The Role of Natural Products from Herbal Medicine in TLR4 Signaling for Colorectal Cancer Treatment
Abstract
1. Introduction
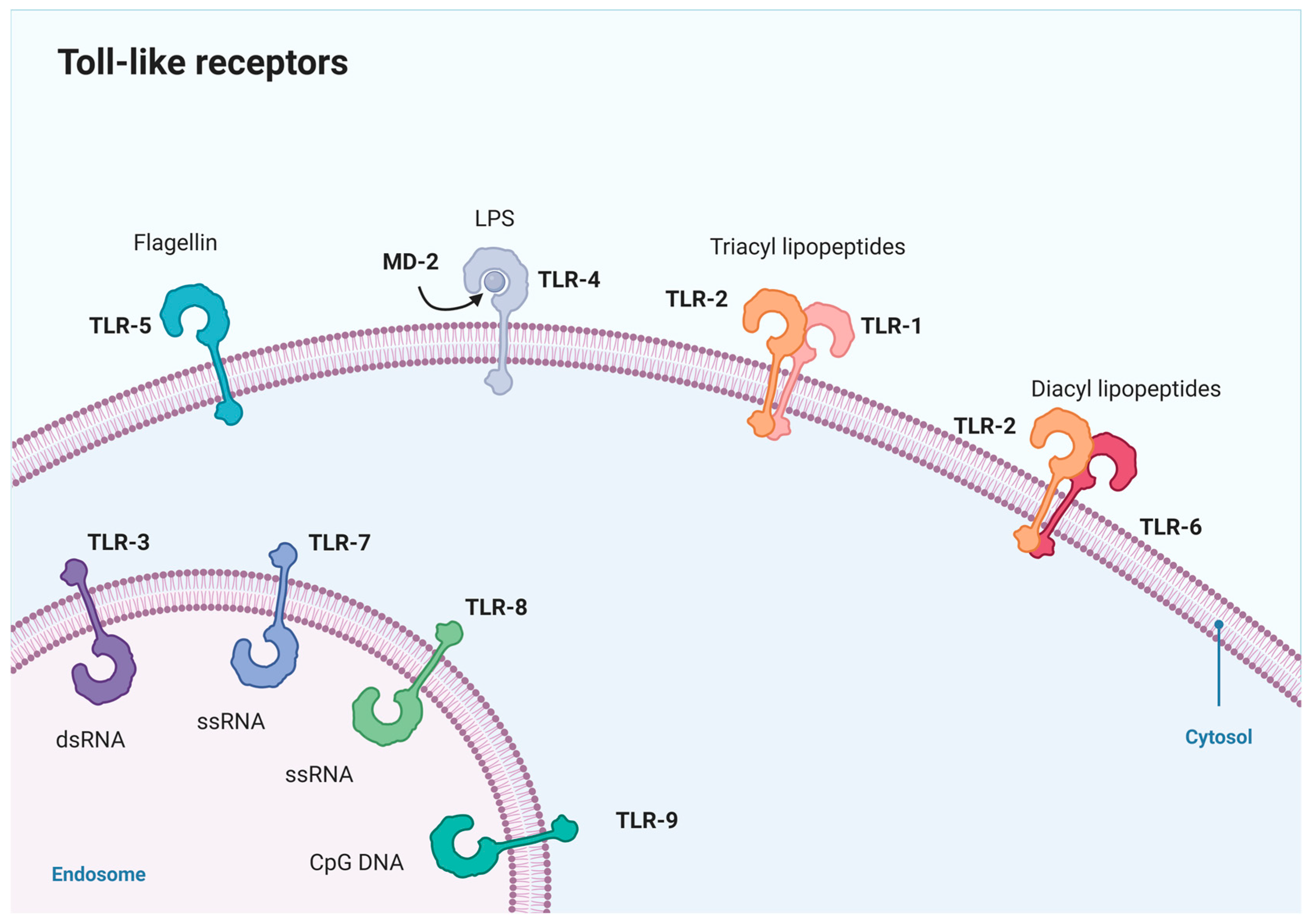
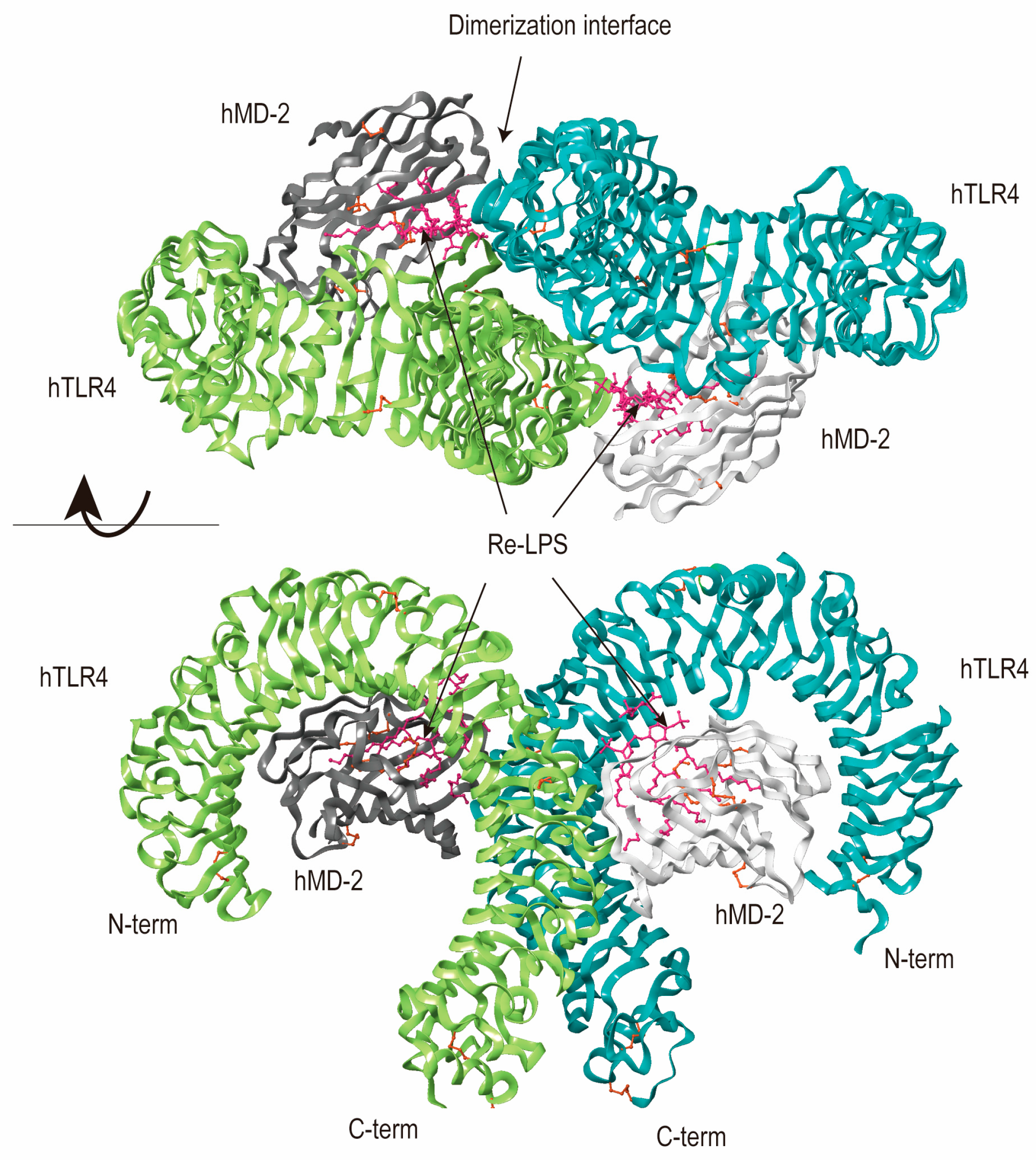
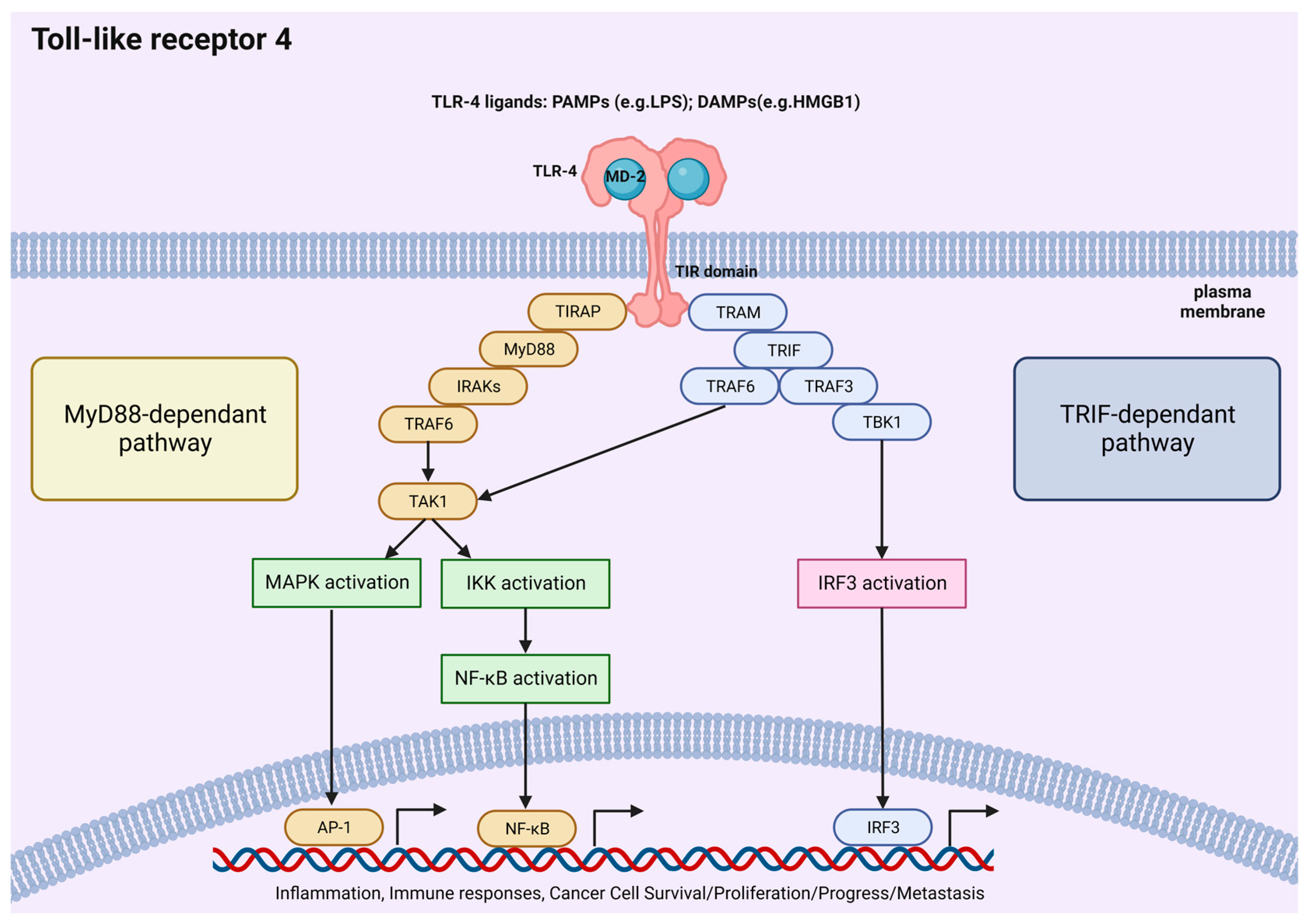
2. TLR4 Pathway
3. TLR4 Pathway-Related Biochemical Processes
3.1. Cancer Cell Proliferation
3.2. Apoptosis
3.3. Metabolism
3.4. Inflammation and Immunization
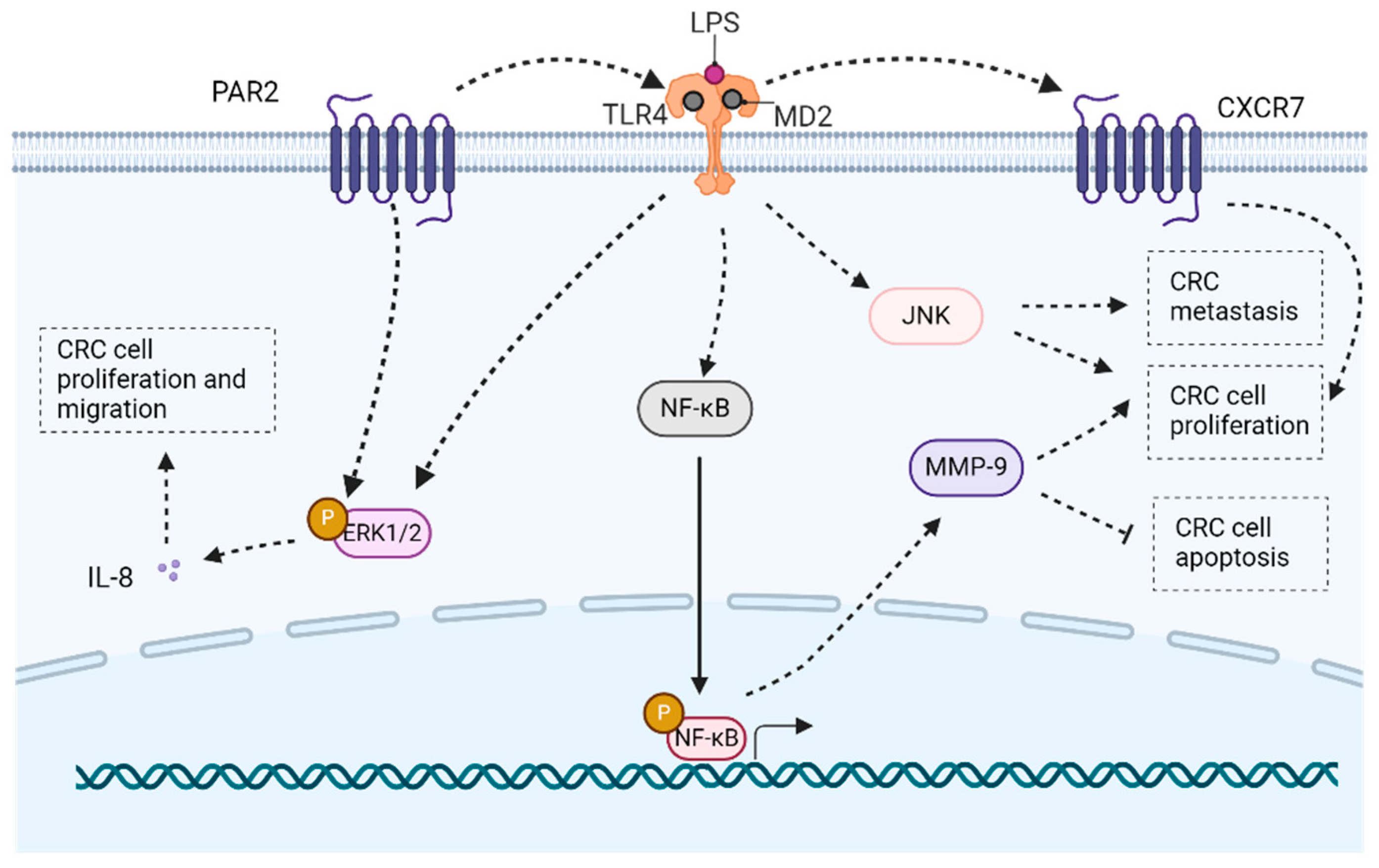
3.5. Tumor Microenvironment
3.6. Drug Resistance
3.7. EMT/Migration/Invasion/Metastasis
4. TCMs with Anti-CRC Effects through TLR4 Signaling Pathway
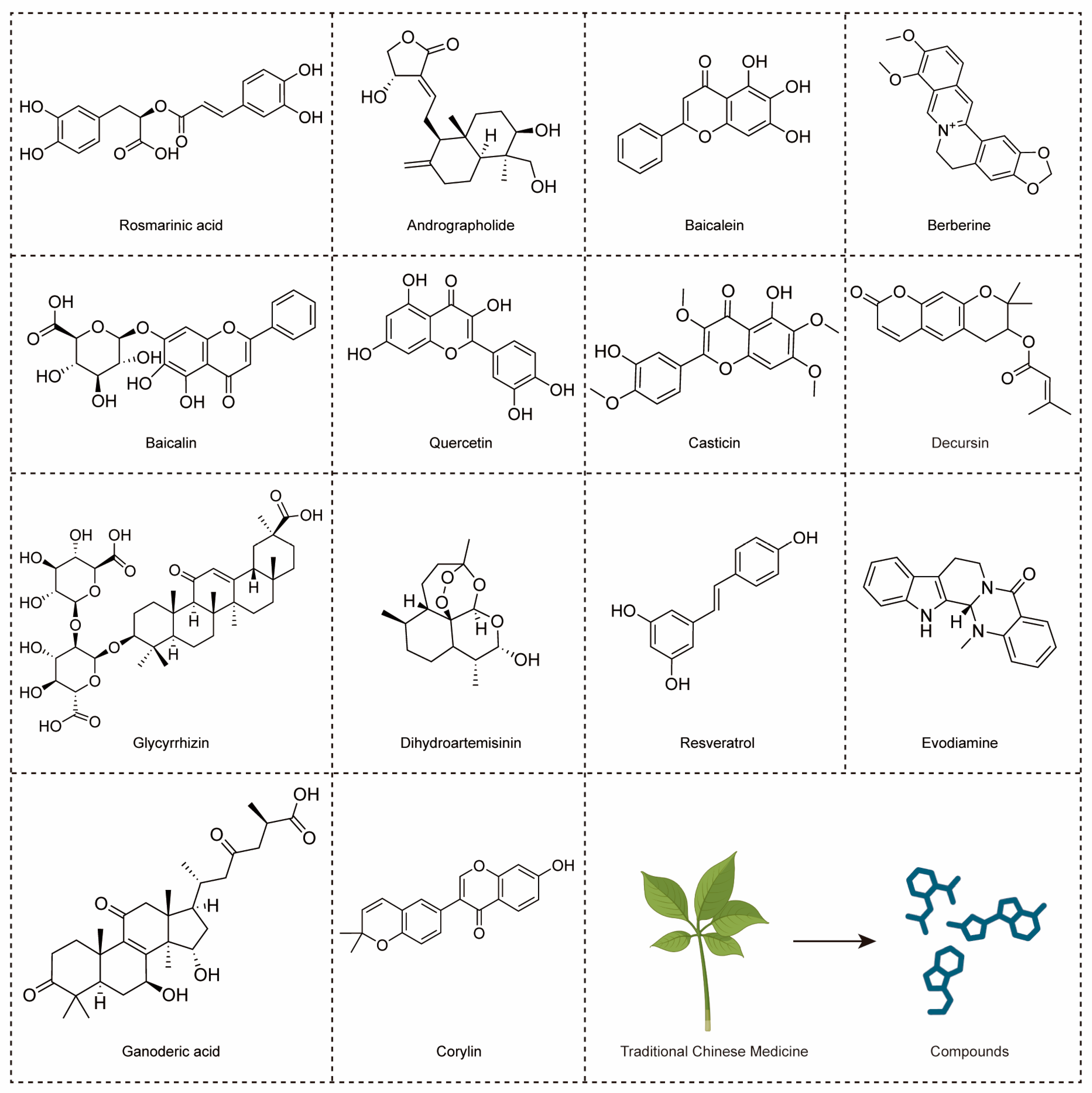
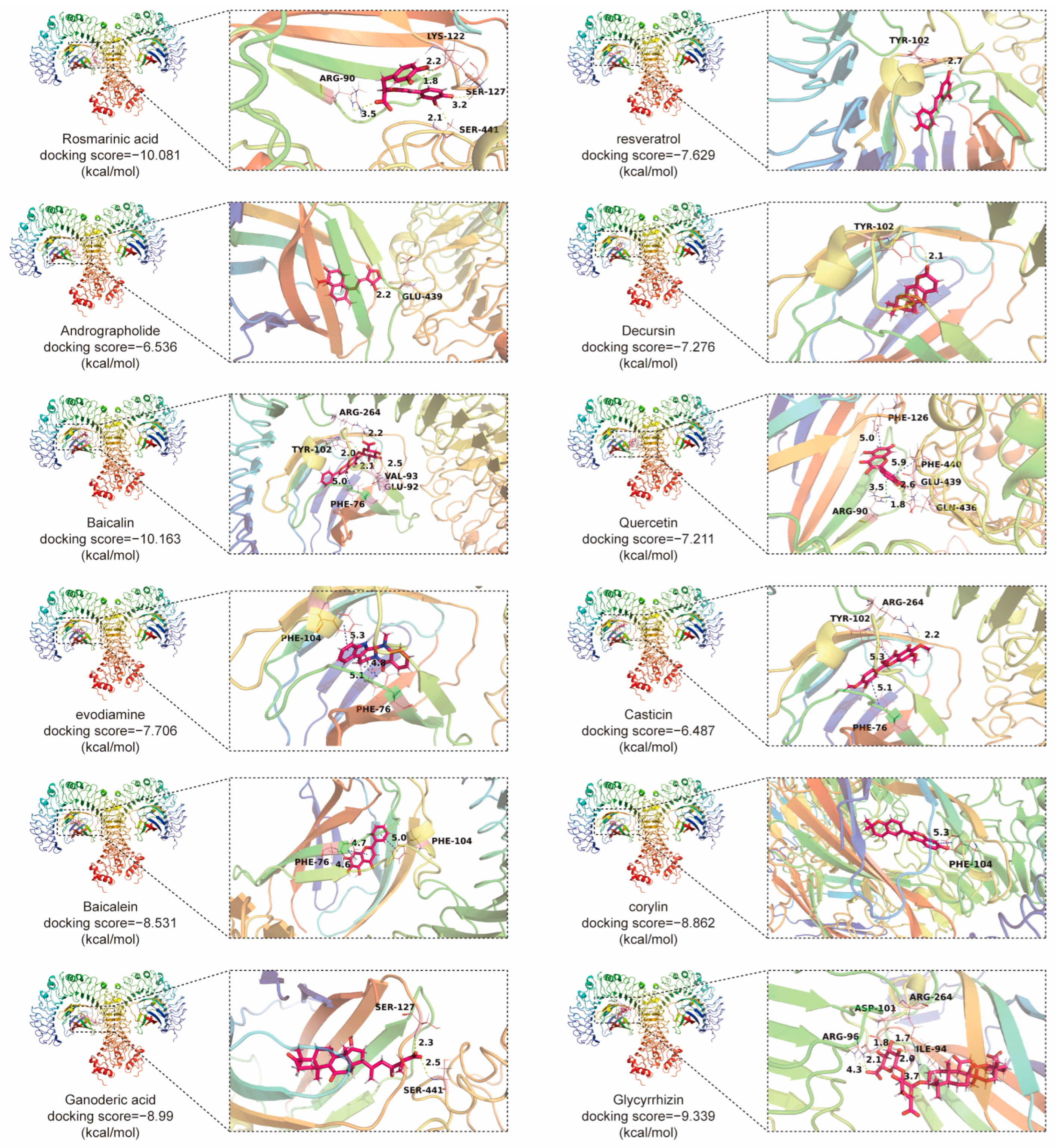
4.1. The Compounds Derived from TCMs with Anti-CRC Effects through TLR4 Signaling Pathway (Table 1)
| Natural Compounds | Sources | Concentration/Dosage | Major Effects | Involved Pathways | Ref. |
|---|---|---|---|---|---|
| Andrographolide | Andrographis paniculata | in vitro: 20 µM | Inhibiting proliferation and inducing apoptosis of SW620 cells. | TLR4/NF-κB/MMP-9 pathway. | [22] |
| Resveratrol | Polygonum multiflorum | in vitro: 30, 40, 50 mM | Reducing LPS-induced inflammatory responses of Caco-2 and SW480 cell. | / | [23] |
| Baicalein | Scutellaria baicalensis | in vitro: 7.5, 15 µM; 3.125, 6.25, 12.5, 25 µM; in vivo: 10, 20 mg/kg; | Inhibiting proliferation, migration and angiogenesis in CRC. | TLR4/HIF-1α/VEGF pathway. | [25] |
| Baicalin | Scutellaria baicalensis | in vitro: 5–80 μg/mL; 0, 5, 10, 20 μM; in vivo: 20, 40 mg/kg; | Triggering apoptosis and anti-tumor immunity, and inhibiting migration in CRC. | TLR4/NF-κB pathway. | [26] |
| Berberine | Coptis chinensis | in vivo: 50, 100 mg/kg | Regulating short-chain fatty acid metabolism and alleviating the CAC. | TLR4/p-NF-κB p65/IL-6/p-STAT3 pathway. | [24] |
| Casticin | Vitex trifolia | in vitro: 10–100 μM; 40 μM | Inducing G2/M-phase arrest and apoptotic, increasing ROS production and decreasing mitochondria membrane potential and Ca2+ of colo 205 cells. | / | [134] |
| Decursin | Angelica sinensis | in vitro: 10 μM | Inhibiting inflammation and metastasis in CRC. | / | [27] |
| Ganoderic acid | Ganoderma lucidum | in vivo: 50 mg/kg | Alleviating chemotherapy-induced fatigue in CRC. | TLR4/Myd88/NF-κB pathway. | [135] |
| Glycyrrhizin | Glycyrrhiza uralensis | in vivo: 15 mg/kg | Inhibiting the inflammation in CRC. | HMGB1/TLR4/NF-κB pathway. | [73] |
| Quercetin | Ginkgo biloba | in vitro: 300, 600 µM | Decreasing chemoresistance in CRC. | TLR4/NLRP3 and ERK/NLRP3 pathway. | [110] |
| Evodiamine | Evodia rutaecarpa | in vitro: 100, 200 µM; in vivo: 10 mg/kg | Inhibiting inflammation in CRC; Inducing G2/M-phase arrest of SW480 cells. | / | [136] |
| Corylin | Psoralea corylifolia | in vivo: 25, 100 mg/kg | Inhibiting inflammation, proliferation of CSCs and colon epithelial cell, improving microbial diversity and community richness, regulating macrophage polarization in CRC. | TLR4/p38/AP-1 pathway. | [137] |
| Rosmarinic acid | Perilla frutescens | in vitro: 25, 50 μM; in vivo: 30 mg/kg | Inhibiting inflammation of CRC. | TLR4/NF-κB/STAT3 pathway. | [100] |
| Dihydroartemisinin | Artemisia annua | in vitro: 1–40 μM; in vivo: 10 mg/kg | Inhibiting inflammation of CRC; Improving cell cycle inhibition and apoptosis in CRC cells. | TLR4 signaling pathway. | [138] |
4.2. Formulas of TCMs with Anti-CRC Effects through TLR4 Signaling Pathway
4.3. The Synergistic Effects of TCM Production with Conventional CRC Therapies
4.3.1. Enhanced Efficacy
4.3.2. Reduced Side Effects
4.3.3. Inhibition of Chemoresistance
4.3.4. Reducing Toxicity
4.4. The Safety/Toxicity of These TCM Compounds
| Natural Compounds | Toxicity or Side Effects | Potential Side Effects in VigiBase | Ref. |
|---|---|---|---|
| Andrographolide | Reproductive toxicity, nephrotoxicity, taste disturbance, headache, fatigue, and diarrhea, anaphylactic reaction | Gastrointestinal disorders, general disorders and administration site conditions | [184] |
| Resveratrol | Reproductive toxicity, cardiac toxicity, nephrotoxicity, increased hepatotoxicity, risk of bleeding and anaphylactic reaction | Eye disorders, gastrointestinal disorders, metabolism and nutrition disorders, etc. | [185] |
| Baicalein | Elevated levels of C-reactive protein and triglycerides, elevated levels of alanine aminotransferase and aspartate aminotransferase, proteinuria and abdominal pain, constipation | / | [186] |
| Baicalin | / | / | / |
| Berberine | Diarrhea and constipation, exacerbation of jaundice in neonates with glucose-6-phosphate dehydrogenase deficiency and uterine stimulation | Blood and lymphatic system disorder, ear and labyrinth disorders and eye disorders, etc. | [187] |
| Casticin | / | / | / |
| Decursin | / | / | / |
| Ganoderic acid | / | / | / |
| Glycyrrhizin | / | Blood and lymphatic system disorders, cardiac disorders and ear and labyrinth disorders, etc. | / |
| Quercetin | / | Blood and lymphatic system disorders, cardiac disorders and ear and labyrinth disorders, etc. | / |
| Evodiamine | / | / | / |
| Corylin | / | / | / |
| Rosmarinic acid | / | / | / |
| Dihydroartemisinin | Impairment of oocyte maturation | Ear and labyrinth disorders, eye disorders and gastrointestinal disorders, etc. | [189] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACAT1 | Acyl coenzyme A cholesterol acyltransferase 1 |
| ADAM | A disintegrin and metalloproteinase |
| AKT | Protein kinase B |
| AOM | Azoxymethane |
| CAC | Colitis-associated colon cancer |
| COX | Cyclooxygenase |
| CRC | Colorectal cancer |
| CSCs | Cancer stem cells |
| CXCR7 | CXC chemokine receptor 7 |
| DAMPs | Damage-associated molecular patterns |
| DCs | Dendritic cells |
| DSS | Dextran sodium sulfate |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial–mesenchymal transition |
| ERK | Extracellular regulated protein kinases |
| Gal-1 | Galectin-1 |
| GSK-3β | Glycogen synthase kinase-3β |
| HMGB1 | High-mobility group box 1 |
| HSP110 | Heat shock protein-110 |
| IBD | Inflammatory bowel disease |
| IECs | Intestinal epithelial cells |
| IL | Interleukin |
| IRAKs | IL-1 receptor-associated kinases |
| IRF | Interferon Regulatory Factor |
| JNK | c-Jun N-terminal |
| LPS | Lipopolysaccharide |
| MANs | Monophosphoryl lipid A- assembled nanovaccines |
| MAPK | Mitogen-associated protein kinase |
| MD2 | Myeloid differentiation protein-2 |
| MDSCs | Myeloid-derived suppressor cells |
| MIF | Macrophage inhibitory factor |
| MyD88 | Myeloid differentiation primary response gene 88 |
| NF-κB | Nuclear factor kappa-B |
| NK | Natural killer |
| PAR2 | Protease-activated receptor 2 |
| PGE-2 | Prostaglandin E2 |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| ROS | Reactive oxygen species |
| STAT3 | Signal transducers and activators of transcription 3 |
| TAMs | Tumor-associated macrophages |
| TAK1 | Transforming growth factor-βactivated protein kinase 1 |
| TAp63 | Transcriptionally active p63 |
| TCMs | Traditional Chinese medicines |
| THBS2 | Thrombospondin 2 |
| TILs | Tumor-infiltrating lymphocytes |
| TIPE2 | The tumor necrosis factor (TNF)-α-induced protein 8-like-2 |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| TOPORS | TOP1-binding arginine/serine-rich protein |
| TRAF6 | Tumor necrosis factor receptor-associated factor 6 |
| TRAM | TRIF-related adaptor molecule |
| TIRAP | Toll/interleukin-1-receptor-domain-containing adaptor protein |
| TRIF | Toll/interleukin-1-receptordomain-containing adaptor inducing interferon-β |
| VEGF | Vascular endothelial growth factor |
References
- Tomii, A.; Higa, M.; Naito, K.; Kurata, K.; Kobayashi, J.; Takei, C.; Yuasa, K.; Koto, Y.; Shimizu, H. Activation of the TLR4-JNK but not the TLR4-ERK pathway induced by indole-3-acetic acid exerts anti-proliferative effects on Caco-2 cells. Biosci. Biotechnol. Biochem. 2023, 87, 839–849. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Yde, J.; Larsen, H.M.; Laurberg, S.; Krogh, K.; Moeller, H.B. Chronic diarrhoea following surgery for colon cancer-frequency, causes and treatment options. Int. J. Color. Dis. 2018, 33, 683–694. [Google Scholar] [CrossRef]
- Yesudhas, D.; Gosu, V.; Anwar, M.A.; Choi, S. Multiple roles of toll-like receptor 4 in colorectal cancer. Front. Immunol. 2014, 5, 334. [Google Scholar] [CrossRef]
- Lu, L.; Dai, M.; Mullins, C.S.; Schafmayer, C.; Linnebacher, M. Global Association of Cause-specific Mortality between the Major Gastrointestinal Cancers and Parkinson’s Disease for the First Two Decades of the New Millennium. Aging Dis. 2022, 13, 534–539. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fedewa, S.A.; Ahnen, D.J.; Meester, R.G.S.; Barzi, A.; Jemal, A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 177–193. [Google Scholar] [CrossRef]
- Ni, Q.; Li, M.; Yu, S. Research Progress of Epithelial-mesenchymal Transition Treatment and Drug Resistance in Colorectal Cancer. Technol. Cancer Res. Treat. 2022, 21, 15330338221081219. [Google Scholar] [CrossRef]
- El-Shami, K.; Oeffinger, K.C.; Erb, N.L.; Willis, A.; Bretsch, J.K.; Pratt-Chapman, M.L.; Cannady, R.S.; Wong, S.L.; Rose, J.; Barbour, A.L.; et al. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J. Clin. 2015, 65, 428–455. [Google Scholar] [CrossRef]
- Hryniuk, A.; Grainger, S.; Savory, J.G.A.; Lohnes, D. Cdx1 and Cdx2 function as tumor suppressors. J. Biol. Chem. 2014, 289, 33343–33354. [Google Scholar] [CrossRef]
- So, E.Y.; Ouchi, T. The application of Toll like receptors for cancer therapy. Int. J. Biol. Sci. 2010, 6, 675–681. [Google Scholar] [CrossRef]
- Keogh, B.; Parker, A.E. Toll-like receptors as targets for immune disorders. Trends Pharmacol. Sci. 2011, 32, 435–442. [Google Scholar] [CrossRef]
- Frolova, L.; Drastich, P.; Rossmann, P.; Klimesova, K.; Tlaskalova-Hogenova, H. Expression of Toll-like receptor 2 (TLR2), TLR4, and CD14 in biopsy samples of patients with inflammatory bowel diseases: Upregulated expression of TLR2 in terminal ileum of patients with ulcerative colitis. J. Histochem. Cytochem. 2008, 56, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhou, H.; Ling, S.; Guo, D.; Yan, Y.; Zhou, F.; Wu, Y. Activation of PAR2 or/and TLR4 promotes SW620 cell proliferation and migration via phosphorylation of ERK1/2. Oncol. Rep. 2011, 25, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wu, Q.; Dang, S.; Jin, M.; Xu, J.; Cheng, Y.; Pan, M.; Wu, Y.; Zhang, C. Alteration of CXCR7 Expression Mediated by TLR4 Promotes Tumor Cell Proliferation and Migration in Human Colorectal Carcinoma. PLoS ONE 2011, 6, e27399. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Roy, D.; Pati, S.; Sa, G. The Adroitness of Andrographolide as a Natural Weapon Against Colorectal Cancer. Front. Pharmacol. 2021, 12, 731492. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Gui, Y.; Li, Q.; An, J.; Wang, D. The signaling pathways and targets of natural products from traditional Chinese medicine treating gastric cancer provide new candidate therapeutic strategies. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2023, 1878, 188998. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Vong, C.T.; Chen, F.; Tan, H.; Zhang, C.; Wang, N.; Cui, L.; Wang, Y.; Feng, Y. Immunomodulatory potential of natural products from herbal medicines as immune checkpoints inhibitors: Helping to fight against cancer via multiple targets. Med. Res. Rev. 2022, 42, 1246–1279. [Google Scholar] [CrossRef]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar]
- Chan, K.I.; Zhang, S.; Li, G.; Xu, Y.; Cui, L.; Wang, Y.; Su, H.; Tan, W.; Zhong, Z. MYC Oncogene: A Druggable Target for Treating Cancers with Natural Products. Aging Dis. 2024, 15, 640–697. [Google Scholar] [CrossRef]
- Kong, M.Y.; Li, L.Y.; Lou, Y.M.; Chi, H.Y.; Wu, J.J. Chinese herbal medicines for prevention and treatment of colorectal cancer: From molecular mechanisms to potential clinical applications. J. Integr. Med. 2020, 18, 369–384. [Google Scholar] [CrossRef]
- Chen, J.F.; Wu, S.W.; Shi, Z.M.; Hu, B. Traditional Chinese medicine for colorectal cancer treatment: Potential targets and mechanisms of action. Chin. Med. 2023, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhao, J.; Xu, J.; Jiao, D.X.; Wang, J.; Gong, Z.Q.; Jia, J.H. Andrographolide suppresses proliferation of human colon cancer SW620 cells through the TLR4/NF-κB/MMP-9 signaling pathway. Oncol. Lett. 2017, 14, 4305–4310. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Carofiglio, V.; Acquafredda, A.; Cavallo, P.; Cianciulli, A. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-κB activation in Caco-2 and SW480 human colon cancer cells. Br. J. Nutr. 2012, 108, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chang, J.; Hao, X.; Liu, J.; Tan, X.; Geng, Z.; Wang, Z. Berberine regulates short-chain fatty acid metabolism and alleviates the colitis-associated colorectal tumorigenesis through remodeling intestinal flora. Phytomedicine 2022, 102, 154217. [Google Scholar] [CrossRef]
- Chen, M.; Zhong, K.; Tan, J.; Meng, M.; Liu, C.M.; Chen, B.; Huang, C.; Wong, H.L.X.; Bian, Z.; Su, T.; et al. Baicalein is a novel TLR4-targeting therapeutics agent that inhibits TLR4/HIF-1α/VEGF signaling pathway in colorectal cancer. Clin. Transl. Med. 2021, 11, e564. [Google Scholar] [CrossRef]
- Song, L.; Zhu, S.; Liu, C.; Zhang, Q.; Liang, X. Baicalin triggers apoptosis, inhibits migration, and enhances anti-tumor immunity in colorectal cancer via TLR4/NF-κB signaling pathway. J. Food Biochem. 2022, 46, e13703. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Islam, S.U.; Lee, Y.S. Decursin negatively regulates LPS-induced upregulation of the TLR4 and JNK signaling stimulated by the expression of PRP4 in vitro. Anim. Cells Syst. 2020, 24, 44–52. [Google Scholar] [CrossRef]
- Miller, S.I.; Ernst, R.K.; Bader, M.W. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 2005, 3, 36–46. [Google Scholar] [CrossRef]
- Li, M.; Wen, J.; Huang, X.; Nie, Q.; Wu, X.; Ma, W.; Nie, S.; Xie, M. Interaction between polysaccharides and toll-like receptor 4: Primary structural role, immune balance perspective, and 3D interaction model hypothesis. Food Chem. 2022, 374, 131586. [Google Scholar] [CrossRef]
- Kim, B.S. Critical role of TLR activation in viral replication, persistence, and pathogenicity of Theiler’s virus. Front. Immunol. 2023, 14, 1167972. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Xu, X.Y.; Lin, W.; Hu, D.D.; Shi, W.; Jia, X.; Wang, H.; Song, N.J.; Zhang, Y.Q.; Zhang, L. Activation of Different Heterodimers of TLR2 Distinctly Mediates Pain and Itch. Neuroscience 2020, 429, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Triantafilou, M.; Uddin, A.; Maher, S.; Charalambous, N.; Hamm, T.S.C.; Alsumaiti, A.; Triantafilou, K. Anthrax toxin evades Toll-like receptor recognition, whereas its cell wall components trigger activation via TLR2/6 heterodimers. Cell Microbiol. 2007, 9, 2880–2892. [Google Scholar] [CrossRef] [PubMed]
- Triantafilou, M.; Gamper, F.G.J.; Haston, R.M.; Mouratis, M.A.; Morath, S.; Hartung, T.; Triantafilou, K. Membrane sorting of toll-like receptor (TLR)-2/6 and TLR2/1 heterodimers at the cell surface determines heterotypic associations with CD36 and intracellular targeting. J. Biol. Chem. 2006, 281, 31002–31011. [Google Scholar] [CrossRef] [PubMed]
- Blasius, A.L.; Beutler, B. Intracellular toll-like receptors. Immunity 2010, 32, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Tsan, M.F. Toll-like receptors, inflammation and cancer. Semin. Cancer Biol. 2006, 16, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, S.; Voulgarelis, M. Toll-Like Receptors, Tissue Injury, and Tumourigenesis. Mediat. Inflamm. 2010, 2010, 581837. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhu, Y. TLR4 Signaling Promotes Immune Escape of Human Colon Cancer Cells by Inducing Immunosuppressive Cytokines and Apoptosis Resistance. Oncol. Res. 2012, 20, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Wu, S.; Yan, C.; Zhao, C.; Jin, H.; Yan, N.; Xu, J.; Wu, Y.; Li, C.; Shao, Q.; et al. Butyrate upregulates the TLR4 expression and the phosphorylation of MAPKs and NK-κB in colon cancer cell in vitro. Oncol. Lett. 2018, 16, 4439–4447. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.P.; Huang, W.C.; Lin, T.J.; Chiu, C.C.; Wang, Y.C.; Chen, Y.H.; Hung, S.W.; Chuang, H.L.; Chen, T.H. Toll-like receptor 4 prevents AOM/DSS-induced colitis-associated colorectal cancer in Bacteroides fragilis gnotobiotic mice. Hum. Exp. Toxicol. 2021, 40, 622–633. [Google Scholar] [CrossRef]
- Matsunaga, N.; Tsuchimori, N.; Matsumoto, T.; Ii, M. TAK-242 (Resatorvid), a Small-Molecule Inhibitor of Toll-Like Receptor (TLR) 4 Signaling, Binds Selectively to TLR4 and Interferes with Interactions between TLR4 and Its Adaptor Molecules. Mol. Pharmacol. 2011, 79, 34–41. [Google Scholar] [CrossRef]
- Li, Y.; Teo, W.L.; Low, M.J.; Meijer, L.; Sanderson, I.; Pettersson, S.; Greicius, G. Constitutive TLR4 signalling in intestinal epithelium reduces tumor load by increasing apoptosis in APC(Min/+) mice. Oncogene 2014, 33, 369–377. [Google Scholar] [CrossRef]
- Ye, K.; Wu, Y.; Sun, Y.; Lin, J.; Xu, J. TLR4 siRNA inhibits proliferation and invasion in colorectal cancer cells by downregulating ACAT1 expression. Life Sci. 2016, 155, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Alvero, A.B.; Silasi, D.A.; Mor, G. Inflammation, cancer and chemoresistance: Taking advantage of the toll-like receptor signaling pathway. Am. J. Reprod. Immunol. 2007, 57, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hao, Y.; Pan, W.; Wang, W.; Min, Y. Monophosphoryl lipid A-assembled nanovaccines enhance tumor immunotherapy. Acta Biomater. 2023, 171, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, W.; Shi, X.; Li, X.; Wang, Y.; Hu, M.; Ma, F.; Tao, N.; Wang, G.; Qin, Z. An Asparagus polysaccharide fraction inhibits MDSCs by inducing apoptosis through toll-like receptor 4. Phytother. Res. 2018, 32, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Al-Obeed, O.; El-Obeid, A.S.; Matou-Nasri, S.; Vaali-Mohammed, M.A.; AlHaidan, Y.; Elwatidy, M.; Al Dosary, H.; Alehaideb, Z.; Alkhayal, K.; Haseeb, A.; et al. Herbal melanin inhibits colorectal cancer cell proliferation by altering redox balance, inducing apoptosis, and modulating MAPK signaling. Cancer Cell Int. 2020, 20, 126. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, X.; Pu, W.; Zhou, C. Sanwu Baisan decoction inhibits colorectal cancer progression in mice by remodeling gut microbiota and tumorigenesis. J. Tradit. Chin. Med. 2023, 43, 466–473. [Google Scholar]
- Zhang, D.; Sun, Y.; Yue, Z.; Li, Q.; Meng, J.; Liu, J. Apple polysaccharides induce apoptosis in colorectal cancer cells. Int. J. Mol. Med. 2012, 30, 100–106. [Google Scholar] [PubMed]
- Tang, X.Y.; Zhu, Y.Q.; Wei, B.; Wang, H. Expression and Functional Research of TLR4 in Human Colon Carcinoma. Am. J. Med. Sci. 2010, 339, 319–326. [Google Scholar] [CrossRef]
- Makkar, S.; Riehl, T.E.; Chen, B.; Yan, Y.; Alvarado, D.M.; Ciorba, M.A.; Stenson, W.F. Hyaluronic Acid Binding to TLR4 Promotes Proliferation and Blocks Apoptosis in Colon Cancer. Mol. Cancer Ther. 2019, 18, 2446–2456. [Google Scholar] [CrossRef]
- Sun, Q.; Zheng, Y.; Liu, Q.; Cao, X. Rapamycin reverses TLR4 signaling-triggered tumor apoptosis resistance by disrupting Akt-mediated Bcl-xL upregulation. Int. Immunopharmacol. 2008, 8, 1854–1858. [Google Scholar] [CrossRef]
- Park, G.B.; Chung, Y.H.; Gong, J.H.; Jin, D.H.; Kim, D. GSK-3β-mediated fatty acid synthesis enhances epithelial to mesenchymal transition of TLR4-activated colorectal cancer cells through regulation of TAp63. Int. J. Oncol. 2016, 49, 2163–2172. [Google Scholar] [CrossRef]
- Xu, C.; Gu, L.; Kuerbanjiang, M.; Wen, S.; Xu, Q.; Xue, H. Thrombospondin 2/Toll-Like Receptor 4 Axis Contributes to HIF-1α-Derived Glycolysis in Colorectal Cancer. Front. Oncol. 2020, 10, 557730. [Google Scholar] [CrossRef]
- Hu, X.; Fatima, S.; Chen, M.; Xu, K.; Huang, C.; Gong, R.H.; Su, T.; Wong, H.L.X.; Bian, Z.; Kwan, H.Y. Toll-like receptor 4 is a master regulator for colorectal cancer growth under high-fat diet by programming cancer metabolism. Cell Death Dis. 2021, 12, 791. [Google Scholar] [CrossRef]
- Park, G.B.; Kim, D. TLR4-mediated galectin-1 production triggers epithelial-mesenchymal transition in colon cancer cells through ADAM10- and ADAM17-associated lactate production. Mol. Cell Biochem. 2017, 425, 191–202. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, L.; Lin, X.; Zhang, J.; Tang, Y.; Zhou, X.; Lu, B.; Lin, X.; Liu, C.; Prochownik, E.V.; et al. Ceramide-mediated gut dysbiosis enhances cholesterol esterification and promotes colorectal tumorigenesis in mice. JCI Insight 2022, 7, e150607. [Google Scholar] [CrossRef]
- Xu, C.; Gu, L.; Hu, L.; Jiang, C.; Li, Q.; Sun, L.; Zhou, H.; Liu, Y.; Xue, H.; Li, J.; et al. FADS1-arachidonic acid axis enhances arachidonic acid metabolism by altering intestinal microecology in colorectal cancer. Nat. Commun. 2023, 14, 2042. [Google Scholar] [CrossRef]
- Kong, C.; Yan, X.; Zhu, Y.; Zhu, H.; Luo, Y.; Liu, P.; Ferrandon, S.; Kalady, M.F.; Gao, R.; He, J.; et al. Fusobacterium Nucleatum Promotes the Development of Colorectal Cancer by Activating a Cytochrome P450/Epoxyoctadecenoic Acid Axis via TLR4/Keap1/NRF2 Signaling. Cancer Res. 2021, 81, 4485–4498. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Borcherding, D.C.; Chandra, L.; Jergens, A.E.; Atherly, T.; Bourgois-Mochel, A.; Ellinwood, N.M.; Snella, E.; Severin, A.J.; Martin, M.; et al. Differential Transcriptomic Profiles Following Stimulation with Lipopolysaccharide in Intestinal Organoids from Dogs with Inflammatory Bowel Disease and Intestinal Mast Cell Tumor. Cancers 2022, 14, 3525. [Google Scholar] [CrossRef]
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.H.; Niu, Y.B.; Sun, Y.; Guo, Z.J.; Li, Q.; Li, C.; Feng, J.; Cao, S.S.; Mei, Q.B. An apple oligogalactan prevents against inflammation and carcinogenesis by targeting LPS/TLR4/NF-κB pathway in a mouse model of colitis-associated colon cancer. Carcinogenesis 2010, 31, 1822–1832. [Google Scholar] [CrossRef]
- Shen, W.; He, J.; Hou, T.; Si, J.; Chen, S. Common Pathogenetic Mechanisms Underlying Aging and Tumor and Means of Interventions. Aging Dis. 2022, 13, 1063–1091. [Google Scholar] [CrossRef]
- Proença, M.A.; Biselli, J.M.; Succi, M.; Severino, F.E.; Berardinelli, G.N.; Caetano, A.; Reis, R.M.; Hughes, D.J.; Silva, A.E. Relationship between Fusobacterium nucleatum, inflammatory mediators and microRNAs in colorectal carcinogenesis. World J. Gastroenterol. 2018, 24, 5351–5365. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W.; Romano, K.A.; Gu, M.; Sanidad, K.Z.; Kim, D.; Yang, J.; Schmidt, B.; Panigrahy, D.; Pei, R.; et al. A common antimicrobial additive increases colonic inflammation and colitis-associated colon tumorigenesis in mice. Sci. Transl. Med. 2018, 10, eaan4116. [Google Scholar] [CrossRef]
- Fukata, M.; Shang, L.; Santaolalla, R.; Sotolongo, J.; Pastorini, C.; España, C.; Ungaro, R.; Harpaz, N.; Cooper, H.S.; Elson, G.; et al. Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm. Bowel Dis. 2011, 17, 1464–1473. [Google Scholar] [CrossRef]
- Pastille, E.; Faßnacht, T.; Adamczyk, A.; Ngo Thi Phuong, N.; Buer, J.; Westendorf, A.M. Inhibition of TLR4 Signaling Impedes Tumor Growth in Colitis-Associated Colon Cancer. Front. Immunol. 2021, 12, 669747. [Google Scholar] [CrossRef]
- Davoodi, H.; Hashemi, S.R.; Seow, H.F. Increased NFκ-B Activity in HCT116 Colorectal Cancer Cell Line Harboring TLR4 Asp299Gly Variant. Iran. J. Allergy Asthma Immunol. 2012, 11, 121–132. [Google Scholar]
- Li, X.M.; Su, J.R.; Yan, S.P.; Cheng, Z.L.; Yang, T.T.; Zhu, Q. A novel inflammatory regulator TIPE2 inhibits TLR4-mediated development of colon cancer via caspase-8. Cancer Biomark. 2014, 14, 233–240. [Google Scholar] [CrossRef]
- Huang, H.C.; Cai, B.H.; Suen, C.S.; Lee, H.Y.; Hwang, M.J.; Liu, F.T.; Kannagi, R. BGN/TLR4/NF-κB Mediates Epigenetic Silencing of Immunosuppressive Siglec Ligands in Colon Cancer Cells. Cells 2020, 9, 397. [Google Scholar] [CrossRef]
- Seçme, M.; Kocoglu, S.S. Investigation of the TLR4 and IRF3 signaling pathway-mediated effects of monensin in colorectal cancer cells. Med. Oncol. 2023, 40, 187. [Google Scholar] [CrossRef]
- Burgueño, J.F.; Fritsch, J.; González, E.E.; Landau, K.S.; Santander, A.M.; Fernández, I.; Hazime, H.; Davies, J.M.; Santaolalla, R.; Phillips, M.C.; et al. Epithelial TLR4 Signaling Activates DUOX2 to Induce Microbiota-Driven Tumorigenesis. Gastroenterology 2021, 160, 797–808.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hiramoto, K.; Ma, N.; Yoshikawa, N.; Ohnishi, S.; Murata, M.; Kawanishi, S. Glycyrrhizin Attenuates Carcinogenesis by Inhibiting the Inflammatory Response in a Murine Model of Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 2609. [Google Scholar] [CrossRef]
- Luo, B.; Song, L.; Chen, L.; Cai, Y.; Zhang, M.; Wang, S. Loss of polarity protein Par3 in the intestinal epithelium promotes colitis-associated colorectal cancer progression by damaging tight junction assembly. Mol. Carcinog. 2023, 62, 1990–2004. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Han, T.; Han, C.; Sun, H.; Yang, X.; Zhang, D.; Ni, X. Propofol Regulates the TLR4/NF-κB Pathway Through miRNA-155 to Protect Colorectal Cancer Intestinal Barrier. Inflammation 2021, 44, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liao, M.; Wang, J. TLR4 signaling in the development of colitis-associated cancer and its possible interplay with microRNA-155. Cell Commun. Signal. 2021, 19, 90. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, Z.; Luo, L.; Chen, Y.; Han, G.; Wang, R.; Xiao, H.; Li, X.; Hou, C.; Feng, J.; et al. Spliceosome protein Eftud2 promotes colitis-associated tumorigenesis by modulating inflammatory response of macrophage. Mucosal Immunol. 2019, 12, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Angelou, A.; Papalois, A.E.; Antoniou, E.; Wang, J.; Amini, N.; Pikouli, A.; Andreatos, N.; Buettner, S.; Munir, M.; Theodoropoulos, G.; et al. The Interplay Between Innate Immunity (TLR-4) and sCD40L in the Context of an Animal Model of Colitis-associated Cancer. Anticancer. Res. 2020, 40, 5457–5462. [Google Scholar] [CrossRef]
- Hernandez, Y.; Sotolongo, J.; Fukata, M. Toll-Like Receptor 4 Signaling Integrates Intestinal Inflammation with Tumorigenesis: Lessons from the Murine Model of Colitis-Associated Cancer. Cancers 2011, 3, 3104–3113. [Google Scholar] [CrossRef]
- Fukata, M.; Chen, A.; Vamadevan, A.S.; Cohen, J.; Breglio, K.; Krishnareddy, S.; Xu, R.; Harpaz, N.; Dannenberg, A.J.; Cooper, H.S.; et al. Toll-like receptor-4 (TLR4) promotes the development of colitis- associated colorectal tumors. Gastroenterology 2008, 133, 1869–1881. [Google Scholar] [CrossRef]
- Amini-Khoei, H.; Momeny, M.; Abdollahi, A.; Dehpour, A.R.; Amiri, S.; Haj-Mirzaian, A.; Tavangar, S.M.; Ghaffari, S.H.; Rahimian, R.; Mehr, S.E. Tropisetron suppresses colitis-associated cancer in a mouse model in the remission stage. Int. Immunopharmacol. 2016, 36, 9–16. [Google Scholar] [CrossRef]
- Lu, C.C.; Kuo, H.C.; Wang, F.S.; Jou, M.H.; Lee, K.C.; Chuang, J.H. Upregulation of TLRs and IL-6 as a Marker in Human Colorectal Cancer. Int. J. Mol. Sci. 2014, 16, 159–177. [Google Scholar] [CrossRef]
- Han, J.X.; Tao, Z.H.; Qian, Y.; Yu, C.Y.; Li, J.; Kang, Z.R.; Lu, S.; Xie, Y.; Hong, J.; Chen, H.; et al. ZFP90 drives the initiation of colitis-associated colorectal cancer via a microbiota-dependent strategy. Gut Microbes 2021, 13, 1917269. [Google Scholar] [CrossRef]
- Xing, S.; Hu, K.; Wang, Y. Tumor Immune Microenvironment and Immunotherapy in Non-Small Cell Lung Cancer: Update and New Challenges. Aging Dis. 2022, 13, 1615–1632. [Google Scholar] [CrossRef]
- Crame, E.E.; Nourmohammadi, S.; Wardill, H.R.; Coller, J.K.; Bowen, J.M. Contribution of TLR4 to colorectal tumor microenvironment, etiology and prognosis. J. Cancer Res. Clin. Oncol. 2023, 149, 3009–3021. [Google Scholar] [CrossRef]
- Sun, Y.; Diao, F.; Niu, Y.; Li, X.; Zhou, H.; Mei, Q.; Li, Y. Apple polysaccharide prevents from colitis-associated carcinogenesis through regulating macrophage polarization. Int. J. Biol. Macromol. 2020, 161, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Chiang, S.F.; Ke, T.W.; Chen, T.W.; Lan, Y.C.; You, Y.S.; Shiau, A.C.; Chen, W.T.L.; Chao, K.S.C. Cytosolic high-mobility group box protein 1 (HMGB1) and/or PD-1+ TILs in the tumor microenvironment may be contributing prognostic biomarkers for patients with locally advanced rectal cancer who have undergone neoadjuvant chemoradiotherapy. Cancer Immunol. Immunother. 2018, 67, 551–562. [Google Scholar] [CrossRef]
- Pucci, M.; Raimondo, S.; Urzì, O.; Moschetti, M.; Di Bella, M.A.; Conigliaro, A.; Caccamo, N.; La Manna, M.P.; Fontana, S.; Alessandro, R. Tumor-Derived Small Extracellular Vesicles Induce Pro-Inflammatory Cytokine Expression and PD-L1 Regulation in M0 Macrophages via IL-6/STAT3 and TLR4 Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 12118. [Google Scholar] [CrossRef]
- Rezasoltani, S.; Ghanbari, R.; Looha, M.A.; Mojarad, E.N.; Yadegar, A.; Stewart, D.; Aghdaei, H.A.; Zali, M.R. Expression of Main Toll-like Receptors in Patients with Different Types of Colorectal Polyps and Their Relationship with Gut Microbiota. Int. J. Mol. Sci. 2020, 21, 8968. [Google Scholar] [CrossRef] [PubMed]
- Beilmann-Lehtonen, I.; Kasurinen, J.; Hagström, J.; Kaprio, T.; Böckelman, C.; Haglund, C. High tissue expression of TLRs combined with high density of tumor infiltrating lymphocytes predicts a better prognosis in colorectal cancer patients. PLoS ONE 2023, 18, e0280085. [Google Scholar] [CrossRef]
- Berthenet, K.; Boudesco, C.; Collura, A.; Svrcek, M.; Richaud, S.; Hammann, A.; Causse, S.; Yousfi, N.; Wanherdrick, K.; Duplomb, L.; et al. Extracellular HSP110 skews macrophage polarization in colorectal cancer. OncoImmunology 2016, 5, e1170264. [Google Scholar] [CrossRef]
- Li, R.; Zhou, R.; Wang, H.; Li, W.; Pan, M.; Yao, X.; Zhan, W.; Yang, S.; Xu, L.; Ding, Y.; et al. Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ. 2019, 26, 2447–2463. [Google Scholar] [CrossRef]
- Firmal, P.; Shah, V.K.; Pant, R.; Chattopadhyay, S. RING finger protein TOPORS modulates the expression of tumor suppressor SMAR1 in colorectal cancer via the TLR4-TRIF pathway. Mol. Oncol. 2022, 16, 1523–1540. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Kong, X.; Wu, R.; Peng, Q.; Zhang, Y.; Zhou, L.; Duan, L. Fusobacterium nucleatum Facilitates M2 Macrophage Polarization and Colorectal Carcinoma Progression by Activating TLR4/NF-κB/S100A9 Cascade. Front. Immunol. 2021, 12, 658681. [Google Scholar] [CrossRef]
- Chen, T.; Li, Q.; Wu, J.; Wu, Y.; Peng, W.; Li, H.; Wang, J.; Tang, X.; Peng, Y.; Fu, X. Fusobacterium nucleatum promotes M2 polarization of macrophages in the microenvironment of colorectal tumours via a TLR4-dependent mechanism. Cancer Immunol. Immunother. 2018, 67, 1635–1646. [Google Scholar] [CrossRef]
- Pham, T.N.N.; Hong, C.Y.; Min, J.J.; Rhee, J.H.; Nguyen, T.A.T.; Park, B.C.; Yang, D.H.; Park, Y.K.; Kim, H.R.; Chung, I.J.; et al. Enhancement of antitumor effect using dendritic cells activated with natural killer cells in the presence of Toll-like receptor agonist. Exp. Mol. Med. 2010, 42, 407–419. [Google Scholar] [CrossRef]
- Aschtgen, M.S.; Fragkoulis, K.; Sanz, G.; Normark, S.; Selivanova, G.; Henriques-Normark, B.; Peuget, S. Enterobacteria impair host p53 tumor suppressor activity through mRNA destabilization. Oncogene 2022, 41, 2173–2186. [Google Scholar] [CrossRef]
- Fukata, M.; Hernandez, Y.; Conduah, D.; Cohen, J.; Chen, A.; Breglio, K.; Goo, T.; Hsu, D.; Xu, R.; Abreu, M.T. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm. Bowel Dis. 2009, 15, 997–1006. [Google Scholar] [CrossRef]
- Sharma, S.; Evans, A.; Hemers, E. Mesenchymal-epithelial signalling in tumour microenvironment: Role of high-mobility group Box 1. Cell Tissue Res. 2016, 365, 357–366. [Google Scholar] [CrossRef]
- Jin, B.R.; Chung, K.S.; Hwang, S.; Hwang, S.N.; Rhee, K.J.; Lee, M.; An, H.J. Rosmarinic acid represses colitis-associated colon cancer: A pivotal involvement of the TLR4-mediated NF-κB-STAT3 axis. Neoplasia 2021, 23, 561–573. [Google Scholar] [CrossRef]
- Huang, M.; Wu, R.; Chen, L.; Peng, Q.; Li, S.; Zhang, Y.; Zhou, L.; Duan, L. S100A9 Regulates MDSCs-Mediated Immune Suppression via the RAGE and TLR4 Signaling Pathways in Colorectal Carcinoma. Front. Immunol. 2019, 10, 2243. [Google Scholar] [CrossRef]
- Cammarota, R.; Bertolini, V.; Pennesi, G.; Bucci, E.O.; Gottardi, O.; Garlanda, C.; Laghi, L.; Barberis, M.C.; Sessa, F.; Noonan, D.M.; et al. The tumor microenvironment of colorectal cancer: Stromal TLR-4 expression as a potential prognostic marker. J. Transl. Med. 2010, 8, 112. [Google Scholar] [CrossRef]
- Fang, H.; Ang, B.; Xu, X.; Huang, X.; Wu, Y.; Sun, Y.; Wang, W.; Li, N.; Cao, X.; Wan, T. TLR4 is essential for dendritic cell activation and anti-tumor T-cell response enhancement by DAMPs released from chemically stressed cancer cells. Cell. Mol. Immunol. 2014, 11, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Eiró, N.; González, L.; González, L.O.; Fernandez-Garcia, B.; Andicoechea, A.; Barbón, E.; García-Muñiz, J.L.; Vizoso, F.J. Toll-like Receptor-4 Expression by Stromal Fibroblasts Is Associated With Poor Prognosis in Colorectal Cancer. J. Immunother. 2013, 36, 342–349. [Google Scholar] [CrossRef]
- Ying, J.; Zhou, H.; Wang, Z.; You, Q.; Chen, J.; Lu, H.; Zhang, J. Aspirin increases chemosensitivity of colorectal cancer cells and inhibits the expression of toll-like receptor 4. Cancer Cell Int. 2023, 23, 6. [Google Scholar] [CrossRef]
- Teng, Z.; Sun, X.; Guo, Y.; Zhang, M.; Liu, Y.; Xu, M. Curcumae longae Rhizoma (Jianghuang) extract reverses the 5-Fluoruracil resistance in colorectal cancer cells via TLR4/PI3K/Akt/mTOR pathway. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101976. [Google Scholar] [CrossRef]
- Davoodi, H.; Hashemi, S.R.; Seow, H.F. 5-Fluorouracil Induce the Expression of TLR4 on HCT116 Colorectal Cancer Cell Line Expressing Different Variants of TLR4. Iran. J. Pharm. Res. 2013, 12, 453–460. [Google Scholar]
- Chung, Y.H.; Kim, D. Enhanced TLR4 Expression on Colon Cancer Cells After Chemotherapy Promotes Cell Survival and Epithelial-Mesenchymal Transition Through Phosphorylation of GSK3β. Anticancer. Res. 2016, 36, 3383–3394. [Google Scholar]
- Deng, Z.; Wu, N.; Suo, Q.; Wang, J.; Yue, Y.; Geng, L.; Zhang, Q. Fucoidan, as an immunostimulator promotes M1 macrophage differentiation and enhances the chemotherapeutic sensitivity of capecitabine in colon cancer. Int. J. Biol. Macromol. 2022, 222 Pt A, 562–572. [Google Scholar] [CrossRef]
- Lee, K.C.; Wu, K.L.; Yen, C.K.; Chang, S.F.; Chen, C.N.; Lu, Y.C. Inhibition of NLRP3 by Fermented Quercetin Decreases Resistin-Induced Chemoresistance to 5-Fluorouracil in Human Colorectal Cancer Cells. Pharmaceuticals 2022, 15, 798. [Google Scholar] [CrossRef]
- Scarpa, M.; Ruffolo, C.; Kotsafti, A.; Canal, F.; Erroi, F.; Basato, S.; Dall’Agnese, L.; Fiorot, A.; Pozza, A.; Brun, P.; et al. MLH1 Deficiency Down-Regulates TLR4 Expression in Sporadic Colorectal Cancer. Front. Mol. Biosci. 2021, 8, 624873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Weng, W.; Guo, B.; Cai, G.; Ma, Y.; Cai, S. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 2019, 38, 14. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, L.; Zhang, N.; Qi, X.; Lu, T.; Xing, J.; Akhtar, M.F.; Li, L.; Liu, G. Donkey Oil-Based Ketogenic Diet Prevents Tumor Progression by Regulating Intratumor Inflammation, Metastasis and Angiogenesis in CT26 Tumor-Bearing Mice. Genes 2023, 14, 1024. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, A.; Watanabe-Takahashi, M.; Mishima, T.; Omori, T.; Ohto, U.; Arashiki, N.; Nakamura, F.; Nishikawa, K.; Maru, Y. Novel multivalent S100A8 inhibitory peptides attenuate tumor progression and metastasis by inhibiting the TLR4-dependent pathway. Cancer Gene Ther. 2023, 30, 973–984. [Google Scholar] [CrossRef]
- Ying, J.; Zhou, H.y.; Liu, P.; You, Q.; Kuang, F.; Shen, Y.n.; Hu, Z.q. Aspirin inhibited the metastasis of colon cancer cells by inhibiting the expression of toll-like receptor 4. Cell Biosci. 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Paarnio, K.; Väyrynen, S.; Klintrup, K.; Ohtonen, P.; Mäkinen, M.J.; Mäkelä, J.; Karttunen, T.J. Divergent expression of bacterial wall sensing Toll-like receptors 2 and 4 in colorectal cancer. World J. Gastroenterol. 2017, 23, 4831–4838. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.L.; Qian, Z.R.; Nakasono, M.; Tanahashi, T.; Yoshimoto, K.; Bando, Y.; Kudo, E.; Shimada, M.; Sano, T. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. Br. J. Cancer 2010, 102, 908–915. [Google Scholar] [CrossRef] [PubMed]
- D’Alterio, C.; Nasti, G.; Polimeno, M.; Ottaiano, A.; Conson, M.; Circelli, L.; Botti, G.; Scognamiglio, G.; Santagata, S.; De Divitiis, C.; et al. CXCR4-CXCL12-CXCR7, TLR2-TLR4, and PD-1/PD-L1 in colorectal cancer liver metastases from neoadjuvant-treated patients. Oncoimmunology 2016, 5, e1254313. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.h.; Mi, M.; Jiang, F.L.; Yue, Z.g.; Sun, Y.; Fan, L.; Meng, J.; Zhang, X.; Liu, L.; et al. Modified apple polysaccharides suppress the migration and invasion of colorectal cancer cells induced by lipopolysaccharide. Nutr. Res. 2013, 33, 839–848. [Google Scholar] [CrossRef]
- Vasquez, M.; Fioravanti, J.; Aranda, F.; Paredes, V.; Gomar, C.; Ardaiz, N.; Fernandez-Ruiz, V.; Méndez, M.; Nistal-Villan, E.; Larrea, E.; et al. Interferon alpha bioactivity critically depends on Scavenger receptor class B type I function. OncoImmunology 2016, 5, e1196309. [Google Scholar] [CrossRef]
- Ye, K.; Chen, Q.; Sun, Y.; Lin, J.; Xu, J. Loss of BMI-1 dampens migration and EMT of colorectal cancer in inflammatory microenvironment through TLR4/MD-2/MyD88-mediated NF-κB signaling. J. Cell Biochem. 2018, 119, 1922–1930. [Google Scholar] [CrossRef]
- Kuo, W.T.; Lee, T.C.; Yang, H.Y.; Chen, C.Y.; Au, Y.C.; Lu, Y.Z.; Wu, L.L.; Wei, S.C.; Ni, Y.H.; Lin, B.R.; et al. LPS receptor subunits have antagonistic roles in epithelial apoptosis and colonic carcinogenesis. Cell Death Differ. 2015, 22, 1590–1604. [Google Scholar] [CrossRef]
- Hsu, R.Y.C.; Chan, C.H.F.; Spicer, J.D.; Rousseau, M.C.; Giannias, B.; Rousseau, S.; Ferri, L.E. LPS-Induced TLR4 Signaling in Human Colorectal Cancer Cells Increases b1 Integrin-Mediated Cell Adhesion and Liver Metastasis. Cancer Res. 2011, 71, 1989–1998. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Q.; Zheng, Y.; Cao, X. Rapamycin suppresses TLR4-triggered IL-6 and PGE2 production of colon cancer cells by inhibiting TLR4 expression and NF-κB activation. Mol. Immunol. 2008, 45, 2929–2936. [Google Scholar] [CrossRef]
- Earl, T.M.; Nicoud, I.B.; Pierce, J.M.; Wright, J.P.; Majoras, N.E.; Rubin, J.E.; Pierre, K.P.; Gorden, D.L.; Chari, R.S. Silencing of TLR4 Decreases Liver Tumor Burden in a Murine Model of Colorectal Metastasis and Hepatic Steatosis. Ann. Surg. Oncol. 2009, 16, 1043–1050. [Google Scholar] [CrossRef]
- O’Leary, D.P.; Bhatt, L.; Woolley, J.F.; Gough, D.R.; Wang, J.H.; Cotter, T.G.; Redmond, H.P. TLR-4 Signalling Accelerates Colon Cancer Cell Adhesion via NF-κB Mediated Transcriptional Up-Regulation of Nox-1. Deb S, editor. PLoS ONE 2012, 7, e44176. [Google Scholar]
- Eyking, A.; Ey, B.; Rünzi, M.; Roig, A.I.; Reis, H.; Schmid, K.W.; Gerken, G.; Podolsky, D.K.; Cario, E. Toll-like Receptor 4 Variant D299G Induces Features of Neoplastic Progression in Caco-2 Intestinal Cells and Is Associated with Advanced Human Colon Cancer. Gastroenterology 2011, 141, 2154–2165. [Google Scholar] [CrossRef]
- Huang, M.; Geng, Y.; Deng, Q.; Li, R.; Shao, X.; Zhang, Z.; Xu, W.; Wu, Y.; Ma, Q. Translationally controlled tumor protein affects colorectal cancer metastasis through the high mobility group box 1-dependent pathway. Int. J. Oncol. 2018, 53, 1481–1492. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, W.; Wu, Z.; Tian, X.; Xiang, J.; Li, L.; Li, Z.; Peng, X.; Wei, S.; Ma, X.; et al. Baicalin and the liver-gut system: Pharmacological bases explaining its therapeutic effects. Pharmacol. Res. 2021, 165, 105444. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.m.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Lai, Z.; Lin, J. Anticancer Properties of Traditional Chinese Medicine. Comb. Chem. High Throughput Screen. 2017, 20, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Naghavi Alhosseini, M.; Mazandarani, M.; Enayati, A.; Saiedi, M.; Davoodi, H. Anticancer Activity of Ethnopharmacological Plants of Golestan Province/Iran against AGS, HT-29 and KYSE-30 Cell Lines through Promoting the Apoptosis and Immunomodulatory Effects. Iran. J. Pharm. Res. 2021, 20, 636–646. [Google Scholar] [PubMed]
- Shang, H.S.; Liu, J.Y.; Lu, H.F.; Chiang, H.S.; Lin, C.H.; Chen, A.; Lin, Y.F.; Chung, J.G. Casticin induced apoptotic cell death and altered associated gene expression in human colon cancer colo 205 cells. Environ. Toxicol. 2017, 32, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Abulizi, A.; Hu, L.; Ma, A.; Shao, F.y.; Zhu, H.z.; Lin, S.m.; Shao, G.y.; Xu, Y.; Ran, J.h.; Li, J.; et al. Ganoderic acid alleviates chemotherapy-induced fatigue in mice bearing colon tumor. Acta Pharmacol. Sin. 2021, 42, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Zhao, Y.; Wu, W.; Meng, W.; Zhou, Y.; Qiu, Y.; Li, C. Protection against ulcerative colitis and colorectal cancer by evodiamine via anti-inflammatory effects. Mol. Med. Rep. 2022, 25, 188. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.Y.; Liu, H.M.; Leu, Y.L.; Hsu, C.H.; Lee, T.Y. Modulation of Gut Microbiota Combined with Upregulation of Intestinal Tight Junction Explains Anti-Inflammatory Effect of Corylin on Colitis-Associated Cancer in Mice. Int. J. Mol. Sci. 2022, 23, 2667. [Google Scholar] [CrossRef]
- Bai, B.; Wu, F.; Ying, K.; Xu, Y.; Shan, L.; Lv, Y.; Gao, X.; Xu, D.; Lu, J.; Xie, B. Therapeutic effects of dihydroartemisinin in multiple stages of colitis-associated colorectal cancer. Theranostics 2021, 11, 6225–6239. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Wen, H.; Jiang, H.; Hou, Q.; Yan, H. Berberine improves negative symptoms and cognitive function in patients with chronic schizophrenia via anti-inflammatory effect: A randomized clinical trial. Chin. Med. 2023, 18, 41. [Google Scholar] [CrossRef]
- Xu, Y.D.; Guo, Y.J.; Mao, H.R.; Xiong, Z.X.; Luo, M.Y.; Luo, R.Q.; Lu, S.; Huang, L.; Hong, Y. Integration of transcriptomics and proteomics to elucidate inhibitory effect and mechanism of rosmarinic acid from Perilla frutescens (L.) Britt. in treating Trichophyton mentagrophytes. Chin. Med. 2023, 18, 67. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, R.; Wu, L.; Tang, S.; Wei, B.; Guo, L.; He, L.; Feng, Y. Atractylodes macrocephala polysaccharides regulate the innate immunity of colorectal cancer cells by modulating the TLR4 signaling pathway. Onco Targets Ther. 2019, 12, 7111–7121. [Google Scholar] [CrossRef]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Y.; Zhu, L.; Wan, J.; Lei, N.; Yao, X.; Duan, X.; Zhang, Y.; Cheng, Y.; Tao, N.; et al. Polysaccharides From Lentinus Edodes Inhibits Lymphangiogenesis via the Toll-Like Receptor 4/JNK Pathway of Cancer-Associated Fibroblasts. Front. Oncol. 2021, 10, 547683. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Zhao, Y.; Zong, S.; Tian, Y.; Chen, S.; Li, M.; Liu, H.; Zhang, Q.; Jing, X.; et al. Therapeutic effects of lentinan on inflammatory bowel disease and colitis-associated cancer. J. Cell. Mol. Med. 2019, 23, 750–760. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, F.; Tao, J.; Wang, H.; Sun, M.; He, Y.; Lai, Z. Effects of Shu Bu Wenshen Guchang recipe on intestinal injury and the TLR4/NF-κB signaling pathways in mice with irinotecan-induced delayed-type diarrhea. Transl. Cancer Res. 2022, 11, 3250–3259. [Google Scholar] [CrossRef]
- Xiang, B.; Geng, R.; Zhang, Z.; Ji, X.; Zou, J.; Chen, L.; Liu, J. Identification of the effect and mechanism of Yiyi Fuzi Baijiang powder against colorectal cancer using network pharmacology and experimental validation. Front. Pharmacol. 2022, 13, 929836. [Google Scholar] [CrossRef]
- Shao, S.; Jia, R.; Zhao, L.; Zhang, Y.; Guan, Y.; Wen, H.; Liu, J.; Zhao, Y.; Feng, Y.; Zhang, Z.; et al. Xiao-Chai-Hu-Tang ameliorates tumor growth in cancer comorbid depressive symptoms via modulating gut microbiota-mediated TLR4/MyD88/NF-κB signaling pathway. Phytomedicine 2021, 88, 153606. [Google Scholar] [CrossRef]
- Chen, M.; May, B.H.; Zhou, I.W.; Xue, C.C.L.; Zhang, A.L. Meta-Analysis of Oxaliplatin-Based Chemotherapy Combined With Traditional Medicines for Colorectal Cancer: Contributions of Specific Plants to Tumor Response. Integr. Cancer Ther. 2016, 15, 40–59. [Google Scholar] [CrossRef]
- Su, M.; Qin, B.; Liu, F.; Chen, Y.; Zhang, R. Andrographolide enhanced 5-fluorouracil-induced antitumor effect in colorectal cancer via inhibition of c-MET pathway. Drug Des. Dev. Ther. 2017, 11, 3333–3341. [Google Scholar] [CrossRef]
- Wang, W.; Guo, W.; Li, L.; Fu, Z.; Liu, W.; Gao, J.; Shu, Y.; Xu, Q.; Sun, Y.; Gu, Y. Andrographolide reversed 5-FU resistance in human colorectal cancer by elevating BAX expression. Biochem. Pharmacol. 2016, 121, 8–17. [Google Scholar] [CrossRef]
- Xu, L.; Cai, P.; Li, X.; Wu, X.; Gao, J.; Liu, W.; Yang, J.; Xu, Q.; Guo, W.; Gu, Y. Inhibition of NLRP3 inflammasome activation in myeloid-derived suppressor cells by andrographolide sulfonate contributes to 5-FU sensitization in mice. Toxicol. Appl. Pharmacol. 2021, 428, 115672. [Google Scholar] [CrossRef]
- Hong, H.; Cao, W.; Wang, Q.; Liu, C.; Huang, C. Synergistic antitumor effect of Andrographolide and cisplatin through ROS-mediated ER stress and STAT3 inhibition in colon cancer. Med. Oncol. 2022, 39, 101. [Google Scholar] [CrossRef]
- Li, X.; Tian, R.; Liu, L.; Wang, L.; He, D.; Cao, K.; Ma, J.K.; Huang, C. Andrographolide enhanced radiosensitivity by downregulating glycolysis via the inhibition of the PI3K-Akt-mTOR signaling pathway in HCT116 colorectal cancer cells. J. Int. Med. Res. 2020, 48, 300060520946169. [Google Scholar] [CrossRef]
- Moutabian, H.; Majdaeen, M.; Ghahramani-Asl, R.; Yadollahi, M.; Gharepapagh, E.; Ataei, G.; Falahatpour, Z.; Bagheri, H.; Farhood, B. A systematic review of the therapeutic effects of resveratrol in combination with 5-fluorouracil during colorectal cancer treatment: With a special focus on the oxidant, apoptotic, and anti-inflammatory activities. Cancer Cell Int. 2022, 22, 142. [Google Scholar] [CrossRef]
- Yu, M.; Tong, X.; Qi, B.; Qu, H.; Dong, S.; Yu, B.; Zhang, N.; Tang, N.; Wang, L.; Zhang, C. Berberine enhances chemosensitivity to irinotecan in colon cancer via inhibition of NF-κB. Mol. Med. Rep. 2014, 9, 249–254. [Google Scholar] [CrossRef]
- Atashpour, S.; Fouladdel, S.; Movahhed, T.K.; Barzegar, E.; Ghahremani, M.H.; Ostad, S.N.; Azizi, E. Quercetin induces cell cycle arrest and apoptosis in CD133(+) cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran. J. Basic. Med. Sci. 2015, 18, 635–643. [Google Scholar]
- Boersma, H.H.; Woerdenbag, H.J.; Bauer, J.; Scheithauer, W.; Kampinga, H.H.; Konings, A.W. Interaction between the cytostatic effects of quercetin and 5-fluorouracil in two human colorectal cancer cell lines. Phytomedicine 1994, 1, 239–244. [Google Scholar] [CrossRef]
- Terana, G.T.; Abd-Alhaseeb, M.M.; Omran, G.A.; Okda, T.M. Quercetin potentiates 5-fluorouracil effects in human colon cancer cells through targeting the Wnt/β-catenin signalling pathway: The role of miR-27a. Contemp. Oncol. 2022, 26, 229–238. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Lima, C.F.; Rohde, M.; Pereira-Wilson, C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother. Pharmacol. 2011, 68, 1449–1457. [Google Scholar] [CrossRef]
- Lee, J.; Jang, C.H.; Kim, Y.; Oh, J.; Kim, J.S. Quercetin-Induced Glutathione Depletion Sensitizes Colorectal Cancer Cells to Oxaliplatin. Foods 2023, 12, 1733. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, H.; Zhao, Y.; Sui, M.; Liu, J.; Li, P.; Liu, N.; Zhang, K. Role of Ginseng, Quercetin, and Tea in Enhancing Chemotherapeutic Efficacy of Colorectal Cancer. Front. Med. 2022, 9, 939424. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, D.; Wu, T.; Lin, H.; Ni, L.; Sui, H.; Xiao, S.; Wang, C.; Jiang, S.; Pan, H.; et al. Dihydroartemisinin enhances the anti-tumor activity of oxaliplatin in colorectal cancer cells by altering PRDX2-reactive oxygen species-mediated multiple signaling pathways. Phytomedicine 2022, 98, 153932. [Google Scholar] [CrossRef]
- Opattova, A.; Horak, J.; Vodenkova, S.; Kostovcikova, K.; Cumova, A.; Macinga, P.; Galanova, N.; Rejhova, A.; Vodickova, L.; Kozics, K.; et al. Ganoderma Lucidum induces oxidative DNA damage and enhances the effect of 5-Fluorouracil in colorectal cancer in vitro and in vivo. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 845, 403065. [Google Scholar] [CrossRef]
- McCulloch, M.; Ly, H.; Broffman, M.; See, C.; Clemons, J.; Chang, R. Chinese Herbal Medicine and Fluorouracil-Based Chemotherapy for Colorectal Cancer: A Quality-Adjusted Meta-Analysis of Randomized Controlled Trials. Integr. Cancer Ther. 2016, 15, 285–307. [Google Scholar] [CrossRef]
- Abulizi, A.; Ran, J.; Ye, Y.; An, Y.; Zhang, Y.; Huang, Z.; Lin, S.; Zhou, H.; Lin, D.; Wang, L.; et al. Ganoderic acid improves 5-fluorouracil-induced cognitive dysfunction in mice. Food Funct. 2021, 12, 12325–12337. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, F.; Li, R.; Liu, Y.; Wang, X.; Zhang, X.; Xu, C.; Li, Y.; Guo, Y.; Yao, Q. Berberine regulates fecal metabolites to ameliorate 5-fluorouracil induced intestinal mucositis through modulating gut microbiota. Biomed. Pharmacother. 2020, 124, 109829. [Google Scholar] [CrossRef]
- Yue, B.; Gao, R.; Lv, C.; Yu, Z.; Wang, H.; Geng, X.; Wang, Z.; Dou, W. Berberine Improves Irinotecan-Induced Intestinal Mucositis Without Impairing the Anti-colorectal Cancer Efficacy of Irinotecan by Inhibiting Bacterial β-glucuronidase. Front. Pharmacol. 2021, 12, 774560. [Google Scholar] [CrossRef]
- Brockmueller, A.; Sajeev, A.; Koklesova, L.; Samuel, S.M.; Kubatka, P.; Büsselberg, D.; Kunnumakkara, A.B.; Shakibaei, M. Resveratrol as sensitizer in colorectal cancer plasticity. Cancer Metastasis Rev. 2024, 43, 55–85. [Google Scholar] [CrossRef]
- Jugait, S.; Areti, A.; Nellaiappan, K.; Narwani, P.; Saha, P.; Velayutham, R.; Kumar, A. Neuroprotective Effect of Baicalein Against Oxaliplatin-Induced Peripheral Neuropathy: Impact on Oxidative Stress, Neuro-inflammation and WNT/β-Catenin Signaling. Mol. Neurobiol. 2022, 59, 4334–4350. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, L.; Chen, Y.; Zheng, X.; Wang, R.; Liu, B.; Zhang, S.; Wang, H. Quercetin reverses 5-fluorouracil resistance in colon cancer cells by modulating the NRF2/HO-1 pathway. Eur. J. Histochem. 2023, 67, 3719. [Google Scholar] [CrossRef]
- Lin, C.; Yu, Y.; Zhao, H.G.; Yang, A.; Yan, H.; Cui, Y. Combination of quercetin with radiotherapy enhances tumor radiosensitivity in vitro and in vivo. Radiother. Oncol. 2012, 104, 395–400. [Google Scholar] [CrossRef]
- Dai, X.; Chen, W.; Qiao, Y.; Chen, X.; Chen, Y.; Zhang, K.; Zhang, Q.; Duan, X.; Li, X.; Zhao, J.; et al. Dihydroartemisinin inhibits the development of colorectal cancer by GSK-3β/TCF7/MMP9 pathway and synergies with capecitabine. Cancer Lett. 2024, 582, 216596. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Chai, Z.; Li, J.; Zhu, C.; Peng, Y.; Qiu, J.; Xu, J.; Liu, C. Dihydroartemisinin synergistically enhances the cytotoxic effects of oxaliplatin in colon cancer by targeting the PHB2-RCHY1 mediated signaling pathway. Mol. Carcinog. 2023, 62, 293–302. [Google Scholar] [CrossRef]
- Chen, M.H.; May, B.H.; Zhou, I.W.; Zhang, A.L.; Xue, C.C. Integrative Medicine for Relief of Nausea and Vomiting in the Treatment of Colorectal Cancer Using Oxaliplatin-Based Chemotherapy: A Systematic Review and Meta-Analysis. Phytother. Res. 2016, 30, 741–753. [Google Scholar] [CrossRef]
- Han, J.; Lai, H.; Li, W.; Liao, H.; Xiao, C.; Li, X.; You, F.; Guo, J. Efficacy and safety of traditional plant-based medicines for preventing chronic oxaliplatin-induced peripheral neurotoxicity in patients with colorectal cancer: A systematic review and meta-analysis with core herb contribution. J. Ethnopharmacol. 2024, 326, 117735. [Google Scholar] [CrossRef]
- Wang, C.; Teng, X.; Wang, C.; Liu, B.; Zhou, R.; Xu, X.; Qiu, H.; Fu, Y.; Sun, R.; Liang, Z.; et al. Insight into the mechanism of Xiao-Chai-Hu-Tang alleviates irinotecan-induced diarrhea based on regulating the gut microbiota and inhibiting Gut β-GUS. Phytomedicine 2023, 120, 155040. [Google Scholar] [CrossRef]
- Liu, Y.F.; Feng, Z.Q.; Chu, T.H.; Yi, B.; Liu, J.; Yu, H.; Xue, J.; Wang, Y.J.; Zhang, C.Z. Andrographolide sensitizes KRAS-mutant colorectal cancer cells to cetuximab by inhibiting the EGFR/AKT and PDGFRβ/AKT signaling pathways. Phytomedicine 2024, 126, 155462. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Z.; Zhu, W.; Chen, Y.; He, X.; Li, J.; Han, Z.; Yang, Y.; Liu, W.; Zhang, K. Dihydroartemisinin inhibited stem cell-like properties and enhanced oxaliplatin sensitivity of colorectal cancer via AKT/mTOR signaling. Drug Dev. Res. 2023, 84, 988–998. [Google Scholar] [CrossRef]
- Yao, Z.; Bhandari, A.; Wang, Y.; Pan, Y.; Yang, F.; Chen, R.; Xia, E.; Wang, O. Dihydroartemisinin potentiates antitumor activity of 5-fluorouracil against a resistant colorectal cancer cell line. Biochem. Biophys. Res. Commun. 2018, 501, 636–642. [Google Scholar] [CrossRef]
- Li, X.; Yang, G.; Li, X.; Zhang, Y.; Yang, J.; Chang, J.; Sun, X.; Zhou, X.; Guo, Y.; Xu, Y.; et al. Traditional Chinese medicine in cancer care: A review of controlled clinical studies published in chinese. PLoS ONE 2013, 8, e60338. [Google Scholar]
- Li, Q.C.; Liang, Y.; Hu, G.R.; Tian, Y. Enhanced therapeutic efficacy and amelioration of cisplatin-induced nephrotoxicity by quercetin in 1,2-dimethyl hydrazine-induced colon cancer in rats. Indian J. Pharmacol. 2016, 48, 168–171. [Google Scholar]
- Schwingel, T.E.; Klein, C.P.; Nicoletti, N.F.; Dora, C.L.; Hadrich, G.; Bica, C.G.; Lopes, T.G.; da Silva, V.D.; Morrone, F.B. Effects of the compounds resveratrol, rutin, quercetin, and quercetin nanoemulsion on oxaliplatin-induced hepatotoxicity and neurotoxicity in mice. Naunyn Schmiedeberg’s Arch. Pharmacol. 2014, 387, 837–848. [Google Scholar] [CrossRef]
- Areti, A.; Komirishetty, P.; Kalvala, A.K.; Nellaiappan, K.; Kumar, A. Rosmarinic Acid Mitigates Mitochondrial Dysfunction and Spinal Glial Activation in Oxaliplatin-induced Peripheral Neuropathy. Mol. Neurobiol. 2018, 55, 7463–7475. [Google Scholar] [CrossRef]
- Zeng, B.; Wei, A.; Zhou, Q.; Yuan, M.; Lei, K.; Liu, Y.; Song, J.; Guo, L.; Ye, Q. Andrographolide: A review of its pharmacology, pharmacokinetics, toxicity and clinical trials and pharmaceutical researches. Phytother. Res. 2022, 36, 336–364. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Froldi, G.; Ragazzi, E. Selected Plant-Derived Polyphenols as Potential Therapeutic Agents for Peripheral Artery Disease: Molecular Mechanisms, Efficacy and Safety. Molecules 2022, 27, 7110. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine and barberry (Berberis vulgaris): A clinical review. Phytother. Res. 2019, 33, 504–523. [Google Scholar] [CrossRef]
- Kumar, A.; Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef]
- Luo, Y.; Che, M.J.; Liu, C.; Liu, H.G.; Fu, X.W.; Hou, Y.P. Toxicity and related mechanisms of dihydroartemisinin on porcine oocyte maturation in vitro. Toxicol. Appl. Pharmacol. 2018, 341, 8–15. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Sun, C.; Peng, F.; Yu, L.; Chen, Y.; Tan, Y.; Cao, X.; Tang, Y.; Xie, X.; et al. Chemistry, pharmacokinetics, pharmacological activities, and toxicity of Quercitrin. Phytother. Res. 2022, 36, 1545–1575. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef]
- Asami, J.; Shimizu, T. Structural and functional understanding of the toll-like receptors. Protein Sci. 2021, 30, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Mineev, K.S.; Goncharuk, S.A.; Goncharuk, M.V.; Volynsky, P.E.; Novikova, E.V.; Aresinev, A.S. Spatial structure of TLR4 transmembrane domain in bicelles provides the insight into the receptor activation mechanism. Sci. Rep. 2017, 7, 6864. [Google Scholar] [CrossRef] [PubMed]
- Ve, T.; Vajjhala, P.R.; Hedger, A.; Croll, T.; DiMaio, F.; Horsefield, S.; Yu, X.; Lavrencic, P.; Hassan, Z.; Morgan, G.P.; et al. Structural basis of TIR-domain-assembly formation in MAL- and MyD88-dependent TLR4 signaling. Nat. Struct. Mol. Biol. 2017, 24, 743–751. [Google Scholar] [CrossRef]
- Raby, A.C.; Holst, B.; Le Bouder, E.; Diaz, C.; Ferran, E.; Conraux, L.; Guillemot, J.C.; Coles, B.; Kift-Morgan, A.; Colmont, C.S.; et al. Targeting the TLR co-receptor CD14 with TLR2-derived peptides modulates immune responses to pathogens. Sci. Transl. Med. 2013, 5, 185ra64. [Google Scholar] [CrossRef] [PubMed]
- Paramo, T.; Piggot, T.J.; Bryant, C.E.; Bond, P.J. The Structural Basis for Endotoxin-induced Allosteric Regulation of the Toll-like Receptor 4 (TLR4) Innate Immune Receptor. J. Biol. Chem. 2013, 288, 36215–36225. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, L.; Yu, P.; Zhang, Y.; Fang, B.; Wu, C.; Luo, W.; Chen, X.; Li, C.; Liang, G. Discovery of 3-(Indol-5-yl)-indazole Derivatives as Novel Myeloid Differentiation Protein 2/Toll-like Receptor 4 Antagonists for Treatment of Acute Lung Injury. J. Med. Chem. 2019, 62, 5453–5469. [Google Scholar] [CrossRef] [PubMed]
- Ain, Q.u.; Batool, M.; Choi, S. TLR4-Targeting Therapeutics: Structural Basis and Computer-Aided Drug Discovery Approaches. Molecules 2020, 25, 627. [Google Scholar] [CrossRef]
- Sameer, A.S.; Nissar, S. Toll-Like Receptors (TLRs): Structure, Functions, Signaling, and Role of Their Polymorphisms in Colorectal Cancer Susceptibility. BioMed Res. Int. 2021, 2021, e1157023. [Google Scholar] [CrossRef]
- Leifer, C.A.; Medvedev, A.E. Molecular mechanisms of regulation of Toll-like receptor signaling. J. Leukoc. Biol. 2016, 100, 927–941. [Google Scholar] [CrossRef] [PubMed]

| Full Official Name | Concentration/Dosage | Major Effects | Involved Pathways | Ref. |
|---|---|---|---|---|
| Asparagus polysaccharide | in vitro: 0.25, 0.5 mg/mL | Inhibiting MDSC activity in CRC. | / | [46] |
| Arctiumlappa root | in vitro: 4 mg/mL | Increasing apoptosis in CRC; Decreasing cancer cell attachment to the surface of CRC. | TLR-4/AKT/ERK pathway. | [133] |
| Atractylodes macrocephala polysaccharides | in vitro: 50, 100, 200, 400 μg/mL | Enhancing the phagocytosis of BMDMs by CRC cells. | TLR4/MyD88 pathway. | [141] |
| Curcumae longae Rhizoma | in vivo: 500 mg/kg | Reversing the 5-Fluoruracil resistance in SW480 cells. | TLR4/PI3K/Akt/mTOR pathway. | [106] |
| Ganoderma lucidum polysaccharide | in vivo: 200, 300 mg/kg | Inhibiting inflammation and tumorigenesis in colon. | TLR4/MyD88/NF-κB pathway. | [142] |
| Lentinus edodes Polysaccharides | in vitro: 30 µg/mL; 0.5, 1, 2 mg/mL in vivo: 30 mg/kg; 5, 10, 20 mg/kg | Inhibiting lymphangiogenesis and metastasis of lymphatic, and inflammation in CRC. | TLR4/JNK and TLR4/NF-κB pathway. | [143,144] |
| Shubu Wenshen Guchang recipe | in vivo: 100, 200 mg/kg | Inhibiting intestinal damage in CRC. | TLR4/NF-κB pathway. | [145] |
| Yiyi Fuzi Baijiang powder | / | Inhibiting inflammation in CRC. | TLR4/NF-κB pathway. | [146] |
| Sanwu Baisan Decoction | in vitro: 5.647 mg/mL in vivo: 5, 10, 50 mg/kg | Remodeling gut microbiota and inducing apoptosis in CRC. | TLR-4/COX-2/PGE-2 pathway. | [48] |
| Xiaochai Hu Decoction | in vivo: 10.27, 20.54 mg/kg | Reducing depressive symptoms and reversing gut dysbiosis in CRC | TLR4/MyD88/NF-κB pathway. | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Zhang, G.; Hu, C.; Huang, L.; Wang, D.; Chen, Z.; Wang, Y. The Role of Natural Products from Herbal Medicine in TLR4 Signaling for Colorectal Cancer Treatment. Molecules 2024, 29, 2727. https://doi.org/10.3390/molecules29122727
Luo Y, Zhang G, Hu C, Huang L, Wang D, Chen Z, Wang Y. The Role of Natural Products from Herbal Medicine in TLR4 Signaling for Colorectal Cancer Treatment. Molecules. 2024; 29(12):2727. https://doi.org/10.3390/molecules29122727
Chicago/Turabian StyleLuo, Yan, Guochen Zhang, Chao Hu, Lijun Huang, Dong Wang, Zhejie Chen, and Yumei Wang. 2024. "The Role of Natural Products from Herbal Medicine in TLR4 Signaling for Colorectal Cancer Treatment" Molecules 29, no. 12: 2727. https://doi.org/10.3390/molecules29122727
APA StyleLuo, Y., Zhang, G., Hu, C., Huang, L., Wang, D., Chen, Z., & Wang, Y. (2024). The Role of Natural Products from Herbal Medicine in TLR4 Signaling for Colorectal Cancer Treatment. Molecules, 29(12), 2727. https://doi.org/10.3390/molecules29122727








