Antimicrobial Composites Based on Methacrylic Acid–Methyl Methacrylate Electrospun Fibers Stabilized with Copper(II)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preliminary Electrospinning Results
2.2. Fiber Stability
2.3. Optimization
2.4. Conductivity and Viscosity
2.5. Adsorption of Cu(II) Ions
2.6. Fiber and Composite Characterization
2.7. Swelling Degree
2.8. Antimicrobial Activity
3. Experimental
3.1. Materials and Methods
3.2. Preliminary Electrospinning Tests and Fiber Stability
3.3. Stability Tests
3.4. Optimizing the Electrospinning Process
3.5. Adsorption Studies of Cu(II) Ions
3.6. Swelling Degree
3.7. Characterization
3.8. Antimicrobial Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, C.; Wang, X.; Wang, G.; Hao, C.; Li, X.; Li, T. Adsorption Performance of a Polysaccharide Composite Hydrogel Based on Crosslinked Glucan/Chitosan for Heavy Metal Ions. Compos. Part B Eng. 2019, 169, 45–54. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Cagnetta, G.; Wang, W.; Meng, P.; Liu, D.; Yu, G. Regeneration of Chitosan-Based Adsorbents Used in Heavy Metal Adsorption: A Review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Zou, X.; Zhao, Y.; Zhang, Z. Preparation of Hydroxyapatite Nanostructures with Different Morphologies and Adsorption Behavior on Seven Heavy Metals Ions. J. Contam. Hydrol. 2019, 226, 103538. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Du, Y.; Duyu, R.; Shang, Y.; Zhang, S.; Han, R. Adsorption of Copper Ion from Solution by Polyethylenimine Modified Wheat Straw. Bioresour. Technol. Rep. 2019, 6, 96–102. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace Elements in Human Physiology and Pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Negrini, A.; Silva, R.F.; Welter, P.D.; da Rocha Giovenardi, A.; Soriani, H.H.; Da Ros, C.O. Swine Wastewater Compost and Arbuscular Mycorrhizal Fungi in the Growth and Accumulation of Copper in Eucalyptus Grandis. Rhizosphere 2022, 24, 100624. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Ni, S.; Bie, C.; Zhi, H.; Sun, X. A Clean Process for Selective Recovery of Copper from Industrial Wastewater by Extraction-Precipitation with p-Tert-Octyl Phenoxy Acetic Acid. J. Environ. Manag. 2022, 304, 114164. [Google Scholar] [CrossRef]
- Siu, P.C.C.; Koong, L.F.; Saleem, J.; Barford, J.; McKay, G. Equilibrium and Kinetics of Copper Ions Removal from Wastewater by Ion Exchange. Chin. J. Chem. Eng. 2016, 24, 94–100. [Google Scholar] [CrossRef]
- Urbina, L.; Guaresti, O.; Requies, J.; Gabilondo, N.; Eceiza, A.; Corcuera, M.A.; Retegi, A. Design of Reusable Novel Membranes Based on Bacterial Cellulose and Chitosan for the Filtration of Copper in Wastewaters. Carbohydr. Polym. 2018, 193, 362–372. [Google Scholar] [CrossRef]
- Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. Performance Evaluation of Reverse Osmosis Process in the Post-Treatment of Mining Wastewaters: Case Study of Costerfield Mining Operations, Victoria, Australia. J. Water Process Eng. 2020, 34, 101116. [Google Scholar] [CrossRef]
- Shao, N.; Li, S.; Yan, F.; Su, Y.; Liu, F.; Zhang, Z. An All-in-One Strategy for the Adsorption of Heavy Metal Ions and Photodegradation of Organic Pollutants Using Steel Slag-Derived Calcium Silicate Hydrate. J. Hazard. Mater. 2020, 382, 121120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, Y.; Chen, H.; Lu, J.; Yu, G.; Möslang, M.; Zhou, Y. Superior Adsorption Capacity of Functionalised Straw Adsorbent for Dyes and Heavy-Metal Ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef]
- Wang, M.; Bai, Y.; Zhang, B.; Zhong, B.; Yu, Y.; Zhang, J.; Huang, X.; Wen, G. Large Scale Fabrication of Porous Boron Nitride Microrods with Tunable Pore Size for Superior Copper (II) Ion Adsorption. Ceram. Int. 2019, 45, 6684–6692. [Google Scholar] [CrossRef]
- Anbinder, P.S.; Macchi, C.; Amalvy, J.; Somoza, A. A Study of the Structural Changes in a Chitosan Matrix Produced by the Adsorption of Copper and Chromium Ions. Carbohydr. Polym. 2019, 222, 114987. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Visanko, M. Lignin-Rich Sulfated Wood Nanofibers as High-Performing Adsorbents for the Removal of Lead and Copper from Water. J. Hazard. Mater. 2020, 383, 121174. [Google Scholar] [CrossRef] [PubMed]
- Mehrani, Z.; Ebrahimzadeh, H.; Asgharinezhad, A.A.; Moradi, E. Determination of Copper in Food and Water Sources Using Poly M-Phenylenediamine/CNT Electrospun Nanofiber. Microchem. J. 2019, 149, 103975. [Google Scholar] [CrossRef]
- Mingjun, C.; Youchen, Z.; Haoyi, L.; Xiangnan, L.; Yumei, D.; Bubakir, M.M.; Weimin, Y. An Example of Industrialization of Melt Electrospinning: Polymer Melt Differential Electrospinning. Adv. Ind. Eng. Polym. Res. 2019, 2, 110–115. [Google Scholar] [CrossRef]
- Karbowniczek, J.E.; Berniak, K.; Knapczyk-Korczak, J.; Williams, G.; Bryant, J.A.; Nikoi, N.D.; Banzhaf, M.; de Cogan, F.; Stachewicz, U. Strategies of Nanoparticles Integration in Polymer Fibers to Achieve Antibacterial Effect and Enhance Cell Proliferation with Collagen Production in Tissue Engineering Scaffolds. J. Colloid Interface Sci. 2023, 650, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Alyamani, A.A.; Al-Musawi, M.H.; Albukhaty, S.; Sulaiman, G.M.; Ibrahim, K.M.; Ahmed, E.M.; Jabir, M.S.; Al-Karagoly, H.; Aljahmany, A.A.; Mohammed, M.K.A. Electrospun Polycaprolactone/Chitosan Nanofibers Containing Cordia Myxa Fruit Extract as Potential Biocompatible Antibacterial Wound Dressings. Molecules 2023, 28, 2501. [Google Scholar] [CrossRef]
- Hussain, Z.; Ullah, S.; Yan, J.; Wang, Z.; Ullah, I.; Ahmad, Z.; Zhang, Y.; Cao, Y.; Wang, L.; Mansoorianfar, M.; et al. Electrospun Tannin-Rich Nanofibrous Solid-State Membrane for Wastewater Environmental Monitoring and Remediation. Chemosphere 2022, 307, 135810. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K. Electrospun Nanofiber Membranes for Air Filtration: A Review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Satilmis, B.; Uyar, T. Electrospinning of Uniform Nanofibers of Polymers of Intrinsic Microporosity (PIM-1): The Influence of Solution Conductivity and Relative Humidity. Polymer 2019, 178, 121610. [Google Scholar] [CrossRef]
- Thakral, S.; Thakral, N.K.; Majumdar, D.K. Eudragit®: A Technology Evaluation. Expert Opin. Drug Deliv. 2013, 10, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.N.; Priya, R.; Swain, S.; Kumar Jena, G.; Panigrahi, K.C.; Ghose, D. Pharmaceutical Significance of Eudragit: A Review. Future J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Critical Attributes of Nanofibers: Preparation, Drug Loading, and Tissue Regeneration. Int. J. Pharm. 2015, 484, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bao, J.; Zhang, X.; Li, W.; Xie, Y.; Sun, S.; Zhao, W.; Zhao, C. Functionalized Polyethersulfone Nanofibrous Membranes with Ultra-High Adsorption Capacity for Organic Dyes by One-Step Electrospinning. J. Colloid Interface Sci. 2019, 533, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Pavezi, K.J.P.; Rocha, A.; Bonafé, E.G.; Martins, A.F. Electrospinning-Electrospraying of Poly(Acid Lactic) Solutions in Binary Chloroform/Formic Acid and Chloroform/Acetic Acid Mixtures. J. Mol. Liq. 2020, 320, 114448. [Google Scholar] [CrossRef]
- Coban, O.; Aytac, Z.; Yildiz, Z.I.; Uyar, T. Colon Targeted Delivery of Niclosamide from β-Cyclodextrin Inclusion Complex Incorporated Electrospun Eudragit® L100 Nanofibers. Colloids Surf. B Biointerfaces 2021, 197, 111391. [Google Scholar] [CrossRef] [PubMed]
- Giram, P.S.; Shitole, A.; Nande, S.S.; Sharma, N.; Garnaik, B. Fast Dissolving Moxifloxacin Hydrochloride Antibiotic Drug from Electrospun Eudragit L-100 Nonwoven Nanofibrous Mats. Mater. Sci. Eng. C 2018, 92, 526–539. [Google Scholar] [CrossRef]
- Lasprilla-Botero, J.; Álvarez-Láinez, M.; Lagaron, J.M. The Influence of Electrospinning Parameters and Solvent Selection on the Morphology and Diameter of Polyimide Nanofibers. Mater. Today Commun. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Easteal, A.J.; Nikolaidis, M.G.; Quek, S.Y. Influence of Solution and Processing Parameters towards the Fabrication of Electrospun Zein Fibers with Sub-Micron Diameter. J. Food Eng. 2012, 109, 645–651. [Google Scholar] [CrossRef]
- Des Ligneris, E.; Dumée, L.F.; Kong, L. Nanofibers for Heavy Metal Ion Adsorption: Correlating Surface Properties to Adsorption Performance, and Strategies for Ion Selectivity and Recovery. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100297. [Google Scholar] [CrossRef]
- Khalili Amand, F.; Esmaeili, A. Investigating the Properties of Electrospun Nanofibers Made of Hybride Polymer Containing Anticoagulant Drugs. Carbohydr. Polym. 2020, 228, 115397. [Google Scholar] [CrossRef] [PubMed]
- Facchi, D.P.; Facchi, S.P.; Souza, P.R.; Bonafé, E.G.; Popat, K.C.; Kipper, M.J.; Martins, A.F. Composite Filter with Antimicrobial and Anti-Adhesive Properties Based on Electrospun Poly(Butylene Adipate-Co-Terephthalate)/Poly(Acid Lactic)/Tween 20 Fibers Associated with Silver Nanoparticles. J. Membr. Sci. 2022, 650, 120426. [Google Scholar] [CrossRef]
- Facchi, D.P.; Souza, P.R.; Almeida, V.C.; Bonafé, E.G.; Martins, A.F. Optimizing the Ecovio® and Ecovio®/Zein Solution Parameters to Achieve Electrospinnability and Provide Thin Fibers. J. Mol. Liq. 2021, 321, 114476. [Google Scholar] [CrossRef]
- Smallwood, I. Handbook of Organic Solvent Properties; Elsevier Ltd.: Amsterdam, The Netherlands, 1996. [Google Scholar] [CrossRef]
- Costa, R.G.F.; Oliveira, J.E.D.; Paula, G.F.D.; Picciani, P.H.D.S.; Medeiros, E.S.D.; Ribeiro, C.; Mattoso, L.H.C. Eletrofiação de Polímeros Em Solução: Parte I: Fundamentação TeÃ3rica. Polímeros 2012, 22, 170–177. [Google Scholar] [CrossRef]
- Ferreira, E.E.; Brandão, P.R.G.; Klein, B.; Peres, A.E.C. Reologia de Suspensões Minerais: Uma Revisão. Rem Rev. Esc. Minas 2005, 58, 83–87. [Google Scholar] [CrossRef]
- Wang, H.-F.; Jia, H.-Z.; Cheng, S.-X.; Feng, J.; Zhang, X.-Z.; Zhuo, R.-X. PEG-Stabilized Micellar System with Positively Charged Polyester Core for Fast pH-Responsive Drug Release. Pharm. Res. 2012, 29, 1582–1594. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Paulino, A.T.; Santos, L.B.; Nozaki, J. Removal of Pb2+, Cu2+, and Fe3+ from Battery Manufacture Wastewater by Chitosan Produced from Silkworm Chrysalides as a Low-Cost Adsorbent. React. Funct. Polym. 2008, 68, 634–642. [Google Scholar] [CrossRef]
- Sahebjamee, N.; Soltanieh, M.; Mousavi, S.M.; Heydarinasab, A. Preparation and Characterization of Porous Chitosan–Based Membrane with Enhanced Copper Ion Adsorption Performance. React. Funct. Polym. 2020, 154, 104681. [Google Scholar] [CrossRef]
- Bashir, M.; Tyagi, S.; Annachhatre, A.P. Adsorption of Copper from Aqueous Solution onto Agricultural Adsorbents: Kinetics and Isotherm Studies. Mater. Today Proc. 2020, 28, 1833–1840. [Google Scholar] [CrossRef]
- Martins, J.G.; Facchi, D.P.; Berton, S.B.R.; Nunes, C.S.; Matsushita, M.; Bonafé, E.G.; Popat, K.C.; Almeida, V.C.; Kipper, M.J.; Martins, A.F. Removal of Cu(II) from Aqueous Solutions Imparted by a Pectin-Based Film: Cytocompatibility, Antimicrobial, Kinetic, and Equilibrium Studies. Int. J. Biol. Macromol. 2020, 152, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Sen Gupta, S.; Bhattacharyya, K.G. Kinetics of Adsorption of Metal Ions on Inorganic Materials: A Review. Adv. Colloid Interface Sci. 2011, 162, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, J.; Zhang, N.; Chen, L.; Zhong, S.; Wang, Y.; Zhang, W.; Ling, Q. Preparation of MgAl-EDTA-LDH Based Electrospun Nanofiber Membrane and Its Adsorption Properties of Copper(II) from Wastewater. J. Hazard. Mater. 2018, 345, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Li, H.; Yang, C. Chitosan Membrane Adsorber for Low Concentration Copper Ion Removal. Carbohydr. Polym. 2016, 146, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Rufato, K.B.; Almeida, V.C.; Kipper, M.J.; Rubira, A.F.; Martins, A.F.; Muniz, E.C. Polysaccharide-Based Adsorbents Prepared in Ionic Liquid with High Performance for Removing Pb(II) from Aqueous Systems. Carbohydr. Polym. 2019, 215, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Facchi, D.P.; Cazetta, A.L.; Canesin, E.A.; Almeida, V.C.; Bonafé, E.G.; Kipper, M.J.; Martins, A.F. New Magnetic Chitosan/Alginate/Fe3O4@SiO2 Hydrogel Composites Applied for Removal of Pb(II) Ions from Aqueous Systems. Chem. Eng. J. 2018, 337, 595–608. [Google Scholar] [CrossRef]
- Chai, H.; Guo, L.; Wang, X.; Fu, Y.; Guan, J.; Tan, L.; Ren, L.; Yang, K. Antibacterial Effect of 317L Stainless Steel Contained Copper in Prevention of Implant-Related Infection in Vitro and in Vivo. J. Mater. Sci. Mater. Med. 2011, 22, 2525–2535. [Google Scholar] [CrossRef]
- Deus, D.; Krischek, C.; Pfeifer, Y.; Sharifi, A.R.; Fiegen, U.; Reich, F.; Klein, G.; Kehrenberg, C. Comparative Analysis of the Susceptibility to Biocides and Heavy Metals of Extended-Spectrum β-Lactamase-Producing Escherichia Coli Isolates of Human and Avian Origin, Germany. Diagn. Microbiol. Infect. Dis. 2017, 88, 88–92. [Google Scholar] [CrossRef] [PubMed]
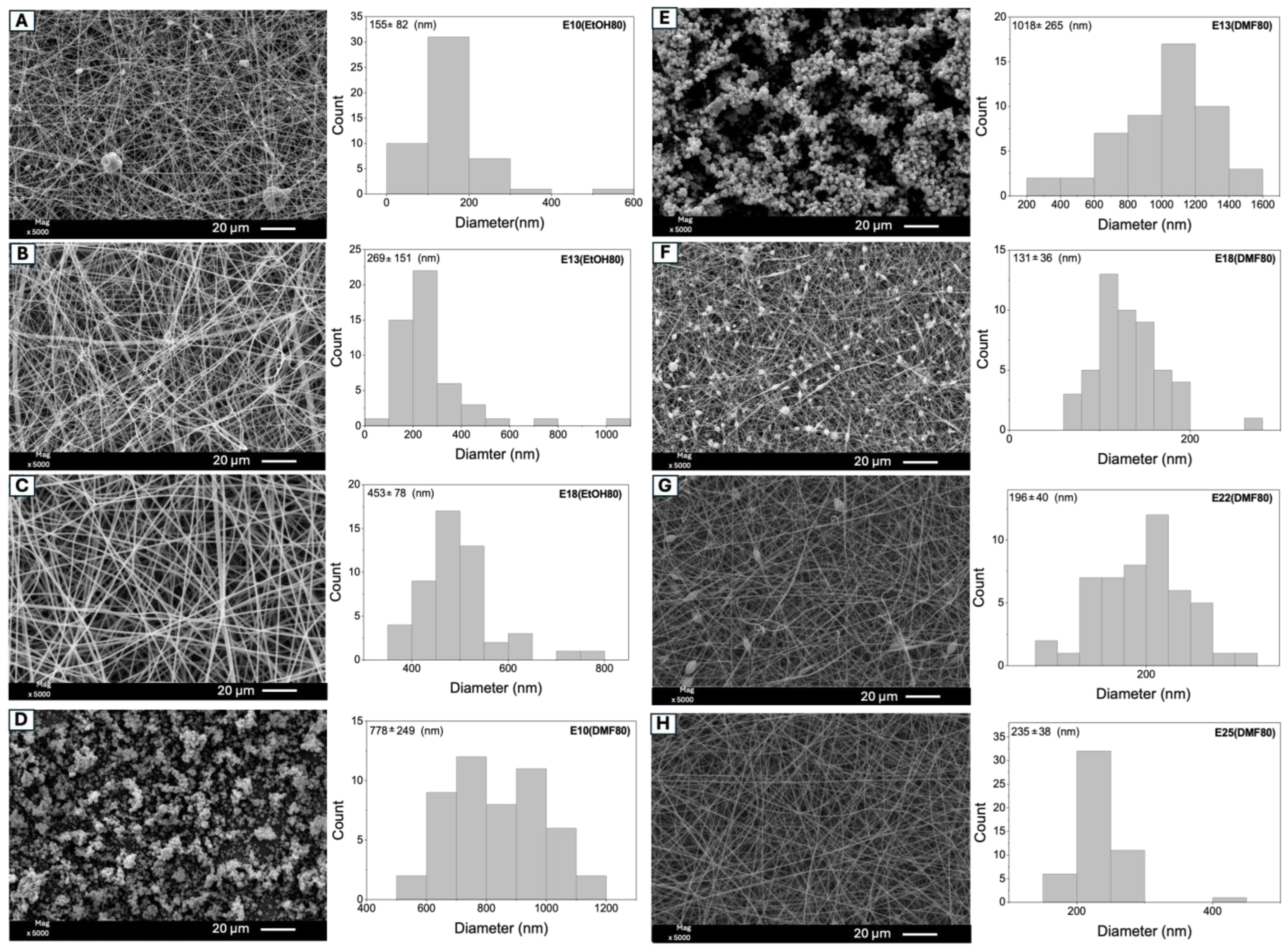
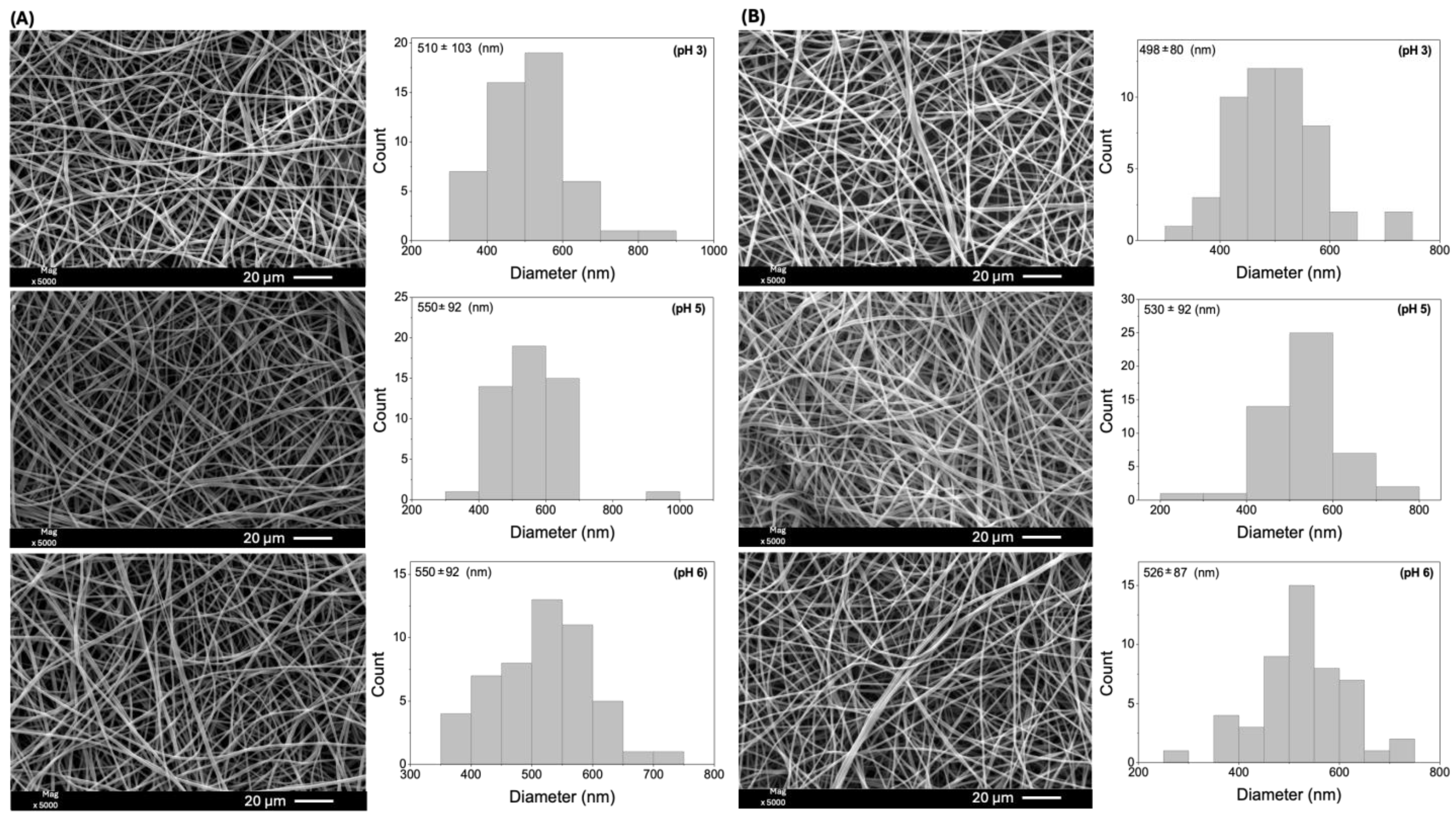


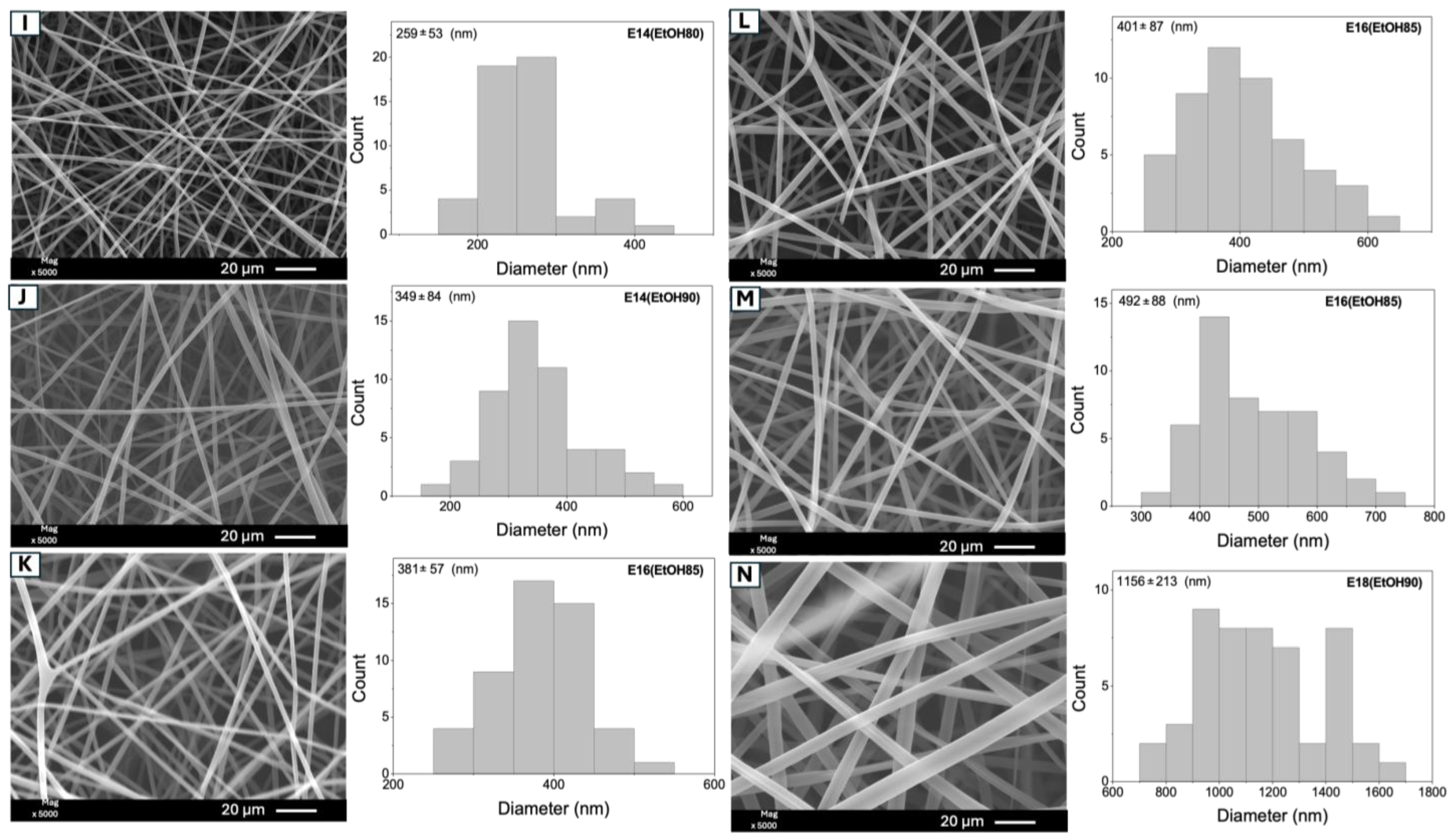



| Samples * | Eudragit L100 (% w/v) | EtOH (mL) | DMF (mL) | Size (nm) ** | |
|---|---|---|---|---|---|
| A | E10(EtOH80) | 10 | 4.0 | 1.0 | 155 ± 82 a |
| B | E13(EtOH80) | 13 | 4.0 | 1.0 | 269 ± 151 b |
| C | E18(EtOH80) | 18 | 4.0 | 1.0 | 453 ± 78 c |
| D | E10(DMF80) | 10 | 1.0 | 4.0 | 778 ± 249 d |
| E | E13(DMF80) | 13 | 1.0 | 4.0 | 1018 ± 265 e |
| F | E18(DMF80) | 18 | 1.0 | 4.0 | 131 ± 37 a |
| G | E22(DMF80) | 22 | 1.0 | 4.0 | 196 ± 40 d |
| H | E25(DMF80) | 25 | 1.0 | 4.0 | 235 ± 38 b |
| Assays | Fibers | X1 (%) * | X2 ** | Size (nm) | |
|---|---|---|---|---|---|
| 1 | I | E14(EtOH80) | (−) (14) | (−) (80/20) | 259 ± 53 a |
| 4 | J | E14(EtOH90) | (−) (14) | (+) (90/10) | 349 ± 84 b |
| 2 | K | E16(EtOH85) | (0) (16) | (0) (85/15) | 381 ± 57 b,c |
| 5 | L | E16(EtOH85) | (0) (16) | (0) (85/15) | 401 ± 87 c |
| 7 | M | E16(EtOH85) | (0) (16) | (0) (85/15) | 492 ± 88 c,d |
| 6 | C | E18(EtOH80) | (+) (18) | (−) (80/20) | 453 ± 78 d |
| 3 | N | E18(EtOH90) | (+) (18) | (+) (90/10) | 1152 ± 213 e |
| Source | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|
| Model | 4.969 × 105 | 1.6 × 105 | 14.06 | 0.0285 |
| X1 (Copolymer concen. (% w/v)) | 2.485 × 105 | 2.485 × 105 | 21.09 | 0.0194 |
| X2 (EtOH/DMF (% v/v)) | 1.556 × 105 | 1.556 × 105 | 13.21 | 0.0359 |
| X1X2 | 92,720.25 | 92,720.25 | 7.87 | 0.0676 |
| Residual | 35,344.11 | 11,781.37 | ||
| Lack of fit | 28,343.44 | 28,343.44 | 8.10 | 0.1045 |
| Pure error | 7000.67 | 3500.33 | ||
| Total | 5.322 × 105 |
| Samples | Conductivity (µS/cm) | Viscosity (mPa·s) | |
|---|---|---|---|
| EtOH | * 1.4 × 10−9 | * 0.82 | |
| DMF | * 6.0 × 10−9 | * 1.08 | |
| EtOH80/DMF20 | 1.36 ** | - | |
| DMF80/EtOH20 | 3.16 ** | - | |
| I | E14(EtOH80) | 47.98 | 52.83 |
| J | E14(EtOH90) | 45.48 | 86.57 |
| K, L, M *** | E16(EtOH85) | 48.08 | 109.45 |
| C | E18(EtOH80) | 53.55 | 137.25 |
| N | E18(EtOH90) | 48.45 | 153.11 |
| Kinetic Parameters | Pseudo-First-Order | Pseudo-Second-Order | Elovich |
|---|---|---|---|
| qe | 29.50 | 43.70 | 0.735 |
| * k/α | k1 = 1264.21 | k2 = 1.62 × 10−4 | = 0.116 |
| R2 | 0.48121 | 0.982 | 0.969 |
| Δqe (%) | 65.72 | 7.94 | 9.54 |
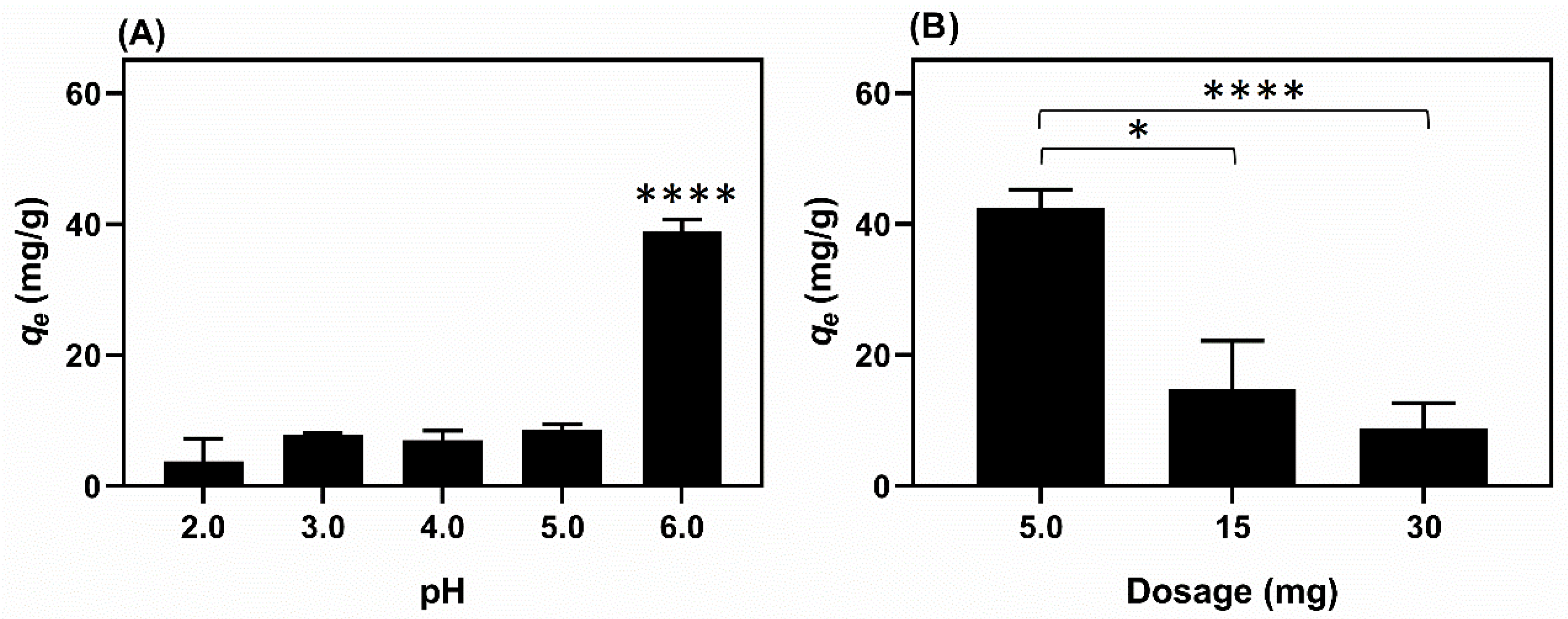
| Columns in Figure 9 | E14(EtOH80/Cu) | CuSO4 | E14(EtOH80) | |||
|---|---|---|---|---|---|---|
| Fiber Disks | Fibers (mg/mL) | Cu(II) in the Fibers (mg) | Cu(II) Conc. in the Fiber Disks (mg/mL) | (mg/mL) | (mg/mL) | |
| 1st * | 6 | 28.5 | 0.3990 | 9.97 × 10−4 | 40 | 28.5 |
| 2nd | 6 | 28.5 | 0.3990 | 9.97 × 10−4 | 40 | 28.5 |
| 3rd | 5 | 23.75 | 0.3325 | 8.31 × 10−4 | 20 | 23.75 |
| 4th | 4 | 19.00 | 0.2660 | 6.65 × 10−4 | 10 | 19.00 |
| 5th | 3 | 14.25 | 0.1995 | 4.98 × 10−4 | 5 | 14.25 |
| 6th | 2 | 9.50 | 0.1330 | 3.32 × 10−4 | 2.5 | 9.50 |
| 7th | 1 | 4.75 | 0.0665 | 1.66 × 10−4 | 1.25 | 4.75 |
| 8th * | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, A.B.; Facchi, S.P.; Bezerra, F.M.; Lis, M.J.; Monteiro, J.P.; Bonafé, E.G.; Rubira, A.F.; Martins, A.F. Antimicrobial Composites Based on Methacrylic Acid–Methyl Methacrylate Electrospun Fibers Stabilized with Copper(II). Molecules 2024, 29, 2835. https://doi.org/10.3390/molecules29122835
da Silva AB, Facchi SP, Bezerra FM, Lis MJ, Monteiro JP, Bonafé EG, Rubira AF, Martins AF. Antimicrobial Composites Based on Methacrylic Acid–Methyl Methacrylate Electrospun Fibers Stabilized with Copper(II). Molecules. 2024; 29(12):2835. https://doi.org/10.3390/molecules29122835
Chicago/Turabian Styleda Silva, Ana B., Suelen P. Facchi, Fabricio M. Bezerra, Manuel J. Lis, Johny P. Monteiro, Elton. G. Bonafé, Adley F. Rubira, and Alessandro F. Martins. 2024. "Antimicrobial Composites Based on Methacrylic Acid–Methyl Methacrylate Electrospun Fibers Stabilized with Copper(II)" Molecules 29, no. 12: 2835. https://doi.org/10.3390/molecules29122835
APA Styleda Silva, A. B., Facchi, S. P., Bezerra, F. M., Lis, M. J., Monteiro, J. P., Bonafé, E. G., Rubira, A. F., & Martins, A. F. (2024). Antimicrobial Composites Based on Methacrylic Acid–Methyl Methacrylate Electrospun Fibers Stabilized with Copper(II). Molecules, 29(12), 2835. https://doi.org/10.3390/molecules29122835











