An Unexpected Synthesis of 2-Sulfonylquinolines via Deoxygenative C2-Sulfonylation of Quinoline N-Oxides with Sulfonyl Chlorides
Abstract
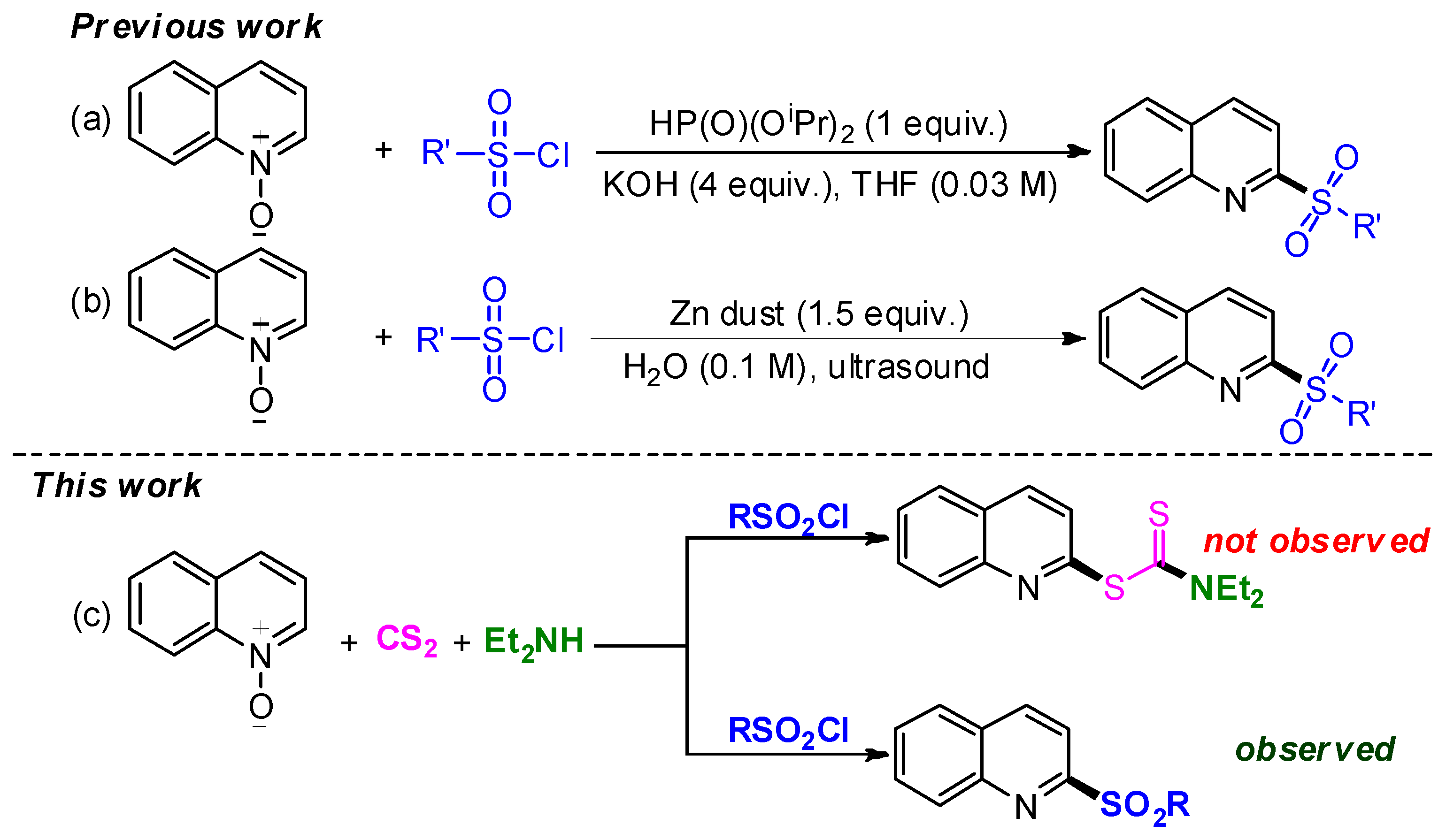
1. Introduction
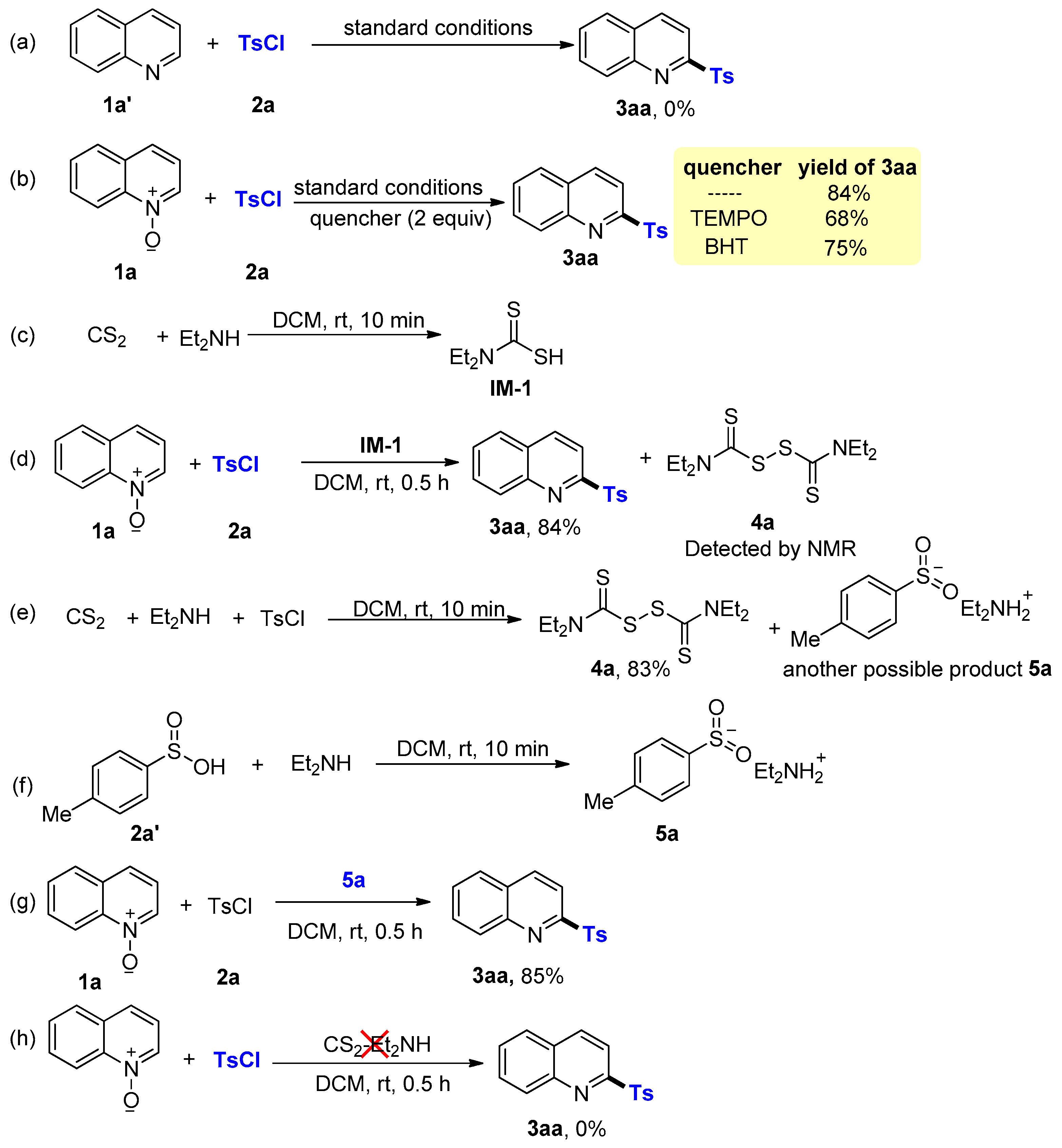
2. Results and Discussion
3. Experimental Section
3.1. General Information
3.2. General Procedure for the Preparation of 2-Sulfonylquinolines 3
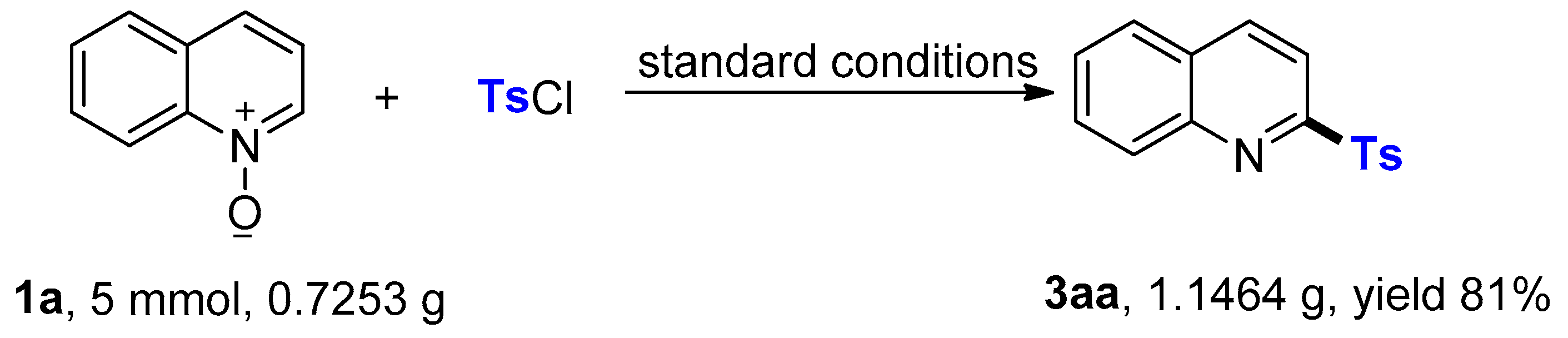
3.3. Gram-Scale Synthesis of 3aa
3.4. Characterization Data of Products 3aa–3ta, 3xa and 3ab–3ap
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andries, K.; Verhasselt, P.; Guillemont, J.; Göhlmann, H.W.H.; Neefs, J.-M.; Winkler, H.; Van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A Diarylquinoline Drug Active on the ATP Synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Galambos, J.; Domány, G.; Nógrádi, K.; Wágner, G.; Keserű, G.M.; Bobok, A.; Kolok, S.; Mikó-Bakk, M.L.; Vastag, M.; Sághy, K.; et al. 4-Aryl-3-arylsulfonyl-quinolines as negative allosteric modulators of metabotropic GluR5 receptors: From HTS hit to development candidate. Bioorg. Med. Chem. Lett. 2016, 26, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Hayat, F.; Moseley, E.; Salahuddin, A.; Van Zyl, R.L.; Azam, A. Antiprotozoal activity of chloroquinoline based chalcones. Eur. J. Med. Chem. 2011, 46, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2008, 25, 166–187. [Google Scholar] [CrossRef] [PubMed]
- Strekowski, L.; Say, M.; Henary, M.; Ruiz, P.; Manzel, L.; Macfarlane, D.E.; Bojarski, A.J. Synthesis and Activity of Substituted 2-Phenylquinolin-4-amines, Antagonists of Immunostimulatory CpG-Oligodeoxynucleotides. J. Med. Chem. 2003, 46, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, P.; Marciniec, K.; Maślankiewicz, A.; Paluchowska, M.H.; Satała, G.; Partyka, A.; Jastrzębska-Więsek, M.; Wróbel, D.; Wesołowska, A.; Duszyńska, B.; et al. Arene- and quinoline-sulfonamides as novel 5-HT7 receptor ligands. Biorg. Med. Chem. 2011, 19, 6750–6759. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Yawer, M.A.; Lalk, M.; Lindequist, U.; Villinger, A.; Fischer, C.; Langer, P. Hetero-Diels–Alder reaction of 1,3-bis(trimethylsilyloxy)-1,3-butadienes with arylsulfonylcyanides. Synthesis and antimicrobial activity of 4-hydroxy-2-(arylsulfonyl)pyridines. Biorg. Med. Chem. 2008, 16, 9898–9903. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Chang, C.-Y.; Su, C.-J.; Huang, H.-L.; Mehndiratta, S.; Chao, Y.-H.; Hsu, C.-M.; Kumar, S.; Sung, T.-Y.; Huang, Y.-Z.; et al. 2-(Phenylsulfonyl)quinoline N-hydroxyacrylamides as potent anticancer agents inhibiting histone deacetylase. Eur. J. Med. Chem. 2016, 122, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Chang, J.-Y.; Nien, C.-Y.; Kuo, C.-C.; Shih, K.-H.; Wu, C.-H.; Chang, C.-Y.; Lai, W.-Y.; Liou, J.-P. 5-Amino-2-aroylquinolines as Highly Potent Tubulin Polymerization Inhibitors. Part 2. The Impact of Bridging Groups at Position C-2. J. Med. Chem. 2011, 54, 8517–8525. [Google Scholar] [CrossRef]
- Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Parisi, L.M.; Bernini, R. Unsymmetrical Diaryl Sulfones and Aryl Vinyl Sulfones through Palladium-Catalyzed Coupling of Aryl and Vinyl Halides or Triflates with Sulfinic Acid Salts. J. Org. Chem. 2004, 69, 5608–5614. [Google Scholar] [CrossRef]
- Li, X.-Y.; Sun, Y.-M.; Yuan, J.-W. Metal-free catalyzed arylsulfonylation of chloroquinoline with sodium arylsulfinates under microwave irradiation. Z. Naturforschung B 2018, 73, 295–303. [Google Scholar] [CrossRef]
- Liu, N.-W.; Hofman, K.; Herbert, A.; Manolikakes, G. Visible-Light Photoredox/Nickel Dual Catalysis for the Cross-Coupling of Sulfinic Acid Salts with Aryl Iodides. Org. Lett. 2018, 20, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-W.; Wang, J.-Q.; Ma, H.; Zhu, Q.; Xie, L.-Y. Ball-milling synthesis of sulfonyl quinolines via coupling of haloquinolines with sulfonic acids. Green Chem. 2021, 23, 7589–7593. [Google Scholar] [CrossRef]
- Maloney, K.M.; Kuethe, J.T.; Linn, K. A Practical, One-Pot Synthesis of Sulfonylated Pyridines. Org. Lett. 2011, 13, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.D.; Nguyen, V.T.; Haug, G.C.; Dang, H.T.; Arman, H.D.; Ermler, W.C.; Larionov, O.V. Rapid and Chemodivergent Synthesis of N-Heterocyclic Sulfones and Sulfides: Mechanistic and Computational Details of the Persulfate-Initiated Catalysis. ACS Catal. 2019, 9, 4015–4024. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Peng, S.; Tan, J.-X.; Sun, R.-X.; Yu, X.; Dai, N.-N.; Tang, Z.-L.; Xu, X.; He, W.-M. Waste-Minimized Protocol for the Synthesis of Sulfonylated N-Heteroaromatics in Water. ACS Sustain. Chem. Eng. 2018, 6, 16976–16981. [Google Scholar] [CrossRef]
- Ai, C.; Liao, X.; Zhou, Y.; Yan, Z.; Lin, S. SO2F2-mediated deoxygenative C2-sulfonylation of quinoline N-oxides with sodium sulfinates. Tetrahedron Lett. 2020, 61, 152586. [Google Scholar] [CrossRef]
- Dong, D.-Q.; Li, L.-X.; Li, G.-H.; Deng, Q.; Wang, Z.-L.; Long, S. Visible-light-induced deoxygenative C2-sulfonylation of quinoline N-oxides with sulfinic acids for the synthesis of 2-sulfonylquinoline via radical reactions. Chin. J. Catal. 2019, 40, 1494–1498. [Google Scholar] [CrossRef]
- Du, B.; Qian, P.; Wang, Y.; Mei, H.; Han, J.; Pan, Y. Cu-Catalyzed Deoxygenative C2-Sulfonylation Reaction of Quinoline N-Oxides with Sodium Sulfinate. Org. Lett. 2016, 18, 4144–4147. [Google Scholar] [CrossRef]
- Jiang, M.; Yuan, Y.; Wang, T.; Xiong, Y.; Li, J.; Guo, H.; Lei, A. Exogenous-oxidant- and catalyst-free electrochemical deoxygenative C2 sulfonylation of quinoline N-oxides. Chem. Commun. 2019, 55, 13852–13855. [Google Scholar] [CrossRef]
- Li, G.-H.; Dong, D.-Q.; Deng, Q.; Yan, S.-Q.; Wang, Z.-L. Copper-Catalyzed Deoxygenative C2-Sulfonylation of Quinoline N-Oxides with DABSO and Phenyldiazonium Tetrafluoroborates for the Synthesis of 2-Sulfonylquinolines via a Radical Reaction. Synthesis 2019, 51, 3313–3319. [Google Scholar] [CrossRef]
- Sumunnee, L.; Buathongjan, C.; Pimpasri, C.; Yotphan, S. Iodine/TBHP-Promoted One-Pot Deoxygenation and Direct 2-Sulfonylation of Quinoline N-Oxides with Sodium Sulfinates: Facile and Regioselective Synthesis of 2-Sulfonylquinolines. Eur. J. Org. Chem. 2017, 2017, 1025–1032. [Google Scholar] [CrossRef]
- Wang, R.; Zeng, Z.; Chen, C.; Yi, N.; Jiang, J.; Cao, Z.; Deng, W.; Xiang, J. Fast regioselective sulfonylation of pyridine/quinoline N-oxides induced by iodine. Org. Biomol. Chem. 2016, 14, 5317–5321. [Google Scholar] [CrossRef] [PubMed]
- You, G.; Xi, D.; Sun, J.; Hao, L.; Xia, C. Transition-metal- and oxidant-free three-component reaction of quinoline N-oxides, sodium metabisulfite and aryldiazonium tetrafluoroborates via a dual radical coupling process. Org. Biomol. Chem. 2019, 17, 9479–9488. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-Y.; Fang, T.-G.; Tan, J.-X.; Zhang, B.; Cao, Z.; Yang, L.-H.; He, W.-M. Visible-light-induced deoxygenative C2-sulfonylation of quinoline N-oxides with sulfinic acids. Green Chem. 2019, 21, 3858–3863. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Li, Y.-J.; Qu, J.; Duan, Y.; Hu, J.; Liu, K.-J.; Cao, Z.; He, W.-M. A base-free, ultrasound accelerated one-pot synthesis of 2-sulfonylquinolines in water. Green Chem. 2017, 19, 5642–5646. [Google Scholar] [CrossRef]
- Sun, K.; Chen, X.-L.; Li, X.; Qu, L.-B.; Bi, W.-Z.; Chen, X.; Ma, H.-L.; Zhang, S.-T.; Han, B.-W.; Zhao, Y.-F.; et al. H-phosphonate-mediated sulfonylation of heteroaromatic N-oxides: A mild and metal-free one-pot synthesis of 2-sulfonyl quinolines/pyridines. Chem. Commun. 2015, 51, 12111–12114. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Song, Y.-X.; He, J.-Y.; Tang, S.-S.; Tan, J.-X.; Cao, Z.; Lin, Y.-W.; He, W.-M. TsCl-promoted sulfonylation of quinoline N-oxides with sodium sulfinates in water. Chin. Chem. Lett. 2019, 30, 2287–2290. [Google Scholar] [CrossRef]
- Malykhin, R.S.; Sukhorukov, A.Y. Nucleophilic Halogenation of Heterocyclic N-Oxides: Recent Progress and a Practical Guide. Adv. Synth. Catal. 2021, 363, 3170–3188. [Google Scholar] [CrossRef]
- Qiao, K.; Wan, L.; Sun, X.; Zhang, K.; Zhu, N.; Li, X.; Guo, K. Regioselective Chlorination of Quinoline N-Oxides and Isoquinoline N-Oxides Using PPh3/Cl3CCN. Eur. J. Org. Chem. 2016, 2016, 1606–1611. [Google Scholar] [CrossRef]
- Wang, D.; Jia, H.; Wang, W.; Wang, Z. A practical and mild chlorination of fused heterocyclic N-oxides. Tetrahedron Lett. 2014, 55, 7130–7132. [Google Scholar] [CrossRef]
- Bi, W.-Z.; Sun, K.; Qu, C.; Chen, X.-L.; Qu, L.-B.; Zhu, S.-H.; Li, X.; Wu, H.-T.; Duan, L.-K.; Zhao, Y.-F. A direct metal-free C2–H functionalization of quinoline N-oxides: A highly selective amination and alkylation strategy towards 2-substituted quinolines. Org. Chem. Front. 2017, 4, 1595–1600. [Google Scholar] [CrossRef]
- Zhang, Z.; Pi, C.; Tong, H.; Cui, X.; Wu, Y. Iodine-Catalyzed Direct C–H Alkenylation of Azaheterocycle N-Oxides with Alkenes. Org. Lett. 2017, 19, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cui, X.; Chen, L.; Jiang, G.; Wu, Y. Palladium-Catalyzed Alkenylation of Quinoline-N-oxides via C−H Activation under External-Oxidant-Free Conditions. J. Am. Chem. Soc. 2009, 131, 13888–13889. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Liu, Y.; Zhao, P.; Gou, S.; Wang, J. Synthesis of 2-Alkenylquinoline by Reductive Olefination of Quinoline N-Oxide under Metal-Free Conditions. Org. Lett. 2016, 18, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Londregan, A.T.; Jennings, S.; Wei, L. Mild Addition of Nucleophiles to Pyridine-N-Oxides. Org. Lett. 2011, 13, 1840–1843. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.-Z.; Qu, C.; Chen, X.-L.; Qu, L.-B.; Liu, Z.-D.; Sun, K.; Li, X.; Zhao, Y.-F. A Direct C2-Selective Phenoxylation and Alkoxylation of Quinoline N-Oxides with Various Phenols and Alcohols in the Presence of H-Phosphonate. Eur. J. Org. Chem. 2017, 2017, 5125–5130. [Google Scholar] [CrossRef]
- Lian, Y.; Coffey, S.B.; Li, Q.; Londregan, A.T. Preparation of Heteroaryl Ethers from Azine N-Oxides and Alcohols. Org. Lett. 2016, 18, 1362–1365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Qiao, K.; Hua, J.; Liu, Z.; Qi, H.; Yang, Z.; Zhu, N.; Fang, Z.; Guo, K. Preparation of fluoroalkoxy or fluorophenoxy substituted N-heterocycles from heterocyclic N-oxides and polyfluoroalcohols. Org. Chem. Front. 2018, 5, 2340–2344. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Qu, Z.; Ke, D.; Qu, L.; Duan, L.; Mai, W.; Yuan, J.; Chen, J.; Zhao, Y. H-Phosphonate-Mediated Amination of Quinoline N-Oxides with Tertiary Amines: A Mild and Metal-Free Synthesis of 2-Dialkylaminoquinolines. Adv. Synth. Catal. 2014, 356, 1979–1985. [Google Scholar] [CrossRef]
- Londregan, A.T.; Jennings, S.; Wei, L. General and Mild Preparation of 2-Aminopyridines. Org. Lett. 2010, 12, 5254–5257. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xiang, B.; Huffman, M.A.; Raab, C.E.; Davies, I.W. A General and Efficient 2-Amination of Pyridines and Quinolines. J. Org. Chem. 2007, 72, 4554–4557. [Google Scholar] [CrossRef] [PubMed]
- Bu, C.; Wang, K.; Gong, C.; Wang, D. Green and fast 2-aryloxylation/amination of quinolines. Green Chem. 2024, 26, 4659–4664. [Google Scholar] [CrossRef]
- Hu, C.; Liu, R.; Ning, Z.; Mou, D.; Fu, Y.; Du, Z. TsCl-promoted thiolation of quinoline N-oxides with thiophenols. Org. Biomol. Chem. 2022, 20, 8280–8284. [Google Scholar] [CrossRef]
- Sarmah, B.K.; Konwar, M.; Bhattacharyya, D.; Adhikari, P.; Das, A. Regioselective Cyanation of Six-Membered N-Heteroaromatic Compounds Under Metal-, Activator-, Base- and Solvent-Free Conditions. Adv. Synth. Catal. 2019, 361, 5616–5625. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Huang, X.; Fang, X.; Li, Z.; Jiang, H.; Qiao, J.; Chu, W.; Sun, Z. Hypervalent Iodine(III)-Mediated Regioselective Cyanation of Quinoline N-Oxides with Trimethylsilyl Cyanide. Adv. Synth. Catal. 2019, 361, 520–525. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Peng, S.; Lu, L.-H.; Hu, J.; Bao, W.-H.; Zeng, F.; Tang, Z.; Xu, X.; He, W.-M. Brønsted Acidic Ionic Liquid-Promoted Amidation of Quinoline N-Oxides with Nitriles. ACS Sustain. Chem. Eng. 2018, 6, 7989–7994. [Google Scholar] [CrossRef]
- Wu, J.; Xia, Z. Gold-Catalyzed Redox-Neutral Reaction of Nitriles with Quinoline N-Oxides. Adv. Synth. Catal. 2023, 365, 3335–3341. [Google Scholar] [CrossRef]
- Chen, X.; Cui, X.; Yang, F.; Wu, Y. Base-Promoted Cross-Dehydrogenative Coupling of Quinoline N-Oxides with 1,3-Azoles. Org. Lett. 2015, 17, 1445–1448. [Google Scholar] [CrossRef]
- Tsunokawa, R.; Karuo, Y.; Tarui, A.; Sato, K.; Hosoya, T.; Agou, T.; Kawai, K.; Omote, M. Metal-Free Arylation of Pyridine/Quinoline N-Oxides with Indoles. Eur. J. Org. Chem. 2023, 26, e202300885. [Google Scholar] [CrossRef]
- Singha, K.; Habib, I.; Hossain, M. Quinoline N-Oxide: A Versatile Precursor in Organic Transformations. ChemistrySelect 2022, 7, e202203537. [Google Scholar] [CrossRef]
- Kutasevich, A.V.; Perevalov, V.P.; Mityanov, V.S. Recent Progress in Non-Catalytic C–H Functionalization of Heterocyclic N-Oxides. Eur. J. Org. Chem. 2021, 2021, 357–373. [Google Scholar] [CrossRef]
- Singh, J.; Patel, R.I.; Sharma, A. Visible-Light-Mediated C-2 Functionalization and Deoxygenative Strategies in Heterocyclic N-Oxides. Adv. Synth. Catal. 2022, 364, 2289–2306. [Google Scholar] [CrossRef]
- Wang, D.; Désaubry, L.; Li, G.; Huang, M.; Zheng, S. Recent Advances in the Synthesis of C2-Functionalized Pyridines and Quinolines Using N-Oxide Chemistry. Adv. Synth. Catal. 2021, 363, 2–39. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Tian, H.; Tian, Z.-Y.; Xu, X.-Q.; Peng, S.; Xie, L.-Y. TsCl promoted deoxygenative phosphorothiolation of quinoline N-oxides towards S-quinolyl phosphorothioates. Org. Biomol. Chem. 2024, 22, 2409–2413. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-Y.; Liu, C.; Wang, S.-Y.; Tian, Z.-Y.; Peng, S. Ts2O mediated deoxygenative C2-dithiocarbamation of quinoline N-oxides with CS2 and amines. RSC Adv. 2024, 14, 14465–14469. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-Y.; Peng, S.; Liu, F.; Chen, G.-R.; Xia, W.; Yu, X.; Li, W.-F.; Cao, Z.; He, W.-M. Metal-free deoxygenative sulfonylation of quinoline N-oxides with sodium sulfinates via a dual radical coupling process. Org. Chem. Front. 2018, 5, 2604–2609. [Google Scholar] [CrossRef]
- Zhao, M.-Y.; Tang, J.-J.; Lin, Y.-J.; Tian, Z.-Y.; Peng, S.; Xie, L.-Y. Ts2O Promoted Deoxygenative C–H Dithiocarbonation of Quinoline N-Oxides with Potassium O-Alkyl Xanthates. J. Org. Chem. 2024, 89, 5560–5572. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.G.; McLeary, J.B.; Sanderson, R.D. Facile preparation of bis(thiocarbonyl)disulfides via elimination. Tetrahedron Lett. 2006, 47, 4771–4774. [Google Scholar] [CrossRef]
- Chen, G.; Xu, B. Divergent Synthesis of Sulfonyl Quinolines, Formyl Indoles, and Quinolones from Ethynyl Benzoxazinanones via AuI Catalysis, AuI-ArI Co-Catalysis, and Silver Catalysis. ACS Catal. 2022, 12, 7134–7141. [Google Scholar] [CrossRef]



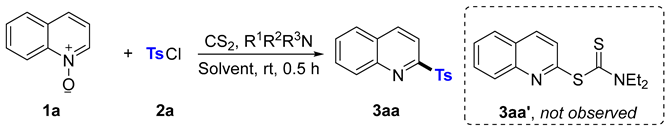 | ||||
|---|---|---|---|---|
| Entry | R1R2R3N | Solvent | Ratios of 2a to 1a | Yield of 3aa % |
| 1 | Et2NH | THF | 2 | 55 |
| 2 | Et2NH | DCM | 2 | 67 |
| 3 | Et2NH | DCE | 2 | 63 |
| 4 | Et2NH | EtOAc | 2 | 42 |
| 5 | Et2NH | Acetone | 2 | 0 |
| 6 | Et2NH | CH3CN | 2 | 47 |
| 7 | Et2NH | DMF | 2 | 24 |
| 8 | Me2NH | DCM | 2 | 43 |
| 9 | IPr2NH | DCM | 2 | 7 |
| 10 | morpholine | DCM | 2 | 12 |
| 11 | piperidine | DCM | 2 | 8 |
| 12 | Et2NH | DCM | 2 | 0 |
| 13 | Et2NH | DCM | 2.5 | 67 |
| 14 | Et2NH | DCM | 3 | 66 |
| 15 | Et2NH | DCM | 1.5 | 53 |
| 16 b | Et2NH | DCM | 2 | 79 |
| 17 c | Et2NH | DCM | 2 | 84 |
| 18 d | Et2NH | DCM | 2 | 82 |
| 19 e | Et2NH | DCM | 2 | 0 |
| 20 | none | DCM | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Tian, Z.-Y.; Lin, Y.-J.; Xie, L.-Y. An Unexpected Synthesis of 2-Sulfonylquinolines via Deoxygenative C2-Sulfonylation of Quinoline N-Oxides with Sulfonyl Chlorides. Molecules 2024, 29, 2863. https://doi.org/10.3390/molecules29122863
Yang W, Tian Z-Y, Lin Y-J, Xie L-Y. An Unexpected Synthesis of 2-Sulfonylquinolines via Deoxygenative C2-Sulfonylation of Quinoline N-Oxides with Sulfonyl Chlorides. Molecules. 2024; 29(12):2863. https://doi.org/10.3390/molecules29122863
Chicago/Turabian StyleYang, Wei, Zhong-Ying Tian, Ying-Jun Lin, and Long-Yong Xie. 2024. "An Unexpected Synthesis of 2-Sulfonylquinolines via Deoxygenative C2-Sulfonylation of Quinoline N-Oxides with Sulfonyl Chlorides" Molecules 29, no. 12: 2863. https://doi.org/10.3390/molecules29122863
APA StyleYang, W., Tian, Z.-Y., Lin, Y.-J., & Xie, L.-Y. (2024). An Unexpected Synthesis of 2-Sulfonylquinolines via Deoxygenative C2-Sulfonylation of Quinoline N-Oxides with Sulfonyl Chlorides. Molecules, 29(12), 2863. https://doi.org/10.3390/molecules29122863







