Green Methods to Recover Bioactive Compounds from Food Industry Waste: A Sustainable Practice from the Perspective of the Circular Economy
Abstract
1. Introduction
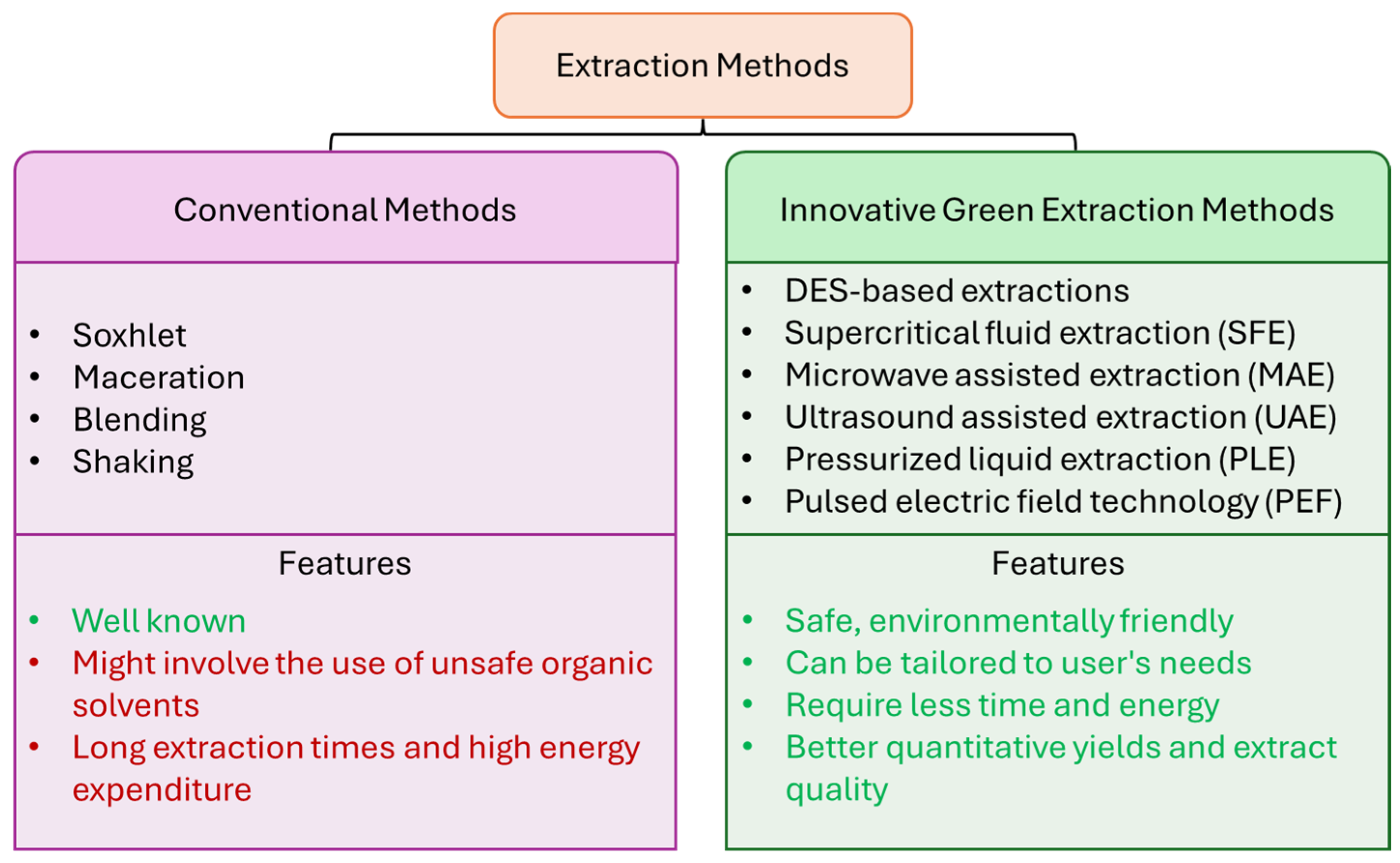
2. Alternative Methods for Green Extraction of Bioactive Compounds from Vegetable/Fruit-Based Waste
2.1. New Sustainable and Innovative Extraction Techniques
- -
- DES-based extraction. DESs are considered green solvents thanks to their non-flammability, chemical and thermal stability, low volatility and toxicity [15]. In some cases, their use can also lead to higher extraction yields and extract quality [15]. Among the most studied DESs, choline-chloride-based DESs are of great interest, since it is possible to modify their physicochemical properties by varying the hydrogen bond donor. Thus, their viscosity, pH and polarity can be tailored to their application [16].
- -
- Supercritical fluid extraction (SCFE). SCFE is a technique that uses a fluid at temperatures and pressures above its critical point [17]. Under these conditions, the fluid exhibits properties that are between those of a liquid and gas, so that higher diffusivity and lower surface tension, density and viscosity are shown compared to conventional solvents [17].
- -
- Microwave-assisted extraction (MAE). This technique consists of internally and externally heating the samples without using any thermal gradient [18,19]. Since microwaves are strongly absorbed by polar molecules, such as ionic solutions, the consequent internal superheating of water molecules of a matrix leads to cellular disruption, with enhanced extraction of compounds of interest from the matrix itself [18,20]. However, microwave power is usually kept under 500 W in order to avoid a considerable decrease in the total flavonoid content and scavenging activity caused by overheating and degradation of antioxidant molecules [18].
- -
- Ultrasound-assisted extraction (UAE). Ultrasound energy and solvents are used to recover target compounds from a wide range of plant matrices [21]. Ultrasound waves induce the formation of cavitation areas in liquids, leading to increased displacement of the molecules from their positions [22]. Ultrasound action results in increased solvent permeability and diffusivity in matrices [23]. It also helps to increase the volume of the plant tissue matrix (swelling index), which is important for the diffusion of solutes during extraction processes [22,24]. A UAE system can be defined as a cost-effective, efficient, environmental friendly and easy-to-use procedure [25].
- -
- -
2.2. Plant Waste Matrices under Study
2.2.1. Banana Peels
2.2.2. Citrus Peels
2.2.3. Pineapple Wastes
2.2.4. Brewery Waste
2.2.5. Cocoa Bean Shells
| Starting Material | Extraction Media | Method | T (°C) | Extraction Time | Ref. |
|---|---|---|---|---|---|
| Banana peels | Ethanol 50% | UAE | 45 °C | 1 h | [50] |
| CO2 with 5% volume of ethanol | SFE | 80 °C | 150 min | [51] | |
| Ethanol 50% (v/v) | HAE | RT | 30 s | [52] | |
| ChCl–ascorbic acid 1:2 DES | Shaking | RT | 20 min | [53] | |
| Kepok banana skin | Water | PEF pre-treatment maceration | RT | 2 min (highest TPC) 4 min (highest antioxidant activity) | [54] |
| Citrus peel | ChCl–LeA–MU 1:1.2:0.8 DES 20% water | UAE | 50 °C | 25 min | [66] |
| Mandarin peel | CO2 (99.97 w/w), water/SC-CO2 pre-treatment | SFE-SWE | 40 °C (pre-treatment) 140–219 °C (extraction) | 90 min (pre-treatment) 9–15 min (extraction) | [67] |
| Lime peel | Ethanol 55% | MAE UAE | Below 60 °C (MAE) | 45 s (MAE) 4 min (UAE) | [68] |
| Kinnow mandarin peel | Methanol 80% | UAE | 45 °C | 60 min | [69] |
| Citrus peels | Ethanol 50% | UAE | Below 40 °C | 30 min | [70] |
| Orange peel | CO2 with 99% purity, ethanol 75% | SFE-PLE | 40 °C (pre-treatment) 65 °C (extraction) | 20 min pre-treatment) 20 min (extraction) | [71] |
| Orange and lime peels | Ethanol 80% | HPE | RT | 10 min (orange peel) 3 min (lemon peel) | [72] |
| Citrus peels | Aqueous enzyme solution (Celluzyme MX) 1.5% (w/w) | Enzyme-assisted aqueous extraction | 50 °C | 3 h | [73] |
| Pineapple skin | Ethanol 50% | MAE | 60 °C | 20 min | [77] |
| Pineapple peel, core and crown | ChCl–glycerol 1:2 DES with 10% water | MAE | 67 °C (drying temperature) | 87 s | [78] |
| Pineapple peel | Ethanol 50% | MAE | 40 min | [79] | |
| Pineapple waste | Ethanol 26% | UAE | 37 °C | 1 min | [80] |
| Pineapple peel | Ethanol 58% | UAE | 62 °C | 30 min | [81] |
| Pineapple skin | Ethanol 50% | UAE | 30 °C | 5.92 min | [82] |
| Pineapple peel | Etanol:solution of 1 mol/L HCl (50:50) | UAE | 60 °C | 30 min | [85] |
| Pineapple crown, peel and core | Water | UAE MAE | Solvent temperature below 10 °C (UAE) | 20.96 min (UAE) 8.99 min (MAE) | [86] |
| Pineapple leaves, peel and stem | Tetrabutyl ammonium chloride:imidazole:glycerol(1:1:1)/sodium sulfate NADES | UA-LPME | 45 °C | 17.5 min | [87] |
| Pineapple core and skin | Water | Autohydrolysis | 200 °C | 30 min | [88] |
| Brewers’ spent grain | NaOH 0.75% | MAE | 100 °C | 15 min | [101] |
| ChCl-Gly (1:2) DES, with 37.46% water | MAE | 100 °C | 13.30 min | [102] | |
| Supercritical CO2 + 60% ethanol | SFE | 40 °C | 240 min | [103] | |
| Ethanol 35% | PLE | 155 °C | 17 min | [104] | |
| Ethanol:water (4:1 v/v) | PEF-assisted extraction | RT | 14.5 s | [105] | |
| Water | UAE | 47 °C | 30 min | [106] | |
| NaOH 2% | Basic hydrolysis | 120 °C | 90 min | [107] | |
| Hot trub | Ethanol 58% | UAE | 36 °C | 30 min | [109] |
| Cocoa bean shell | CO2 with 99.9% purity, ethanol 99.8% | SFE-PLE | 40 °C (SFE pre-treatment) 70 °C (PLE) (extraction) | 20 min (SFE pre-treatment) 20 min (PLE) (extraction) | [118] |
| Absolute ethanol | PLE | 90 °C | 50 min | [119] | |
| Water | SWE | 220 °C | 75 min | [120] | |
| ChCl-based DES with 30% water | MAE | 60 °C | 10 min | [121] | |
| Coffee and cocoa wastes | ChCl-lactic acid 1:2 DES with 10% water | UPAE | 72 °C | 3 min | [122] |
| Cocoa bean shell and coffee silver skin | Ethanol 62.67% (coffee silver skin) Ethanol 39.15% (cocoa bean shell) | PEF-assisted extraction | 25 °C | 75 min (coffee silver skin) 118.54 min (Cocoa bean shell) | [123] |
| Cocoa bean shell | Water:ethanol:hexane (30:49:21) | UAE and HC | 40 °C | 15 min | [124] |
| Malaysian cocoa bean shell | Ethanol 80% | UAE | 55 °C | 45 min | [125] |
| Cocoa bean shell (different varieties) | Water | Heat-assisted extraction | 100 °C | 90 min | [126] |
| (1) Water (2) Ethanol 60 wt.% (3) 40 wt.% citrate buffer and a 30 wt.% phosphate buffer (70/30 phase ratio) | (1) PHWE (2) Precipitation (3) Liquid–liquid extraction from precipitation supernatant | 140 °C (PHWE) | 1 L/h for 0.1 L of solvent (PHWE) | [127] | |
| Ethanol 15% | PHWE | 90 °C | 5 cycles with static time 6 min | [128] |
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Alsaffar, A.A. Sustainable Diets: The Interaction between Food Industry, Nutrition, Health and the Environment. Food Sci. Technol. Int. 2016, 22, 102–111. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.; Hoang, S.A.; Bradney, L.; Dutta, S.; Xiong, X.; Tsang, D.C.W.; Ramadass, K.; Vinu, A.; Kirkham, M.B.; Bolan, N.S. A Review on the Valorisation of Food Waste as a Nutrient Source and Soil Amendment. Environ. Pollut. 2021, 272, 115985. [Google Scholar] [CrossRef]
- Li, H.; Zhou, M.; Mohammed, A.E.A.Y.; Chen, L.; Zhou, C. From Fruit and Vegetable Waste to Degradable Bioplastic Films and Advanced Materials: A Review. Sustain. Chem. Pharm. 2022, 30, 100859. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive Potential of Fruit and Vegetable Wastes. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 91, pp. 157–225. ISBN 978-0-12-820470-2. [Google Scholar]
- De Laurentiis, V.; Corrado, S.; Sala, S. Quantifying Household Waste of Fresh Fruit and Vegetables in the EU. Waste Manag. 2018, 77, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, L.; Vitucci, G.; Catto, M.; Laghezza, A.; Perna, F.; Rullo, M.; Loiodice, F.; Capriati, V.; Solfrizzo, M. Natural Scaffolds with Multi-Target Activity for the Potential Treatment of Alzheimer’s Disease. Molecules 2018, 23, 2182. [Google Scholar] [CrossRef] [PubMed]
- Leuci, R.; Brunetti, L.; Poliseno, V.; Laghezza, A.; Loiodice, F.; Tortorella, P.; Piemontese, L. Natural Compounds for the Prevention and Treatment of Cardiovascular and Neurodegenerative Diseases. Foods 2020, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, L. Historic Recurrences in Medicinal Chemistry: Nature-Inspired Structures as a New Opportunity for Novel Multi-Target Anti-Alzheimer’s Drugs. Neural Regen. Res. 2023, 18, 2671–2672. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, L.; Brunetti, L.; Leuci, R. Can Foods Influence the Onset and Progress of Neurodegenerative Diseases? Neural Regen. Res. 2022, 17, 2443. [Google Scholar] [CrossRef]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and Human Health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Chocolate: Modern Science Investigates an Ancient Medicine; Mary Ann Liebert, Inc.: Larchmont, NY, USA, 2018. [Google Scholar]
- Piemontese, L. Plant Food Supplements with Antioxidant Properties for the Treatment of Chronic and Neurodegenerative Diseases: Benefits or Risks? J. Diet. Suppl. 2017, 14, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Leuci, R.; Colonna, M.A.; Carrieri, R.; Celentano, F.E.; Bozzo, G.; Loiodice, F.; Selvaggi, M.; Tufarelli, V.; Piemontese, L. Food Industry Byproducts as Starting Material for Innovative, Green Feed Formulation: A Sustainable Alternative for Poultry Feeding. Molecules 2022, 27, 4735. [Google Scholar] [CrossRef] [PubMed]
- Dheyab, A.S.; Abu Bakar, M.F.; AlOmar, M.; Sabran, S.F.; Muhamad Hanafi, A.F.; Mohamad, A. Deep Eutectic Solvents (DESs) as Green Extraction Media of Beneficial Bioactive Phytochemicals. Separations 2021, 8, 176. [Google Scholar] [CrossRef]
- Lu, W.; Liu, S. Choline Chloride–Based Deep Eutectic Solvents (Ch-DESs) as Promising Green Solvents for Phenolic Compounds Extraction from Bioresources: State-of-the-Art, Prospects, and Challenges. Biomass Conv. Bioref. 2022, 12, 2949–2962. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Supercritical CO2 Extraction and Purification of Compounds with Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 2441–2469. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Tobón, J.F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- de la Calle, I.; Costas-Rodríguez, M. Microwaves for Greener Extraction. In The Application of Green Solvents in Separation Processes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 253–300. [Google Scholar]
- Yahya, N.A.; Attan, N.; Wahab, R.A. An Overview of Cosmeceutically Relevant Plant Extracts and Strategies for Extraction of Plant-Based Bioactive Compounds. Food Bioprod. Process. 2018, 112, 69–85. [Google Scholar] [CrossRef]
- Freitas De Oliveira, C.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Ferreira Marczak, L.D. Extraction of Pectin from Passion Fruit Peel Assisted by Ultrasound. LWT Food Sci. Technol. 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound Assisted Extraction (UAE) of Bioactive Compounds from Fruit and Vegetable Processing by-Products: A Review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.-S.; Bourvellec, C.L.; Renard, C.M.G.C.; Chemat, F. Lab and Pilot-Scale Ultrasound-Assisted Water Extraction of Polyphenols from Apple Pomace. J. Food Eng. 2012, 111, 73–81. [Google Scholar] [CrossRef]
- Dezhkunov, N.V.; Leighton, T.G. Study into Correlation between the Ultrasonic Capillary Effect and Sonoluminescence. J. Eng. Phys. Thermophys. 2004, 77, 53–61. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Panja, P. Green Extraction Methods of Food Polyphenols from Vegetable Materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Zeng, X.; Han, Z.; Brennan, C.S. Non-thermal Technologies and Its Current and Future Application in the Food Industry: A Review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Pulsed Electric Field Assisted Extraction of Natural Food Pigments and Colorings from Plant Matrices. Food Chem. X 2022, 15, 100398. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green Alternative Methods for the Extraction of Antioxidant Bioactive Compounds from Winery Wastes and By-Products: A Review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R.; Jayasuriya, H.; Al-Attabi, Z. Postharvest Quality, Technologies, and Strategies to Reduce Losses along the Supply Chain of Banana: A Review. Trends Food Sci. Technol. 2023, 134, 177–191. [Google Scholar] [CrossRef]
- Alaa El-Din, G.; Amer, A.A.; Malsh, G.; Hussein, M. Study on the Use of Banana Peels for Oil Spill Removal. Alex. Eng. J. 2018, 57, 2061–2068. [Google Scholar] [CrossRef]
- Tock, J.Y.; Lai, C.L.; Lee, K.T.; Tan, K.T.; Bhatia, S. Banana Biomass as Potential Renewable Energy Resource: A Malaysian Case Study. Renew. Sustain. Energy Rev. 2010, 14, 798–805. [Google Scholar] [CrossRef]
- Sharma, P.; Mishra, A.A. Biofuel Production from Banana Peel by Using Micro Wave. Int. J. Sci. Eng. Technol. 2015, 3, 1015–1018. [Google Scholar] [CrossRef]
- Palma, C.; Contreras, E.; Urra, J.; Martínez, M.J. Eco-Friendly Technologies Based on Banana Peel Use for the Decolourization of the Dyeing Process Wastewater. Waste Biomass Valorization 2011, 2, 77–86. [Google Scholar] [CrossRef]
- Karne, H.U.; Gaydhane, P.; Gohokar, V.; Deshpande, K.; Dunung, P.; Bendkule, G. Synthesis of Biodegradable Material from Banana Peel. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Grover, S.; Aggarwal, P.; Kumar, A.; Kaur, S.; Yadav, R.; Babbar, N. Utilizing Citrus Peel Waste: A Review of Essential Oil Extraction, Characterization, and Food-Industry Potential. Biomass Conv. Bioref. 2024. [Google Scholar] [CrossRef]
- Kumar, A. Utilization of Bioactive Components Present in Pineapple Waste: A Review. J. Pharm. Innov. 2021, 10, 954–961. [Google Scholar] [CrossRef]
- Tran, T.V.; Nguyen, D.T.C.; Nguyen, T.T.T.; Nguyen, D.H.; Alhassan, M.; Jalil, A.A.; Nabgan, W.; Lee, T. A Critical Review on Pineapple (Ananas comosus) Wastes for Water Treatment, Challenges and Future Prospects towards Circular Economy. Sci. Total Environ. 2023, 856, 158817. [Google Scholar] [CrossRef] [PubMed]
- Otieno, E.O.; Kiplimo, R.; Mutwiwa, U. Optimization of Anaerobic Digestion Parameters for Biogas Production from Pineapple Wastes Co-Digested with Livestock Wastes. Heliyon 2023, 9, e14041. [Google Scholar] [CrossRef] [PubMed]
- Sarangi, P.K.; Srivastava, R.K.; Sahoo, U.K.; Singh, A.K.; Parikh, J.; Bansod, S.; Parsai, G.; Luqman, M.; Shadangi, K.P.; Diwan, D.; et al. Biotechnological Innovations in Nanocellulose Production from Waste Biomass with a Focus on Pineapple Waste. Chemosphere 2024, 349, 140833. [Google Scholar] [CrossRef] [PubMed]
- Chetrariu, A.; Dabija, A. Brewer’s Spent Grains: Possibilities of Valorization, a Review. Appl. Sci. 2020, 10, 5619. [Google Scholar] [CrossRef]
- Baiano, A.; La Gatta, B.; Rutigliano, M.; Fiore, A. Functional Bread Produced in a Circular Economy Perspective: The Use of Brewers’ Spent Grain. Foods 2023, 12, 834. [Google Scholar] [CrossRef]
- Wagner, E.; Pería, M.E.; Ortiz, G.E.; Rojas, N.L.; Ghiringhelli, P.D. Valorization of Brewer’s Spent Grain by Different Strategies of Structural Destabilization and Enzymatic Saccharification. Ind. Crops Prod. 2021, 163, 113329. [Google Scholar] [CrossRef]
- Franzen, M.; Borgerhoff Mulder, M. Ecological, Economic and Social Perspectives on Cocoa Production Worldwide. Biodivers. Conserv. 2007, 16, 3835–3849. [Google Scholar] [CrossRef]
- Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Cocoa Bean Shell: A By-Product with High Potential for Nutritional and Biotechnological Applications. Antioxidants 2023, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Aurore, G.; Parfait, B.; Fahrasmane, L. Bananas, Raw Materials for Making Processed Food Products. Trends Food Sci. Technol. 2009, 20, 78–91. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Optimization of Ultrasound-Assisted Extraction Conditions for Recovery of Phenolic Compounds and Antioxidant Capacity from Banana (Musa Cavendish) Peel. J. Food Process. Preserv. 2017, 41, e13148. [Google Scholar] [CrossRef]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic Compounds within Banana Peel and Their Potential Uses: A Review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Faizal, A.N.M.; Che Yunus, M.A. Methods and Potential in Valorization of Banana Peels Waste by Various Extraction Processes: In Review. Sustainability 2022, 14, 10571. [Google Scholar] [CrossRef]
- Chaudhry, F.; Ahmad, M.L.; Hayat, Z.; Ranjha, M.M.A.N.; Chaudhry, K.; Elboughdiri, N.; Asmari, M.; Uddin, J. Extraction and Evaluation of the Antimicrobial Activity of Polyphenols from Banana Peels Employing Different Extraction Techniques. Separations 2022, 9, 165. [Google Scholar] [CrossRef]
- Bello, U.; Amran, N.A.; Samsuri, S.; Ruslan, M.S.H. Kinetics, Thermodynamic Studies, and Parametric Effects of Supercritical CO2 Extraction of Banana Peel Wastes. Sustain. Chem. Pharm. 2023, 31, 100912. [Google Scholar] [CrossRef]
- Pereira, G.A.; Molina, G.; Arruda, H.S.; Pastore, G.M. Optimizing the Homogenizer-Assisted Extraction (HAE) of Total Phenolic Compounds from Banana Peel. J. Food Process Eng. 2017, 40, e12438. [Google Scholar] [CrossRef]
- Aziz, A.H.A.; Engliman, N.S.; Mansor, M.F.; Nasaruddin, R.R. Extraction of Phenolic Compound Using Natural Deep Eutectic Solvent from Biomass Waste. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1192, 012001. [Google Scholar] [CrossRef]
- Hendrawan, Y.; Larasati, R.; Wibisono, Y.; Umam, C.; Sutan, S.M.; Choviya Hawa, L. Extraction of Phenol and Antioxidant Compounds from Kepok Banana Skin with PEF Pre-Treatment. IOP Conf. Ser. Earth Environ. Sci. 2019, 305, 012065. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus Waste as Feedstock for Bio-Based Products Recovery: Review on Limonene Case Study and Energy Valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Citrus Reticulata Blanco Peels as a Source of Antioxidant and Anti-Proliferative Phenolic Compounds. Ind. Crops Prod. 2018, 111, 141–148. [Google Scholar] [CrossRef]
- Nair, A.; Sr, R.K.; Nair, A.S.; Baby, S. Citrus Peels Prevent Cancer. Phytomedicine 2018, 50, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Mahin-Syed-Ismail, P.; Varghese, S.; Thomas-George, B.; Kandathil- Thajuraj, P.; Baby, D.; Haleem, S.; Sreedhar, S.; Devang-Divakar, D. Antimicrobial Effects of Citrus Sinensis Peel Extracts against Dental Caries Bacteria: An In Vitro Study. J. Clin. Exp. Dent. 2016, 8, e71. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Kaur, A.; Singh, N.; Nim, L.; Shevkani, K.; Kaur, H.; Arora, D.S. In Vitro Antioxidant and Antimicrobial Properties of Jambolan (Syzygium cumini) Fruit Polyphenols. LWT Food Sci. Technol. 2016, 65, 1025–1030. [Google Scholar] [CrossRef]
- Sridharan, B.; Mehra, Y.; Ganesh, R.N.; Viswanathan, P. Regulation of Urinary Crystal Inhibiting Proteins and Inflammatory Genes by Lemon Peel Extract and Formulated Citrus Bioflavonoids on Ethylene Glycol Induced Urolithic Rats. Food Chem. Toxicol. 2016, 94, 75–84. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Lebovka, N.; Foucart, F.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed Electric Field Treatment of Citrus Fruits: Improvement of Juice and Polyphenols Extraction. Innov. Food Sci. Emerg. Technol. 2018, 46, 153–161. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Boudhrioua, N.M. Extraction Methods of Citrus Peel Phenolic Compounds. Food Rev. Int. 2014, 30, 265–290. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Ghoul, M.; Mihoubi Boudhrioua, N. Phytochemical Characteristics of Citrus Peel and Effect of Conventional and Nonconventional Processing on Phenolic Compounds: A Review. Food Rev. Int. 2017, 33, 587–619. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.; Cravotto, G.; Binello, A.; Lorenzo, J.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citrus Wastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-Dependent Extraction of Flavonoids from Citrus Peel Waste Using a Tailor-Made Deep Eutectic Solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef]
- Šafranko, S.; Ćorković, I.; Jerković, I.; Jakovljević, M.; Aladić, K.; Šubarić, D.; Jokić, S. Green Extraction Techniques for Obtaining Bioactive Compounds from Mandarin Peel (Citrus unshiu Var. Kuno): Phytochemical Analysis and Process Optimization. Foods 2021, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Extraction of Phenolic Compounds from Lime Peel Waste Using Ultrasonic-Assisted and Microwave-Assisted Extractions. Food Biosci. 2019, 28, 66–73. [Google Scholar] [CrossRef]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and Quantification of Polyphenols from Kinnow (Citrus reticulate L.) Peel Using Ultrasound and Maceration Techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Green Solvents and Ultrasound-Assisted Extraction of Bioactive Orange (Citrus sinensis) Peel Compounds. Sci. Rep. 2019, 9, 16120. [Google Scholar] [CrossRef]
- Barrales, F.M.; Silveira, P.; Barbosa, P.D.P.M.; Ruviaro, A.R.; Paulino, B.N.; Pastore, G.M.; Macedo, G.A.; Martinez, J. Recovery of Phenolic Compounds from Citrus By-Products Using Pressurized Liquids—An Application to Orange Peel. Food Bioprod. Process. 2018, 112, 9–21. [Google Scholar] [CrossRef]
- Casquete, R.; Castro, S.M.; Villalobos, M.C.; Serradilla, M.J.; Queirós, R.P.; Saraiva, J.A.; Córdoba, M.G.; Teixeira, P. High Pressure Extraction of Phenolic Compounds from Citrus Peels†. High Press. Res. 2014, 34, 447–451. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of Phenolics from Citrus Peels. Sep. Purif. Technol. 2006, 48, 189–196. [Google Scholar] [CrossRef]
- Chen, H.; Hu, B.; Zhao, L.; Shi, D.; She, Z.; Huang, X.; Priyadarshani, S.V.G.N.; Niu, X.; Qin, Y. Differential Expression Analysis of Reference Genes in Pineapple (Ananas comosus L.) during Reproductive Development and Response to Abiotic Stress, Hormonal Stimuli. Trop. Plant Biol. 2019, 12, 67–77. [Google Scholar] [CrossRef]
- Yabor, L.; Pérez, L.; Gómez, D.; Villalobos-Olivera, A.; Mendoza, J.R.; Martínez, J.; Escalante, D.; Garro, G.; Hajari, E.; Lorenzo, J.C. Histological Evaluation of Pineapple Transgenic Plants Following 8 Years of Field Growth. Euphytica 2020, 216, 23. [Google Scholar] [CrossRef]
- Pavan, R.; Jain, S.; Shraddha; Kumar, A. Properties and Therapeutic Application of Bromelain: A Review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef] [PubMed]
- Alias, N.H.; Abbas, Z. Microwave-Assisted Extraction of Phenolic Compound from Pineapple Skins: The Optimum Operating Condition and Comparison with Soxhlet Extraction. MJAS 2017, 21, 690–699. [Google Scholar] [CrossRef]
- Vargas-Serna, C.L.; Ochoa-Martínez, C.I.; Vélez-Pasos, C. Microwave-Assisted Extraction of Phenolic Compounds from Pineapple Peel Using Deep Eutectic Solvents. Horticulturae 2022, 8, 791. [Google Scholar] [CrossRef]
- Bansod, S.P.; Parikh, J.K.; Sarangi, P.K. Pineapple Peel Waste Valorization for Extraction of Bio-Active Compounds and Protein: Microwave Assisted Method and Box Behnken Design Optimization. Environ. Res. 2023, 221, 115237. [Google Scholar] [CrossRef] [PubMed]
- Paz-Arteaga, S.; Cadena-Chamorro, E.; Serna-Cock, L.; Torres-Castañeda, H.; Pabón-Rodríguez, O.; Agudelo-Morales, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Aguilar, C.; Torres-León, C. Dual Emerging Applications of Solid-State Fermentation (SSF) with Aspergillus Niger and Ultrasonic-Assisted Extraction (UAE) for the Obtention of Antimicrobial Polyphenols from Pineapple Waste. Fermentation 2023, 9, 706. [Google Scholar] [CrossRef]
- Polanía, A.M.; Londoño, L.; Ramírez, C.; Bolívar, G. Influence of Ultrasound Application in Fermented Pineapple Peel on Total Phenolic Content and Antioxidant Activity. Fermentation 2022, 8, 314. [Google Scholar] [CrossRef]
- Yahya, N.A.; Wahab, R.A.; Xine, T.L.S.; Hamid, M.A. Ultrasound-Assisted Extraction of Polyphenols from Pineapple Skin. In Proceedings of the 2nd International Conference on Biosciences and Medical Engineering (ICBME2019): Towards Innovative Research and Cross-Disciplinary Collaborations, Bali, Indonesia, 11–12 April 2019; p. 020002. [Google Scholar]
- Li, T.; Shen, P.; Liu, W.; Liu, C.; Liang, R.; Yan, N.; Chen, J. Major Polyphenolics in Pineapple Peels and Their Antioxidant Interactions. Int. J. Food Prop. 2014, 17, 1805–1817. [Google Scholar] [CrossRef]
- Hossain, M.A.; Rahman, S.M.M. Total Phenolics, Flavonoids and Antioxidant Activity of Tropical Fruit Pineapple. Food Res. Int. 2011, 44, 672–676. [Google Scholar] [CrossRef]
- Zampar, G.G.; Zampar, I.C.; Beserra Da Silva De Souza, S.; Da Silva, C.; Bolanho Barros, B.C. Effect of Solvent Mixtures on the Ultrasound-Assisted Extraction of Compounds from Pineapple by-Product. Food Biosci. 2022, 50, 102098. [Google Scholar] [CrossRef]
- Mala, T.; Sadiq, M.B.; Anal, A.K. Comparative Extraction of Bromelain and Bioactive Peptides from Pineapple Byproducts by Ultrasonic- and Microwave-assisted Extractions. J. Food Process. Eng. 2021, 44, e13709. [Google Scholar] [CrossRef]
- Balaraman, H.B.; Umasekar, S.; Rajmohan, K.S.; Rathnasamy, S.K. Sustainable Eutectic Mixture-Based Ultrasound Assisted Multifaceted Valorisation of Pineapple Waste for Bromelain and Bioethanol Production. Sustain. Chem. Pharm. 2022, 30, 100876. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Romaní, A.; Aguilar, C.N.; Teixeira, J. Valorization of Pineapple Waste for the Extraction of Bioactive Compounds and Glycosides Using Autohydrolysis. Innov. Food Sci. Emerg. Technol. 2018, 47, 38–45. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Bilbao, A.; Vilches, P.; Angulo, I.; LLuis, J.; Fité, B.; Paseiro-Losada, P.; Cruz, J.M. Brewery Waste as a Potential Source of Phenolic Compounds: Optimisation of the Extraction Process and Evaluation of Antioxidant and Antimicrobial Activities. Food Chem. 2014, 145, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Rachwał, K.; Waśko, A.; Gustaw, K.; Polak-Berecka, M. Utilization of Brewery Wastes in Food Industry. PeerJ 2020, 8, e9427. [Google Scholar] [CrossRef] [PubMed]
- Kubeš, J. Geography of World Hop Production 1990–2019. J. Am. Soc. Brew. Chem. 2022, 80, 84–91. [Google Scholar] [CrossRef]
- Vicente De Andrade Silva, G.; Demaman Arend, G.; Antonio Ferreira Zielinski, A.; Di Luccio, M.; Ambrosi, A. Xanthohumol Properties and Strategies for Extraction from Hops and Brewery Residues: A Review. Food Chem. 2023, 404, 134629. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.M.; Camina, J.L.; Borroni, V.; Pérez, E.E. Protein Recovery from Brewery Solid Wastes. Food Chem. 2023, 407, 134810. [Google Scholar] [CrossRef]
- Thiago, R.D.S.M.; Pedro, P.M.D.M.; Eliana, F.C.S. Solid Wastes in Brewing Process: A Review. J. Brew. Distill. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ Spent Grain: A Review with an Emphasis on Food and Health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Del Río, J.C.; Prinsen, P.; Gutiérrez, A. Chemical Composition of Lipids in Brewer’s Spent Grain: A Promising Source of Valuable Phytochemicals. J. Cereal Sci. 2013, 58, 248–254. [Google Scholar] [CrossRef]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I. Brewer’s Spent Grain: A Valuable Feedstock for Industrial Applications: Brewer’s Spent Grain and Its Potential Applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.L.; O’Callaghan, Y.C.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Brewers’ Spent Grain; Bioactivity of Phenolic Component, Its Role in Animal Nutrition and Potential for Incorporation in Functional Foods: A Review. Proc. Nutr. Soc. 2013, 72, 117–125. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Moreira, M.M.; Morais, S.; Barros, A.A.; Delerue-Matos, C.; Guido, L.F. A Novel Application of Microwave-Assisted Extraction of Polyphenols from Brewer’s Spent Grain with HPLC-DAD-MS Analysis. Anal. Bioanal. Chem. 2012, 403, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- López-Linares, J.C.; Campillo, V.; Coca, M.; Lucas, S.; García-Cubero, M.T. Microwave-assisted Deep Eutectic Solvent Extraction of Phenolic Compounds from Brewer’s Spent Grain. J. Chem. Technol. Biotechnol. 2021, 96, 481–490. [Google Scholar] [CrossRef]
- Spinelli, S.; Conte, A.; Del Nobile, M.A. Microencapsulation of Extracted Bioactive Compounds from Brewer’s Spent Grain to Enrich Fish-Burgers. Food Bioprod. Process. 2016, 100, 450–456. [Google Scholar] [CrossRef]
- González-García, E.; Marina, M.L.; García, M.C. Impact of the Use of Pressurized Liquids on the Extraction and Functionality of Proteins and Bioactives from Brewer’s Spent Grain. Food Chem. 2021, 359, 129874. [Google Scholar] [CrossRef]
- Martín-García, B.; Tylewicz, U.; Verardo, V.; Pasini, F.; Gómez-Caravaca, A.M.; Caboni, M.F.; Dalla Rosa, M. Pulsed Electric Field (PEF) as Pre-Treatment to Improve the Phenolic Compounds Recovery from Brewers’ Spent Grains. Innov. Food Sci. Emerg. Technol. 2020, 64, 102402. [Google Scholar] [CrossRef]
- Alonso-Riaño, P.; Sanz Diez, M.T.; Blanco, B.; Beltrán, S.; Trigueros, E.; Benito-Román, O. Water Ultrasound-Assisted Extraction of Polyphenol Compounds from Brewer’s Spent Grain: Kinetic Study, Extract Characterization, and Concentration. Antioxidants 2020, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Ferulic and P-Coumaric Acids Extraction by Alkaline Hydrolysis of Brewer’s Spent Grain. Ind. Crops Prod. 2007, 25, 231–237. [Google Scholar] [CrossRef]
- Macias-Garbett, R.; Serna-Hernández, S.O.; Sosa-Hernández, J.E.; Parra-Saldívar, R. Phenolic Compounds from Brewer’s Spent Grains: Toward Green Recovery Methods and Applications in the Cosmetic Industry. Front. Sustain. Food Syst. 2021, 5, 681684. [Google Scholar] [CrossRef]
- Gandolpho, B.; Almeida, A.; Gandolpho, G.; Freitas, D.; Gasparini, O.; Machado, M.; Barreto, P. Optimization of brewing waste’s (trub) phenolic compounds extraction by ultrasound assisted using response surface methodology. Quím. Nova 2020, 44, 478–483. [Google Scholar] [CrossRef]
- Manzano, P.; Quijano, M.; Barragn, A.; Viteri, R.; Chez, I.; Hernndez, J.; Valle, O. Polyphenols Extracted from Theobroma Cacao Waste and Its Utility as Antioxidant for Food-Grade Vegetal Oil. Emir. J. Food Agric. 2017, 29, 45. [Google Scholar] [CrossRef]
- Vásquez, Z.S.; De Carvalho Neto, D.P.; Pereira, G.V.M.; Vandenberghe, L.P.S.; De Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.G.; Góes Neto, A.; Soccol, C.R. Biotechnological Approaches for Cocoa Waste Management: A Review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Barišić, V.; Jozinović, A.; Flanjak, I.; Šubarić, D.; Babić, J.; Miličević, B.; Doko, K.; Ačkar, Đ. Difficulties with Use of Cocoa Bean Shell in Food Production and High Voltage Electrical Discharge as a Possible Solution. Sustainability 2020, 12, 3981. [Google Scholar] [CrossRef]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Rupérez, P.; Goya, L.; Bravo, L. Dietary Fibre Composition, Antioxidant Capacity and Physico-Chemical Properties of a Fibre-Rich Product from Cocoa (Theobroma Cacao L.). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Zhong, F.; Mothibe, K.J. In Vitro Hypoglycemic and Cholesterol Lowering Effects of Dietary Fiber Prepared from Cocoa (Theobroma Cacao L.) Shells. Food Funct. 2012, 3, 1044. [Google Scholar] [CrossRef]
- Jozinović, A.; Panak Balentić, J.; Ačkar, Đ.; Babić, J.; Pajin, B.; Miličević, B.; Guberac, S.; Vrdoljak, A.; Šubarić, D. Cocoa Husk Application in the Enrichment of Extruded Snack Products. J. Food Process. Preserv. 2019, 43, e13866. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Jordà, R.E. Constituents of Cocoa Husks. Z. Für Naturforschung C 1998, 53, 785–792. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Viera-Alcaide, I.; Morales-Sillero, A.M.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Bioactive Compounds in Mexican Genotypes of Cocoa Cotyledon and Husk. Food Chem. 2018, 240, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Mazzutti, S.; Rodrigues, L.G.G.; Mezzomo, N.; Venturi, V.; Ferreira, S.R.S. Integrated Green-Based Processes Using Supercritical CO2 and Pressurized Ethanol Applied to Recover Antioxidant Compouds from Cocoa (Theobroma Cacao) Bean Hulls. J. Supercrit. Fluids 2018, 135, 52–59. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.B.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E.C. Pressurized Liquid Extraction of Flavanols and Alkaloids from Cocoa Bean Shell Using Ethanol as Solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Jokić, S.; Gagić, T.; Knez, Ž.; Šubarić, D.; Škerget, M. Separation of Active Compounds from Food By-Product (Cocoa Shell) Using Subcritical Water Extraction. Molecules 2018, 23, 1408. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, N.; Jokić, S.; Jakovljević, M.; Blažić, M.; Molnar, M. Green Extraction Methods for Active Compounds from Food Waste—Cocoa Bean Shell. Foods 2020, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Ruesgas-Ramón, M.; Suárez-Quiroz, M.L.; González-Ríos, O.; Baréa, B.; Cazals, G.; Figueroa-Espinoza, M.C.; Durand, E. Biomolecules Extraction from Coffee and Cocoa By- and Co-Products Using Deep Eutectic Solvents. J. Sci. Food Agric. 2020, 100, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed Electric Field Assisted Extraction of Bioactive Compounds from Cocoa Bean Shell and Coffee Silverskin. Food Bioprocess Technol. 2018, 11, 818–835. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Binello, A.; Mantegna, S.; Cravotto, G.; Chemat, F.; Dizhbite, T.; Lauberte, L.; Telysheva, G. Cocoa Bean Shell Waste Valorisation; Extraction from Lab to Pilot-Scale Cavitational Reactors. Food Res. Int. 2019, 115, 200–208. [Google Scholar] [CrossRef]
- Md Yusof, A.; Abd Gani, S.; Zaidan, U.; Halmi, M.; Zainudin, B. Optimization of an Ultrasound-Assisted Extraction Condition for Flavonoid Compounds from Cocoa Shells (Theobroma Cacao) Using Response Surface Methodology. Molecules 2019, 24, 711. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Cañas, S.; Taladrid, D.; Segovia, Á.; Bartolomé, B.; Aguilera, Y.; Martín-Cabrejas, M.A. Extraction of Phenolic Compounds from Cocoa Shell: Modeling Using Response Surface Methodology and Artificial Neural Networks. Sep. Purif. Technol. 2021, 270, 118779. [Google Scholar] [CrossRef]
- Jensch, C.; Schmidt, A.; Strube, J. Versatile Green Processing for Recovery of Phenolic Compounds from Natural Product Extracts towards Bioeconomy and Cascade Utilization for Waste Valorization on the Example of Cocoa Bean Shell (CBS). Sustainability 2022, 14, 3126. [Google Scholar] [CrossRef]
- Pagliari, S.; Celano, R.; Rastrelli, L.; Sacco, E.; Arlati, F.; Labra, M.; Campone, L. Extraction of Methylxanthines by Pressurized Hot Water Extraction from Cocoa Shell By-Product as Natural Source of Functional Ingredient. LWT 2022, 170, 114115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roselli, V.; Pugliese, G.; Leuci, R.; Brunetti, L.; Gambacorta, L.; Tufarelli, V.; Piemontese, L. Green Methods to Recover Bioactive Compounds from Food Industry Waste: A Sustainable Practice from the Perspective of the Circular Economy. Molecules 2024, 29, 2682. https://doi.org/10.3390/molecules29112682
Roselli V, Pugliese G, Leuci R, Brunetti L, Gambacorta L, Tufarelli V, Piemontese L. Green Methods to Recover Bioactive Compounds from Food Industry Waste: A Sustainable Practice from the Perspective of the Circular Economy. Molecules. 2024; 29(11):2682. https://doi.org/10.3390/molecules29112682
Chicago/Turabian StyleRoselli, Vincenzo, Gianluca Pugliese, Rosalba Leuci, Leonardo Brunetti, Lucia Gambacorta, Vincenzo Tufarelli, and Luca Piemontese. 2024. "Green Methods to Recover Bioactive Compounds from Food Industry Waste: A Sustainable Practice from the Perspective of the Circular Economy" Molecules 29, no. 11: 2682. https://doi.org/10.3390/molecules29112682
APA StyleRoselli, V., Pugliese, G., Leuci, R., Brunetti, L., Gambacorta, L., Tufarelli, V., & Piemontese, L. (2024). Green Methods to Recover Bioactive Compounds from Food Industry Waste: A Sustainable Practice from the Perspective of the Circular Economy. Molecules, 29(11), 2682. https://doi.org/10.3390/molecules29112682







