Asymmetrically Wettable, PET/PA6, Hollow, Segmented-Pie, Microfiber Nonwovens for a Synthetic Leather Base
Abstract
1. Introduction
2. Results and Discussion
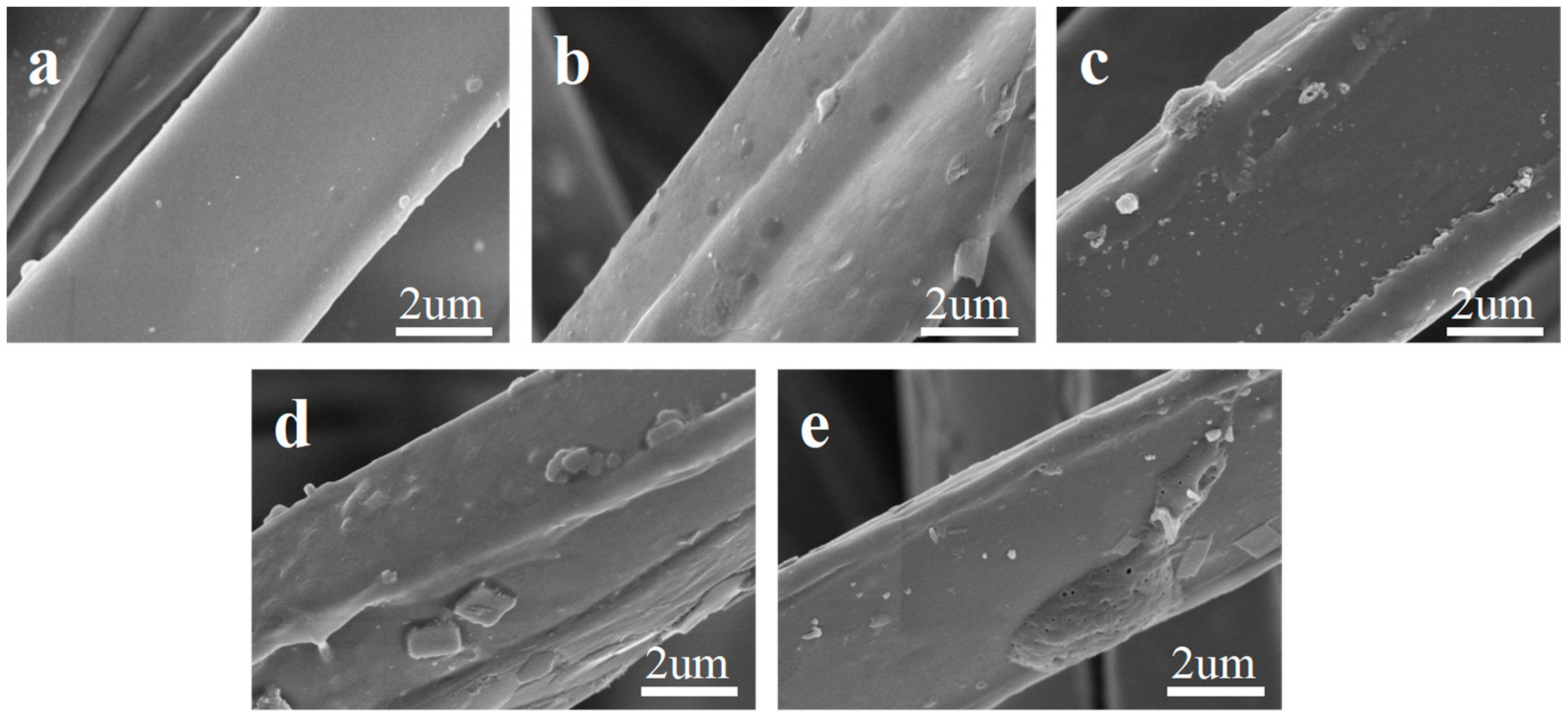
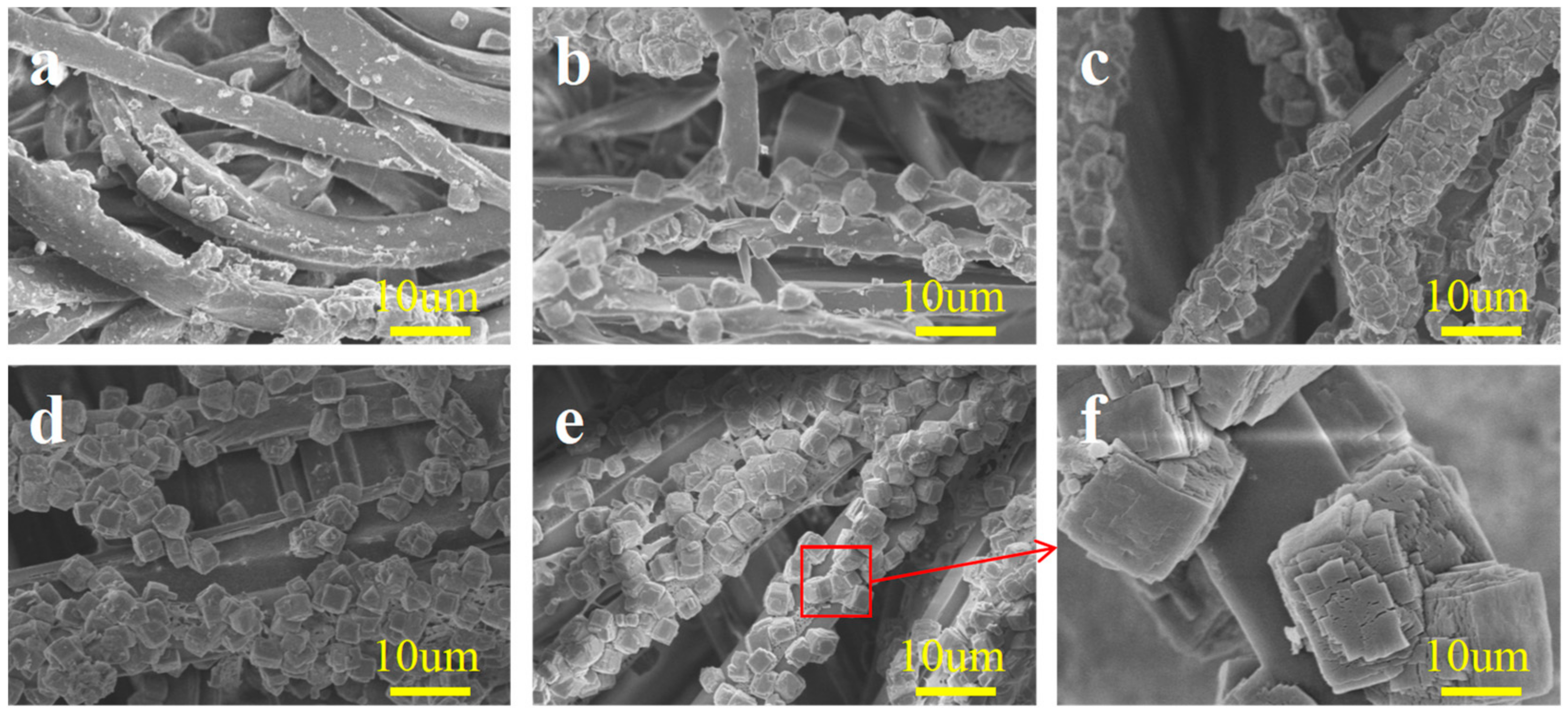
2.1. Micromorphology Analysis
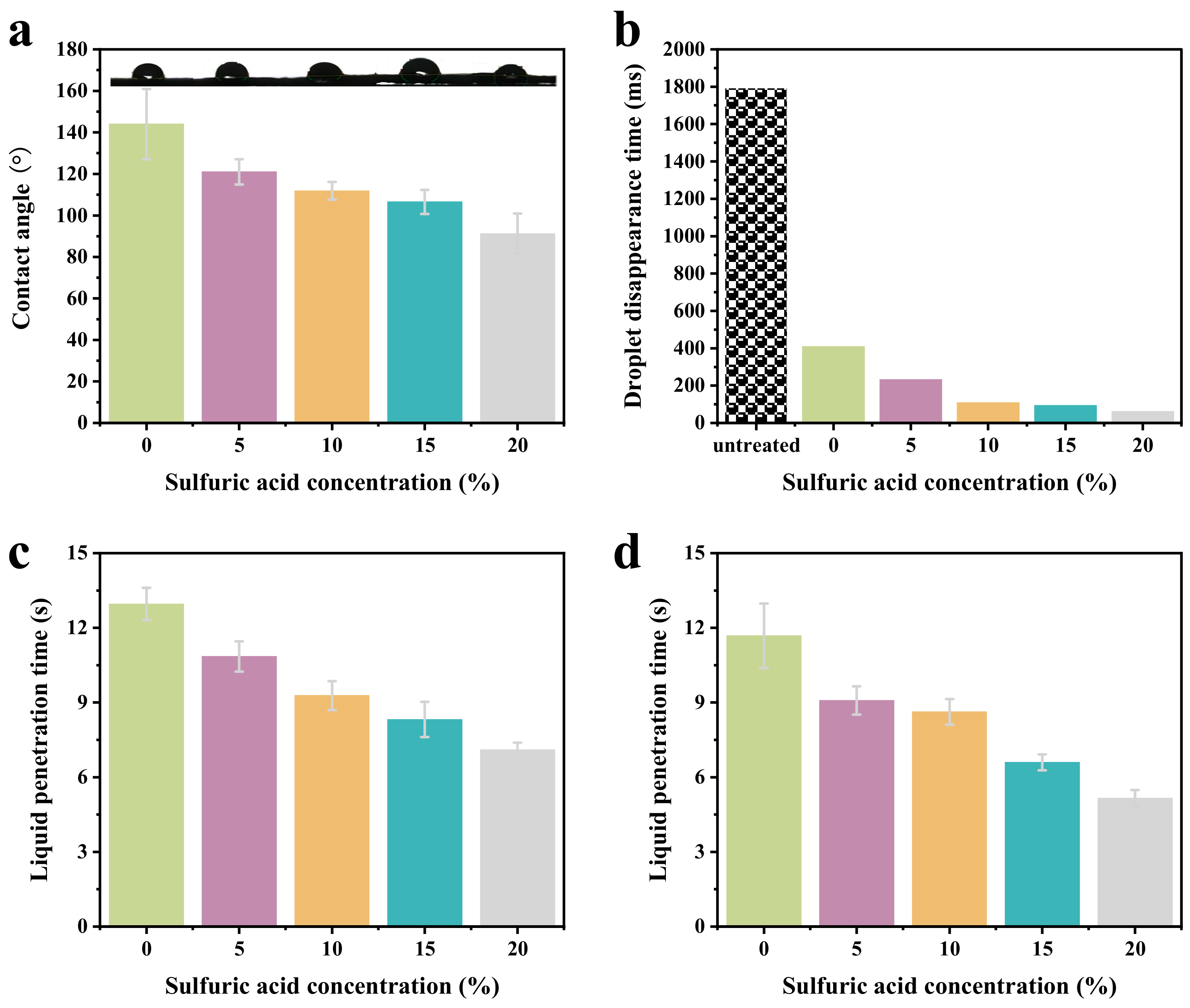
2.2. Wetting Behavior
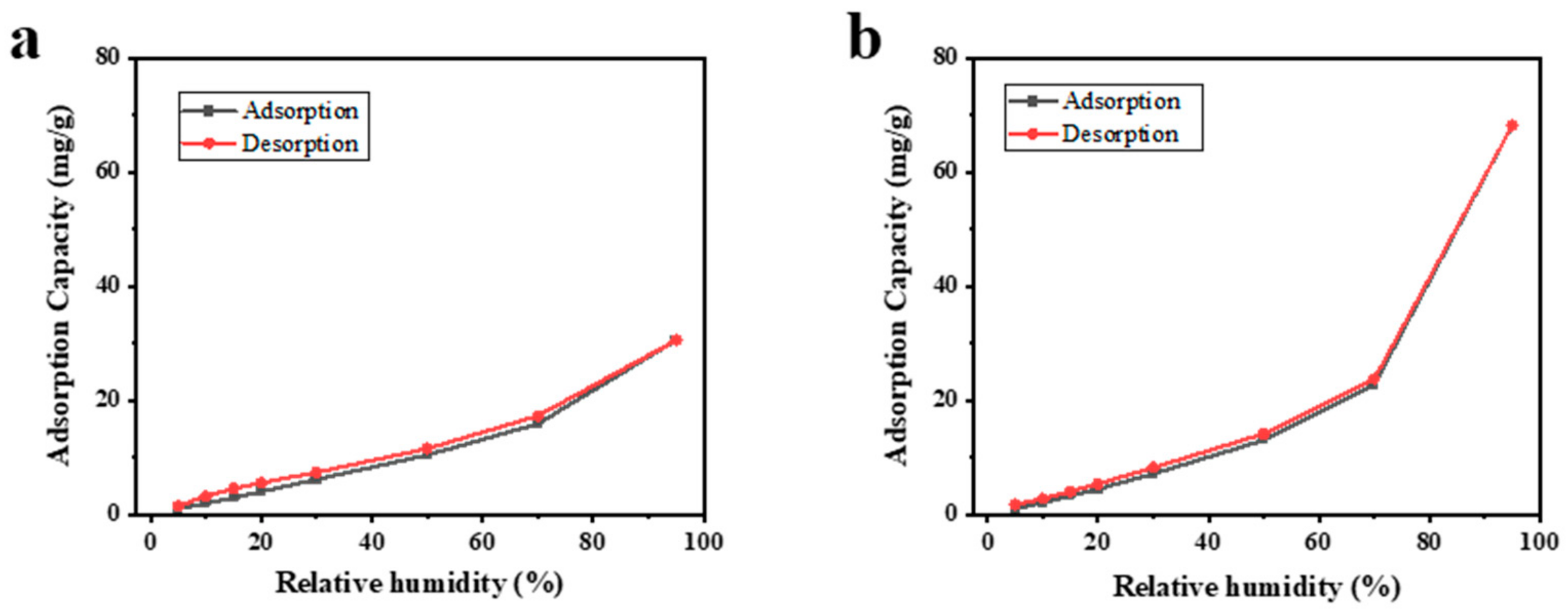
2.3. Water Vapor Adsorption and Desorption
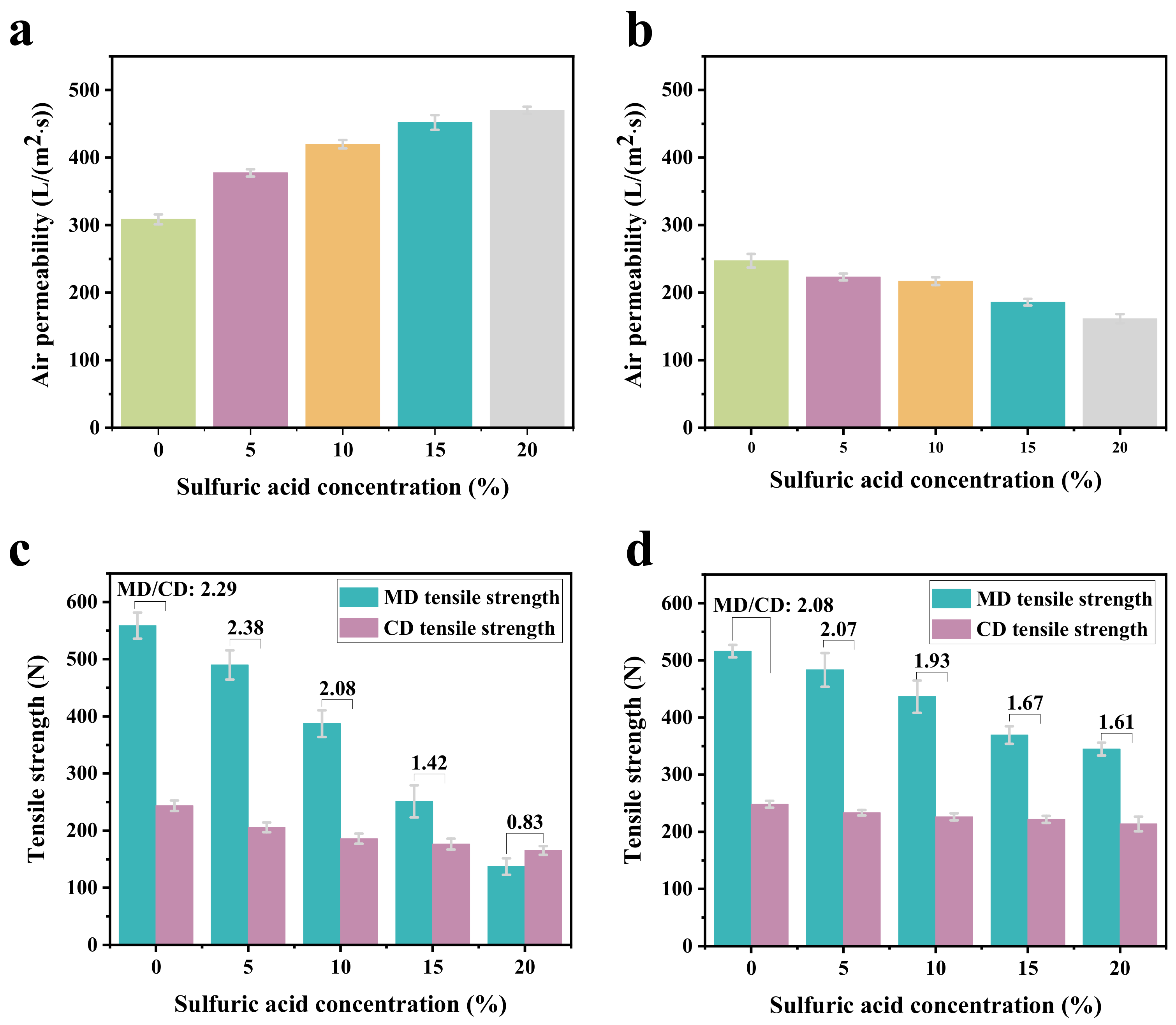
2.4. Air Permeability
2.5. Tensile Strength
3. Materials and Methods
3.1. Materials
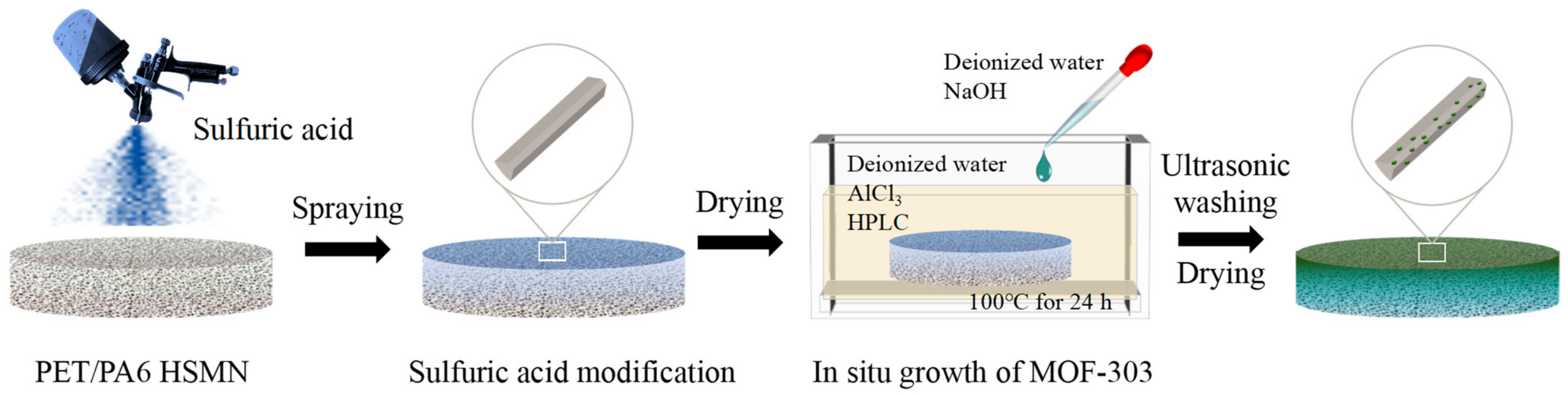
3.2. Preparation of Asymmetrically Wettable PET/PA6 HSMNs
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, A.; Narusue, S. Isotactic polypropylene microfiber prepared by continuous laser-thinning method. J. Appl. Polym. Sci. 2006, 99, 27–31. [Google Scholar] [CrossRef]
- Suzuki, A.; Narusue, S. Mechanical properties and microstructure of poly(ethylene terephthalate) microfiber prepared by carbon dioxide laser heating. J. Appl. Polym. Sci. 2003, 90, 1955–1958. [Google Scholar] [CrossRef]
- Sugawara, K.; Ikaga, T.; Kim, K.H.; Ohkoshi, Y.; Okada, K.; Masunaga, H.; Kanaya, T.; Masuda, M.; Maeda, Y. Fiber structure development in PS/PET sea-island conjugated fiber during continuous laser drawing. Polymer 2015, 79, 37–46. [Google Scholar] [CrossRef]
- Won, J.S.; Lim, S.C.; Park, J.; Hahm, W.G.; Park, J.K.; Lee, S.G.; Jeong, Y.G. Microstructures and mechanical properties of thermoplastic composites based on polyarylate/nylon6 islands-in-sea fibers. Polym. Compos. 2018, 40, E484–E492. [Google Scholar] [CrossRef]
- Xu, N.; Tao, Y.; Wang, X.; Luo, Z. Construction of a Novel Substrate of Unfigured Islands-in-Sea Microfiber Synthetic Leather Based on Waste Collagen. ACS Omega 2021, 6, 16086–16097. [Google Scholar] [CrossRef] [PubMed]
- Tabors, J.; Wust, C.; Pourdeyhimi, B. The role of staple fiber length on the performance of carded, hydroentangled nonwovens produced with splittable fibers. J. Eng. Fibers Fabr. 2019, 14, 1558925019832526. [Google Scholar] [CrossRef]
- Duo, Y.; Qian, X.; Zhao, B.; Gao, L.; Guo, X.; Zhang, S.; Bai, H.; Tang, L. Easily splittable hollow segmented-pie microfiber nonwoven material with excellent filtration and thermal-wet comfort for energy savings. J. Mater. Res. Technol. 2022, 17, 876–887. [Google Scholar] [CrossRef]
- Piyanut, J.; Natee, S. Spinning of Photocatalytic Fiber as Splittable Segmented-Pie Bi-Component Fibers for Antibacterial Textiless. J. Nanosci. 2019, 19, 1554–1561. [Google Scholar]
- Wu, F.; Liu, P.; Wang, J.; Shah, T.; Ahmad, M.; Zhang, Q.; Zhang, B. Fabrication of magnetic tubular fiber with multi-layer heterostructure and its microwave absorbing properties. J. Colloid Interface Sci. 2020, 577, 242–255. [Google Scholar] [CrossRef]
- Seong, D.G.; Kim, S.; Lee, D.; Yi, J.W.; Kim, S.W.; Kim, S.Y. Prediction of Defect Formation during Resin Impregnation Process through a Multi-Layered Fiber Preform in Resin Transfer Molding by a Proposed Analytical Model. Materials 2018, 11, 2055. [Google Scholar] [CrossRef]
- Kara, Y.; Molnár, K. A review of processing strategies to generate melt-blown nano/microfiber mats for high-efficiency filtration applications. J. Ind. Text. 2022, 51, 137S–180S. [Google Scholar] [CrossRef]
- Drabek, J.; Zatloukal, M. Meltblown technology for production of polymeric microfibers/nanofibers: A review. Phys. Fluids 2019, 31, 091301. [Google Scholar] [CrossRef]
- Hao, X.; Zeng, Y. A Review on the Studies of Air Flow Field and Fiber Formation Process during Melt Blowing. Ind. Eng. Chem. Res. 2019, 58, 11624–11637. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, W.; Lin, M.; Qi, H.; Zhang, C. Preparation of PLA-based SMS nonwoven composites and its applications in protective apparel. J. Text. Inst. 2023, 1–9. [Google Scholar] [CrossRef]
- Ke, Z.; Yu, L.; Wang, G.; Sun, R.; Zhu, M.; Dong, H.; Xu, Y.; Ren, M.; Fu, S.; Zhi, C. Three-Dimensional Modeling of Spun-Bonded Nonwoven Meso-Structures. Polymers 2023, 15, 600. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, H.; Kajiwara, K.; Morioka, H. Creation of Permanently Hydrophilized Polyester Spun-Bonded Nonwoven Fabric. Sen-I Gakkaishi 2023, 78, 511–513. [Google Scholar] [CrossRef]
- Wee, J.H.; Bae, Y.; Cho, N.P. Enhancing mechanical properties of flash-spun filaments by pressure-induced phase separation control in supercritical high-density polyethylene solution. Sci. Rep. 2022, 12, 18030. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Sota, H.; Hirogaki, T.; Aoyama, E. Investigation of air filter properties of nanofiber non-woven fabric manufactured by a modified melt-blowing method along with flash spinning method. Precis. Eng. 2021, 68, 187–196. [Google Scholar] [CrossRef]
- Lee, J.; Moon, S.; Lahann, J.; Lee, K.J. Recent Progress in Preparing Nonwoven Nanofibers via Needleless Electrospinning. Macromol. Mater. Eng. 2023, 308, 2300057. [Google Scholar] [CrossRef]
- Malakhov, S.N.; Chvalun, S.N. Nonwoven materials produced by melt electrospinning of commodity polymers. Russ. J. Gen. Chem. 2017, 87, 1364–1370. [Google Scholar] [CrossRef]
- Fadil, F.; Affandi, N.D.N.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on Electrospun Nanofiber-Applied Products. Polymers 2021, 13, 2087. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Ramakrishnan, G. Microfibres. Text. Prog. 2008, 40, 1–86. [Google Scholar] [CrossRef]
- Cadiau, A.; Belmabkhout, Y.; Adil, K.; Bhatt, P.M.; Pillai, R.S.; Shkurenko, A.; Martineau-Corcos, C.; Maurin, G.; Eddaoudi, M. Hydrolytically stable fluorinated metal-organic frameworks for energy-efficient dehydration. Science 2017, 356, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, P.; Zhang, X.; Li, P.; Wasson, M.C.; Islamoglu, T.; Stoddart, J.F.; Farha, O.K. Reticular Access to Highly Porous acs-MOFs with Rigid Trigonal Prismatic Linkers for Water Sorption. J. Am. Chem. Soc. 2019, 141, 2900–2905. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yang, S.; Rao, S.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Fathieh, F.; Kalmutzki, M.J.; Kapustin, E.A.; Waller, P.J.; Yang, J.J.; Yaghi, O.M. Practical water production from desert air. Sci. Adv. 2018, 4, eaat3198. [Google Scholar] [CrossRef]
- Shanmugasundaram, O.L.; Gowda, R.V.M. Development and characterization of bamboo and organic cotton fibre blended baby diapers. Indian J. Fibre Text. Res. 2011, 35, 201–205. [Google Scholar]
- GB/T 5453—1997; Textiles—Determination of the Permeability of Fabrics to Air. Shanghai The State Bureau of Quality and Technical Supervision: Beijing, China, 1997.
- GB/T 24218.3-2010; Textiles—Test Methods for Nonwovens—Part 3: Determination of Tensile Strength and Elongation (Strip Method). General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2011.

| Element | Atomic Percent/% | ||||
|---|---|---|---|---|---|
| 0%+ MOF-303 | 5%+ MOF-303 | 10%+ MOF-303 | 15%+ MOF-303 | 20%+ MOF-303 | |
| C | 74.66 | 68.14 | 56.95 | 57.85 | 65.07 |
| O | 22.62 | 30.57 | 33.81 | 34.19 | 27.33 |
| N | 2.14 | 0.72 | 7.71 | 5.33 | 3.83 |
| Al | 0.58 | 0.98 | 1.74 | 2.76 | 4.56 |
| Sample | Microfiber Nonwovens: (a) Untreated PET/PA6 HSMN (b) PET/PA6 HSMN Treated with Sulfuric Acid of 20% and MOF-303 | ||||
|---|---|---|---|---|---|
| Test temperature | 20 °C | Saturated vapor pressure | 2.337 kPa | Adsorbent | Water vapor |
| Degassing method | Heating + constant pressure | Pre-treatment temperature | 150 °C | Pre-treatment time | 180 min |
| Carrier gas | Nitrogen gas | Adsorption equilibrium conditions | 0.1 mg/h | Total flow rate | 400 sccm |
| Pressure point | 5%, 10%, 15%, 20%, 30%, 50%, 70%, 95% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Liu, C.; Wang, Z.; Feng, Q.; Han, X.; Zhang, J.; Hu, C.; Han, D. Asymmetrically Wettable, PET/PA6, Hollow, Segmented-Pie, Microfiber Nonwovens for a Synthetic Leather Base. Molecules 2024, 29, 2891. https://doi.org/10.3390/molecules29122891
Zhao B, Liu C, Wang Z, Feng Q, Han X, Zhang J, Hu C, Han D. Asymmetrically Wettable, PET/PA6, Hollow, Segmented-Pie, Microfiber Nonwovens for a Synthetic Leather Base. Molecules. 2024; 29(12):2891. https://doi.org/10.3390/molecules29122891
Chicago/Turabian StyleZhao, Baobao, Chunbiao Liu, Zhen Wang, Quan Feng, Xu Han, Jin Zhang, Chenggong Hu, and Dongxu Han. 2024. "Asymmetrically Wettable, PET/PA6, Hollow, Segmented-Pie, Microfiber Nonwovens for a Synthetic Leather Base" Molecules 29, no. 12: 2891. https://doi.org/10.3390/molecules29122891
APA StyleZhao, B., Liu, C., Wang, Z., Feng, Q., Han, X., Zhang, J., Hu, C., & Han, D. (2024). Asymmetrically Wettable, PET/PA6, Hollow, Segmented-Pie, Microfiber Nonwovens for a Synthetic Leather Base. Molecules, 29(12), 2891. https://doi.org/10.3390/molecules29122891







