75As Nuclear Magnetic Resonance Spectroscopic Investigation of the Thioarsenate Speciation in Strongly Alkaline Sulfidic Leaching Solutions
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stauder, S.; Raue, B.; Sacher, F. Thioarsenates in sulfidic waters. Environ. Sci. Technol. 2005, 39, 5933–5939. [Google Scholar] [CrossRef] [PubMed]
- Herath, I.; Vithanage, M.; Seneweera, S.; Bundschuh, J. Thiolated arsenic in natural systems: What is current, what is new and what needs to be known. Environ. Int. 2018, 115, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Stock, T.; Lohs, K. The Challenge of Old Chemical Munitions and Toxic Armament Wastes; Stockholm International Peace Research Institute, Oxford University Press: New York, NY, USA, 1997; 337p, ISBN 0-19-829190-6. [Google Scholar]
- Pitten, F.A.; Müller, G.; König, P.; Schmidt, D.; Thurow, K.; Kramer, A. Risk assessment of a former military base contaminated with organoarsenic-based warfare agents: Uptake of arsenic by terrestrial plants. Sci. Total Environ. 1999, 226, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Dilda, P.J.; Hogg, P.J. Arsenical-based cancer drugs. Cancer Treat. Rev. 2007, 33, 542–564. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.H.; Schipper, H.M.; Lee, J.S.; Singer, J.; Waxman, S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002, 62, 3893–3903. [Google Scholar] [PubMed]
- Ammann, A.A. Arsenic Speciation Analysis by Ion Chromatography—A Critical Review of Principles and Applications. Am. J. Anal. Chem. 2011, 02, 27–45. [Google Scholar] [CrossRef]
- Schwedt, G.; Rieckhoff, M. Separation of thio- and oxothioarsenates by capillary zone electrophoresis and ion chromatography. J. Chromatogr. A 1996, 736, 341–350. [Google Scholar] [CrossRef]
- Tossell, J.A. Calculation of the visible-UV absorption spectra of hydrogen sulfide, bisulfide, polysulfides, and As and Sb sulfides, in aqueous solution. Geochem. Trans. 2003, 4, 28. [Google Scholar] [CrossRef]
- Wood, S.A.; Tait, C.D.; Janecky, D.R. A Raman spectroscopic study of arsenite and thioarsenite species in aqueous solution at 25 C. Geochem. Trans. 2002, 3, 31. [Google Scholar] [CrossRef]
- Leermakers, M.; Baeyens, W.; de Gieter, M.; Smedts, B.; Meert, C.; de Bisschop, H.C.; Morabito, R.; Quevauviller, P. Toxic arsenic compounds in environmental samples: Speciation and validation. TrAC Trends Anal. Chem. 2006, 25, 1–10. [Google Scholar] [CrossRef]
- Radke, B.; Jewell, L.; Namieśnik, J. Analysis of Arsenic Species in Environmental Samples. Crit. Rev. Anal. Chem. 2012, 42, 162–183. [Google Scholar] [CrossRef]
- Gupta, T.; Agarwal, A.K.; Agarwal, R.A.; Labhsetwar, N.K. (Eds.) Environmental Contaminants; Springer: Singapore, 2018; ISBN 978-981-10-7331-1. [Google Scholar]
- Delnomdedieu, M.; Basti, M.M.; Otvos, J.D.; Thomas, D.J. Reduction and binding of arsenate and dimethylarsinate by glutathione: A magnetic resonance study. Chem. Biol. Interact. 1994, 90, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, J.; Brendler, E.; Wagler, J.; Schmidt, A.-C. Kinetics and activation parameters of the reaction of organoarsenic(V) compounds with glutathione. J. Hazard. Mater. 2014, 280, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, J.; Brendler, E.; Wagler, J. Phenylarsonic acid-DMPS redox reaction and conjugation investigated by NMR spectroscopy and X-ray diffraction. Environ. Toxicol. Pharmacol. 2022, 92, 103837. [Google Scholar] [CrossRef]
- Nakayama, T.; Isobe, T.; Nakamiya, K.; Edmonds, J.S.; Shibata, Y.; Morita, M. Complexes of diphenylarsinic acid and phenylarsonic acid with thiols: A 1H and 13C NMR study. Magn. Reson. Chem. 2005, 43, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.K.; Becker, E.D.; Cabral de Menezes, S.M.; Goodfellow, R.; Granger, P. NMR nomenclature. Nuclear spin properties and conventions for chemical shifts (IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1795–1818. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Nöth, H.; Schütt, T.; Warchhold, M. Tetraphenylphosphonium Hexaazidoarsenate(V): The First Structurally Characterized Binary As(V)-Azide Species. Angew. Chem. Int. Ed. 2000, 39, 2108–2109. [Google Scholar] [CrossRef]
- Pettinari, C.; Marchetti, F.; Rafaiani, G. NMR Spectroscopy, Heteronuclei, As, Sb, Bi. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Lindon, J.C., Tranter, G.E., Koppendaal, D.W., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 313–317. [Google Scholar] [CrossRef]
- Balimann, G.; Pregosin, P. Arsenic-75 nuclear magnetic resonance. A study of some arsenic salts. J. Magn. Reson. 1977, 26, 283–289. [Google Scholar] [CrossRef]
- Collins, M.J.; Schrobilgen, G.J. Study of the OTeF5 donor properties of Te(OTeF5)4 by 75As and 125Te NMR spectroscopy. Preparation and characterization of the [TeFx(OTeF5)3-x]+ cations, TeFx(OTeF5)4-x, As(OTeF5)5 and [As(OTeF5)6]-. Inorg. Chem. 1985, 24, 2608–2614. [Google Scholar] [CrossRef]
- McGarvey, G.; Moffat, J. An arsenic-75 nuclear magnetic resonance study of heteropoly oxometalates in solution. J. Magn. Reson. 1990, 88, 305–310. [Google Scholar] [CrossRef]
- Bowers, G.M.; Kirkpatrick, R.J. High-field 75As NMR study of arsenic oxysalts. J. Magn. Reson. 2007, 188, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, C.F.; Saraiva, M.E.; Dias, B.A. Arsenic-75 nuclear magnetic resonance: Study of the interaction of arsenate with various molecules of biological interest. J. Inorg. Biochem. 1992, 46, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Lehmann-Horn, J.A.; Miljak, D.G.; Bastow, T.J. 75As, 63Cu NMR and NQR characterization of selected arsenic minerals. Solid State Nucl. Magn. Reson. 2013, 54, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Kinouchi, H.; Mukuda, H.; Kitaoka, Y.; Shirage, P.M.; Fujihisa, H.; Gotoh, Y.; Eisaki, H.; Iyo, A. Emergent phases of nodeless and nodal superconductivity separated by antiferromagnetic order in iron-based superconductor (Ca4Al2O6)Fe2(As1−xPx)2: 75As- and 31P-NMR studies. Phys. Rev. B 2013, 87, 121101(R). [Google Scholar] [CrossRef]
- Goswami, M.; Knijn, P.J.; Bauhuis, G.J.; Janssen, J.W.G.; van Bentum, P.J.M.; de Wijs, G.A.; Kentgens, A.P.M. Stripline 75As NMR Study of Epitaxial III–V Semiconductor Al0.5Ga0.5As. J. Phys. Chem. C 2014, 118, 13394–13405. [Google Scholar] [CrossRef]
- Salvail, J.Z.; Dluhy, P.; Morse, K.J.; Szech, M.; Saeedi, K.; Huber, J.; Riemann, H.; Abromisov, N.V.; Becker, P.; Pohl, H.-J. Optically enabled magnetic resonance study of As75 and Sb121 in Si28. Phys. Rev. B 2015, 92, 195203. [Google Scholar] [CrossRef]
- Apperley, D.C.; Harris, R.K.; Hodgkinson, P. Solid-State NMR: Basic Principles & Practice; Momentum Press LLC: New York, NY, USA, 2012; ISBN 978-1606503508. [Google Scholar]
- Haga, K.; Batnasan, A.; Shibayama, A. Development of Arsenic and/or Antimony Removal Process from Tennantite/Tetrahedrite via Alkaline Leaching and Precipitation Process. J. MMIJ 2015, 131, 27–32. [Google Scholar] [CrossRef]
- Tongamp, W.; Takasaki, Y.; Shibayama, A. Arsenic removal from copper ores and concentrates through alkaline leaching in NaHS media. Hydrometallurgy 2009, 98, 213–218. [Google Scholar] [CrossRef]
- Tongamp, W.; Takasaki, Y.; Shibayama, A. Precipitation of arsenic as Na3AsS4 from Cu3AsS4–NaHS–NaOH leach solutions. Hydrometallurgy 2010, 105, 42–46. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, H.; Abashina, T.; Vainshtein, M. Review on arsenic removal from sulfide minerals: An emphasis on enargite and arsenopyrite. Miner. Eng. 2021, 172, 107133. [Google Scholar] [CrossRef]
- Meiner, K.; Weigelt, A.; Charitos, A.; Stelter, M.; Wrobel, M.; Hammerschmidt, J. Alkaline sulfide leaching and partial roasting for treatment of As-rich concentrates. In World of Metallurgy—Erzmetall; GDMB Verlag: Clausthal-Zellerfeld, Germany, 2022; Volume 75, pp. 100–108. ISSN 1613-2394. [Google Scholar]
- Meiner, K.; Khulan, B.; Weigelt, A.; Thiere, A.; Vogt, D.; Stelter, M.; Kassahun, A.; Meima, J.; Charitos, A. Investigations on the selective arsenic reduction from copper concentrates by alkaline sulfide leaching and arsenic precipitation. In Proceedings of the 22nd International Copper Conference Copper 2022, Santiago de, Chile, Chile, 13–17 November 2022; p. 336. [Google Scholar]
- Holleman, A.F.; Wiberg, E. Lehrbuch der Anorganischen Chemie, 101st ed.; De Gruyter: Berlin, Germany; New York, NY, USA, 1995; ISBN 3-11-012641-9. [Google Scholar]
- Herbig, M.; Wagler, J. Qualitative Anorganische Analyse; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-662-57849-0. [Google Scholar]
- Stauder, S. Schwefelhaltige Arsenspezies in Grundwässern- Strukturaufklärung, Analytik und Sanierungsstrategien. Ph.D. Dissertation, TU Dresden, Dresden, Germany, 2007. [Google Scholar]
- Gow, R.N.; Young, C.; Huang, H.; Hope, G.; Takasaki, Y. Spectroelectrochemistry of enargite I: Reactivity in alkaline solutions. Min. Metall. Explor. 2015, 32, 6–13. [Google Scholar] [CrossRef]
- Helz, G.R.; Tossell, J.A. Thermodynamic model for arsenic speciation in sulfidic waters: A novel use of ab initio computations. Geochim. Cosmochim. Acta 2008, 72, 4457–4468. [Google Scholar] [CrossRef]
- Li, W. Synthesis and Solubility of Arsenic Tri-sulfide and Sodium Arsenic Oxy-sulfide Complexes in Alkaline Sulfide Solutions. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2013. [Google Scholar]
- Brookins, D.G. Arsenic. In Eh-pH Diagrams for Geochemistry; Springer: Berlin/Heidelberg, Germany, 1988; pp. 28–29. [Google Scholar] [CrossRef]
- Thilo, E.; Hertzog, K.; Winkler, A. Über Vorgänge bei der Bildung des Arsen(V)-sulfids beim Ansäuern von Tetrathioarsenatlösungen. Z. Anorg. Allg. Chem. 1970, 373, 111–121. [Google Scholar] [CrossRef]
- Akitt, J.W.; Kettle, D. 71Ga nuclear magnetic resonance investigation of aqueous gallium(III) and its hydrolysis. Magn. Reson. Chem. 1989, 27, 377–379. [Google Scholar] [CrossRef]
- Ocken, E.; Thomas, B.; Görz, H.; Schönherr, S. Vergleichende Untersuchungen zur Protolyse von Aluminium-, Gallium- und Indiumsalzlösungen. Z. Phys. Chem. 1990, 271O, 647–652. [Google Scholar] [CrossRef]
- Akitt, J.W. An Introduction to Modern NMR Spectroscopy, 3rd ed.; Chapman & Hall: New York, NY, USA, 1992; p. 87. ISBN 0-412-37260-6. [Google Scholar]
- Claridge, T.D.W. Introducing High-Resolution NMR. In High-Resolution NMR Techniques in Organic Chemistry, 3rd ed.; Claridge, T.D.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 2; p. 33. [Google Scholar] [CrossRef]
- Williams, E.A. NMR Spectroscopy of Organosilicon Compounds. In Organic Silicon Compounds; Patai, S., Rappoport, R., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1989; Volume 1, Chapter 8; pp. 511–554. [Google Scholar] [CrossRef]
- Berger, S.; Braun, S.; Kalinowski, H.-O. NMR-Spektroskopie von Nichtmetallen, Band 3-31P-NMR-Spektroskopie; Georg Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 1993; pp. 57–58. ISBN 9783137692010. [Google Scholar]
- Brauer, G.; Baudler, M. Handbuch der Präparativen Anorganischen Chemie, 3rd ed.; Brauer, G., Ed.; Ferdinand Enke Verlag: Stuttgart, Germany, 1975; Volume 1, ISBN 3-432-02328-6. [Google Scholar]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Durand, J.-O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In U.S. Geological Survey Techniques and Methods; Book 6; United States Geological Survey: Reston, VA, USA, 2013; Chapter A43; p. 497. [Google Scholar]

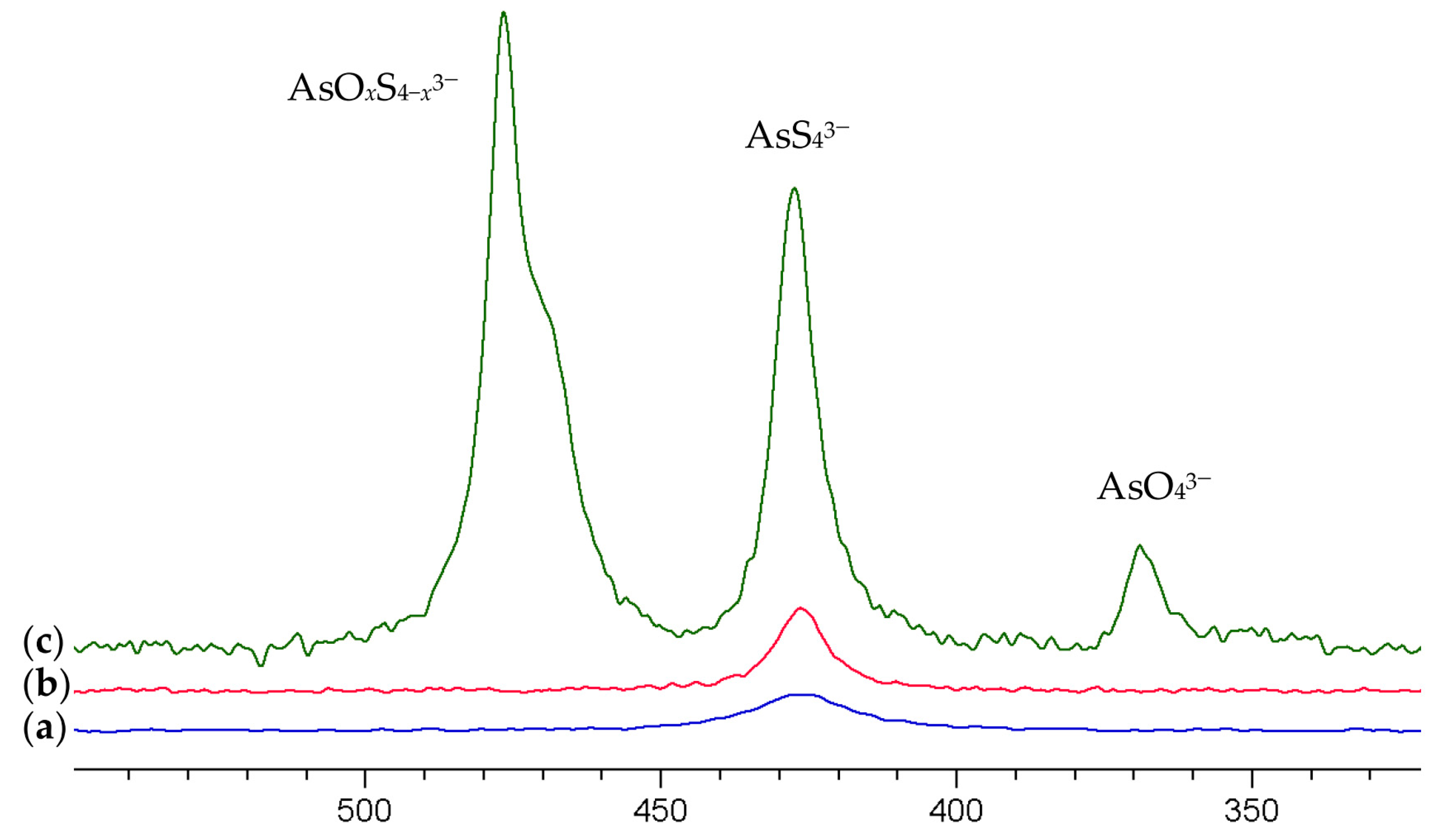
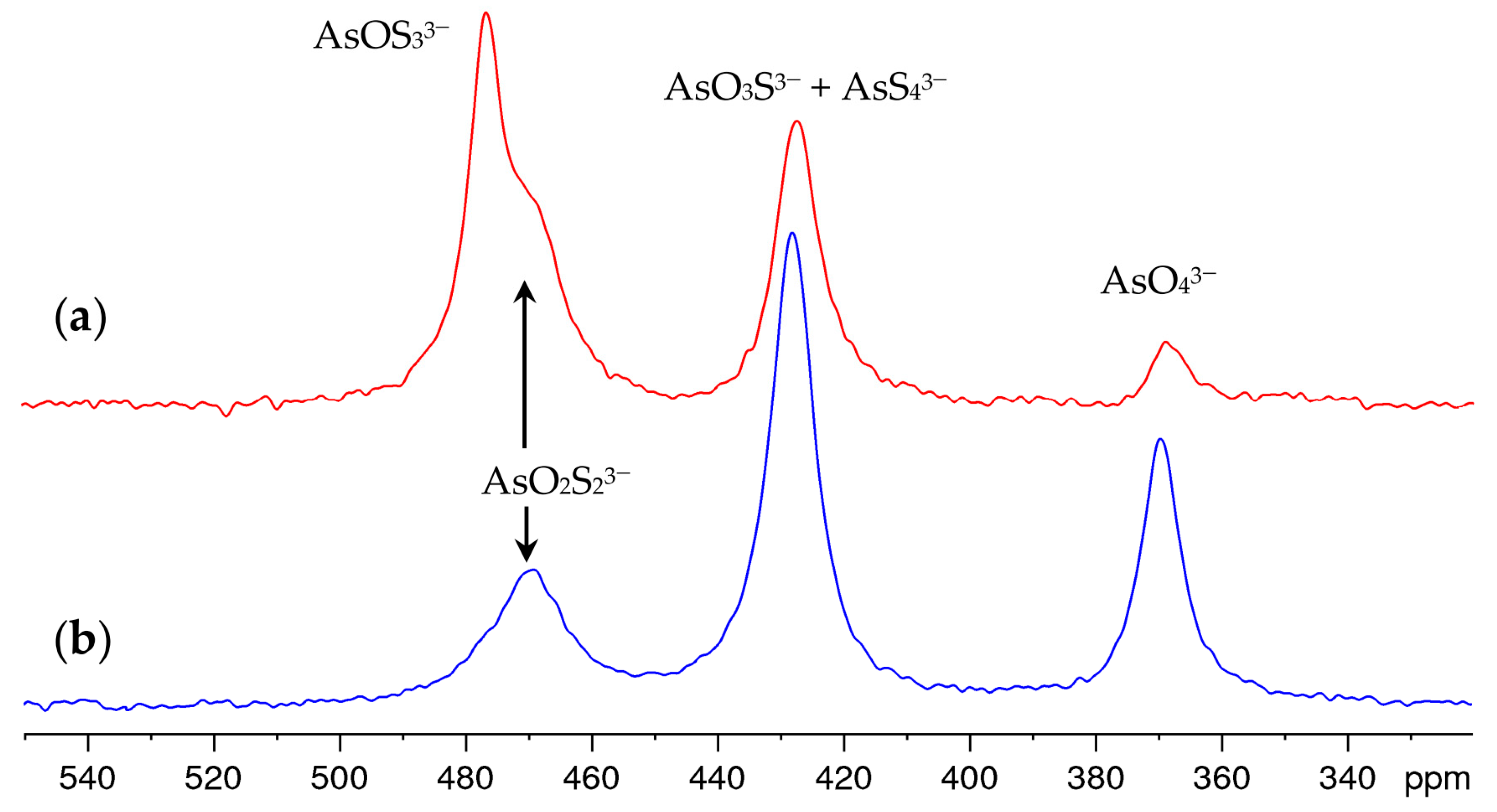



| Arsenate Anion | δ (75As) in ppm |
|---|---|
| AsO43− | 369 1 |
| AsO3S3− | 430 |
| AsO2S23− | 469 |
| AsOS33− | 476 |
| AsS43− | 427 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brendler, E.; Meiner, K.; Wagler, J.; Thiere, A.; Charitos, A.; Stelter, M. 75As Nuclear Magnetic Resonance Spectroscopic Investigation of the Thioarsenate Speciation in Strongly Alkaline Sulfidic Leaching Solutions. Molecules 2024, 29, 2848. https://doi.org/10.3390/molecules29122848
Brendler E, Meiner K, Wagler J, Thiere A, Charitos A, Stelter M. 75As Nuclear Magnetic Resonance Spectroscopic Investigation of the Thioarsenate Speciation in Strongly Alkaline Sulfidic Leaching Solutions. Molecules. 2024; 29(12):2848. https://doi.org/10.3390/molecules29122848
Chicago/Turabian StyleBrendler, Erica, Karsten Meiner, Jörg Wagler, Alexandra Thiere, Alexandros Charitos, and Michael Stelter. 2024. "75As Nuclear Magnetic Resonance Spectroscopic Investigation of the Thioarsenate Speciation in Strongly Alkaline Sulfidic Leaching Solutions" Molecules 29, no. 12: 2848. https://doi.org/10.3390/molecules29122848







