Nanoliposomal Encapsulation of Capparis spinosa Extract and Its Application in Jelly Formulation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Efficiency, Phenolic Content, and Characterization of the Extract
2.2. Particle Size, Particle Size Distribution, and Zeta Potential of Nanoliposomes
2.3. Encapsulation Efficiency (EE) and Loading Capacity (LC)
2.4. The Stability of Polyphenols during Storage
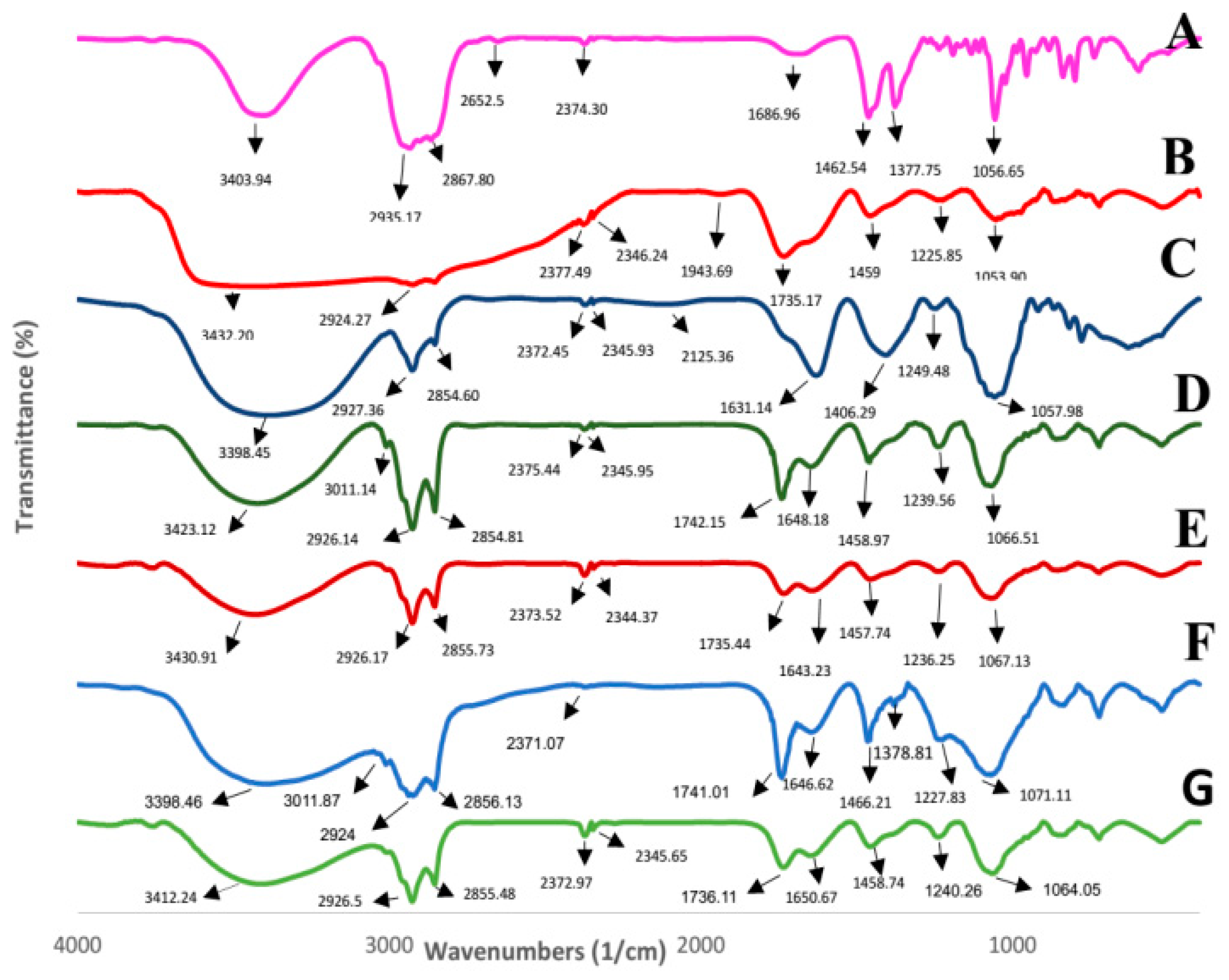
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Field Emission Scanning Electron Microscopy (FE-SEM)
2.7. Physicochemical and Sensory Properties of the Jelly Samples
2.7.1. Moisture Content (MC), pH, and Soluble Solid Content
2.7.2. Color
2.7.3. Texture Profile Analysis (TPA)
2.7.4. Syneresis
2.7.5. Sensorial Evaluation
2.8. Jelly Powders Attributes
2.8.1. Water Solubility Index (WSI)
2.8.2. Hygroscopicity of Jelly Powders
3. Materials and Methods
3.1. Materials
3.2. Extract Preparation
3.3. Phenolic Content Measurement
3.4. Preparation of Nanoliposomes Containing Capparis spinosa Fruit Extract
3.5. Evaluation of Nanoliposomes Containing Capparis spinosa Extract
3.5.1. Particle Size, Particle Size Distribution, and Zeta Potential
3.5.2. Encapsulation Efficiency (EE) of Polyphenols and Loading Capacity (LC) of Nanoliposomes
3.5.3. Stability of Polyphenols during Storage
3.5.4. Fourier Transform Infrared (FTIR) Spectroscopy
3.5.5. Field Emission Scanning Electron Microscopy (FE-SEM)
3.6. Production of Jelly Containing Capparis spinosa Fruit Extract
3.7. Jelly Evaluations
3.7.1. Moisture Content
3.7.2. pH
3.7.3. Soluble Solid Content
3.7.4. Color
3.7.5. Texture Profile Analysis (TPA)
3.7.6. Jelly Syneresis
3.7.7. Sensorial Evaluation
3.8. Powder Production
3.8.1. WSI (Water Solubility Index)
3.8.2. Hygroscopicity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anwar, F.; Muhammad, G.; Hussain, M.A.; Zengin, G.; Alkharfy, K.M.; Ashraf, M.; Gilani, A.H. Capparis spinosa L.: A Plant with High Potential for Development of Functional Foods and Nutraceuticals/Pharmaceuticals. Int. J. Pharmacol. 2016, 12, 201–219. [Google Scholar] [CrossRef]
- Aliyazicioglu, R.; Eyupoglu, O.E.; Sahin, H.; Yildiz, O.; Baltas, N. Phenolic Components, Antioxidant Activity, and Mineral Analysis of Capparis spinosa L. African J. Biotechnol. 2013, 12, 6643–6649. [Google Scholar]
- Rahnavard, R.; Razavi, N. A Review on the Medical Effects of Capparis spinosa L. Adv. Herb. Med. 2017, 3, 44–53. [Google Scholar]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef]
- Tavakoli, H.; Hosseini, O.; Jafari, S.M.; Katouzian, I. Evaluation of Physicochemical and Antioxidant Properties of Yogurt Enriched by Olive Leaf Phenolics within Nanoliposomes. J. Agric. Food Chem. 2018, 66, 9231–9240. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Flanagan, J.; Matia-Merino, L.; Awati, A.; Omri, A.; Suntres, Z.E.; Singh, H. Recent Trends in the Lipid-based Nanoencapsulation of Antioxidants and Their Role in Foods. J. Sci. Food Agric. 2006, 86, 2038–2045. [Google Scholar] [CrossRef]
- Amjadi, S.; Ghorbani, M.; Hamishehkar, H.; Roufegarinejad, L. Improvement in the Stability of Betanin by Liposomal Nanocarriers: Its Application in Gummy Candy as a Food Model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef]
- Babazadeh, A.; Ghanbarzadeh, B.; Hamishehkar, H. Phosphatidylcholine-Rutin Complex as a Potential Nanocarrier for Food Applications. J. Funct. Foods 2017, 33, 134–141. [Google Scholar] [CrossRef]
- Jafari, S.M.; Ghalenoei, M.G.; Dehnad, D. Influence of Spray Drying on Water Solubility Index, Apparent Density, and Anthocyanin Content of Pomegranate Juice Powder. Powder Technol. 2017, 311, 59–65. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Khosravi-Darani, K.; Borazan, G.G.; Cui, J.; Pardakhty, A.; Yurdugul, S. Encapsulation of Food Ingredients Using Nanoliposome Technology. Int. J. Food Prop. 2008, 11, 833–844. [Google Scholar] [CrossRef]
- Thompson, A.K.; Singh, H. Preparation of Liposomes from Milk Fat Globule Membrane Phospholipids Using a Microfluidizer. J. Dairy Sci. 2006, 89, 410–419. [Google Scholar] [CrossRef]
- Rubio-Arraez, S.; Capella, J.V.; Castelló, M.L.; Ortolá, M.D. Physicochemical Characteristics of Citrus Jelly with Non Cariogenic and Functional Sweeteners. J. Food Sci. Technol. 2016, 53, 3642–3650. [Google Scholar] [CrossRef]
- Bhoyar, M.S.; Mishra, G.P.; Naik, P.K.; Singh, S.B. Evaluation of Antioxidant Capacities and Total Polyphenols in Various Edible Parts of Capparis spinosa L. Collected from Trans-Himalayas. Def. Life Sci. J. 2018, 3, 140–145. [Google Scholar] [CrossRef]
- Allaith, A.A.A. Assessment of the Antioxidant Properties of the Caper Fruit (Capparis spinosa L.) from Bahrain. J. Assoc. Arab. Univ. Basic Appl. Sci. 2016, 19, 1–7. [Google Scholar] [CrossRef]
- Ghani, A.; Salehi, S.; Mohtashami, S. Antioxidant Activity, Phenolics Content and Total Flavonoids of Caper Fruit (Caparis Spinosa) at Different Maturity Stages. Res. J. Pharmacogn. 2017, 4, 83. [Google Scholar]
- Tlili, N.; Mejri, H.; Anouer, F.; Saadaoui, E.; Khaldi, A.; Nasri, N. Phenolic profile and antioxidant activity of Capparis spinosa seeds harvested from different wild habitats. Ind. Crop. Prod. 2015, 76, 930–935. [Google Scholar]
- Arrar, L.; Benzidane, N.; Krache, I.; Charef, N.; Khennouf, S.; Baghiani, A. Comparison between Polyphenol Contents and Antioxidant Activities of Different Parts of Capparis spinosa L. Pharmacogn. Commun. 2013, 3, 70. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Plant of the Millennium, Caper (Capparis spinosa L.), chemical composition and medicinal uses. Bull. Natl. Res. Cent. 2021, 45, 131. [Google Scholar] [CrossRef]
- Mohebali, N.; Shahzadeh-Fazeli, S.A.; Ghafoori, H.; Farahmand, Z.; MohammadKhani, E.; Vakhshiteh, F.; Ghamarian, A.; Farhangniya, M.; Sanati, M.H. Effect of flavonoids rich extract of Capparis spinosa on inflammatory involved genes in amyloid-beta peptide injected rat model of Alzheimer’s diseas. Nutr. Neurosci. 2018, 21, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Optimisation and stability assessment of solid lipid nanoparticles using particle size and zeta potential. J. Phys. Sci. 2014, 25, 59. [Google Scholar]
- Gharehbeglou, P.; Jafari, S.M.; Hamishekar, H.; Homayouni, A.; Mirzaei, H. Pectin-Whey Protein Complexes vs. Small Molecule Surfactants for Stabilization of Double Nano-Emulsions as Novel Bioactive Delivery Systems. J. Food Eng. 2019, 245, 139–148. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Hong, C.-T.; Chiu, W.-T.; Wang, Y.-Y. Effect of Liposomes and Niosomes on Skin Permeation of Enoxacin. Int. J. Pharm. 2001, 219, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.R.; Weston, N.; Coombes, A.G.A.; Fitzgerald, M.; Perrie, Y. Liposome Formulation of Poorly Water Soluble Drugs: Optimisation of Drug Loading and ESEM Analysis of Stability. Int. J. Pharm. 2004, 285, 23–34. [Google Scholar] [CrossRef]
- Malheiros, P.D.S.; Sant-Anna, V.; Barbosa, M.D.S.; Brandelli, A.; Franco, B.D.G.D.M. Effect of liposome-encapsulated nisin and bacteriocin-like substance P34 on Listeria monocytogenes growth in Minas frescal cheese. Int. J. Food Microbiol. 2012, 156, 272–277. [Google Scholar] [CrossRef]
- Najaf Najafi, M.; Arianmehr, A.; Mohammadi Sani, A. Encapsulation of Barije (Ferula Gummosa) Essential Oil in Nanoliposomal System and Evaluation of Its Physical and Antimicrobial Properties. Innov. Food Technol. 2019, 7, 71–83. [Google Scholar]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of Solid Lipid Nanoparticles in Brain Targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar] [CrossRef]
- Lu, Q.; Li, D.-C.; Jiang, J.-G. Preparation of a Tea Polyphenol Nanoliposome System and Its Physicochemical Properties. J. Agric. Food Chem. 2011, 59, 13004–13011. [Google Scholar] [CrossRef]
- Liu, D.-Z.; Chen, W.-Y.; Tasi, L.-M.; Yang, S.-P. Microcalorimetric and Shear Studies on the Effects of Cholesterol on the Physical Stability of Lipid Vesicles. Colloids Surf. A Physicochem. Eng. Asp. 2000, 172, 57–67. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of Nanoliposomal Vitamin D3 for Potential Application in Beverage Fortification. Adv. Pharm. Bull. 2014, 4, 569. [Google Scholar] [PubMed]
- Soleimanifard, M.; Sadeghi Mahoonak, A.R.; Ghorbani, M.; Azizi, K.; Sepahvand, A. Optimization Of Khorramabad Olive Leaf Extract Extraction And Investigating The Physical Properties Of Nano-Structured Lipid Carriers Containing It. J. Nutr. Sci. Food Tech. 2018, 13, 81–92. [Google Scholar]
- Fan, M.; Xu, S.; Xia, S.; Zhang, X. Preparation of Salidroside Nano-Liposomes by Ethanol Injection Method and in Vitro Release Study. Eur. Food Res. Technol. 2008, 227, 167–174. [Google Scholar] [CrossRef]
- Alexander, M.; Lopez, A.A.; Fang, Y.; Corredig, M. Incorporation of Phytosterols in Soy Phospholipids Nanoliposomes: Encapsulation Efficiency and Stability. LWT-Food Sci. Technol. 2012, 47, 427–436. [Google Scholar] [CrossRef]
- Brandl, M. Liposomes as drug carriers: A technological approach. Biotechnol. Annu. Rev. 2001, 7, 59–85. [Google Scholar]
- Rafiee, Z.; Barzegar, M.; Sahari, M.A.; Maherani, B. Nanoliposomal Carriers for Improvement the Bioavailability of High–Valued Phenolic Compounds of Pistachio Green Hull Extract. Food Chem. 2017, 220, 115–122. [Google Scholar] [CrossRef]
- Laridi, R.; Kheadr, E.E.; Benech, R.O.; Vuillemard, J.C.; Lacroix, C.; Fliss, I. Liposome encapsulated nisin Z: Optimization, stability and release during milk fermentation. Int. Dairy. J. 2003, 13, 325–336. [Google Scholar] [CrossRef]
- Gupta, U.; Singh, V.K.; Kumar, V.; Khajuria, Y. Spectroscopic Studies of Cholesterol: Fourier Transform Infra-Red and Vibrational Frequency Analysis. Mater. Focus. 2014, 3, 211–217. [Google Scholar] [CrossRef]
- Mohan, V.; Naske, C.D.; Britten, C.N.; Karimi, L.; Walters, K.B. Hydroxide-Catalyzed Cleavage of Selective Ester Bonds in Phosphatidylcholine: An FTIR Study. Vib. Spectrosc. 2020, 109, 103055. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Fu, X.P.; Aisa, H.A.; Abdurahim, M.; Yili, A.; Aripova, S.F.; Tashkhodzhaev, B. Chemical Composition of Capparis spinosa Fruit. Chem. Nat. Compd. 2007, 43, 181–183. [Google Scholar] [CrossRef]
- Hosseini-Nezhad, M.; Mohtashami, M.; Kamali, S.; Elahi, M. Optimizing the Formula of a Low Calorie Fruit Powder Jelly Using Sucralose and Isomalt. Res. Innov. Food Sci. Technol. 2015, 4, 65–74. [Google Scholar]
- Khouryieh, H.A.; Aramouni, F.M.; Herald, T.J. Physical, Chemical and Sensory Properties of Sugar-free Jelly. J. Food Qual. 2005, 28, 179–190. [Google Scholar] [CrossRef]
- Karimi, F.; Hosseini-Ghaboos, S.H. Investigation of Physico-Chemical and Sensory Properties of Jelly Powder Contain Pumpkin Powder. J. Food Sci. Technol. 2018, 15, 319–328. [Google Scholar]
- Mo, E.-K.; Kim, H.-H.; Kim, S.-M.; Jo, H.-H.; Sung, C. Production of Sedum Extract Adding Jelly and Assessment of Its Physicochemical Properties. Korean J. Food Sci. Tech. 2007, 39, 619–624. [Google Scholar]
- Zahedi, Y.; Mazaheri-Tehrani, M. Development of Spreadable Halva Fortified with Soy Flour and Optimization of Formulation Using Mixture Design. J. Food Qual. 2012, 35, 390–400. [Google Scholar] [CrossRef]
- Akhavan-Mahdavi, S.; Jafari, S.M.; Assadpour, E.; Ghorbani, M. Storage Stability of Encapsulated Barberry’s Anthocyanin and Its Application in Jelly Formulation. J. Food Eng. 2016, 181, 59–66. [Google Scholar] [CrossRef]
- Mizrahi, S. Syneresis in Food Gels and Its Implications for Food Quality. In Chemical Deterioration and Physical Instability of Food and Beverages; Elsevier: Amsterdam, The Netherlands, 2010; pp. 324–348. [Google Scholar]
- Kim, Y.M.; Kim, J.-M.; Youn, K.-S. Quality and Textural Properties of Jelly Prepared with Different Gelling Agents. Korean J. Food Preserv. 2020, 27, 566–573. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of Process Conditions on the Physicochemical Properties of Açai (Euterpe Oleraceae Mart.) Powder Produced by Spray Drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar]
- Cai, Y.Z.; Corke, H. Production and Properties of Spray-Dried Amaranthus Betacyanin Pigments. J. Sens. Nutr. Qual. Food. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Wang, J.; Sun, B.; Cao, Y.; Tian, Y.; Li, X. Optimisation of Ultrasound-Assisted Extraction of Phenolic Compounds from Wheat Bran. Food Chem. 2008, 106, 804–810. [Google Scholar] [CrossRef]
- Chi, J.; Ge, J.; Yue, X.; Liang, J.; Sun, Y.; Gao, X.; Yue, P. Preparation of Nanoliposomal Carriers to Improve the Stability of Anthocyanins. LWT-Food Sci. Technol. 2019, 109, 101–107. [Google Scholar] [CrossRef]
- Shaddel, R.; Rajabi-Moghaddam, S. Encapsulation of caffeine in chitosan-coated nanoliposomes and its application in drink formulation. Food Hydrocolloid 2024, 149, 109598. [Google Scholar] [CrossRef]
- ISIRI. Jelly Products–Specifications and Test Methods; ISIRI: Tehran, Iran, 2011. [Google Scholar]
- Periche, A.; Heredia, A.; Escriche, I.; Andrés, A.; Castelló, M.L. Potential Use of Isomaltulose to Produce Healthier Marshmallows. LWT-Food Sci. Technol. 2015, 62, 605–612. [Google Scholar] [CrossRef]
- Akesowan, A. Syneresis and Texture Stability of Hydrogel Complexes Containing Konjac Flour over Multiple Freeze-Thaw Cycles. Life Sci. J. 2012, 9, 1363–1367. [Google Scholar]



| Lecithin: Cholesterol (w:w) | Z-Average (nm) | Polydispersity Index (P.I) | Zeta Potential (mv) |
|---|---|---|---|
| 60:0 | 95.05 ± 5.3 c | 0.517 ± 0.06 a | −60.40 ± 0.4 b |
| 50:10 | 118.5 ± 0.3 b | 0.329 ± 0.02 b | −63.05 ± 2.7 b |
| 40:20 | 130.05 ± 5.6 b | 0.382 ± 0.02 b | −68.55 ± 0.3 a |
| 30:30 | 164.25 ± 14.5 a | 0.364 ± 0.05 b | −68.45 ± 1.2 a |
| Sample ** | Moisture Content (%) | pH | Brix (o) | L* (Lightness) | a* (Redness) | b* (Yellowness) | Hardness (N) | Cohesiveness | Springiness (%) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 7.95 ± 0.1 b | 3.93 ± 0.001 a | 33.25 ± 0.1 a | 44.99 ± 2.1 a | 0.01 ± 0.1 c | 13.68 ± 0.2 ab | 21.47 ± 0.4 a | 0.48 ± 0.08 a | 1.01 ± 0.03 a |
| N3 | 6.68 ± 0.9 b | 3.92 ± 0.005 a | 32.00 ± 0.2 a | 43.04 ± 3.6 a | 0.39 ± 0.2 b | 14.65 ± 1.5 a | 24.59 ± 0.6 a | 0.52 ± 0.06 a | 1.00 ± 0.01 a |
| F3 | 12.65 ± 0.1 a | 3.91 ± 0.005 a | 32.75 ± 0.2 a | 45.62 ± 0.9 a | 0.37 ± 0.0 b | 14.92 ± 0.6 a | 26.90 ± 0.9 a | 0.46 ± 0.14 a | 0.91 ± 0.18 a |
| N7 | 7.12 ± 0.2 b | 3.91 ± 0.010 a | 31.25 ± 0.0 a | 42.57 ± 0.7 a | 0.85 ± 0.1 a | 14.78 ± 0.3 a | 22.30 ± 0.9 a | 0.47 ± 0.08 a | 0.99 ± 0.01 a |
| F7 | 10.95 ± 0.2 a | 3.91 ± 0.002 a | 32.00 ± 0.1 a | 46.11 ± 3.4 a | 0.05 ± 0.1 c | 12.64 ± 0.3 b | 24.91 ± 0.6 a | 0.42 ± 0.13 a | 1.00 ± 0.02 a |
| Sample ** | Color | Flavor | Texture | Overall Acceptability |
|---|---|---|---|---|
| Control | 3.87 ± 0.9 a | 3.87 ± 0.8 a | 3.62 ± 0.5 a | 3.75 ± 1.0 a |
| N3 | 3.75 ± 0.9 a | 2.75 ± 1.0 b | 3.62 ± 0.7 a | 3.75 ± 0.7 a |
| F3 | 4.12 ± 0.6 a | 4.12 ± 0.6 a | 3.87 ± 0.6 a | 4.12 ± 0.6 a |
| N7 | 3.62 ± 1.1 a | 3.37 ± 0.7 ab | 4.25 ± 0.7 a | 3.75 ± 0.5 a |
| F7 | 3.75 ± 1.4 a | 3.50 ± 1.1 ab | 4.37 ± 0.7 a | 3.75 ± 0.9 a |
| Sample ** | Water Solubility Index (%) | Hygroscopicity (g/100 g) |
|---|---|---|
| Control | 41.12 ± 2.6 a | 9.36 ± 3.1 a |
| N3 | 43.74 ± 2.1 a | 5.09 ± 1.6 b |
| F3 | 42.52 ± 1.7 a | 3.41 ± 0.6 b |
| N7 | 42.42 ± 1.7 a | 1.22 ± 0.6 b |
| F7 | 39.59 ± 2.4 a | 1.52 ± 1.4 b |
| Ingredients (% w/w) | Control | N3 | F3 | N7 | F7 |
|---|---|---|---|---|---|
| Gelatin | 15 | 15 | 15 | 15 | 15 |
| Sugar | 75 | 75 | 75 | 75 | 75 |
| Maltodextrin | 7.5 | 4.5 | 4.5 | 1 | 1 |
| Extract of Capparis spinosa | 0 | 3 | 3 | 7 | 7 |
| Fruit essence | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahedi, Y.; Shaddel, R.; Salamatian, M.; Szumny, A. Nanoliposomal Encapsulation of Capparis spinosa Extract and Its Application in Jelly Formulation. Molecules 2024, 29, 2804. https://doi.org/10.3390/molecules29122804
Zahedi Y, Shaddel R, Salamatian M, Szumny A. Nanoliposomal Encapsulation of Capparis spinosa Extract and Its Application in Jelly Formulation. Molecules. 2024; 29(12):2804. https://doi.org/10.3390/molecules29122804
Chicago/Turabian StyleZahedi, Younes, Rezvan Shaddel, Masoumeh Salamatian, and Antoni Szumny. 2024. "Nanoliposomal Encapsulation of Capparis spinosa Extract and Its Application in Jelly Formulation" Molecules 29, no. 12: 2804. https://doi.org/10.3390/molecules29122804







