Organosiloxane-Modified Auricularia Polysaccharide (Si-AP): Improved High-Temperature Resistance and Lubrication Performance in WBDFs
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Characterizations of Si-AP
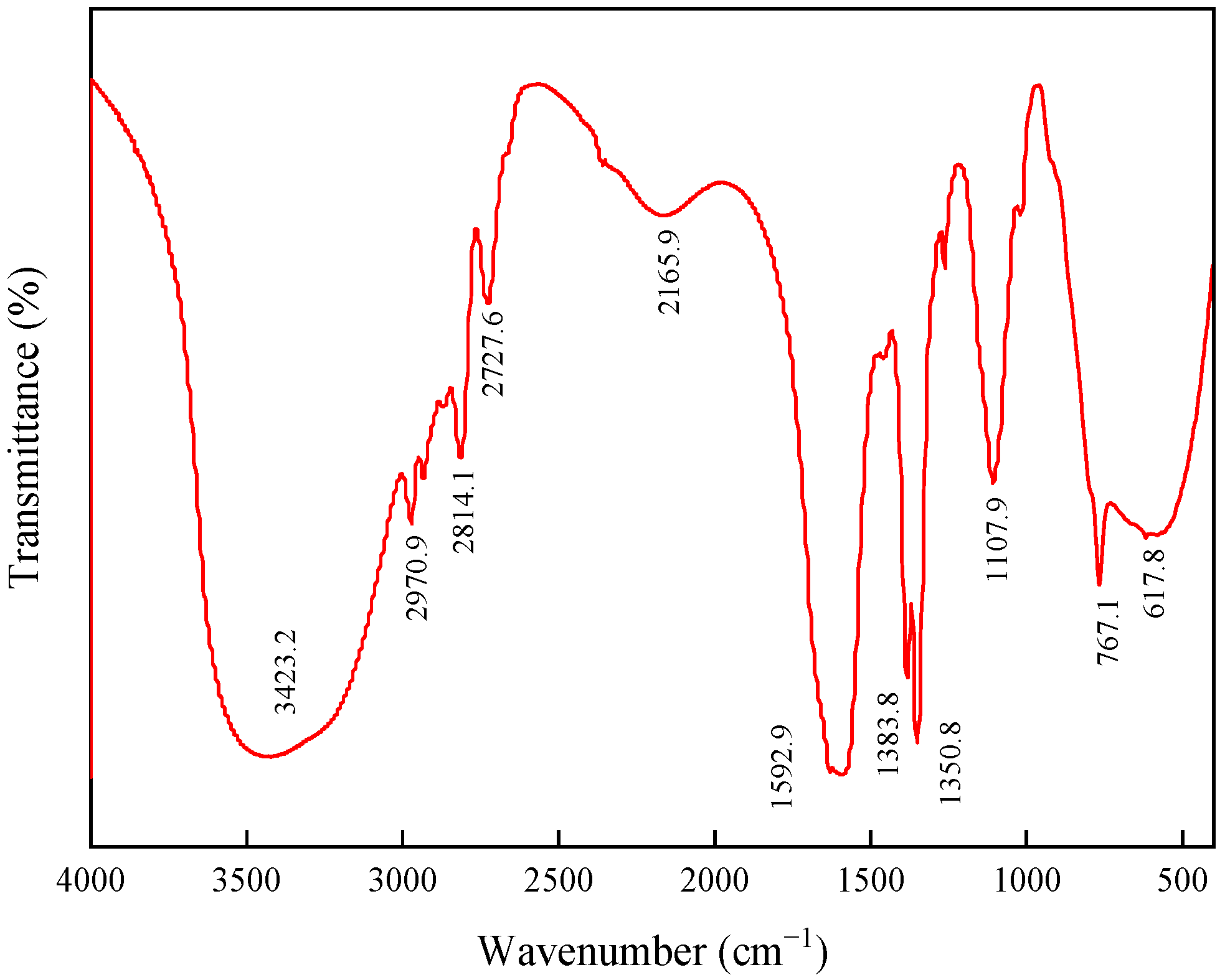
2.1.1. FTIR Spectroscopy Analysis
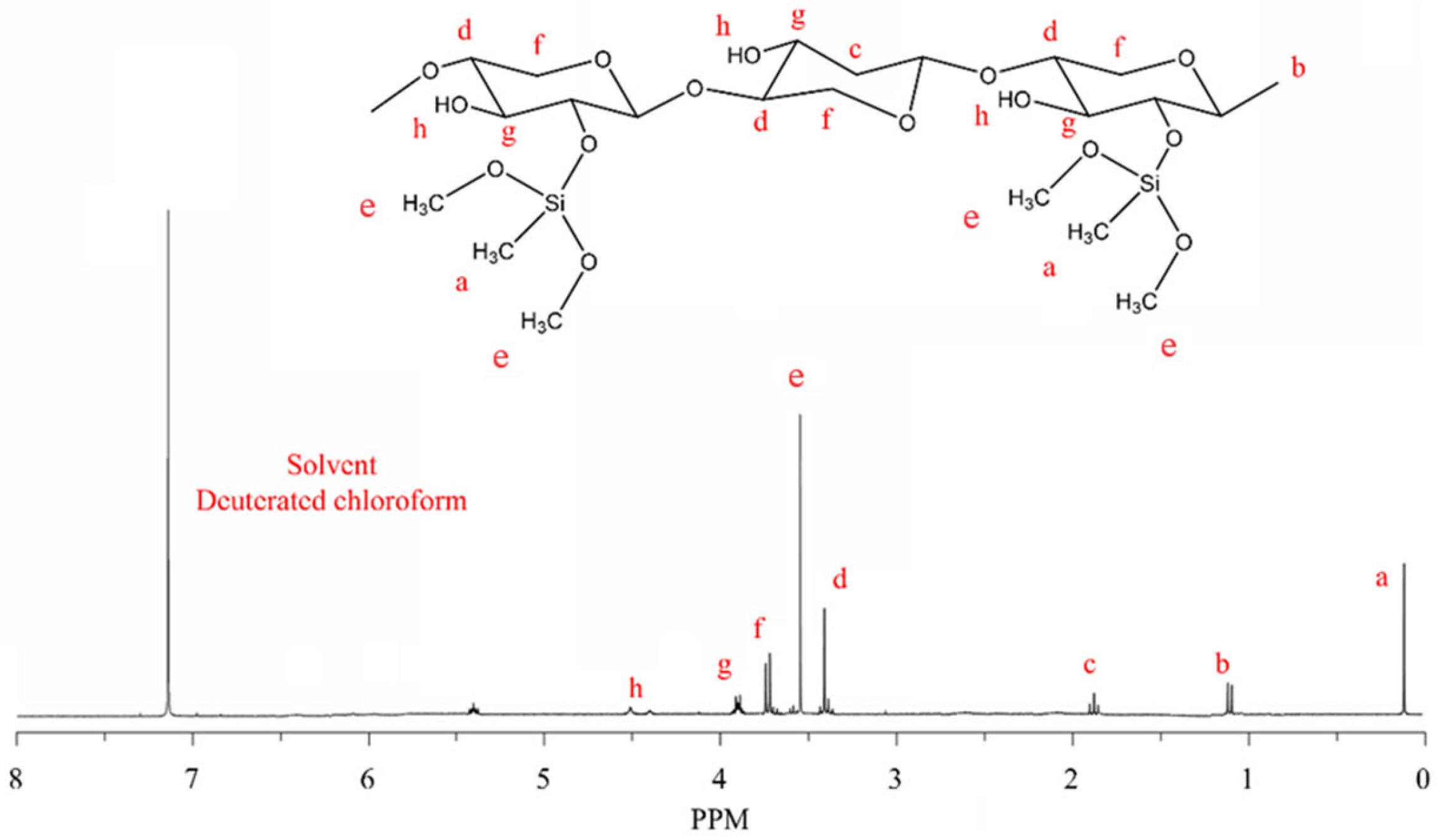
2.1.2. 1H-NMR Analysis
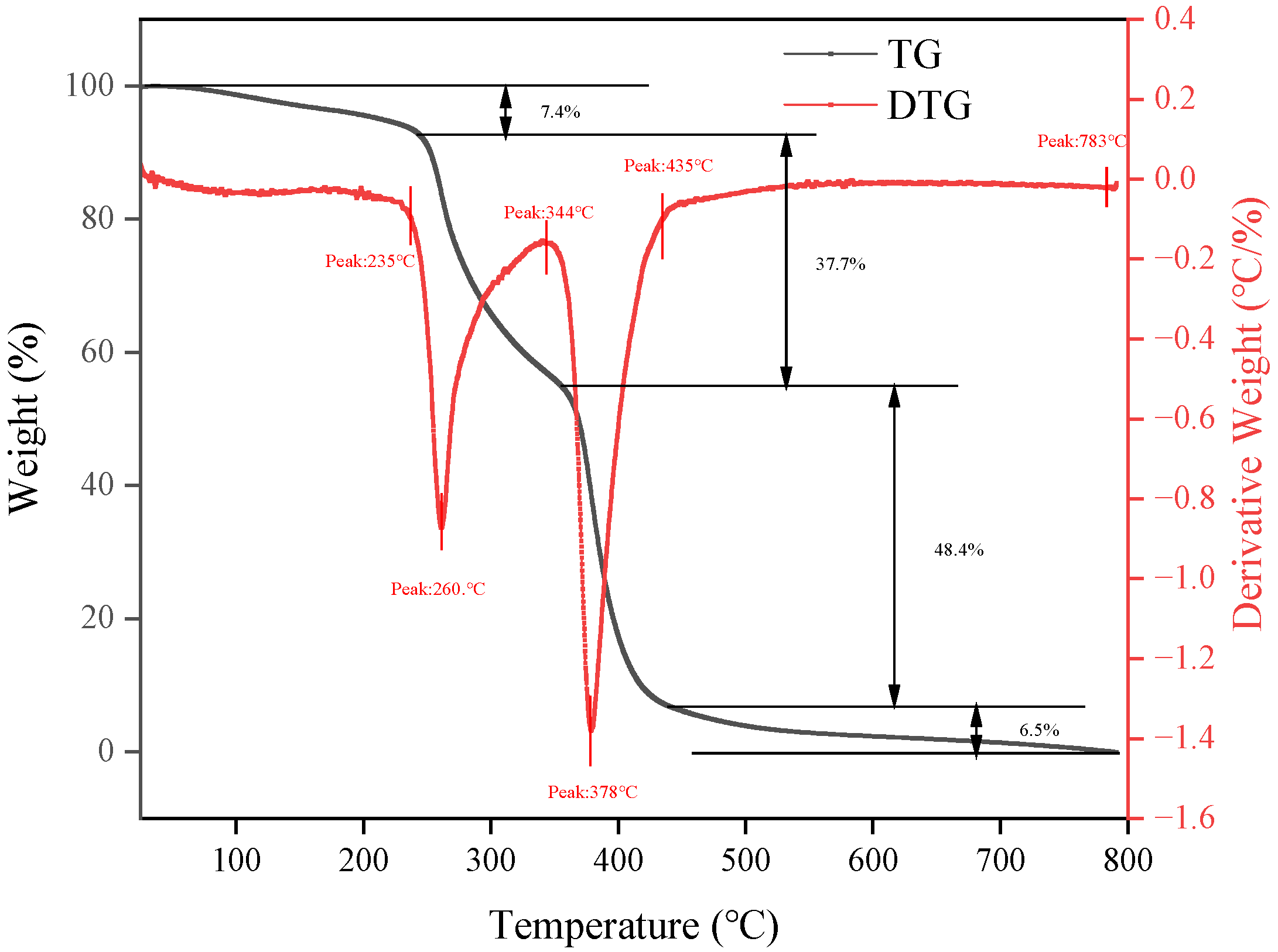
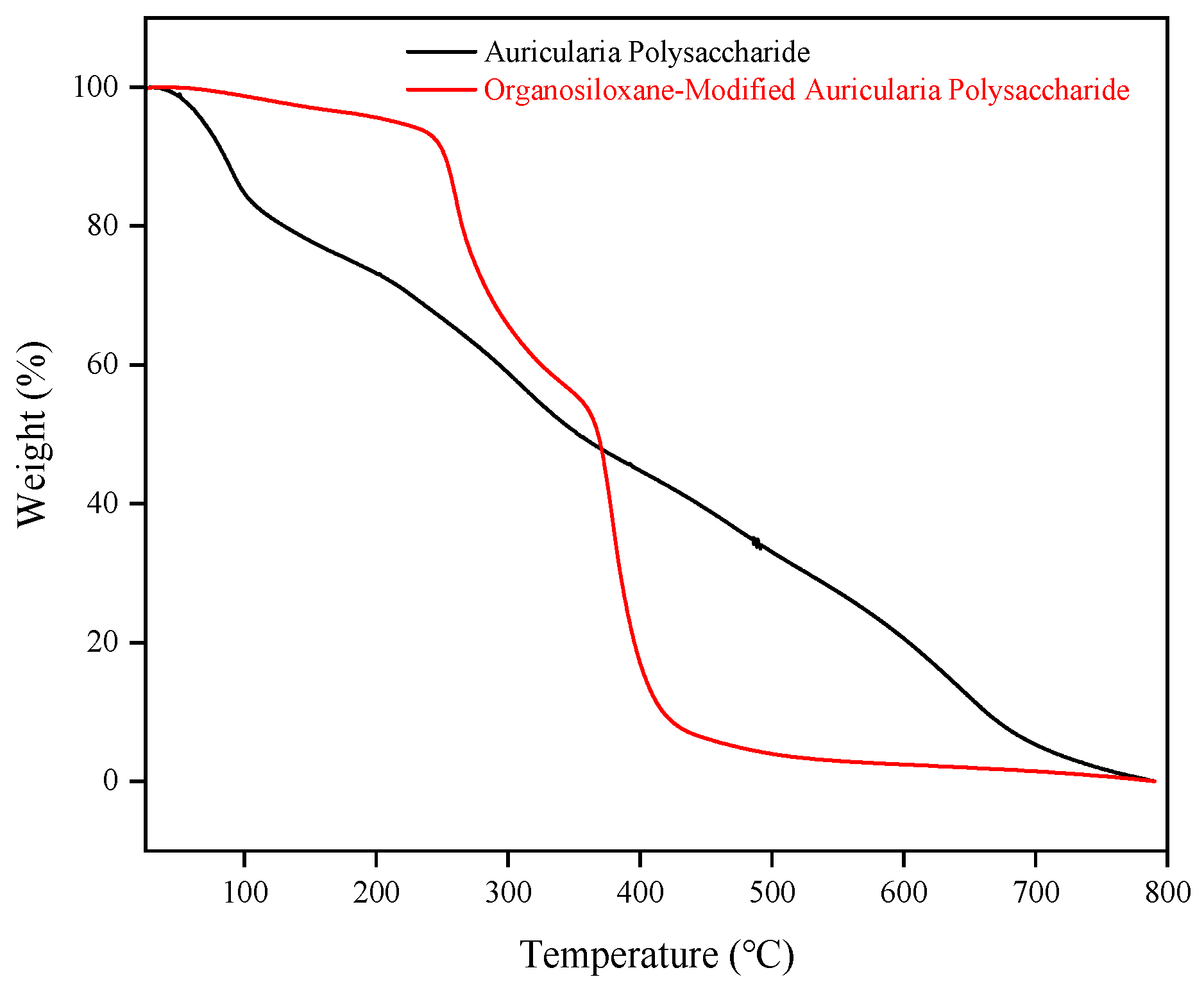
2.1.3. Thermogravimetric Analysis
2.1.4. GPC Analysis
2.2. Performance Evaluations
2.2.1. Rheological Properties and Filtration Characteristics Tests
2.2.2. Lubrication Performance Tests
2.2.3. Inhibition Performance Tests
2.3. Environmental Performance Tests
2.4. Mechanism Analysis
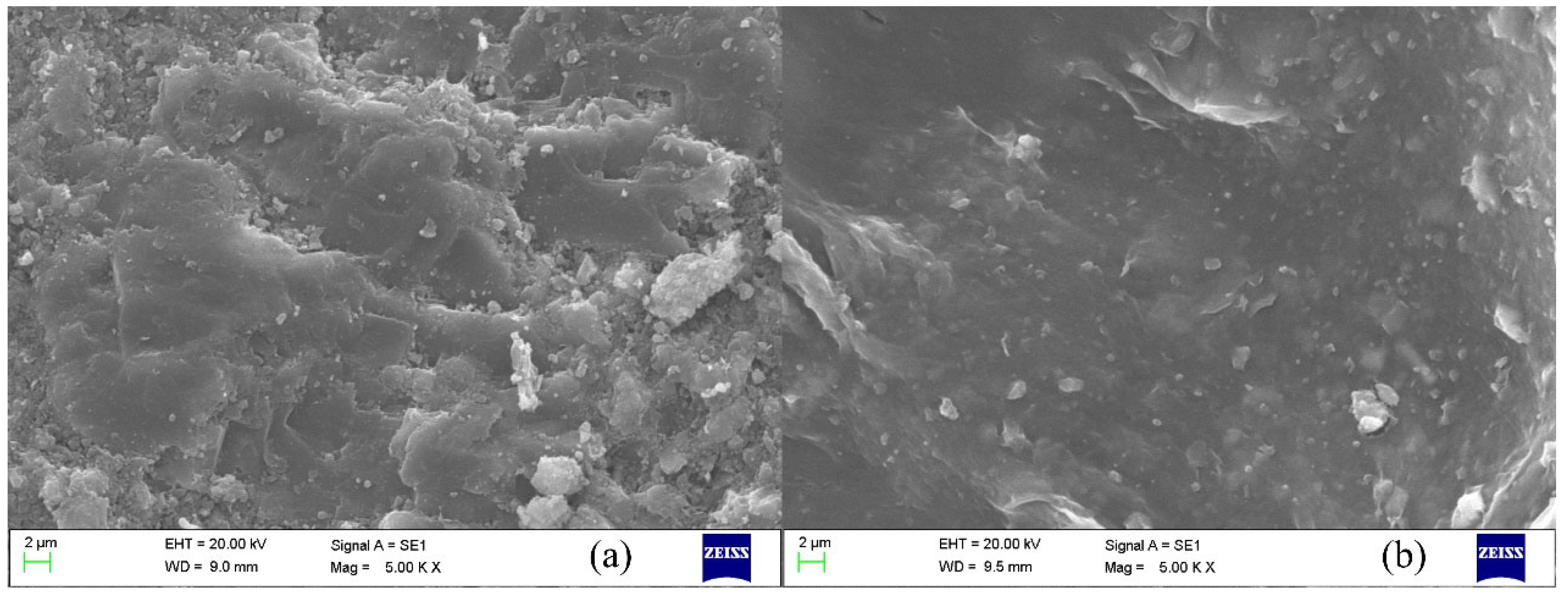
2.4.1. Micromorphology Analysis
2.4.2. Mechanistic Study of Thermal Decomposition
3. Materials and Methods
3.1. Materials
3.2. Preparation of Si-AP
3.3. Structural Characterization and Mechanism Analysis Methods of Si-AP
3.4. Methods
3.4.1. Preparation of Base Mud
3.4.2. Rheological Properties Tests
3.4.3. API Filtration Tests
3.4.4. Lubrication Performance Tests
3.4.5. Inhibition Performance Tests
3.4.6. Environmental Performance Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| AR | Analytical purity |
| WBDFs | Water-based drilling fluids |
| FTIR | Fourier-transform infrared spectroscopy |
| AP | Auricularia polysaccharide |
| Si-AP | Organosiloxane-modified Auricularia polysaccharide |
| PPM | Part per million |
| Na-MT | Sodium bentonite |
| AV | Apparent viscosity |
| PV | Plastic viscosity |
| YP | Yield point |
| YP/PV | Ratio yield point to plastic viscosity |
| EC50 | Median effect concentration |
| BOD5 | Biochemical oxygen demand at 5 days |
| COD | Chemical oxygen demand |
| LC50 | Lethal concentration 50% |
References
- Deng, C.; Du, G.; Li, X.; Xi, J.; Lin, C. Effect of High Temperature and High Pressure on the Biodegradability and Biotoxicity of Typical Additives in Drilling Fluid. J. Petrol. Sci. Eng. 2022, 208, 109773. [Google Scholar] [CrossRef]
- Yang, J.; Sun, J.; Wang, R.; Liu, F.; Wang, J.; Qu, Y.; Wang, P.; Huang, H.; Liu, L.; Zhao, Z. Laponite-Polymer Composite as a Rheology Modifier and Filtration Loss Reducer for Water-Based Drilling Fluids at High Temperature. Colloids Surf. A 2022, 655, 130261. [Google Scholar] [CrossRef]
- Kontorovich, A.E.; Epov, M.I.; Eder, L.V. Long-Term and Medium-Term Scenarios and Factors in World Energy Perspectives for the 21st Century. Russ. Geol. Geophys. 2014, 55, 534–543. [Google Scholar] [CrossRef]
- Gautam, S.; Guria, C.; Rajak, V.K. A State of the Art Review on the Performance of High-Pressure and High-Temperature Drilling Fluids: Towards Understanding the Structure-Property Relationship of Drilling Fluid Additives. J. Petrol. Sci. Eng. 2022, 213, 110318. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, X.; Ji, C.; Zhan, Q.; Li, Z.; Guan, J.; Huang, J. Modification Method of High-Efficiency Organic Bentonite for Drilling Fluids: A Review. Molecules 2023, 28, 7866. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiu, Z.; Zhong, H.; Zhao, X.; Huang, W.; Chen, G. Effects of Water-Based Drilling Fluid on Properties of Mudcake and Wellbore Stability in Fractured Formations. J. Pet. Sci. Eng. 2021, 208, 109704. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Olayiwola, T.; Elkatatny, S.; Haq, B.; Patil, S. Insights into the Application of Surfactants and Nanomaterials as Shale Inhibitors for Water-Based Drilling Fluid: A Review. J. Nat. Gas Sci. Eng. 2021, 92, 103987. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Shan, W.; Wang, W.; Zhang, J.; Chen, G. Synthesis, Performance and Inhibition Mechanism of Modified Peanut Shell Nanocellulose as Shale Hydration Inhibitor. Polym. Bull. 2023, 80, 263–277. [Google Scholar] [CrossRef]
- Su, J.; Liu, M.; Lin, L.; Pu, X.; Ge, C.; Zhang, T.; Liu, G. Sulfonated Lignin Modified with Silane Coupling Agent as Biodegradable Shale Inhibitor in Water-Based Drilling Fluid. J. Petrol. Sci. Eng. 2022, 208, 109618. [Google Scholar] [CrossRef]
- Kong, X.; Chen, M.; Zhang, C.; Liu, Z.; Jin, Y.; Wang, X.; Liu, M.; Li, S. Optimization of High Temperature-Resistant Modified Starch Polyamine Anti-Collapse Water-Based Drilling Fluid System for Deep Shale Reservoir. Molecules 2022, 27, 8936. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, X.; Chen, B.; Egwu, S.B.; Huang, Z. Polyanionic Cellulose/Hydrophilic Monomer Copolymer Grafted Silica Nanocomposites as HTHP Drilling Fluid-Loss Control Agent for Water-Based Drilling Fluids. Appl. Surf. Sci. 2022, 578, 152089. [Google Scholar] [CrossRef]
- Djouonkep, L.D.W.; Tamo, A.K.; Doench, I.; Selabi, N.B.S.; Ilunga, E.M.; Lenwoue, A.R.K.; Gauthier, M.; Cheng, Z.; Osorio-Madrazo, A. Synthesis of High Performance Thiophene–Aromatic Polyesters from Bio-Sourced Organic Acids and Polysaccharide-Derived Diol: Characterization and Degradability Studies. Molecules 2022, 27, 325. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.-F.; Zhang, Q.; Cheng, C.; Zhang, J.; Chen, G. Sulfonation and Application of a Polysaccharide as High Inhibitive Drilling Fluid Additive. DEStech Trans. Eng. Technol. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, G.; Xia, Y. Technical Progress on Environmental-Friendly, High-Performance Water-Based Drilling Fluids. Environ. Earth Sci. Res. J. 2020, 7, 121. [Google Scholar] [CrossRef]
- Cescon, L.D.S.; Quartarone, P.; Ribeiro, S.P.D.S.; Nascimento, R.S.V. Cationic Starch Derivatives as Reactive Shale Inhibitors for Water-Based Drilling Fluids: Research Article. J. Appl. Polym. Sci. 2018, 135, 46621. [Google Scholar] [CrossRef]
- Luo, T.; Li, J.; Xu, J.; Wang, J.; Zhang, L.; Yu, Z. The Effects of Organically Modified Lithium Magnesium Silicate on the Rheological Properties of Water-Based Drilling Fluids. Materials 2024, 17, 1564. [Google Scholar] [CrossRef]
- Rana, A.; Arfaj, M.K.; Saleh, T.A. Advanced Developments in Shale Inhibitors for Oil Production with Low Environmental Footprints—A Review. Fuel 2019, 247, 237–249. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Kamal, M.S.; Al-Harthi, M. Polymeric and Low Molecular Weight Shale Inhibitors: A Review. Fuel 2019, 251, 187–217. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.; Zhang, W.; Wang, Y.; Ai, E.; Liu, Z.; Wei, L.; Li, Q. Synthesis of an Eco-Friendly Xylooligosaccharides and Its Mechanistic Evaluation in Water-Based Drilling Fluids. Sustainability 2023, 15, 15993. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, L.; Davletshin, A.; Li, Z.; You, J.; Tan, S. Application of Polysaccharide Biopolymer in Petroleum Recovery. Polymers 2020, 12, 1860. [Google Scholar] [CrossRef]
- Lv, K.; Shen, H.; Sun, J.; Huang, X.; Du, H. Acylated Inulin as a Potential Shale Hydration Inhibitor in Water Based Drilling Fluids for Wellbore Stabilization. Molecules 2024, 29, 1456. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-C.; Liu, X.; Lv, K.; Sun, J.; Dai, C.; Liao, B.; Liu, C.; Mei, C.; Wu, Q.; Hubbe, M. Cellulose Nanomaterials in Oil and Gas Industry: Current Status and Future Perspectives. Prog. Mater Sci. 2023, 139, 101187. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Tan, X.; An, Y.; Liu, H.; Gao, K.; Guo, M. Application of Hybrid Silicate as a Film-Forming Agent in High-Temperature Water-Based Drilling Fluids. ACS Omega 2021, 6, 20577–20589. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, J.; Jin, J.; Lv, K.; Li, H.; Rong, K.; Zhang, C.; Meng, X. Use of Silicone Quaternary Ammonium Salt for Hydrophobic Surface Modification to Inhibit Shale Hydration and Mechanism Study. J. Mol. Liq. 2021, 341, 117369. [Google Scholar] [CrossRef]
- Chu, Q.; Lin, L.; Su, J. Amidocyanogen Silanol as a High-Temperature-Resistant Shale Inhibitor in Water-Based Drilling Fluid. Appl. Clay Sci. 2020, 184, 105396. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, H.; Yuan, S.; Chen, Z. Film Morphology and Orientation of Amino Silicone Adsorbed onto Cellulose Substrate. Appl. Surf. Sci. 2009, 255, 8435–8442. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, J.; Li, Q.; Lv, K.; Wang, J.; Wang, Z. Mechanism of Organosilicate Polymer as High-Temperature Resistant Inhibitor in Water-Based Drilling Fluids. Colloids Surf. A 2022, 641, 128489. [Google Scholar] [CrossRef]
- Elam, J.H.; Nygren, H.; Stenberg, M. Covalent Coupling of Polysaccharides to Silicon and Silicon Rubber Surfaces. J. Biomed. Mater. Res. 2018, 18, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Q.; Chen, G.; Zhao, J.R.; Xu, R.J. Cationization and Application of Polysaccharide as High Inhibitive Drilling Fluid Additive. Appl. Mech. Mater. 2014, 488–489, 795–798. [Google Scholar] [CrossRef]
- Zheng, N.; Xu, Y.; Zhao, Q.; Xie, T. Dynamic Covalent Polymer Networks: A Molecular Platform for Designing Functions beyond Chemical Recycling and Self-Healing. Chem. Rev. 2021, 121, 1716–1745. [Google Scholar] [CrossRef]
- Medved, I.; Gaurina-Međimurec, N.; Novak Mavar, K.; Mijić, P. Waste Mandarin Peel as an Eco-Friendly Water-Based Drilling Fluid Additive. Energies 2022, 15, 2591. [Google Scholar] [CrossRef]
- Quan, X.; Jiang, G.; Luo, X.; He, Y.; Dong, T. Research and Application of New Technology of Bionic Enhanced Wellbore and Strong Lubrication Water-Based Drilling Fluid. Sustainability 2020, 12, 8387. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, F.; Lv, K.; Chang, X. A Novel Film-Forming Silicone Polymer as Shale Inhibitor for Water-Based Drilling Fluids. e-Polymers 2019, 19, 574–578. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, J.; Dai, Z.; Chang, X.; Huang, X.; Liu, J.; Wang, Z.; Lv, K. Organosilicate Polymer as High Temperature Resistent Inhibitor for Water-Based Drilling Fluids. J. Polym. Res. 2020, 27, 107. [Google Scholar] [CrossRef]
- API Specification 13A; Specification for Drilling Fluid Materials; American Petroleum Institute: Washington, DC, USA, 1993.
- Medved, I.; Gaurina-Međimurec, N.; Pašić, B.; Mijić, P. Green Approach in Water-Based Drilling Mud Design to Increase Wellbore Stability. Appl. Sci. 2022, 12, 5348. [Google Scholar] [CrossRef]
- Pašić, B.; Gaurina-Međimurec, N.; Mijić, P.; Medved, I. Experimental Research of Shale Pellet Swelling in Nano-Based Drilling Muds. Energies 2020, 13, 6246. [Google Scholar] [CrossRef]
- Swigart, J.; Heo, J.; Wolf, D. Soil Contamination Assessments from Drilling Fluids and Produced Water Using Combined Field and Laboratory Investigations: A Case Study of Arkansas, USA. Int. J. Environ. Res. Public Health 2021, 18, 2421. [Google Scholar] [CrossRef]
- Shadfar, D.; Mohammed, A.; David, A.W.; Valeriy, S.R.; Konstantin, M.M. Synthetic polymers: A review of applications in drilling fluids. Pet. Sci. 2024, 21, 475–518. [Google Scholar] [CrossRef]
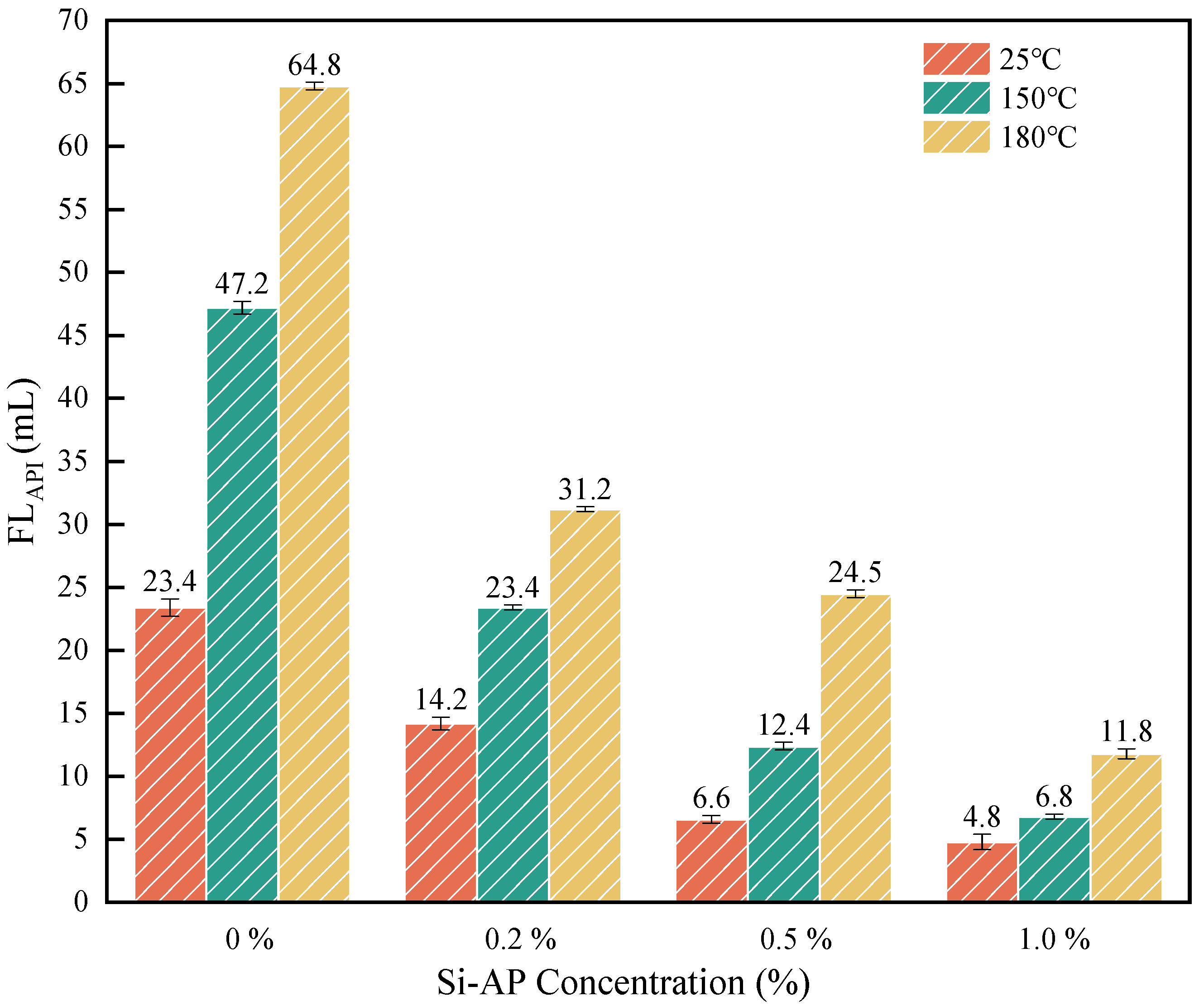

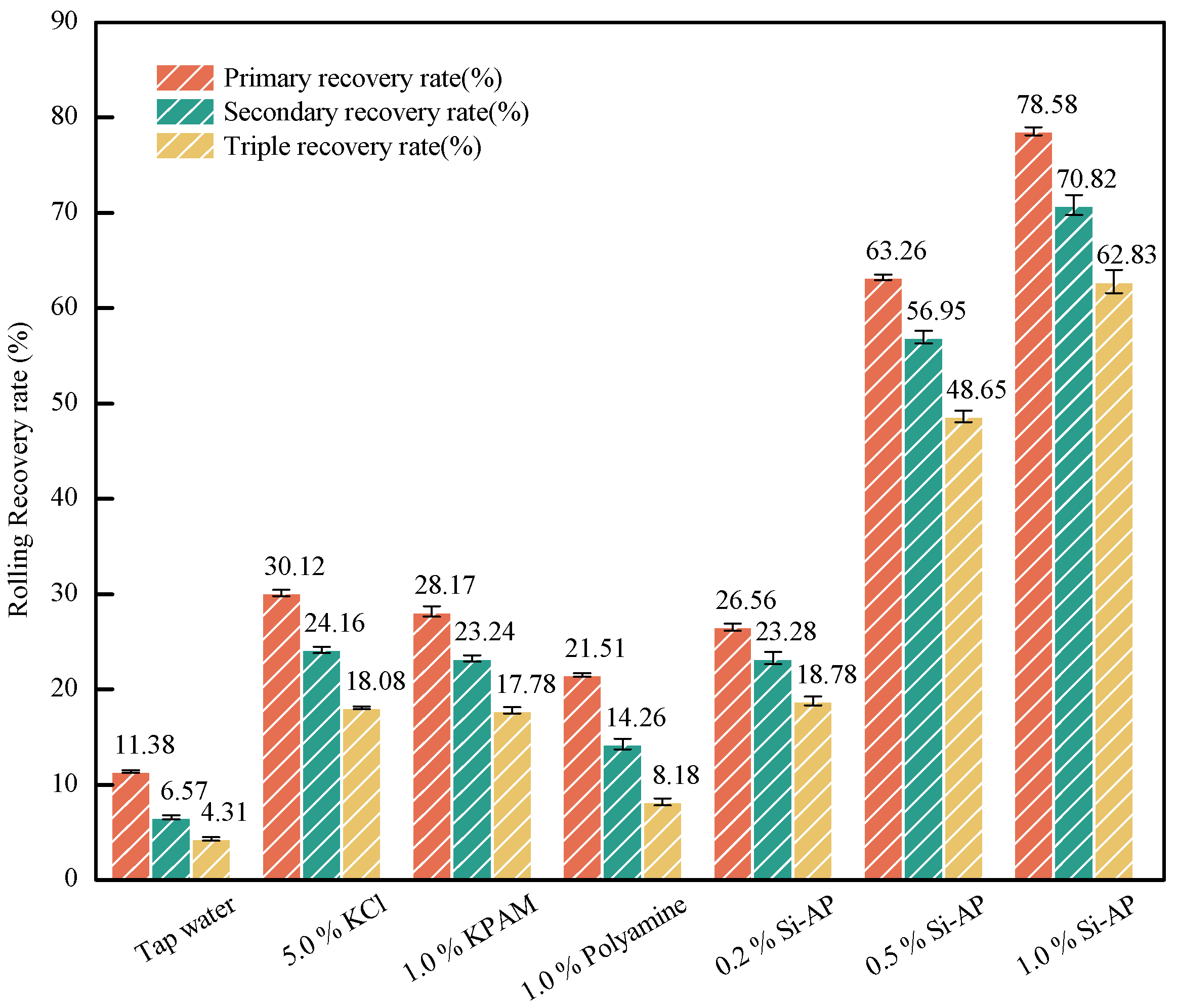
| Mn (Daltons) | Mw (Daltons) | MP (Daltons) | Mz (Daltons) | Mz + 1 (Daltons) | Polydispersity | Mz/Mw | Mz + 1/Mw |
|---|---|---|---|---|---|---|---|
| 40,966 | 106,619 | 79,995 | 226,530 | 382,090 | 2.602604 | 2.124668 | 3.583697 |
| Si-AP | AV (mPa·s) | PV (mPa·s) | YP (mPa·s) | YP/PV | Mud Cake Thickness (mm) |
|---|---|---|---|---|---|
| 0% | 7.5 | 3 | 4.5 | 1.5 | 2.5 |
| 0.2% | 14.2 | 9 | 5.2 | 0.57 | 2.2 |
| 0.5% | 22 | 13 | 9 | 0.69 | 1.9 |
| 1.0% | 42.5 | 24.5 | 18 | 0.73 | 1.8 |
| 0% (180 °C) | 10 | 9 | 1 | 0.11 | 2.8 |
| 1.0% (180 °C) | 35.2 | 22 | 13.2 | 0.6 | 2.0 |
| Formula | Friction Coefficient | Reduction Rate of Friction Coefficient/% |
|---|---|---|
| Tap water | 0.3500 | / |
| 4.0% Base mud | 0.5412 | / |
| 4.0% Base mud + 0.2% Si-AP | 0.1980 | 63.41% |
| 4.0% Base mud + 0.5% Si-AP | 0.1579 | 70.82% |
| 4.0% Base mud + 1.0% Si-AP | 0.1276 | 76.42% |
| Aging Temperature/°C | Formula | Friction Coefficient | Reduction Rate of Friction Coefficient/% |
|---|---|---|---|
| 25 | 4.0% base mud | 0.5412 | 76.42 |
| 4.0% base mud + 1.0% Si-AP | 0.1276 | ||
| 150 | 4.0% base mud | 0.5853 | 69.83 |
| 4.0% base mud + 1.0% Si-AP | 0.1766 | ||
| 180 | 4.0% base mud | 0.6294 | 67.56 |
| 4.0% base mud + 1.0% Si-AP | 0.2042 | ||
| 200 | 4.0% base mud | 0.6536 | 51.59 |
| 4.0% base mud + 1.0% Si-AP | 0.3164 |
| Items | Measured Value | Reference Range |
|---|---|---|
| EC50 (mg/L) | 38,000 | ≥30,000 |
| COD (mg/L) | 63 | 60–100 |
| BOD5 (mg/L) | 18.2 | ≤20 |
| BOD5:COD (%) | 28.89 | ≥10 |
| LC50 (mg/L) | 46,000 | ≥30,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Wang, Y.; Wang, B.; Geng, Y.; Chang, X.; Zhang, W.; Li, Y.; Zhang, W. Organosiloxane-Modified Auricularia Polysaccharide (Si-AP): Improved High-Temperature Resistance and Lubrication Performance in WBDFs. Molecules 2024, 29, 2689. https://doi.org/10.3390/molecules29112689
Zhang F, Wang Y, Wang B, Geng Y, Chang X, Zhang W, Li Y, Zhang W. Organosiloxane-Modified Auricularia Polysaccharide (Si-AP): Improved High-Temperature Resistance and Lubrication Performance in WBDFs. Molecules. 2024; 29(11):2689. https://doi.org/10.3390/molecules29112689
Chicago/Turabian StyleZhang, Fan, Yu Wang, Bo Wang, Yuan Geng, Xiaofeng Chang, Wenzhe Zhang, Yutong Li, and Wangyuan Zhang. 2024. "Organosiloxane-Modified Auricularia Polysaccharide (Si-AP): Improved High-Temperature Resistance and Lubrication Performance in WBDFs" Molecules 29, no. 11: 2689. https://doi.org/10.3390/molecules29112689
APA StyleZhang, F., Wang, Y., Wang, B., Geng, Y., Chang, X., Zhang, W., Li, Y., & Zhang, W. (2024). Organosiloxane-Modified Auricularia Polysaccharide (Si-AP): Improved High-Temperature Resistance and Lubrication Performance in WBDFs. Molecules, 29(11), 2689. https://doi.org/10.3390/molecules29112689






