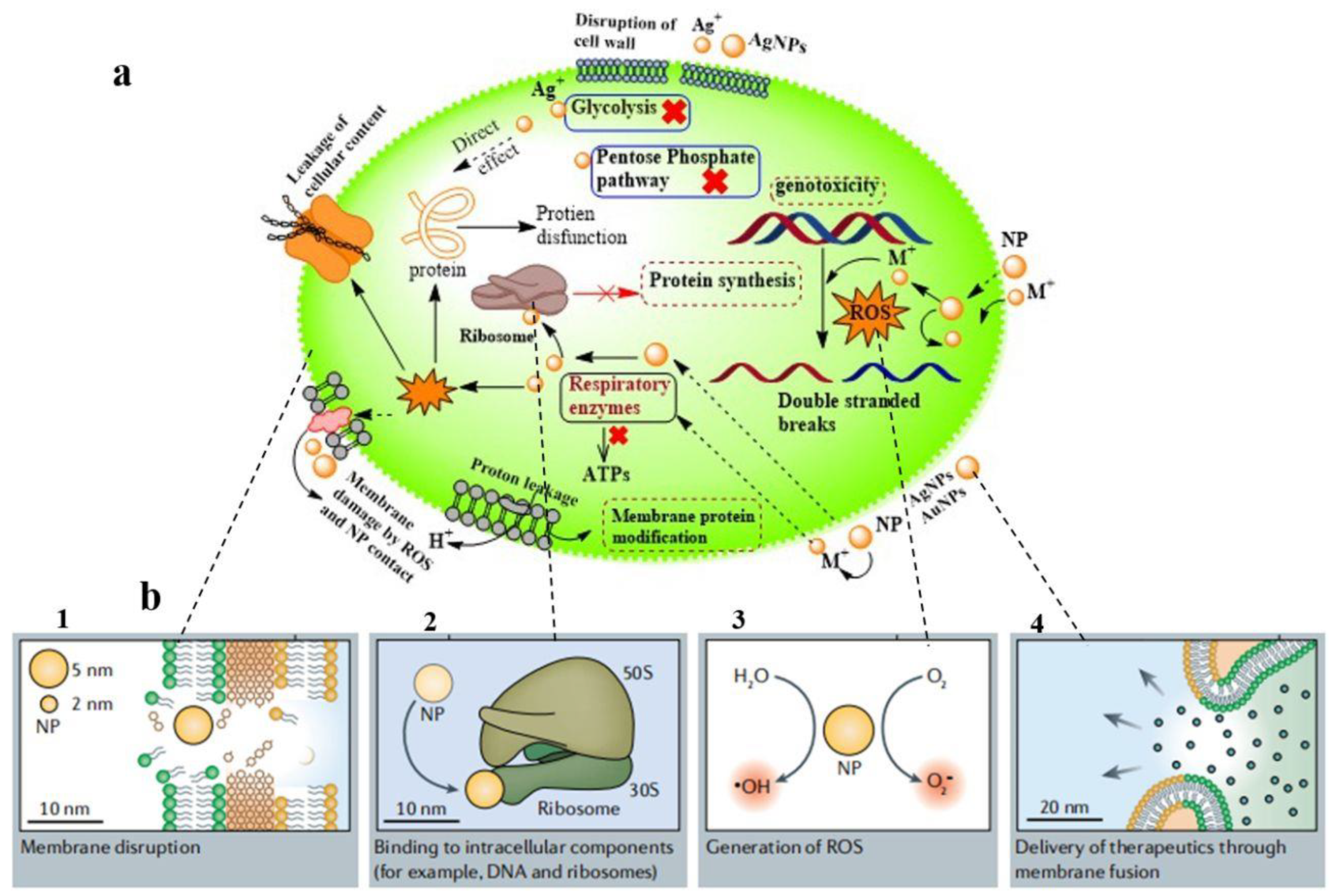
2.2.1. Antibacterial Potency of the Prepared NPs@AL
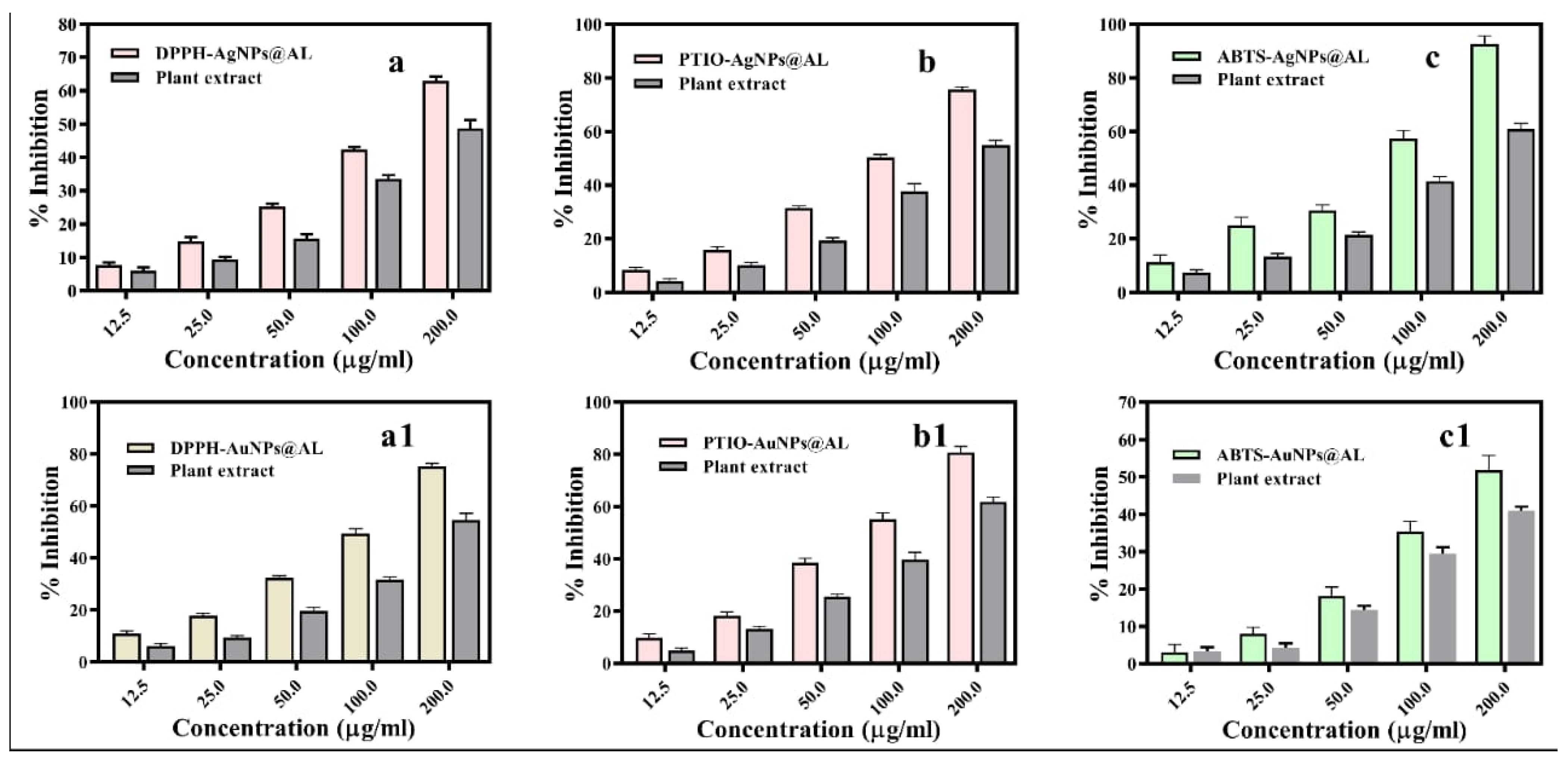
2.2.2. DPPH Radical Scavenging Activity
2.2.3. PTIO Radical Scavenging Assay
2.2.4. ABTS Radical Scavenging Assay
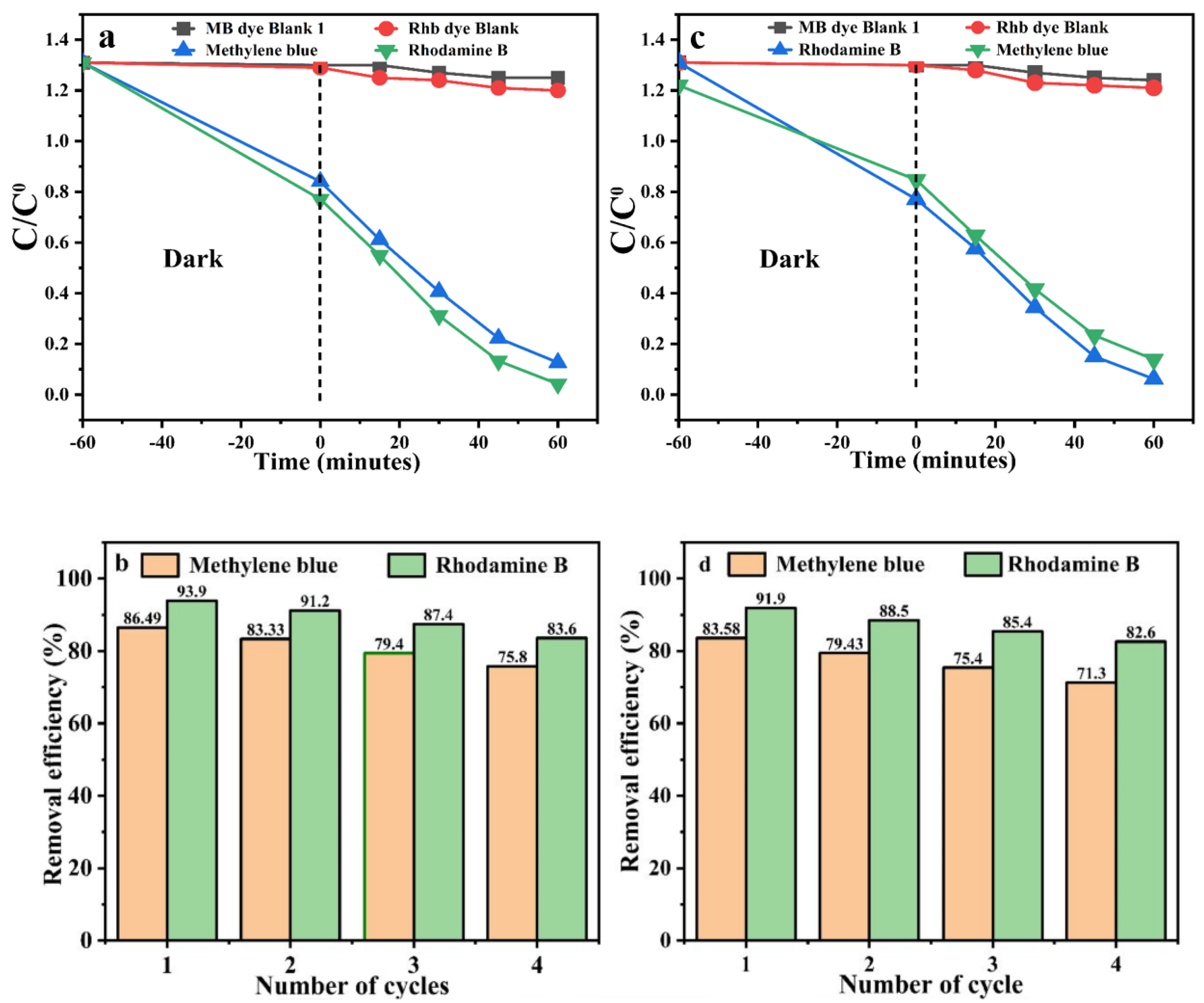
2.3. Photocatalytic Activity of AuNP@AL
3. Materials and Methods
3.1. Preparation of Plant Extract
3.2. Synthesis of AgNPs@AL
3.3. Synthesis of AuNP@AL
3.4. Characterization of Prepared NPs
3.5. Antioxidant Assays of the Prepared NPs
3.5.1. 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Assay
3.5.2. 2-Phenyl-4,4,5,5-Tetramethylimidazoline-1-Oxyl 3-Oxide (PTIO) Assay
3.5.3. The ABTS Assay
3.5.4. Antibacterial Activity
3.5.5. Photocatalytic Activity of NPs@AL
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suhag, D.; Thakur, P.; Thakussr, A. Introduction to nanotechnology. In Integrated Nanomaterials and Their Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–17. [Google Scholar]
- Restrepo, C.V.; Villa, C.C. Synthesis of silver nanoparticles, influence of capping agents, and dependence on size and shape: A review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- House, J.S.; Bouzos, E.; Fahy, K.M.; Francisco, V.M.; Lloyd, D.T.; Wright, F.A.; Motsinger-Reif, A.A.; Asuri, P.; Wheeler, K.E. Low-Dose Silver Nanoparticle Surface Chemistry and Temporal Effects on Gene Expression in Human Liver Cells. Small 2020, 16, 2000299. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.T.J.; Nyam, K.L. Evaluation of silver nanoparticles in cosmeceutical and potential biosafety complications. Saudi J. Biol. Sci. 2022, 29, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Hillyer, M.B.; Condon, B.D.; Lum, J.S.; Richards, M.N.; Zhang, Q. Silver nanoparticle-infused cotton fiber: Durability and aqueous release of silver in laundry water. J. Agric. Food Chem. 2020, 68, 13231–13240. [Google Scholar] [CrossRef]
- Basova, T.V.; Vikulova, E.S.; Dorovskikh, S.I.; Hassan, A.; Morozova, N.B. The use of noble metal coatings and nanoparticles for the modification of medical implant materials. Mater. Des. 2021, 204, 109672. [Google Scholar] [CrossRef]
- Istiqola, A.; Syafiuddin, A. A review of silver nanoparticles in food packaging technologies: Regulation, methods, properties, migration, and future challenges. J. Chin. Chem. Soc. 2020, 67, 1942–1956. [Google Scholar] [CrossRef]
- Dahm, H. Silver nanoparticles in wound infections: Present status and future prospects. In Nanotechnology in Skin, Soft Tissue, and Bone Infections; Springer: Cham, Switzerland, 2020; pp. 151–168. [Google Scholar]
- Cai, L.; Zhu, X.; Ruan, H.; Yang, J.; Wei, W.; Wu, Y.; Zhou, L.; Jiang, H.; Ji, M.; Chen, J. Curcumin-stabilized silver nanoparticles encapsulated in biocompatible electrospun nanofibrous scaffold for sustained eradication of drug-resistant bacteria. J. Hazard. Mater. 2023, 452, 131290. [Google Scholar] [CrossRef]
- Ashkezari, S.; Abtahi, M.S.; Sattari, Z.; Yaraki, M.T.; Hosseini, F.; Salehi, R.I.; Afzali, E.; Hajihosseini, S.; Mousavi-Niri, N. Antibiotic and inorganic nanoparticles co-loaded into carboxymethyl chitosan-functionalized niosome: Synergistic enhanced antibacterial and anti-biofilm activities. J. Drug Deliv. Sci. Technol. 2023, 83, 104386. [Google Scholar] [CrossRef]
- Camargo, L.d.O.; Fontoura, I.; Veriato, T.S.; Raniero, L.; Castilho, M.L. Antibacterial activity of silver nanoparticles functionalized with amikacin applied against multidrug-resistant Acinetobacter baumannii. Am. J. Infect. Control 2023, 51, 871–878. [Google Scholar] [CrossRef]
- Qu, R.; Chen, M.; Liu, J.; Xie, Q.; Liu, N.; Ge, F. Blockage of ATPase-mediated energy supply inducing metabolic disturbances in algal cells under silver nanoparticles stress. J. Environ. Sci. 2023, 131, 141–150. [Google Scholar] [CrossRef]
- Sahoo, B.; Rath, S.K.; Champati, B.B.; Panigrahi, L.L.; Pradhan, A.K.; Nayak, S.; Kar, B.R.; Jha, S.; Arakha, M. Photocatalytic activity of biosynthesized silver nanoparticle fosters oxidative stress at nanoparticle interface resulting in antimicrobial and cytotoxic activities. Environ. Toxicol. 2023, 38, 1577–1588. [Google Scholar] [CrossRef]
- Tripathi, N.; Goshisht, M.K. Recent advances and mechanistic insights into antibacterial activity, antibiofilm activity, and cytotoxicity of silver nanoparticles. ACS Appl. Bio Mater. 2022, 5, 1391–1463. [Google Scholar] [CrossRef] [PubMed]
- Skrzyniarz, K.; Sanchez-Nieves, J.; de la Mata, F.J.; Łysek-Gładysińska, M.; Lach, K.; Ciepluch, K. Mechanistic insight of lysozyme transport through the outer bacteria membrane with dendronized silver nanoparticles for peptidoglycan degradation. Int. J. Biol. Macromol. 2023, 237, 124239. [Google Scholar] [CrossRef] [PubMed]
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical applications of functionalized gold nanoparticles: A review. J. Clust. Sci. 2021, 33, 1–16. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, W.; Qin, Z.; Chen, Y.; Jiang, H.; Wang, X. Mercaptopyrimidine-conjugated gold nanoclusters as nanoantibiotics for combating multidrug-resistant superbugs. Bioconjugate Chem. 2018, 29, 3094–3103. [Google Scholar] [CrossRef] [PubMed]
- Bresee, J.; Bond, C.M.; Worthington, R.J.; Smith, C.A.; Gifford, J.C.; Simpson, C.A.; Carter, C.J.; Wang, G.; Hartman, J.; Osbaugh, N.A. Nanoscale structure–activity relationships, mode of action, and biocompatibility of gold nanoparticle antibiotics. J. Am. Chem. Soc. 2014, 136, 5295–5300. [Google Scholar] [CrossRef]
- Simpson, C.A.; Huffman, B.J.; Gerdon, A.E.; Cliffel, D.E. Unexpected toxicity of monolayer protected gold clusters eliminated by PEG-thiol place exchange reactions. Chem. Res. Toxicol. 2010, 23, 1608–1616. [Google Scholar] [CrossRef]
- De, M.; You, C.-C.; Srivastava, S.; Rotello, V.M. Biomimetic interactions of proteins with functionalized nanoparticles: A thermodynamic study. J. Am. Chem. Soc. 2007, 129, 10747–10753. [Google Scholar] [CrossRef]
- Shaker, M.A.; Shaaban, M.I. Formulation of carbapenems loaded gold nanoparticles to combat multi-antibiotic bacterial resistance: In vitro antibacterial study. Int. J. Pharm. 2017, 525, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tang, H.; Jiang, X. Deploying gold nanomaterials in combating multi-drug-resistant bacteria. ACS Nano 2022, 16, 10066–10087. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, D.G. Synergistic antibacterial activity of gold nanoparticles caused by apoptosis-like death. J. Appl. Microbiol. 2019, 127, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Vasam, M.; Punagoti, R.A.; Mourya, R. Biomedical applications of gold nanoparticles. In Smart Nanomaterials in Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 41–59. [Google Scholar]
- Guo, Z.; Yu, G.; Zhang, Z.; Han, Y.; Guan, G.; Yang, W.; Han, M.Y. Intrinsic optical properties and emerging applications of gold nanostructures. Adv. Mater. 2023, 35, e2206700. [Google Scholar] [CrossRef] [PubMed]
- Zayadi, R.A.; Bakar, F.A. Comparative study on stability, antioxidant and catalytic activities of bio-stabilized colloidal gold nanoparticles using microalgae and cyanobacteria. J. Environ. Chem. Eng. 2020, 8, 103843. [Google Scholar] [CrossRef]
- Qiao, J.; Qi, L. Recent progress in plant-gold nanoparticles fabrication methods and bio-applications. Talanta 2021, 223, 121396. [Google Scholar] [CrossRef]
- Hulikere, M.M.; Joshi, C.G. Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus-Cladosporium cladosporioides. Process Biochem. 2019, 82, 199–204. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A brief overview on antioxidant activity determination of silver nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Dridi, R.; Essghaier, B.; Hannachi, H.; Khedher, G.B.; Chaffei, C.; Zid, M.F. Biosynthesized silver nanoparticles using Anagallis monelli: Evaluation of antioxidant activity, antibacterial and antifungal effects. J. Mol. Struct. 2022, 1251, 132076. [Google Scholar] [CrossRef]
- Khalil, S.; Mehmood, A.; Khan, M.A.R.; Ahmad, K.S.; Abasi, F.; Raffi, M.; Ali, K.; Khan, M.E.H.; Jones, D.A.; Abdelkarim, M. Antibacterial, antioxidant and photocatalytic activity of novel Rubus ellipticus leaf mediated silver nanoparticles. J. Saudi Chem. Soc. 2023, 27, 101576. [Google Scholar] [CrossRef]
- Elemike, E.E.; Fayemi, O.E.; Ekennia, A.C.; Onwudiwe, D.C.; Ebenso, E.E. Silver nanoparticles mediated by Costus afer leaf extract: Synthesis, antibacterial, antioxidant and electrochemical properties. Molecules 2017, 22, 701. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Yang, W.; Gao, K.; Tang, J. Concave gold nano-arrows (AuCNAs) for efficient catalytic reduction of 4-nitrophenol. Chemosphere 2023, 310, 136800. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Yan, Y.; Zhang, D.; Xia, Y.; Chen, X.; Lei, L.; Shi, S. Synergistic Bonding of Poly (N-isopropylacrylamide)-Based Hybrid Microgels and Gold Nanoparticles Used for Temperature-Responsive Controllable Catalysis of p-Nitrophenol Reduction. Langmuir 2023, 39, 2408–2421. [Google Scholar] [CrossRef] [PubMed]
- Wagh, S.S.; Kadam, V.S.; Jagtap, C.V.; Salunkhe, D.B.; Patil, R.S.; Pathan, H.M.; Patole, S.P. Comparative Studies on Synthesis, Characterization and Photocatalytic Activity of Ag Doped ZnO Nanoparticles. ACS Omega 2023, 8, 7779–7790. [Google Scholar] [CrossRef] [PubMed]
- Sahin, M.; Gubbuk, I.H. Green synthesis of palladium nanoparticles and investigation of their catalytic activity for methylene blue, methyl orange and rhodamine B degradation by sodium borohydride. React. Kinet. Mech. Catal. 2022, 135, 999–1010. [Google Scholar] [CrossRef]
- Rana, A.; Pathak, S.; Lim, D.-K.; Kim, S.-K.; Srivastava, R.; Sharma, S.N.; Verma, R. Recent Advancements in Plant-and Microbe-Mediated Synthesis of Metal and Metal Oxide Nanomaterials and Their Emerging Antimicrobial Applications. ACS Appl. Nano Mater. 2023, 6, 8106–8134. [Google Scholar] [CrossRef]
- Behzad, F.; Naghib, S.M.; Tabatabaei, S.N.; Zare, Y.; Rhee, K.Y. An overview of the plant-mediated green synthesis of noble metal nanoparticles for antibacterial applications. J. Ind. Eng. Chem. 2021, 94, 92–104. [Google Scholar] [CrossRef]
- Ramanathan, S.; Gopinath, S.C.; Arshad, M.M.; Poopalan, P.; Perumal, V. Nanoparticle synthetic methods: Strength and limitations. In Nanoparticles in Analytical and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2021; pp. 31–43. [Google Scholar]
- Bhardwaj, P.; Singh, B.; Behera, S.P. Green approaches for nanoparticle synthesis: Emerging trends. Nanomaterials 2021, 167–193. [Google Scholar] [CrossRef]
- Verma, S.; Ojha, S.; Raish, M. Anti-inflammatory activity of Aconitum heterophyllum on cotton pellet-induced granuloma in rats. J. Med. Plants Res 2010, 4, 1566–1569. [Google Scholar]
- Ahmad, H.; Ahmad, S.; Shah, S.A.A.; Khan, H.U.; Khan, F.A.; Ali, M.; Latif, A.; Shaheen, F.; Ahmad, M. Selective dual cholinesterase inhibitors from Aconitum laeve. J. Asian Nat. Prod. Res. 2018, 20, 172–181. [Google Scholar] [CrossRef]
- Kumar, S.; Javed, M.S.; Kumar, P.; Gupta, S.; Kumar, R.; Singh, P.K. In-vitro antifungal and anti-bacterial activity of chloroform extract from tubers of Aconitum laeve Royle: Endangered species, India. Mater. Today Proc. 2021, 34, 563–568. [Google Scholar] [CrossRef]
- Nengroo, Z.R.; Ganie, A.S.; Azeem, M. Aconitum heterophylum from Kashmir: Evaluation of fatty acid profile, antibacterial, antioxidant activities and functional group analysis. Carbohydr. Polym. Technol. Appl. 2021, 2, 100105. [Google Scholar] [CrossRef]
- Hu, Z.-X.; Tang, H.-Y.; Yan, X.-H.; Zeng, Y.-R.; Aisa, H.A.; Zhang, Y.; Hao, X.-J. Five new alkaloids from Aconitum apetalum (Ranunculaceae). Phytochem. Lett. 2019, 29, 6–11. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Elshafie, H.S.; Pohl, P. Green synthesis of silver nanoparticles (AgNPs) by Lallemantia royleana leaf Extract: Their Bio-Pharmaceutical and catalytic properties. J. Photochem. Photobiol. A Chem. 2024, 448, 115318. [Google Scholar] [CrossRef]
- Dilipan, E.; Sivaperumal, P.; Kamala, K.; Ramachandran, M.; Vivekanandhan, P. Green synthesis of silver nanoparticles using seagrass Cymodocea serrulata (R. Br.) Asch. & Magnus, characterization, and evaluation of anticancer, antioxidant, and antiglycemic index. Biotechnol. Appl. Biochem. 2023, 70, 1346–1356. [Google Scholar] [PubMed]
- Hou, J.; Lartey, J.A.; Lee, C.Y.; Kim, J.-H. Light-enhanced catalytic activity of stable and large gold nanoparticles in homocoupling reactions. Sci. Rep. 2024, 14, 1352. [Google Scholar] [CrossRef] [PubMed]
- Tavan, M.; Hanachi, P.; Mirjalili, M.H.; Dashtbani-Roozbehani, A. Comparative assessment of the biological activity of the green synthesized silver nanoparticles and aqueous leaf extract of Perilla frutescens (L.). Sci. Rep. 2023, 13, 6391. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.Z.; Guan, Z.-H.; Din, A.U.; Ali, A.; Rehman, A.U.; Jan, K.; Faisal, S.; Saud, S.; Adnan, M.; Wahid, F. Synthesis of silver nanoparticles using Plantago lanceolata extract and assessing their antibacterial and antioxidant activities. Sci. Rep. 2021, 11, 20754. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.A.; Al Othman, M.R.; Mohammed, A.E. Bio fabrication of silver nanoparticles with antibacterial and cytotoxic abilities using lichens. Sci. Rep. 2020, 10, 16781. [Google Scholar] [CrossRef]
- Mahmood, M.; Abid, M.; Nazar, M.F.; Zafar, M.N.; Raza, M.A.; Ashfaq, M.; Khan, A.M.; Sumrra, S.H.; Zubair, M. The wet chemical synthesis of surfactant-capped quasi-spherical silver nanoparticles with enhanced antibacterial activity. Mater. Adv. 2020, 1, 2332–2338. [Google Scholar] [CrossRef]
- Soto, K.M.; López-Romero, J.M.; Mendoza, S.; Peza-Ledesma, C.; Rivera-Muñoz, E.; Velazquez-Castillo, R.R.; Pineda-Piñón, J.; Méndez-Lozano, N.; Manzano-Ramírez, A. Rapid and facile synthesis of gold nanoparticles with two Mexican medicinal plants and a comparison with traditional chemical synthesis. Mater. Chem. Phys. 2023, 295, 127109. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Seyghalan, H.N.; Hashemi, Z.; Ebrahimzadeh, M.A. Scrophularia striata extract mediated synthesis of gold nanoparticles; their antibacterial, antileishmanial, antioxidant, and photocatalytic activities. Inorg. Chem. Commun. 2023, 156, 111138. [Google Scholar] [CrossRef]
- Ahmad, A.; Syed, F.; Shah, A.; Khan, Z.; Tahir, K.; Khan, A.U.; Yuan, Q. Silver and gold nanoparticles from Sargentodoxa cuneata: Synthesis, characterization and antileishmanial activity. RSC Adv. 2015, 5, 73793–73806. [Google Scholar] [CrossRef]
- AbdelHamid, A.A.; Al-Ghobashy, M.A.; Fawzy, M.; Mohamed, M.B.; Abdel-Mottaleb, M.M. Phytosynthesis of Au, Ag, and Au–Ag bimetallic nanoparticles using aqueous extract of sago pondweed (Potamogeton pectinatus L.). ACS Sustain. Chem. Eng. 2013, 1, 1520–1529. [Google Scholar] [CrossRef]
- Hemlata; Meena, P.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of silver nanoparticles using Cucumis prophetarum aqueous leaf extract and their antibacterial and antiproliferative activity against cancer cell lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Yan, L.; Kuang, W.; Kaffash, A.; Mahdavi, B.; Baghayeri, M.; Liu, W. Novel biosynthesis of gold nanoparticles for multifunctional applications: Electrochemical detection of hydrazine and treatment of gastric cancer. Environ. Res. 2023, 238, 117081. [Google Scholar] [CrossRef] [PubMed]
- Vidhu, V.; Philip, D. Catalytic degradation of organic dyes using biosynthesized silver nanoparticles. Micron 2014, 56, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Wei, Y.; Syed, F.; Khan, S.; Khan, G.M.; Tahir, K.; Khan, A.U.; Raza, M.; Khan, F.U.; Yuan, Q. Isatis tinctoria mediated synthesis of amphotericin B-bound silver nanoparticles with enhanced photoinduced antileishmanial activity: A novel green approach. J. Photochem. Photobiol. B Biol. 2016, 161, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Wei, Y.; Syed, F.; Tahir, K.; Rehman, A.U.; Khan, A.; Ullah, S.; Yuan, Q. The effects of bacteria-nanoparticles interface on the antibacterial activity of green synthesized silver nanoparticles. Microb. Pathog. 2017, 102, 133–142. [Google Scholar] [CrossRef]
- Maitra, B.; Khatun, M.H.; Ahmed, F.; Ahmed, N.; Kadri, H.J.; Rasel, M.Z.U.; Saha, B.K.; Hakim, M.; Kabir, S.R.; Habib, M.R. Biosynthesis of Bixa orellana seed extract mediated silver nanoparticles with moderate antioxidant, antibacterial and antiproliferative activity. Arab. J. Chem. 2023, 16, 104675. [Google Scholar] [CrossRef]
- Ahmad, A.; Wei, Y.; Syed, F.; Tahir, K.; Taj, R.; Khan, A.U.; Hameed, M.U.; Yuan, Q. Amphotericin B-conjugated biogenic silver nanoparticles as an innovative strategy for fungal infections. Microb. Pathog. 2016, 99, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Mojaddami, A.; Koolivand, Z.; Panahimehr, M.; Chamkouri, N. Green synthesis, phytochemical analysis, characterization and cytotoxic evaluation of gold–zinc oxide nanocomposites using Russian Artemisia leaf extract. Nano-Struct. Nano-Objects 2023, 34, 100979. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, A.; Wang, A.; Raza, M.; Jan, A.U.; Tahir, K.; Rahman, A.U.; Qipeng, Y. Bio-fabrication of catalytic platinum nanoparticles and their in vitro efficacy against lungs cancer cells line (A549). J. Photochem. Photobiol. B Biol. 2017, 173, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Vilas, V.; Philip, D.; Mathew, J. Phytochemical-capped biogenic gold nanocrystals with chemocatalytic and radical scavenging potential. J. Mol. Liq. 2014, 200, 390–397. [Google Scholar] [CrossRef]
- Reddy, N.J.; Vali, D.N.; Rani, M.; Rani, S.S. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mater. Sci. Eng. C 2014, 34, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Yasin, G.; Ahmad, A.; Qin, L.; Yuan, Q.; Khan, A.U.; Khan, U.A.; Rahman, A.U.; Slimani, Y. Construction of well-designed 1D selenium–tellurium nanorods anchored on graphene sheets as a high storage capacity anode material for lithium-ion batteries. Inorg. Chem. Front. 2020, 7, 1750–1761. [Google Scholar] [CrossRef]
- Chen, M.; Kang, H.; Gong, Y.; Guo, J.; Zhang, H.; Liu, R. Bacterial cellulose supported gold nanoparticles with excellent catalytic properties. ACS Appl. Mater. Interfaces 2015, 7, 21717–21726. [Google Scholar] [CrossRef] [PubMed]
- Hepperle, P.; Herman, A.; Khanbabaee, B.; Baek, W.Y.; Nettelbeck, H.; Rabus, H. XPS examination of the chemical composition of PEGMUA-coated gold nanoparticles. Part. Part. Syst. Charact. 2022, 39, 2200070. [Google Scholar] [CrossRef]
- Song, J.; Kang, H.; Lee, C.; Hwang, S.H.; Jang, J. Aqueous synthesis of silver nanoparticle embedded cationic polymer nanofibers and their antibacterial activity. ACS Appl. Mater. Interfaces 2012, 4, 460–465. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Yi, Z.; Liu, Y.; Huang, Y. Confined selenium within porous carbon nanospheres as cathode for advanced Li–Se batteries. Nano Energy 2014, 9, 229–236. [Google Scholar] [CrossRef]
- Banerjee, M.; Sharma, S.; Chattopadhyay, A.; Ghosh, S.S. Enhanced antibacterial activity of bimetallic gold-silver core–shell nanoparticles at low silver concentration. Nanoscale 2011, 3, 5120–5125. [Google Scholar] [CrossRef]
- Elbehiry, A.; Al-Dubaib, M.; Marzouk, E.; Moussa, I. Antibacterial effects and resistance induction of silver and gold nanoparticles against Staphylococcus aureus-induced mastitis and the potential toxicity in rats. Microbiologyopen 2019, 8, e00698. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Cao, Z.; Liu, X.; Cui, Z.; Li, Z.; Liang, Y.; Zhu, S.; Wu, S. Noble metal-based nanomaterials as antibacterial agents. J. Alloys Compd. 2022, 904, 164091. [Google Scholar] [CrossRef]
- Luo, L.; Huang, W.; Zhang, J.; Yu, Y.; Sun, T. Metal-Based Nanoparticles as Antimicrobial Agents: A Review. ACS Appl. Nano Mater. 2024, 7, 2529–2545. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small molecule-capped gold nanoparticles as potent antibacterial agents that target gram-negative bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, M.H.; Tahar, L.B.; Harrath, A.H. Catalytic, antioxidant and anticancer activities of gold nanoparticles synthesized by kaempferol glucoside from Lotus leguminosae. Arab. J. Chem. 2020, 13, 3112–3122. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Souto, E.B. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Tao, Y.; Ju, E.; Ren, J.; Qu, X. Bifunctionalized mesoporous silica-supported gold nanoparticles: Intrinsic oxidase and peroxidase catalytic activities for antibacterial applications. Adv. Mater. 2015, 27, 1097–1104. [Google Scholar] [CrossRef]
- Bernardos, A.; Piacenza, E.; Sancenón, F.; Hamidi, M.; Maleki, A.; Turner, R.J.; Martínez-Máñez, R. Mesoporous silica-based materials with bactericidal properties. Small 2019, 15, 1900669. [Google Scholar] [CrossRef] [PubMed]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Li, W.; Ma, X.; Dong, M.; Geng, L.; Li, Y.; Fan, Y.; Khan, S.; Yasin, G. Novel and facile strategy for immobilization of Ag3PW12 crystals on graphitic carbon nitride (g-C3-N4) sheets, a superior photocatalyst for decomposition of organic waste. J. Solid State Chem. 2024, 329, 124405. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, S.; Xu, Q.; Cao, X.; Khan, I.; Yanga, R.; Yan, H. Green synthesis of Gold and Silver Nanoparticles Using Crude Extract of Aconitum violaceum and Evaluation of their Antibacterial, Antioxidant and Photocatalytic Activities. Front. Bioeng. Biotechnol. 2023, 11, 1320739. [Google Scholar] [CrossRef] [PubMed]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef] [PubMed]
- Karan, T.; Gonulalan, Z.; Erenler, R.; Kolemen, U.; Eminagaoglu, O. Green synthesis of silver nanoparticles using Sambucus ebulus leaves extract: Characterization, quantitative analysis of bioactive molecules, antioxidant and antibacterial activities. J. Mol. Struct. 2024, 1296, 136836. [Google Scholar] [CrossRef]
- Li, X. 2-Phenyl-4, 4, 5, 5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) radical scavenging: A new and simple antioxidant assay in vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef]
- Moosavy, M.-H.; de la Guardia, M.; Mokhtarzadeh, A.; Khatibi, S.A.; Hosseinzadeh, N.; Hajipour, N. Green synthesis, characterization, and biological evaluation of gold and silver nanoparticles using Mentha spicata essential oil. Sci. Rep. 2023, 13, 7230. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Xu, Q.; Tariq, M.; Song, M.; Liu, C.; Yan, H. Assessing the Potential of Aconitum Laeve Extract for Biogenic Silver and Gold Nanoparticle Synthesis and Their Biological and Catalytic Applications. Molecules 2024, 29, 2640. https://doi.org/10.3390/molecules29112640
Ahmad S, Xu Q, Tariq M, Song M, Liu C, Yan H. Assessing the Potential of Aconitum Laeve Extract for Biogenic Silver and Gold Nanoparticle Synthesis and Their Biological and Catalytic Applications. Molecules. 2024; 29(11):2640. https://doi.org/10.3390/molecules29112640
Chicago/Turabian StyleAhmad, Shahbaz, Qianqian Xu, Muhammad Tariq, Meijie Song, Chao Liu, and Hai Yan. 2024. "Assessing the Potential of Aconitum Laeve Extract for Biogenic Silver and Gold Nanoparticle Synthesis and Their Biological and Catalytic Applications" Molecules 29, no. 11: 2640. https://doi.org/10.3390/molecules29112640




