Structural Design, Anticancer Evaluation, and Molecular Docking of Newly Synthesized Ni(II) Complexes with ONS-Donor Dithiocarbazate Ligands
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Analyses
2.2. Hirshfeld Surface
2.3. Infrared Spectra
2.4. Electronic Spectra
2.5. 1H NMR Spectra
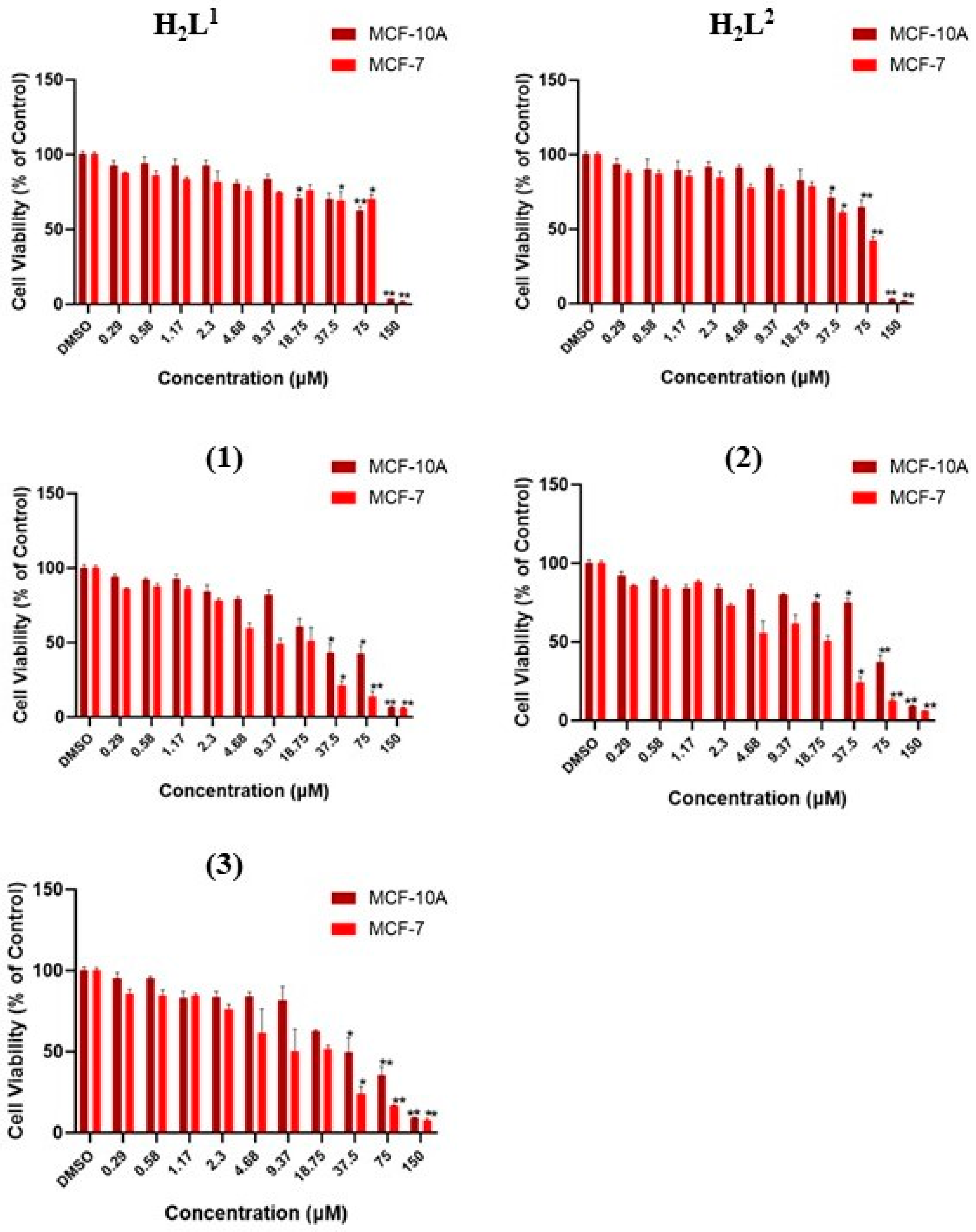
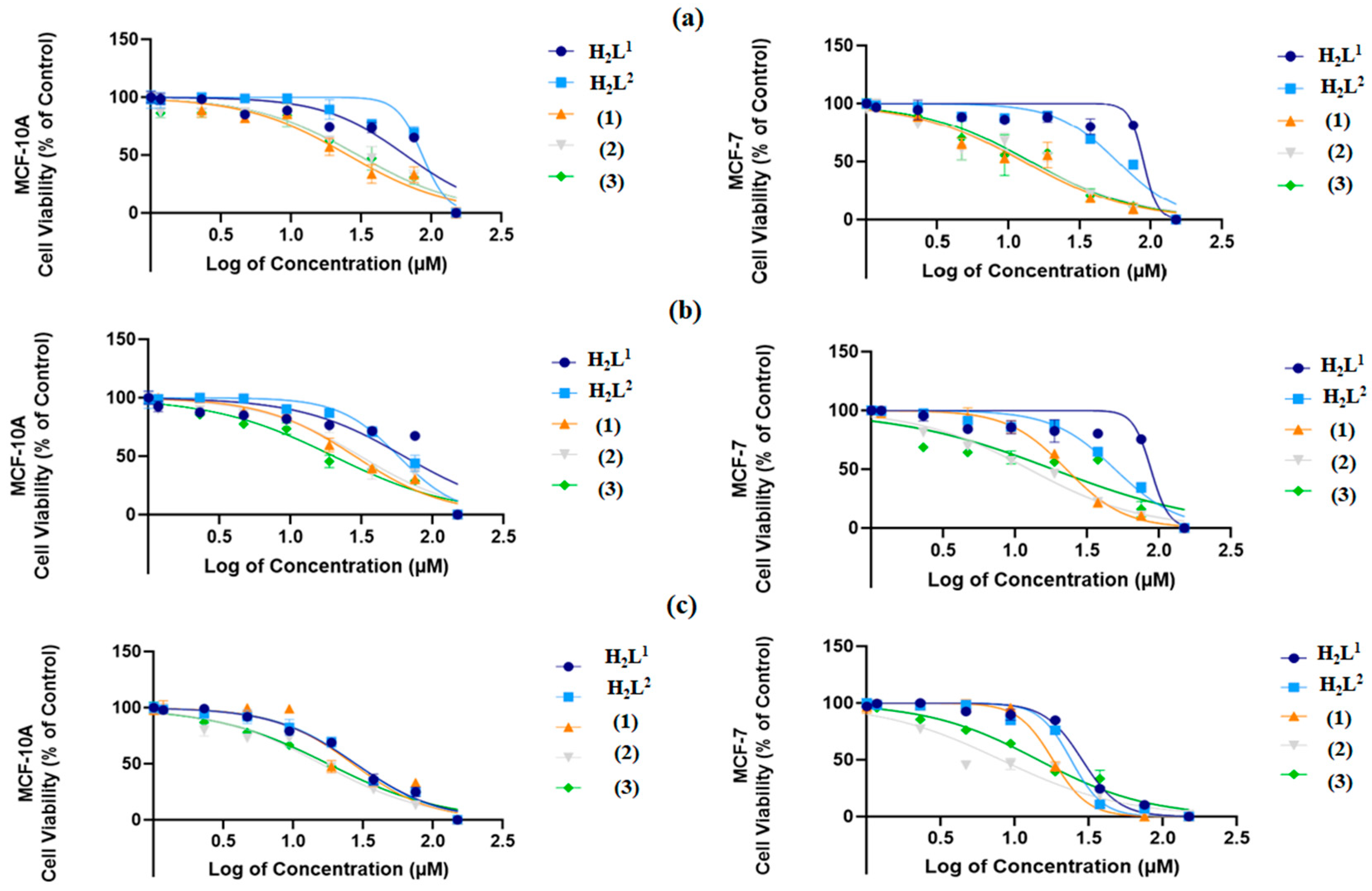
2.6. Biological Activity Analysis
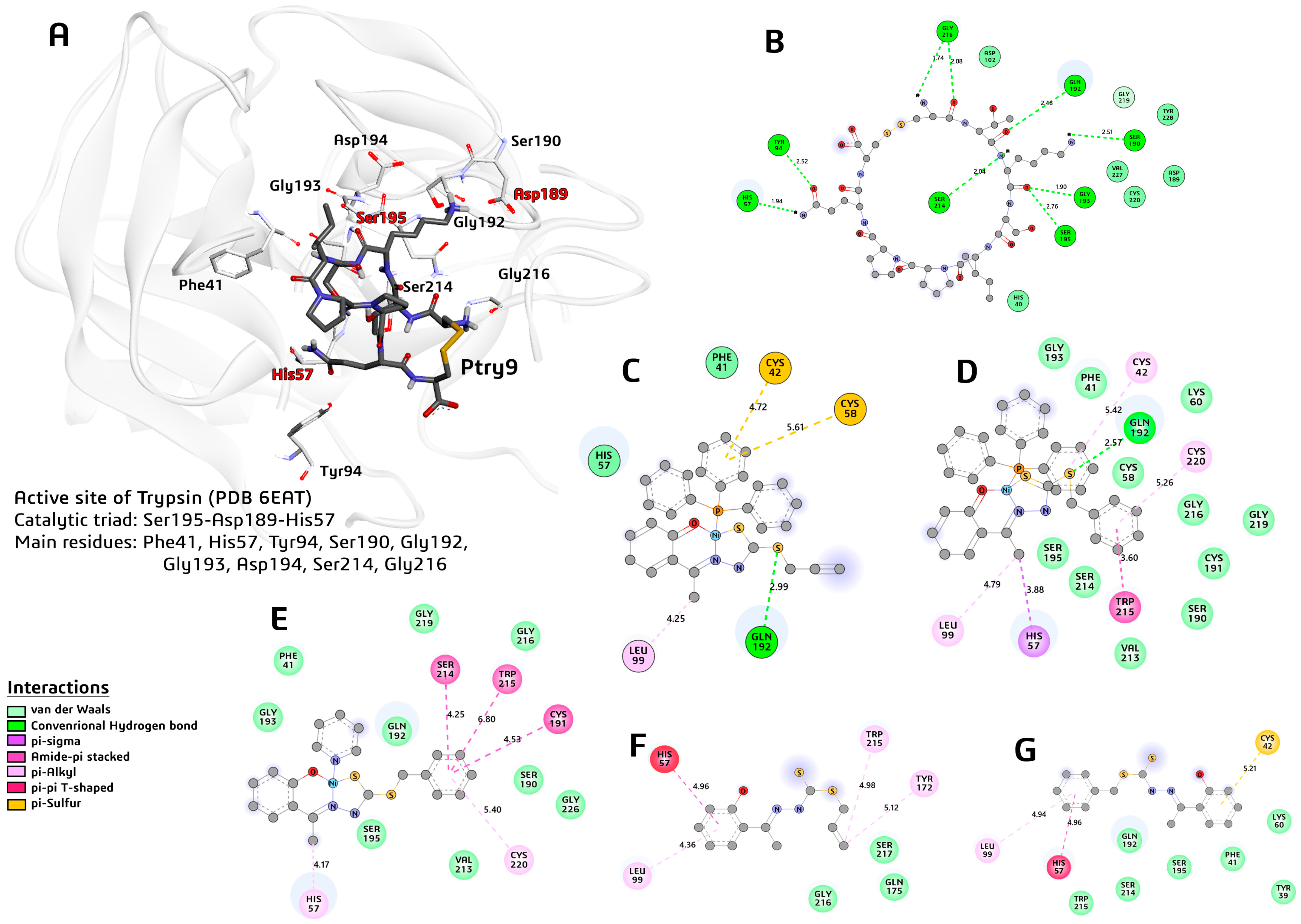
2.7. Molecular Docking
3. Materials and Methods
3.1. Materials, Methods, and Instruments
3.2. Synthesis of S-Allyl-2-(2-hydroxyphenyl-ethylidene)dithiocarbazato (H2L1)
3.3. Synthesis of S-Benzyl-2-(2-hydroxyphenyl-ethylidene)dithiocarbazato (H2L2)
3.4. Synthesis of [Ni(L1)PPh3] (1)
3.5. Synthesis of [Ni(L2)PPh3] (2)
3.6. Synthesis of [Ni(L2)Py] (3)
3.7. Crystal Structure Determination
3.8. Computational Details
3.9. Biological Activity
3.10. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Montesdeoca, N.; Karges, J.; Xiao, H. Immunogenic Cell Death Inducing Metal Complexes for Cancer Therapy. Angew. Chemie-Int. Ed. 2023, 62, e202300662. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, T.; Ali, B.; Qayyum, H.; Haroone, M.S.; Shabbir, G. Pharmacological Aspects of Schiff Base Metal Complexes: A Critical Review. Inorg. Chem. Commun. 2023, 150, 110449. [Google Scholar] [CrossRef]
- Lee, L.C.C.; Lo, K.K.W. Luminescent and Photofunctional Transition Metal Complexes: From Molecular Design to Diagnostic and Therapeutic Applications. J. Am. Chem. Soc. 2022, 144, 14420–14440. [Google Scholar] [CrossRef] [PubMed]
- Boulechfar, C.; Ferkous, H.; Delimi, A.; Djedouani, A.; Kahlouche, A.; Boublia, A.; Darwish, A.S.; Lemaoui, T.; Verma, R.; Benguerba, Y. Schiff Bases and Their Metal Complexes: A Review on the History, Synthesis, and Applications. Inorg. Chem. Commun. 2023, 150, 110451. [Google Scholar] [CrossRef]
- Paprocka, R.; Wiese-Szadkowska, M.; Janciauskiene, S.; Kosmalski, T.; Kulik, M.; Helmin-Basa, A. Latest Developments in Metal Complexes as Anticancer Agents. Coord. Chem. Rev. 2022, 452, 214307. [Google Scholar] [CrossRef]
- Pellei, M.; Bello, F.D.; Porchia, M.; Santini, C. Zinc Coordination Complexes as Anticancer Agents. Coord. Chem. Rev. 2021, 445, 214088. [Google Scholar] [CrossRef]
- Yusof, E.N.M.; Ravoof, T.B.S.A.; Page, A.J. Cytotoxicity of Tin(IV)-Based Compounds A Review. Polyhedron 2021, 198, 115069. [Google Scholar] [CrossRef]
- Chaudhary, N.K.; Mishra, P. Metal Complexes of a Novel Schiff Base Based on Penicillin: Characterization, Molecular Modeling, and Antibacterial Activity Study. Bioinorg. Chem. Appl. 2017, 2017, 6927675. [Google Scholar] [CrossRef]
- Kelland, L. The Resurgence of Platinum-Based Cancer Chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry 2020, 2, 849–899. [Google Scholar] [CrossRef]
- Cepeda, V.; Fuertes, M.A.; Castilla, J.; Alonso, C.; Quevedo, C.; Perez, J.M. Biochemical Mechanisms of Cisplatin Cytotoxicity. Anticancer. Agents Med. Chem. 2007, 7, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.A.B.; da Cunha Júnior, A.D. Acute Nephrotoxicity of Cisplatin: Molecular Mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Shakya, B.; Yadav, P.N. Thiosemicarbazones as Potent Anticancer Agents and Their Modes of Action. Mini-Reviews Med. Chem. 2020, 20, 638. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, C.d.Q.O.; da Mota, T.H.A.; de Oliveira, D.M.; Nascimento, É.C.M.; Martins, J.B.L.; Pittella-Silva, F.; Gatto, C.C. Dithiocarbazate Ligands and Their Ni(II) Complexes with Potential Biological Activity: Structural, Antitumor and Molecular Docking Study. Front. Mol. Biosci. 2023, 10, 1146820. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalt. Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- El-Afify, M.E.; Elsayed, S.A.; Shalaby, T.I.; Toson, E.A.; El-Hendawy, A.M. Synthesis, Characterization, DNA Binding/Cleavage, Cytotoxic, Apoptotic, and Antibacterial Activities of V(IV), Mo(VI), and Ru(II) Complexes Containing a Bioactive ONS-Donor Chelating Agent. Appl. Organomet. Chem. 2021, 35, e6082. [Google Scholar] [CrossRef]
- Zahan, R.; Rahi, M.S.; Sheikh, M.C.; Miyatake, R.; Zangrando, E.; Naz, T.; Islam, M.A.A.A.A.; Reza, M.A. Design, Synthesis and X-Ray Structural Studies of Novel [Acetonitrile-Benzyl-3-N-(2, 4 Dihydroxyphenylmethylene) Hydrazinecarbodithioato-Κ3-N′, S, O] Nickel(Ll) Complex that Potently Inhibit Cell Proliferation through Regulation of Apoptosis Related Genes. Appl. Organomet. Chem. 2019, 33, e4601. [Google Scholar] [CrossRef]
- Qiu, X.Y.; Zhang, C.; Li, S.Z.; Cao, G.X.; Qu, P.; Zhang, F.Q.; Ma, J.G.; Zhai, B. Synthesis, Crystal Structures and Cytotoxic Activity of Mononuclear Nickel(II) and Dinuclear Zinc(II) Complexes with Ligand Derived from S-Benzyldithiocarbazate. Inorg. Chem. Commun. 2014, 46, 202–206. [Google Scholar] [CrossRef]
- Lima, F.C.; Silva, T.S.; Martins, C.H.G.; Gatto, C.C. Synthesis, Crystal Structures and Antimicrobial Activity of Dimeric Copper(II) Complexes with 2-Hydroxyphenyl-Ethylidene-Dithiocarbazates. Inorganica Chim. Acta 2018, 483, 464–472. [Google Scholar] [CrossRef]
- Malik, M.A.; Lone, S.A.; Wani, M.Y.; Talukdar, M.I.A.; Dar, O.A.; Ahmad, A.; Hashmi, A.A. S-Benzyldithiocarbazate Imine Coordinated Metal Complexes Kill Candida Albicans by Causing Cellular Apoptosis and Necrosis. Bioorg. Chem. 2020, 98, 103771. [Google Scholar] [CrossRef] [PubMed]
- Yekke-Ghasemi, Z.; Takjoo, R.; Ramezani, M.; Mague, J.T. Molecular Design and Synthesis of New Dithiocarbazate Complexes; Crystal Structure, Bioactivities and Nano Studies. RSC Adv. 2018, 8, 41795–41809. [Google Scholar] [CrossRef] [PubMed]
- Sohtun, W.P.; Khamrang, T.; Kannan, A.; Balakrishnan, G.; Saravanan, D.; Akhbarsha, M.A.; Velusamy, M.; Palaniandavar, M. Iron(III) Bis-Complexes of Schiff Bases of S-Methyldithiocarbazates: Synthesis, Structure, Spectral and Redox Properties and Cytotoxicity. Appl. Organomet. Chem. 2020, 34, e5593. [Google Scholar] [CrossRef]
- Yusof, E.N.M.; Azam, M.; Sirat, S.S.; Ravoof, T.B.S.A.; Page, A.J.; Veerakumarasivam, A.; Karunakaran, T.; Razali, M.R. Dithiocarbazate Ligand-Based Cu(II), Ni(II), and Zn(II) Complexes: Synthesis, Structural Investigations, Cytotoxicity, DNA Binding, and Molecular Docking Studies. Bioinorg. Chem. Appl. 2022, 2022, 2004052. [Google Scholar] [CrossRef] [PubMed]
- Paulus, G.; Policar, C.; Low, M.L.; Rosli, R.; Guillot, R.; Dorlet, P.; Crouse, K.A.; Delsuc, N. Synthesis, Characterization and Biological Activity of Cu(II), Zn(II) and Re(I) Complexes Derived from S-Benzyldithiocarbazate and 3-Acetylcoumarin. BioMetals 2015, 28, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Ramilo-Gomes, F.; Addis, Y.; Tekamo, I.; Cavaco, I.; Campos, D.L.; Pavan, F.R.; Gomes, C.S.B.; Brito, V.; Santos, A.O.; Domingues, F.; et al. Antimicrobial and Antitumor Activity of S-Methyl Dithiocarbazate Schiff Base Zinc(II) Complexes. J. Inorg. Biochem. 2021, 216, 111331. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, S.A.; Badr, H.E.; di Biase, A.; El-Hendawy, A.M. Synthesis, Characterization of Ruthenium(II), Nickel(II), Palladium(II), and Platinum(II) Triphenylphosphine-Based Complexes Bearing an ONS-Donor Chelating Agent: Interaction with Biomolecules, Antioxidant, in Vitro Cytotoxic, Apoptotic Activity and Cell. J. Inorg. Biochem. 2021, 223, 111549. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Chen, M.; Li, S.; Deng, J.; Li, J.; Fang, G.; Yang, F.; Huang, G. Dithiocarbazate-Copper Complexes for Bioimaging and Treatment of Pancreatic Cancer. J. Med. Chem. 2021, 64, 5485–5499. [Google Scholar] [CrossRef]
- Cavalcante, C.D.Q.O.; Arcanjo, D.D.S.; Silva, G.G.D.; Oliveira, D.M.D.; Gatto, C.C. Solution and Solid Behavior of Mono and Binuclear Zinc(Ii) and Nickel(Ii) Complexes with Dithiocarbazates: X-Ray Analysis, Mass Spectrometry and Cytotoxicity against Cancer Cell Lines. New J. Chem. 2019, 43, 11209–11221. [Google Scholar] [CrossRef]
- Manan, M.A.F.A.; Mohammat, M.F. Synthesis, Structural Studies and Antimicrobial Evaluation of Nickel (II) Bis-Complex of Schiff Base of S-Benzyldithiocarbazate. Trends Sci. 2022, 19, 1500. [Google Scholar] [CrossRef]
- Sabina, M.; Das, D.; Zangrando, E.; Rahman, S.; Alodhayb, A.; Khurshida, M.; Miya, C.; Miyatake, R.; Hossain, B.; Karim, R.; et al. A Dithiocarbazate N, S Schiff Base Ligand with a Long Alkyl Chain: Synthesis, Characterization, DFT Study and Antimicrobial Activity of Its Ni (II) Complex. J. Mol. Struct. 2023, 1277, 134808. [Google Scholar] [CrossRef]
- Lima, F.C.; Só, Y.A.O.; Gargano, R.; de Oliveira, D.M.; Gatto, C.C. Structural, Theoretical and Biological Activity of Mono and Binuclear Nickel(II) Complexes with Symmetrical and Asymmetrical 4,6-Diacetylresorcinol-Dithiocarbazate Ligands. J. Inorg. Biochem. 2021, 224, 111559. [Google Scholar] [CrossRef] [PubMed]
- Biswal, D.; Pramanik, N.R.; Chakrabarti, S.; Chakraborty, N.; Acharya, K.; Mandal, S.S.; Ghosh, S.; Drew, M.G.B.; Mondal, T.K.; Biswas, S. Lewis Base Controlled Supramolecular Architectures via Non-Covalent Interactions of Dioxomolybdenum(VI) Complexes with an ONS Donor Ligand: DFT Calculations and Biological Study. New J. Chem. 2015, 39, 2778–2794. [Google Scholar] [CrossRef]
- Okuniewski, A.; Rosiak, D.; Chojnacki, J.; Becker, B. Coordination Polymers and Molecular Structures among Complexes of Mercury(II) Halides with Selected 1-Benzoylthioureas. Polyhedron 2015, 90, 47–57. [Google Scholar] [CrossRef]
- Yusof, E.N.M.; Nasri, N.M.; Ravoof, T.B.S.A.; Tiekink, E.R.T. A Ternary Nickel(II) Schiff Base Complex Containing Di-Anionic and Neutral Forms of a Dithiocarbazate Schiff Base. Molbank 2019, 2019, M1057. [Google Scholar] [CrossRef]
- El-Barasi, N.M.; Miloud, M.M.; El-ajaily, M.M.; Mohapatra, R.K.; Sarangi, A.K.; Das, D.; Mahal, A.; Parhi, P.K.; Pintilie, L.; Barik, S.R.; et al. Synthesis, Structural Investigations and Antimicrobial Studies of Hydrazone Based Ternary Complexes with Cr(III), Fe(III) and La(III) Ions. J. Saudi Chem. Soc. 2020, 24, 492–503. [Google Scholar] [CrossRef]
- Santiago, P.H.O.; Santiago, M.B.; Martins, C.H.G.; Gatto, C.C. Copper(II) and Zinc(II) Complexes with Hydrazone: Synthesis, Crystal Structure, Hirshfeld Surface and Antibacterial Activity. Inorganica Chim. Acta 2020, 508, 119632. [Google Scholar] [CrossRef]
- Turner, M.J.; Mckinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, Australia, 2017; Volume 5. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Delgado, G.E.; Liew, S.M.; Jamalis, J.; Cisterna, J.; Cárdenas, A.; Brito, I. Structural Characterization and Hirshfeld Surface Analysis of the Pyrazoline 1-(3-(4-Iodophenyl)-5-(3-Methylthiophen-2-Yl)-4,5-Dihydro-1H-Pyrazol-1-Yl)Ethan-1-One. J. Mol. Struct. 2020, 1210, 128044. [Google Scholar] [CrossRef]
- Murugan, K.; Vijayapritha, S.; Kavitha, V.; Viswanathamurthi, P. Versatile Formation of Ru (II) Hydrazone Complexes: Structure, Theoretical Studies and Catalytic Activity in α-Alkylation. Polyhedron 2020, 190, 114737. [Google Scholar] [CrossRef]
- Takjoo, R.; Takjoo, R.; Yazdanbakhsh, M.; Aghaei Kaju, A.; Chen, Y. Mixed Ligand Palladium(II) Complex with NS-Bidentate S-Allyldithiocarbazate Schiff Base: Synthesis, Spectral Characterization, Crystal Structure and Ecoding Intermolecular Interactions with Hirshfeld Surface Analysis. Chin. J. Chem. 2010, 28, 221–228. [Google Scholar] [CrossRef]
- Boshaala, A.; Flörke, U.; Yamin, B.M.; Amer, Y.O.B.; Ghaith, G.S.H.; Almughery, A.A.; Zarrouk, A.; Warad, I. Crystal Interaction, Hirshfeld Surface Analysis, and Spectral Analysis of New Dithiocarbazate Schiff Bases Derivative (LH) and Its Neutral Cis-Cu(L)2 Complex. J. Mol. Struct. 2021, 1224, 129207. [Google Scholar] [CrossRef]
- Samy, F.; Omar, F.M. Synthesis, Characterization, Antitumor Activity, Molecular Modeling and Docking of New Ligand, (2,5-Pyrrole)-Bis(5,6-Diphenyl-[1,2,4]-Triazin-3-Yl)Hydrazone and Its Complexes. J. Mol. Struct. 2020, 1222, 128910. [Google Scholar] [CrossRef]
- Muthu Tamizh, M.; Mereiter, K.; Kirchner, K.; Ramachandra Bhat, B.; Karvembu, R. Synthesis, Crystal Structures and Spectral Studies of Square Planar Nickel(II) Complexes Containing an ONS Donor Schiff Base and Triphenylphosphine. Polyhedron 2009, 28, 2157–2164. [Google Scholar] [CrossRef]
- Nadia, E.; Ravoof, T.B.S.A.; Jamsari, J.; Tiekink, E.R.T.; Veerakumarasivam, A.; Crouse, K.A.; Tahir, M.I.M.; Ahmad, H. Inorganica Chimica Acta Synthesis, Characterization and Biological Studies of S-4-Methylbenzyl- and Ni2+ Complexes. Inorganica Chim. Acta 2015, 438, 85–93. [Google Scholar] [CrossRef]
- Kılıç-Cıkla, I.; Güveli, Ş.; Bal-Demirci, T.; Aygün, M.; Ülküseven, B.; Yavuz, M. X-ray Diffraction, Spectroscopic and DFT Studies on Nickel(II)-Triphenylphosphine Complexes of 2-Hydroxyacetophenone Thiosemicarbazones. Polyhedron 2017, 130, 1–12. [Google Scholar] [CrossRef]
- Priyarega, S.; Kalaivani, P.; Prabhakaran, R.; Hashimoto, T.; Endo, A.; Natarajan, K. Nickel(II) Complexes Containing Thiosemicarbazone and Triphenylphosphine: Synthesis, Spectroscopy, Crystallography and Catalytic Activity. J. Mol. Struct. 2011, 1002, 58–62. [Google Scholar] [CrossRef]
- Suganthy, P.K.; Prabhu, R.N.; Sridevi, V.S. Synthesis, Structural Characterization and Catalytic Transfer Hydrogenation of Ruthenium(II) Carbonyl Complexes Bearing N,N,O Pincer Type Benzoylhydrazone Ligands. Polyhedron 2015, 88, 57–62. [Google Scholar] [CrossRef]
- Santra, A.; Brandao, P.; Mondal, G.; Bera, P.; Jana, A.; Bhattacharyya, I.; Pramanik, C.; Bera, P. The Role of Methyl and Benzyl Substituted Dithiocarbazate of 2-Acetyl Pyridine for the Formation of Bridged Dimeric and Unbridged Monomeric Copper(II) Complexes and Catecholase Mimetic Activity of the Complexes. Polyhedron 2020, 176, 114277. [Google Scholar] [CrossRef]
- Takjoo, R.; Centore, R. Synthesis, X-ray Structure, Spectroscopic Properties and DFT Studies of Some Dithiocarbazate Complexes of Nickel(II). J. Mol. Struct. 2013, 1031, 180–185. [Google Scholar] [CrossRef]
- Bhat, R.A.; Kumar, D.; Alam, A.; Mir, B.A.; Srivastava, A.; Malla, M.A.; Mir, M.A. Synthesis, Characterization, Thermal and DFT Studies of S-Methyl-β-N-(3-(2-Nitrophenyl)Allylidene)Dithiocarbazate as Anti-Bacterial Agent. J. Mol. Struct. 2018, 1173, 72–80. [Google Scholar] [CrossRef]
- Latif, M.A.; Ahmed, T.; Hossain, M.S.; Chaki, B.M.; Abdou, A.; Kudrat-E-Zahan, M. Synthesis, Spectroscopic Characterization, DFT Calculations, Antibacterial Activity, and Molecular Docking Analysis of Ni(II), Zn(II), Sb(III), and U(VI) Metal Complexes Derived from a Nitrogen-Sulfur Schiff Base. Russ. J. Gen. Chem. 2023, 93, 389–397. [Google Scholar] [CrossRef]
- Kalaiarasi, G.; Jain, R.; Shanmugapriya, A.; Puschman, H.; Kalaivani, P.; Prabhakaran, R. New Binuclear Ni(II) Metallates as Potent Antiproliferative Agents against MCF-7 and HeLa Cells. Inorganica Chim. Acta 2017, 462, 174–187. [Google Scholar] [CrossRef]
- Cavalcante, C.D.Q.O.; Garcia, E.; da Mota, T.H.A.; de Oliveira, D.M.; Gatto, C.C. Comparative Investigation of Cu(II) Complexes with Dithiocarbazate: Structural Design, Theoretical Calculation, and in Vitro Antitumor Activity. J. Inorg. Biochem. 2022, 237, 112015. [Google Scholar] [CrossRef]
- Lima, F.C.; Só, Y.A.O.; Gargano, R.; Fujimori, M.; França, E.L.; Honorio-França, A.C.; Gatto, C.C. Synthesis, Theoretical Calculation and Anticancer Activity of 4,6-Diacetylresorcinol-Dithiocarbazates and Their Copper(II) Complexes. J. Mol. Struct. 2020, 1212, 128083. [Google Scholar] [CrossRef]
- Enikeeva, K.R.; Kasimov, A.I.; Litvinov, I.A.; Lyubina, A.P.; Voloshina, A.D.; Musina, E.I.; Karasik, A.A. Synthesis of Nickel(II) Complexes Based on Dialkylphosphorylpyridines and Their Cytotoxic Activity. Russ. J. Inorg. Chem. 2023, 68, 1117–1124. [Google Scholar] [CrossRef]
- Break, M.K.B.; Fung, T.Y.; Koh, M.Z.; Ho, W.Y.; Tahir, M.I.M.; Elfar, O.A.; Syed, R.U.; Khojali, W.M.A.; Alluhaibi, T.M.; Huwaimel, B.; et al. Synthesis, Crystal Structure, Antibacterial and In Vitro Anticancer Activity of Novel Macroacyclic Schiff Bases and Their Cu(II) Complexes Derived from S-Methyl and S-Benzyl Dithiocarbazate. Molecules 2023, 28, 5009. [Google Scholar] [CrossRef]
- Li, Y.S.; Peng, B.; Ma, L.; Cao, S.L.; Bai, L.L.; Yang, C.R.; Wan, C.Q.; Yan, H.J.; Ding, P.P.; Li, Z.F.; et al. Synthesis, Crystal Structures and Antitumor Activity of Two Platinum(II) Complexes with Methyl Hydrazinecarbodithioate Derivatives of Indolin-2-One. Eur. J. Med. Chem. 2017, 127, 137–146. [Google Scholar] [CrossRef]
- Mahavorasirikul, W.; Chaijaroenkul, W.; Itharat, A.; Na-Bangchang, K. Screening of Cytotoxic Activity of Thai Medicinal Plants against Human Cholangiocarcinoma Cells In Vitro. Drug Metab. Rev. 2010, 41, 85. [Google Scholar]
- Fernandes, J.P.C.; Mehdad, A.; Valadares, N.F.; Mourão, C.B.F.; Ventura, M.M.; Barbosa, J.A.R.G.; de Freitas, S.M. Crystallographic Structure of a Complex between Trypsin and a Nonapeptide Derived from a Bowman-Birk Inhibitor Found in Vigna Unguiculata Seeds. Arch. Biochem. Biophys. 2019, 665, 79–86. [Google Scholar] [CrossRef]
- Clemente, A.; Moreno, F.J.; Marín-Manzano, M.d.C.; Jiménez, E.; Domoney, C. The Cytotoxic Effect of Bowman-Birk Isoinhibitors, IBB1 and IBBD2, from Soybean (Glycine Max) on HT29 Human Colorectal Cancer Cells Is Related to Their Intrinsic Ability to Inhibit Serine Proteases. Mol. Nutr. Food Res. 2010, 54, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Day, R.; Seidah, N.; Moody, T.W.; Cuttitta, F.; Davis, T.P. Protease Inhibitors Suppress in Vitro Growth of Human Small Cell Lung Cancer. Peptides 1993, 14, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- An, W.G.; Hwang, S.G.; Trepel, J.B.; Blagosklonny, M.V. Protease Inhibitor-Induced Apoptosis: Accumulation of Wt P53, P21WAF1/CIP1, and Induction of Apoptosis Are Independent Markers of Proteasome Inhibition. Leukemia 2000, 14, 1276–1283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Gatto, C.C.; Dias, L.M.; Paiva, C.A.; da Silva, I.C.R.; Freire, D.O.; Tormena, R.P.I.; Nascimento, É.C.M.; Martins, J.B.L. Effects of Changing Ions on the Crystal Design, Non-Covalent Interactions, Antimicrobial Activity, and Molecular Docking of Cu(II) Complexes with a Pyridoxal-Hydrazone Ligand. Front. Chem. 2024, 12, 1347370. [Google Scholar] [CrossRef] [PubMed]
- Biovia, D.S. Biovia Discovery Studio Visualizer; Dassault Systèmes: San Diego, CA, USA, 2020. [Google Scholar]


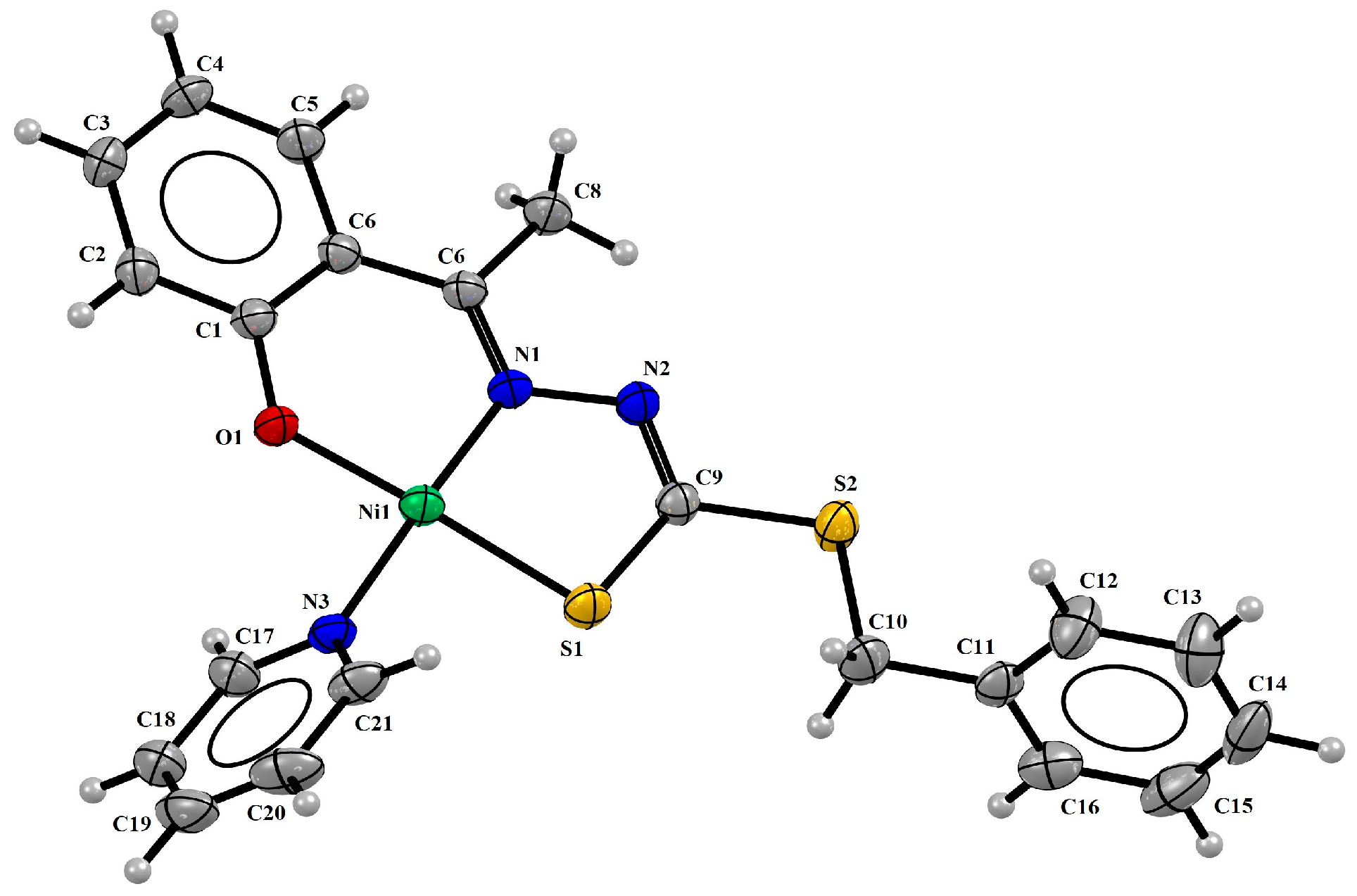
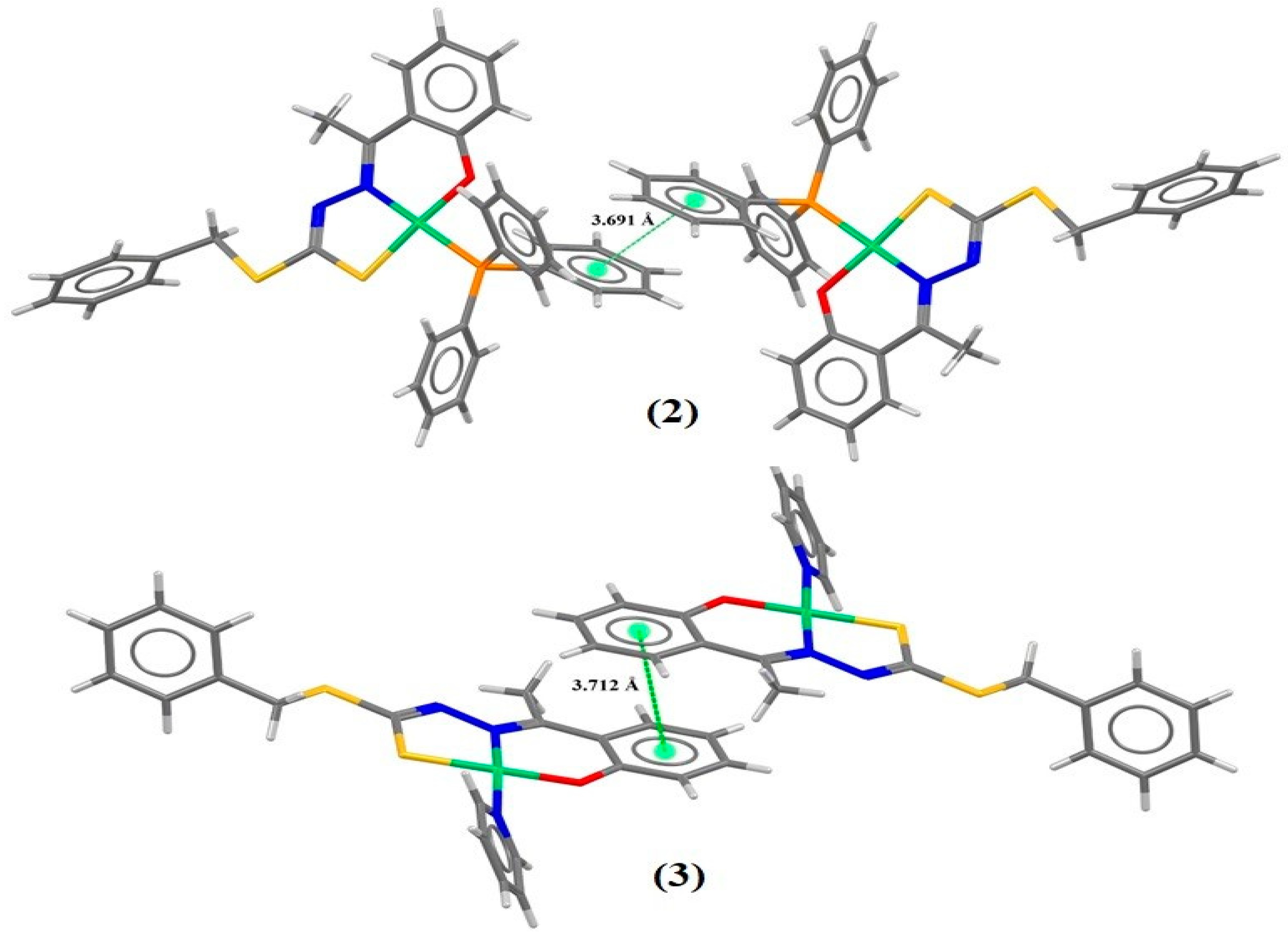

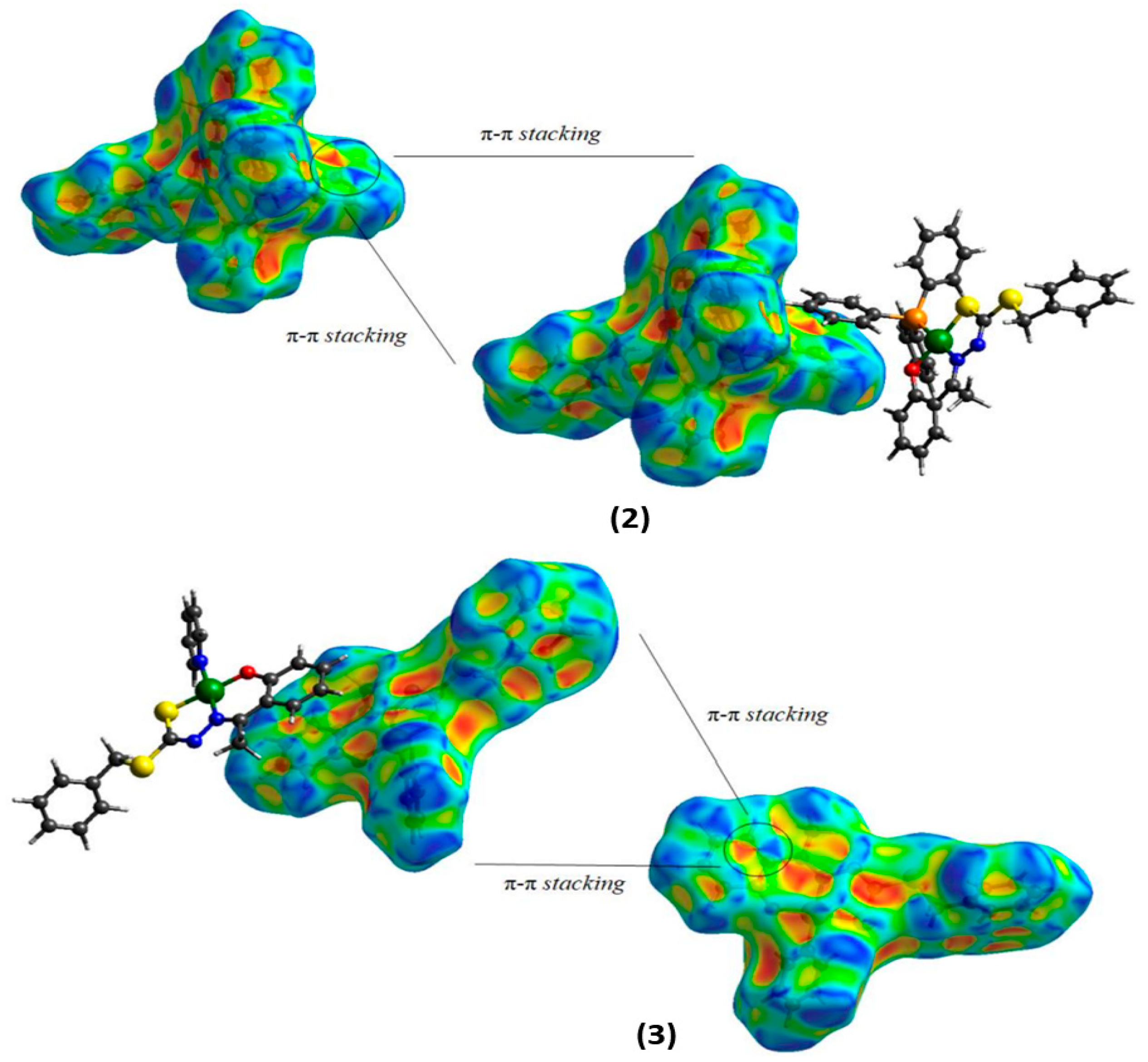
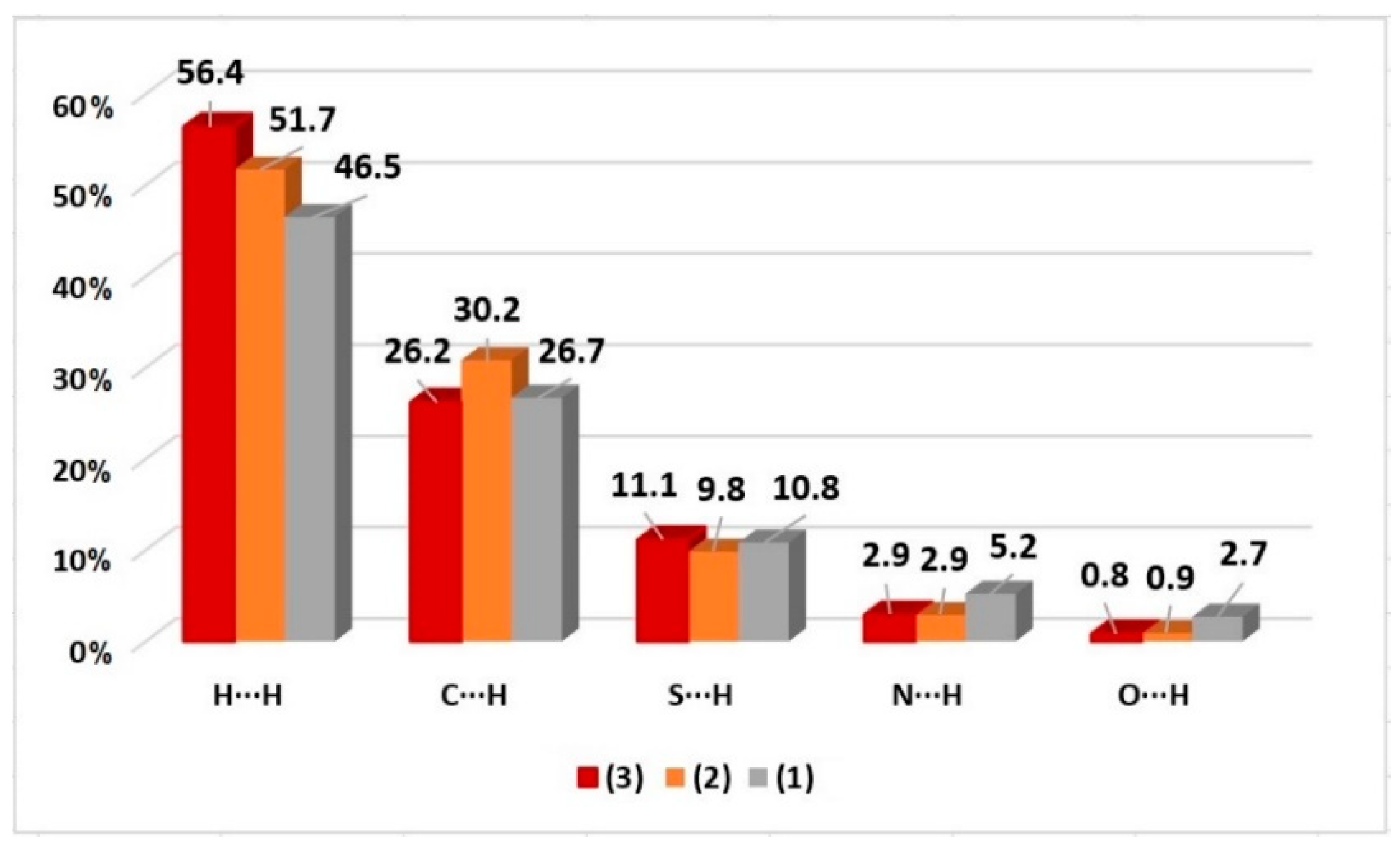
| Bond Lengths (Å) | |||
|---|---|---|---|
| (1) | (2) | (3) | |
| C1-O1 | 1.311(5) | 1.313(3) | 1.325(4) |
| C7-N1 | 1.330(5) | 1.313(3) | 1.328(4) |
| N1-N2 | 1.407(4) | 1.414(3) | 1.412(4) |
| N2-C9 | 1.277(5) | 1.289(3) | 1.295(4) |
| C9-S1 | 1.735(4) | 1.729(3) | 1.713(4) |
| C9-S2 | 1.743(4) | 1.725(3) | 1.756(4) |
| Ni1-O1 | 1.820(3) | 1.836(17) | 1.823(2) |
| Ni1-N1 | 1.896(3) | 1.895(2) | 1.851(3) |
| Ni1-S1 | 2.122(12) | 2.127(8) | 2.133(12) |
| Ni1-X | 2.205(11) | 2.200(8) | 1.907(3) |
| Bond Angles (°) | |||
| O1-Ni1-N1 | 94.11(12) | 94.28(8) | 96.12(12) |
| O1-Ni1-X | 84.51(9) | 86.19(6) | 85.80(12) |
| X-Ni1-S1 | 93.23(4) | 90.88(3) | 89.73(10) |
| S1-Ni1-N1 | 88.77(10) | 88.55(6) | 88.62(10) |
| O1-Ni1-S1 | 173.95(10) | 176.56(6) | 174.12(9) |
| N1-Ni1-X | 173.24(10) | 176.92(6) | 175.25(13) |
| H2L1 | H2L2 | (1) | (2) | (3) | |
|---|---|---|---|---|---|
| ν(O-H) | 3245 | 3418 | - | - | - |
| ν(N-H) | 3184 | 3179 | - | - | - |
| ν(CSS) | 1101 | 1057 | 982 | 939 | 984 |
| ν(C=N) | 1601 | 1601 | 1523 | 1561 | 1522 |
| ν(C-O) | 1219 | 1228 | 1239 | 1156 | 1239 |
| ν(N-N) | 936 | 987 | 1023 | 1014 | 1070 |
| ν(C=N)py | - | - | - | - | 1603 |
| δ(Py) | - | - | 690 | ||
| Ν(CPPh3-PPPh3) | - | - | 1070 | 1094 | - |
| ν(CPPh3-CPPh3) | 1435 | 1434 | |||
| ν(Ni-PPPh3) | 692 | 693 |
| IC50 (Inhibitory Concentration 50%) ± SD at 24 h | |||||
|---|---|---|---|---|---|
| Cell Line | H2L1 | H2L2 | (1) | (2) | (3) |
| MCF-10A | 65.44 ± 0.034 | 86.10 ± 0.082 | 25.47 ± 0.031 | 30.24 ± 0.014 | 30.13 ± 0.018 |
| MCF-7 | 88.85 ± 0.097 | 58.59 ± 0.018 | 12.82 ± 0.029 | 13.76 ± 0.025 | 14.37 ± 0.032 |
| SI * | 0.73 | 1.47 | 2.03 | 2.19 | 2.10 |
| IC50 (Inhibitory Concentration 50%) ± SD at 48 h | |||||
| MCF-10A | 62.86 ± 0.013 | 57.46 ± 0.032 | 28.43 ± 0.021 | 31.05 ± 0.010 | 20.11 ± 0.022 |
| MCF-7 | 87.73 ± 0.042 | 49.47 ± 0.012 | 22.71 ± 0.016 | 12.53 ± 0.015 | 18.34 ± 0.012 |
| SI * | 0.71 | 1.16 | 1.25 | 2.48 | 1.10 |
| IC50 (Inhibitory Concentration 50%) ± SD at 72 h | |||||
| MCF-10A | 62.86 ± 0.025 | 57.46 ± 0.012 | 28.43 ± 0.011 | 31.05 ± 0.024 | 20.11 ± 0.012 |
| MCF-7 | 28.49 ± 0.023 | 23.97 ± 0.015 | 18.07 ± 0.017 | 8.073 ± 0.011 | 14.38 ± 0.014 |
| SI * | 2.21 | 2.40 | 1.57 | 3.85 | 1.40 |
| Molecule | Score | #HB | Distance |
|---|---|---|---|
| (1) | −6.60 | 1 | 2.99S2 |
| (2) | −9.20 | 1 | 2.57S2 |
| (3) | −7.80 | 0 | - |
| H2L1 | −5.40 | 0 | - |
| H2L2 | −5.80 | 0 | - |
| Ptry9L | −10.70 | 9 | 2.48Thr2:0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatto, C.C.; Cavalcante, C.d.Q.O.; Lima, F.C.; Nascimento, É.C.M.; Martins, J.B.L.; Santana, B.L.O.; Gualberto, A.C.M.; Pittella-Silva, F. Structural Design, Anticancer Evaluation, and Molecular Docking of Newly Synthesized Ni(II) Complexes with ONS-Donor Dithiocarbazate Ligands. Molecules 2024, 29, 2759. https://doi.org/10.3390/molecules29122759
Gatto CC, Cavalcante CdQO, Lima FC, Nascimento ÉCM, Martins JBL, Santana BLO, Gualberto ACM, Pittella-Silva F. Structural Design, Anticancer Evaluation, and Molecular Docking of Newly Synthesized Ni(II) Complexes with ONS-Donor Dithiocarbazate Ligands. Molecules. 2024; 29(12):2759. https://doi.org/10.3390/molecules29122759
Chicago/Turabian StyleGatto, Claudia C., Cássia de Q. O. Cavalcante, Francielle C. Lima, Érica C. M. Nascimento, João B. L. Martins, Brunna L. O. Santana, Ana C. M. Gualberto, and Fabio Pittella-Silva. 2024. "Structural Design, Anticancer Evaluation, and Molecular Docking of Newly Synthesized Ni(II) Complexes with ONS-Donor Dithiocarbazate Ligands" Molecules 29, no. 12: 2759. https://doi.org/10.3390/molecules29122759
APA StyleGatto, C. C., Cavalcante, C. d. Q. O., Lima, F. C., Nascimento, É. C. M., Martins, J. B. L., Santana, B. L. O., Gualberto, A. C. M., & Pittella-Silva, F. (2024). Structural Design, Anticancer Evaluation, and Molecular Docking of Newly Synthesized Ni(II) Complexes with ONS-Donor Dithiocarbazate Ligands. Molecules, 29(12), 2759. https://doi.org/10.3390/molecules29122759







