Efficient Preparation of Biodiesel Using Sulfonated Camellia oleifera Shell Biochar as a Catalyst
Abstract
:1. Introduction
2. Results and Discussion
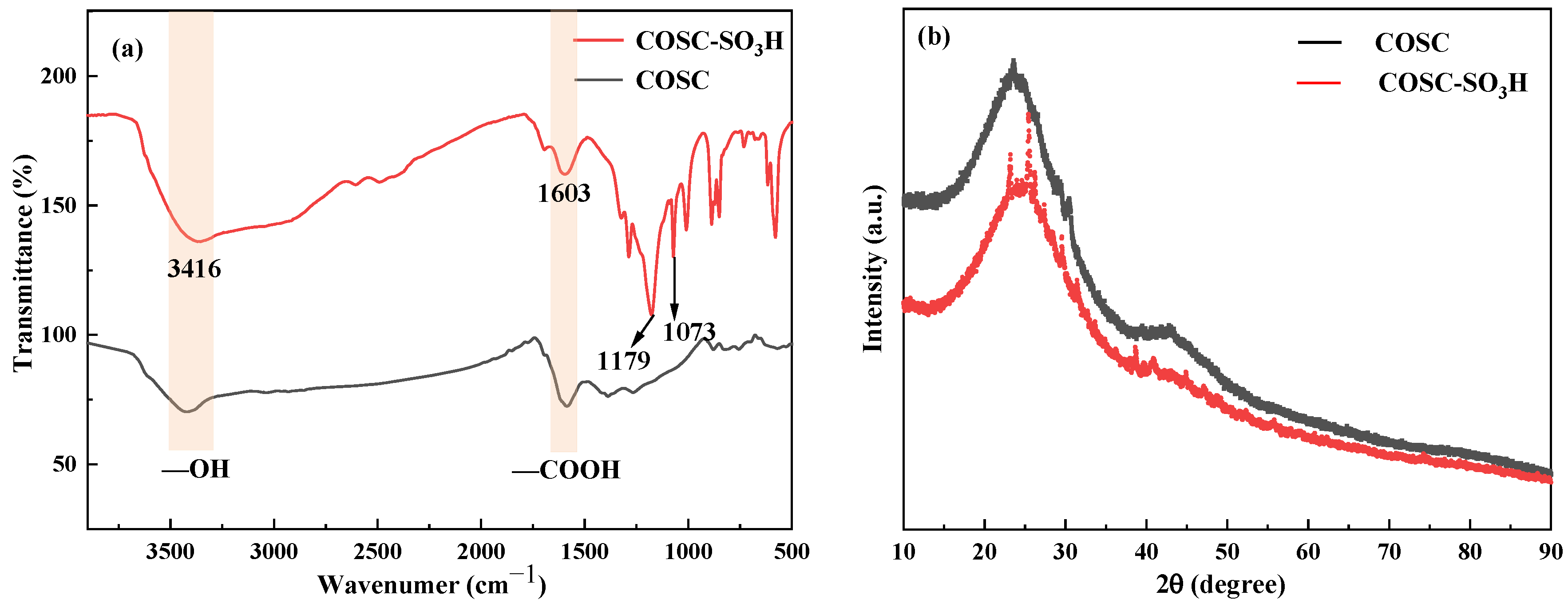
2.1. FT-IR and XRD Analysis
2.2. Pore Structure Analysis
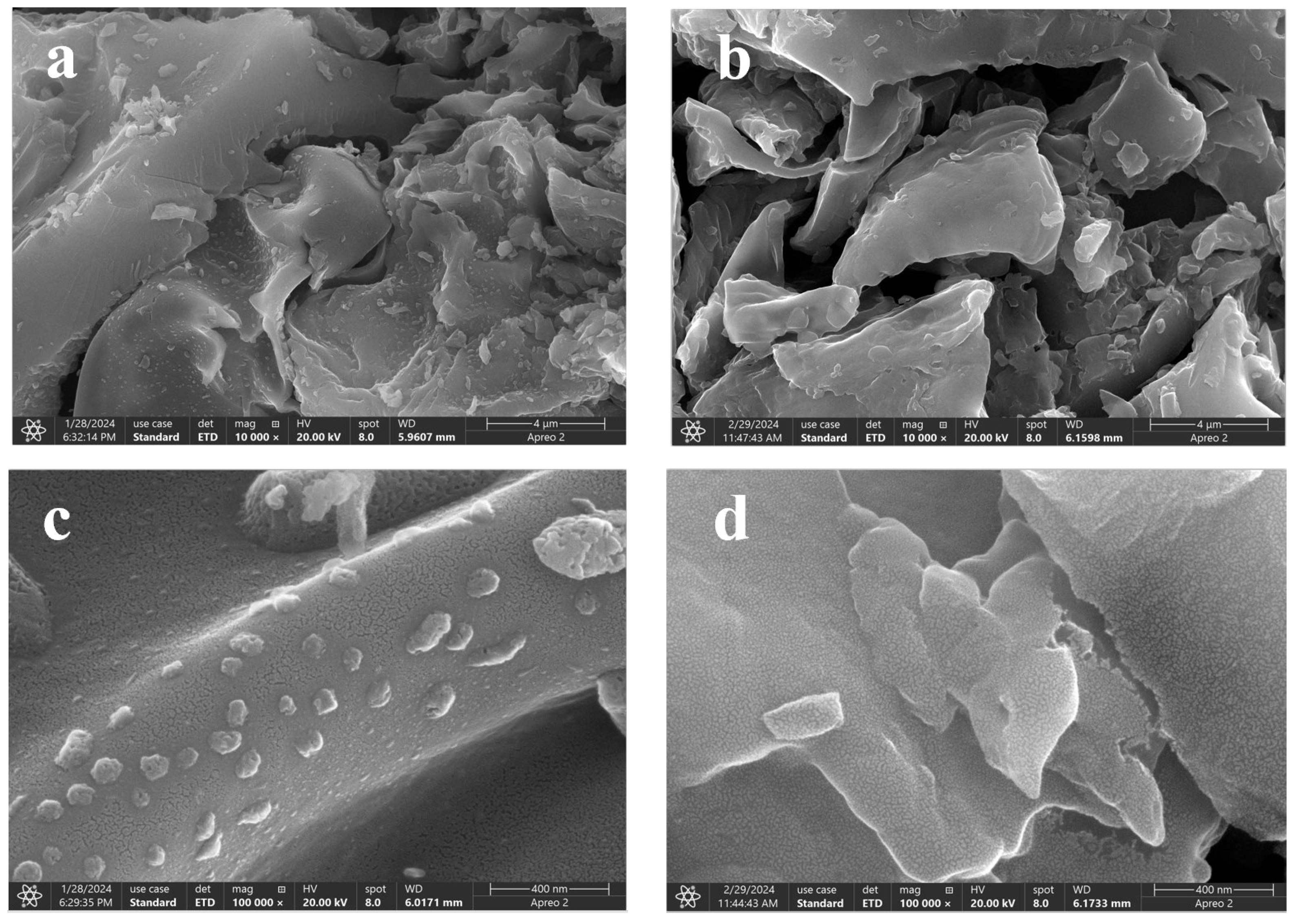
2.3. SEM Analysis
2.4. Biodiesel Yield Analysis
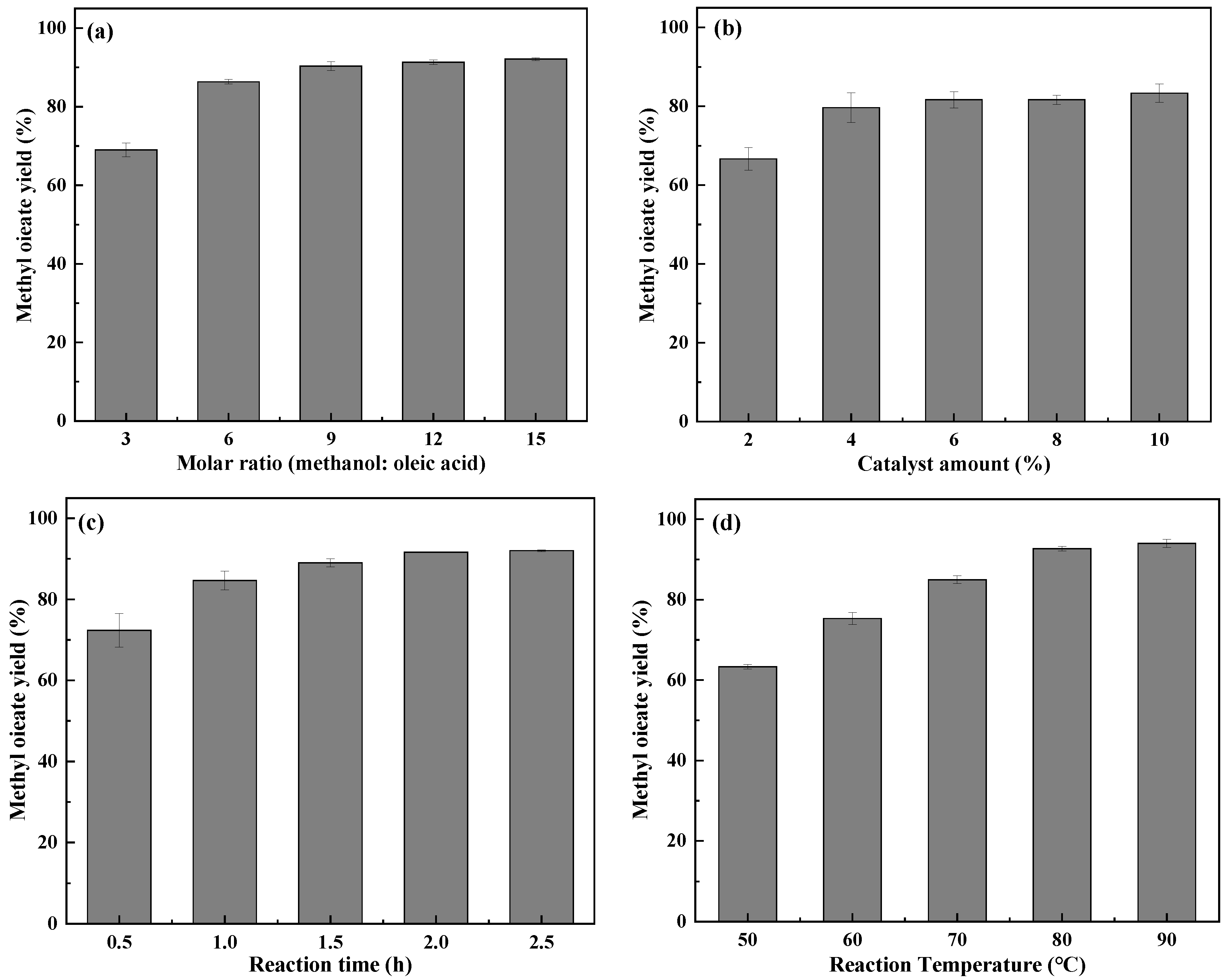
2.5. Optimization of the Esterification Reaction Process
2.6. Water Tolerance and Thermal Filtration Analysis
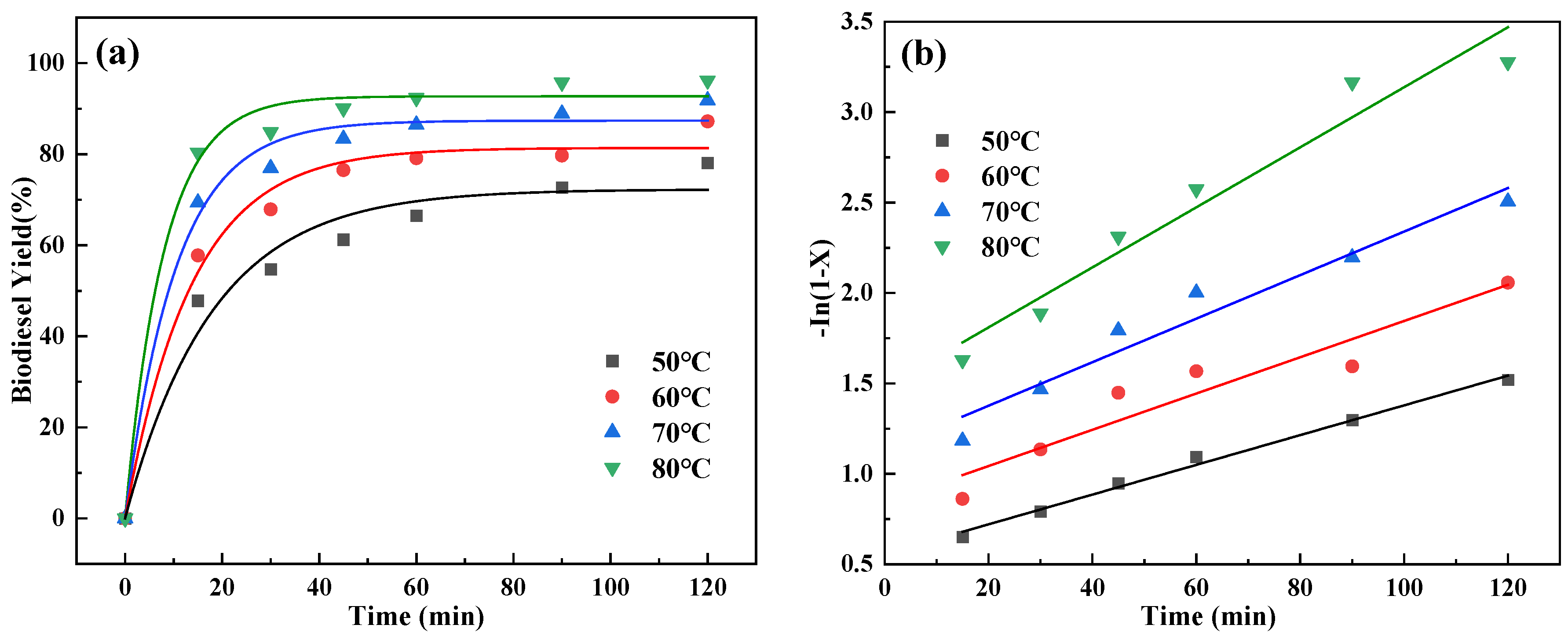
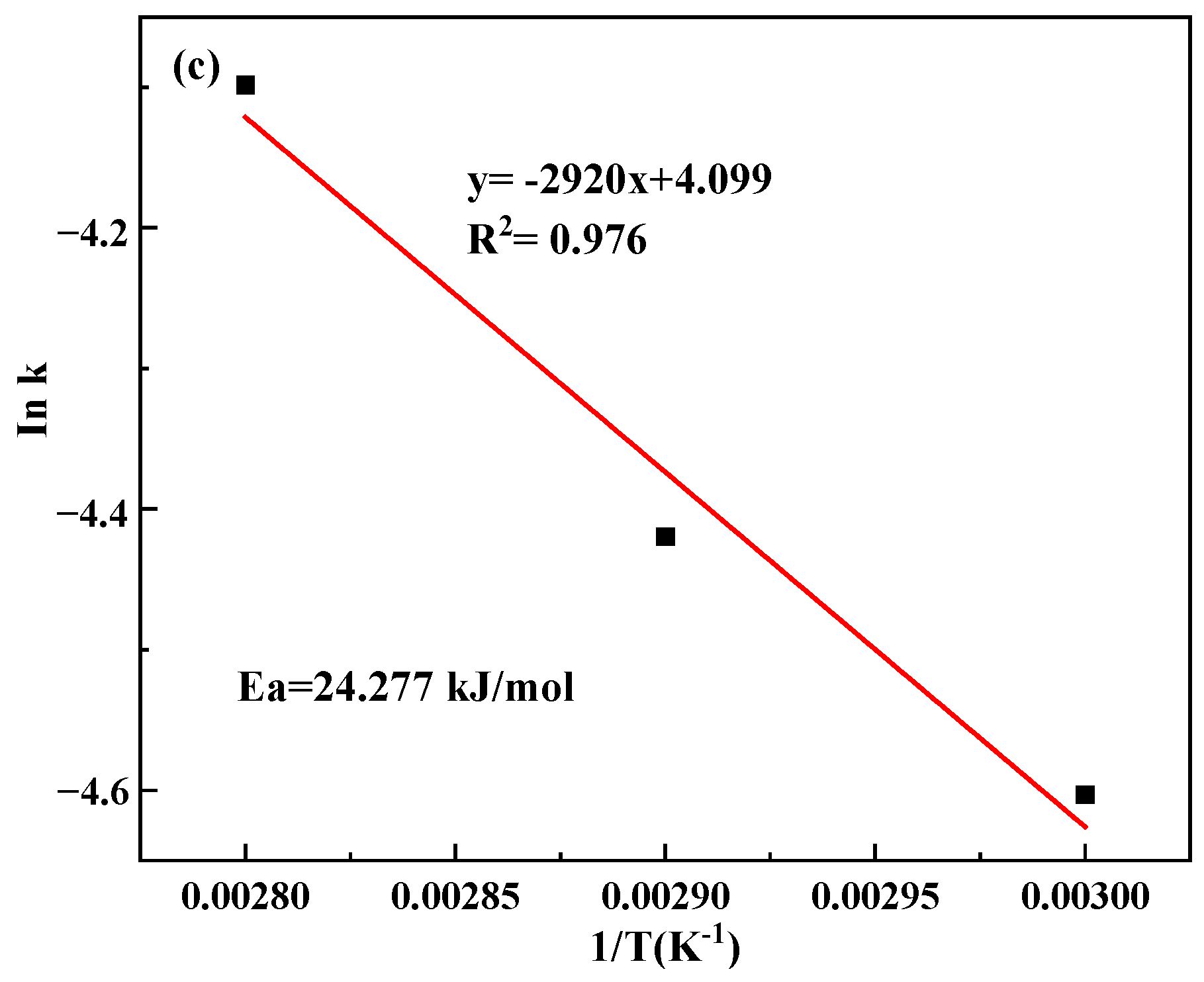
2.7. Reaction Kinetics Studies
3. Experimental Materials and Method
3.1. Materials
3.2. Sulfonation of Biochar from Camellia oleifera Fruit Shell
3.3. Determination of the Catalyst Acid Density
3.4. Esterification Reactions and Analytical Methods
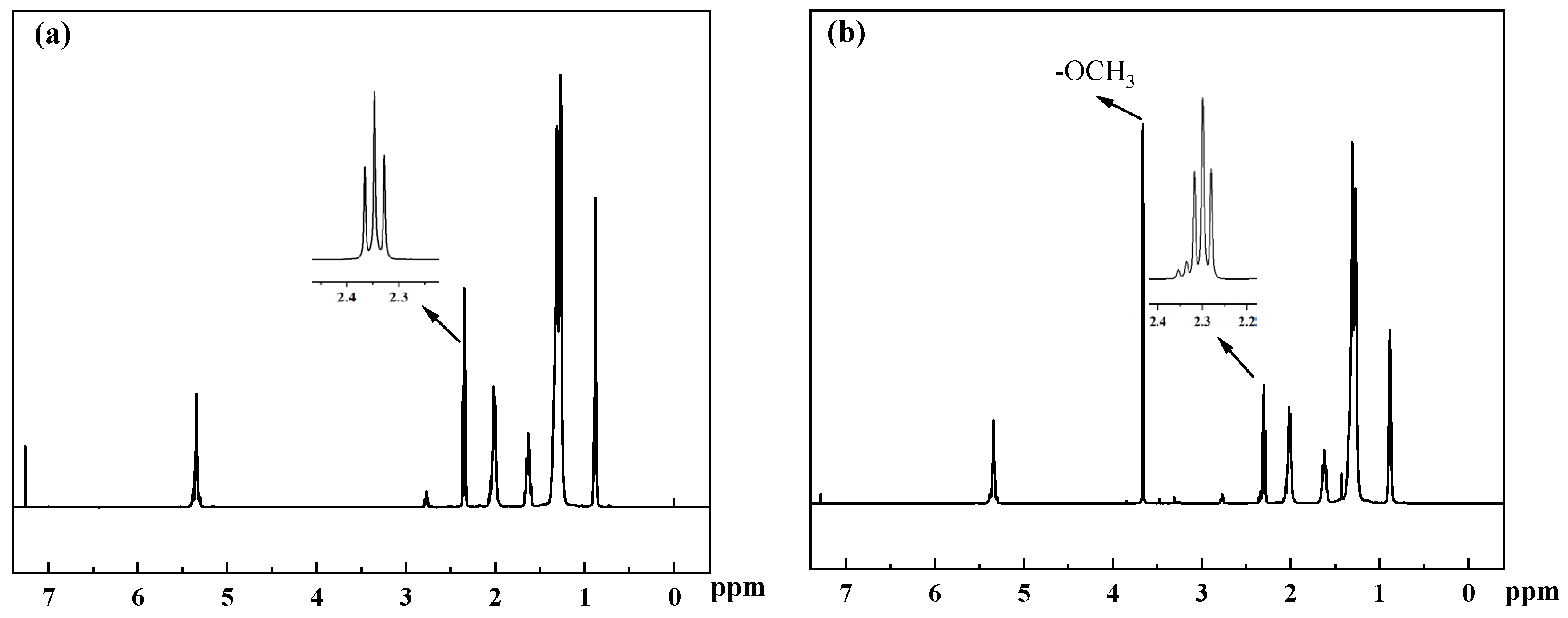
3.5. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, A.; Murad, S.M.W.; Mohsin, A.K.M.; Wang, X. Does renewable energy proactively contribute to mitigating carbon emissions in major fossil fuels consuming countries? J. Clean. Prod. 2024, 452, 142113. [Google Scholar] [CrossRef]
- Wang, A.; Sudarsanam, P.; Xu, Y.; Zhang, H.; Li, H.; Yang, S. Functionalized magnetic nano-sized materials for efficient biodiesel synthesis via acid-base/enzyme catalysis. Green Chem. Int. J. Green Chem. Resour. GC 2020, 22, 2977–3012. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, C.B.; Zhou, K.C.; Yang, S. Chemo-catalytic esterification and transesterification over organic Polymer-Based catalysts for biodiesel synthesis. Curr. Org. Chem. 2019, 23, 2190–2203. [Google Scholar] [CrossRef]
- Wang, A.; Quan, W.; Zhang, H.; Li, H.; Yang, S. Heterogeneous ZnO-containing catalysts for efficient biodiesel production. RSC Adv. 2021, 11, 20465–20478. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Li, Z.; Zhang, H.; Li, H.; Yang, S. Green synthesis of heterogeneous polymeric bio-based acid decorated with hydrophobic regulator for efficient catalytic production of biodiesel at low temperatures. Fuel 2022, 329, 125467. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Li, Y.; Hu, Y.; Li, H.; Xu, C.C.; Yang, S. Functionalized organic–inorganic hybrid porous coordination polymer-based catalysts for biodiesel production via trans/esterification. Green Chem. Int. J. Green Chem. Resour. GC 2022, 24, 7763–7786. [Google Scholar] [CrossRef]
- Pan, H.; Xia, Q.; Li, H.; Wang, Y.; Shen, Z.; Wang, Y.; Li, L.; Li, X.; Xu, H.; Zhou, Z.; et al. Direct production of biodiesel from crude Euphorbia lathyris L. Oil catalyzed by multifunctional mesoporous composite materials. Fuel 2022, 309, 122172. [Google Scholar] [CrossRef]
- Daimary, N.; Eldiehy, K.S.H.; Boruah, P.; Deka, D.; Bora, U.; Kakati, B.K. Potato peels as a sustainable source for biochar, bio-oil and a green heterogeneous catalyst for biodiesel production. J. Environ. Chem. Eng. 2022, 10, 107108. [Google Scholar] [CrossRef]
- He, L.; Chen, L.; Zheng, B.; Zhou, H.; Wang, H.; Li, H.; Zhang, H.; Xu, C.C.; Yang, S. Deep eutectic solvents for catalytic biodiesel production from liquid biomass and upgrading of solid biomass into 5-hydroxymethylfurfural. Green Chem. Int. J. Green Chem. Resour. GC 2023, 25, 741–744. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, K.; Jiang, Y.; Chen, L.; Zhang, H.; Li, H.; Yang, S. Biomass-derived hydrophobic metal-organic frameworks solid acid for green efficient catalytic esterification of oleic acid at low temperatures. Fuel Process. Technol. 2023, 239, 107558. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Tan, X.; Li, H.; Yang, S. Catalytic high-yield biodiesel production from fatty acids and non-food oils over a magnetically separable acid nanosphere. Ind. Crop. Prod. 2021, 173, 114126. [Google Scholar] [CrossRef]
- İnan, B.; Koçer, A.T.; Özçimen, D.B. Valorization of lignocellulosic wastes for low-cost and sustainable algal biodiesel production using biochar-based solid acid catalyst. J. Anal. Appl. Pyrol. 2023, 173, 106095. [Google Scholar] [CrossRef]
- Wang, H.; Peng, X.; Zhang, H.; Yang, S.; Li, H. Microorganisms-promoted biodiesel production from biomass: A review. Energy Convers. Manag. X 2021, 12, 100137. [Google Scholar] [CrossRef]
- Sahu, G.; Gupta, N.K.; Kotha, A.; Saha, S.; Datta, S.; Chavan, P.; Kumari, N.; Dutta, P. A Review on Biodiesel Production through Heterogeneous Catalysis Route. ChemBioEng Rev. 2018, 5, 231–252. [Google Scholar] [CrossRef]
- Jung, J.; Oh, J.; Baek, K.; Lee, J.; Kwon, E.E. Biodiesel production from waste cooking oil using biochar derived from chicken manure as a porous media and catalyst. Energy Convers. Manag. 2018, 165, 628–633. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Kim, H.J.; Yang, S.Y.; Song, H.S.; Park, J.Y.; Park, Y.L.; Han, Y.H.; Choi, Y.K.; et al. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef]
- Cao, M.; Peng, L.; Xie, Q.; Xing, K.; Lu, M.; Ji, J. Sulfonated Sargassum horneri carbon as solid acid catalyst to produce biodiesel via esterification. Bioresour. Technol. 2021, 324, 124614. [Google Scholar] [CrossRef]
- Karmakar, B.; Ghosh, B.; Halder, G. Sulfonated Mesua ferrea Linn seed shell catalyzed biodiesel synthesis from castor oil—Response surface optimization. Front. Energy Res. 2020, 8, 576792. [Google Scholar] [CrossRef]
- Xie, W.; Wang, H. Immobilized polymeric sulfonated ionic liquid on core-shell structured Fe3O4/SiO2 composites: A magnetically recyclable catalyst for simultaneous transesterification and esterifications of low-cost oils to biodiesel. Renew. Energy 2020, 145, 1709–1719. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, P.; Yang, L.; Xing, S.; Luo, W.; Wang, Z. Biodiesel synthesis over biochar-based catalyst from biomass waste pomelo peel. Energy Convers. Manag. 2018, 160, 477–485. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Tan, Y.H.; Kansedo, J.; Mubarak, N.M.; Liew, R.K.; Yek, P.N.Y.; Aghbashlo, M.; Ng, H.S.; Chong, W.W.F.; Lam, S.S.; et al. Assessing biodiesel production using palm kernel shell-derived sulfonated magnetic biochar from the life cycle assessment perspective. Energy 2023, 282, 128758. [Google Scholar] [CrossRef]
- Chong, C.C.; Cheng, Y.W.; Lam, M.K.; Setiabudi, H.D.; Vo, D.N. State-of-the-art of the synthesis and applications of sulfonated carbon-based catalysts for biodiesel production: A review. Energy Technol. 2021, 9, 21003. [Google Scholar] [CrossRef]
- Bastos, R.R.C.; Da Luz Corrêa, A.P.; Da Luz, P.T.S.; Da Rocha Filho, G.N.; Zamian, J.R.; Da Conceição, L.R.V. Optimization of biodiesel production using sulfonated carbon-based catalyst from an amazon agro-industrial waste. Energy Convers. Manag. 2020, 205, 112457. [Google Scholar] [CrossRef]
- Yadav, N.; Yadav, G.; Ahmaruzzaman, M. Biomass-derived sulfonated polycyclic aromatic carbon catalysts for biodiesel production by esterification reaction. Biofuels, bioproducts and biorefining 2023, 17, 1343–1367. [Google Scholar] [CrossRef]
- Balajii, M.; Niju, S. Biochar-derived heterogeneous catalysts for biodiesel production. Environ. Chem. Lett. 2019, 17, 1447–1469. [Google Scholar] [CrossRef]
- Hussain, Z.; Lingareddy, J.R.; Sulthana, N.; Boddu, S.K. Novel corncob-based catalytic biodiesel production process: Experiments, modeling, and simulation. Chem. Eng. Technol. 2024, 47, 289–296. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Tang, W.; Peng, S.; Li, M.; Deng, N.; Chen, Y. Predicting potential distribution and evaluating suitable soil condition of oil tea camellia in china. Forests 2018, 9, 487. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, J.; Yang, T.; Chang, S. Anatomy and lignin deposition of stone cell in Camellia oleifera shell during the young stage. Protoplasma 2021, 258, 361–370. [Google Scholar] [CrossRef]
- Rashid, U.; Ahmad, J.; Ibrahim, M.L.; Nisar, J.; Hanif, M.A.; Shean, T.Y.C. Single-Pot synthesis of biodiesel using efficient Sulfonated-Derived tea Waste-Heterogeneous catalyst. Materials 2019, 12, 2293. [Google Scholar] [CrossRef]
- Li, K.; Liu, S.; Shu, T.; Yan, L.; Guo, H.; Dai, Y.; Luo, X.; Luo, S. Fabrication of carbon microspheres with controllable porous structure by using waste Camellia oleifera shells. Mater. Chem. Phys. 2016, 181, 518–528. [Google Scholar] [CrossRef]
- Niu, S.; Ning, Y.; Lu, C.; Han, K.; Yu, H.; Zhou, Y. Esterification of oleic acid to produce biodiesel catalyzed by sulfonated activated carbon from bamboo. Energy Convers. Manag. 2018, 163, 59–65. [Google Scholar] [CrossRef]
- Rhithuparna, D.; Ghosh, N.; Khatoon, R.; Rokhum, S.L.; Halder, G. Evaluating the commercial potential of Cocos nucifera derived biochar catalyst in biodiesel synthesis from Kanuga oil: Optimization, kinetics, thermodynamics, and process cost analysis. Process Saf. Environ. 2024, 183, 859–874. [Google Scholar] [CrossRef]
- Li, Y.; Niu, S.; Hao, Y.; Zhou, W.; Wang, J.; Liu, J. Role of oxygen vacancy on activity of Fe-doped SrTiO3 perovskite bifunctional catalysts for biodiesel production. Renew. Energy 2022, 199, 1258–1271. [Google Scholar] [CrossRef]
- Zheng, B.; Chen, L.; He, L.; Wang, H.; Li, H.; Zhang, H.; Yang, S. Facile synthesis of chitosan-derived sulfonated solid acid catalysts for realizing highly effective production of biodiesel. Ind. Crop. Prod. 2024, 210, 118058. [Google Scholar] [CrossRef]
- Chen, L.; He, L.; Zheng, B.; Wei, G.; Li, H.; Zhang, H.; Yang, S. Bifunctional acid-activated montmorillonite catalyzed biodiesel production from non-food oil: Characterization, optimization, kinetic and thermodynamic studies. Fuel Process. Technol. 2023, 250, 107903. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Tan, M.; Jiang, B.; Zheng, J.; Tsubaki, N.; Wu, M. Monodispersed hollow SO3H-Functionalized Carbon/Silica as efficient solid acid catalyst for esterification of oleic acid. ACS Appl. Mater. Inter. 2015, 7, 26767–26775. [Google Scholar] [CrossRef]
- Pan, H.; Li, H.; Zhang, H.; Wang, A.; Yang, S. Acidic ionic liquid-functionalized mesoporous melamine-formaldehyde polymer as heterogeneous catalyst for biodiesel production. Fuel 2019, 239, 886–895. [Google Scholar] [CrossRef]
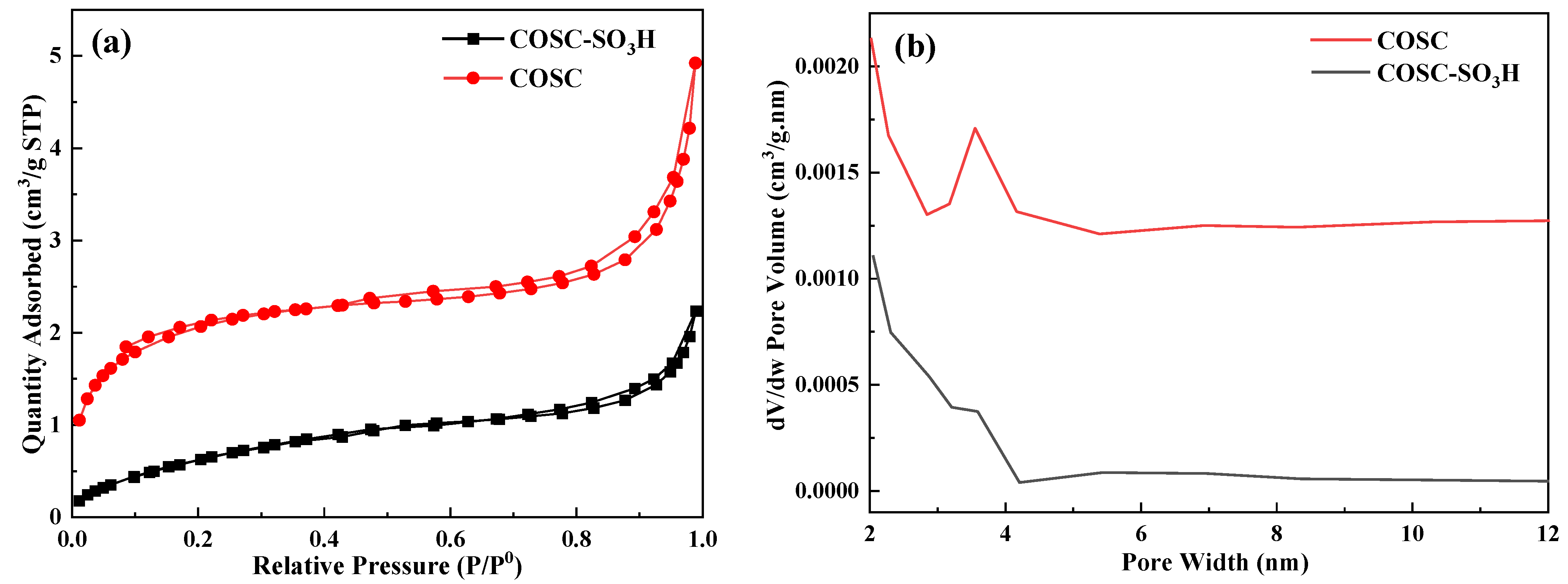

| Sample | BET Surface Area (m2/g) | Pore Volume (mm3/g) | Pore Size (nm) |
|---|---|---|---|
| COSC | 7.6 | 5.2 | 10.5 |
| COSC-SO3H | 2.7 | 3.4 | 6.0 |
| Temperature (°C) | Reaction Rate Constant, k′ (1/min) | Coefficient of Determination (R2) |
|---|---|---|
| 50 | 0.0166 | 0.951 |
| 60 | 0.0121 | 0.949 |
| 70 | 0.0100 | 0.907 |
| 80 | 0.00822 | 0.993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Wang, Y.; Wu, X.; Quan, W.; Chen, Q.; Wang, A. Efficient Preparation of Biodiesel Using Sulfonated Camellia oleifera Shell Biochar as a Catalyst. Molecules 2024, 29, 2752. https://doi.org/10.3390/molecules29122752
Yang Z, Wang Y, Wu X, Quan W, Chen Q, Wang A. Efficient Preparation of Biodiesel Using Sulfonated Camellia oleifera Shell Biochar as a Catalyst. Molecules. 2024; 29(12):2752. https://doi.org/10.3390/molecules29122752
Chicago/Turabian StyleYang, Zhimin, Yu Wang, Xichang Wu, Wenxuan Quan, Qi Chen, and Anping Wang. 2024. "Efficient Preparation of Biodiesel Using Sulfonated Camellia oleifera Shell Biochar as a Catalyst" Molecules 29, no. 12: 2752. https://doi.org/10.3390/molecules29122752






