Metal-Free Cascade Formation of C–C and C–N Bond for the Construction of 3-Cyano-2-Pyridones with Insecticidal Properties
Abstract
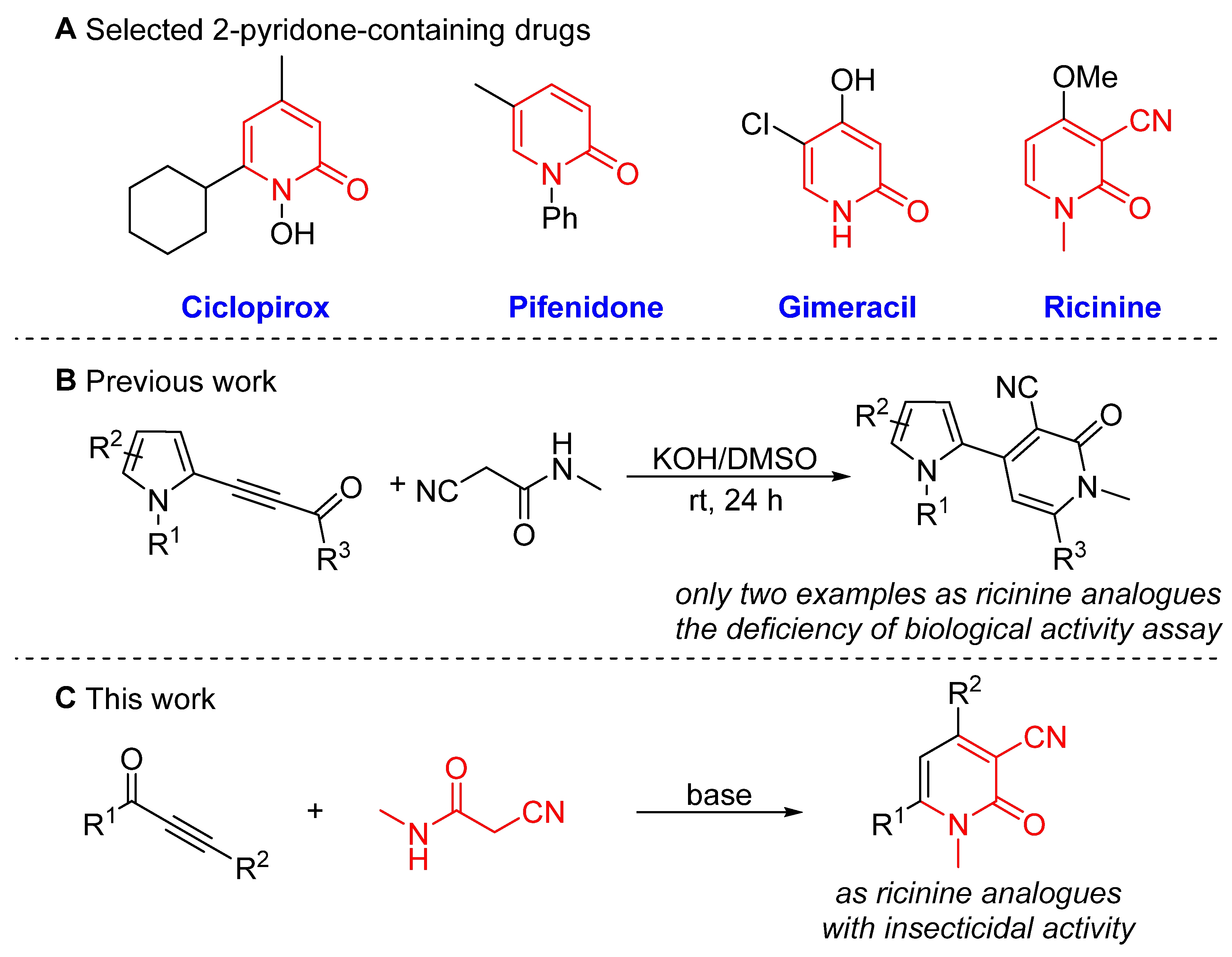
:1. Introduction
2. Results and Discussion
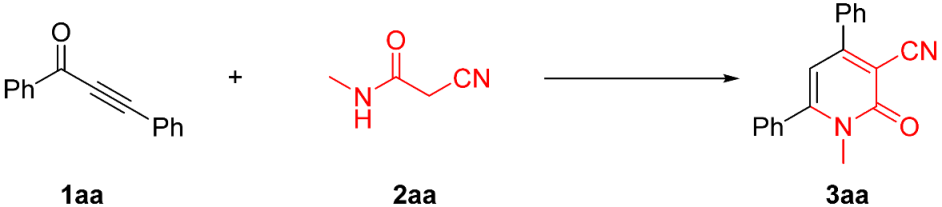
2.1. Optimization of the Reaction Conditions
2.2. Scope of the Reaction
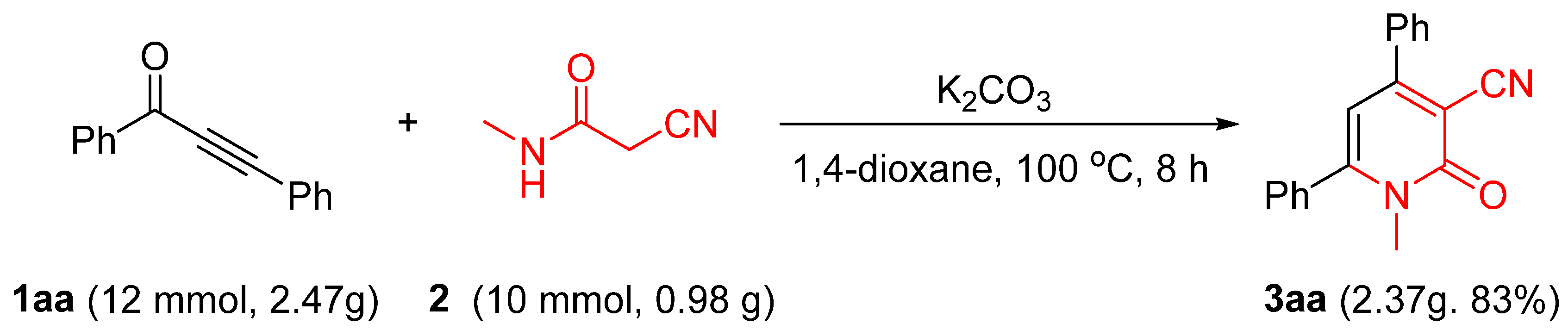
2.3. Gram-Scale of the Reaction
2.4. Plausible Mechanism of the Reaction
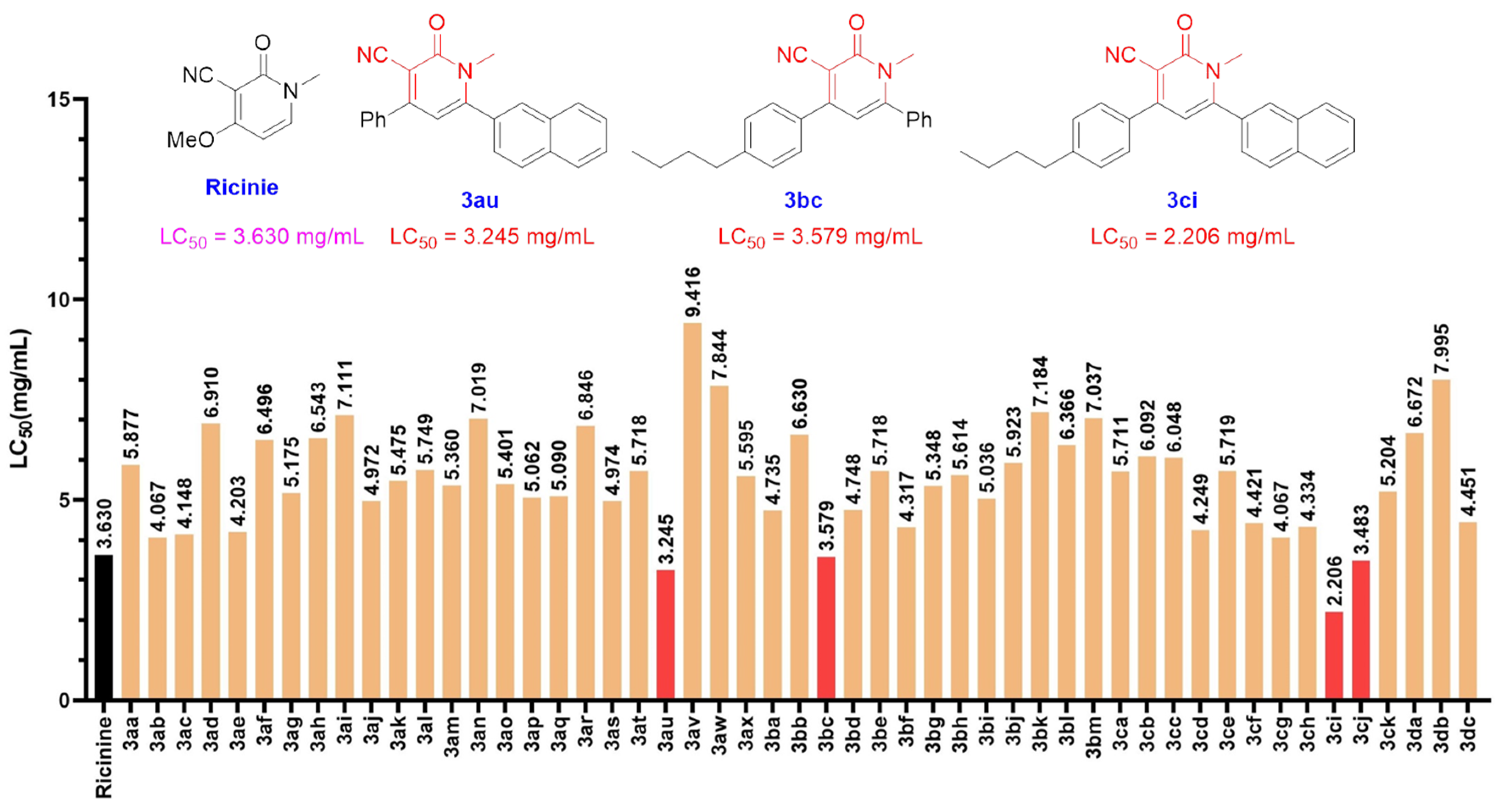
2.5. Study of the Insecticidal Properties of 3-Cyano-2-Pyridones
3. Experimental Section
3.1. General Methods and Materials for the Synthesis of 3-Cyano-2-Pyridones 3
3.2. Insecticidal Properties Study Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Misra, R.; Pandey, R.C.; Silverton, J.V. Fredericamycin A, an Antitumor Antibiotic of a Novel Skeletal Type. J. Am. Chem. Soc. 1982, 104, 4478–4479. [Google Scholar] [CrossRef]
- Bryan, M.C.; Burdick, D.J.; Chan, B.K.; Chen, Y.; Clausen, S.; Dotson, J.; Eigenbrot, C.; Elliott, R.; Hanan, E.J.; Heald, R.; et al. Pyridones as Highly Selective, Noncovalent Inhibitors of T790M Double Mutants of EGFR. ACS Med. Chem. Lett. 2016, 7, 100–104. [Google Scholar] [CrossRef]
- Fioravanti, R.; Stazi, G.; Zwergel, C.; Valente, S.; Mai, A. Six Years (2012–2018) of Researches on Catalytic Ezh2 Inhibitors: The Boom of the 2-Pyridone Compounds. Chem. Rec. 2018, 18, 1818–1832. [Google Scholar] [CrossRef]
- Dragovich, P.S.; Prins, T.J.; Zhou, R.; Brown, E.L.; Maldonado, F.C.; Fuhrman, S.A.; Zalman, L.S.; Tuntland, T.; Lee, C.A.; Patick, A.K.; et al. Structure–Based Design, Synthesis, and Biological Evaluation of Irreversible Human Rhinovirus 3C Protease Inhibitors. 6. Structure-Activity Studies of Orally Bioavailable, 2-Pyridone-Containing Peptidomimetics. J. Med. Chem. 2002, 45, 1607–1623. [Google Scholar] [CrossRef]
- Hibi, S.; Ueno, K.; Nagato, S.; Kawano, K.; Ito, K.; Norimine, Y.; Takenaka, O.; Hanada, T.; Yonaga, M. Discovery of 2-(2-Oxo-1-phenyl-5-pyridin-2-yl-1,2-dihydropyridin-3-yl)benzonitrile (Perampanel): A Novel, Noncompetitive α-Amino-3-hydroxy-5-methyl-4-isoxazolepropanoic Acid (AMPA) Receptor Antagonist. J. Med. Chem. 2012, 55, 10584–10600. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Morrell, A.; Dexheimer, T.S.; Scher, E.S.; Pommier, Y.; Cushman, M. Design, Synthesis, and Biological Evaluation of 14-Substituted Aromathecins as Topoisomerase I Inhibitors. J. Med. Chem. 2008, 51, 4609–4619. [Google Scholar] [CrossRef]
- Du, W. Towards new anticancer drugs: A decade of advances in synthesis of camptothecins and related alkaloids. Tetrahedron 2003, 59, 8649–8687. [Google Scholar] [CrossRef]
- Ravinder, M.; Mahendar, B.; Mattapally, S.; Hamsini, K.V.; Reddy, T.N.; Rohit, C.; Srinivas, K.; Banerjee, S.K.; Rao, V.J. Synthesis and evaluation of novel 2-pyridone derivatives as inhibitors of phosphodiesterase3 (PDE3): A target for heart failure and platelet aggregation. Bioorg. Med. Chem. Lett. 2012, 22, 6010–6015. [Google Scholar] [CrossRef]
- Pfefferkorn, J.A.; Lou, J.H.; Minich, M.L.; Filipski, K.J.; He, M.Y.; Zhou, R.; Ahmed, S.; Benbow, J.; Perez, A.G.; Tu, M.; et al. Pyridones as glucokinase activators: Identification of a unique metabolic liability of the 4-sulfonyl-2-pyridone heterocycle. Bioorg. Med. Chem. Lett. 2009, 19, 3247–3252. [Google Scholar] [CrossRef]
- Chen, J.; Lu, M.M.; Liu, B.; Chen, Z.; Li, Q.B.; Tao, L.J.; Hu, G.Y. Synthesis and structure-activity relationship of 5-substituent-2(1H)-pyridone derivatives as anti-fibrosis agents. Bioorg. Med. Chem. Lett. 2012, 22, 2300–2302. [Google Scholar] [CrossRef]
- Mizutani, S.; Komori, K.; Taniguchi, T.; Monde, K.; Kuramochi, K.; Tsubaki, K. A Bioinspired Synthesis of (±)-Rubrobramide, (±)-Flavipucine, and (±)-Isoflavipucine. Angew. Chem. Int. Ed. 2016, 55, 9553–9556. [Google Scholar] [CrossRef]
- Wech, F.; Hasenbeck, M.; Gellrich, U. Semihydrogenation of Alkynes Catalyzed by a Pyridone Borane Complex: Frustrated Lewis Pair Reactivity and Boron–Ligand Cooperation in Concert. Chem.–Eur. J. 2020, 26, 13445–13450. [Google Scholar] [CrossRef]
- Kumar, V.; Keshavayya, J.; Matada, M.N.; Srinivasa, S.M.; Rangappa, S. Synthesis, Characterization and Biological Potency of Butyl-Pyridone Based Azo Dyes. ChemistrySelect 2020, 5, 5460–5464. [Google Scholar] [CrossRef]
- Brown, T.R.; Lange, J.P.; Mortimer, M.J.; Berry, J.F. New Oxypyridinate Paddlewheel Ligands for Alkane-Soluble, Sterically-Protected Ru2(II,III) and Ru2(II,II) Complexes. Inorg. Chem. 2018, 57, 10331–10340. [Google Scholar] [CrossRef]
- Fotiadou, A.D.; Zografos, A.L. Accessing the Structural Diversity of Pyridone Alkaloids: Concise Total Synthesis of Rac–Citridone A. Org. Lett. 2011, 13, 4592–4595. [Google Scholar] [CrossRef]
- Nakao, Y.; Idei, H.; Kanyiva, K.S.; Hiyama, T. Direct Alkenylation and Alkylation of Pyridone Derivatives by Ni/AlMe3 Catalysis. J. Am. Chem. Soc. 2009, 131, 15996–15997. [Google Scholar] [CrossRef]
- Phakdeeyothin, K.; Yotphan, S. Metal-free regioselective direct thiolation of 2-pyridones. Org. Biomol. Chem. 2019, 17, 6432–6440. [Google Scholar] [CrossRef]
- Maity, S.; Das, D.; Sarkar, S.; Samanta, R. Direct Pd(II)-Catalyzed Site-Selective C5-Arylation of 2-Pyridone Using Aryl Iodides. Org. Lett. 2018, 20, 5167–5171. [Google Scholar] [CrossRef]
- Tao, S.Q.; Xiao, J.; Li, Y.D.; Sun, F.X.; Du, Y.F. PhICl2/NH4SCN-Mediated Oxidative Regioselective Thiocyanation of Pyridin-2(1H)-ones. Chin. J. Chem. 2021, 39, 2536–2546. [Google Scholar] [CrossRef]
- Kittikool, T.; Phakdeeyothin, K.; Chantarojsiri, T.; Yotphan, S. Manganese-Promoted Regioselective Direct C3-Phosphinoylation of 2-Pyridones. Eur. J. Org. Chem. 2021, 21, 3071–3078. [Google Scholar] [CrossRef]
- Kamali, M.; Ebrahimi, A. One-pot multicomponent green synthesis of novel series of 4-hydroxy-2-pyridone-fused spiropyrans catalyzed by acetic acid. Synthetic Commun. 2023, 53, 1439–1450. [Google Scholar] [CrossRef]
- Li, J.; Tan, H.R.; An, Y.L.; Shao, Z.Y.; Zhao, S.Y. Synthesis and DABCO-induced demethylation of 3-cyano-4-methoxy-2-pyridone derivatives. J. Heterocyclic Chem. 2019, 57, 486–496. [Google Scholar] [CrossRef]
- Burger, B.S.; Cherioux, F.; Monnier-Jobe, K.; Laude, B.; Maillotte, H. 2-Pyridones as a New Photochemically Stable Structural Design for the Off-Resonant Optical Kerr Effect. Adv. Funct. Mater. 2022, 12, 339–347. [Google Scholar] [CrossRef]
- Jayasinghe, L.; Abbas, H.K.; Jacob, M.R.; Herath, W.H.M.W.; Nanayakkara, N.P.D. N-Methyl-4-hydroxy-2-pyridinone Analogues from Fusarium oxysporum. J. Nat. Prod. 2006, 69, 439–442. [Google Scholar] [CrossRef]
- Breinholt, J.; Ludvigsen, S.; Rassing, B.R.; Rosendahl, C.N. Oxysporidinone: A Novel, Antifungal N-Methyl-4-hydroxy-2-pyridone from Fusarium oxysporum. J. Nat. Prod. 1997, 60, 33–35. [Google Scholar] [CrossRef]
- Singh, S.B.; Liu, W.G.; Li, X.H.; Chen, T.; Shafiee, A.; Card, D.; Abruzzo, G.; Flattery, A.; Gill, C.; Thompson, J.R.; et al. Antifungal Spectrum, In Vivo Efficacy, and Structure—Activity Relationship of Ilicicolin H. ACS Med. Chem. Lett. 2012, 3, 814–817. [Google Scholar] [CrossRef]
- Storck, P.; Aubertin, A.M.; Grierson, D.S. Tosylation/mesylation of 4-hydroxy-3-nitro-2-pyridinones as an activation step in the construction of dihydropyrido[3,4-b] benzo[f][1,4]thiazepin-1-one based anti-HIV agents. Tetrahedron Lett. 2005, 46, 2919–2922. [Google Scholar] [CrossRef]
- Cocco, M.T.; Congiu, C.; Onnis, V. New bis(pyridyl)methane derivatives from 4-hydroxy-2-pyridones: Synthesis and antitumoral activity. Eur. J. Med. Chem. 2003, 38, 37–47. [Google Scholar] [CrossRef]
- Cocco, M.T.; Congiu, C.; Onnis, V. Synthesis and antitumour activity of 4-hydroxy-2-pyridone derivatives. Eur. J. Med. Chem. 2000, 35, 545–552. [Google Scholar] [CrossRef]
- Jessen, H.J.; Gademann, K. 4-Hydroxy-2-pyridone alkaloids: Structures and synthetic approaches. Nat. Prod. Rep. 2010, 27, 1168–1185. [Google Scholar] [CrossRef]
- Tohda, C.; Kuboyama, T.; Komatsu, K. Search for Natural Products Related to Regeneration of the Neuronal Network. Neurosignals 2005, 14, 34–35. [Google Scholar] [CrossRef]
- Wilson, R.M.; Danishefsky, S.J. Applications of Total Synthesis to Problems in Neurodegeneration: Fascinating Chemistry along the Way. Acc. Chem. Res. 2006, 39, 539–549. [Google Scholar] [CrossRef]
- Metifiot, M.; Johnson, B.; Smith, S.; Zhao, X.Z.; Marchand, C.; Burke, T.; Hughes, S.; Pommier, Y. MK-0536 Inhibits HIV-1 Integrases Resistant to Raltegravir. Antimicrob. Agents Ch. 2011, 55, 127–133. [Google Scholar] [CrossRef]
- Manfroni, G.; Meschini, F.; Barreca, M.L.; Leyssen, P.; Samuele, A.; Iraci, N.; Sabatini, S.; Massari, S.; Maga, G.; Neyts, J.; et al. Pyridobenzothiazole derivatives as new chemotype targeting the HCV NS5B polymerase. Bioorg. Med. Chem. 2012, 20, 866–876. [Google Scholar] [CrossRef]
- Yan, T.Y.; Ding, W.J.; Liu, H.W.; Wang, P.M.; Zheng, D.Q.; Xu, J.Z. New pyridone alkaloids from marine-derived fungus Penicillium sp. Tetrahedron Lett. 2020, 61, 151843–151848. [Google Scholar] [CrossRef]
- Gonçalves, D.S.; Melo, S.M.S.; Jacomini, A.P.; Silva, M.J.V.; Pianoski, K.E.; Ames, F.Q.; Aguiar, R.P.; Oliveira, A.F.; Volpato, H.; Bidóia, D.L.; et al. Synthesis of novel 3,5,6-trisubstituted 2-pyridone derivatives and evaluation for their anti-inflammatory activity. Bioorg. Med. Chem. 2020, 28, 115549–115590. [Google Scholar] [CrossRef]
- Xie, W.L.; Wu, Y.Q.; Zhang, J.G.; Qi, H.M.; Zhang, Y.H.; Zhu, N.; Liu, R.Z.; Zhang, H.L. Design, synthesis and biological evaluations of novel pyridonethiazole hybrid molecules as antitumor agents. Eur. J. Med. Chem. 2018, 145, 35–51. [Google Scholar] [CrossRef]
- Jue, S.G.; Dawson, G.W.; Brogden, R.N. Ciclopirox olamine 1% cream a preliminary review of its antimicrobial activity and therapeutic use. Drugs 1985, 29, 330–341. [Google Scholar] [CrossRef]
- Kim, E.S.; Keating, G.M. Pirfenidone: A review of its use in idiopathic pulmonary fibrosis. Drugs 2015, 75, 219–230. [Google Scholar] [CrossRef]
- Yang, Z.H.; Ren, J.; Yi, L.J.; Zheng, J.H.; Wei, H. Tegafur gimeracil oter combined with oxaliplatin for advanced colorectal cancer. Eur. Rev. Med. Pharmaco. 2015, 19, 3391–3396. [Google Scholar]
- Hamelin, E.I.; Johnson, R.C.; Osterloh, J.D.; Howard, D.J.; Thomas, J.D. Evaluation of Ricinine, a Ricin Biomarker, from a Non-Lethal Castor Bean Ingestion. J. Anal. Toxicol. 2012, 36, 660–662. [Google Scholar] [CrossRef]
- Rana, M.; Dhamija, H.; Prashar, B.; Sharma, S. Ricinus communis L.–A Review. Int. J. Pharm Tech Res. 2012, 4, 1706–1711. [Google Scholar]
- Moshiri, M.; Hamid, F.; Etemad, L. Ricin Toxicity: Clinical and Molecular Aspects. Rep. Biochem. Mol. Biol. 2016, 4, 60–66. [Google Scholar]
- Duong, H.A.; Cross, M.J.; Louie, J. Nickel-Catalyzed Cycloaddition of Alkynes and Isocyanates. J. Am. Chem. Soc. 2004, 126, 11438–11439. [Google Scholar] [CrossRef]
- Tanaka, K.; Wada, A.; Noguchi, K. Rhodium-Catalyzed Chemo-, Regio-, and Enantioselective [2+2+2] Cycloaddition of Alkynes with Isocyanates. Org. Lett. 2005, 7, 4737–4739. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kinpara, K.; Saigoku, T.; Takagishi, H.; Okuda, S.; Nishiyama, H.; Itoh, K. Cp*RuCl-Catalyzed [2+2+2] Cycloadditions of r,ω-Diynes with Electron-Deficient Carbon-Heteroatom Multiple Bonds Leading to Heterocycles. J. Am. Chem. Soc. 2005, 127, 605–613. [Google Scholar] [CrossRef]
- Yamamoto, K.; Takagishi, H.; Itoh, K. Ruthenium(II)-Catalyzed Cycloaddition of 1,6-Diynes with Isocyanates Leading to Bicyclic Pyridones. Org. Lett. 2001, 3, 2117–2119. [Google Scholar] [CrossRef]
- Sengupta, T.; Gayen, K.S.; Pandit, P.; Maiti, D.K. FeCl3.6H2O-Catalyzed Intermolecular-Cascade Cyclization of Acetoacetanilide: Aldehyde-Tuned Synthesis to Valuable 2-Pyridone Analogues. Chem. Eur. J. 2012, 18, 1905–1909. [Google Scholar] [CrossRef]
- Takahashi, T.; Tsai, F.Y.; Li, Y.Z.; Wang, H.; Kondo, Y.; Yamanaka, M.; Nakajima, K.; Kotora, M. Selective Preparation of Pyridines, Pyridones, and Iminopyridines from Two Different Alkynes via Azazirconacycles. J. Am. Chem. Soc. 2002, 124, 5059–5067. [Google Scholar] [CrossRef]
- Imase, H.; Noguchi, K.; Hirano, M.; Tanaka, K. Convergent and Rapid Assembly of Substituted 2-Pyridones through Formation of N-Alkenyl Alkynylamides Followed by Gold-Catalyzed Cycloisomerization. Org. Lett. 2008, 10, 3563–3566. [Google Scholar] [CrossRef]
- Poomathi, N.; Perumal, P.T.; Ramakrishna, S. An efficient and eco-friendly synthesis of 2-pyridones and functionalized azaxanthone frameworks via indium triflate catalyzed domino reaction. Green Chem. 2017, 19, 2524–2529. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhang, H.J.; Nan, F.J. Construction of a 3-Amino-2-pyridone Library by Ring-Closing Metathesis of r-Amino Acrylamide. J. Comb. Chem. 2004, 6, 684–687. [Google Scholar] [CrossRef]
- Carles, L.; Narkunan, K.; Penlou, S.B.; Rousset, L.; Bouchu, D.; Ciufolini, M.A. 2-Pyridones from Cyanoacetamides and Enecarbonyl Compounds: Application to the Synthesis of Nothapodytine B. J. Org. Chem. 2002, 67, 4304–4308. [Google Scholar] [CrossRef]
- Pintiala, C.; Lawson, A.M.; Comesse, S.; Daïch, A. A versatile domino process for the synthesis of substituted 3-aminomethylene-chromanones and 2-pyridones catalyzed by CsF. Tetrahedron Lett. 2013, 54, 2853–2857. [Google Scholar] [CrossRef]
- Chun, Y.S.; Ryu, K.Y.; Ko, Y.O.; Hong, J.Y.; Hong, J.K.; Shin, H.; Lee, S.G. One-Pot Synthesis of 2-Pyridones via Chemo-and Regioselective Tandem Blaise Reaction of Nitriles with Propiolates. J. Org. Chem. 2009, 74, 7556–7558. [Google Scholar] [CrossRef]
- Samzadeh-Kermani, A. Heteropolyacid-Catalyzed One-Pot Synthesis of 2-Pyridone Derivatives. Synlett 2016, 27, 461–464. [Google Scholar] [CrossRef]
- Patel, B.H.; Mason, A.M.; Barrett, A.G.M. Synthesis of 6-Substituted-4-Hydroxy-2-pyridinones via Intramolecular Ketene Trapping of Functionalized Enamine-Dioxinones. Org. Lett. 2011, 13, 5156–5159. [Google Scholar] [CrossRef]
- Hachiya, I.; Ogura, K.; Shimizu, M. Novel 2-Pyridone Synthesis via Nucleophilic Addition of Malonic Esters to Alkynyl Imines. Org. Lett. 2002, 4, 2755–2757. [Google Scholar] [CrossRef]
- Fujii, M.; Nishimura, T.; Koshiba, T.; Yokoshima, S.; Fukuyama, T. 2-Pyridone Synthesis Using 2-(Phenylsulfinyl)acetamide. Org. Lett. 2013, 15, 232–234. [Google Scholar] [CrossRef]
- Kumar, M. A review on phytochemical constituents and pharmacological activities of ricinus communis L. Plant. Int. J. Pharmco. Phytochem. Res. 2017, 4, 466–472. [Google Scholar] [CrossRef]
- Bernini, R.; Fabrizi, G.; Sferrazza, A.; Cacchi, S. Copper-Catalyzed C–C Bond Formation through C–H Functionalization: Synthesis of Multisubstituted Indoles from N-Aryl Enaminones. Angew. Chem. Int. Ed. 2009, 48, 8078–8081. [Google Scholar] [CrossRef]
- Shen, J.; Cheng, G.; Cui, X. “One pot” regiospecific synthesis of polysubstituted pyrroles from benzylamines and ynones under metal free conditions. Chem. Commun. 2013, 49, 10641–10643. [Google Scholar] [CrossRef]
- Cheng, G.; Xue, L.; Weng, Y.; Cui, X. Transition-Metal-Free Cascade Approach toward 2-Alkoxy/2-Sulfenylpyridines and Dihydrofuro[2,3-b]pyridines by Trapping In Situ Generated 1,4-Oxazepine. J. Org. Chem. 2017, 82, 9515–9524. [Google Scholar] [CrossRef]
- Cheng, G.; Weng, Y.; Yang, X.; Cui, X. Base-Promoted N-Pyridylation of Heteroarenes Using N-Propargyl Enaminones as Equivalents of Pyridine Scaffolds. Org. Lett. 2015, 17, 3790–3793. [Google Scholar] [CrossRef]
- Cheng, G.; Zeng, X.; Shen, J.; Wang, X.; Cui, X. A Metal-Free Multicomponent Cascade Reaction for the Regiospecific Synthesis of 1,5-Disubstituted 1,2,3-Triazoles. Angew. Chem. Int. Ed. 2013, 52, 13265–13268. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, X.; Wang, W.; Zhong, X.; Tan, Y.; Wang, Y.; Zhang, J.; Li, Y.; Wang, X. Regitz Diazo Transfer Reaction for the Synthesis of 1,4,5-Trisubstituted 1,2,3-Triazoles and Subsequent Regiospecific Construction of 1,4-Disubstituted 1,2,3-Triazoles via C–C Bond Cleavage. J. Org. Chem. 2021, 86, 4071–4080. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, X.; Wang, W.; Zeng, T.; Wang, Y.; Tan, Y.; Liu, D.; Wang, X.; Li, Y. Base-Promoted Regiospecific Synthesis of Fully Substituted 1,2,3-Triazoles and 1,5-Disubstituted 1,2,3-Triazoles. Asian J. Org. Chem. 2020, 9, 2176–2183. [Google Scholar] [CrossRef]
- Xia, X.F.; He, W.; Wang, D. Metal-Free Oxidative Annulation/Cyclization of 1,6-Enynes for the Synthesis of 4-Carbonylquinolines. Adv. Synth. Catal. 2019, 361, 2959–2964. [Google Scholar] [CrossRef]
- Xia, X.F.; Zhang, L.L.; Song, X.R.; Liu, X.Y.; Liang, Y.M. Copper-Catalyzed Oxidative Cyclization of Enynes for the Synthesis of 4-Carbonyl-quinolines with O2. Org. Lett. 2012, 14, 2480–2483. [Google Scholar] [CrossRef]
- Wu, M.; Jiang, Y.; An, Z.; Qi, Z.; Yan, R. Iron-Catalyzed Synthesis of Substituted Thiazoles from Enamines and Elemental Sulfur through C−S Bond Formation. Adv. Synth. Catal. 2018, 360, 4236–4240. [Google Scholar] [CrossRef]
- Liu, J.; Ba, D.; Lv, W.; Chen, Y.; Zhao, Z.; Cheng, G. Base-Promoted Michael Addition/Smiles Rearrangement/N-Arylation Cascade: One-Step Synthesis of 1,2,3-Trisubstituted 4-Quinolones from Ynones and Sulfonamides. Adv. Synth. Catal. 2020, 362, 213–223. [Google Scholar] [CrossRef]
- Cui, X.; Ma, J.; Zeng, T.; Xu, J.; Li, Y.; Wang, X. Metal-free cascade synthesis of unsymmetrical 2-aminopyrimidines from imidazolate enaminones. RSC Adv. 2021, 11, 24247–24253. [Google Scholar] [CrossRef]
- Ma, J.; Tan, Y.; Tang, Y.; Cui, X.; Xu, J.; Li, Y.; Wang, X. Base-Promoted Cascade C–N and C–C Formation: An Approach to Pyrido[1,2-a] pyrimidinones from Ynones and 2-Methylpyrimidin-4-ols. Asian J. Org. Chem. 2022, 11, e202200451. [Google Scholar] [CrossRef]
- Saliy, I.V.; Gotsko, M.D.; Sobenina, L.N.; Ushakov, I.A.; Trofimov, B.A. Bio-inspired Functionalized Pyrrole-Pyridone Ensembles: Synthesis on the Platform of Acylethynylpyrroles. Synthesis 2020, 52, 2698–2704. [Google Scholar]
- Al Nakib, T.; Meegan, M.J. A novel synthetic route to 2,3,4-trisubstituted 6-phenyl-pyridines. J. Chem. Res. Synop. 1988, 5, 146–147. [Google Scholar]
- Kuthan, J.; Nesvadba, P.; Popl, M.; Fähnrich, J. Some 3-cyano-4, 6-diaryl-2-pyridones with luminiscent properties. Collect. Czech. Chem. Commun. 1979, 44, 2409–2416. [Google Scholar] [CrossRef]
- Sharma, S.; Vasudevan, P.; Madan, M. Insecticidal value of castor (Ricinus communis) against termites. Int. Biodeter. 1990, 27, 249–254. [Google Scholar] [CrossRef]

 | |||
| Entry | Base | Solvent | Yield(%) b |
| 1 | K2CO3 | 1,4-dioxane | 81 |
| 2 | Na2CO3 | 1,4-dioxane | 70 |
| 3 | Cs2CO3 | 1,4-dioxane | 74 |
| 4 | NaOH | 1,4-dioxane | Trace |
| 5 | NaH | 1,4-dioxane | Trace |
| 6 | EtONa | 1,4-dioxane | Trace |
| 7 | LiOtBu | 1,4-dioxane | NR |
| 8 | NaOtBu | 1,4-dioxane | Trace |
| 9 | KOtBu | 1,4-dioxane | Trace |
| 10 | Et3N | 1,4-dioxane | NR |
| 11 | DBU | 1,4-dioxane | NR |
| 12 | DABCO | 1,4-dioxane | NR |
| 13 | K2CO3 | DMSO | 54 |
| 14 | K2CO3 | DMF | 66 |
| 15 | K2CO3 | NMP | 50 |
| 16 | K2CO3 | CH3CN | Trace |
| 17 | K2CO3 | Toluene | Trace |
| 18 | K2CO3 | THF | Trace |
| 19 | K2CO3 | CH3OH | Trace |
| 20 c | K2CO3 | 1,4-dioxane | 45 |
| 21 d | K2CO3 | 1,4-dioxane | 83 |
| 22 e | K2CO3 | 1,4-dioxane | 85 |
 | |||||
| Entry | R1 | R2 | R3 | 3 | Yield(%) b |
| 1 | Ph | Ph | Me | 3aa | 81 |
| 2 | 4-MeC6H4 | Ph | Me | 3ab | 87 |
| 3 | 3-MeC6H4 | Ph | Me | 3ac | 83 |
| 4 | 2-MeC6H4 | Ph | Me | 3ad | 73 |
| 5 | 4-OMeC6H4 | Ph | Me | 3ae | 86 |
| 6 | 2-OMeC6H4 | Ph | Me | 3af | 75 |
| 7 | 4-FC6H4 | Ph | Me | 3ag | 75 |
| 8 | 3-FC6H4 | Ph | Me | 3ah | 73 |
| 9 | 2-FC6H4 | Ph | Me | 3ai | 64 |
| 10 | 4-ClC6H4 | Ph | Me | 3aj | 76 |
| 11 | 3-ClC6H4 | Ph | Me | 3ak | 74 |
| 12 | 2-ClC6H4 | Ph | Me | 3al | 67 |
| 13 | 4-BrC6H4 | Ph | Me | 3am | 75 |
| 14 | 2-BrC6H4 | Ph | Me | 3an | 62 |
| 15 | 4-CF3C6H4 | Ph | Me | 3ao | 65 |
| 16 | 2-Me-4-FC6H3 | Ph | Me | 3ap | 70 |
| 17 | 2,4-ClC6H3 | Ph | Me | 3aq | 55 |
| 18 | 2,3-ClC6H3 | Ph | Me | 3ar | 58 |
| 19 | 2-Cl-4-FC6H3 | Ph | Me | 3as | 56 |
| 20 | 2,4-FC6H3 | Ph | Me | 3at | 57 |
| 21 | 2-naphthyl | Ph | Me | 3au | 90 |
| 22 | cyclopropyl | Ph | Me | 3av | 83 |
| 23 | Me | Ph | Me | 3aw | 76 |
| 24 | 2-thienyl | Ph | Me | 3ax | 84 |
| 25 | Ph | 4-MeC6H4 | Me | 3ba | 88 |
| 26 | Ph | 3-MeC6H4 | Me | 3bb | 84 |
| 27 | Ph | 4-nButylC6H4 | Me | 3bc | 85 |
| 28 | Ph | 4-OMeC6H4 | Me | 3bd | 95 |
| 29 | Ph | 3-OMeC6H4 | Me | 3be | 92 |
| 30 | Ph | 4-FC6H4 | Me | 3bf | 74 |
| 31 | Ph | 4-ClC6H4 | Me | 3bg | 75 |
| 32 | Ph | 2-ClC6H4 | Me | 3bh | 60 |
| 33 | Ph | 4-BrC6H4 | Me | 3bi | 78 |
| 34 | Ph | 4-COOMeC6H4 | Me | 3bj | 70 |
| 35 | Ph | cyclopropyl | Me | 3bk | 87 |
| 36 | Ph | n-hexyl | Me | 3bl | 82 |
| 37 | Ph | 2-thienyl | Me | 3bm | 80 |
| 38 | 4-MeC6H4 | 4-OMeC6H4 | Me | 3ca | 96 |
| 39 | 4-MeC6H4 | 4-nButylC6H4 | Me | 3cb | 90 |
| 40 | 4-OMeC6H4 | 4-OMeC6H4 | Me | 3cc | 98 |
| 41 | 2-FC6H4 | 2-ClC6H4 | Me | 3cd | 64 |
| 42 | 2-BrC6H4 | 4-OMeC6H4 | Me | 3ce | 68 |
| 43 | 4-cynaoC6H4 | 4-OMeC6H4 | Me | 3cf | 70 |
| 44 | 2,4-FC6H3 | 2-ClC6H4 | Me | 3cg | 53 |
| 45 | 2,3-ClC6H3 | 2-ClC6H4 | Me | 3ch | 59 |
| 46 | 2-naphthyl | 4-nButylC6H4 | Me | 3ci | 88 |
| 47 | 2-naphthyl | 2-ClC6H4 | Me | 3cj | 80 |
| 48 | 2-BrC6H4 | cyclopropyl | Me | 3ck | 60 |
| 49 | 3-MeC6H4 | Ph | Ph | 3da | 53 |
| 50 | 3-MeC6H4 | Ph | Bn | 3db | 69 |
| 51 | 3-MeC6H4 | Ph | H | 3dc | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Wu, N.; Xu, J.; Zhang, X.; Li, Y.; Wang, X. Metal-Free Cascade Formation of C–C and C–N Bond for the Construction of 3-Cyano-2-Pyridones with Insecticidal Properties. Molecules 2024, 29, 2792. https://doi.org/10.3390/molecules29122792
Tang Y, Wu N, Xu J, Zhang X, Li Y, Wang X. Metal-Free Cascade Formation of C–C and C–N Bond for the Construction of 3-Cyano-2-Pyridones with Insecticidal Properties. Molecules. 2024; 29(12):2792. https://doi.org/10.3390/molecules29122792
Chicago/Turabian StyleTang, Yao, Nvjiang Wu, Junyu Xu, Xiaopo Zhang, Youbin Li, and Xuesong Wang. 2024. "Metal-Free Cascade Formation of C–C and C–N Bond for the Construction of 3-Cyano-2-Pyridones with Insecticidal Properties" Molecules 29, no. 12: 2792. https://doi.org/10.3390/molecules29122792





