An Overview of the Synthesis of 3,4-Fused Pyrrolocoumarins of Biological Interest
Abstract
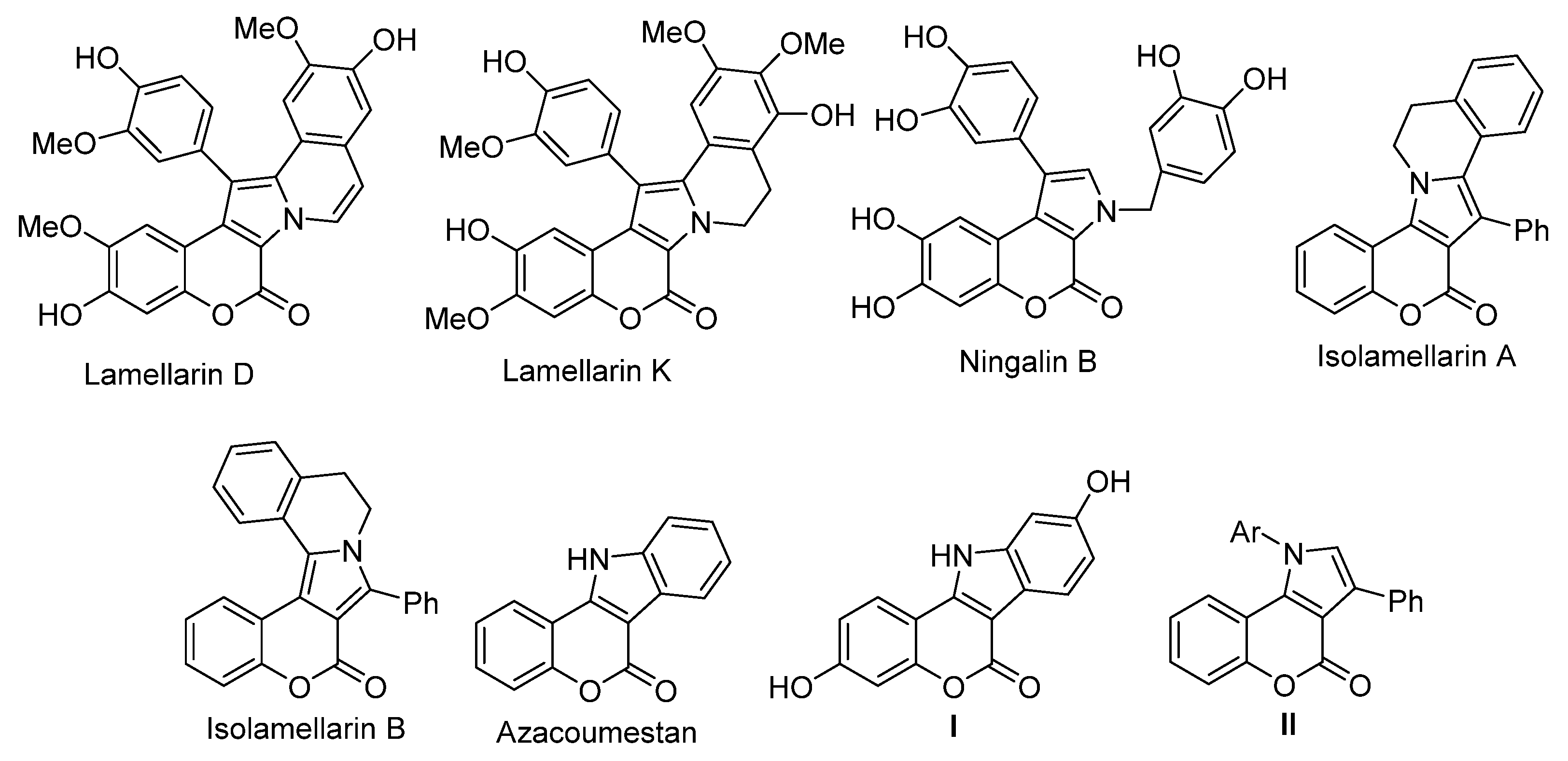
:1. Introduction
2. Synthetic Strategies for the Preparation of [3,4]-Fused Pyrrolocoumarin Derivatives
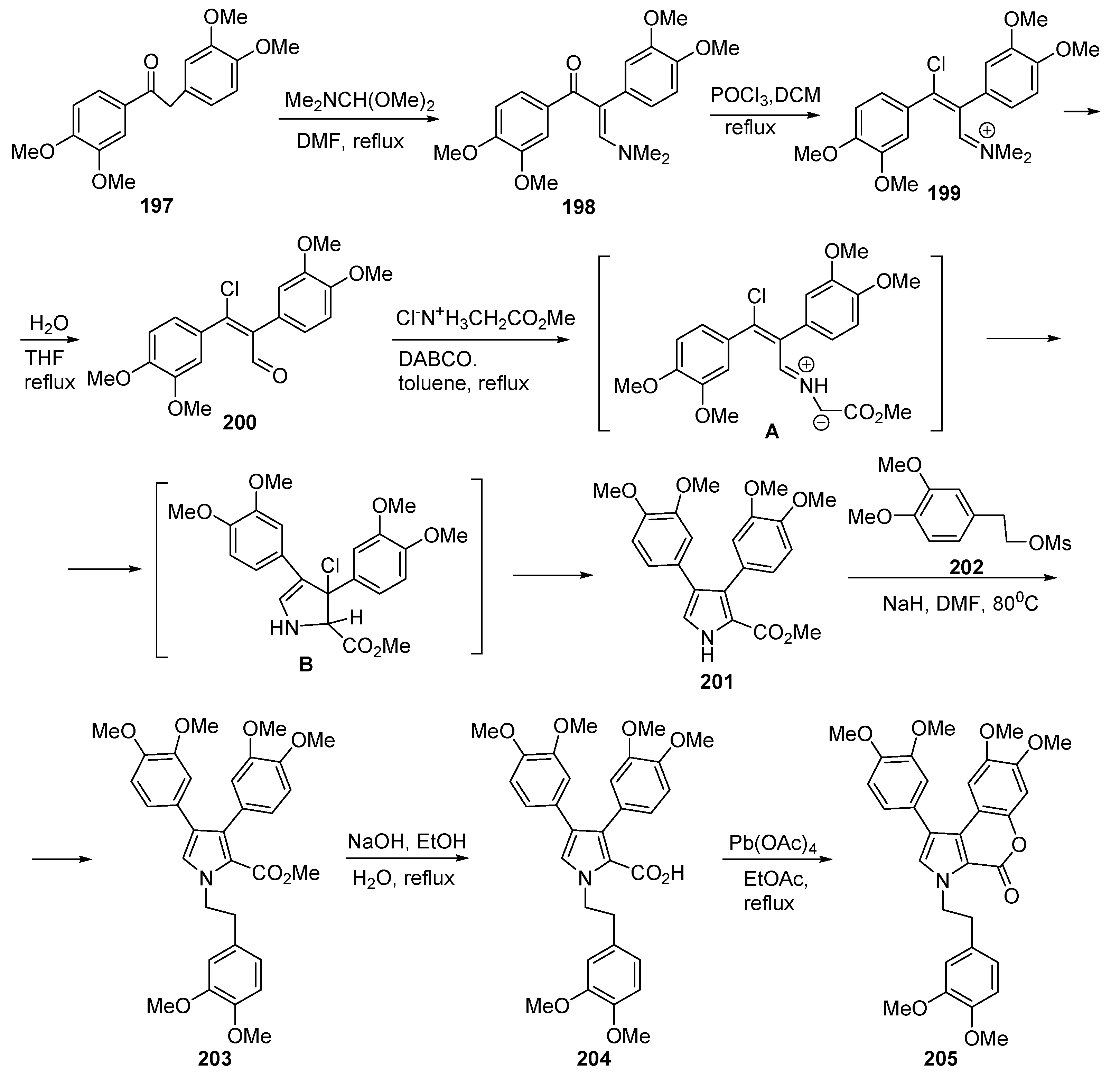
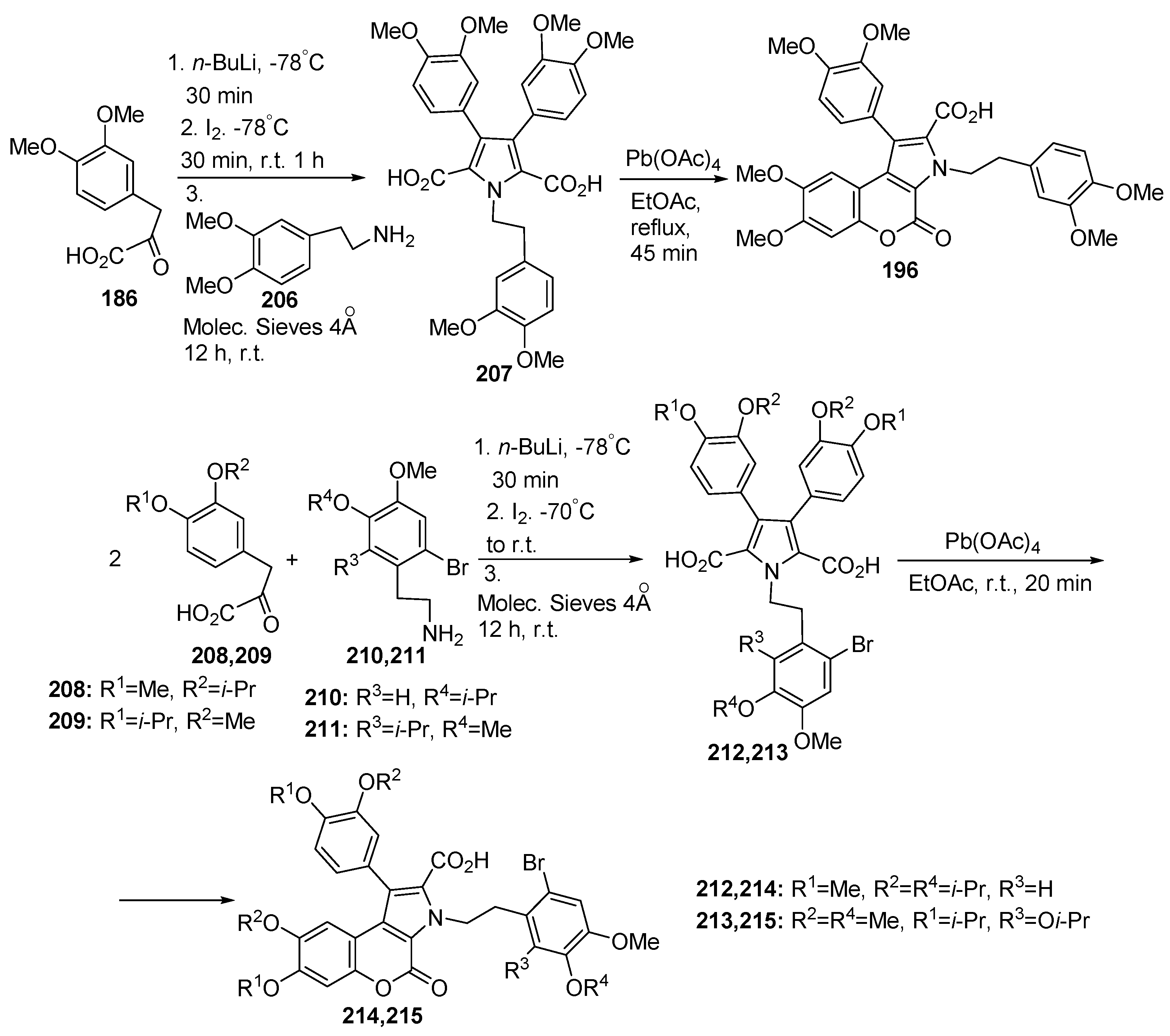
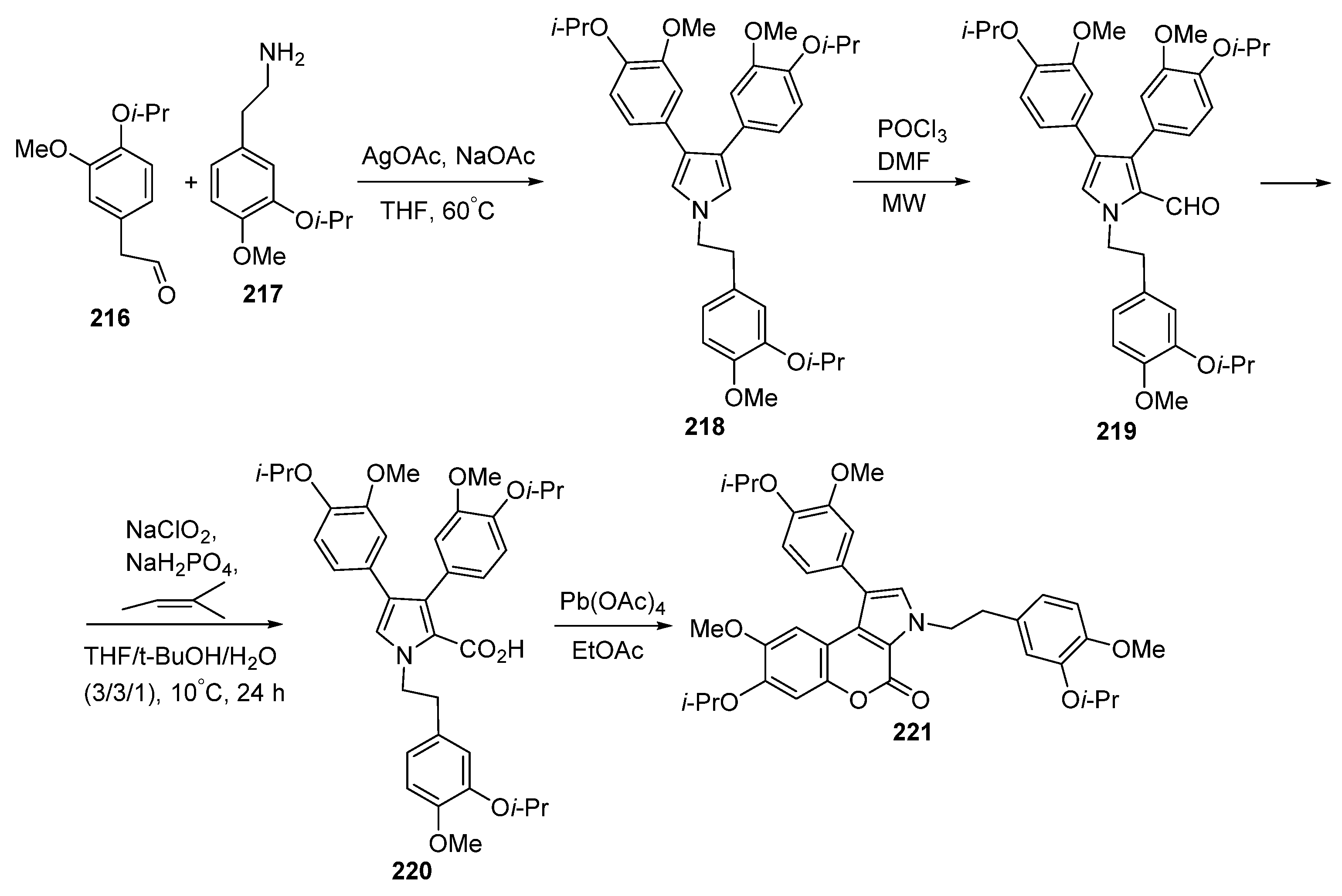
2.1. Pyrrole Ring Formation
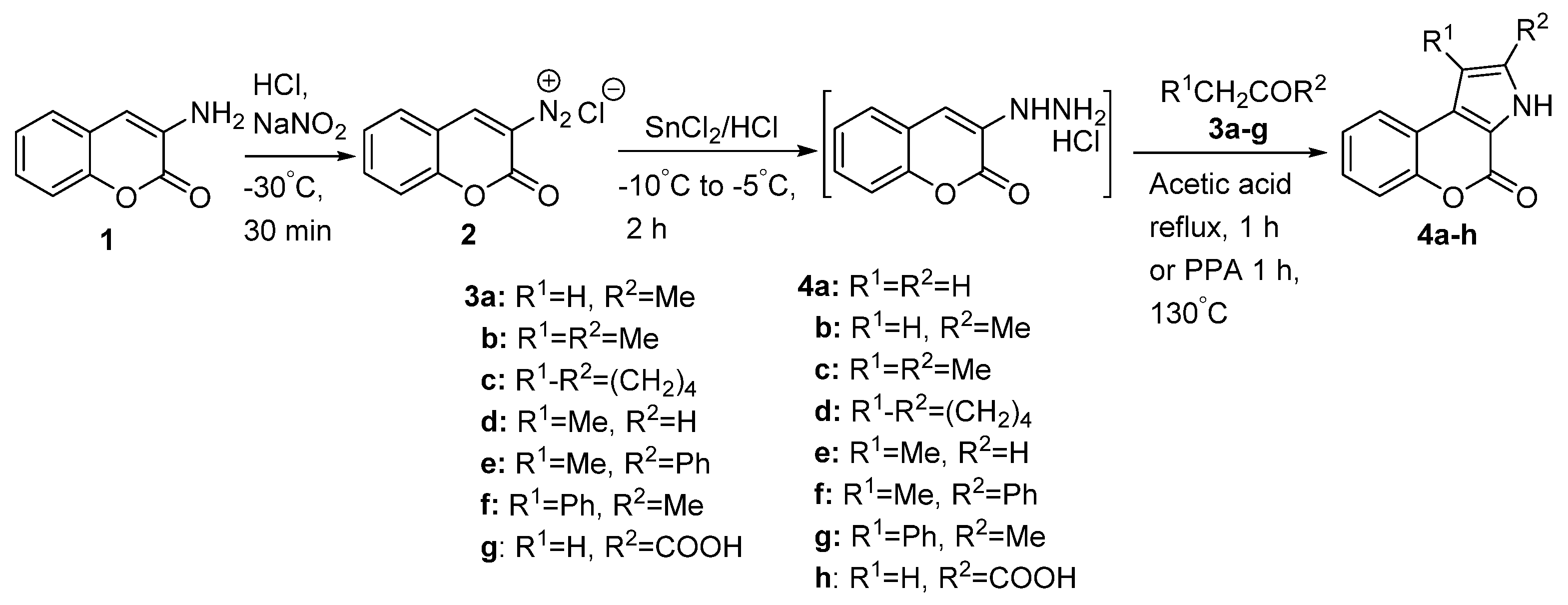
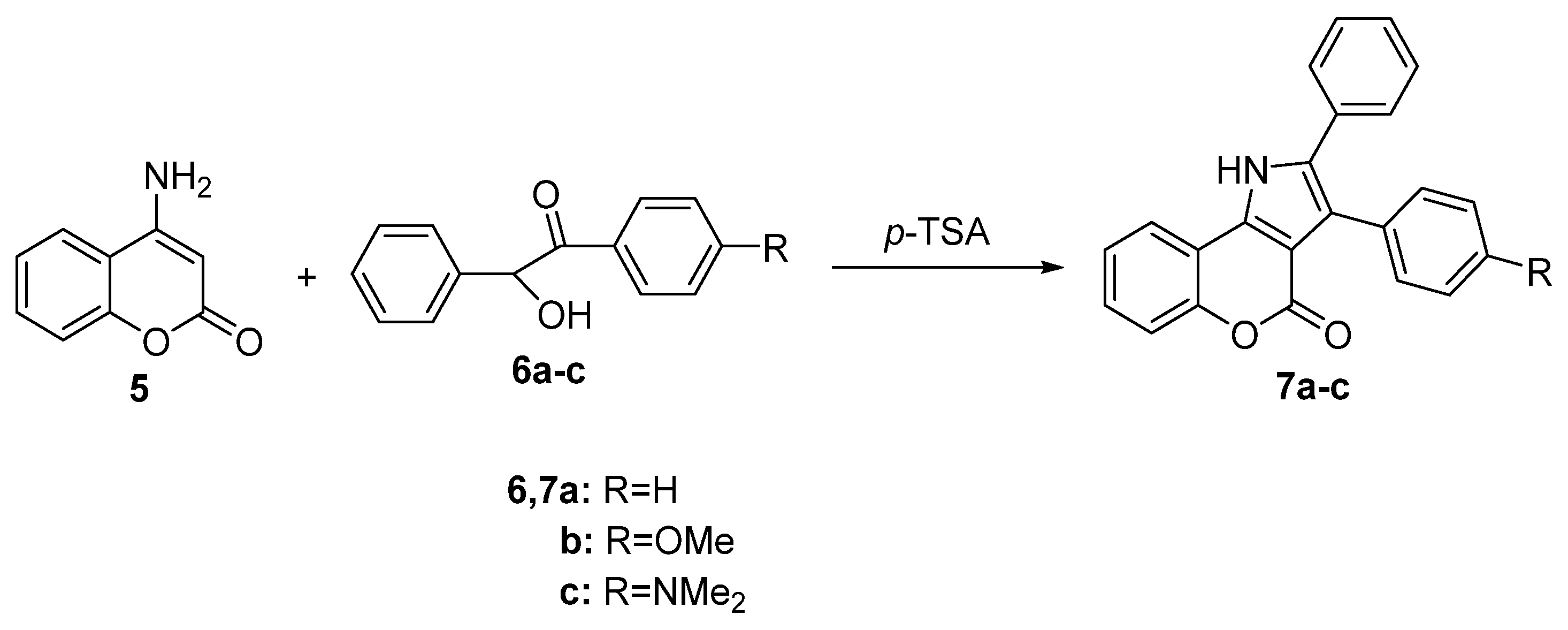
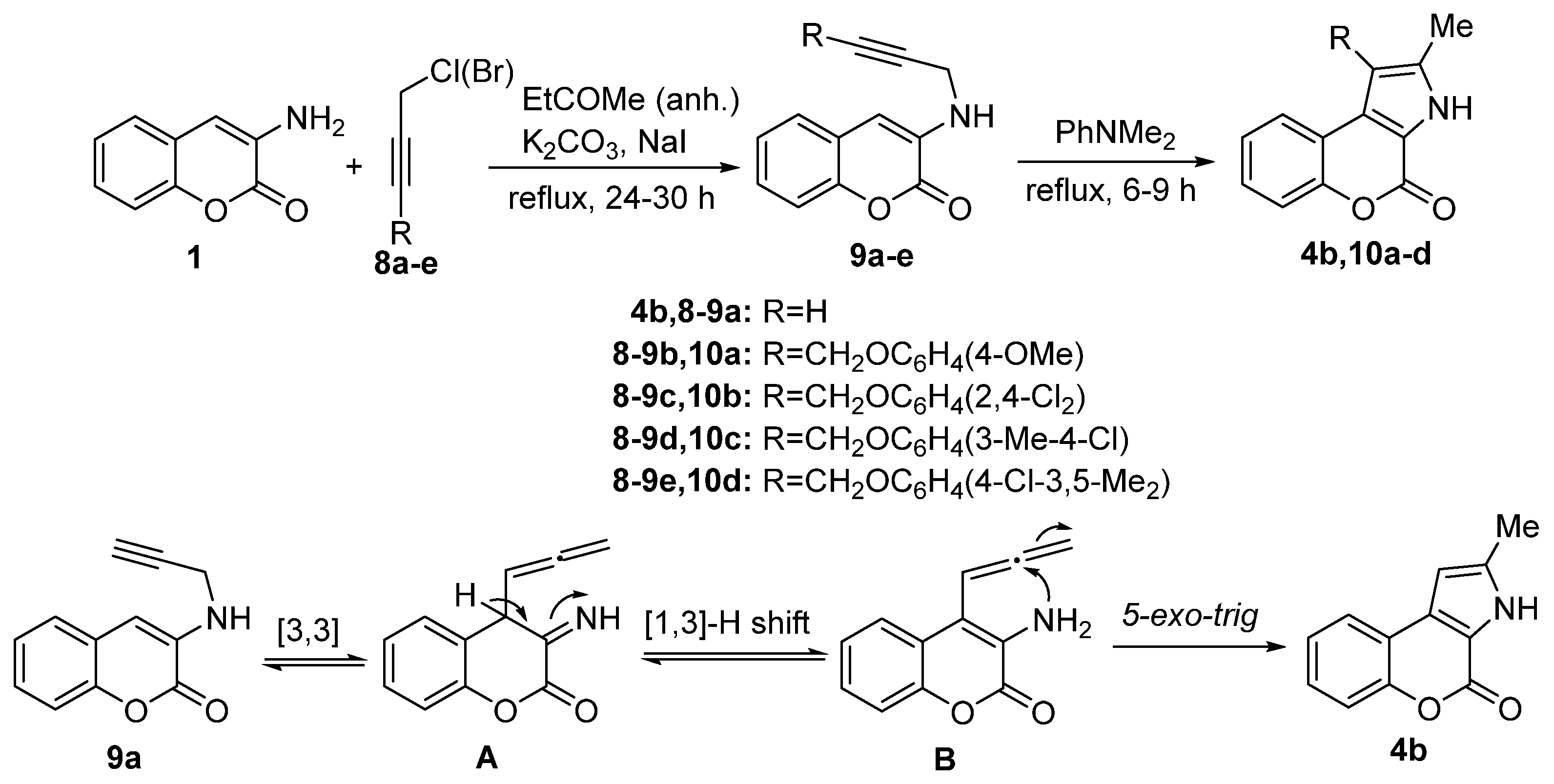
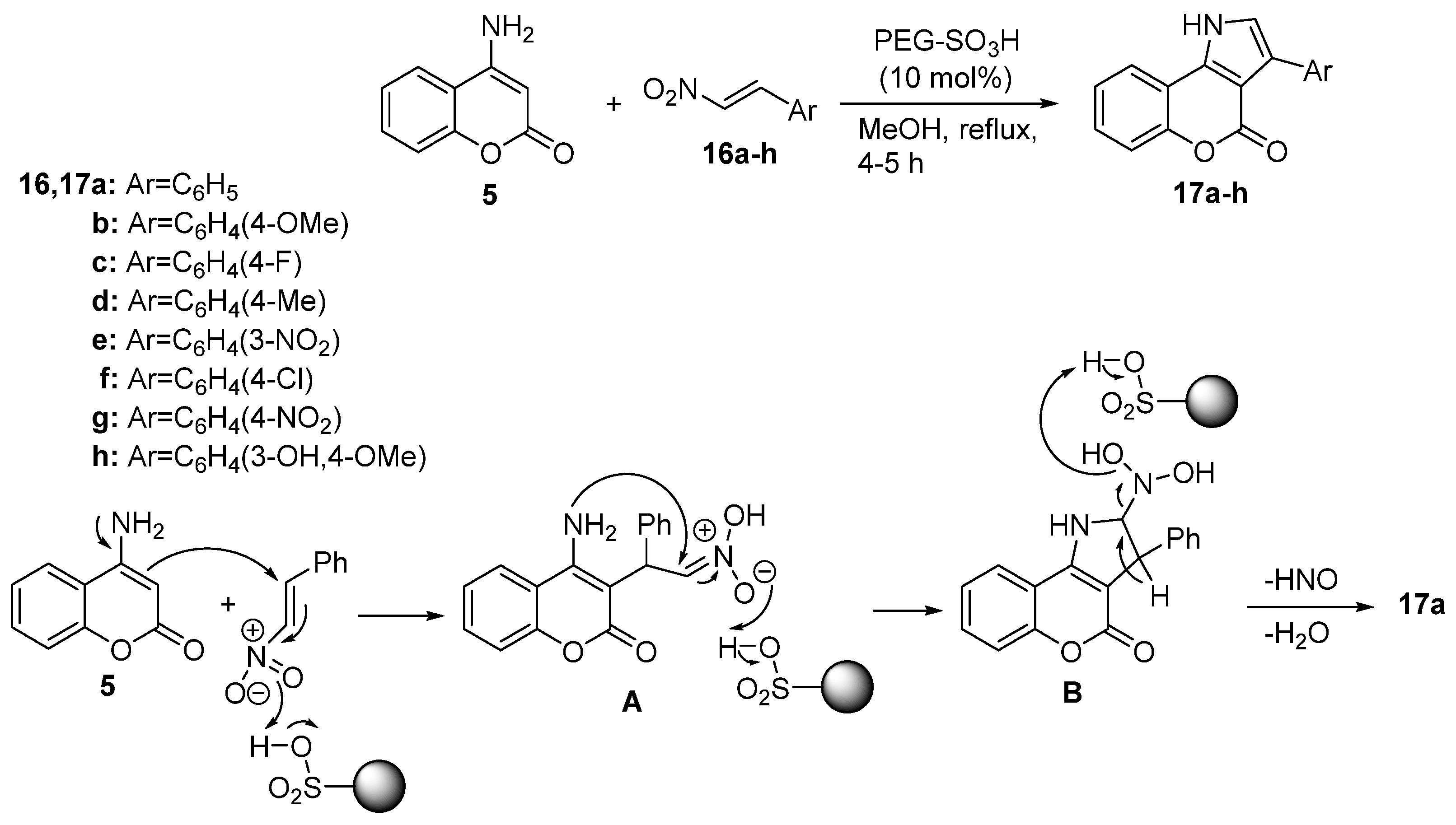
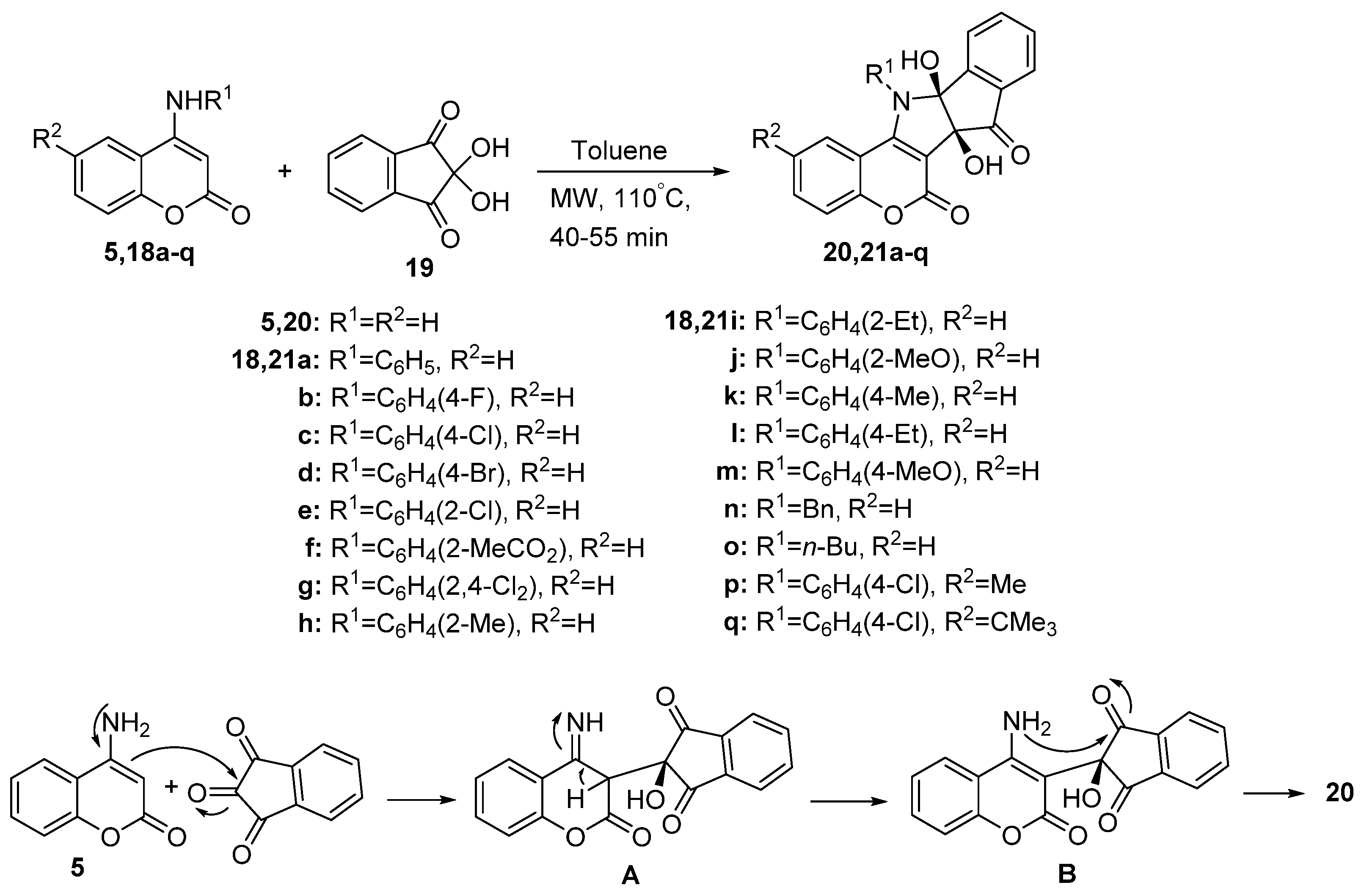
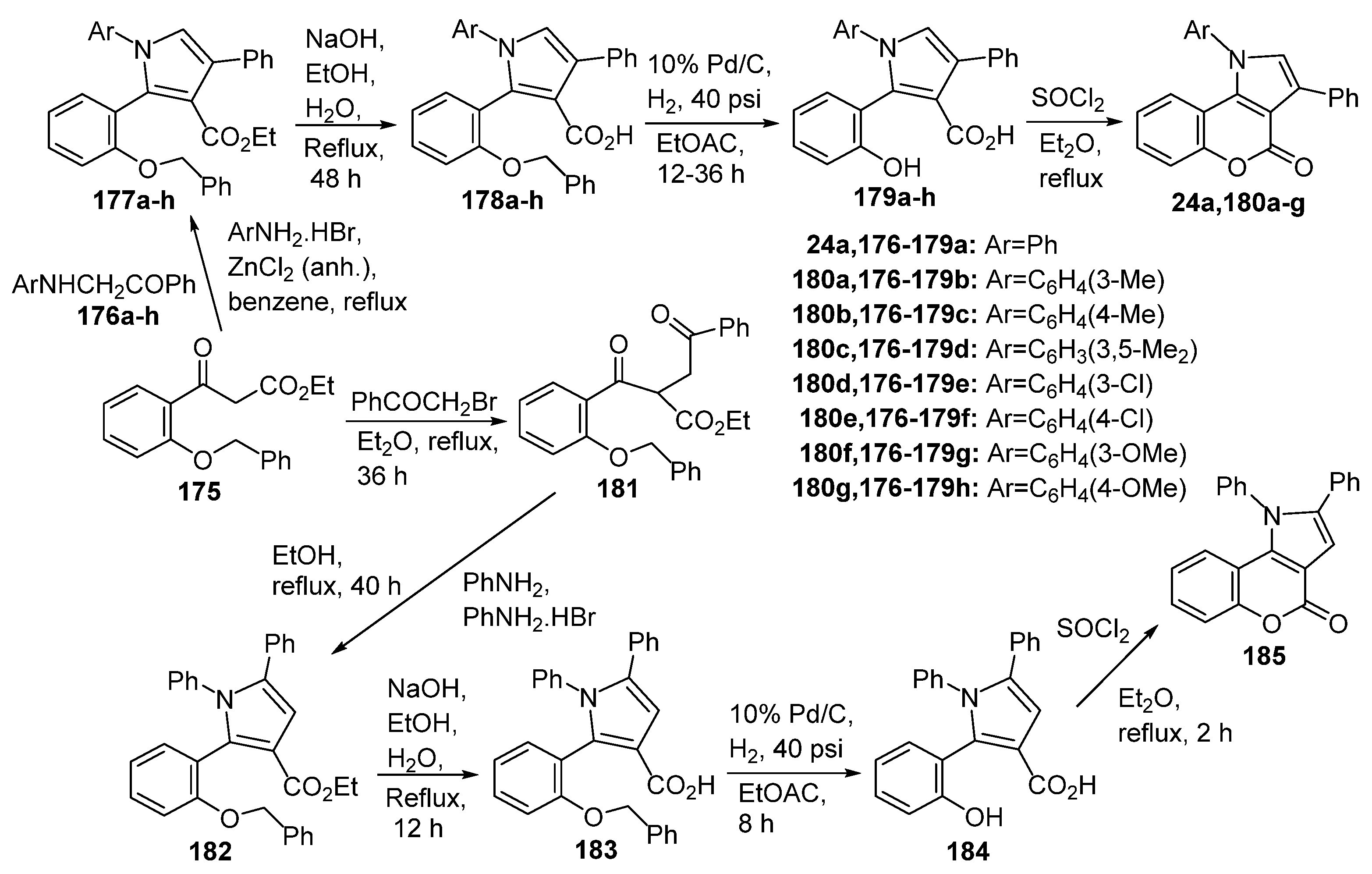
2.1.1. Synthesis from Aminocoumarin Derivatives
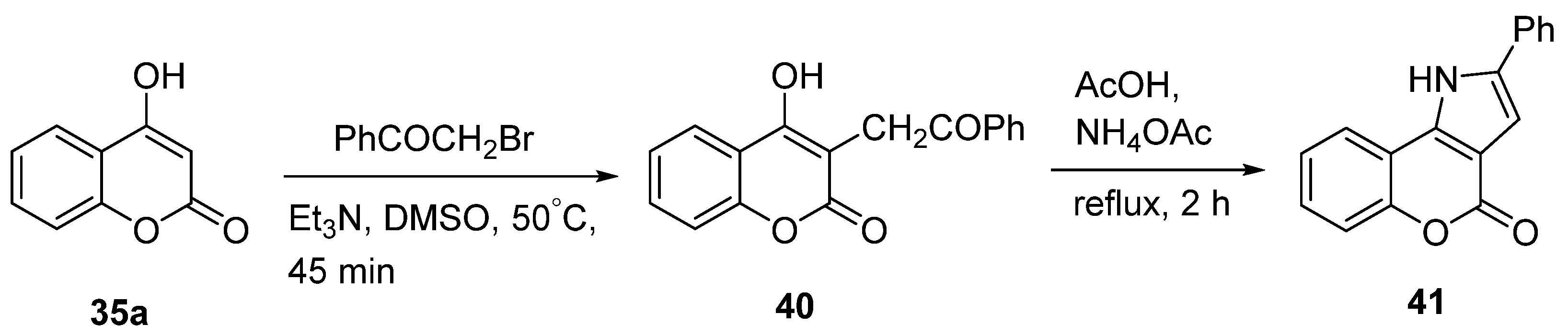
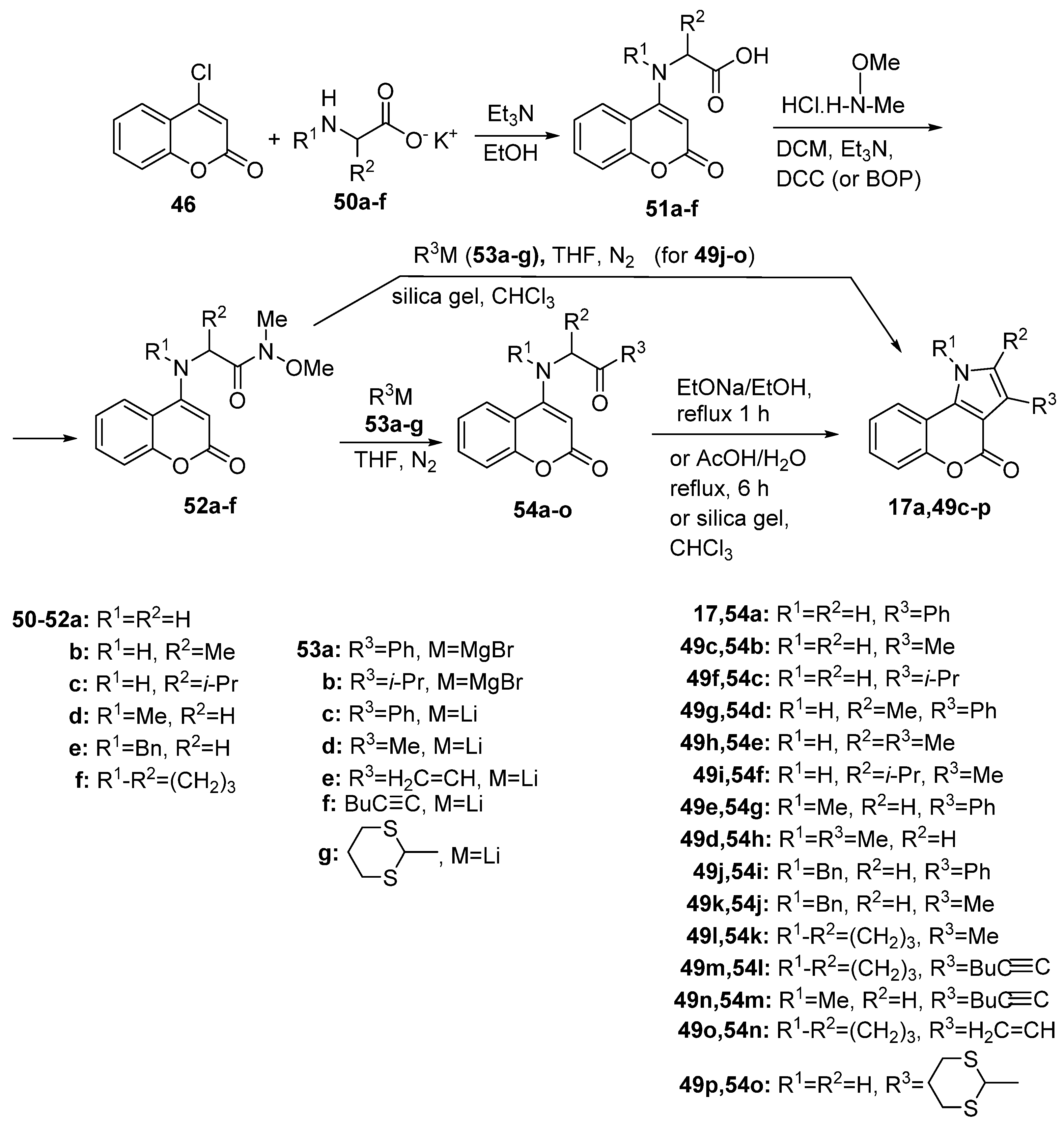
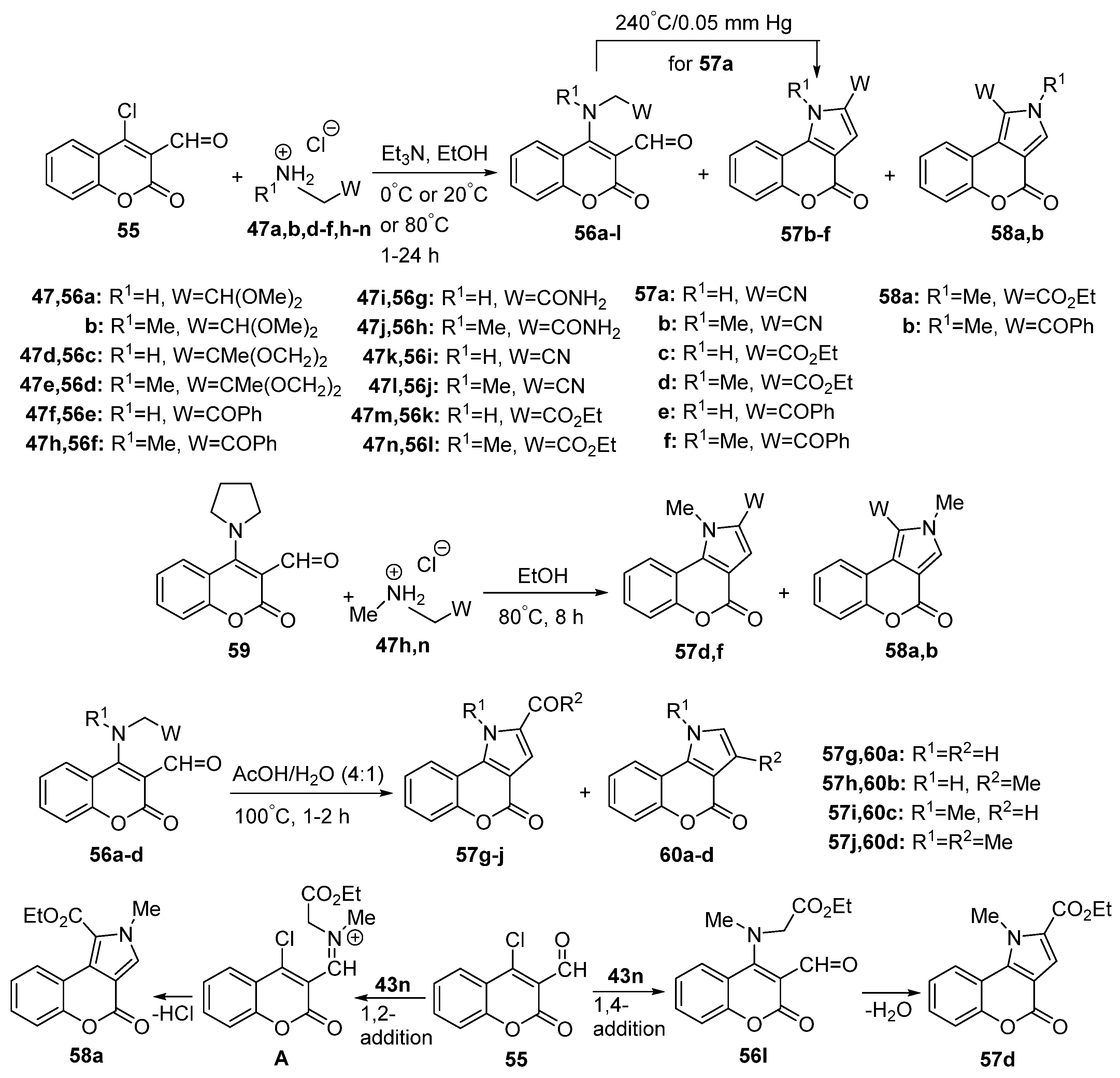
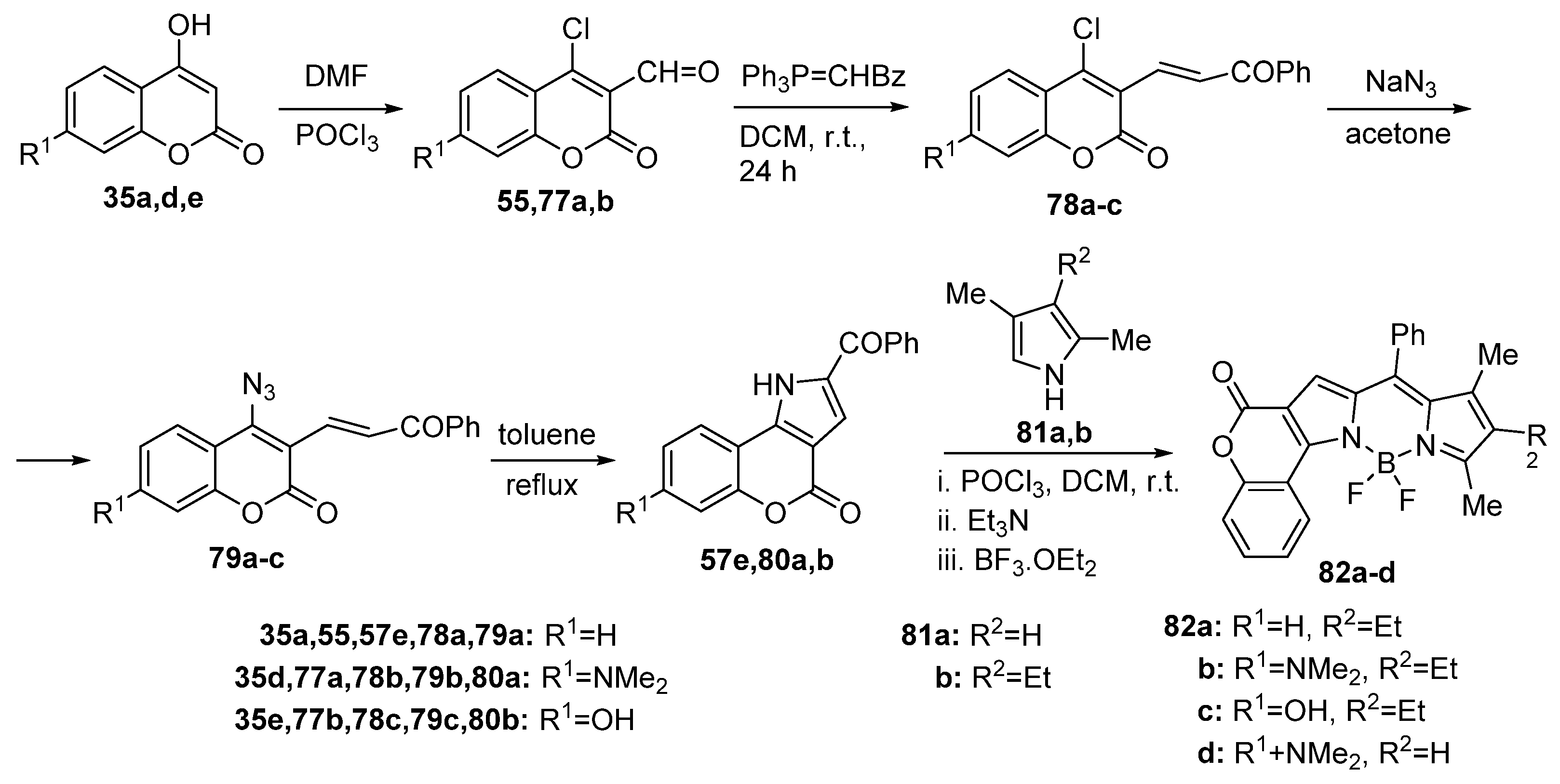
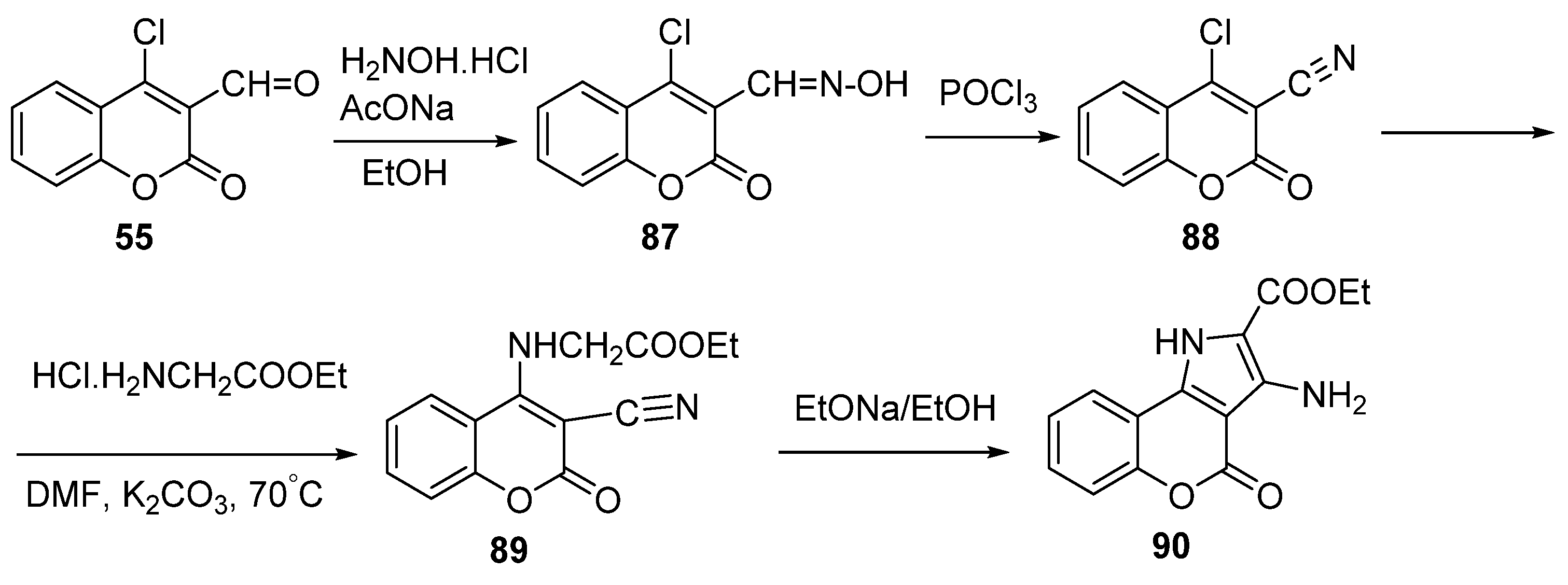
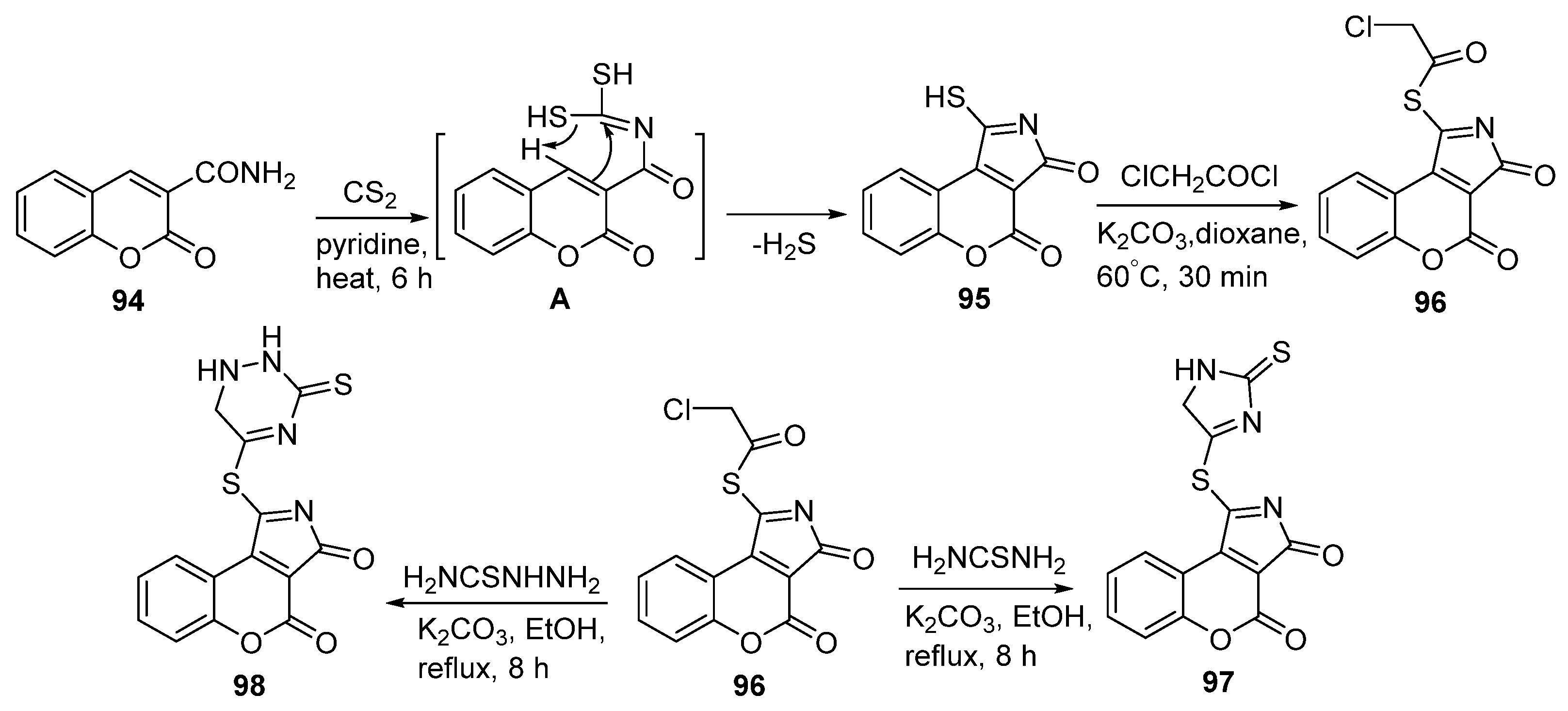
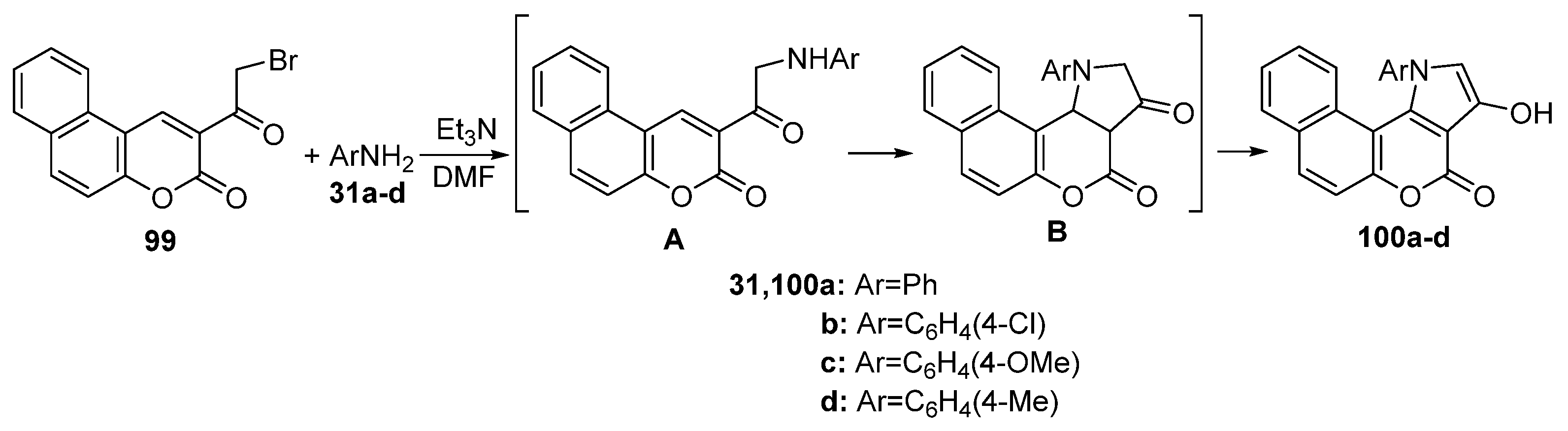
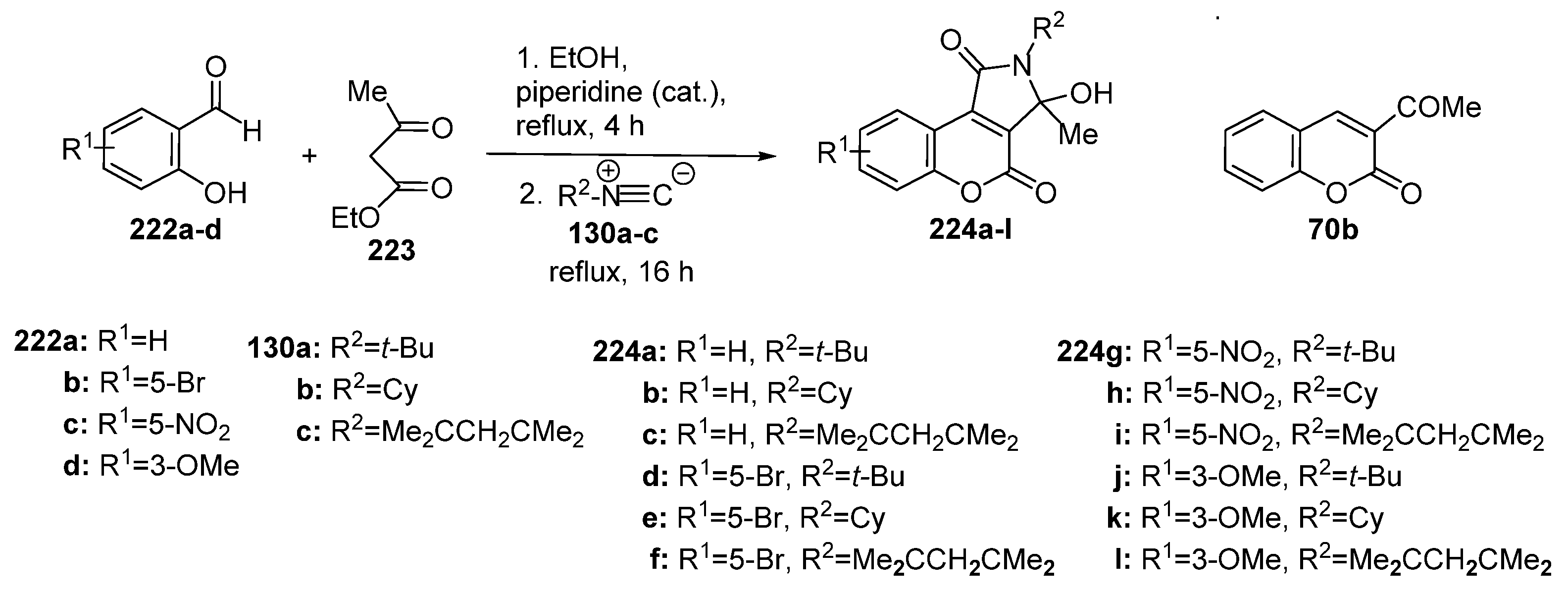
2.1.2. Synthesis from Other Coumarin Derivatives
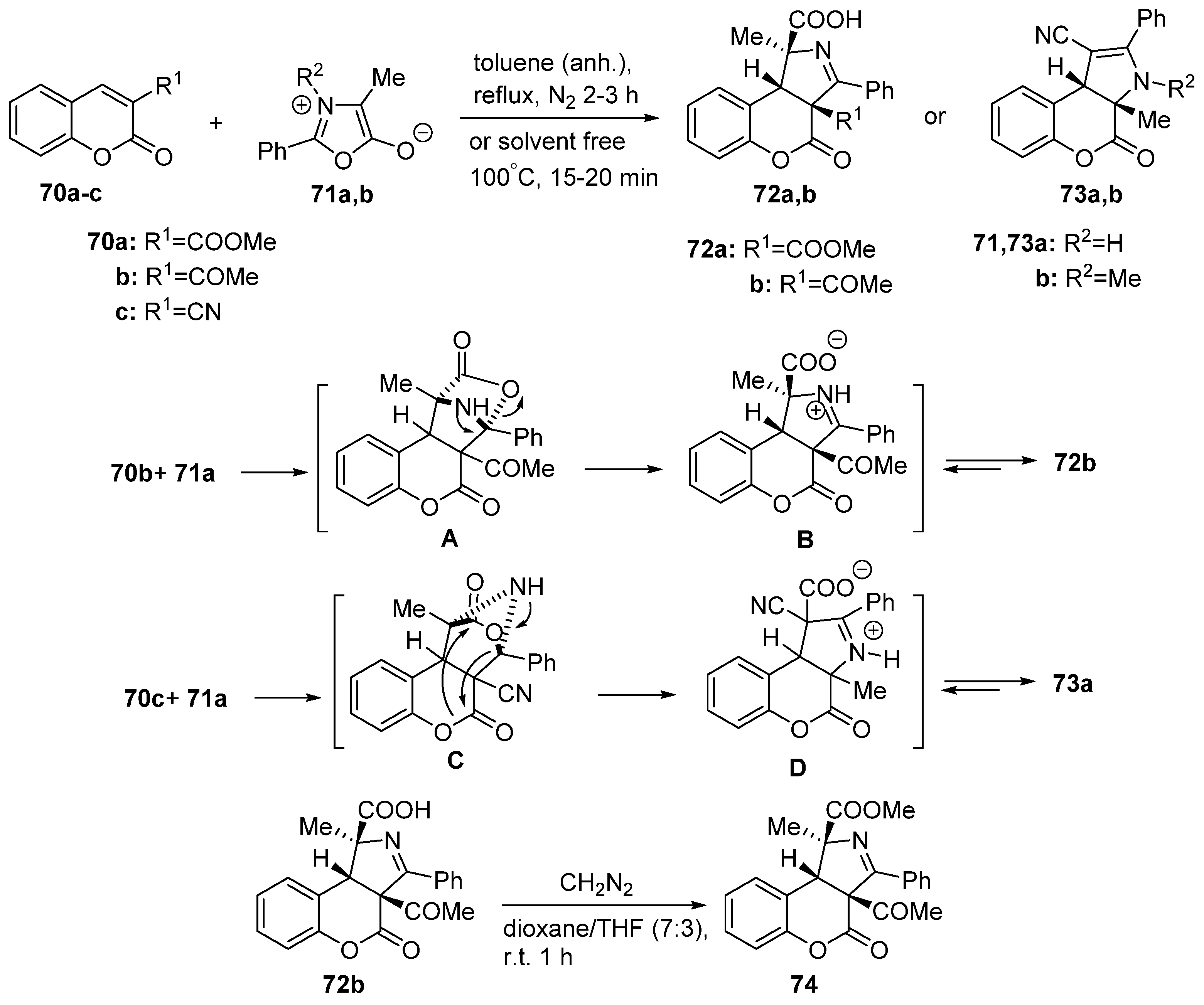
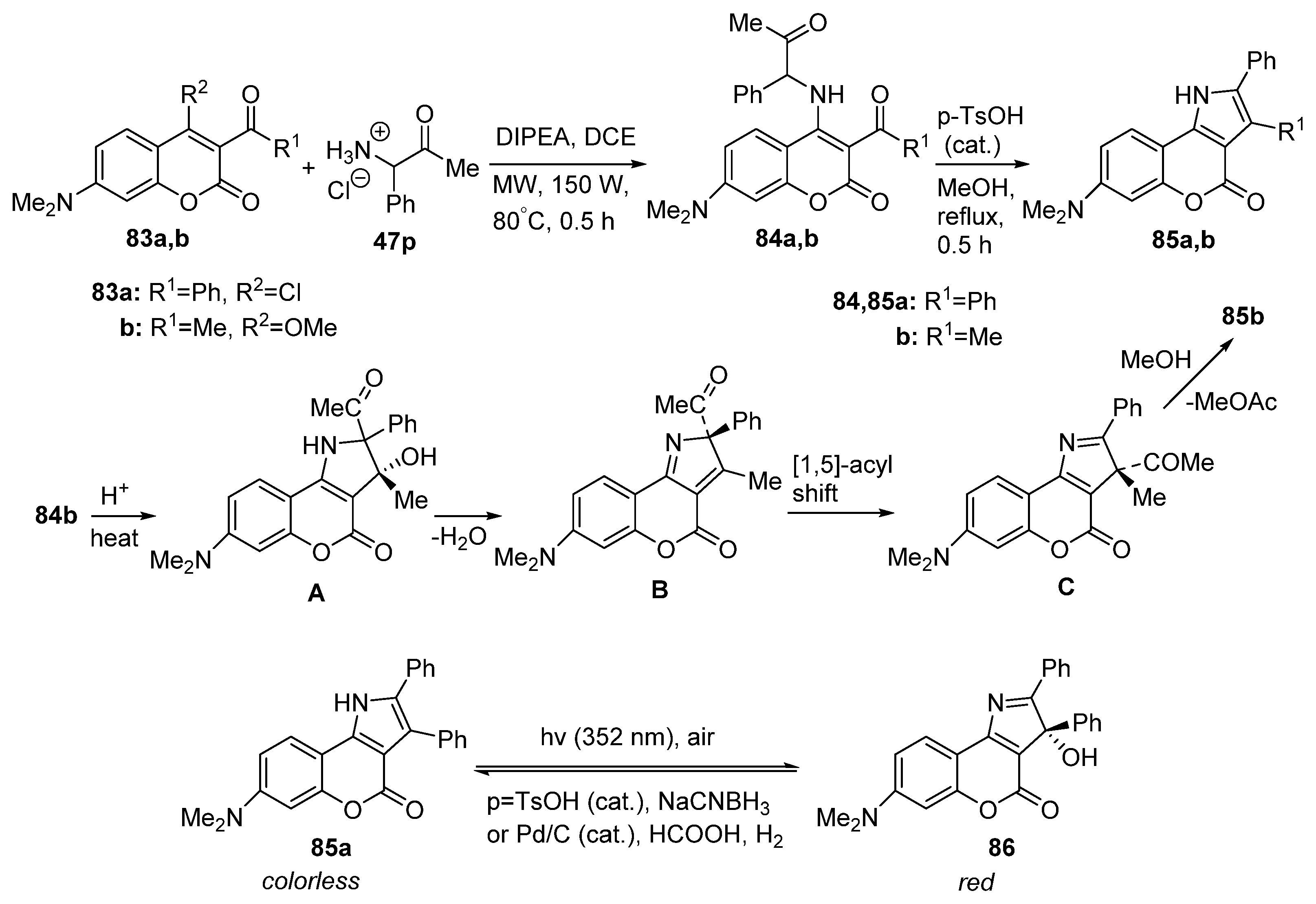
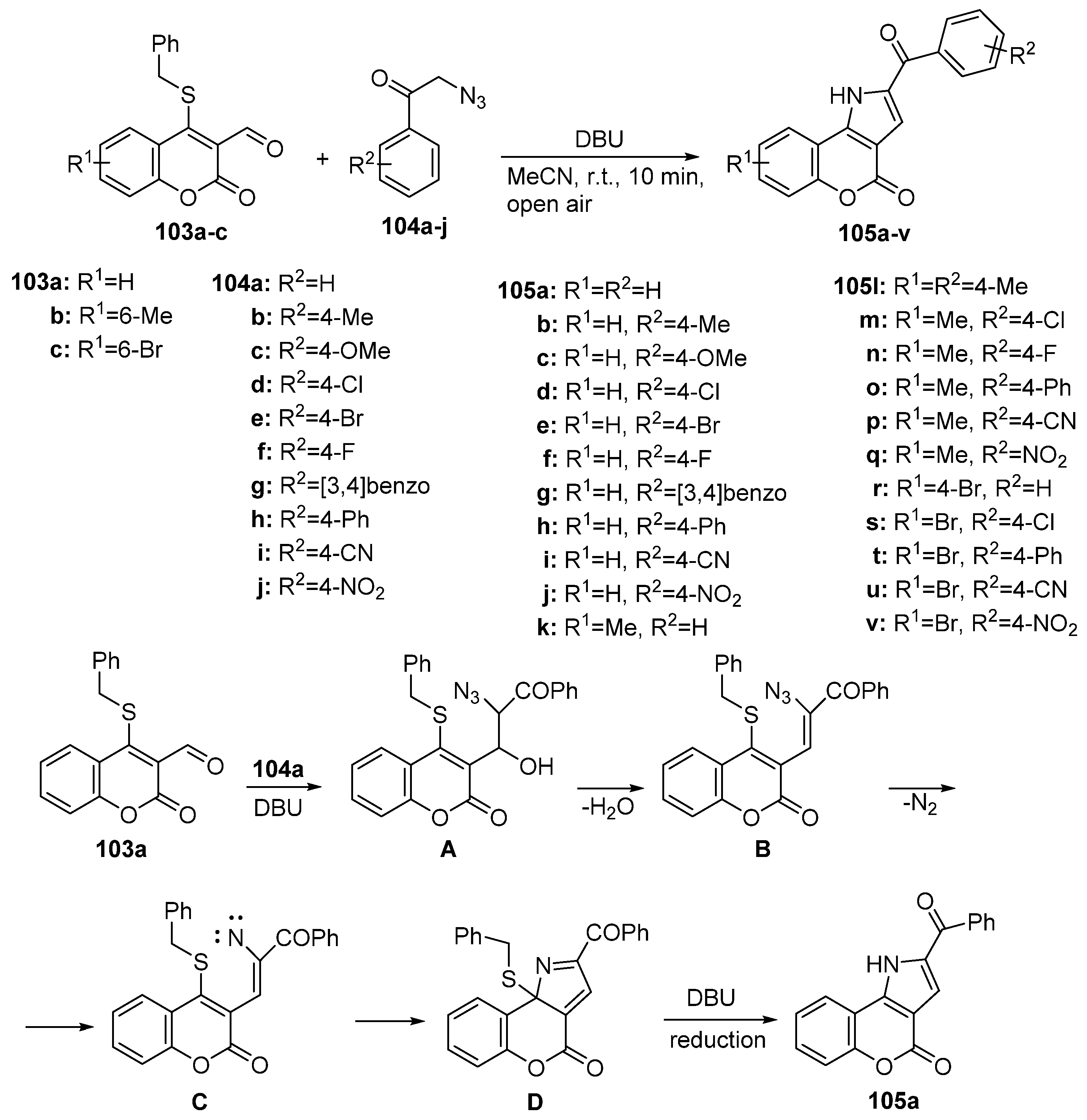
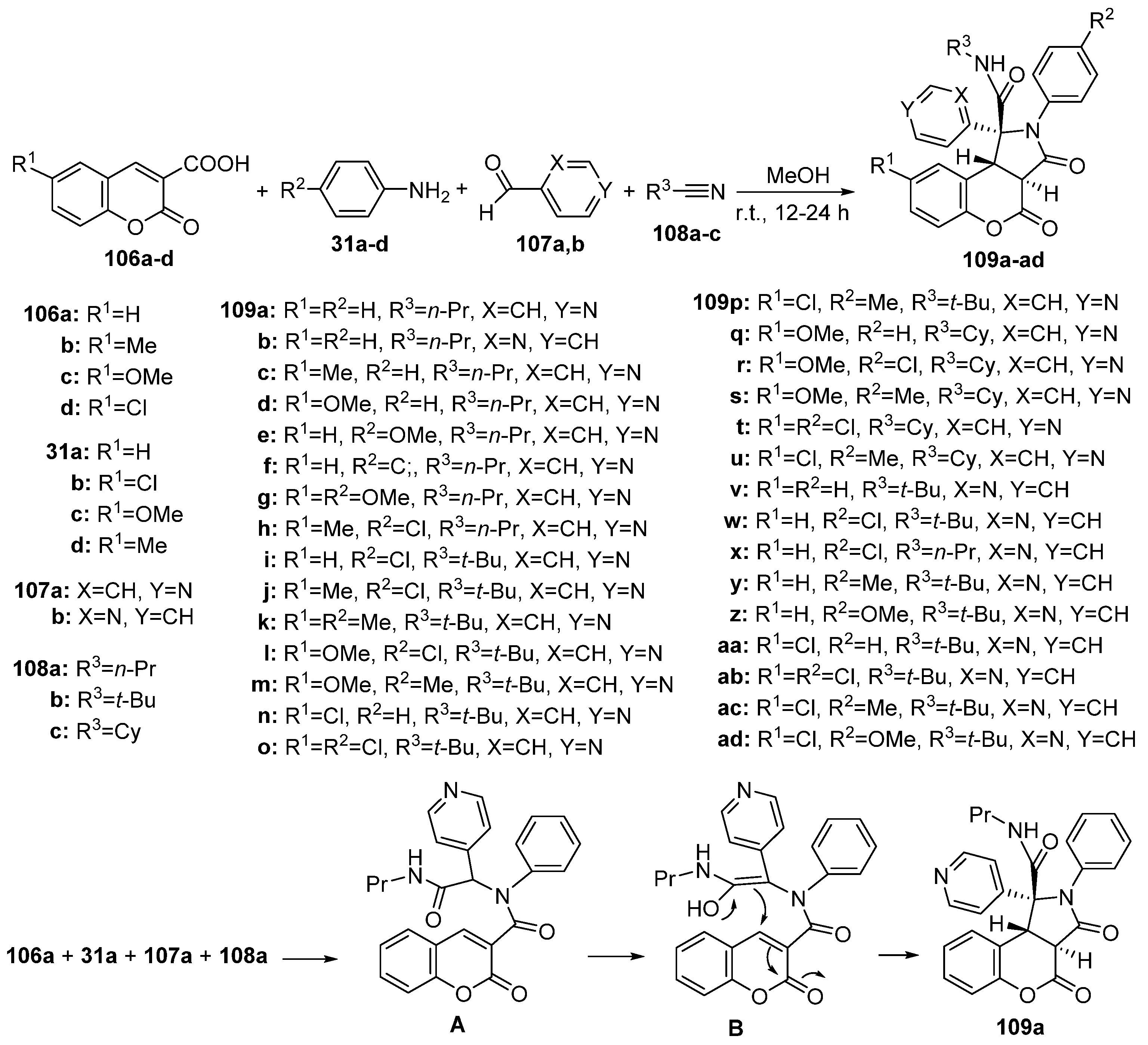
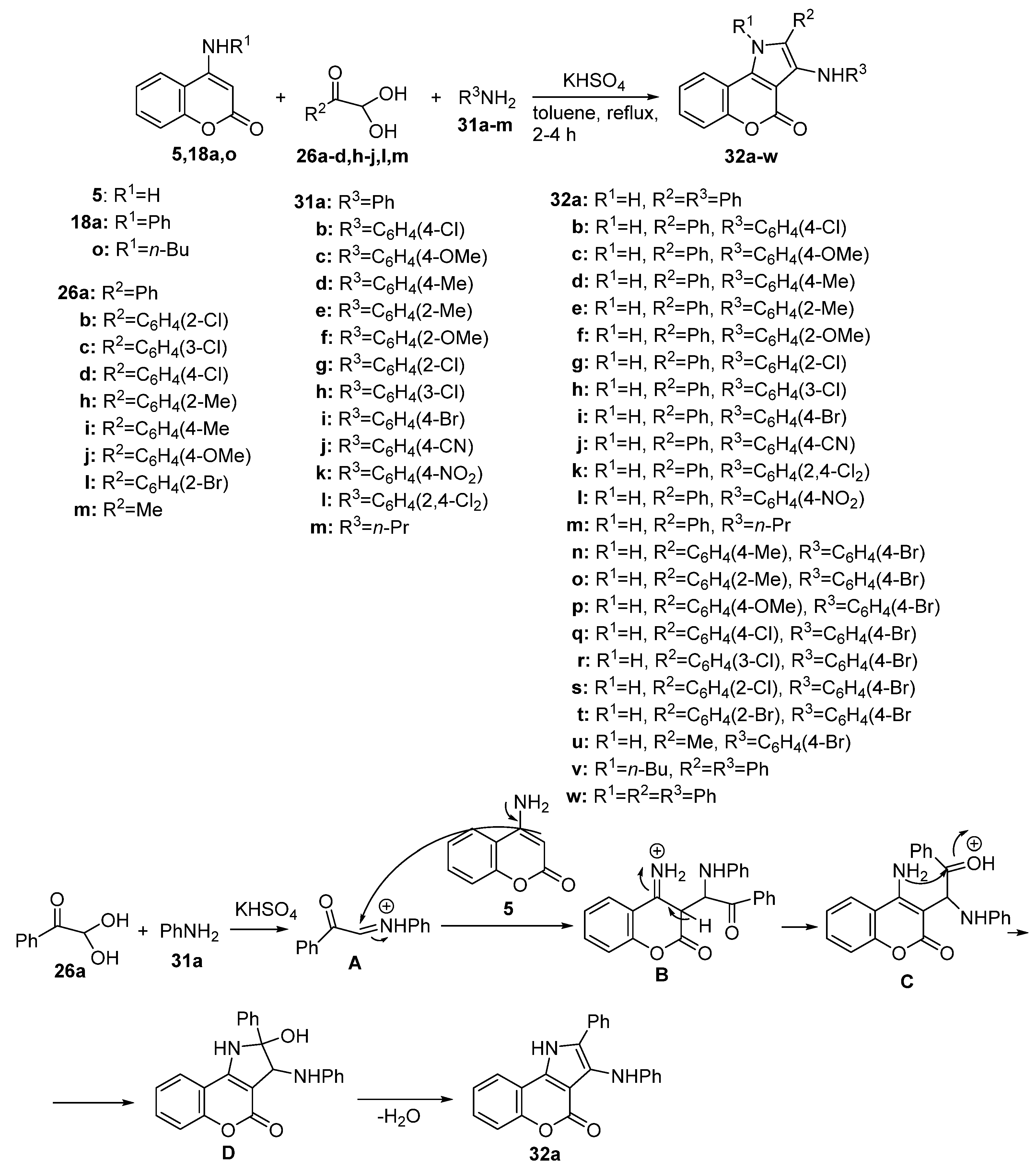
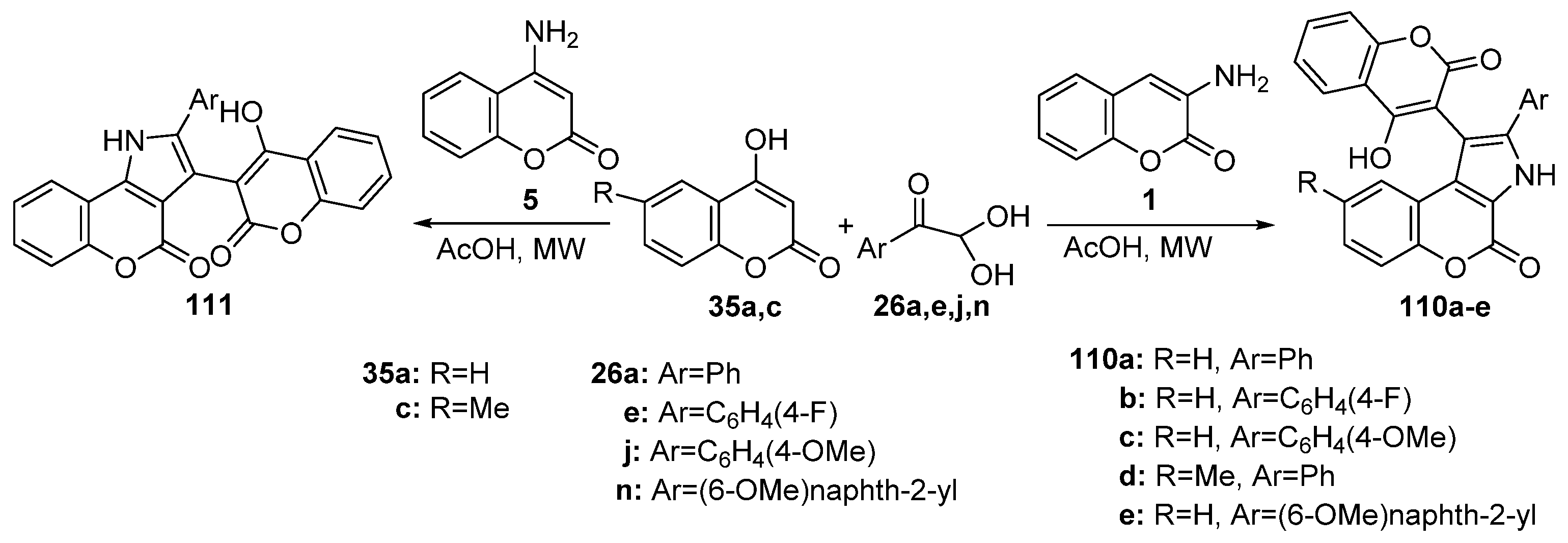
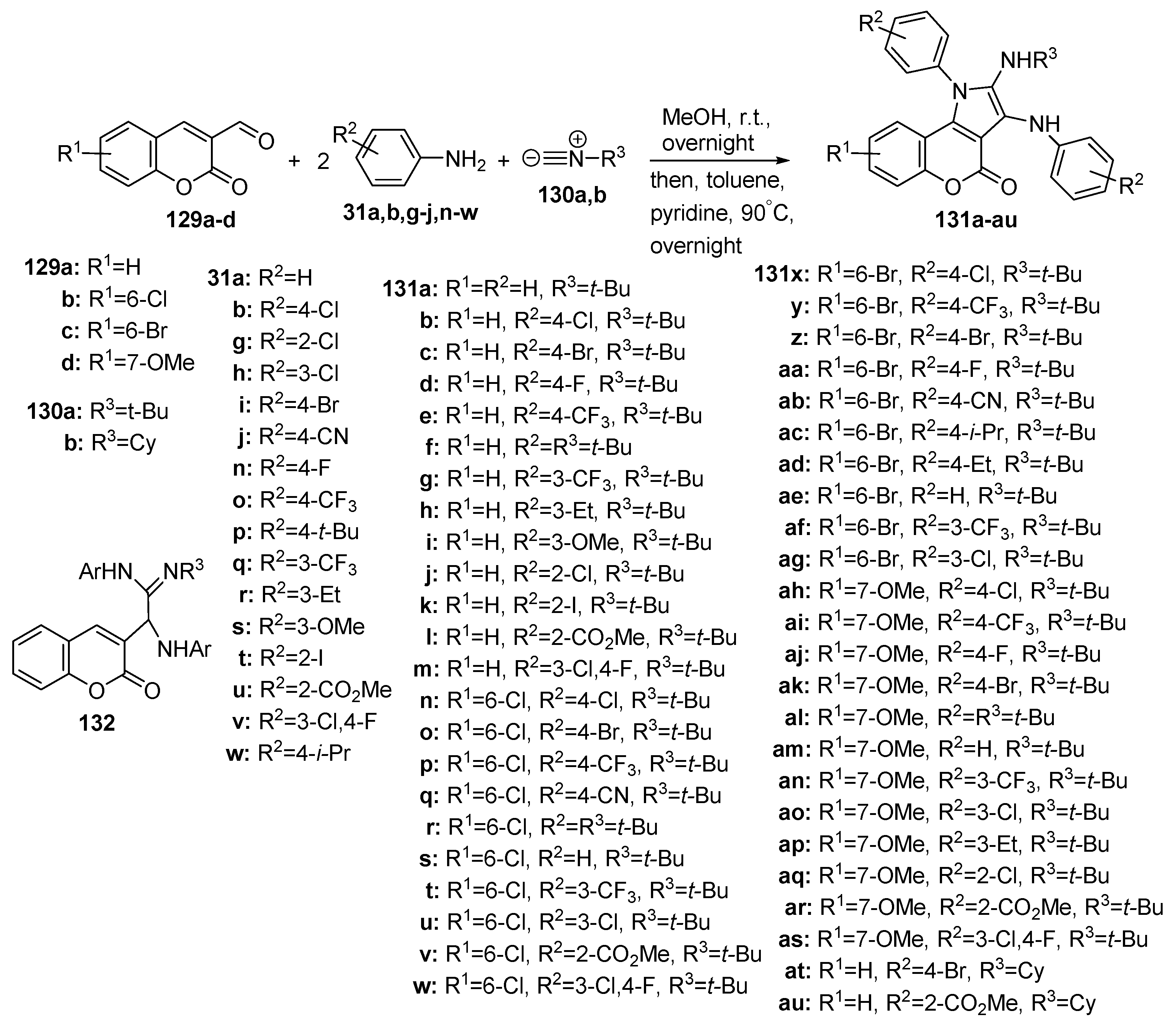
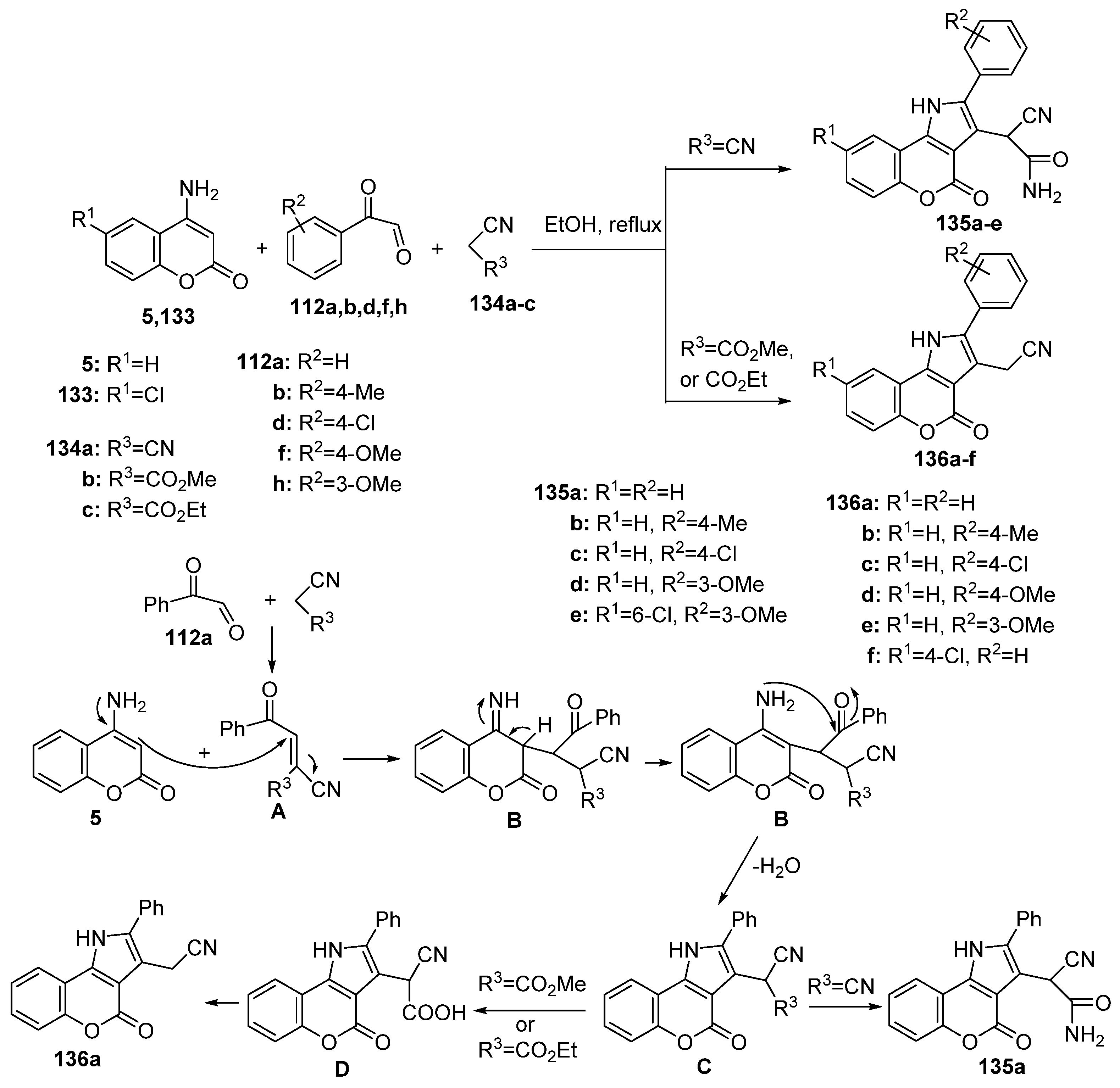
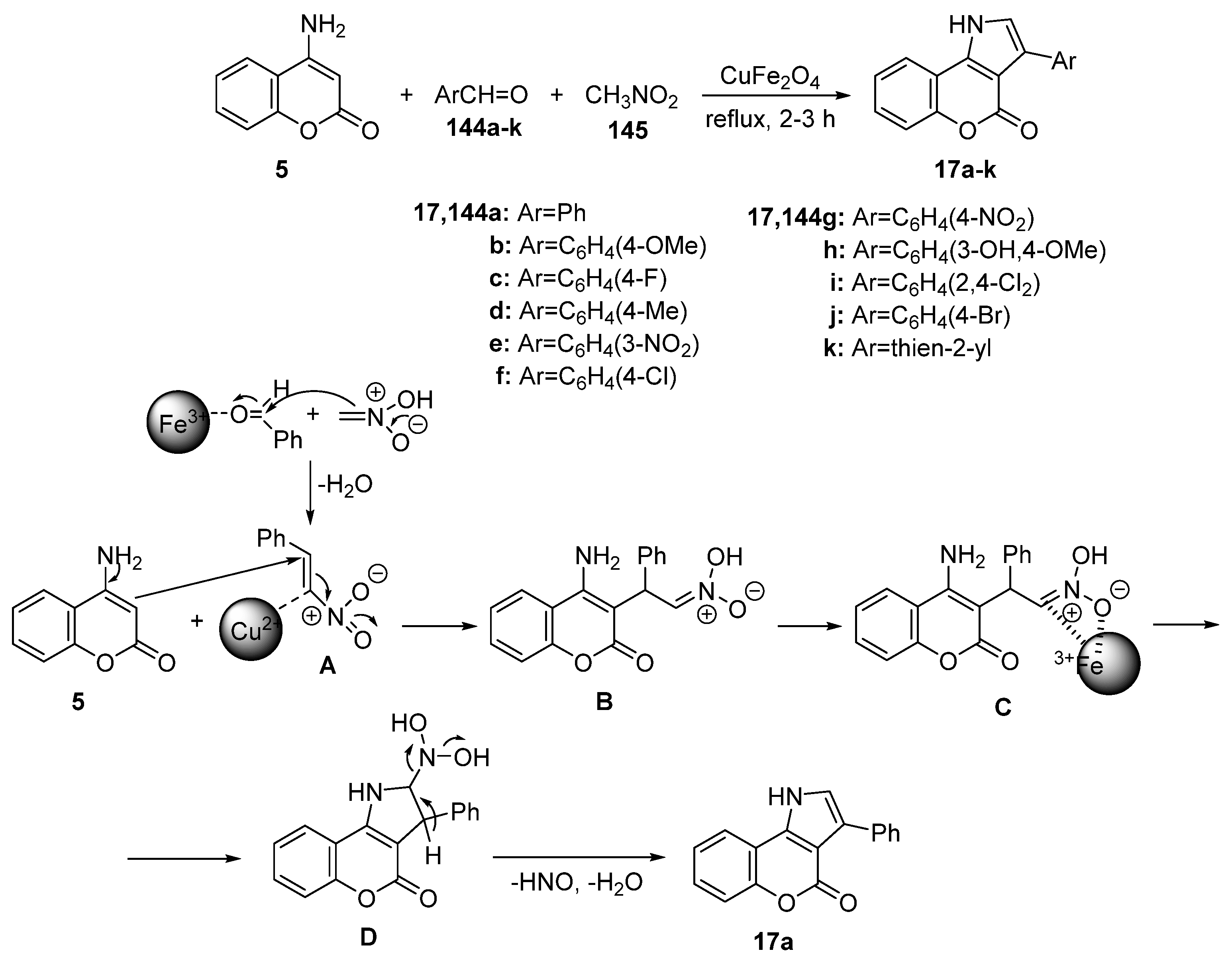
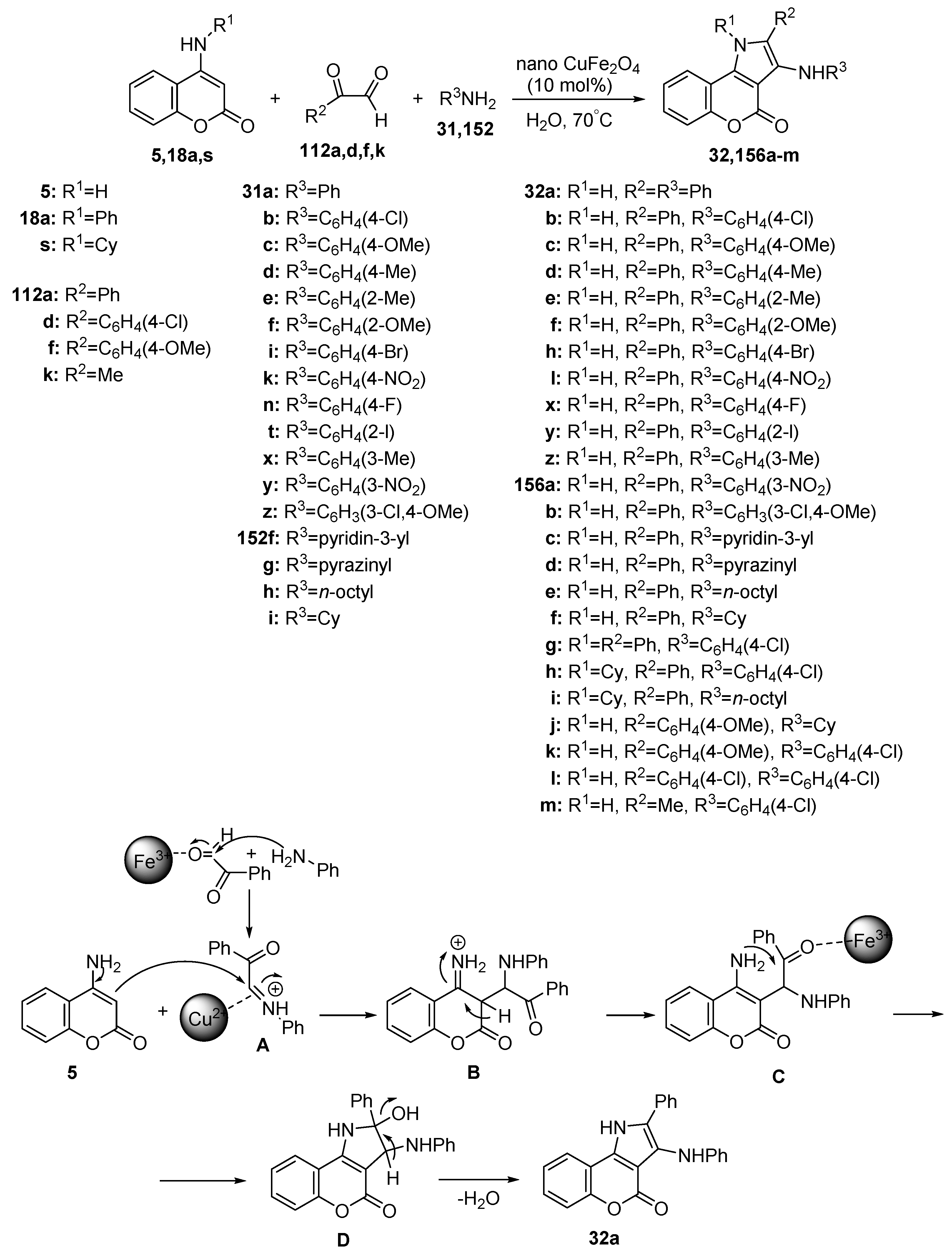
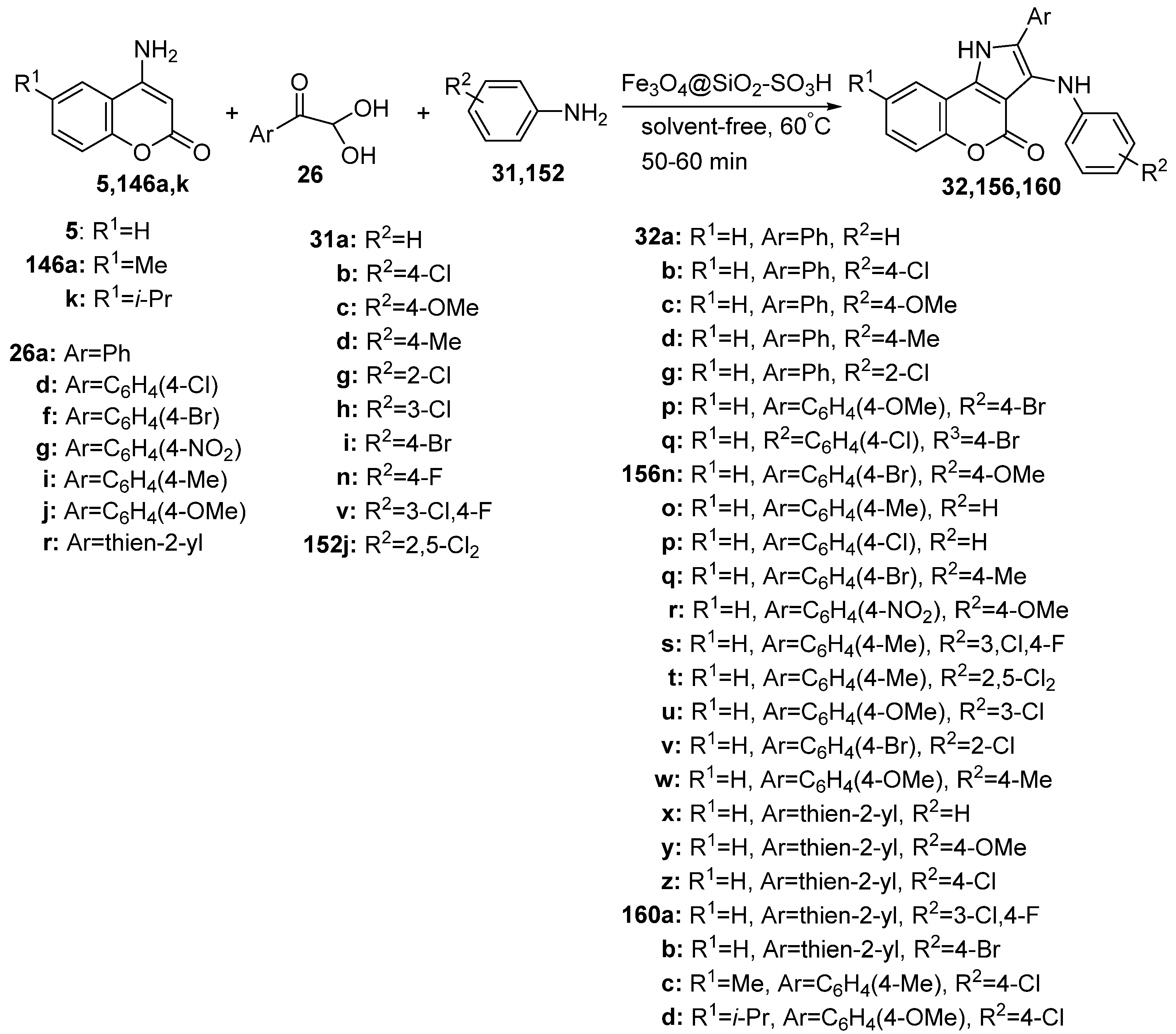
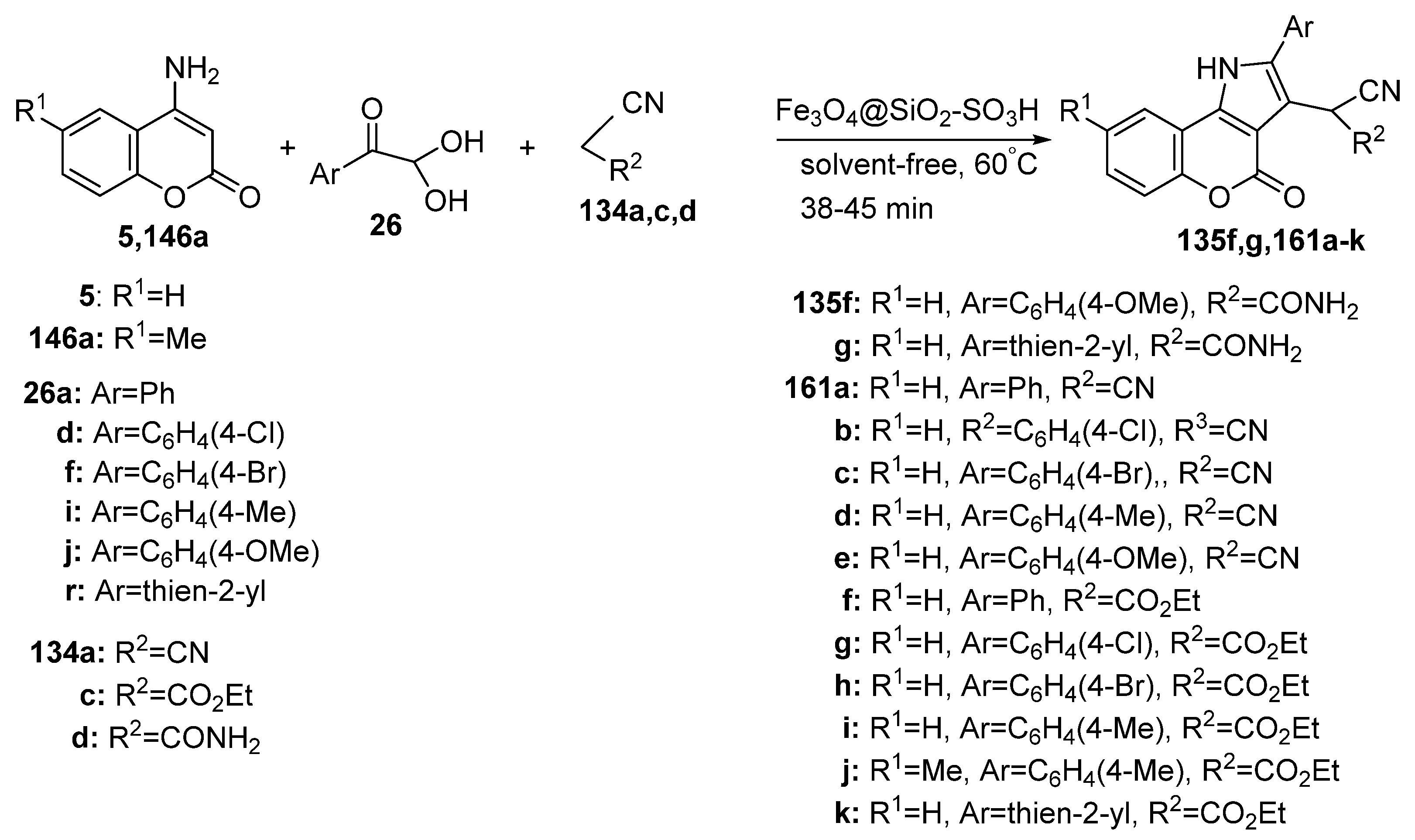
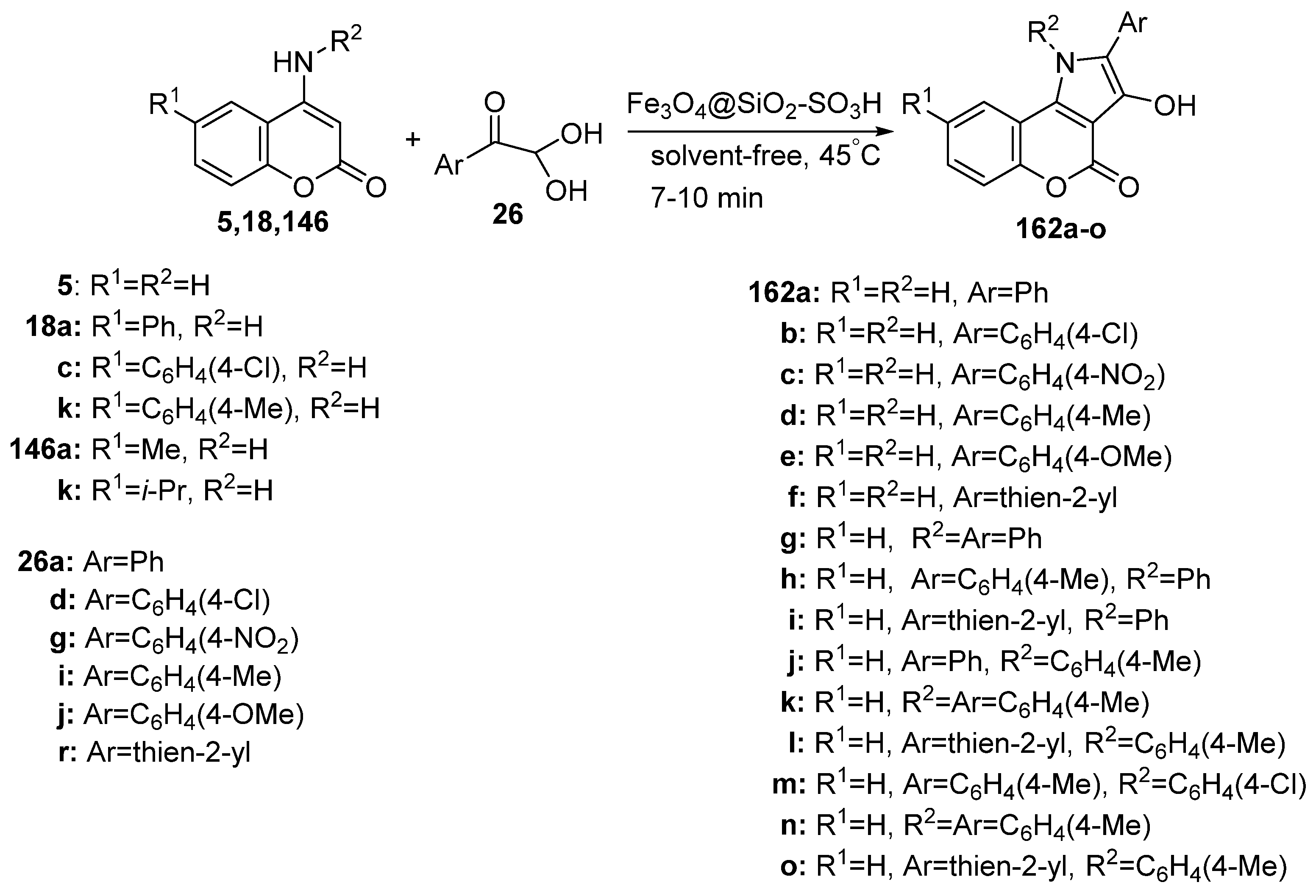
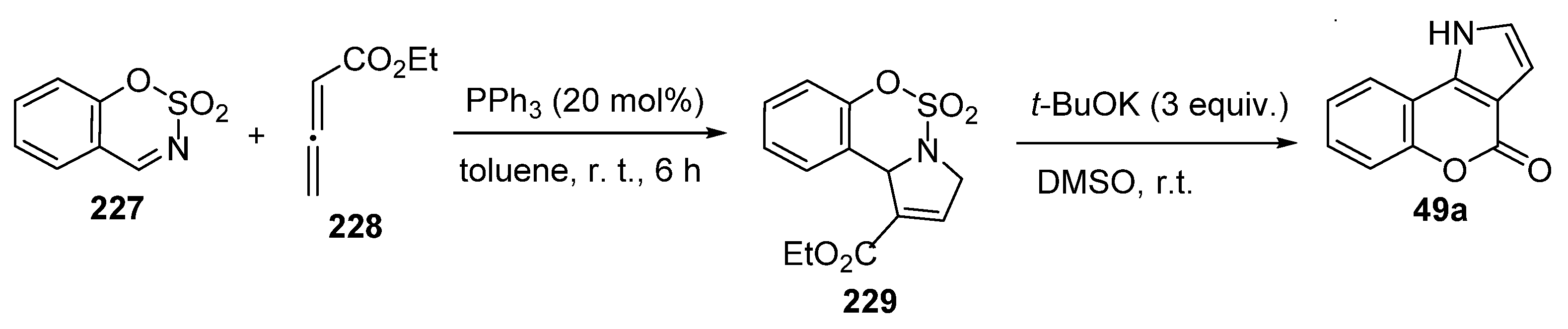
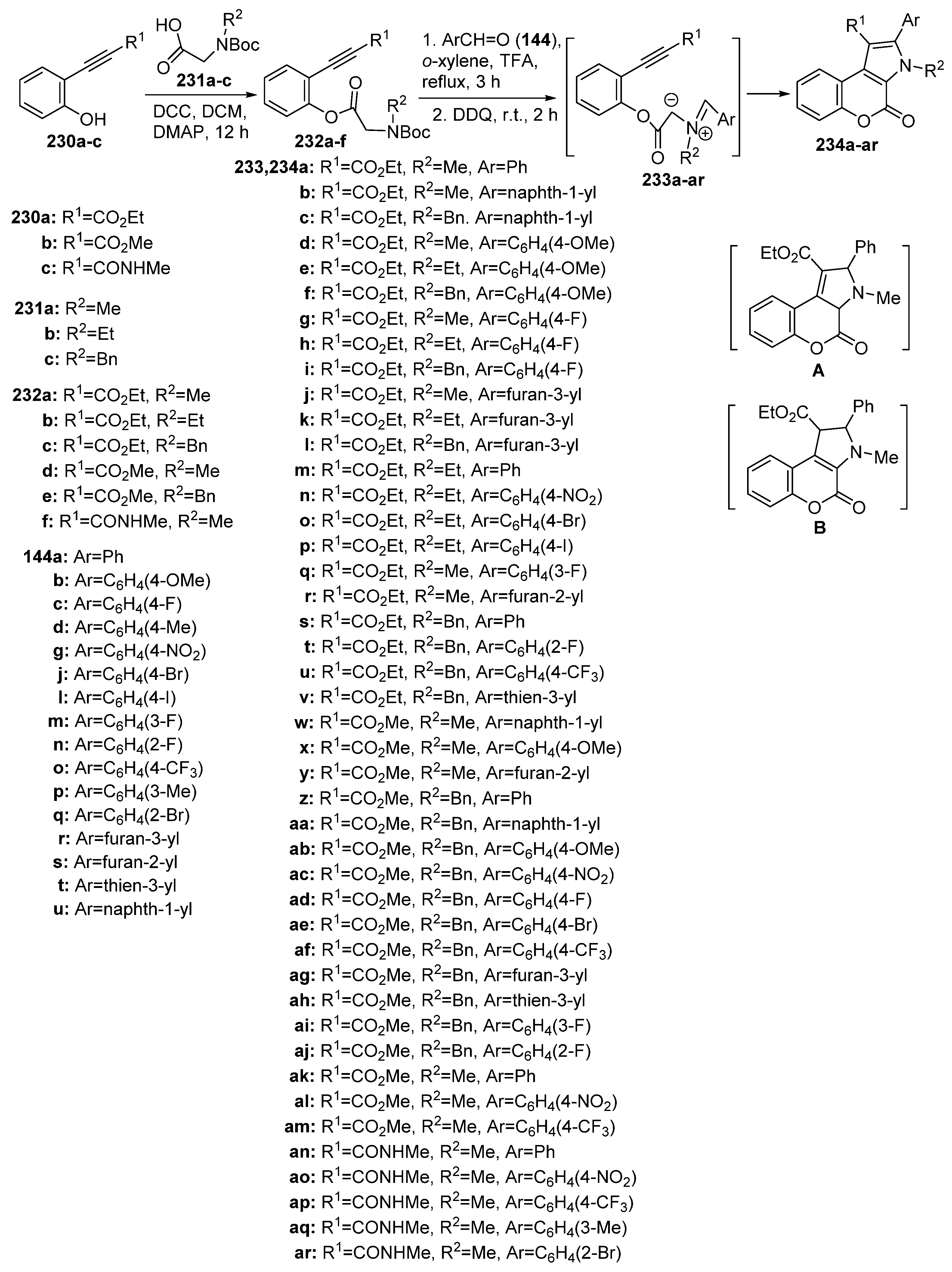
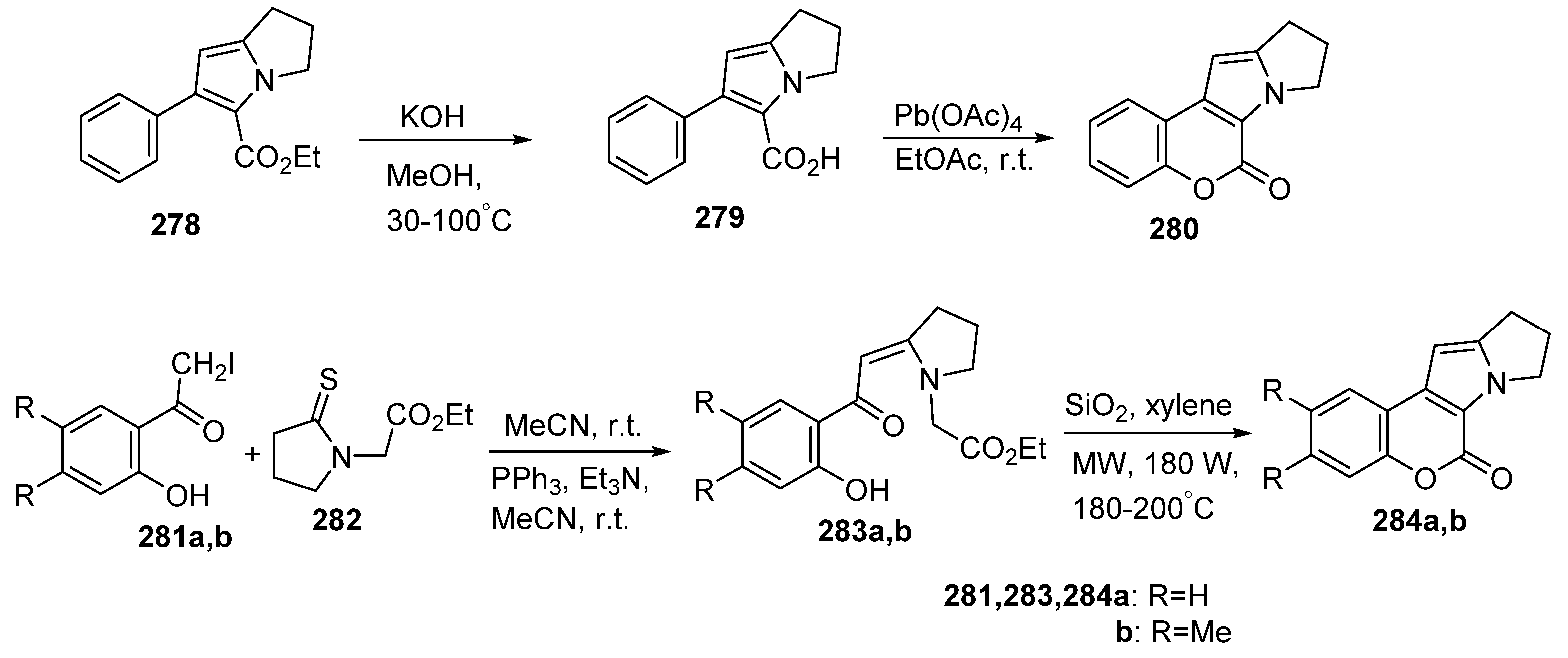
2.1.3. Synthesis through MCR Reactions
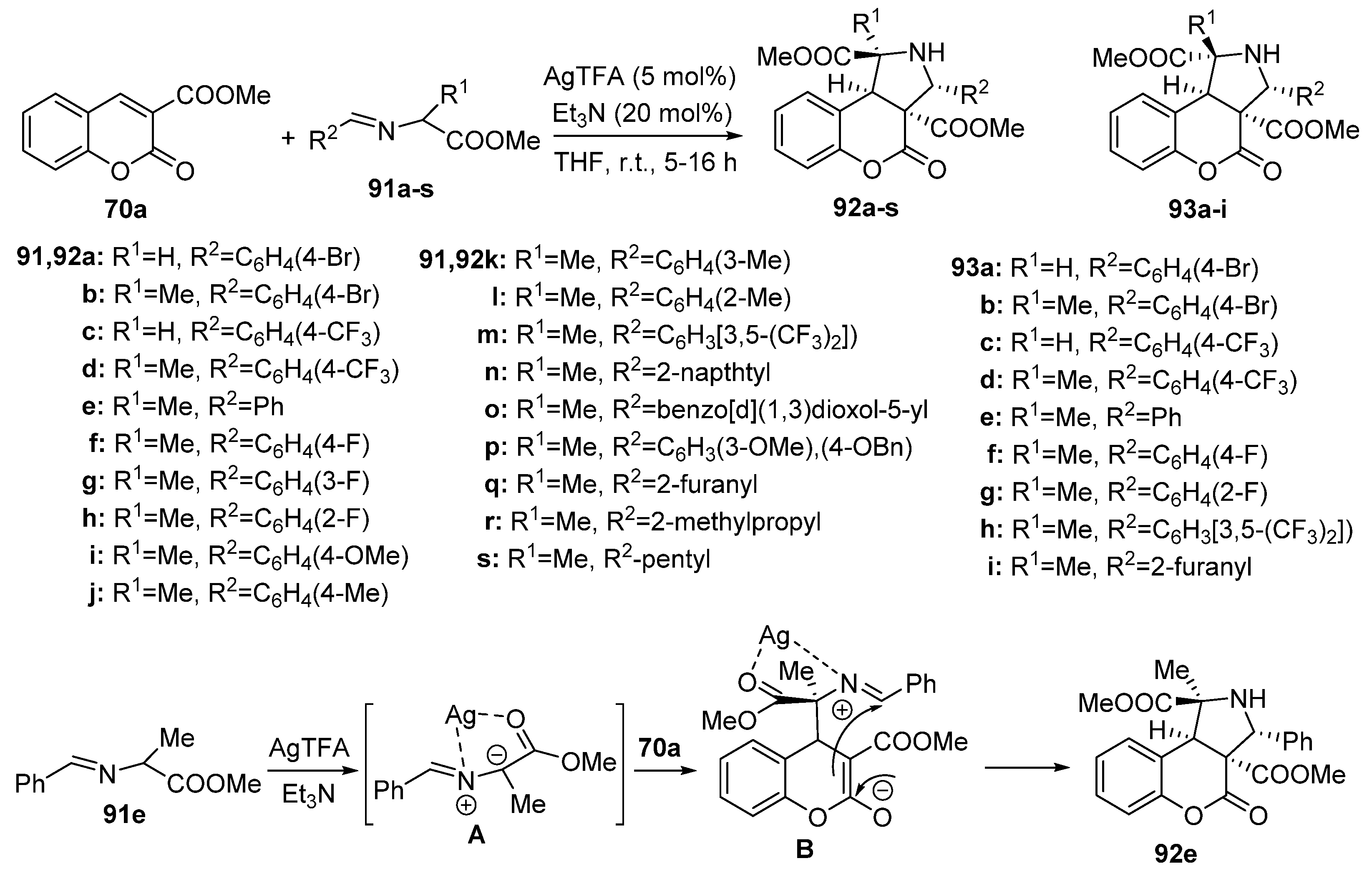
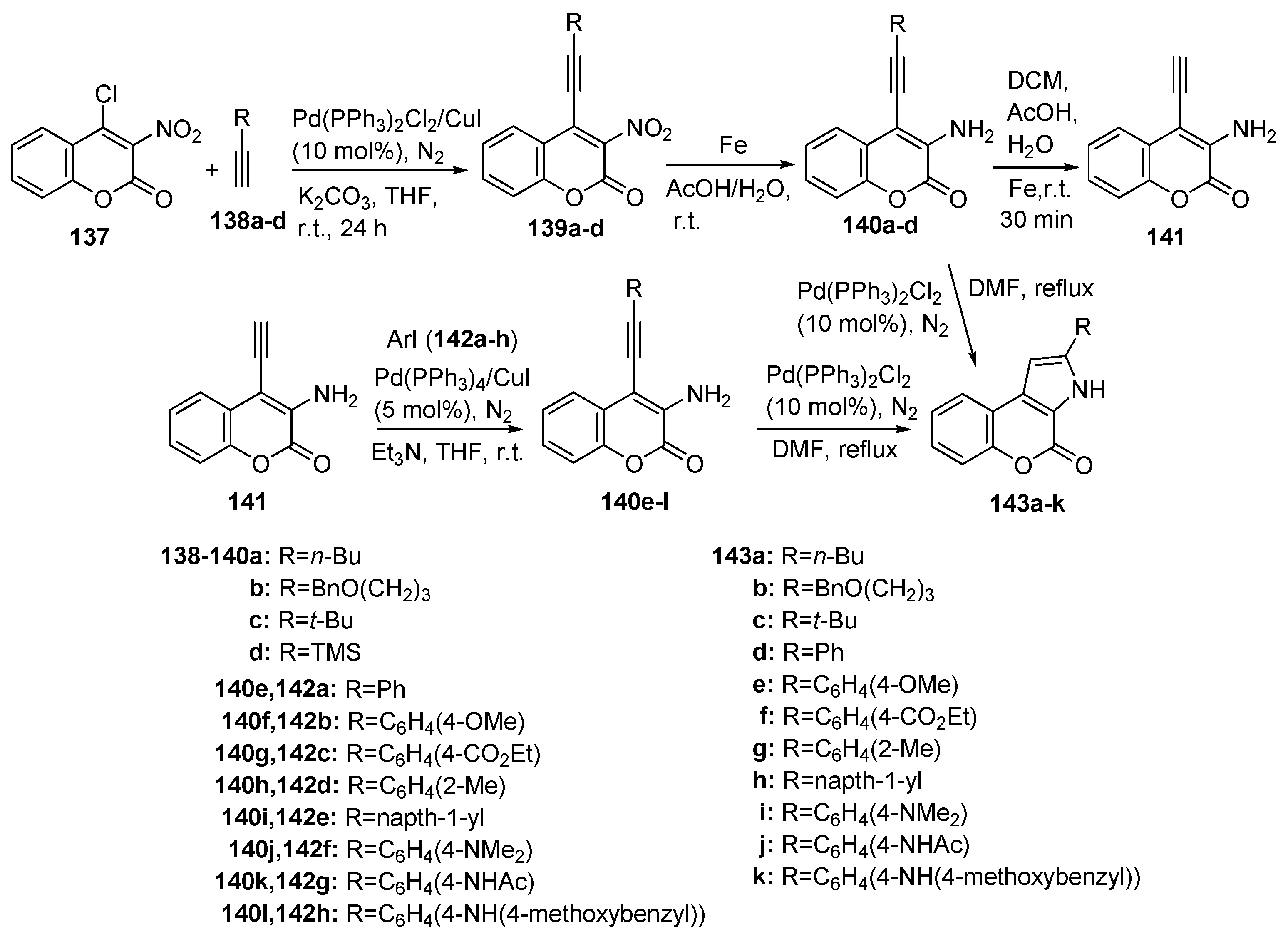
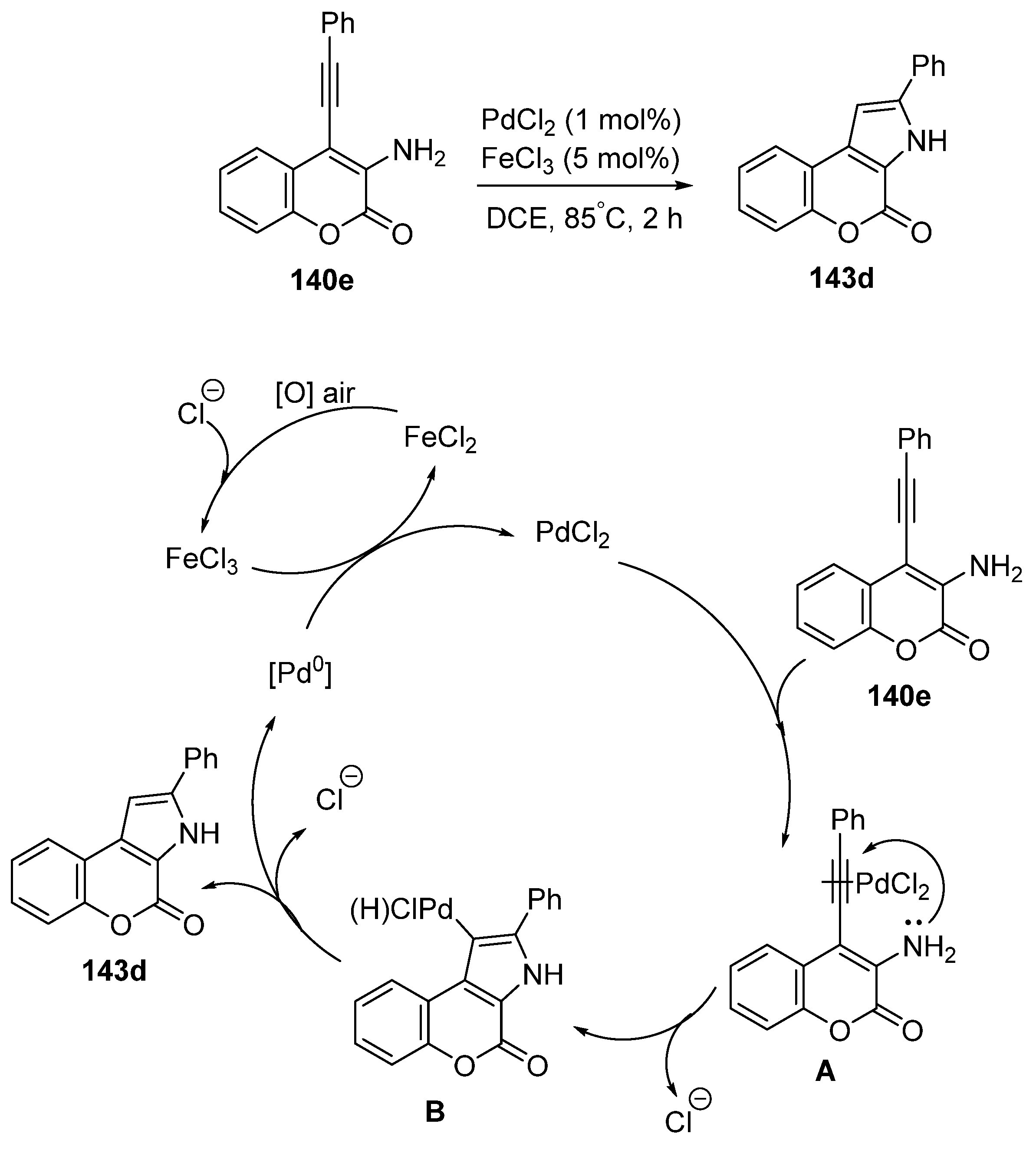
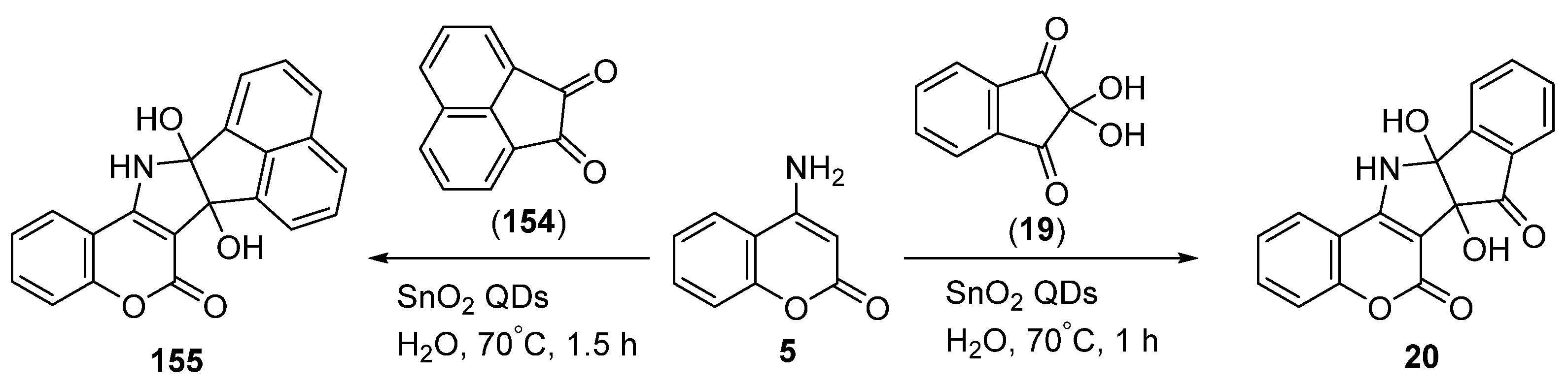
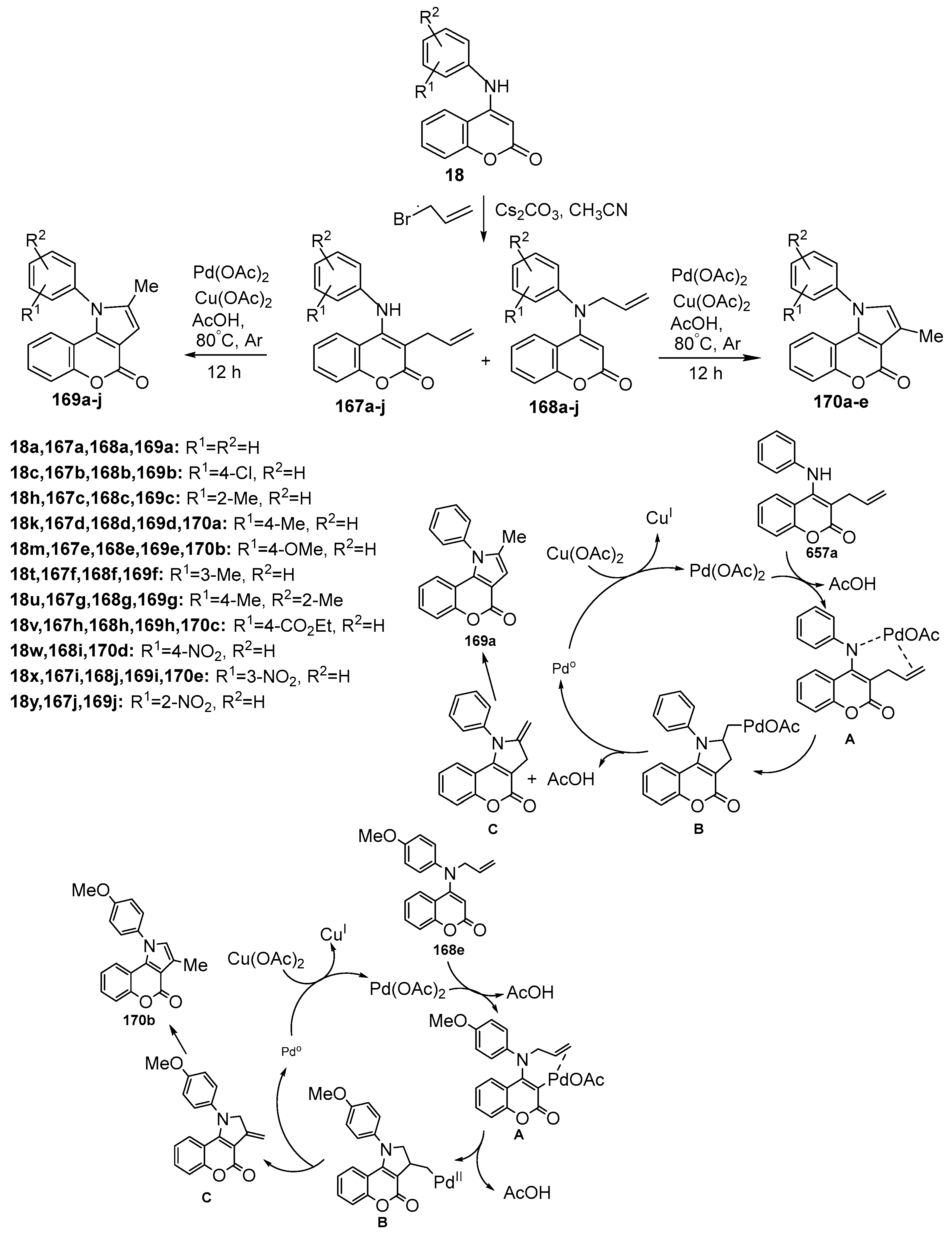
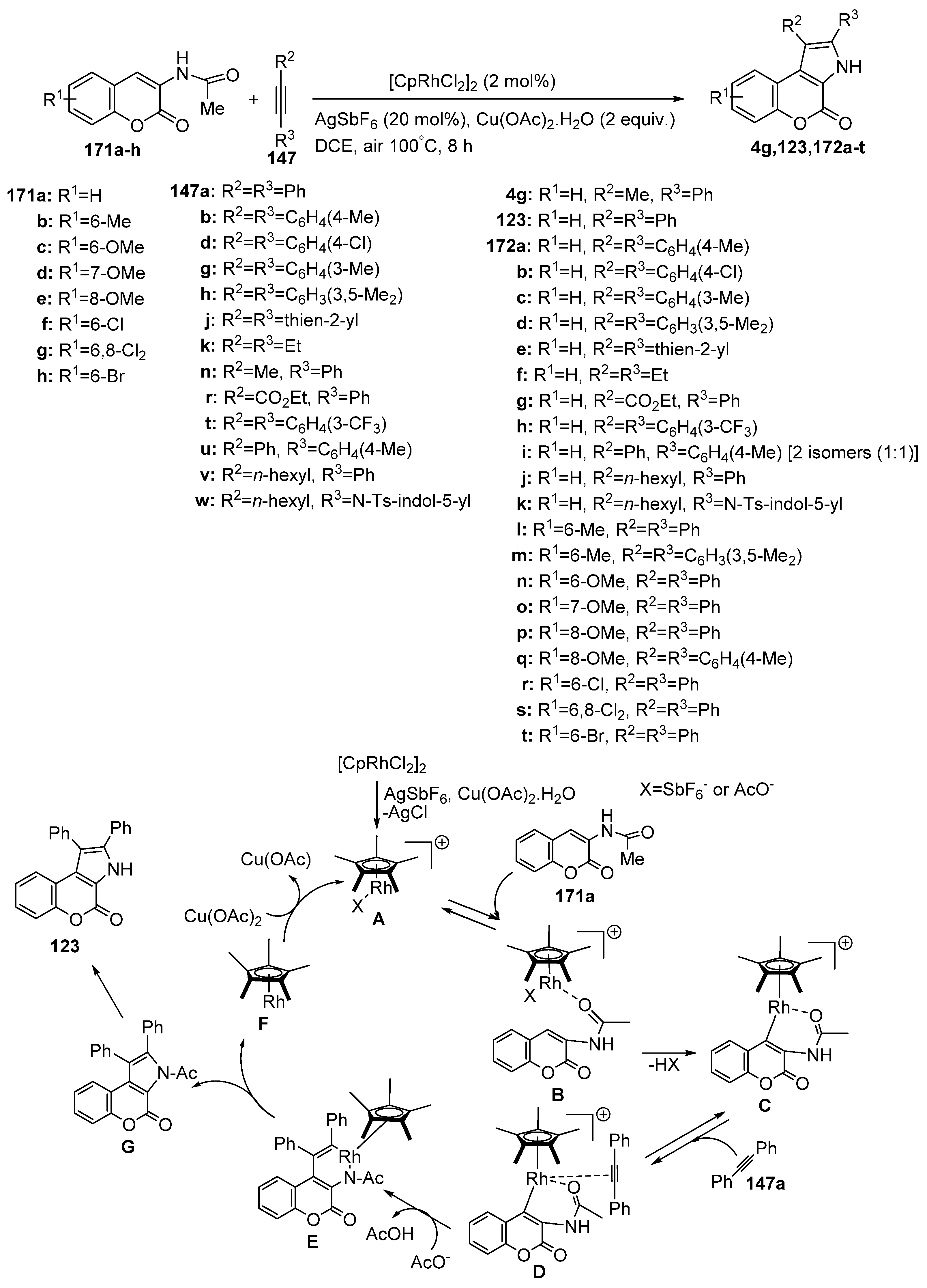
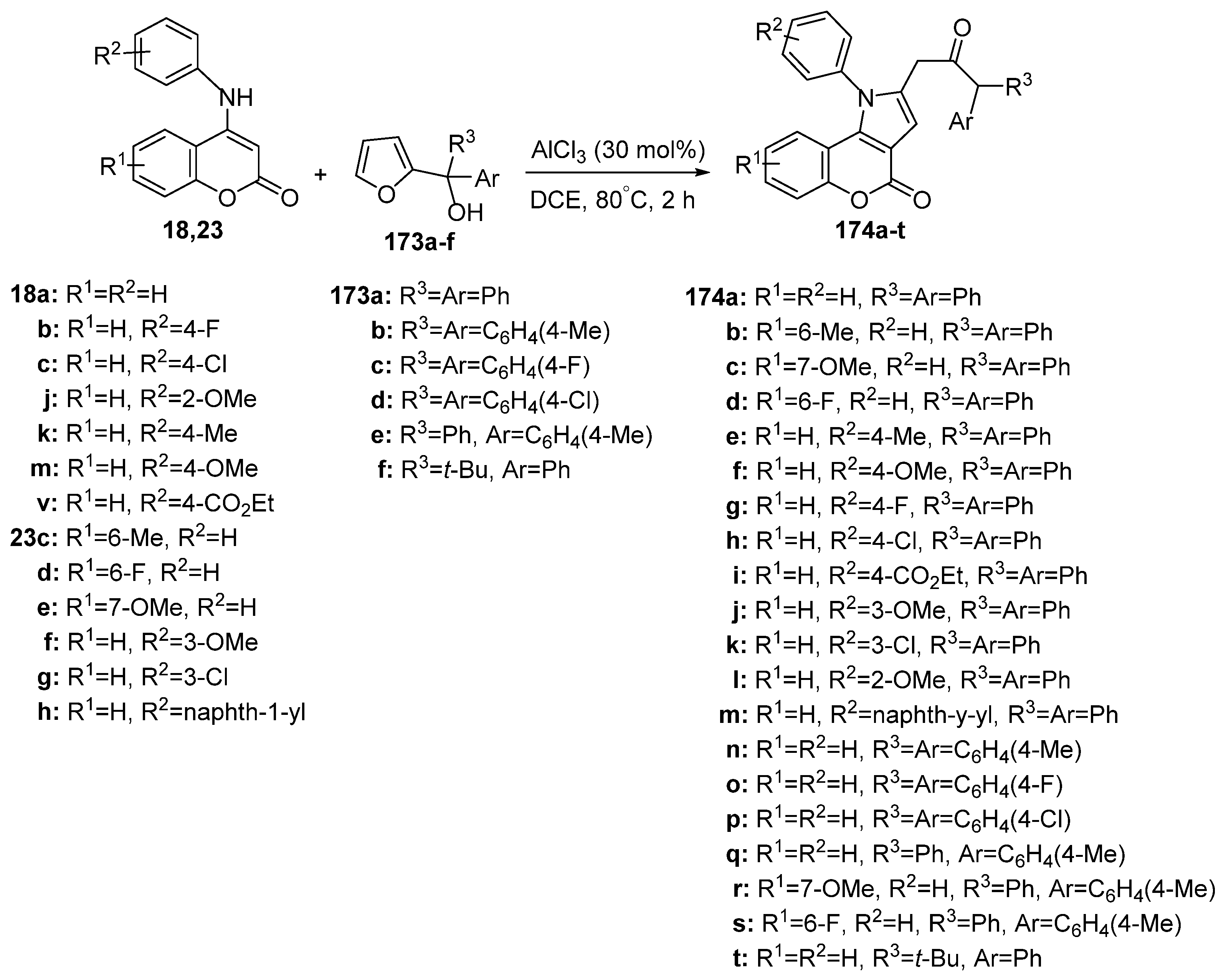
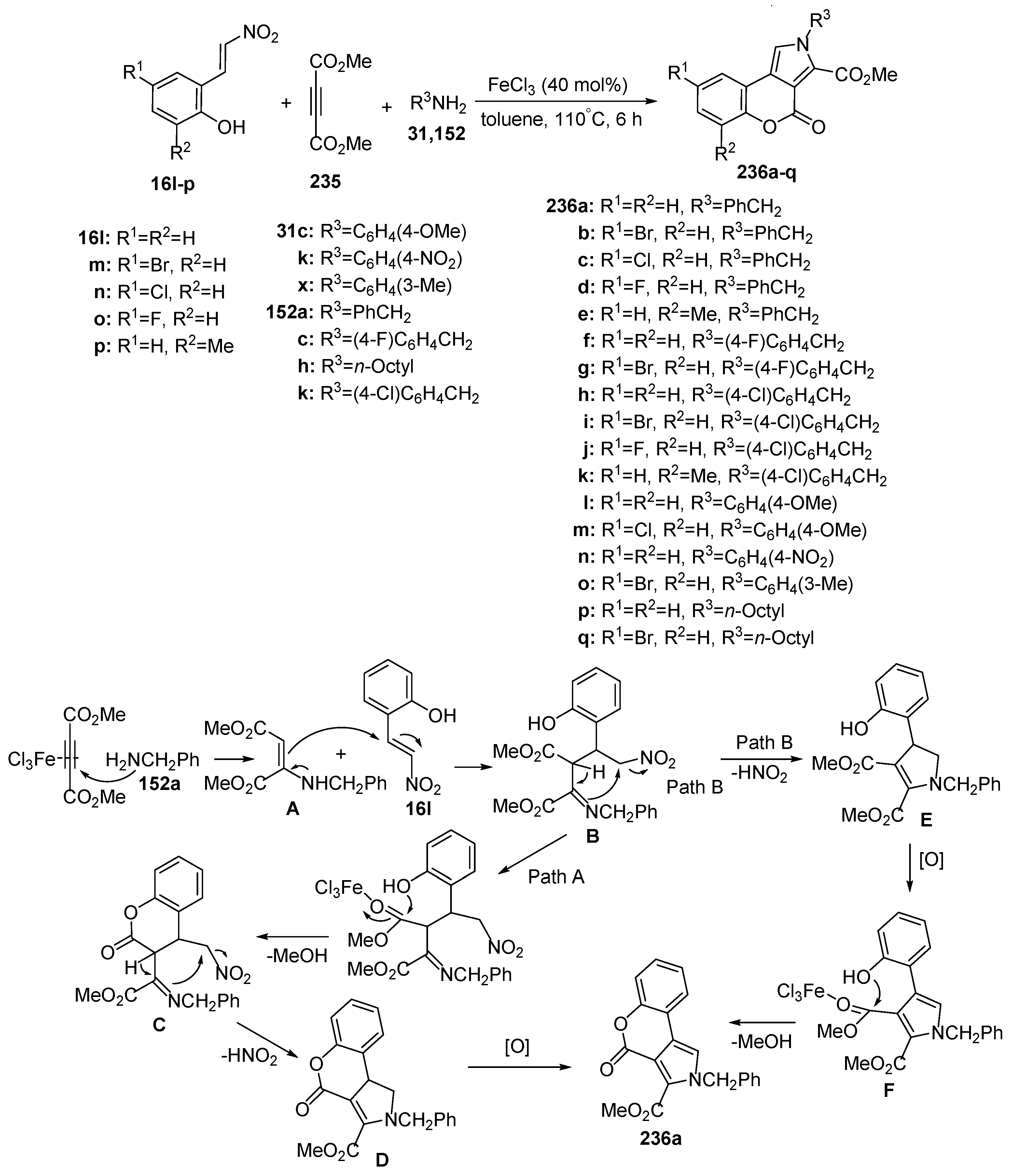
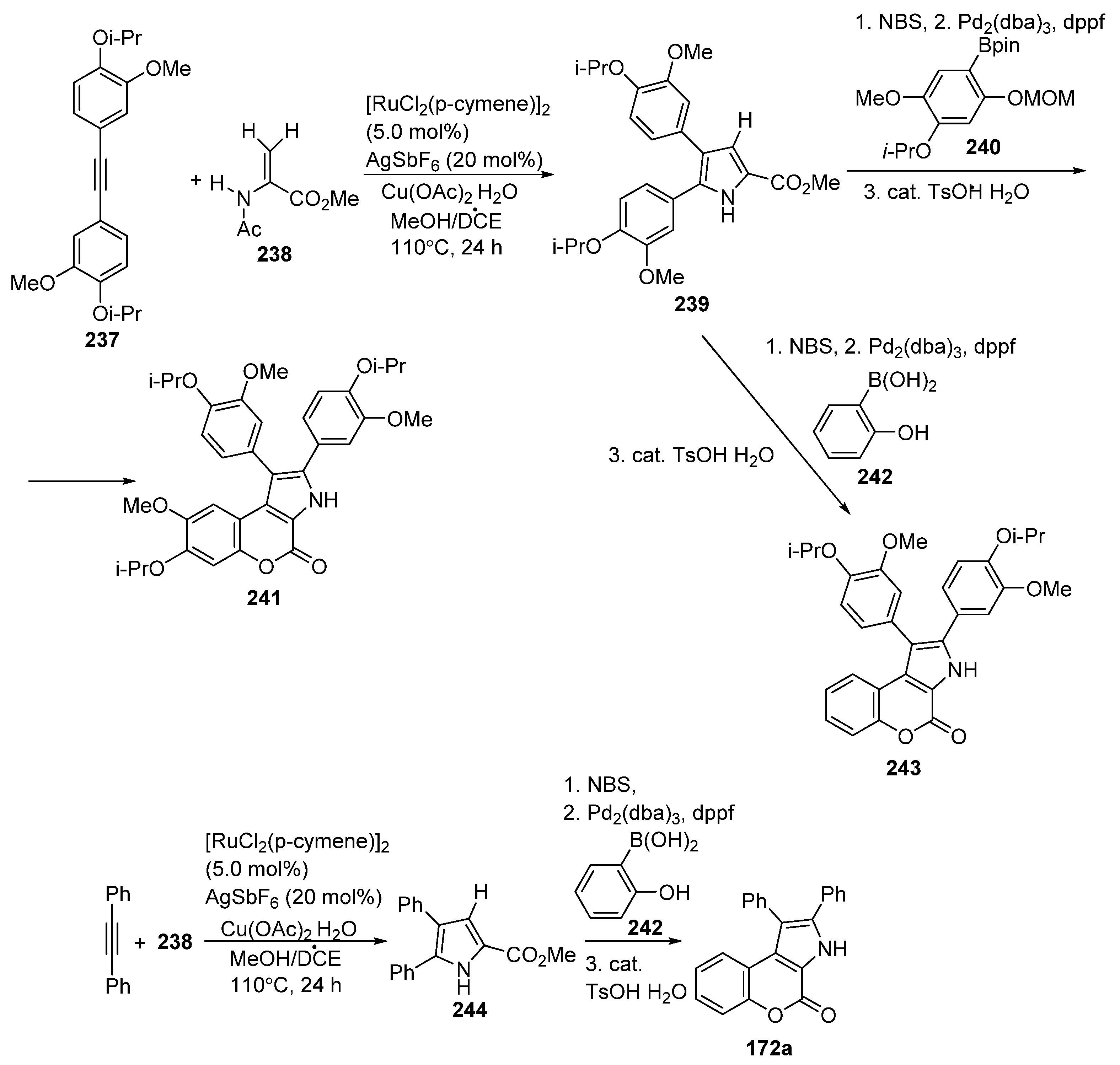
2.1.4. Synthesis through Metal-Catalyzed Reactions
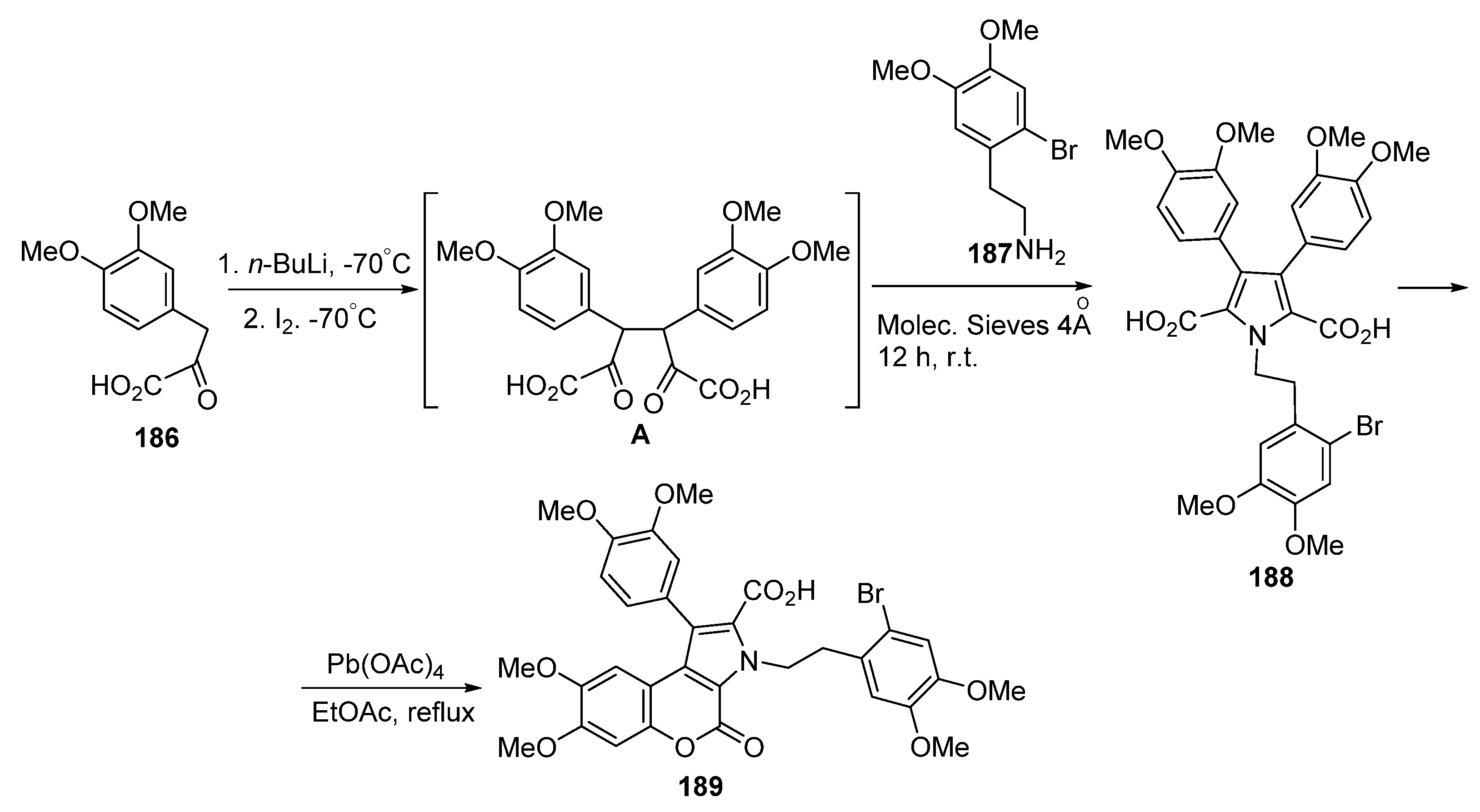
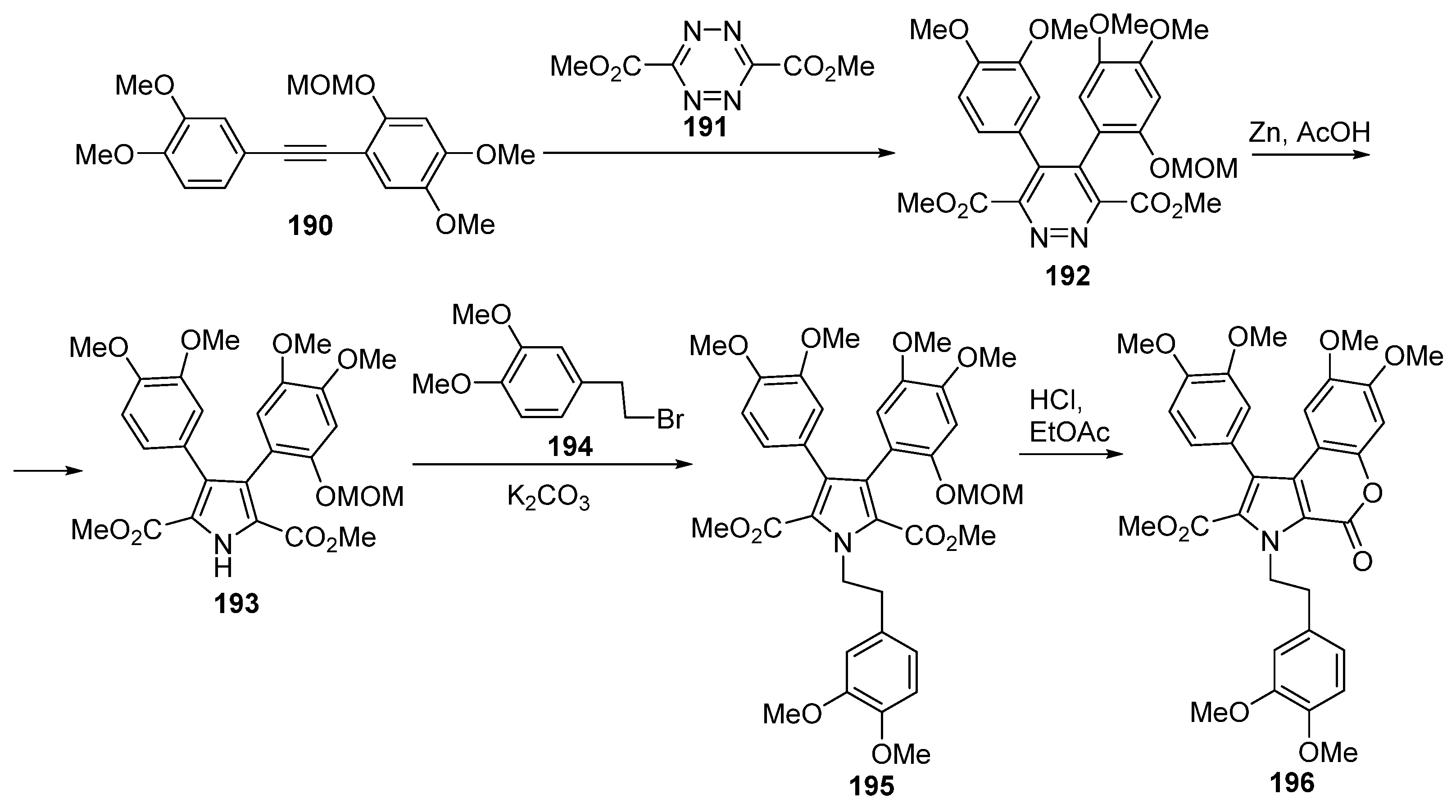
2.2. Pyranone Ring Formation
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fernández-Peña, L.; Matos, M.J.; López, E. Recent Advances in Biologically Active Coumarins from Marine Sources: Synthesis and Evaluation. Mar. Drugs 2023, 21, 37. [Google Scholar] [CrossRef]
- Heghes, S.C.; Vostinaru, O.; Mogosan, C.; Miere, D.; Iuga, C.A.; Filip, L. Safety Profile of Nutraceuticals Rich in Coumarins: An Update. Front. Pharmacol. 2022, 13, 803338. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Albohy, A.; Abdulrazik, B.S.; Bayoumi, S.A.L.; Malak, L.G.; Khallaf, I.S.A.; Bringmann, G.; Farag, S.F. Natural coumarins as potential anti-SARS-CoV-2 agents supported by docking analysis. RSC Adv. 2021, 11, 16970–16979. [Google Scholar] [CrossRef]
- Carneiro, A.; Matos, M.J.; Uriarte, E.; Santana, L. Trending Topics on Coumarin and Its Derivatives in 2020. Molecules 2021, 26, 501. [Google Scholar] [CrossRef]
- Stringlis, I.A.; de Jonge, R.; Pieterse, C.M.J. The age of coumarins in plant-microbe interactions. Plant Cell Physiol. 2019, 60, 1405–1419. [Google Scholar] [CrossRef]
- Hussain, M.I.; Syed, Q.A.; Khattak, M.N.K.; Hafez, B.; Reigosa, M.J.; El-Keblawy, A. Natural Product Coumarins: Biological and Pharmacological Perspectives. Biologia 2019, 74, 863–888. [Google Scholar] [CrossRef]
- Voges, M.J.E.E.E.; Baic, Y.; Schulze-Lefert, P.; Sattely, E.S. Plant-derived coumarins shape the composition of an Arabidopsis synthetic root microbiome. Proc. Natl. Acad. Sci. USA 2019, 116, 12558–12565. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yord, E.G. Coumarins—An Important Class of Phytochemicals. In Phytochemicals—Isolation, Characterisation and Role in Human Health; Rao, V., Rao, L., Eds.; IntechOpen: Rijeka, Croatia, 2015; Chapter 5. [Google Scholar]
- Murray, D.H.; Mendez, J.; Brown, S.A. The Natural Coumarins: Occurrence, Chemistry and Biochemistry; J. Wiley & Sons: New York, NY, USA, 1982. [Google Scholar]
- Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules 2023, 28, 2413. [Google Scholar] [CrossRef]
- Pereira, T.M.; Franco, D.P.; Vitorio, F.; Kummerle, A.E. Coumarin Compounds in Medicinal Chemistry: Some Important Examples from the Last Years. Curr. Top. Med. Chem. 2018, 18, 124–148. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 25, 250. [Google Scholar] [CrossRef]
- Al-Majedy, Y.; Al-Amiery, A.; Kadhum, A.A.; Mohamad, A.B. Antioxidant activity of coumarins. Sys. Rev. Pharm. 2017, 8, 24–30. [Google Scholar] [CrossRef]
- Medina, F.G.; Marrero, J.G.; Macías-Alonso, M.; González, M.C.; Córdova-Guerrero, I.; Teissier García, A.G.; Osegueda-Robles, S. Coumarin heterocyclic derivatives: Chemical synthesis and biological activity. Nat. Prod. Rep. 2015, 32, 1472–1507. [Google Scholar] [CrossRef]
- O’Kennedy, R.; Thornes, R.D. Coumarins: Biology, Applications and Mode of Action; J. Wiley & Sons: Chichester, UK, 1997. [Google Scholar]
- Rubab, L.; Afroz, S.; Ahmad, S.; Hussain, S.; Nawaz, I.; Irfan, A.; Batool, F.; Kotwica-Mojzych, K.; Mojzych, M. An Update on Synthesis of Coumarin Sulfonamides as Enzyme Inhibitors and Anticancer Agents. Molecules 2022, 27, 1604. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Q.; Zhang, Y.; Liang, C. Coumarin-based derivatives with potential anti-HIV activity. Fitoterapia 2021, 150, 104863. [Google Scholar] [CrossRef]
- Yu, D.; Suzuki, M.; Xie, L.; Morris-Natschke, S.L.; Lee, K.H. Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Res. Rev. 2003, 23, 322–345. [Google Scholar] [CrossRef]
- Pana, Y.; Liua, T.; Wang, X.; Suna, J. Research progress of coumarins and their derivatives in the treatment of diabetes. J. Enz. Inh. Med. Chem. 2022, 37, 616–628. [Google Scholar] [CrossRef]
- Li, H.; Yao, Y.; Li, L. Coumarins as potential antidiabetic agents. J. Pharm. Pharmacol. 2017, 69, 1253–1264. [Google Scholar] [CrossRef]
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiba, M.E.; Alkahtani, H.M.; Awad, G.E.A.; Mahmoud, A.H.; Ibrahim, B.M.M.; Bari, A.; Villinger, A. Synthesis and Evaluation of New Coumarin Derivatives as Antioxidant, Antimicrobial, and Anti-Inflammatory Agents. Molecules 2020, 25, 3251. [Google Scholar] [CrossRef]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Design 2004, 10, 3813–3833. [Google Scholar] [CrossRef]
- Kasperkiewicz, K.; Ponczek, M.B.; Owczarek, J.; Guga, P.; Budzisz, E. Antagonists of Vitamin K—Popular Coumarin Drugs and New Synthetic and Natural Coumarin Derivatives. Molecules 2020, 25, 1465. [Google Scholar] [CrossRef]
- Lowenthal, J.; Birnbaum, H. Vitamin K and coumarin anticoagulants: Dependence of anticoagulant effect on inhibition of vitamin K transport. Science 1969, 164, 181–183. [Google Scholar] [CrossRef]
- Martin, A.L.A.R.; De Menezes, I.R.A.; Sousa, A.K.; Farias, P.A.M.; dos Santos, F.A.V.; Freitas, T.S.; Figueredo, F.G.; Ribeiro-Filho, J.; Carvalho, D.T.; Coutinho, H.D.M.; et al. In vitro and in silico antibacterial evaluation of coumarin derivatives against MDR strains of Staphylococcus aureus and Escherichia coli. Microb. Pathogen. 2023, 177, 106058. [Google Scholar] [CrossRef]
- Zou, J.; Liu, Y.; Guo, R.; Tang, Y.; Shi, Z.; Zhang, M.; Wu, W.; Chen, Y.; Hou, K. An in-vitro coumarin antibiotic combination treatment of Pseudomonas aeruginosa biofilms. Nat. Prod. Commun. 2021, 16, 1–7. [Google Scholar] [CrossRef]
- Reddy, D.S.; Kongot, M.; Kumar, A. Coumarin hybrid derivatives as promising leads to treat tuberculosis: Recent developments and critical aspects of structural design to exhibit anti-tubercular activity. Tuberculosis 2021, 127, 102050. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Xu, Z.; Zhang, S.; Wu, X.; Ding, J.-W.; Lv, Z.S.; Feng, L.S. Recent developments of coumarin-containing derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 136, 122–130. [Google Scholar] [CrossRef]
- Goncalves de Oliveira, L.M.; Carreira, R.B.; de Oliveira, J.V.R.; do Nascimento, R.P.; dos Santos Souza, C.; Trias, E.; da Silva, V.D.A.; Costa, S.L. Impact of Plant-Derived Compounds on Amyotrophic Lateral Sclerosis. Neurotox. Res. 2023, 41, 288–309. [Google Scholar] [CrossRef]
- Lin, T.-H.; Chang, K.-H.; Chiu, Y.-J.; Weng, Z.-K.; Sun, Y.-C.; Lin, W.; Lee-Chen, G.-J.; Chen, C.-M. Neuroprotective Action of Coumarin Derivatives through Activation of TRKB-CREB-BDNF Pathway and Reduction of Caspase Activity in Neuronal Cells Expressing Pro-Aggregated Tau Protein. Int. J. Mol. Sci. 2022, 23, 12734. [Google Scholar] [CrossRef]
- Hu, Y.; Shan, L.; Qiu, T.; Liu, L.; Chen, J. Synthesis and biological evaluation of novel coumarin derivatives in rhabdoviral clearance. Eur. J. Med. Chem. 2021, 223, 113739. [Google Scholar] [CrossRef]
- El-Sawy, E.R.; Abdelwahab, A.B.; Kirsch, G. Synthetic Routes to Coumarin(Benzopyrone)-Fused Five-Membered Aromatic Heterocycles Built on the α-Pyrone Moiety. Part 1: Five-Membered Aromatic Rings with One Heteroatom. Molecules 2021, 26, 483. [Google Scholar] [CrossRef]
- Wozniak, L.; Połaska, M.; Marszałek, K.; Skapska, S. Photosensitizing Furocoumarins: Content in Plant Matrices and Kinetics of Supercritical Carbon Dioxide Extraction. Molecules 2020, 25, 3805. [Google Scholar] [CrossRef]
- Vlachou, E.-E.N.; Litinas, K.E. An overview on pyranocoumarins: Synthesis and biological activities. Curr. Org. Chem. 2019, 23, 2679–2721. [Google Scholar] [CrossRef]
- Douka, M.; Litinas, K.E. An overview on the synthesis of fused pyridocoumarins with biological interest. Molecules 2022, 27, 7256. [Google Scholar] [CrossRef]
- Duc, D.X.; Quoc, N.V. Isolation, Bioactivities, and Synthesis of Lamellarin Alkaloids: A Review. Curr. Org. Chem. 2022, 26, 961–990. [Google Scholar] [CrossRef]
- Mohamed, K.S.; Elbialy, E.E. Synthesis, Characterization, and Cytotoxicity Evaluation of Some New Benzo[f]coumarin Derivatives. J. Heterocycl. Chem. 2018, 55, 893–898. [Google Scholar] [CrossRef]
- Soman, S.S.; Thaker, T.H.; Rajput, R.A. Novel synthesis and cytotoxic activity of some chromeno [3,4-b]pyrrol-4(3H)-ones. Chem. Heterocycl. Comp. 2011, 46, 1514–1519. [Google Scholar] [CrossRef]
- Fukuda, T.; Ishibashi, F.; Iwao, M. Synthesis and biological activity of lamellarin alkaloids: An overview. Heterocycles 2011, 83, 491–529. [Google Scholar] [CrossRef]
- Chittchang, M.; Batsomboon, P.; Ruchirawat, S.; Ploypradith, P. Cytotoxicities and Structure–Activity Relationships of Natural and Unnatural Lamellarins toward Cancer Cell Lines. ChemMedChem 2009, 4, 457–465. [Google Scholar] [CrossRef]
- Fan, H.; Peng, J.; Hamann, M.T.; Hu, J.-F. Lamellarins and Related Pyrrole-Derived Alkaloids from Marine Organisms. Chem. Rev. 2008, 108, 264–287. [Google Scholar] [CrossRef]
- Ridley, C.P.; Reddy, M.V.R.; Rocha, G.; Bushman, F.D.; Faulkner, D.J. Total Synthesis and Evaluation of Lamellarin α- 20-Sulfate Analogues. Bioorg. Med. Chem. 2002, 10, 3285–3290. [Google Scholar] [CrossRef]
- Reddy, M.V.R.; Rao, M.R.; Rhodes, D.; Hansen, M.S.T.; Rubins, K.; Bushman, F.D.; Venkateswarlu, Y.; Faulkner, D.J. Lamellarin α 20-Sulfate, an Inhibitor of HIV-1 Integrase Active against HIV-1 Virus in Cell Culture. J. Med. Chem. 1999, 42, 1901–1907. [Google Scholar] [CrossRef]
- Salehian, F.; Nadri, H.; Jalili-Baleh, L.; Youseftabar-Miri, L.; Bukhari, S.N.A.; Foroumadi, A.; Küçükkilinç, T.T.; Sharifzadeh, M.; Khoobi, M. A review: Biologically active 3,4-heterocycle-fused coumarins. Eur. J. Med. Chem. 2021, 212, 113034. [Google Scholar] [CrossRef]
- Balalas, T.D.; Kanelli, M.G.; Gabriel, C.; Pontiki, E.; Hadjipavlou-Litina, D.J.; Litinas, K.E. Pd Catalyzed N–H or C–H functionalization/oxidative cyclization for the efficient synthesis of N-aryl-substituted [3,4]-fused pyrrolocoumarins. Synthesis 2022, 54, 2894–2906. [Google Scholar] [CrossRef]
- Kontogiorgis, C.; Litinas, K.E.; Makri, A.; Nicolaides, D.N.; Vronteli, A.; Hadjipavlou-Litina, D.J.; Pontiki, E.; Siohou, A.A. Synthesis and biological evaluation of novel angularly fused pyrrolocoumarins. J. Enz. Inh. Med. Chem. 2008, 23, 43–49. [Google Scholar] [CrossRef]
- Thakur, A.; Thakur, M.; Khadikar, P. Topological Modeling of Benzodiazepine Receptor Binding. Bioorg. Med. Chem. 2003, 11, 5203–5207. [Google Scholar] [CrossRef]
- Colotta, V.; Cecchi, L.; Melani, F.; Filacchioni, G.; Martini, C.; Giannaccini, G.; Lucacchini, A. Tricyclic Heteroaromatic Systems. [1]Benzopyranopyrrol-4-ones and [1]Benzopyrano-l,2,3-triazol-4-ones as Benzodiazepine Receptor Ligands. Synthesis and Structure-Activity Relationships. J. Med. Chem. 1990, 33, 2646–2651. [Google Scholar] [CrossRef]
- Dakshanamurthy, S.; Kim, M.; Brown, M.L.; Byers, S.W. In-silico fragmentbased identification of novel angiogenesis inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 4551–4556. [Google Scholar] [CrossRef]
- Neagoie, C.; Vedrenne, E.; Buron, F.; Mérour, J.-Y.; Rosca, S.; Bourg, S.; Lozach, O.; Meijer, L.; Baldeyrou, B.; Lansiaux, A.; et al. Synthesis of chromeno [3,4-b]indoles as Lamellarin D analogues: A novel DYRK1A inhibitor class. Eur. J. Med. Chem. 2012, 49, 379–396. [Google Scholar] [CrossRef]
- Friese, A.; Kapoor, S.; Schneidewind, T.; Vidadala, S.R.; Sardana, J.; Brause, A.; Fçrster, T.; Bischoff, M.; Wagner, J.; Janning, P.; et al. Chemical Genetics Reveals a Role of dCTP Pyrophosphatase 1 in Wnt Signaling. Angew. Chem. Int. Ed. 2019, 58, 13009–13013. [Google Scholar] [CrossRef]
- Mukherjee, S.; Hazra, S.; Chowdhury, S.; Sarkar, S.; Chattopadhyay, K.; Pramanik, A. A novel pyrrole fused coumarin based highly sensitive and selective fluorescence chemosensor for detection of Cu2+ ions and applications towards live cell imaging. J. Photochem. Photobiol. A Chem. 2018, 364, 635–644. [Google Scholar] [CrossRef]
- Andersen, R.J.; Faulkner, D.J.; Cun-heng, H.; Van Duyne, G.D.; Clardy, J. Metabolites of the marine prosobranch mollusk Lamellaria sp. J. Am. Chem. Soc. 1985, 107, 5492–5495. [Google Scholar] [CrossRef]
- Reddy, S.M.; Srinivasulu, M.; Satyanarayana, N.; Kondapi, A.K.; Venkateswarlu, Y. New potent cytotoxic lamellarin alkaloids from Indian ascidian Didemnum obscurum. Tetrahedron 2005, 61, 9242–9247. [Google Scholar] [CrossRef]
- Kang, H.; Fenical, W. Ningalins A-D: Novel Aromatic Alkaloids from a Western Australian Ascidian of the Genus Didemnum. J. Org. Chem. 1997, 62, 3254–3262. [Google Scholar] [CrossRef]
- Patra, P. A short review on the synthesis of pyrrolo [3,4-c]coumarins an isolamellarin-B Scaffolds. Synth. Commun. 2022, 52, 1999–2018. [Google Scholar] [CrossRef]
- Samanta, K.; Patra, P.; Kar, G.K.; Dinda, S.K.; Mahanty, D.S. Diverse synthesis of pyrrolo/indolo [3,2-c]coumarins as isolamellarin-A scaffolds: A brief update. New J. Chem. 2021, 45, 7450–7485. [Google Scholar] [CrossRef]
- Stadlbauer, W.; Kappe, T. Simple and effective approaches to coumestans and azacoumestans. Heterocycles 1993, 35, 1425–1440. [Google Scholar]
- Darbarwar, M.; Sundaramurthy, V. Synthesis of Coumarins with 3:4-Fused Ring Systems and Their Physiological Activity. Synthesis 1982, 1982, 337–388. [Google Scholar] [CrossRef]
- Chaudhary, A. Arylglyoxals as Versatile Synthons for Heterocycles Through Multi-Component Reactions. Curr. Org. Chem. 2019, 23, 1945–1983. [Google Scholar] [CrossRef]
- Patel, G.; Banerjee, S. Review on Synthesis of Bio-active Coumarin-fused Heterocyclic Molecules. Curr. Org. Chem. 2020, 24, 2566–2587. [Google Scholar] [CrossRef]
- Dalpozzo, R.; Mancuso, R. Copper-Catalyzed Synthesis of Coumarins. A Mini-Review. Catalysts 2021, 11, 1382. [Google Scholar] [CrossRef]
- Patra, P. 4-Chloro-3-formylcoumarin as a multifaceted building block for the development of various bio-active substituted and fused coumarin heterocycles: A brief review. New J. Chem. 2021, 45, 14269–14327. [Google Scholar] [CrossRef]
- Cui, H.-L. Recent progress in the synthesis of pyrrolo[2,1-a]Isoquinolines. Org. Biomol. Chem. 2022, 20, 2779–2801. [Google Scholar] [CrossRef]
- Patra, P.; Patra, S. 4-Aminocoumarin Derivatives as Multifaceted Building Blocks for the Development of Various Bioactive Fused Coumarin Heterocycles: A Brief Review. Curr. Org. Chem. 2022, 26, 1585–1614. [Google Scholar] [CrossRef]
- Nasab, N.H.; Azimian, F.; Kruger, H.G.; Kim, S.J. Acetylcoumarin in cyclic and heterocyclic-containing coumarins: Synthesis and biological applications. Tetrahedron 2022, 129, 133158. [Google Scholar] [CrossRef]
- Patra, P.; Manna, S.; Patra, S.; Samanta, K.; Roy, D. A Brief Review on the Synthesis of Pyrrolo [2,3-c]coumarins, including Lamellarin and Ningalin Scaffolds. Org. Prepar. Proc. Int. 2023, 55, 63–83. [Google Scholar] [CrossRef]
- Hamama, W.S.; Ibrahim, M.E.; Metwalli, A.E.; Zoorob, H.H. Recent synthetic aspects on the chemistry of aminocoumarins. Res. Chem. Intermed. 2017, 43, 5943–5983. [Google Scholar] [CrossRef]
- Khan, M.A.; de Brito Morley, M.L. Condensed benzopyrans III. 3H,4H[1[benzopyrano [3,4-b]pyrrol-4-ones. J. Heterocycl. Chem. 1978, 15, 1399–1401. [Google Scholar] [CrossRef]
- Joshi, S.D.; Sakhardande, V.D.; Seshadri, S. Synthesis of 3-substituted 5(H)-oxo[1]benzopyrano[4,3-b]pyridines and 2,3-diaryl-4(H)-oxo-[1]benzopyrano[4,3-b]pyrroles. Indian J. Chem. Sect. B 1984, 23, 206–208. [Google Scholar]
- Majumdar, K.C.; Chattopadhyay, B. Amino-Claisen versus Oxy-Claisen rearrangement: Regioselective synthesis of pyrrolocoumarin derivatives. Synthesis 2008, 2008, 921–924. [Google Scholar] [CrossRef]
- Paul, S.; Das, A.R. A new application of polymer supported, homogeneous and reusable catalyst PEG-SO3H in the synthesis of coumarin and uracil fused pyrrole derivatives. Catal. Sci. Technol. 2012, 2, 1130–1135. [Google Scholar] [CrossRef]
- Chen, Z.-W.; Hou, J.-B.; Dai, Z.-R.; Yang, X.-F. A regioselective synthesis of pentacyclic compounds containing coumarin, pyrrole, indene without catalysts under microwave irradiation. Chin. Chem. Lett. 2016, 27, 1622–1625. [Google Scholar] [CrossRef]
- Ibrahim, M.E.; Hamama, W.S.; Metwalli, A.E.; Zoorob, H.H. Chemoselective synthesis of enamino-coumarin derivatives as potent antitumor agents. J. Heterocyclic Chem. 2016, 53, 1318–1323. [Google Scholar] [CrossRef]
- Padilha, G.; Inglesias, B.A.; Back, D.F.; Kaufman, T.S.; Silveira, C.C. Synthesis of chromeno [4,3-b]pyrrol-4(1H)-ones, from β-nitroalkenes and 4-phenylaminocoumarins, under solvent–free conditions. ChemistrySelect 2017, 2, 1297–1304. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Zhong, W. Synthesis of chromeno [3,4-b]pyrrol-4(3H)-ones through the domino cyclization of 3-aminocoumarins with aryl glyoxal monohydrates. Eur. J. Org. Chem. 2017, 2258–2265. [Google Scholar] [CrossRef]
- Yang, X.; Jing, L.; Chen, Z. An efficient method for one-pot synthesis of 3-alkoxy-substituted chromeno[4,3-b]pyrrol-4(1H)-one derivatives. Chem. Heterocycl. Comp. 2018, 54, 1065–1069. [Google Scholar] [CrossRef]
- Pandya, M.K.; Chhasatia, M.R.; Vala, N.D.; Parekh, T.H. Synthesis of furano [2,3-c}/pyrrolo [2,3-c]coumarins and synthesis of 1(H)[1]benzopyrano [3,4-b][1]benzopyrano [3′,4′-b]furan-7(H)-ones/1(H)[1]benzopyrano [3,4-b][1]benzopyrano [3′,4′-b]pyrrole-7(H)-ones. J. Drug. Deliv. Ther. 2019, 9, 32–42. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Sharma, V.L.; Bhaduri, A.P. Studies on the Nef reaction induced by organic bases. J. Heterocycl. Chem. 1987, 24, 23–25. [Google Scholar] [CrossRef]
- Islam, S.; Dhara, S.; Das, D.; Das, A.R. I2/DMSO mediated cascade cyclization reaction of aminocoumarins with aryl methyl ketones: Access to dicoumarino-fused [1,5]-diazocine and pyrrolo-coumarin scaffolds. ChemistrySelect 2023, 8, e202300126. [Google Scholar] [CrossRef]
- Trkovnik, M.; Djudjic, R.; Tabakovic, I.; Kule, M. Syntheses of furo-, pyrrolo-, and thieno[3,2-c]coumarins. Org. Prep. Proced. Int. 1982, 14, 21–29. [Google Scholar] [CrossRef]
- Grigg, R.; Vipond, D. 4-Phenylsulfinyl- and 4-phenylsulfonylcoumarins as 2π-components in cycloaddition reactions. Tetrahedron 1989, 45, 7587–7592. [Google Scholar] [CrossRef]
- Alberola, A.; Alvaro, R.; Andres, J.M.; Calvo, B.; Gonzales, A. Synthesis of [1]benzopyrano[4,3-b]pyrrol-4(1H)-ones from 4-chlorocoumarin. Synthesis 1994, 1994, 279–281. [Google Scholar] [CrossRef]
- Alberola, A.; Alvaro, R.; Gonzales-Ortega, A.; Sadaba, M.L.; Sanudo, M.C. Synthesis of [1]benzopyrano[4,3-b]pyrrol-4(1H)-ones from N(α)-(2-oxo-2H-1-benzopyran-4-yl) Weinreb α-aminoamides. Tetrahedron 1999, 55, 13211–13224. [Google Scholar] [CrossRef]
- Alberola, A.; Calvo, L.; Gonzales-Ortega, A.; Encabo, A.P.; Sanudo, M.C. Synthesis of [1]benzopyrano[4,3-b]pyrrol-4(1H)-ones from 4-chloro-3-formylcoumarin. Synthesis 2001, 2001, 1941–1948. [Google Scholar] [CrossRef]
- Liao, Y.-X.; Kuo, P.-Y.; Yang, D.-Y. Efficient synthesis of trisubstituted [1]benzopyrano[4,3-b]pyrrol-4(1H)-ones from 4-hydroxycoumarin. Tetrahedron Lett. 2003, 44, 1509–1602. [Google Scholar] [CrossRef]
- Cordaro, M.; Grassi, G.; Risitano, F.; Scala, A. N-Substituted and N-unsubstituted 1,3-oxazolium-5-olates cycloaddition reactions with 3-substituted coumarins. Tetrahedron 2010, 66, 2713–2717. [Google Scholar] [CrossRef]
- Fan, L.-P.; Yang, W.-J.; Xu, D.-C.; Li, X.-S.; Xie, J.-W. Efficient methods for the synthesis of benzopyran [3,4-c]pyrrolidines by catalyzed 1,3-dipolar cycloaddition reaction of azomethine ylides with 3-substituted coumarins. Synth. Commun. 2011, 41, 3376–3384. [Google Scholar] [CrossRef]
- Bochkov, A.Y.; Akchurin, I.O.; Dyachenko, O.A.; Traven, V.F. NIT-fluorescent coumarin-fused BODIPY dyes with large Stokes shifts. Chem. Commun. 2013, 49, 11653–11655. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, D.-Y. Synthesis of coumarin/pyrrole-fused heterocycles and their photochemical and redox-switching properties. Org. Lett. 2013, 15, 2802–2805. [Google Scholar] [CrossRef]
- Kamal El-Dean, A.M.; Zaki, R.M.; Geies, A.A.; Radwan, S.M.; Tolba, M.S. Synthesis and antimicrobial activity of new heterocyclic compounds containing thieno[3,2-c]coumarin and pyrazolo[4,3-c]coumarin frameworks. Russ. J. Bioorg. Chem. 2013, 39, 553–564. [Google Scholar] [CrossRef]
- Potowski, M.; Golz, C.; Strohmann, C.; Antonchick, A.P.; Waldmann, H. Biology-oriented synthesis of benzopyran[3,4-c]pyrrolidines. Bioorg. Med. Chem. 2015, 23, 2895–2903. [Google Scholar] [CrossRef]
- El Azab, I.H.; Elkanzi, N.A.A. Synthesis and pharmacological evaluation of some new chromeno [3,4-c[pyrrol-3,4-dione-based N-heterocycles as antimicrobial agents. J. Heterocycl. Chem. 2017, 54, 1404–1414. [Google Scholar] [CrossRef]
- Shaabani, A.; Sepahvand, H.; Bazgir, A.; Khavasi, H.R. Tosylmethylisocyanide (TosMIC) [3+2]cycloaddition reactions: A facile Val Leusen protocol for the synthesis of new class of spirooxazolines, spyropyrrolines, and chromen[3,4-c]pyrrols. Tetrahedron 2018, 74, 7058–7067. [Google Scholar] [CrossRef]
- Borkotoki, L.; Borra, S.; Maurya, R.A. Access to pyrrolocoumarins through DBU-mediated coupling of 2-oxo 2H-chromene-3-carbaldehydes and phenacyl azides. Eur. J. Org. Chem. 2022, 2022, e202101237. [Google Scholar] [CrossRef]
- Che, C.; Li, S.; Jiang, X.; Quan, J.; Lin, S.; Yang, Z. One-pot syntheses of chromeno [3,4-c]pyrrole-2,4-diones via Ugi-4CR and intramolecular Michae; addition. Org. Lett. 2010, 12, 4682–4685. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Su, W. An efficient protocol for the multicomponent synthesis of functionalized chromeno [4,3-b]pyrrol-4(H)-one derivatives. Tetrahedron Lett. 2015, 56, 2476–2479. [Google Scholar] [CrossRef]
- Mishra, R.; Panday, A.K.; Choudhur, L.H.; Subramanian, R.; Verma, A. Multicomponent reactions of aryl glyoxal, 4-hydroxycoumarin and cyclic 1,3-C,N binucleophiles: Binucleophile-directed synthesis of fused five- and six-membered N-heterocycles. Eur. J. Org. Chem. 2017, 2017, 2789–2800. [Google Scholar] [CrossRef]
- Yahyavi, H.; Heravi, M.M.; Mahdavi, M.; Foroumadi, A. Iodine-catalyzed tandem oxidative coupling reaction: A one-pot strategy for the synthesis of new coumarin-fused pyrroles. Tetrahedron Lett. 2018, 59, 94–98. [Google Scholar] [CrossRef]
- Yang, X.; Zheng, L.; Chen, Z.; Zhong, W. Catalyst-free three-component approach to efficient synthesis of chromeno[4,3-b]pyrrol-4(H)-one derivatives. Synth. Commun. 2018, 48, 929–935. [Google Scholar] [CrossRef]
- Belal, M.; Khan, A.T. Iodine-catalyzed synthesis of pyrrolo[2,3-c]coumarin derivatives using 3-aminocoumarins, arylglyoxals, and 4-hydroxycoumarin through one-pot three-component reaction. ChemistrySelect 2018, 3, 2431–2434. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, S.; Zhang, X. Bronsted acid-promoted multicomponent reaction for the construction of pyrrolocoumarin derivatives. Heterocycles 2018, 96, 501–508. [Google Scholar] [CrossRef]
- Wu, C.-K.; Weng, Z.; Yang, D.-Y. One-pot construction of 1-phenylchromeno [3,4-b]pyrrol-4(3H)-one: Application to total synthesis of ningalin B and a pyrrolocoumarin-based electrochromic switch. Org. Lett. 2019, 21, 5225–5228. [Google Scholar] [CrossRef]
- Lai, X.; Che, C. Synthesis of chromeno [4,3-b]pyrrol-4(H)-ones through multicomponent reaction and cyclization strategy. ACS Omega 2020, 5, 21968–21977. [Google Scholar] [CrossRef]
- Karami, M.; Hasaninejad, A.; Mahdavi, H.; Iraji, A.; Mojtavabi, S.; Faramarzi, M.A.; Mahdavi, M. One-pot multi-component synthesis of novel chromeno [4,3-b]pyrrol-3-yl derivatives as alpha-glucosidase inhibitors. Mol. Divers. 2022, 26, 2393–2405. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.-H. A new approach to pyrrolocoumarin derivativesby Palladium-catalyzed reactions: Expedient construction of polycyclic lamellarin scaffold. Adv. Synth. Catal. 2009, 351, 2005–2012. [Google Scholar] [CrossRef]
- Majumdar, K.C.; De, N.; Roy, B. Iron/Palladium-catalyzed intramolecular hydroamination: An expedient synthesis of pyrrole-annulated coumarin and quinolone derivatives. Synthesis 2010, 2010, 4207–4212. [Google Scholar] [CrossRef]
- Paul, S.; Pal, G.; Das, A.R. Three-component synthesis of a polysubstituted pyrrole core containing heterocyclic scaffolds over magnetically separable nanocrystalline copper ferrite. RSC Adv. 2013, 3, 8637–8644. [Google Scholar] [CrossRef]
- Peng, S.; Wang, L.; Huang, J.; Sun, S.; Guo, H.; Wang, J. Palladium-catalyzed oxidative annulation via C–H/N–H functionalization: Access to substituted pyrroles. Adv. Synth. Catal. 2013, 355, 2550–2557. [Google Scholar] [CrossRef]
- Ngo, T.N.; Akrawi, O.A.; Dang, T.T.; Villinger, A.; Langer, P. Synthesis of pyrrolocoumarins via Pd-catalyzed domino C–N coupling/hydroamination reactions. Tetrahedron Lett. 2015, 56, 86–88. [Google Scholar] [CrossRef]
- Pradhan, K.; Paul, S.; Das, A.R. Synthesis of indeno and acenaphtheno cores containing dihydroxy indolone, pyrrole, coumarin and uracil fused heterocyclic motifs under sustainable conditions exploring the catalytic role of the SnO2 quantum dot. RSC Adv. 2015, 5, 12062–12070. [Google Scholar] [CrossRef]
- Saha, M.; Pradhan, K.; Das, A.R. Facile and eco-friendly synthesis of chromeno [4,3-b]pyrrol-4(H)-one derivatives applying magnetically recoverable nano crystalline CuFe2O4 involving a domino three-component reaction in aqueous media. RSC Adv. 2016, 6, 55033–55038. [Google Scholar] [CrossRef]
- Jin, S.-J.; Guo, J.-M.; Zhu, Y.-S.; Wang, Q.-L.; Bu, Z.-W. A copper-catalyzed tandem reaction for the construction of coumarin fused 9H-pyrrolo[1,2-a]indoles. Org. Biomol. Chem. 2017, 15, 8729–8737. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sarkar, S.; Pramanik, A. A sustainable synthesis of functionalized pyrrole fused coumarins under solvent-free conditions using magnetic nanocatalyst and a new route to polyaromatic indolocoumarins. ChemistrySelect 2018, 3, 1537–1544. [Google Scholar] [CrossRef]
- Yadav, A.; Gudimella, S.K.; Samanta, S. A, expedient Lewis-acid-catalyzed microwave-assisted domino approach to coumarin-fused pyrroles and related heterocycles under neat conditions. ChemistrySelect 2019, 4, 12768–12773. [Google Scholar] [CrossRef]
- Watanabe, T.; Mutoh, Y.; Saito, S. Synthesis of lactone-fused pyrroles by ruthenium-catalyzed 1,2-carbon migration-cycloisomerization. Org. Biomol. Chem. 2020, 18, 81–85. [Google Scholar] [CrossRef]
- Das, D.; Das, A.R. Access to π-extended heterocycles containing pyrrolo-coumarin cores involving –COCH3 as a traceless directing group and materializing two successive sp2C–H/sp3N–H and sp2C–H/sp2N–H activations. J. Org. Chem. 2022, 87, 11443–11456. [Google Scholar] [CrossRef]
- Guan, P.; Ma, R.; Liu, M.; Kong, L.; Han, Y.; Wang, C. AlCl3-catalyzed chemoselective cascade vreactions of 4-anilinocoumarins with 2-furylcarbinols: Access to densely funcionalized chromeno[4,3-b]pyrrol-4(1H)-one derivatives. Org. Biomol. Chem. 2023, 21, 1379–1383. [Google Scholar] [CrossRef]
- Colotta, V.; Cecchi, L.; Melani, F.; Filacchioni, G.; Martini, C.; Gelli, S.; Lucacchini, A. Tricyclic heteroaromatic systems. Synthesis of 1,3 and 1,2 disubstituted [1]benzopyrano [4,3-b]pyrrol-4-ones and structure-activity relationships as benzodiazepine receptor ligands. Il Farmaco 1991, 46, 1139–1154. [Google Scholar]
- Heim, A.; Terpin, A.; Steglich, W. Biomimetic synthesis of lamellarin G trimethyl ether. Angew. Chem. Int. Ed. Engl. 1997, 36, 155–156. [Google Scholar] [CrossRef]
- Boger, D.L.; Soenen, D.R.; Boyce, C.W.; Hedrick, M.P.; Jin, Q. Total synthesis of ningalin B utilizing a heterocyclic azadiene Diels-Alder reaction and discovery of a new class of potent muti drug resistant (MDR) reversal agents. J. Org. Chem. 2000, 65, 2479–2483. [Google Scholar] [CrossRef]
- Gupton, J.T.; Clough, S.C.; Miller, R.B.; Lukens, J.R.; Henry, C.A.; Kanters, R.P.F.; Sikorski, J.A. The application of vinylogous iminium salt derivatives to the synthesis of ningalin B hexamethyl ether. Tetrahedron 2003, 59, 207–215. [Google Scholar] [CrossRef]
- Peschko, C.; Winklhofer, C.; Terpin, A.; Steglich, W. Biomimetic synthesis of lamellarin and lukianol-type alkaloids. Synthesis 2006, 2006, 3048–3057. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, J.; Fan, A.; Cui, Y.; Jia, Y. Total synthesis of lamellarins D, H, and R and ningalin B. Org. Lett. 2011, 13, 312–315. [Google Scholar] [CrossRef]
- Ghandi, M.; Ghomi, A.T.; Kubicki, M. Synthesis of pyrrole-fused chromanones via one-pot multicomponent reactions. Tetrahedron 2013, 69, 3054–3060. [Google Scholar] [CrossRef]
- Alizadeh, A.; Ghanbaripour, R.; Zhu, L.-G. An approach to the synthesis 2-acylchromeno [3,4-c]pyrrol-4(2H)-one derivatives via a sequential three-component reaction. Synlett 2013, 24, 2124–2126. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Yang, Z.; Li, Z.; Zhao, Y.; Xiao, Y.; Guo, H. Phosphine-catalyzed [3 + 2] cycloaddition of sulfamate derived cyclic imines with allenoates: Synthesis of sulfamate-fused dihydropyrroles. J. Org. Chem. 2013, 78, 8427–8436. [Google Scholar] [CrossRef]
- Vidadala, S.R.; Waldmann, H. One-pot synthesis of a natural product inspired pyrrolocoumarine compound collection by means of an intramolecular 1,3-cycloaddition as key step. Tetrahedron Lett. 2015, 56, 3358–3360. [Google Scholar] [CrossRef]
- Xue, S.; Yao, J.; Liu, J.; Wang, L.; Liu, X.; Wang, C. Three-component reaction between substituted 2-(2-nitrovinyl)phenols, acetylenedicarboxylate and amines: Diversity-oriented synthesis of novel pyrrolo[3,4-c]coumarins. RSC Adv. 2016, 6, 1700–1704. [Google Scholar] [CrossRef]
- Mei, R.; Zhang, S.-K.; Ackermann, L. Concise synthesis of lamellarin alkaloids by C-H/N-H activation: Evaluation of metal catalysts in oxidative alkyne annulation. Synlett 2017, 28, 1715–1718. [Google Scholar] [CrossRef]
- Lade, D.M.; Pawar, A.B.; Mainkar, P.S.; Chandrasekhar, S. Total synthesis of lamellarin D trimethyl ether, and lamellarin H. J. Org. Chem. 2017, 82, 4998–5004. [Google Scholar] [CrossRef]
- Reddy, E.P.; Sumankumar, A.; Sridhar, B.; Hemasri, Y.; Rao, Y.J.; Reddy, B.V.S. 1,5-Electrocyclization of conjugated azomethine ylides derived from 3-formyl chromene and N-alkyl amino acids/esters. Org. Biomol. Chem. 2017, 15, 7580–7583. [Google Scholar] [CrossRef]
- Morikawa, D.; Morii, K.; Yasuda, Y.; Mori, A.; Okano, K. Convergent total synthesis of lamellarins and their congeners. J. Org. Chem. 2020, 85, 8603–8617. [Google Scholar] [CrossRef]
- Silyanova, E.A.; Samet, A.V.; Salamandra, L.K.; Khrustalev, V.N.; Semenov, V.V. Formation of 2,3-diarylpyrrole- and pyrrolocoumarin core of natural marine products via Barton-Zard reaction and selective O-demethylation. Eur. J. Org. Chem. 2020, 2020, 2093–2100. [Google Scholar] [CrossRef]
- Rusanov, D.A.; Samet, A.V.; Rusak, V.V.; Semenov, V.V. Synthesis of functionalized 1-methylchromeno [3,4-b]pyrrole-4(3H)-ones via the Barton-Zard reaction starting from pseudonitrosites. Chem. Heterocycl. Comp. 2021, 57, 944–948. [Google Scholar] [CrossRef]
- Das, B.; Dahiya, A.; Chakraborty, N.; Patel, B.K. Synte4sis of chromenopyrroles (azacoumestans) from funcionalized enones and alkyl isocyanoacetates. Org. Lett. 2023, 25, 5209–5213. [Google Scholar] [CrossRef]
- Scalzullo, S.M.; Morgans, G.L.; Klintworth, L.; de Koening, C.B.; Fernandes, M.A.; van Otterlo, W.A.L.; Michael, J.P. Chromenone-fused pyrrolizines and pyrrolizine analogues of lamellarins: Expanding the lamellarin family. Eur. J. Org. Chem. 2024, 27, e202301230. [Google Scholar] [CrossRef]

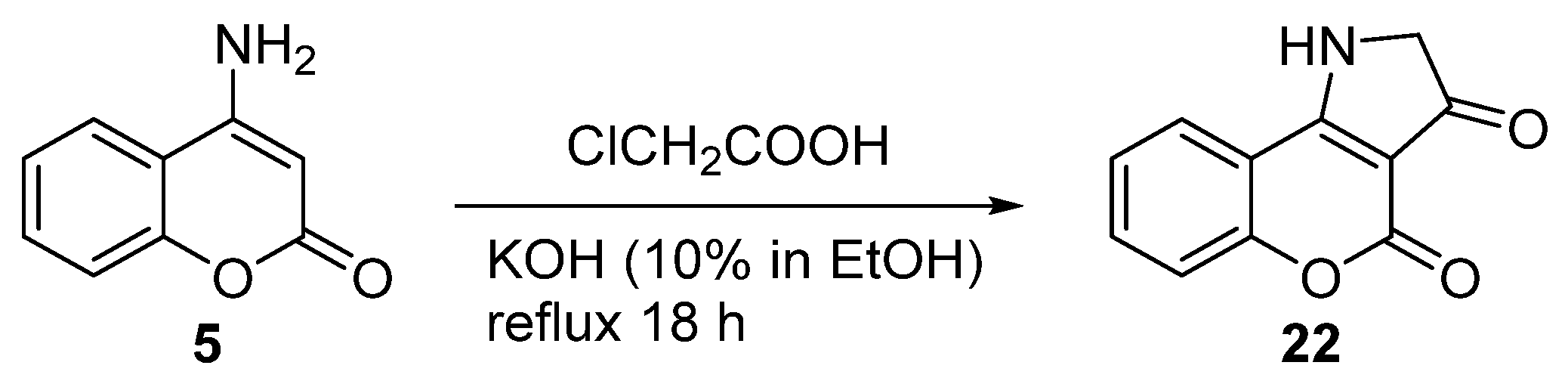
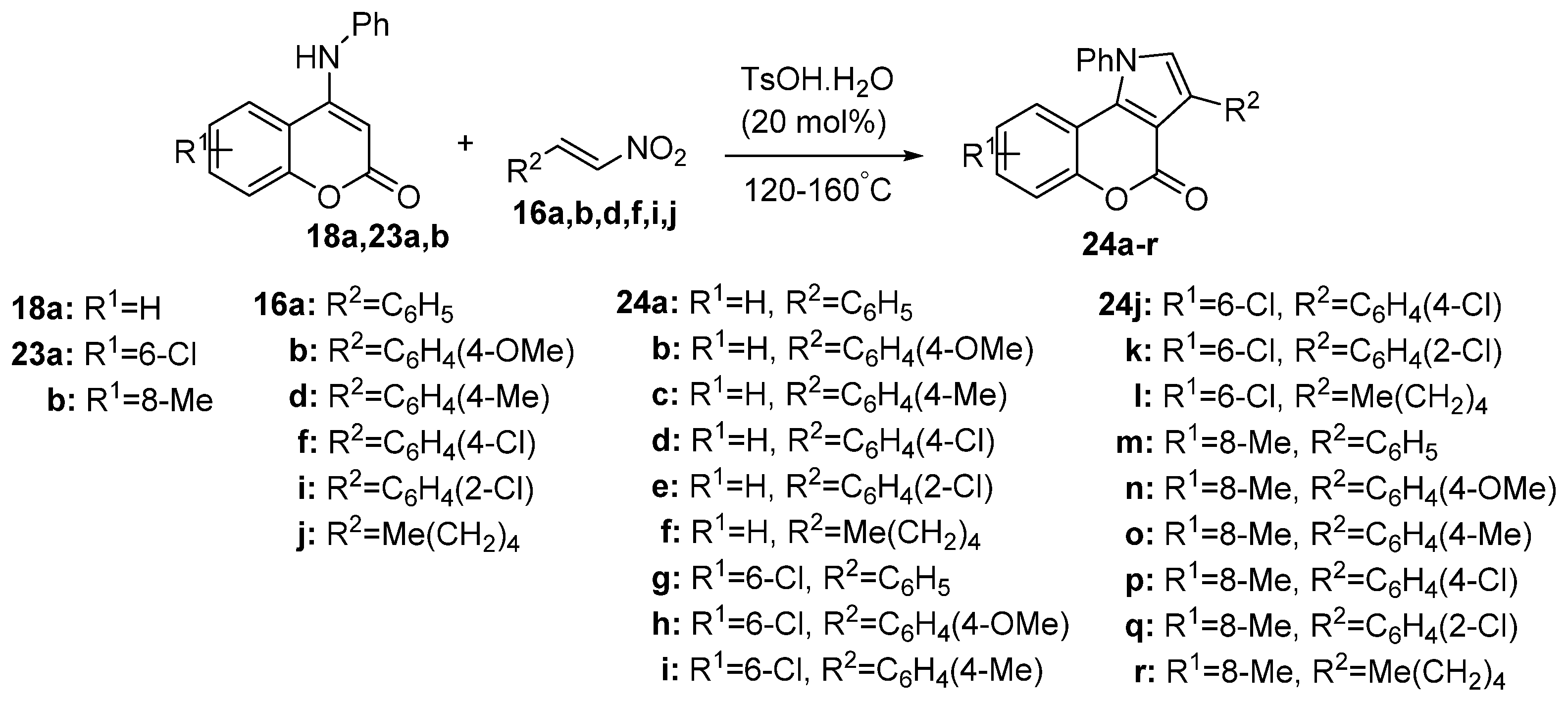

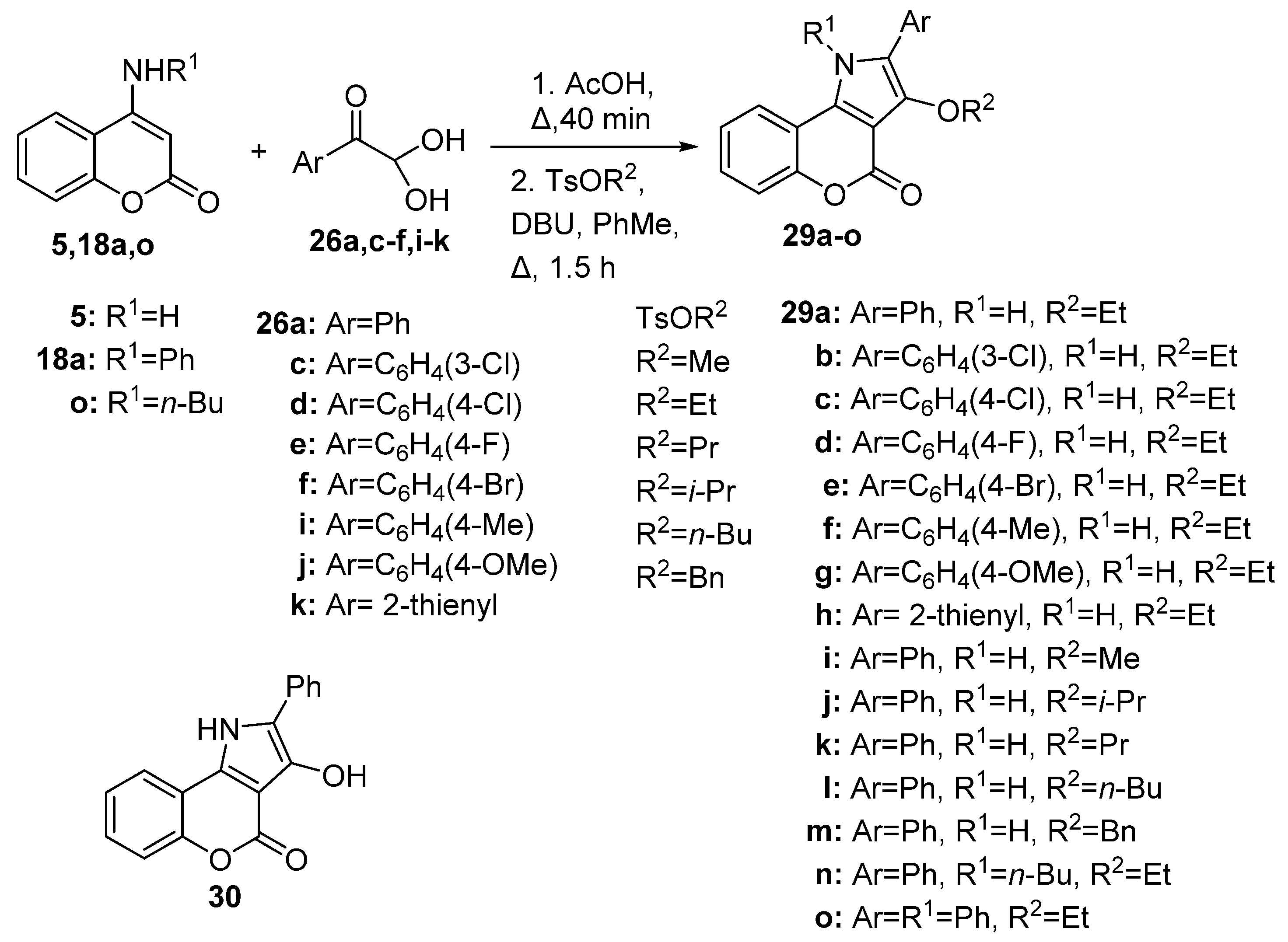
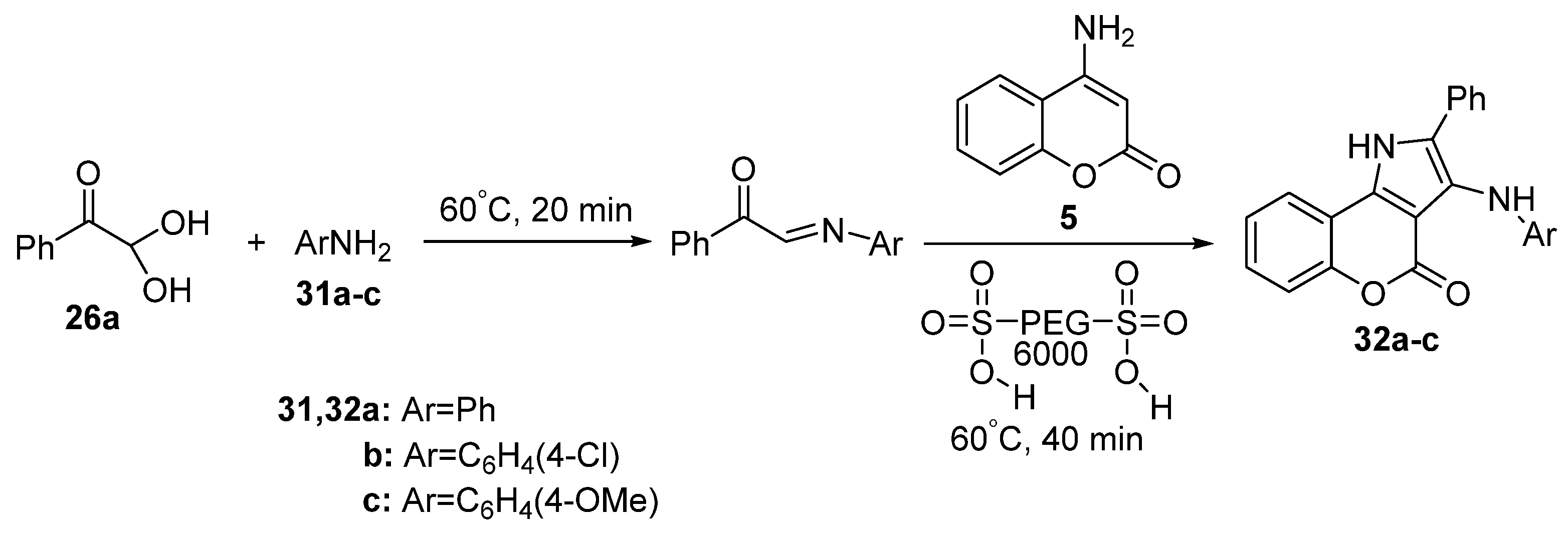
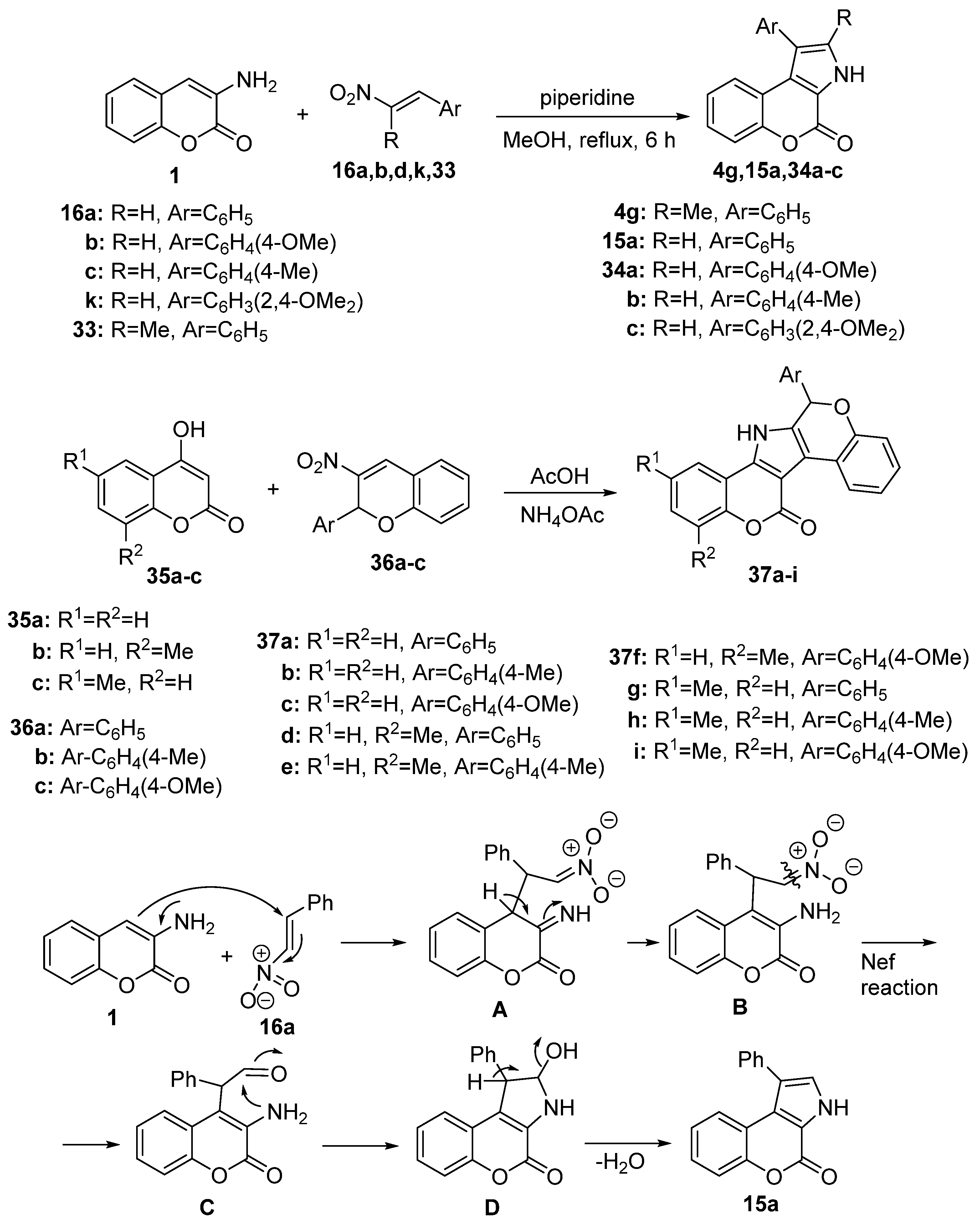
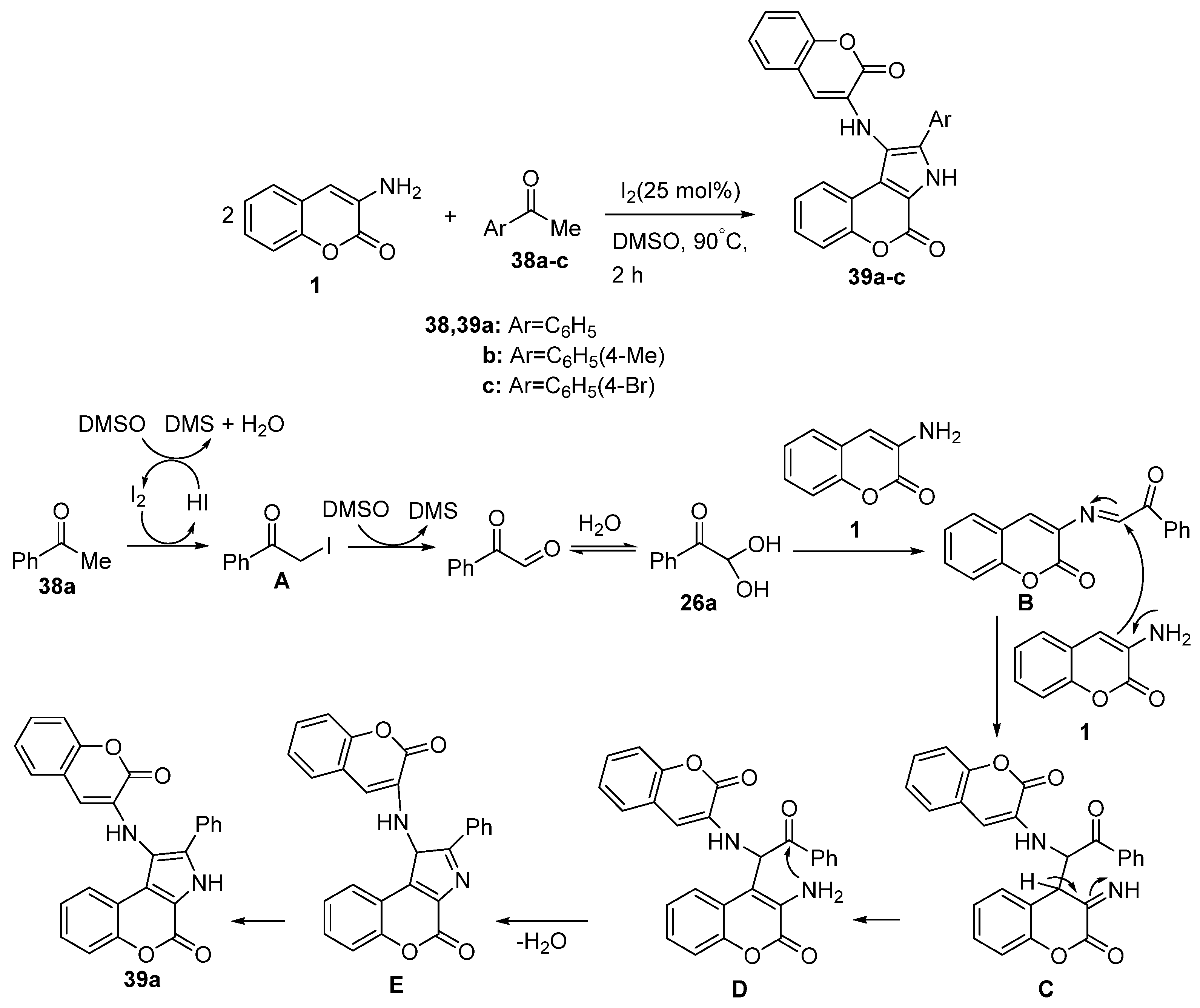



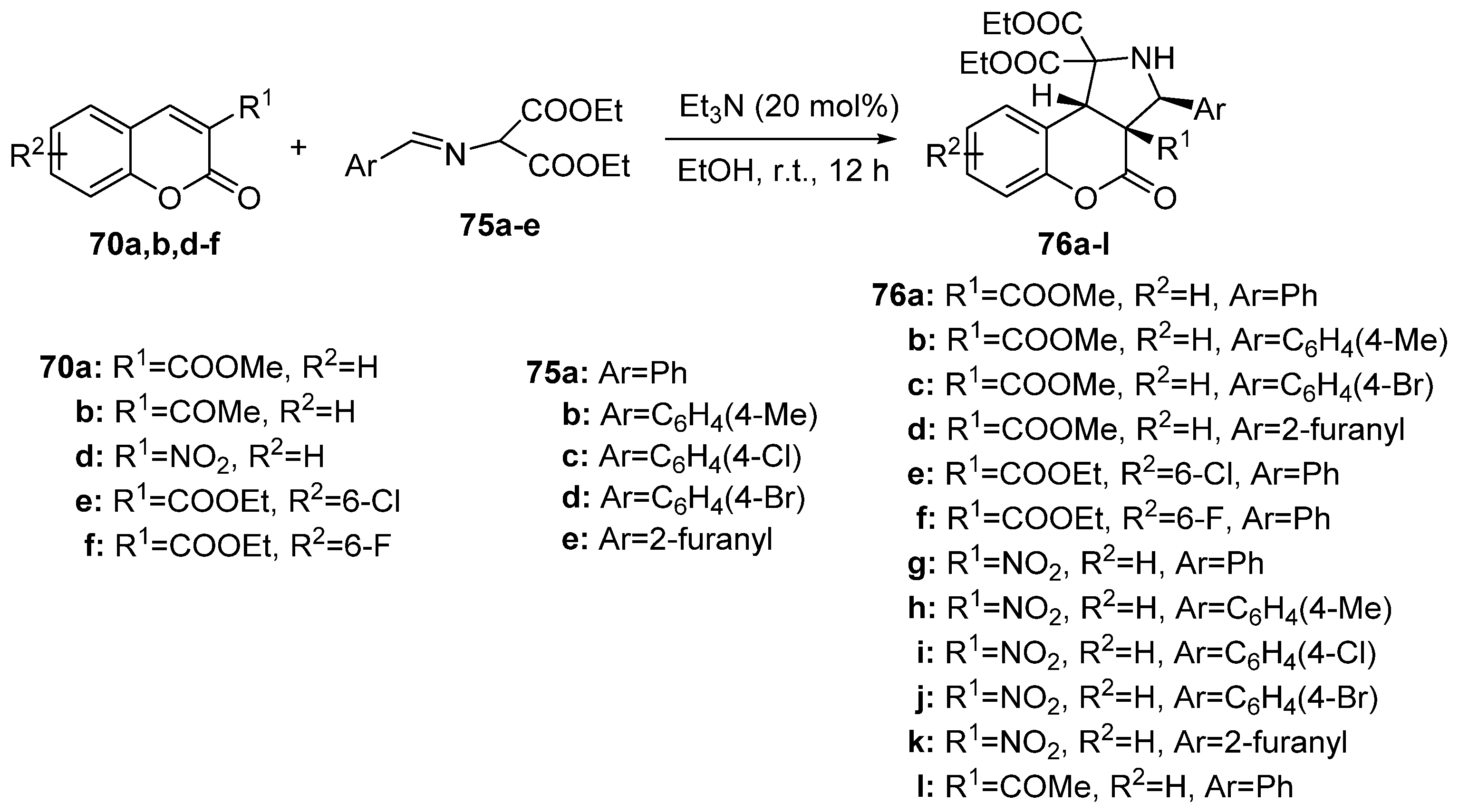
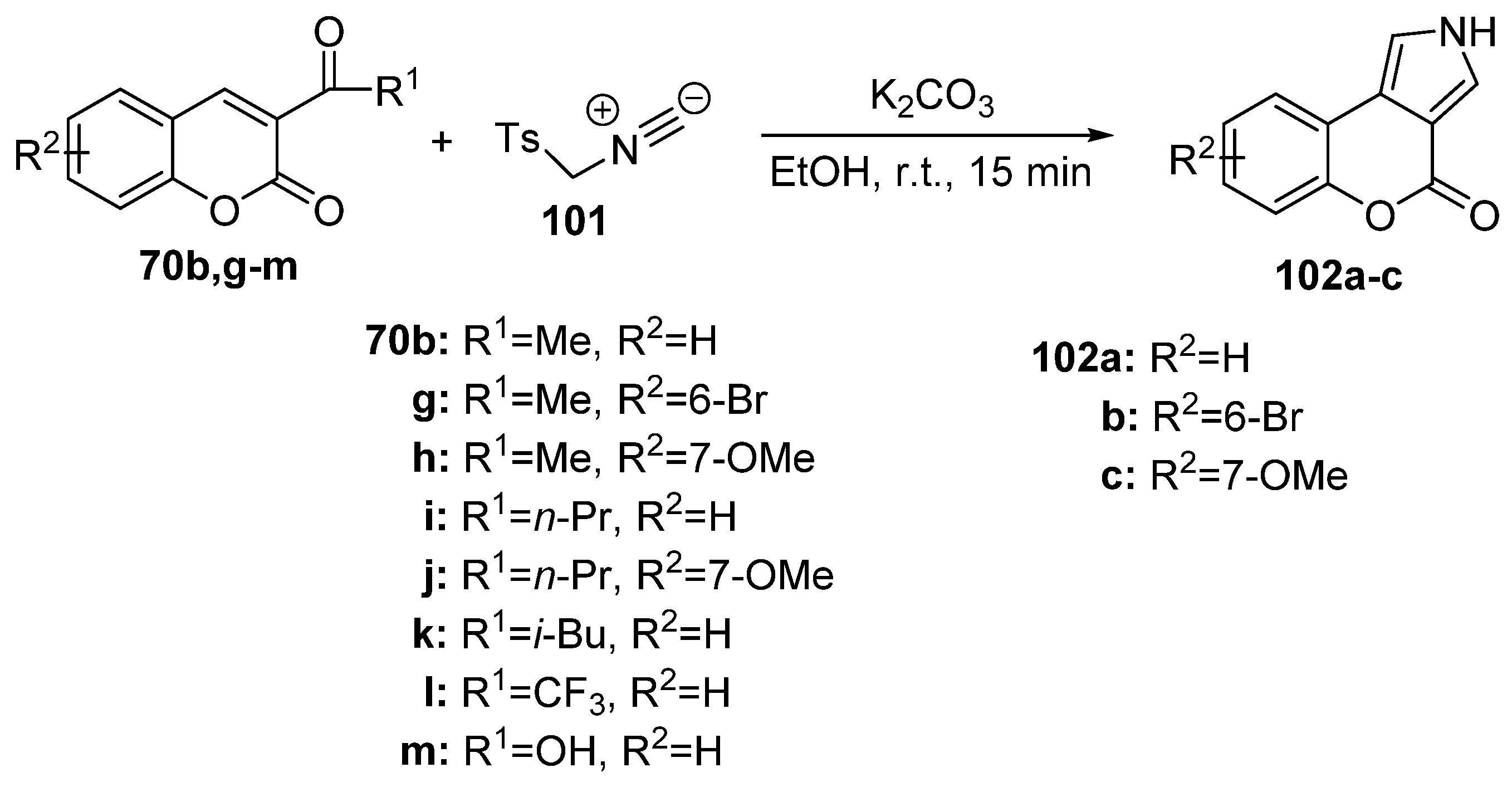
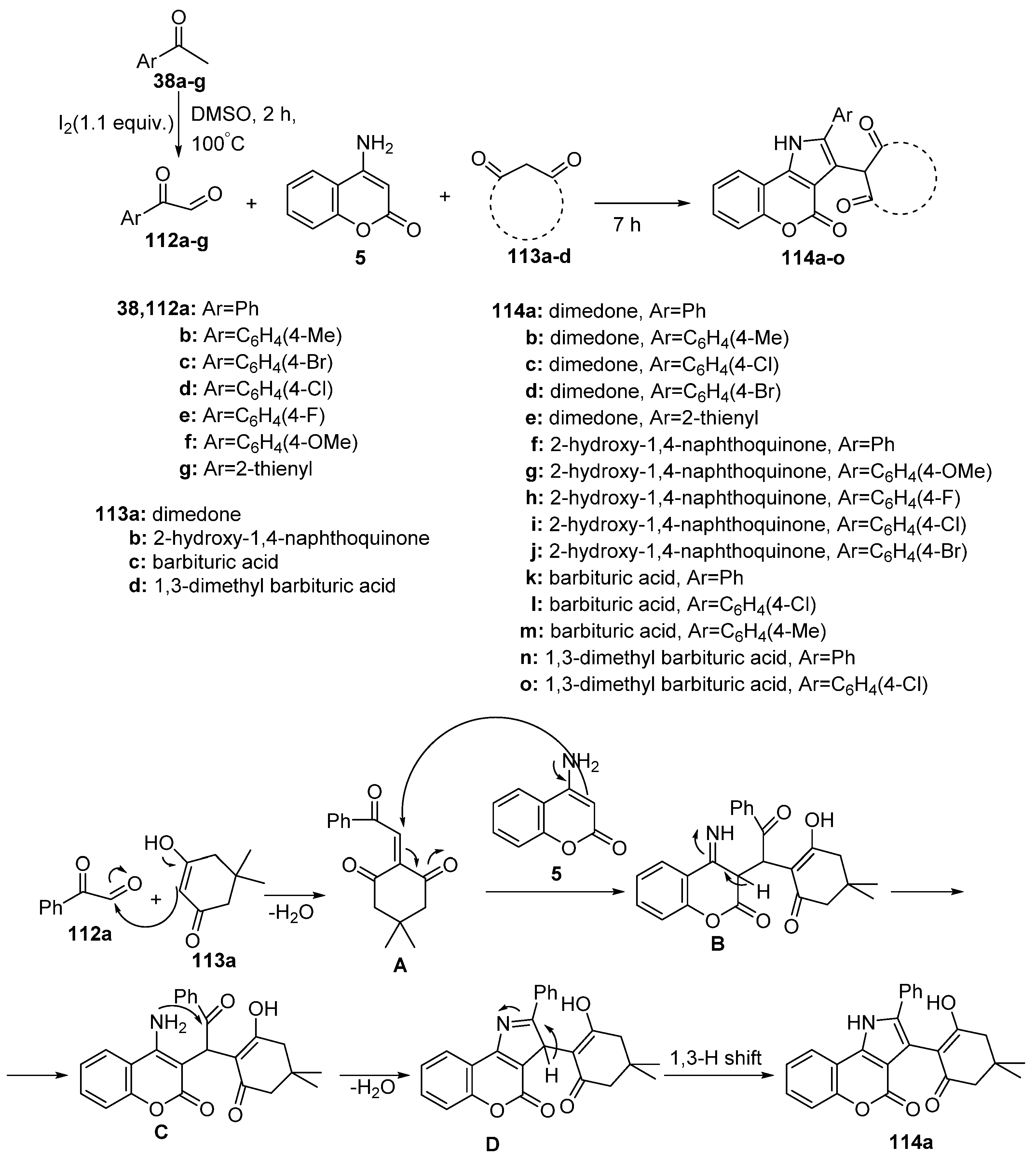
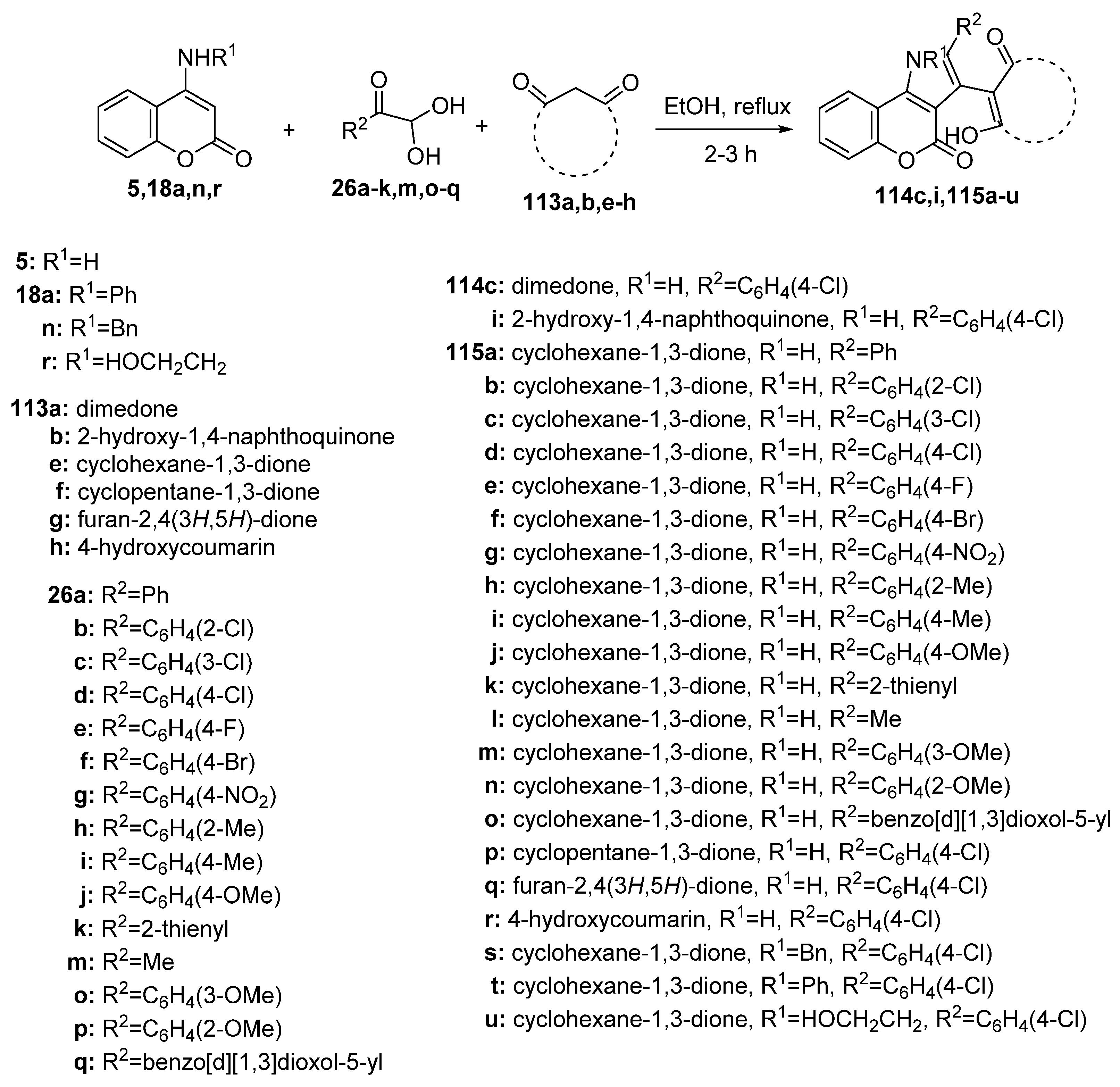
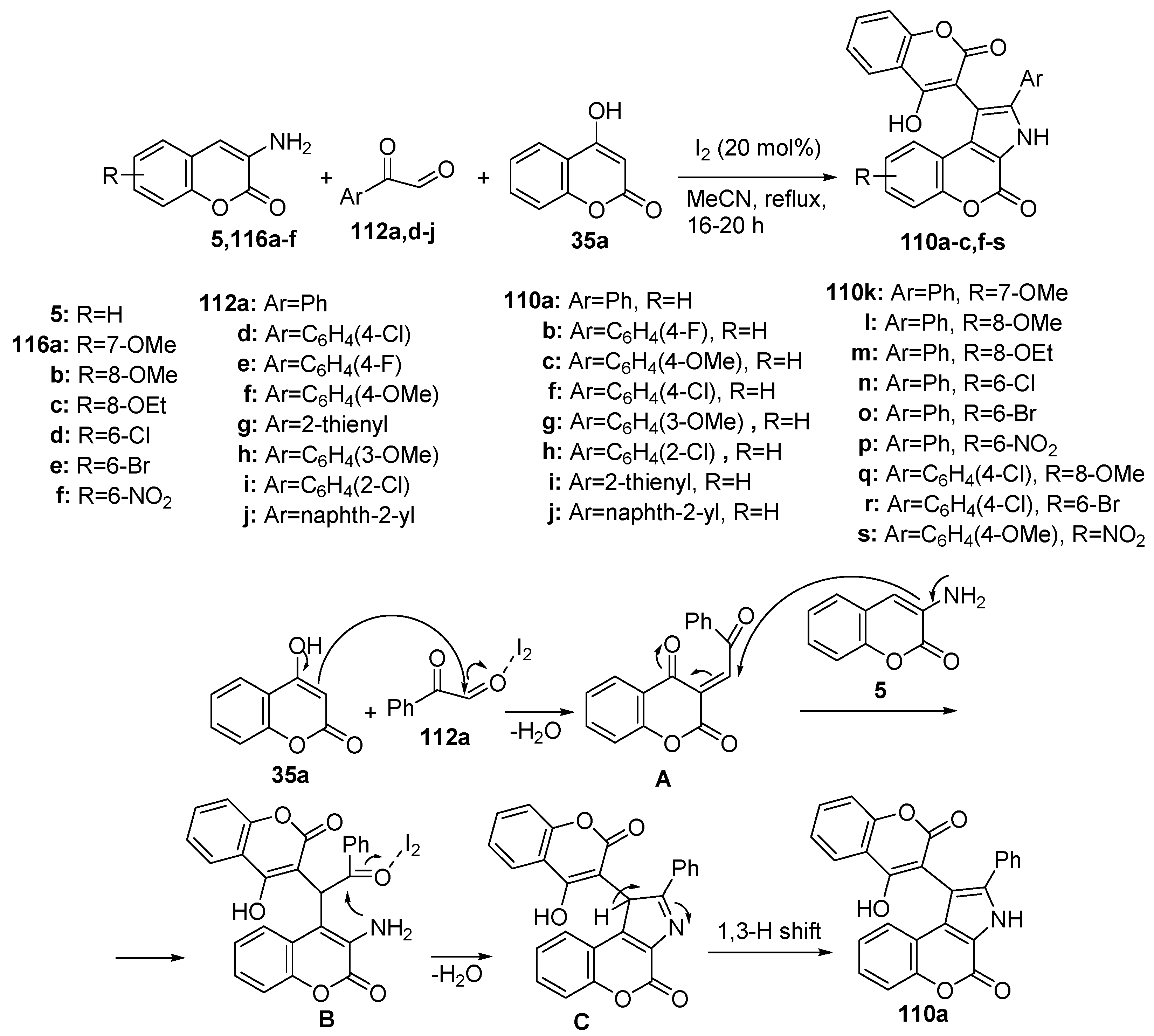
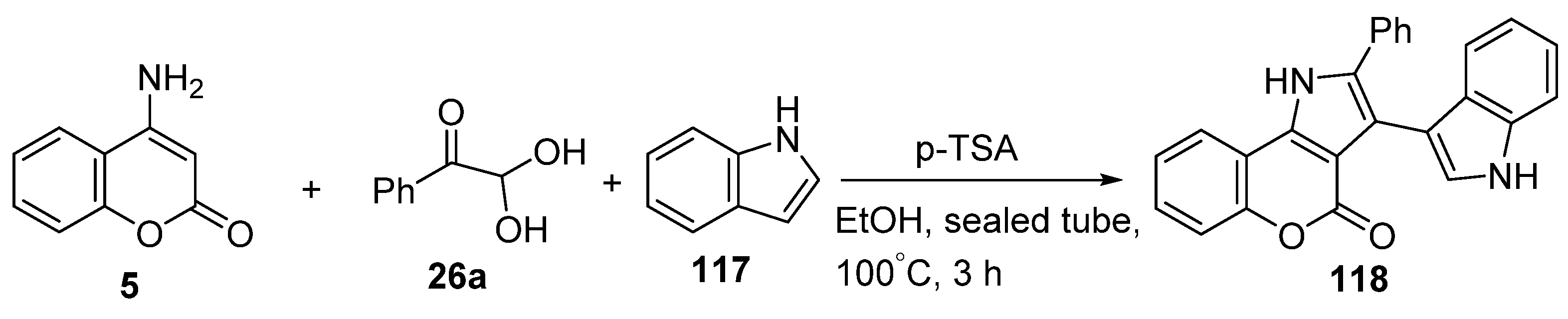










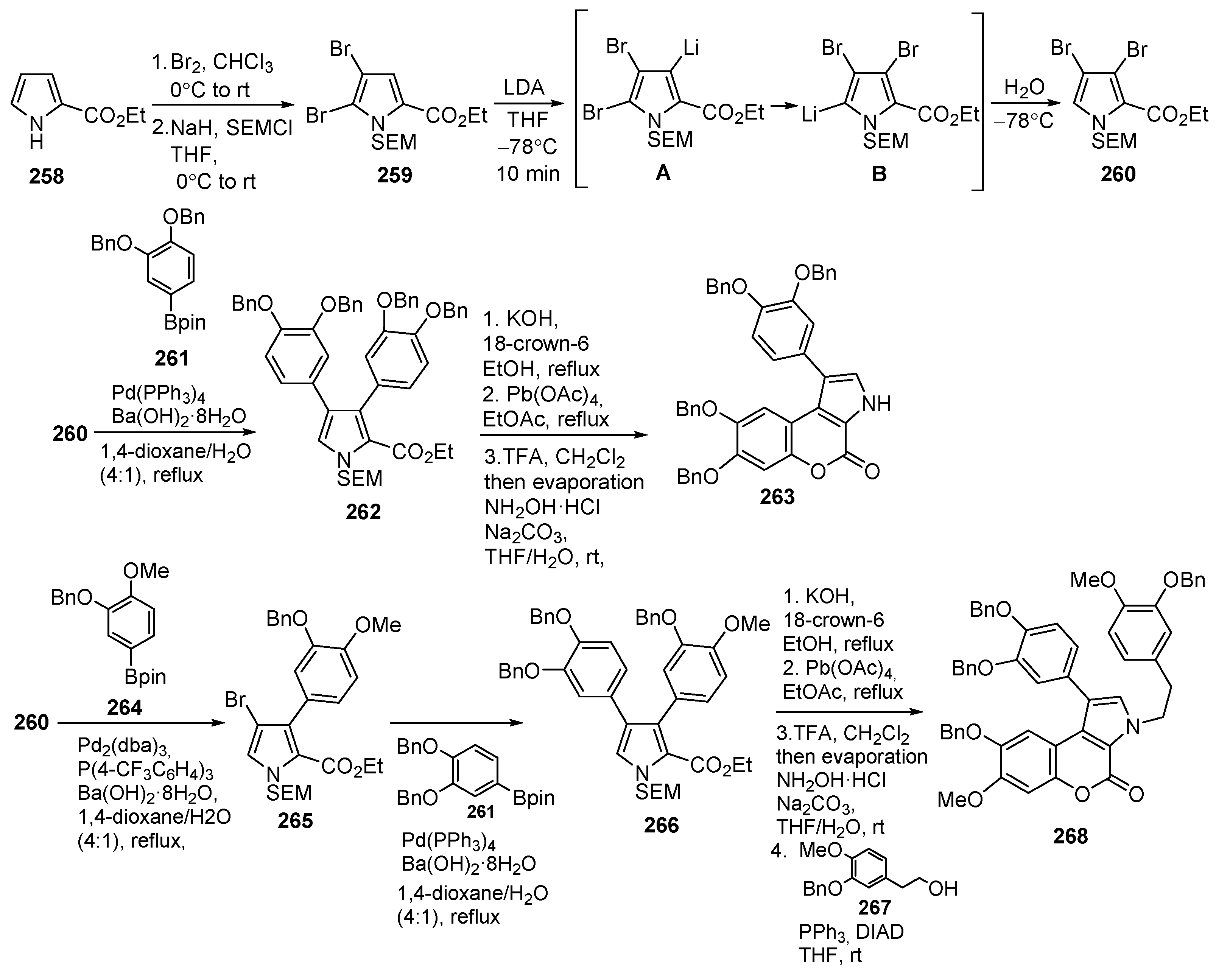
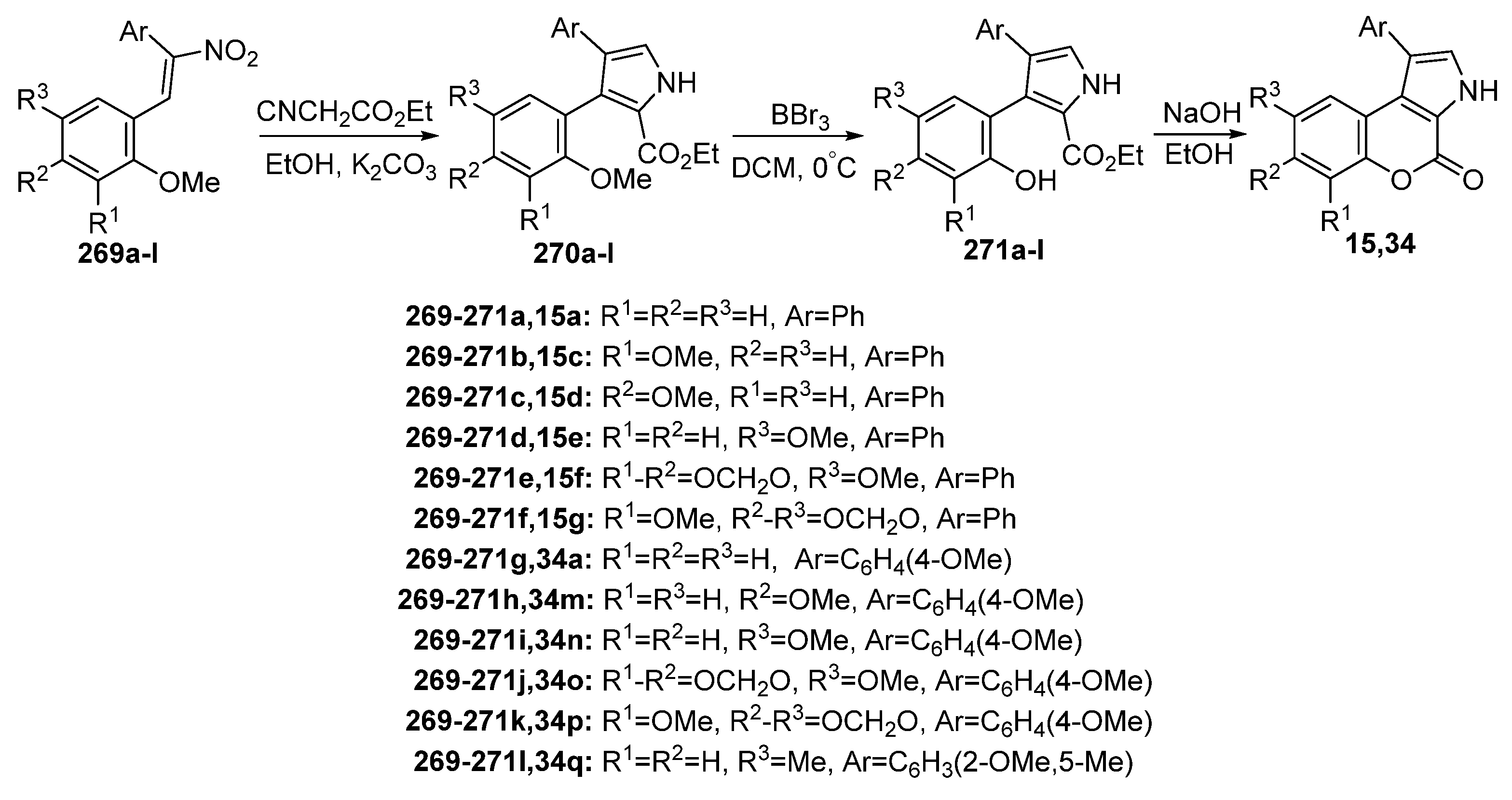
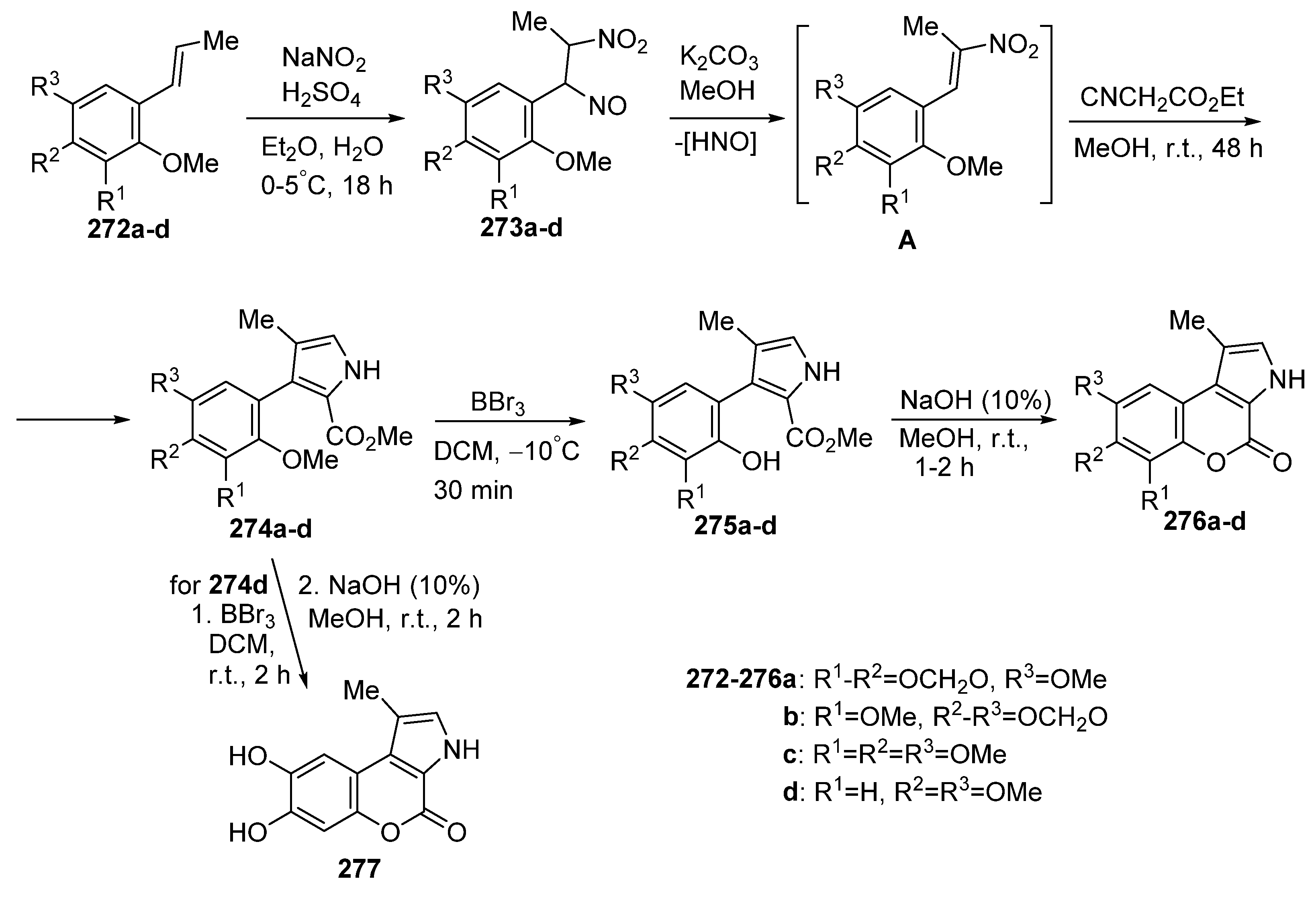
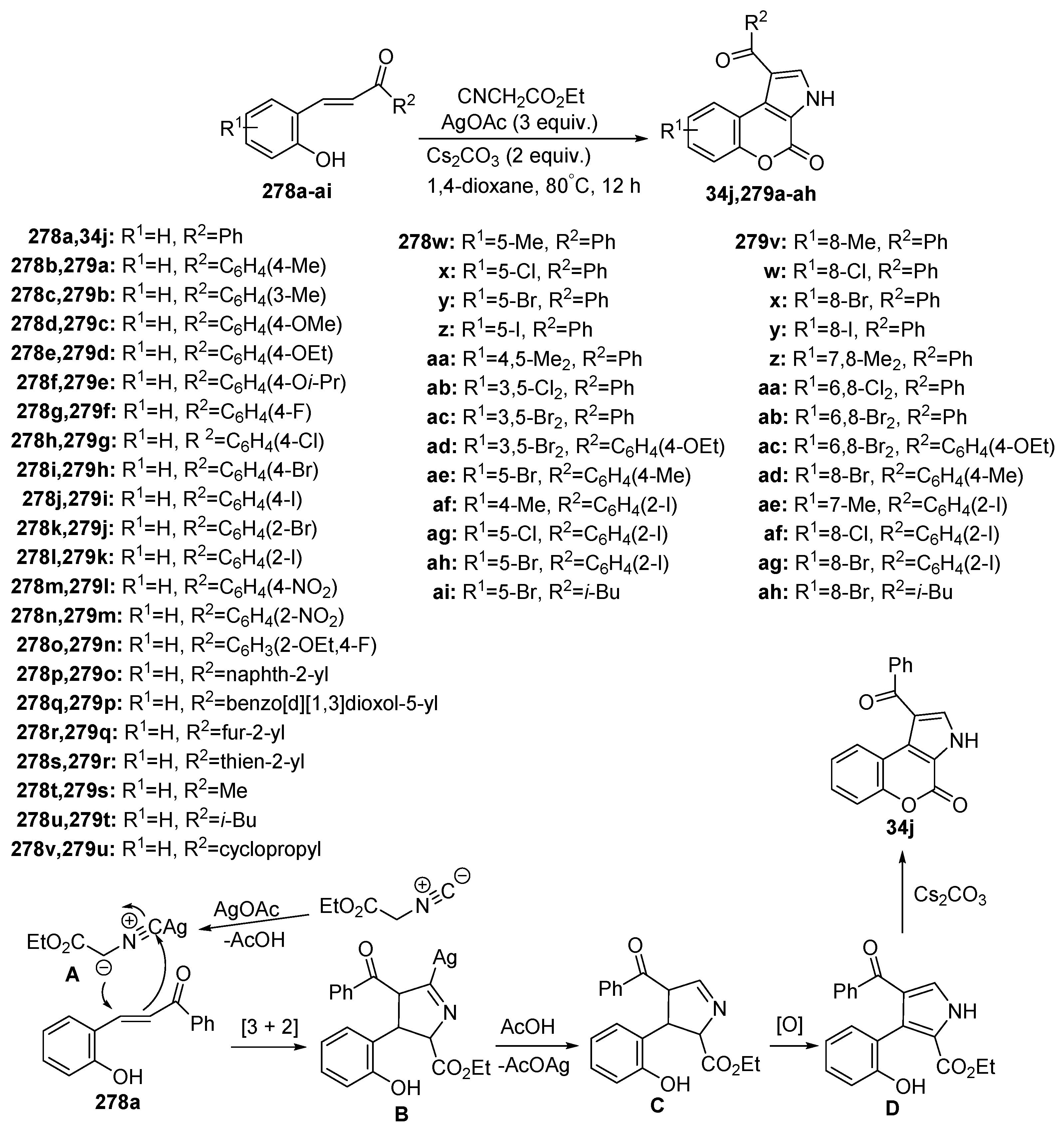
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapidou, E.; Litinas, K.E. An Overview of the Synthesis of 3,4-Fused Pyrrolocoumarins of Biological Interest. Molecules 2024, 29, 2748. https://doi.org/10.3390/molecules29122748
Kapidou E, Litinas KE. An Overview of the Synthesis of 3,4-Fused Pyrrolocoumarins of Biological Interest. Molecules. 2024; 29(12):2748. https://doi.org/10.3390/molecules29122748
Chicago/Turabian StyleKapidou, Eleni, and Konstantinos E. Litinas. 2024. "An Overview of the Synthesis of 3,4-Fused Pyrrolocoumarins of Biological Interest" Molecules 29, no. 12: 2748. https://doi.org/10.3390/molecules29122748
APA StyleKapidou, E., & Litinas, K. E. (2024). An Overview of the Synthesis of 3,4-Fused Pyrrolocoumarins of Biological Interest. Molecules, 29(12), 2748. https://doi.org/10.3390/molecules29122748





