Carbon Dioxide Solubility in Three Bis Tri (Fluromethylsulfonyl) Imide-Based Ionic Liquids
Abstract
1. Introduction
2. Results and Discussion
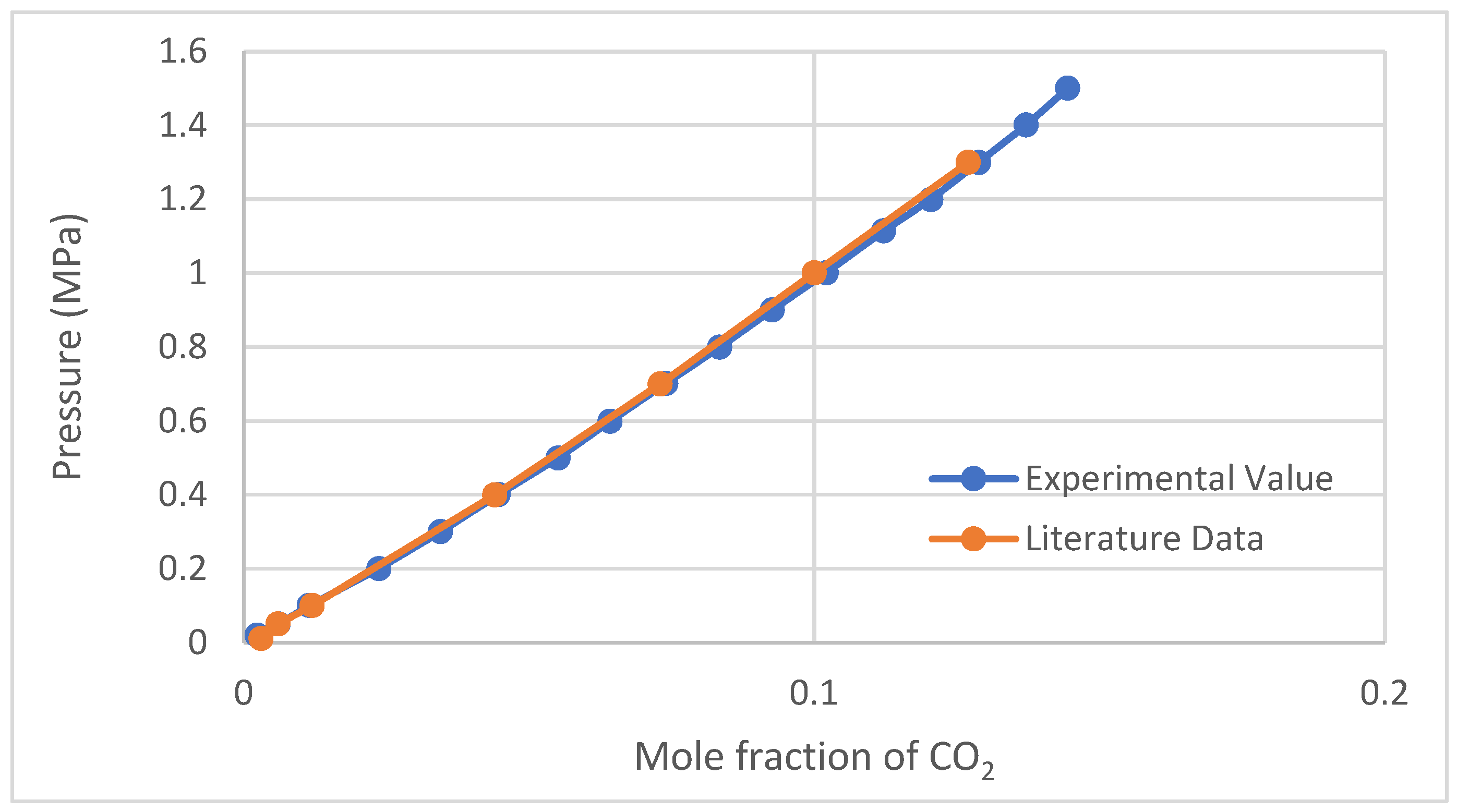
2.1. CO2 Solubility Validation Test
2.2. ILs CO2 Solubility
2.3. Simulation Interaction Parameters
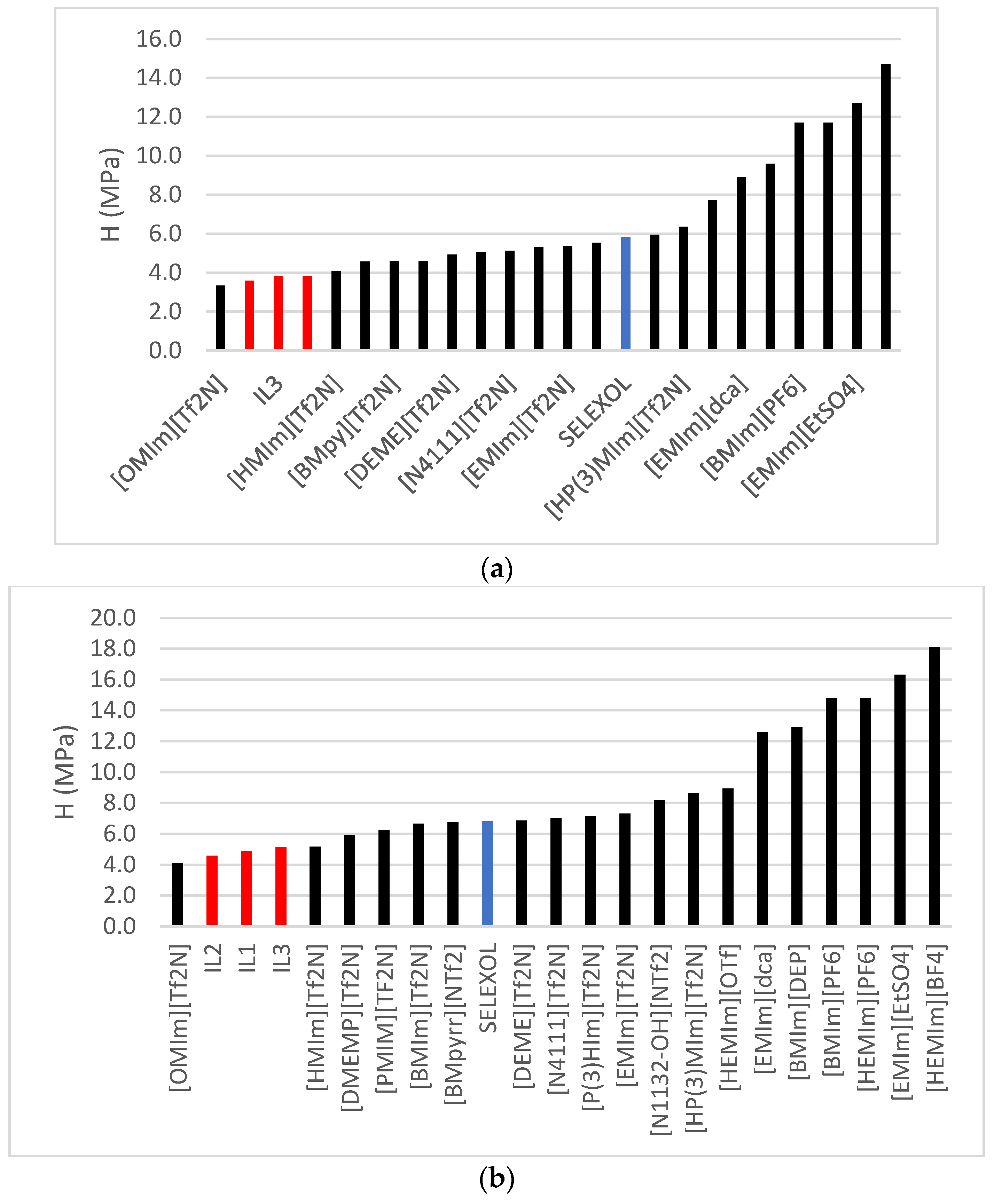
2.4. Henry’s Law Constant, Enthalpies and Entropies
3. Materials and Method
3.1. Materials
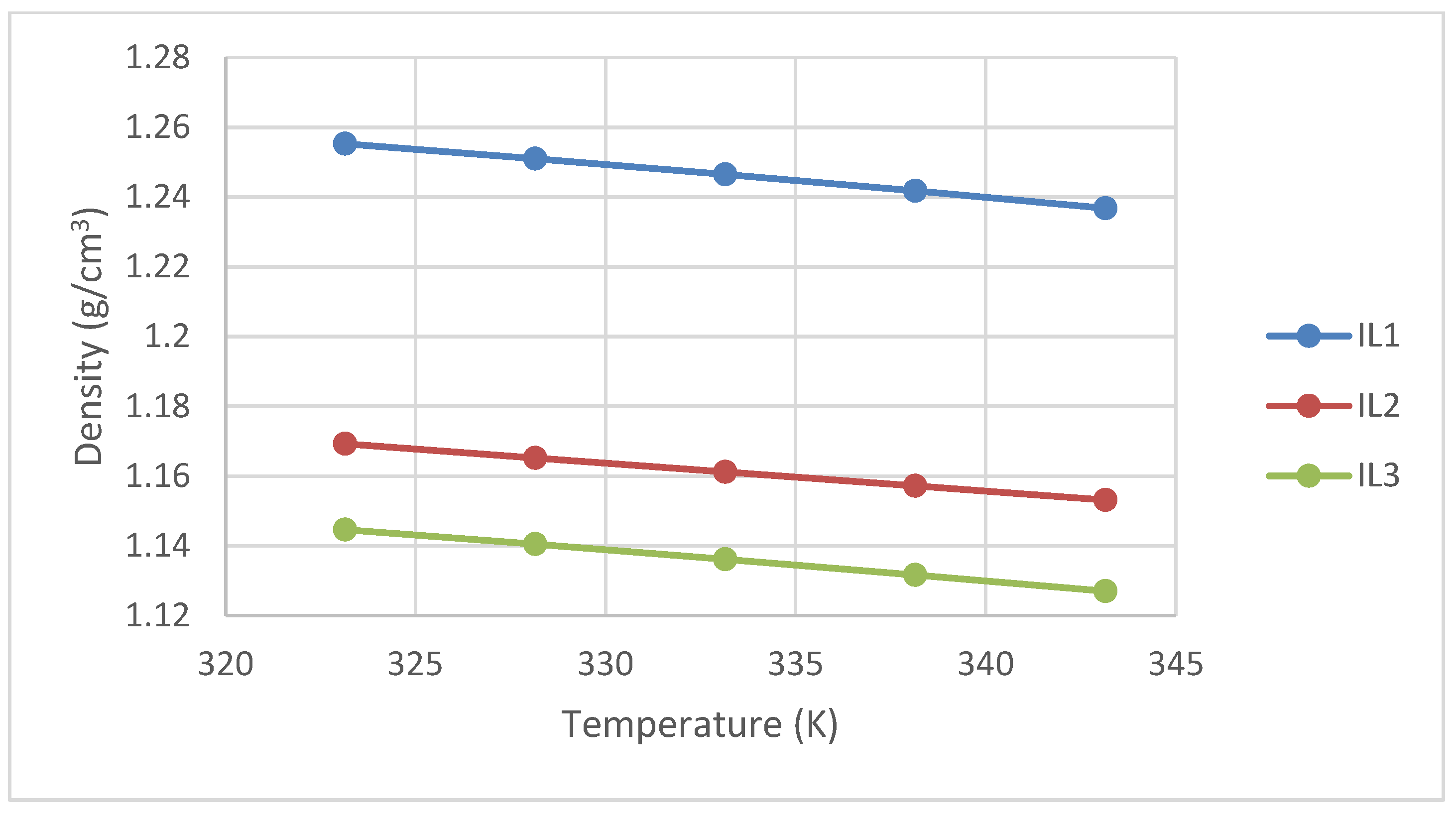
3.2. Density Measurement
3.3. Solubility Analysis
3.4. Thermodynamic Modelling
- van der Waals one single binary interaction parameter;
- van der Waals two binary interaction parameters;
- NRTL model combined with Wong–Sandler mixing rules (WS-NRTL).
3.4.1. van der Waals Mixing Rules
3.4.2. Critical Properties Calculations
3.5. Binary Interaction Parameter Optimization
3.6. Henry’s Law Constant, Enthalpy of Absorption and Entropy of Solvation
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pachauri, R.; Meyer, L. Intergovernmental Panel on Climate Change Synthesis Report; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Zhang, C. Absorption principle and techno-economic analysis of CO2 absorption technologies. IOP Conf. Ser. Earth Environ. Sci. 2021, 657, 012045. [Google Scholar] [CrossRef]
- Yethiraj, A.; Phys, J. Structure of room temperature ionic liquids. J. Phys. Condens. Matter 2016, 28, 414020. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.; Maginn, E.; Brennecke, J. Solubilities and thermodynamic properties of gases. J. Phys. Chem. B 2002, 109, 7315–7320. [Google Scholar] [CrossRef]
- Vadillo, J.; Diaz-Sainz, G.; Gomez-Coma, L.; Garea, A.; Irabien, A. Chemical and Physical Ionic Liquids in CO2 Capture Systems Using Membrane Vacuum Regeneration. Membrane 2022, 12, 785. [Google Scholar] [CrossRef] [PubMed]
- Shiflett, M.; Yokozeki, A. Solubilities and diffusivities of carbon dioxide in ionic liquids. Ind. Eng. Chem. Res. 2005, 44, 4453–4464. [Google Scholar] [CrossRef]
- Karunarathne, S.; Eimer, D.; Lars, E. Density, Viscosity, and Excess Properties of MDEA + H2O, DMEA + H2O, and DEEA + H2O Mixture. Appl. Sci. 2020, 10, 3196. [Google Scholar] [CrossRef]
- Aravind, V.; Amr, H.; Paitoon, T. High Pressure Physical Solubility of Carbon Dioxide (CO2) in Mixed Polyethylene Glycol Dimethyl Ethers (Genosorb 1753). Can. J. Chem. Eng. 2011, 90, 567–583. [Google Scholar]
- Nath, D.; Henni, A. Solubility of carbon dioxide (CO2) in four bis (trifluoromethylsulfonyl)imide ([Tf2N]) based ionic liquids. Fluid Phase Equilibria 2020, 524, 112757. [Google Scholar] [CrossRef]
- Valderrama, J.; Rojas, R. Critical properties of ionic liquids. Revisited. Ind. Eng. Chem. Res. 2009, 48, 6890–6900. [Google Scholar] [CrossRef]
- Huseynov, M. Thermodynamic and Experimental Studies of Ethane Solubility in Promising Ionic Liquids for CO2 Capture. Master’s Thesis, University of Regina, Regina, SK, Canada, 2015. [Google Scholar]


| IL1 | |||||
|---|---|---|---|---|---|
| 303.15 K | 323.15 K | 343.15 K | |||
| Pressure (MPa) | Pressure (MPa) | Pressure (MPa) | |||
| 0.034 | 0.0997 | 0.026 | 0.1003 | 0.020 | 0.0999 |
| 0.065 | 0.1998 | 0.051 | 0.2002 | 0.040 | 0.1999 |
| 0.094 | 0.2994 | 0.073 | 0.2999 | 0.057 | 0.2994 |
| 0.122 | 0.4002 | 0.092 | 0.4003 | 0.073 | 0.3997 |
| 0.148 | 0.5004 | 0.111 | 0.4989 | 0.090 | 0.4999 |
| 0.172 | 0.5988 | 0.130 | 0.5990 | 0.106 | 0.5994 |
| 0.195 | 0.7003 | 0.149 | 0.7006 | 0.121 | 0.7011 |
| 0.217 | 0.8003 | 0.167 | 0.8002 | 0.136 | 0.7994 |
| 0.238 | 0.9000 | 0.184 | 0.8995 | 0.151 | 0.9007 |
| 0.259 | 0.9999 | 0.201 | 0.9998 | 0.164 | 1.0004 |
| 0.278 | 1.1000 | 0.217 | 1.0990 | 0.176 | 1.1005 |
| 0.296 | 1.1990 | 0.231 | 1.1990 | 0.189 | 1.1998 |
| 0.314 | 1.3000 | 0.246 | 1.3010 | 0.200 | 1.2992 |
| 0.329 | 1.4000 | 0.262 | 1.3990 | 0.213 | 1.3989 |
| 0.345 | 1.4995 | 0.276 | 1.4991 | 0.224 | 1.5003 |
| IL2 | |||||
| 323.15 K | 333.15 K | 343.15 K | |||
| Pressure (MPa) | Pressure (MPa) | Pressure (MPa) | |||
| 0.026 | 0.0998 | 0.024 | 0.0999 | 0.022 | 0.1003 |
| 0.053 | 0.1999 | 0.049 | 0.1997 | 0.044 | 0.2009 |
| 0.077 | 0.2999 | 0.070 | 0.2985 | 0.061 | 0.2993 |
| 0.098 | 0.3999 | 0.089 | 0.4001 | 0.078 | 0.3996 |
| 0.118 | 0.5005 | 0.108 | 0.5008 | 0.094 | 0.4988 |
| 0.139 | 0.5991 | 0.127 | 0.5987 | 0.111 | 0.5990 |
| 0.157 | 0.7006 | 0.143 | 0.6992 | 0.127 | 0.7008 |
| 0.174 | 0.8010 | 0.160 | 0.8009 | 0.143 | 0.7998 |
| 0.193 | 0.8993 | 0.176 | 0.8997 | 0.158 | 0.8997 |
| 0.211 | 1.0006 | 0.193 | 0.9996 | 0.172 | 1.0007 |
| 0.228 | 1.0998 | 0.207 | 1.1006 | 0.187 | 1.0997 |
| 0.245 | 1.2002 | 0.223 | 1.1998 | 0.202 | 1.1997 |
| 0.261 | 1.2999 | 0.240 | 1.2995 | 0.216 | 1.2991 |
| 0.272 | 1.3999 | 0.255 | 1.4165 | 0.227 | 1.3989 |
| 0.289 | 1.5071 | 0.264 | 1.4991 | 0.238 | 1.5003 |
| IL3 | |||||
| 303.15 K | 323.15 K | 343.15 K | |||
| Pressure (MPa) | Pressure (MPa) | Pressure (MPa) | |||
| 0.034 | 0.0999 | 0.025 | 0.0999 | 0.016 | 0.0986 |
| 0.066 | 0.1998 | 0.051 | 0.1998 | 0.037 | 0.1997 |
| 0.096 | 0.3004 | 0.075 | 0.2996 | 0.056 | 0.2999 |
| 0.124 | 0.4004 | 0.097 | 0.4003 | 0.074 | 0.4000 |
| 0.151 | 0.5009 | 0.115 | 0.5001 | 0.089 | 0.4941 |
| 0.176 | 0.5994 | 0.136 | 0.6005 | 0.106 | 0.6003 |
| 0.200 | 0.7012 | 0.155 | 0.6987 | 0.124 | 0.6991 |
| 0.222 | 0.8006 | 0.173 | 0.7992 | 0.138 | 0.7956 |
| 0.243 | 0.8988 | 0.192 | 0.9007 | 0.154 | 0.8949 |
| 0.267 | 0.9937 | 0.208 | 0.9996 | 0.169 | 1.0003 |
| 0.284 | 1.1005 | 0.226 | 1.1007 | 0.186 | 1.1251 |
| 0.303 | 1.2000 | 0.243 | 1.2004 | 0.198 | 1.2005 |
| 0.321 | 1.3002 | 0.257 | 1.3005 | 0.211 | 1.3053 |
| 0.341 | 1.4084 | 0.276 | 1.4219 | 0.227 | 1.4093 |
| 0.353 | 1.5013 | 0.288 | 1.5003 | 0.238 | 1.4990 |
| Ionic Liquids + CO2 | Temperature (°C) | Binary Interaction Parameter (k12) | % AAD | |||
|---|---|---|---|---|---|---|
| IL1 | 30 | −0.0711 | 7.52 | |||
| 50 | −0.0857 | 6.37 | ||||
| 70 | −0.1090 | 5.68 | ||||
| IL2 | 50 | −0.1030 | 6.39 | |||
| 60 | −0.1200 | 6.57 | ||||
| 70 | −0.1310 | 5.67 | ||||
| IL3 | 30 | −0.0737 | 6.85 | |||
| 50 | −0.0956 | 5.43 | ||||
| 70 | −0.1160 | 3.14 | ||||
| Ionic Liquids + CO2 | Temperature (°C) | Binary Interaction Parameter | % AAD | |||
| (k12) | (l12) | |||||
| IL1 | 30 | −0.0029 | 0.0139 | 0.38 | ||
| 50 | −0.0081 | 0.0149 | 1.01 | |||
| 70 | −0.0107 | 0.0173 | 0.63 | |||
| IL2 | 50 | −0.0372 | 0.0134 | 0.96 | ||
| 60 | −0.0455 | 0.0143 | 1.04 | |||
| 70 | −0.0493 | 0.0149 | 1.49 | |||
| IL3 | 30 | −0.0166 | 0.0103 | 6.85 | ||
| 50 | −0.0347 | 0.0099 | 0.87 | |||
| 70 | −0.0717 | 0.0066 | 1.48 | |||
| Ionic Liquids + CO2 | Temperature (°C) | Binary Interaction Parameter | % AAD | |||
| (k12) | (τ12) | (τ21) | ||||
| IL1 | 30 | 0.8930 | −0.4706 | 0.0017 | 0.41 | |
| 50 | 0.8619 | −0.6007 | 0.1619 | 0.87 | ||
| 70 | 0.8039 | −0.9079 | 0.6283 | 0.59 | ||
| IL2 | 50 | 0.7568 | −0.4833 | −0.1106 | 0.97 | |
| 60 | 0.7212 | −0.4676 | −0.1439 | 1.00 | ||
| 70 | 0.7013 | −0.5794 | 0.0076 | 1.34 | ||
| IL3 | 30 | 0.9156 | 0.1346 | −0.6906 | 0.43 | |
| 50 | 0.8161 | −0.4606 | −0.0019 | 0.85 | ||
| 70 | 0.6969 | −0.3518 | −0.0001 | 1.53 | ||
| Ionic Liquid (ILs) | Henry’s Law Constant (MPa) | (kJ/mol) | (KJ/Kmol·K) | ||
|---|---|---|---|---|---|
| T = 30 °C | T = 50 °C | T = 70 °C | |||
| IL1 | 2.79 | 3.83 | 4.90 | −12.80 | −37.78 |
| T = 50 °C | T = 60 °C | T = 70 °C | |||
| IL2 | 3.66 | 4.05 | 4.58 | −10.33 | −31.04 |
| IL3 | T = 30 °C | T = 50 °C | T = 70 °C | ||
| 2.71 | 3.82 | 5.13 | −13.79 | −42.80 | |
| Ionic Liquid | Nomenclature | Acronym | Structure |
|---|---|---|---|
| 1-Decyl-3-MethylimidazoliumBis (Trifluromethylsulfonyl Imide) (≥98.0%, water: 8 ppm) * CAS Number: 433337-23-6 | C15H26F6N3O4S2 | IL1 |  |
| 1-Hexadecyl-3-Methyl imidazoliumBis (Trifluromethylsulfonyl Imide) (≥98.0%, water: 146 ppm) * CAS Number: 404001-50-9 | C21H40F6N3O4S2 | IL2 | 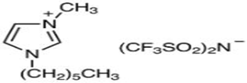 |
| Triethyl tetradecyl Ammonium Bis Bis (Trifluromethylsulfonyl Imide) (≥98.0%, water: 102 ppm) * CAS Number: n/a | C20H44F6N2O4S2 | IL3 | 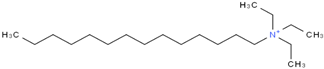 |
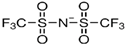 |
| Temperature (K) | Density (g/cm3) | ||
|---|---|---|---|
| IL1 | IL2 | IL3 | |
| 303.15 | 1.2727 | - | 1.1608 |
| 308.15 | 1.2684 | - | 1.1569 |
| 313.15 | 1.2640 | - | 1.1529 |
| 318.15 | 1.2597 | - | 1.1488 |
| 323.15 | 1.2553 | 1.1693 | 1.1446 |
| 328.15 | 1.2510 | 1.1652 | 1.1405 |
| 333.15 | 1.2465 | 1.1612 | 1.1362 |
| 338.15 | 1.2418 | 1.1572 | 1.1317 |
| 343.15 | 1.2368 | 1.1532 | 1.1270 |
| Component | Tc (K) | Pc (Bar) | ω |
|---|---|---|---|
| IL1 | 1345.1 | 18.700 | 0.5741 |
| IL2 | 1195.4 | 18.347 | 0.9176 |
| IL3 | 1207.7 | 14.265 | 1.1367 |
| CO2 | 304.1 | 73.800 | 0.2390 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quaye, E.; Henni, A.; Shirif, E. Carbon Dioxide Solubility in Three Bis Tri (Fluromethylsulfonyl) Imide-Based Ionic Liquids. Molecules 2024, 29, 2784. https://doi.org/10.3390/molecules29122784
Quaye E, Henni A, Shirif E. Carbon Dioxide Solubility in Three Bis Tri (Fluromethylsulfonyl) Imide-Based Ionic Liquids. Molecules. 2024; 29(12):2784. https://doi.org/10.3390/molecules29122784
Chicago/Turabian StyleQuaye, Eric, Amr Henni, and Ezeddin Shirif. 2024. "Carbon Dioxide Solubility in Three Bis Tri (Fluromethylsulfonyl) Imide-Based Ionic Liquids" Molecules 29, no. 12: 2784. https://doi.org/10.3390/molecules29122784
APA StyleQuaye, E., Henni, A., & Shirif, E. (2024). Carbon Dioxide Solubility in Three Bis Tri (Fluromethylsulfonyl) Imide-Based Ionic Liquids. Molecules, 29(12), 2784. https://doi.org/10.3390/molecules29122784






