Preparative Fractionation of Brazilian Red Propolis Extract Using Step-Gradient Counter-Current Chromatography
Abstract
:1. Introduction
2. Results
2.1. CCC Method Development
2.2. UHPLC–MS Analysis, HPLC Purification
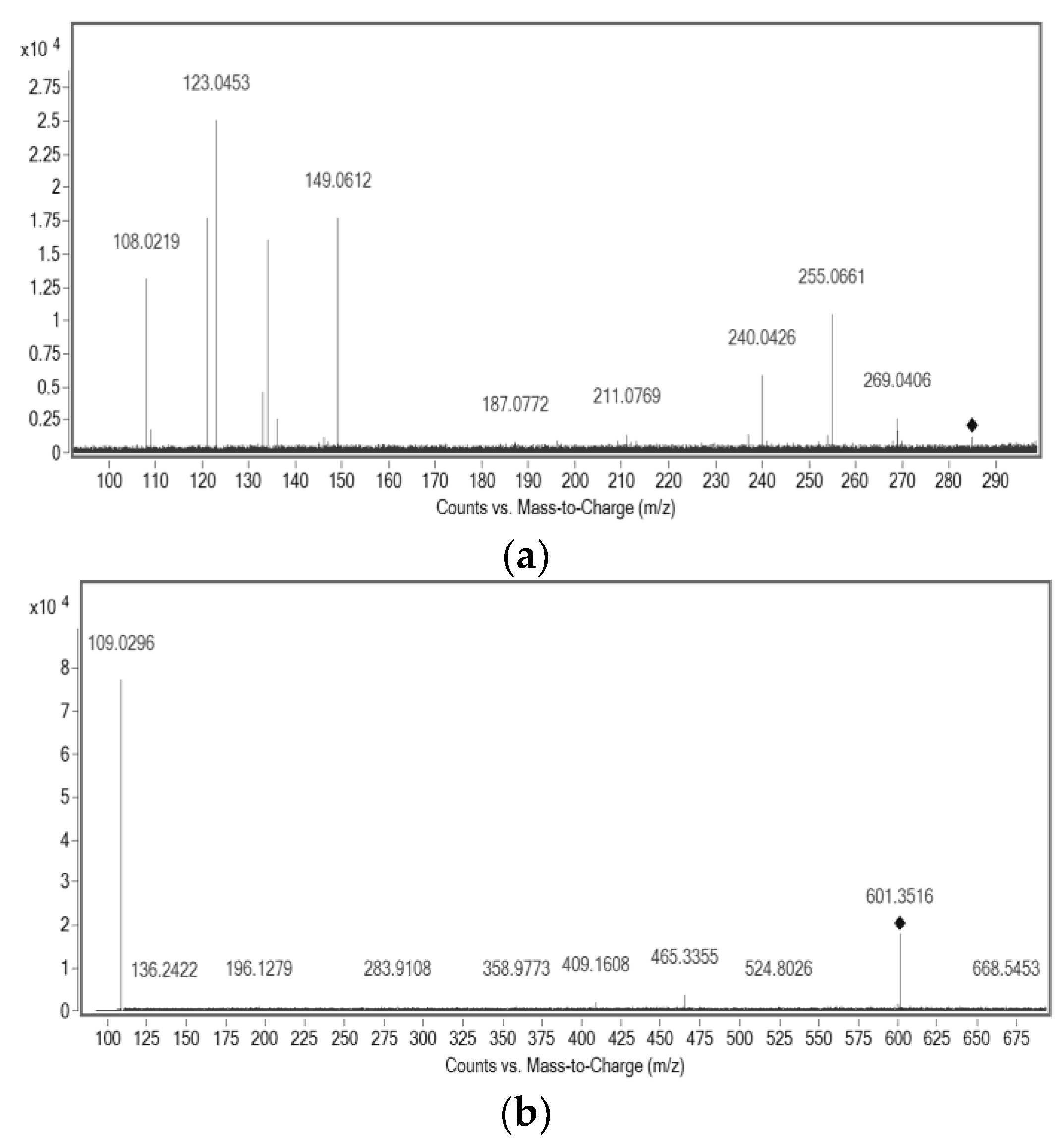
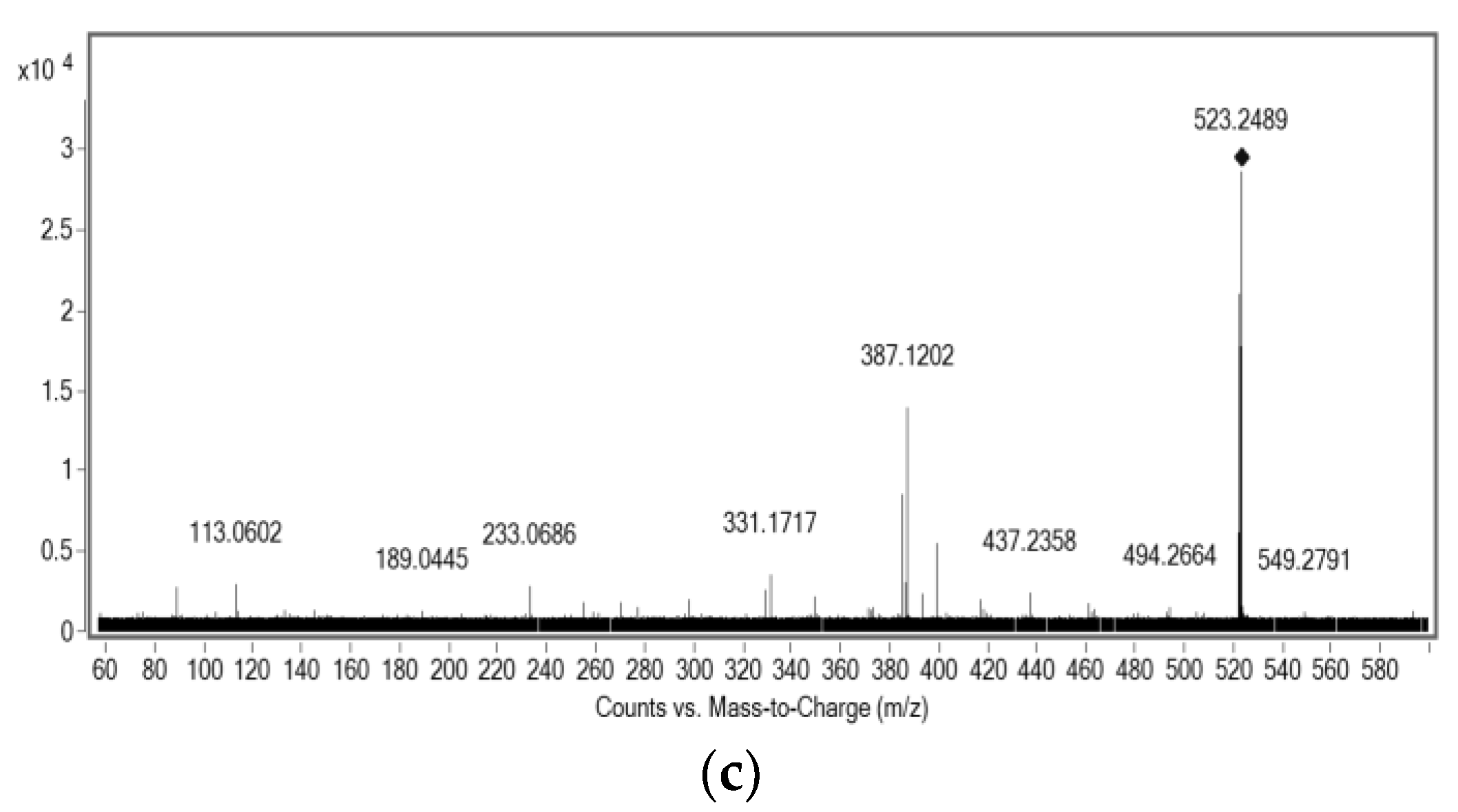
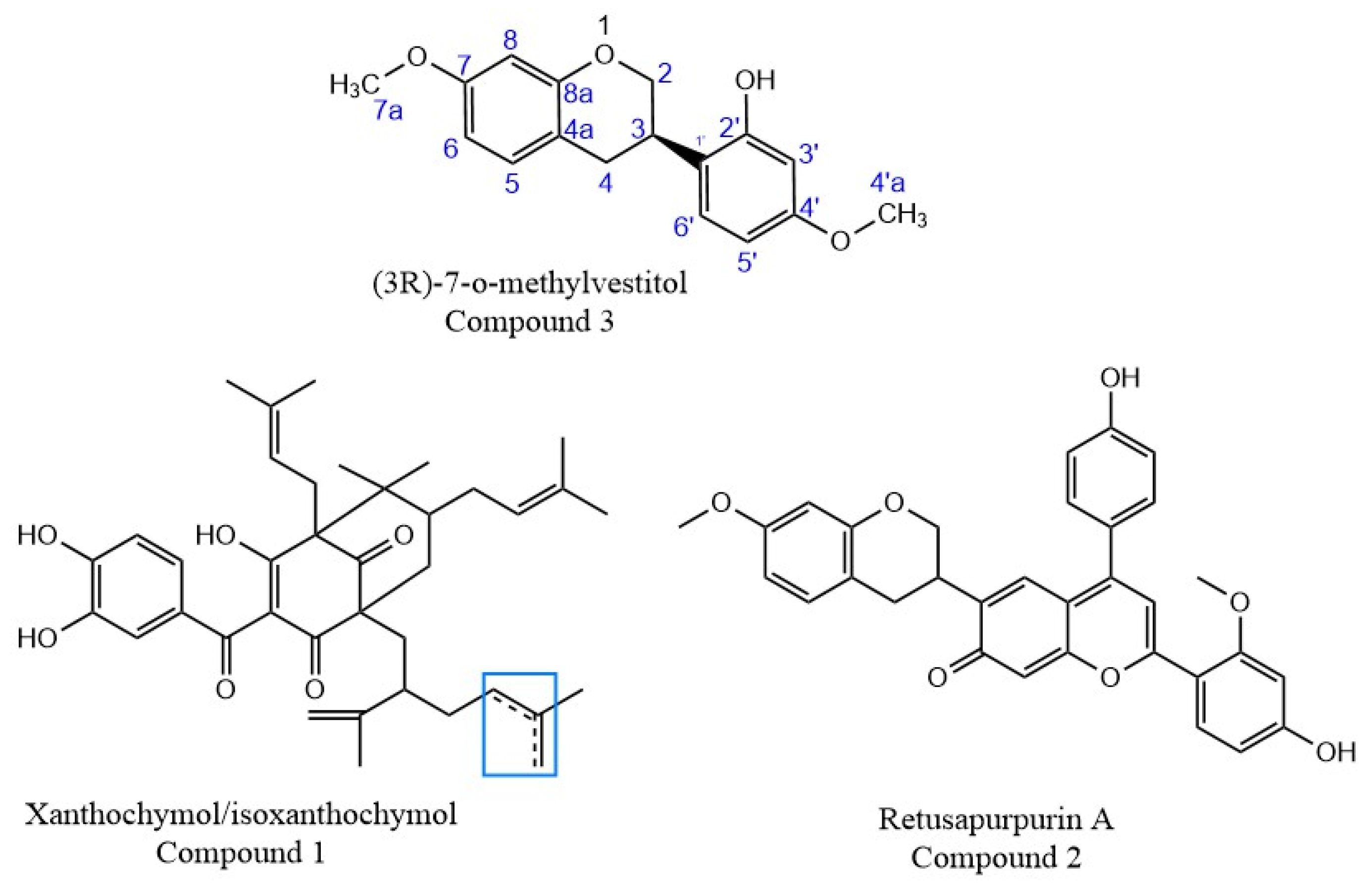
2.3. Structural Characterization of Purified Molecules
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Counter-Current Chromatography
4.3. Preparative HPLC
4.4. UHPLC–MS
4.5. High Resolution Mass Spectrometry ESI-QTOF–MS (QTOF)
4.6. Nuclear Magnetic Resonance (NMR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Spivak, M. Propolis and bee health: The natural history and significance of resin use by honeybees. Apidologie 2010, 41, 295–311. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Alencar, S.M.; Koo, H.; Ikegaki, M.; Silva, G.V.; Napimoga, M.H.; Rosalen, P.L. Anti-inflammatory and antimicrobial evaluation of neovestitol and vestitol isolated from Brazilian red propolis. J. Agric. Food Chem. 2013, 61, 4546–4550. [Google Scholar] [CrossRef] [PubMed]
- Cabral, I.S.R.; Oldoni, T.L.C.; Prado, A.; Bezerra, R.M.N.; Alencar, S.M.D.; Ikegaki, M.; Rosalen, P.L. Composição fenólica, atividade antibacteriana e antioxidante da própolis vermelha brasileira. Química Nova 2009, 32, 1523–1527. [Google Scholar] [CrossRef]
- Righi, A.A.; Alves, T.R.; Negri, G.; Marques, L.M.; Breyer, H.; Salatino, A. Brazilian red propolis: Unreported substances, antioxidant and antimicrobial activities. J. Sci. Food Agric. 2011, 91, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Lopez, B.; De Lourenço, C.; Alves, D.; Machado, D.; Lancellotti, M.; Sawaya, A.C.H.F. Antimicrobial and cytotoxic activity of red propolis: An alert for its safe use. J. Appl. Microbiol. 2015, 119, 677–687. [Google Scholar] [CrossRef]
- Bezerra, A.M.F.; Bezerra, K.K.S.; Albuquerque, F.G.F.; Fernandes Filho, A.; Casimiro, G.S.; Nunes, E.M.; da Silva, E.M.L.; Bezerra, W.K.T.; de Almeida, P.B.; de Araújo, T.L.M. Red propolis antifungal action on species of Candida of the oral cavity. Int. Arch. Med. 2015, 8. [Google Scholar]
- Park, Y.K.; Ikegaki, M. Preparation of water and ethanolic extracts of propolis and evaluation of the preparations. Biosci. Biotechnol. Biochem. 1998, 62, 2230–2232. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.J.H.; Barreto, G.d.A.; Cerqueira, J.C.; dos Anjos, J.P.; Andrade, L.N.; Padilha, F.F.; Druzian, B.A.S.; Machado, J.I. Evaluation of the antioxidant profile and cytotoxic activity of red propolis extracts from different regions of northeastern Brazil obtained by conventional and ultrasound assisted extraction. PLoS ONE 2019, 14, e0219063. [Google Scholar] [CrossRef]
- Sawaya, A.C.H.F.; Tomazela, D.M.; Cunha, I.B.; Bankova, V.S.; Marcucci, M.C.; Custodio, A.R.; Eberlin, M.N. Electrospray ionization mass spectrometry fingerprinting of propolis. Analyst 2004, 129, 739–744. [Google Scholar] [CrossRef]
- Trusheva, B.; Popova, M.; Bankova, V.; Simova, S.; Marcucci, M.C.; Miorin, P.L.; da Rocha Pasin, F.; Tsvetkova, I. Bioactive constituents of Brazilian red propolis. Evid.-Based Complement. Altern. Med. 2006, 3, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Rubio, O.; Piccinelli, A.L.; Campo Fernandez, M.; Marquez Hernandez, I.; Rosado, A.; Rastrelli, L. Chemical characterization of Cuban propolis by HPLC−PDA, HPLC−MS, and NMR: The brown, red, and yellow Cuban varieties of propolis. J. Agric. Food Chem. 2007, 55, 7502–7509. [Google Scholar] [CrossRef] [PubMed]
- Oldoni, T.L.C.; Cabral, I.S.; d’Arce, M.A.R.; Rosalen, P.L.; Ikegaki, M.; Nascimento, A.M.; Alencar, S.M. Isolation and analysis of bioactive isoflavonoids and chalcone from a new type of Brazilian propolis. Sep. Pur. Technol. 2011, 77, 208–213. [Google Scholar] [CrossRef]
- Sticher, O. Natural product isolation. Nat.Prod. Rep. 2008, 25, 517–554. [Google Scholar] [CrossRef] [PubMed]
- McChesney, J.D.; Rodenburg, D.L. Preparative chromatography and natural products discovery. Curr. Opin. Biotechnol. 2014, 25, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.; Zou, D.; Chen, C.; You, J.; Zhou, G.; Sun, J.; Li, Y. Application of an efficient strategy based on liquid–liquid extraction, high-speed counter-current chromatography, and preparative HPLC for the rapid enrichment, separation, and purification of four anthraquinones from Rheum tanguticum. J. Sep. Sci. 2014, 37, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wei, Y.; Yuan, Q. Preparative separation of gallic acid from Chinese traditional medicine by high-speed counter-current chromatography and followed by preparative liquid chromatography. Sep. Pur. Technol. 2007, 55, 40–43. [Google Scholar] [CrossRef]
- Sutherland, I.A.; Fisher, D. Role of counter-current chromatography in the modernization of Chinese herbal medicines. J. Chromatogr. A 2009, 1216, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.; Thickitt, C.; Douillet, N.; Freebairn, K.; Johns, D.; Mountain, C.; Wood, P.; Edwards, N.; Rooke, D.; Harris, G. Scalable Technology for the Extraction of Pharmaceutics: Outcomes from a 3-year collaborative industry/academia research programme. J. Chromatogr. A 2013, 1282, 84–94. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Garrard, I. A comprehensive classification of solvent systems used for natural product purifications in countercurrent and centrifugal partition chromatography. Nat. Prod. Rep. 2015, 32, 1556–1561. [Google Scholar] [CrossRef]
- Jerz, G.; Elnakady, Y.A.; Braun, A.; Jäckel, K.; Sasse, F.; Al Ghamdi, A.A.; Omar, M.O.; Winterhalter, P. Preparative mass-spectrometry profiling of bioactive metabolites in Saudi-Arabian propolis fractionated by high-speed countercurrent chromatography and off-line atmospheric pressure chemical ionization mass-spectrometry injection. J. Chromatogr. A 2014, 1347, 17–29. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.C.A.; Heringer, A.P.; Figueiredo, M.R.; de Paiva, S.R. Separation of clusianone from Clusia fluminensis Planch. and Triana (Clusiaceae) by high-speed counter-current chromatography. J. Liq. Chromatogr. Rel. Technol. 2012, 35, 2313–2321. [Google Scholar] [CrossRef]
- Queiroga, C.; Cavalcante, M.; Ferraz, P.; Coser, R.; Sartoratto, A.; De Magalhaes, P. High-speed countercurrent chromatography as a tool to isolate nerolidol from the Baccharis dracunculifolia volatile oil. J. Essent. Oil Res. 2014, 26, 334–337. [Google Scholar] [CrossRef]
- Ignatova, S.; Sumner, N.; Colclough, N.; Sutherland, I.A. Gradient elution in Counter-Current Chromatography: A new layout for an old path. J. Chromatogr. A 2011, 1218, 6053–6060. [Google Scholar] [CrossRef] [PubMed]
- Foucault, A.P. (Ed.) Centrifugal Partition Chromatography; Marcel Dekker: New York, NY, USA, 1995. [Google Scholar]
- Peng, A.; Hewitson, P.; Ye, H.; Zu, L.; Garrard, I.; Sutherland, I.; Chen, L.; Ignatova, S. Sample injection strategy to increase throughput in counter-current chromatography: Case study of Honokiol purification. J. Chromatogr. A 2016, 1476, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Fasolo, D.; Bergold, A.M.; von Poser, G.; Teixeira, H.F. Determination of benzophenones in lipophilic extract of Brazilian red propolis, nanotechnology-based product and porcine skin and mucosa: Analytical and Bioanalytical assays. J. Pharm. Biomed. Anal. 2016, 124, 57–66. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.; Chattopadhyay, S. Rapid and sensitive HPLC-PDA method for simultaneous identification and quantification of dietary weight reducing compound hydroxy citric acid lactone and chemo preventive compounds isoxanthochymol and xanthochymol in Garcinia indica. Intern. Food Res. J. 2013, 20, 397–402. [Google Scholar]
- Piccinelli, A.L.; Lotti, C.; Campone, L.; Cuesta-Rubio, O.; Campo Fernandez, M.; Rastrelli, L. Cuban and Brazilian red propolis: Botanical origin and comparative analysis by high-performance liquid chromatography–photodiode array detection/electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 6484–6491. [Google Scholar] [CrossRef] [PubMed]
- Shahi, M.; Javadi, A.; Mogaddam, M.R.A.; Mirzaei, H.; Nemati, M. Extraction of some antibiotics from propolis samples using homogenous liquid–liquid extraction coupled with deep eutectic solvent–based hollow fibre protected preconcentration. Int.J. Environ. Anal. Chem. 2022, 102, 6422. [Google Scholar] [CrossRef]
- Arruda, C.; Ribeiro, V.P.; Mejia, J.A.A.; Almeida, M.O.; Goulart, M.O.; Candido, A.C.B.B.; dos Santos, R.A.; Magalhães, L.G.; Martins, C.H.G.; Bastos, J.K. Green Propolis: Cytotoxic and Leishmanicidal Activities of Artepillin C, p-Coumaric Acid, and Their Degradation Products. Braz. J. Pharmacog. 2020, 30, 169. [Google Scholar] [CrossRef]
- Silva, V.C.; Silva, A.M.G.S.; Basílio, J.A.D.; Xavier, J.A.; do Nascimento, T.G.; Naal, R.M.Z.G.; del Lama, M.P.; Leonelo, L.A.D.; Mergulhão, N.L.O.N.; Maranhão, F.C.A.; et al. New Insights for Red Propolis of Alagoas—Chemical Constituents, Topical Membrane Formulations and Their Physicochemical and Biological Properties. Molecules 2020, 25, 5811. [Google Scholar] [CrossRef] [PubMed]
- Ccana-Ccapatinta, G.V.; Mejía, J.A.A.; Tanimoto, M.H.; Groppo, M.; de Carvalho, J.C.A.S.; Bastos, J.K. Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera L.f.: The Botanical Sources of Isoflavonoids and Benzophenones in Brazilian Red Propolis. Molecules 2020, 25, 2060. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.B.; Leandro, L.F.; Rosa, R.B.; Silva, M.V.; Teixeira, S.C.; Servato, J.P.S.; Ambrósio, S.R.; Veneziani, R.C.S.; Aldana-Mejía, J.A.; Bastos, J.K.; et al. Brazilian Red Propolis Presents Promising Anti-H. pylori Activity in In Vitro and In Vivo Assays with the Ability to Modulate the Immune Response. Molecules 2022, 27, 7310. [Google Scholar] [CrossRef] [PubMed]
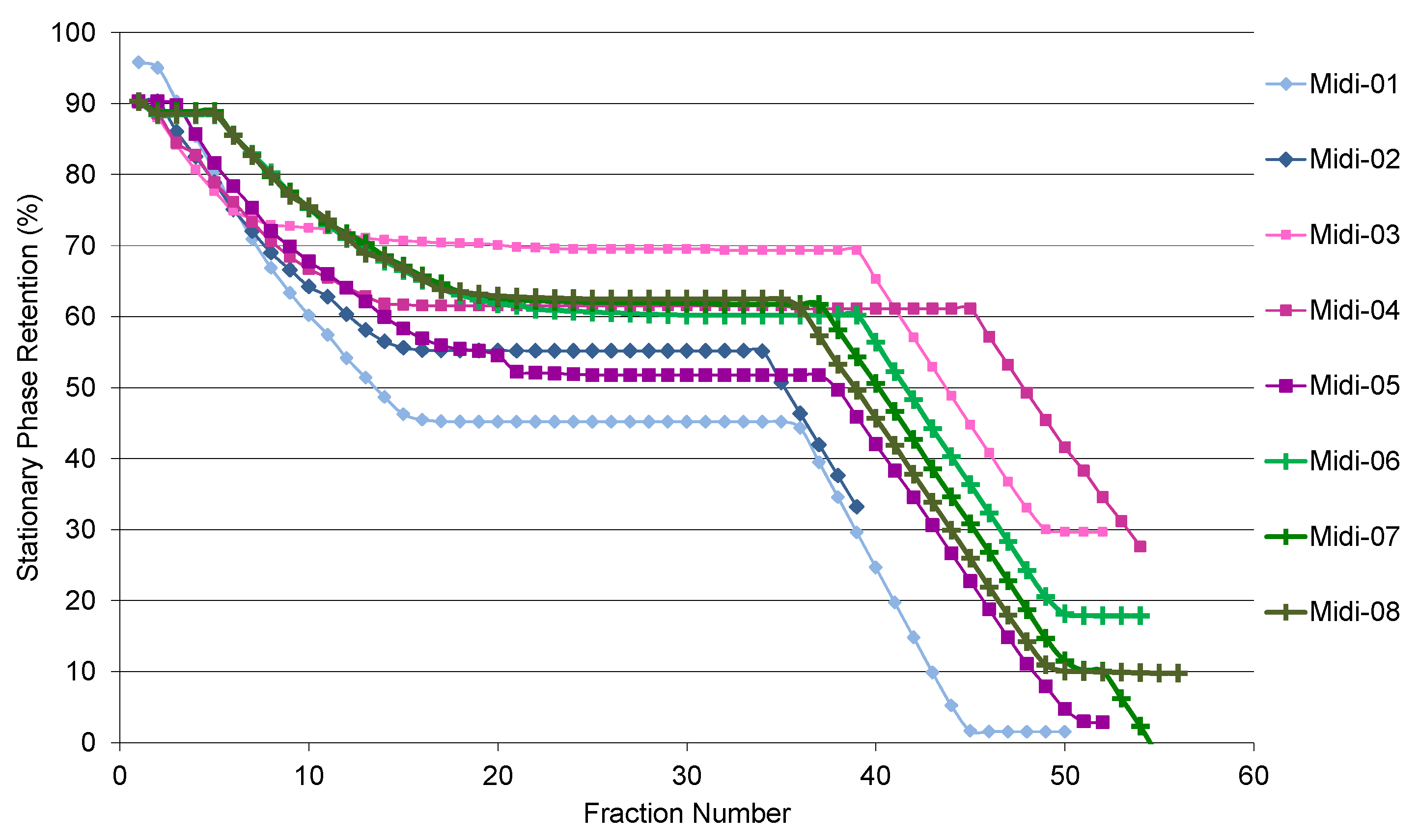

| Solvent | 17 | 20A | 23 | 25 | 26 | 27 | 2 | 3 | 4 |
| Hexane | 1 | 2.3 | 4 | 6 | 9 | 19 | 5 | 8 | 1 |
| Ethyl acetate | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| Methanol | 1 | 2.3 | 4 | 6 | 9 | 19 | 4 | 2 | 0 |
| Water | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| Acetonitrile | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 1 |
| KD NP a | 0.1 | 0.1 | 0.6 | 2.0 | 3.3 | 5.6 | 1.2 | 17.5 | 16.7 |
| Retention Time (min) | Solvent System HMWat b (5:4:1) | Solvent System HEMWat c (1:1:1:1) |
|---|---|---|
| LP/UP | LP/UP | |
| 2.466 | 5.9 | 21.9 |
| 2.739 | 30.2 | 37.1 |
| 4.014 | 353.0 | 286.2 |
| 4.311 | 362.1 | 2.8 |
| 4.901 | 742.9 | 3.1 |
| 5.200 | 509.3 | 2.2 |
| 5.615 | 760.0 | 1.2 |
| 5.841 | 618.3 | 1.2 |
| 6.009 | 546.4 | 1.1 |
| 6.160 | 511.3 | 0.8 |
| 6.279 | 279.9 | 0.9 |
| 6.520 | 108.9 | 1.1 |
| 6.626 | 684.0 | 0.0 |
| 6.786 | 290.6 | 1.2 |
| 6.972 | 180.5 | 2.2 |
| 7.079 | 140.8 | 0.0 |
| 7.335 | 4.1 | 0.7 |
| 7.479 | 451.5 | 1.0 |
| 7.719 | 6.3 | 0.0 |
| 8.132 | 29.2 | 0.6 |
| 8.453 | 165.6 | 0.3 |
| 8.690 | 29.1 | 0.0 |
| 9.278 | 0.7 | 0.1 |
| 9.919 | 128.6 | 0.1 |
| 10.759 | 4.4 | 0.0 |
| 10.974 | 3.0 | 0.4 |
| 11.836 | 57.2 | 0.0 |
| 13.075 | 2.7 | 0.0 |
| 14.080 | 0.1 | 0.0 |
| 14.418 | 1.2 | 0.1 |
| 14.985 | 0.6 | 0.0 |
| CCC Midi Run No. | Sample Solvent | Sample Volume (mL) | Sample Concentration (mg·mL−1) | Sample Loading (g) | Mobile Phase Flow Rate (mL·min−1) |
|---|---|---|---|---|---|
| 1 a | LP | 20 | 100 | 2.00 | 50 |
| 2 | MeOH b | 20 | 100 | 2.00 | 50 |
| 3 | MeOH | 10 | 100 | 1.00 | 40 |
| 4 | MeOH | 10 | 200 | 2.00 | 40 |
| 5 | MeOH | 10 | 400 | 4.00 | 40 |
| 6 | MeOH | 10 | 254 | 2.54 | 40 |
| 7 | MeOH | 10 | 254 | 2.54 | 40 |
| 8 | MeOH | 10 | 254 | 2.54 | 40 |
| Compound | Molecular Formula | m/z Experimental | m/z Theoretical a | Mass Error (ppm) | |
|---|---|---|---|---|---|
| 1 | C38H50O6 | 601.3522 | 601.3535 | Precursor ion | 1.78 |
| [C6H5O2]− | 109.0296 | 109.0290 | Product ion | −0.82 | |
| 2 | C32H26O7 | 523.2852 | 523.1727 | Precursor ion | 4.07 |
| [C24H17O5]+ | 385.0912 | 385.1037 | Product ion | 5.57 | |
| 3 | C17H18O4 | 285.1139 | 285.1132 | Precursor ion | −2.85 |
| [C7H7O2]− | 123.0453 | 123.0446 | Product ion | −1.19 | |
| [C9H9O2]− | 149.0611 | 149.0603 | Product ion | −2.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, B.G.-C.; Marcucci, M.C.; Rocco, S.A.; Sforça, M.L.; Eberlin, M.N.; Hewitson, P.; Ignatova, S.; Sawaya, A.C.H.F. Preparative Fractionation of Brazilian Red Propolis Extract Using Step-Gradient Counter-Current Chromatography. Molecules 2024, 29, 2757. https://doi.org/10.3390/molecules29122757
Lopez BG-C, Marcucci MC, Rocco SA, Sforça ML, Eberlin MN, Hewitson P, Ignatova S, Sawaya ACHF. Preparative Fractionation of Brazilian Red Propolis Extract Using Step-Gradient Counter-Current Chromatography. Molecules. 2024; 29(12):2757. https://doi.org/10.3390/molecules29122757
Chicago/Turabian StyleLopez, Begoña Gimenez-Cassina, Maria Cristina Marcucci, Silvana Aparecida Rocco, Maurício Luís Sforça, Marcos Nogueira Eberlin, Peter Hewitson, Svetlana Ignatova, and Alexandra Christine Helena Frankland Sawaya. 2024. "Preparative Fractionation of Brazilian Red Propolis Extract Using Step-Gradient Counter-Current Chromatography" Molecules 29, no. 12: 2757. https://doi.org/10.3390/molecules29122757





