An Amphiphilic Surface with Improved Thermal Radiation for Water Harvesting
Abstract
:1. Introduction
2. Theoretical Basis
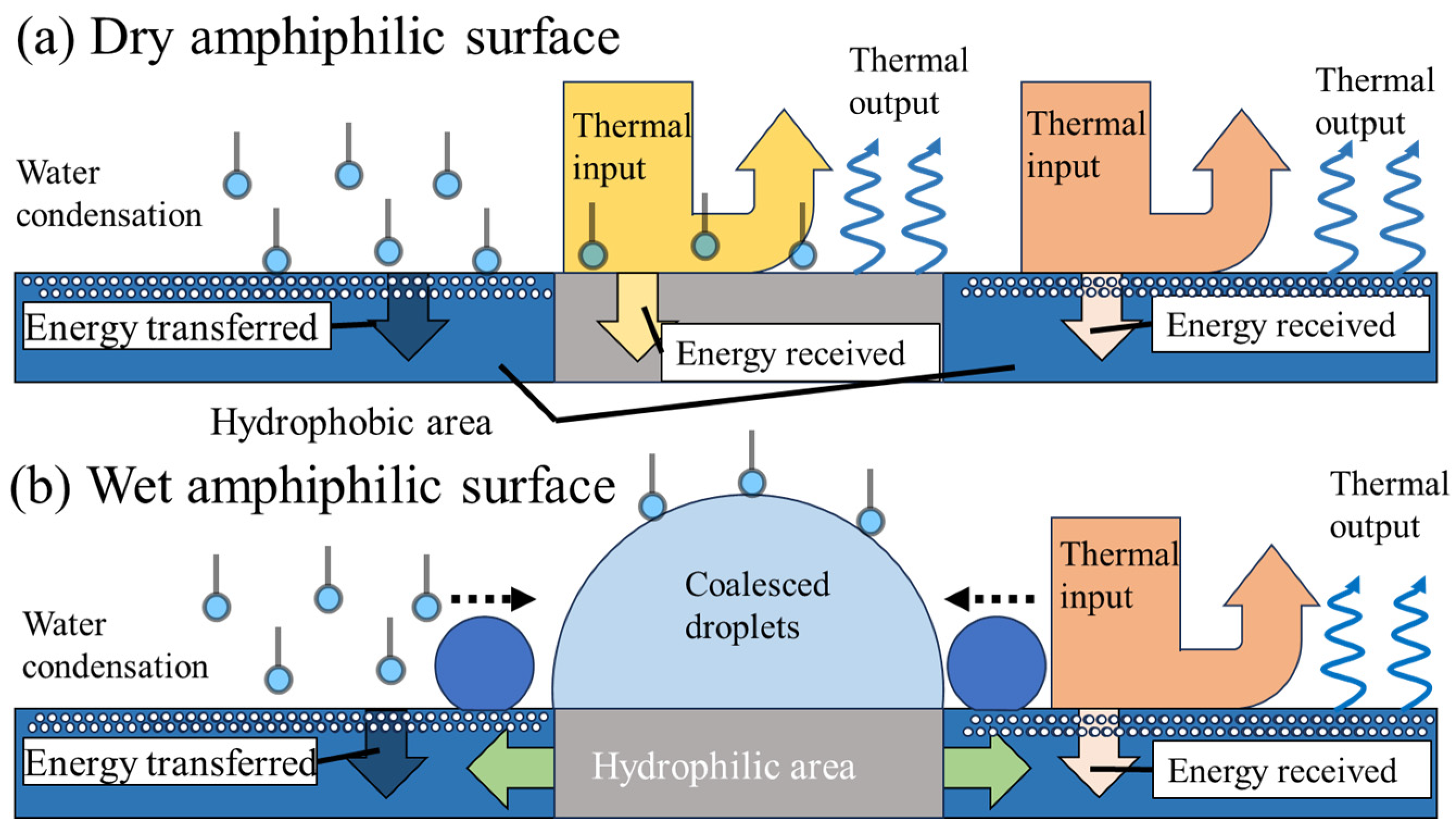
2.1. Design Rationale 1: Thermal Radiation and Emission Features on Amphiphilic Surfaces
2.2. Design Rationale 1: Surface Mechanicss
3. Results and Discussion
3.1. SEM Results
3.2. AFM Result
3.3. Static Contact Angle Test and XRD Results
3.4. Droplet Coalescence Test Results
3.5. Water-Harvesting Test Results
4. Materials and Methods
4.1. Design Rationale 3: Amphiphilc Surface Assmbly Method
- (1)
- Hydrophilic particle pinning: First, PVP semi-solid glue was uniformly applied on the surface of a glass plate. Then, size-screened hydrophilic particles were sprinkled onto the glass glazing plate, which was put into an oven at 60 °C for 30 min to obtain prefabricated templates with fixed hydrophilic islands. The diameter range of the hydrophilic particles was limited to 100–125 μm to eliminate the influence of the amphiphilic surface’s morphology due to the different diameters of the hydrophilic particles.
- (2)
- The preparation and solidification of the hydrophobic prepolymer: The PDMS pre-polymer, crosslinker, diluent, and nanoparticles were mixed at different ratios and mechanically stirred for 10 min at a low temperature until they formed a homogeneous dispersion, which was poured onto the prefabricated templates. The samples were placed into a vacuum-drying oven to perform a two-step solidification process. Firstly, a vacuum was drawn for 30 min at room temperature. After all of the air bubbles were removed, the pre-polymer was cured at 85 °C for 30 min. Finally, solid PDMS composites with an internal amphiphilic structure were obtained.
- (3)
- The purification of the amphiphilic surface: The glass plate applied with solid PDMS was immersed in alcoholic liquid for two hours until the PVP colloid melted and separated from the glass plate. Then, the fallen PDMS solid was placed in an ultrasonic cleaner to clean off the PVP colloid adhered to the PDMS surface to obtain an island-type amphiphilic surface.
4.2. Water-Harvesting Performance Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Overpeck, J.T. The challenge of hot drought. Nature 2013, 503, 350–351. [Google Scholar] [CrossRef]
- McGrath, G.S.; Sadler, R.; Fleming, K.; Tregoning, P.; Hinz, C.; Veneklaas, E.J. Tropical cyclones and the ecohydrology of Australia’s recent continental-scale drought. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Grubert, E.A.; Stillwell, A.S.; Webber, M.E. Where does solar-aided seawater desalination make sense? A method for identifying sustainable sites. Desalination 2014, 339, 10–17. [Google Scholar] [CrossRef]
- Song, Y.-Y.; Liu, Y.; Jiang, H.-B.; Li, S.-Y.; Kaya, C.; Stegmaier, T.; Han, Z.-W.; Ren, L.-Q. Temperature-tunable wettability on a bioinspired structured graphene surface for fog collection and unidirectional transport. Nanoscale 2018, 10, 3813–3822. [Google Scholar] [CrossRef]
- Hou, B.; Wu, C.; Li, X.; Huang, J.; Chen, M. Contact line-based model for the Cassie-Wenzel transition of a sessile droplet on the hydrophobic micropillar-structured surfaces. Appl. Surf. Sci. 2021, 542, 148611. [Google Scholar] [CrossRef]
- Qin, S.; Jin, Y.; Yin, F.; Wang, Z.; Bai, G. Can solid surface energy be a predictor of ice nucleation ability? Appl. Surf. Sci. 2022, 602, 154193. [Google Scholar] [CrossRef]
- Bao, X.; Zhang, M.; Li, P.; Lu, J.; Yuan, G.; Zuo, Y. Investigating the surface wettability and surface free energy of sodium silicate-impregnated poplar wood. Wood Mater. Sci. Eng. 2021, 18, 141–150. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, J.-W.; Kim, J.Y.; Moon, J.W.; Jang, H.S.; Jung, S.Y. Effect of Mesh Wettability Modification on Atmospheric and Industrial Fog Harvesting. Front. Phys. 2021, 9, 1–6. [Google Scholar] [CrossRef]
- Wang, S.-W.; Peng, L.; Chen, J.-W.; Li, L. A comparative study of the self-propelled jumping capabilities of coalesced droplets on RTV surfaces and superhydrophobic surfaces. Chin. Phys. B 2021, 30, 046501. [Google Scholar] [CrossRef]
- Wu, S.-C.; Lin, Z.-H.; Lo, C.-K. Study of super-hydrophilic nanoscale bilayer assembly surface modification and its application to enhance evaporation. Therm. Sci. Eng. Prog. 2022, 27, 101133. [Google Scholar] [CrossRef]
- Pogorzelski, S.; Boniewicz-Szmyt, K.; Grzegorczyk, M.; Rochowski, P. Wettability of Metal Surfaces Affected by Paint Layer Covering. Materials 2022, 15, 1830. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-W.; Fan, L.-W. Understanding the effects of surface roughness on the temperature and pressure relevancy of water contact angles. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130391. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y. Contact angle hysteresis of a water droplet on a hydrophobic fuel cell surface. J. Colloid Interface Sci. 2019, 545, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Shi, Z.; Tan, X.; Zhang, J.; Zhang, S.; Zhang, X. Accelerated wetting transition from hydrophilic to hydrophobic of sputtered Cu films with micro-scale patterns. Appl. Surf. Sci. 2020, 527, 146741. [Google Scholar] [CrossRef]
- Khan, S.A.; Boltaev, G.S.; Iqbal, M.; Kim, V.; Ganeev, R.A.; Alnaser, A.S. Ultrafast fiber laser-induced fabrication of superhydrophobic and self-cleaning metal surfaces. Appl. Surf. Sci. 2021, 542, 148560. [Google Scholar] [CrossRef]
- Liu, M.; Li, M.-T.; Xu, S.; Yang, H.; Sun, H.-B. Bioinspired Superhydrophobic Surfaces via Laser-Structuring. Front. Chem. 2020, 8, 835. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, R.A.; Rosa, F.M.; Volú, R.M.; de Vasconcelos, G.; Trava-Airoldi, V.J.; Corat, E.J. Vertically aligned carbon nanotubes (VACNT) surfaces coated with polyethylene for enhanced dew harvesting. Diam. Relat. Mater. 2020, 107, 107837. [Google Scholar] [CrossRef]
- Yan, Y.-L.; Cai, Y.-X.; Liu, X.-C.; Ma, G.-W.; Lv, W.; Wang, M.-X. Hydrophobic Modification on the Surface of SiO2 Nanoparticle: Wettability Control. Langmuir 2020, 36, 14924–14932. [Google Scholar] [CrossRef]
- Deng, Z.; Gao, S.; Wang, H.; Liu, X.; Zhang, C. Visualization study on the condensation heat transfer on vertical surfaces with a wettability gradient. Int. J. Heat Mass Transf. 2022, 184, 122331. [Google Scholar] [CrossRef]
- Kim, D.E.; Oh, J.S. Local phase and thermal behaviors in pool boiling on different wettability surfaces. Exp. Therm. Fluid Sci. 2022, 139, 110728. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Lu, L.; Li, J. Liquid bridges between particles and the hydrophobic or hydrophilic surfaces of solar photovoltaic glass. Sci. Total Environ. 2022, 822, 153552. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.R.; Lawrence, C.R. Water capture by a desert beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef]
- Yu, Z.; Yun, F.F.; Wang, Y.; Yao, L.; Dou, S.; Liu, K.; Jiang, L.; Wang, X. Desert Beetle-Inspired Superwettable Patterned Surfaces for Water Harvesting. Small 2017, 13, 1701403. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Z. Recent progress in beetle-inspired superhydrophilic-superhydrophobic micropatterned water-collection materials. Water Sci. Technol. 2020, 82, 207–226. [Google Scholar] [CrossRef]
- Zhu, H.; Huang, Y.; Lou, X.; Xia, F. Beetle-inspired wettable materials: From fabrications to applications. Mater. Today Nano 2019, 6, 100034. [Google Scholar] [CrossRef]
- Dai, X.; Sun, N.; Steven, O.N.; Birgitt, B.S.; Wang, J.; Yang, S.; Wong, T.-S. Hydrophilic directional slippery rough surfaces for water harvesting. Sci. Adv. 2018, 4, eaaq0919. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Zeng, Q.; Huang, J.; Guo, Z.; Liu, W. Fog collection behavior of bionic surface and large fog collector: A review. Adv. Colloid Interface Sci. 2022, 300, 102583. [Google Scholar] [CrossRef]
- Jarimi, H.; Powell, R.; Riffat, S. Review of sustainable methods for atmospheric water harvesting. Int. J. Low-Carbon Technol. 2020, 15, 253–276. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, X.; Guo, Z.; Liu, W. Recent advances in atmosphere water harvesting: Design principle, materials, devices, and applications. Nano Today 2021, 40, 101283. [Google Scholar] [CrossRef]
- Benda, J.; Narikiyo, H.; Stafslien, S.J.; VanderWal, L.J.; Finlay, J.A.; Aldred, N.; Clare, A.S.; Webster, D.C. Studying the Effect of Pre-Polymer Composition and Incorporation of Surface-Modifying Amphiphilic Additives on the Fouling-Release Performance of Amphiphilic Siloxane-Polyurethane Coatings. ACS Appl. Mater. Interfaces 2022, 14, 37229–37247. [Google Scholar] [CrossRef]
- Yan, D.; Chen, Y.; Liu, J.; Song, J. Super-Fast Fog Collector Based on Self-Driven Jet of Mini Fog Droplets. Small 2023, 19, e2301745. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, X.; Yao, Z. Condensation on solid surfaces with amphiphilic micro-nanostructures by lattice Boltzmann simulation. Chem. Phys. 2018, 513, 258–265. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, J.; Liu, C.; Yu, N.; Mo, J.; Pan, Y.; Luo, D. Design and optimization of hybrid superhydrophobic–hydrophilic pattern surfaces for improving fog harvesting efficiency. Prog. Org. Coat. 2022, 171, 107016. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, T.; Yu, C.; Liu, Q.; Wang, G.; Yang, H.; Yang, M.; Jiang, L.; Liu, M. Amphiphilic Pd@micro-organohydrogels with controlled wettability for enhancing gas-liquid-solid triphasic catalytic performance. Nano Res. 2022, 15, 557–563. [Google Scholar] [CrossRef]
- Zhang, S.; Chi, M.; Mo, J.; Liu, T.; Liu, Y.; Fu, Q.; Wang, J.; Luo, B.; Qin, Y.; Wang, S.; et al. Bioinspired asymmetric amphiphilic surface for triboelectric enhanced efficient water harvesting. Nat. Commun. 2022, 13, 4168. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.; Shi, J.; Yamanaka, S.; Morikawa, H. Bioinspired Composite Materials used for Efficient Fog Harvesting with Structures that Consist of Fungi-Mycelia Networks. ACS Sustain. Chem. Eng. 2022, 10, 12529–12539. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Park, E.; Nguyen, N.-A.; Omelianovych, O.; Larina, L.L.; Hussain, S.S.; Choi, H.-S. 3D-printed plasma-treated super-amphiphilic microgroove surface for outperformance of liquid vertical transportation. Appl. Surf. Sci. 2023, 615, 156418. [Google Scholar] [CrossRef]
- Kozbial, A.; Trouba, C.; Liu, H.; Li, L. Characterization of the Intrinsic Water Wettability of Graphite Using Contact Angle Measurements: Effect of Defects on Static and Dynamic Contact Angles. Langmuir 2017, 33, 959–967. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, D.; Liu, R.; Lu, Y.; Zhao, D.; Deng, X.; Song, J. Green self-propelling swimmer driven by rain droplets. Nano Energy 2022, 101, 107543. [Google Scholar] [CrossRef]
- Suaste, E.; Castillo, V.; González, R. Determination of the phase transition in Pb0.88Ln0.08Ti0.98Mn0.02O3 (Ln=La, Sm, Eu) piezoceramics based on the Stefan–Boltzmann law. Mater. Charact. 2004, 52, 279–282. [Google Scholar] [CrossRef]
- Wang, H.; Wang, D.T.; Zhang, X.Y.; Zhang, Z.Z. Modified PDMS with inserted hydrophilic particles for water harvesting. Compos. Sci. Technol. 2021, 213, 108954. [Google Scholar] [CrossRef]
- Cai, Q.; Scullion, D.; Gan, W.; Falin, A.; Zhang, S.; Watanabe, K.; Taniguchi, T.; Chen, Y.; Santos, E.J.G.; Li, L.H. High thermal conductivity of high-quality monolayer boron nitride and its thermal expansion. Sci. Adv. 2019, 5, eaav0129. [Google Scholar] [CrossRef] [PubMed]
- Brodu, E.; Balat-Pichelin, M. Emissivity of Boron Nitride and Metals for the Solar Probe Plus Mission. J. Spacecr. Rocket. 2016, 53, 1119–1127. [Google Scholar] [CrossRef]
- Vukovic, T.; Røstad, J.; Farooq, U.; Torsæter, O.; van der Net, A. Systematic Study of Wettability Alteration of Glass Surfaces by Dichlorooctamethyltetrasiloxane Silanization—A Guide for Contact Angle Modification. ACS Omega 2023, 8, 36662–36676. [Google Scholar] [CrossRef]
- He, H.; Qu, N.; Zeng, Y. Lotus-leaf-like microstructures on tungsten surface induced by one-step nanosecond laser irradiation. Surf. Coat. Technol. 2016, 307, 898–907. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Li, S.; Zhang, Y.; Wu, W.; Ali, K.A.M.; Li, C. An Amphiphilic Surface with Improved Thermal Radiation for Water Harvesting. Molecules 2024, 29, 2672. https://doi.org/10.3390/molecules29112672
Wang H, Li S, Zhang Y, Wu W, Ali KAM, Li C. An Amphiphilic Surface with Improved Thermal Radiation for Water Harvesting. Molecules. 2024; 29(11):2672. https://doi.org/10.3390/molecules29112672
Chicago/Turabian StyleWang, Han, Shengtao Li, Ye Zhang, Weihui Wu, Khaled Abdeen Mousa Ali, and Changyou Li. 2024. "An Amphiphilic Surface with Improved Thermal Radiation for Water Harvesting" Molecules 29, no. 11: 2672. https://doi.org/10.3390/molecules29112672







