Advancements in Characterization Techniques for Microemulsions: From Molecular Insights to Macroscopic Phenomena
Abstract
:1. Introduction
2. The Formation Mechanism of Microemulsions
2.1. The Instantaneous Negative Interfacial Tension Theory
2.2. The Double-Layer Membrane Theory System
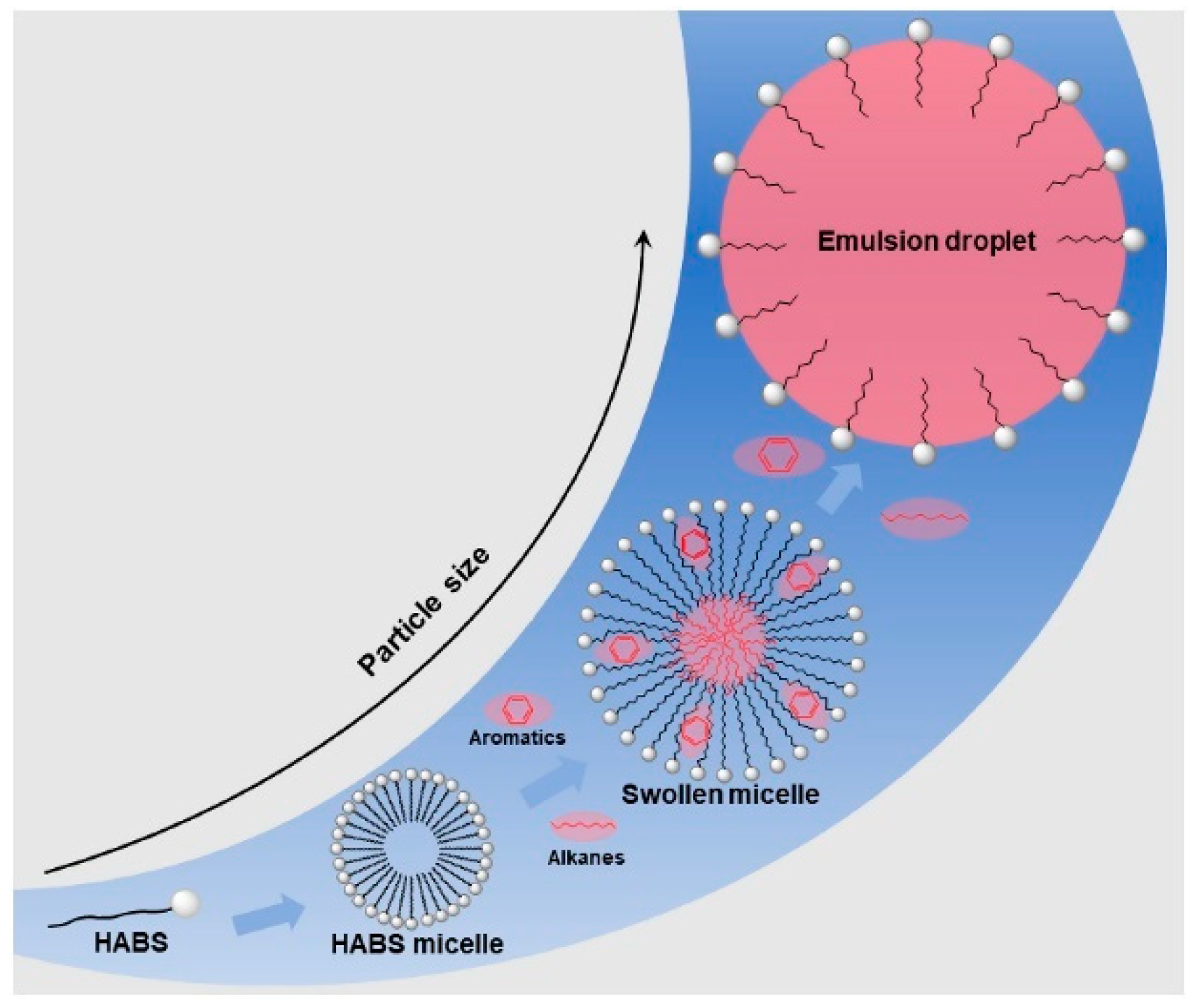
2.3. The Micellar Solubilization Theory
2.4. Electrochemical Theory and System Stability
3. The Types of Microemulsions and Their Microstructures
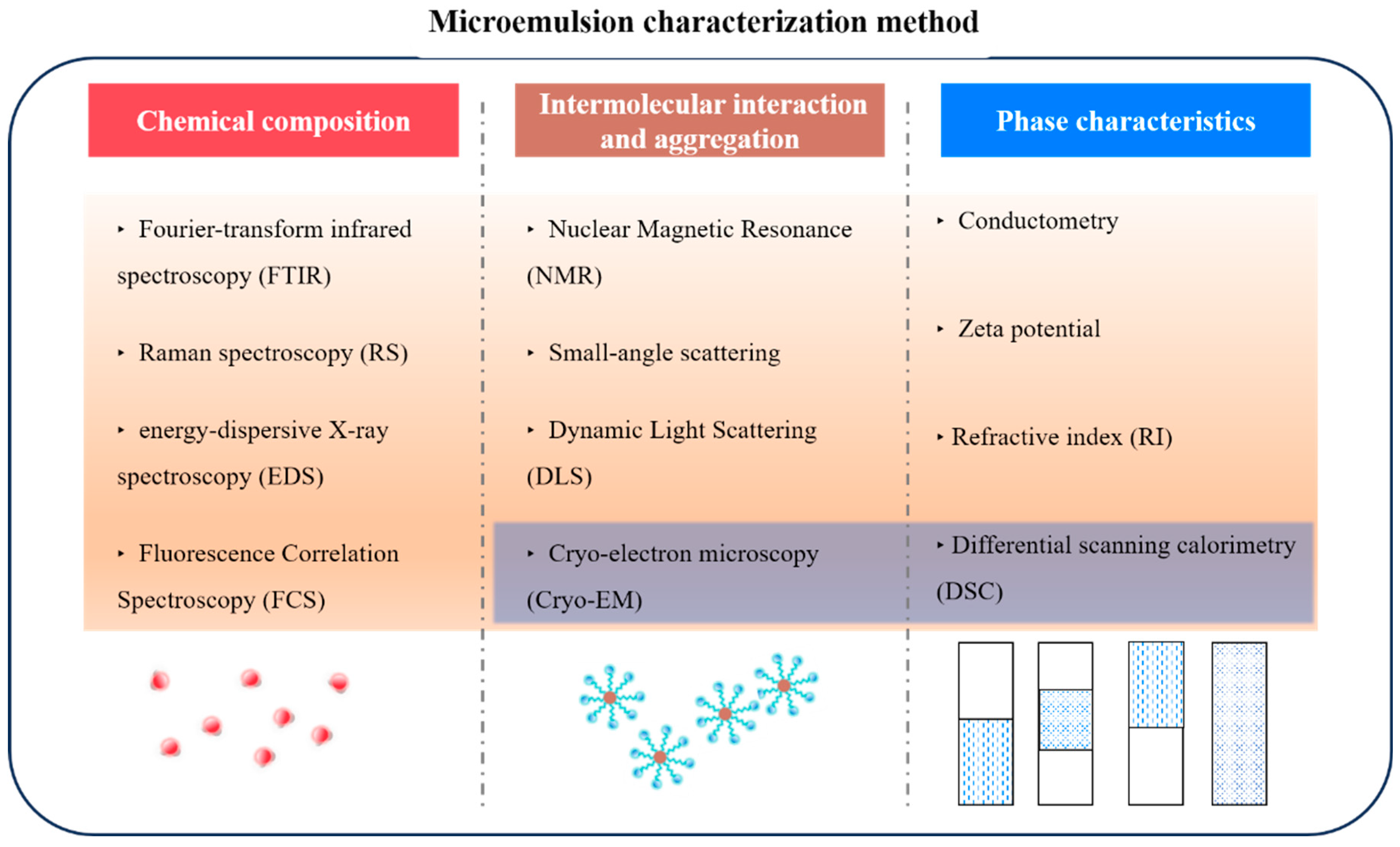
4. Microemulsion Characterization Techniques
4.1. Chemical Composition—Component Analysis
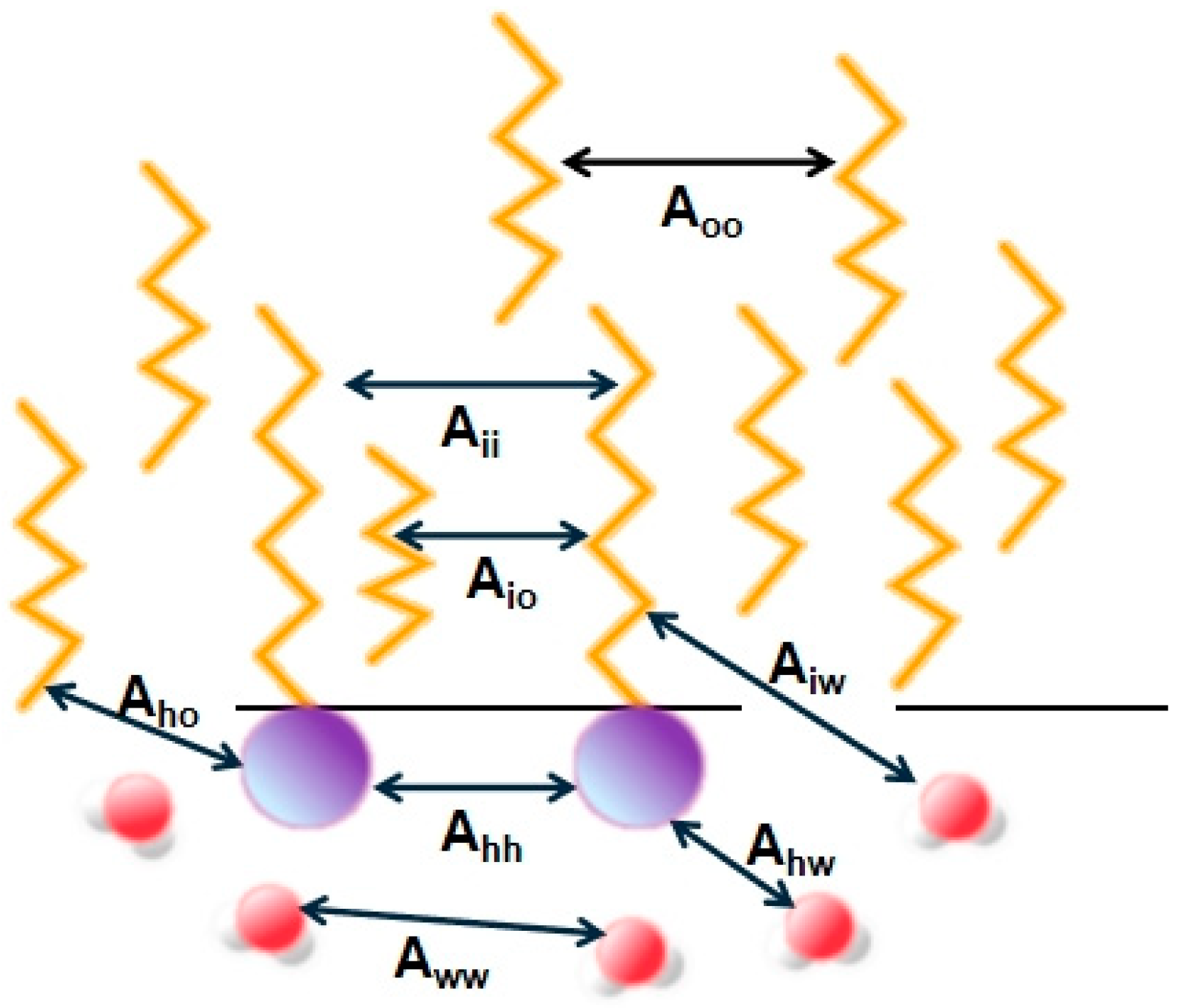
4.2. Intermolecular Interaction and Aggregation
4.2.1. Nuclear Magnetic Resonance (NMR)

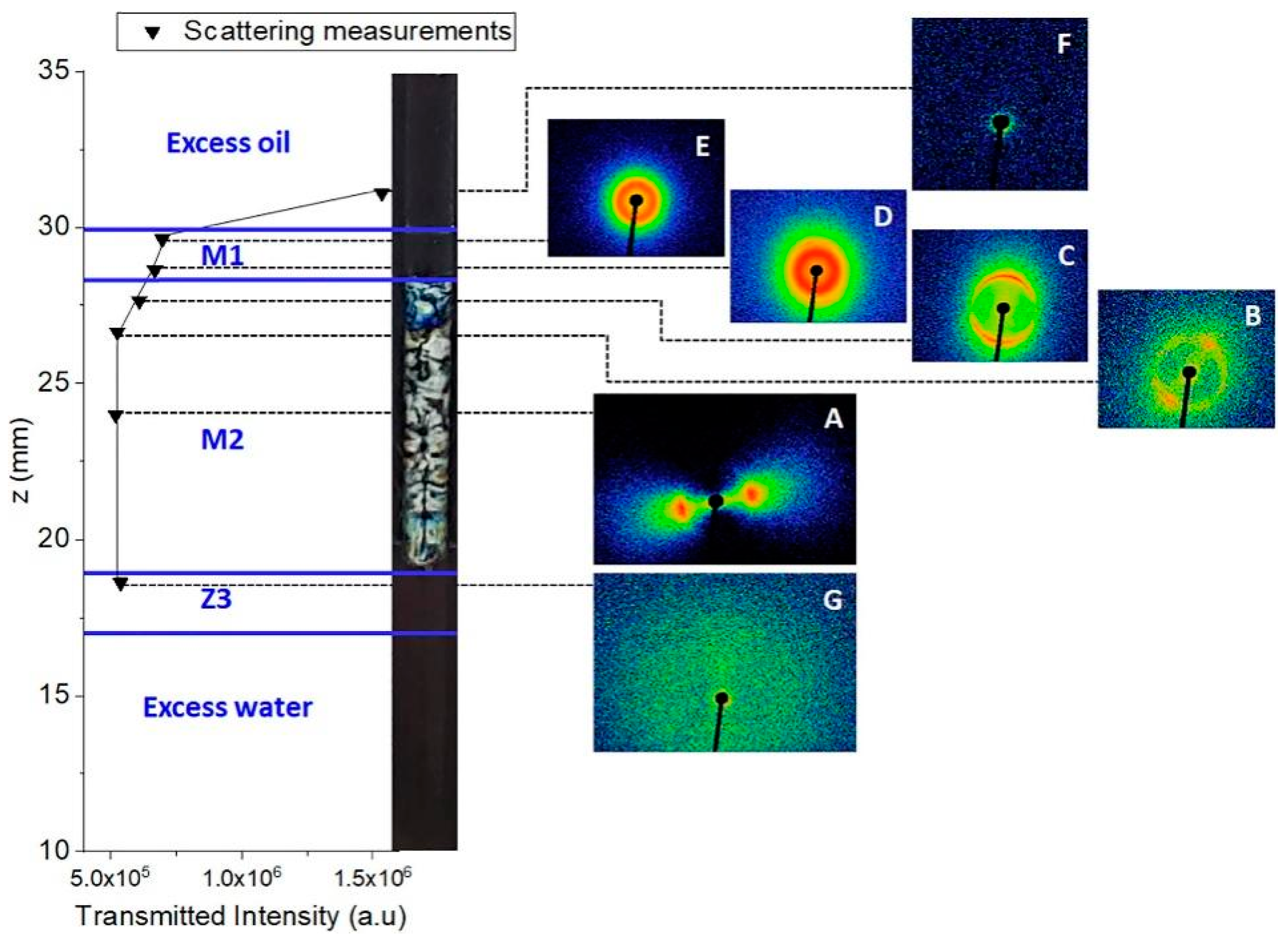
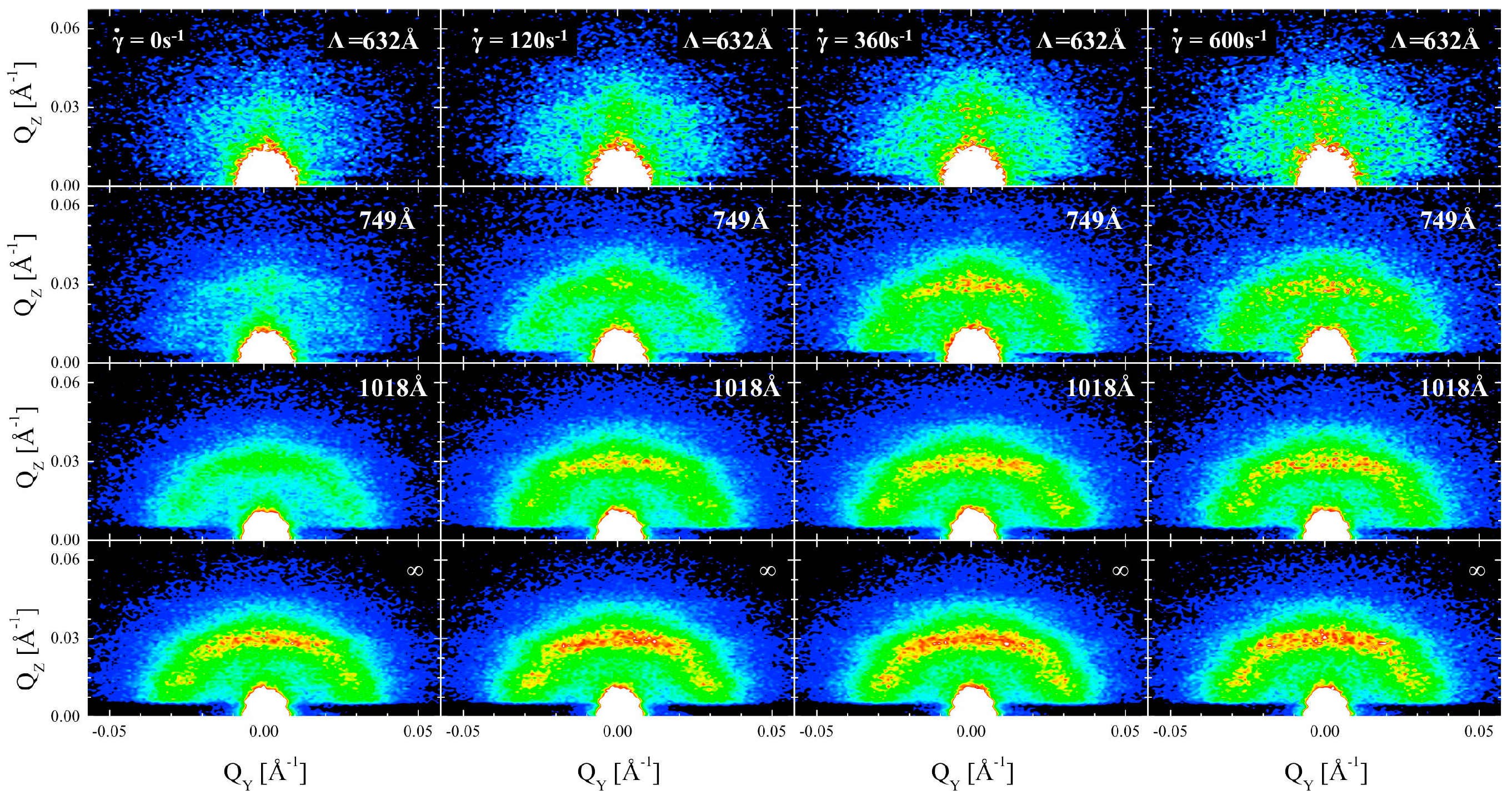
4.2.2. Small-Angle Scattering
4.2.3. Dynamic Light Scattering (DLS)

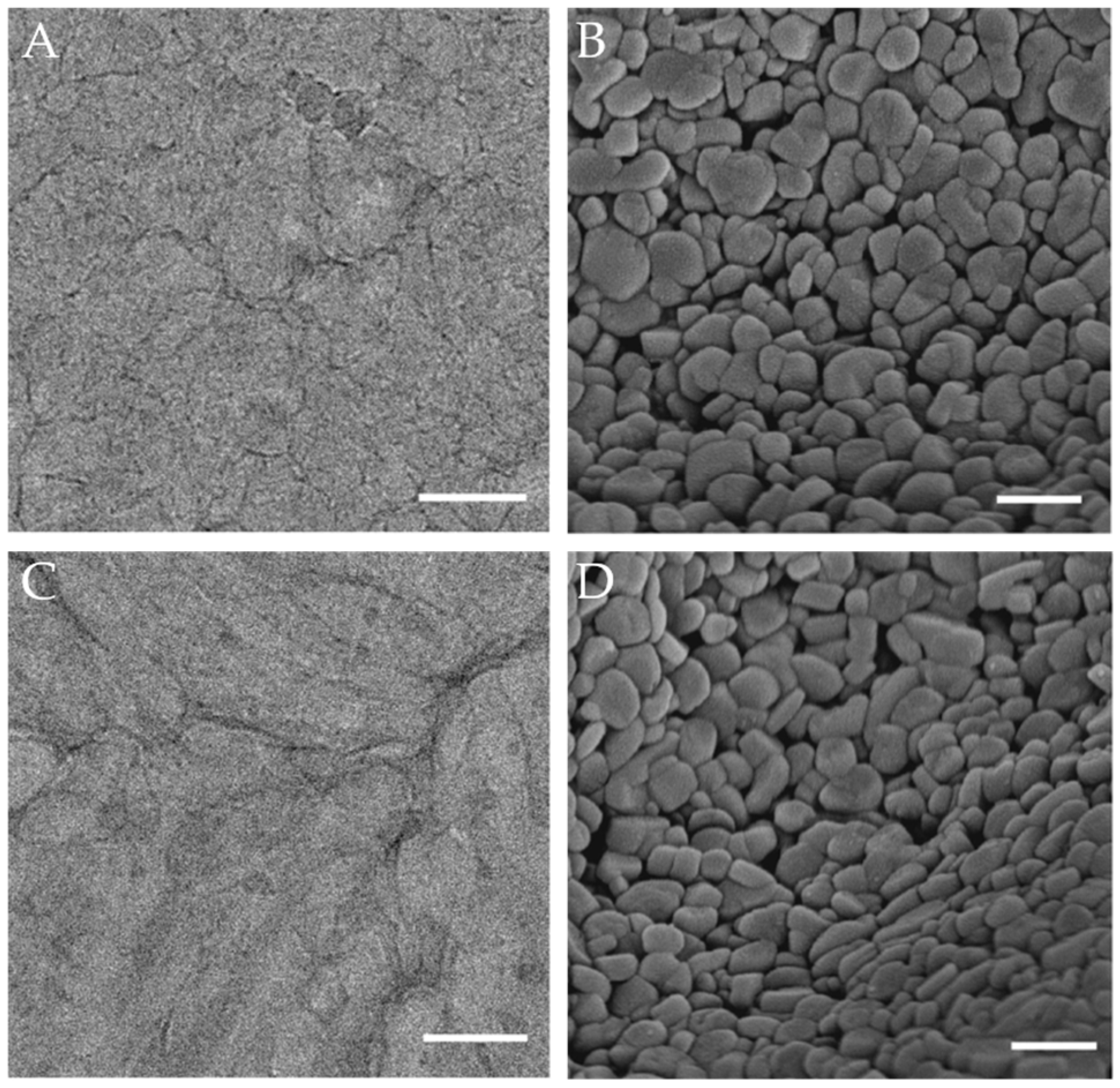
4.2.4. Cryo-Electron Microscopy (Cryo-EM)
4.3. Phase Characteristics
4.3.1. Conductometry
4.3.2. Zeta Potential
4.3.3. Refractive Index (RI)
4.3.4. Differential Scanning Calorimetry (DSC)
5. Conclusions and Prospects
5.1. Conclusions
5.2. Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, M.K.; Shah, D.O. Introduction to Macro- and Microemulsions. In Macro- and Microemulsions; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1985; Volume 272, pp. 1–18. ISBN 978-0-8412-0896-4. [Google Scholar]
- Danielsson, I.; Lindman, B. The Definition of Microemulsion. Colloids Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Sandhu, R.K.; Kaur, A.; Kaur, P.; Rajput, J.K.; Khullar, P.; Bakshi, M.S. Solubilization of Surfactant Stabilized Gold Nanoparticles In Oil–in–Water and Water–in–Oil Microemulsions. J. Mol. Liq. 2021, 336, 116305. [Google Scholar] [CrossRef]
- Guo, R.; Qian, S.; Qian, J. Hydrotrope and Hydrotrope-Solubilization Action of Cephanone in Ctab/N-C5h11oh/H2o System. Colloid Polym. Sci. 2004, 283, 15–23. [Google Scholar] [CrossRef]
- Mo, Y.; Han, Y.; Dong, J.; Liang, X.; Zhang, W. Mechanisms of Simultaneous Removal of Multiple Chlorinated Hydrocarbons in Aquifers by In-Situ Microemulsion. Sep. Purif. Technol. 2024, 336, 126342. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Sheng, L.; Wang, M.; Liu, H. The Phase Behavior and Solubilization Ability of Nonionic Surfactant-Distillate Fraction of Crude Oil Microemulsion System. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125181. [Google Scholar] [CrossRef]
- Dong, X.; Ke, X.; Liao, Z. The Microstructure Characterization of Meloxicam Microemulsion and Its Influence on the Solubilization Capacity. Drug Dev. Ind. Pharm. 2011, 37, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Ott, T.M.; Schweins, R.; Frielinghaus, H.; Lade, O.; Sottmann, T. Phase Behavior and Microstructure of Symmetric Nonionic Microemulsions with Long-Chain N-Alkanes and Waxes. Ind. Eng. Chem. Res. 2019, 58, 2583–2595. [Google Scholar] [CrossRef]
- Tenjarla, S. Microemulsions: An Overview and Pharmaceutical Applications. Crit. Rev. Ther. Drug Carr. Syst. 1999, 16, 461–521. [Google Scholar] [CrossRef]
- Obuebite, A.A.; Okwonna, O.O.; Eke, W.I.; Akaranta, O. Orange Mesocarp Extract as a Natural Surfactant: Impact on Fluid–Fluid and Fluid–Rock Interactions During Chemical Flooding. ACS Omega 2024, 9, 4263–4276. [Google Scholar] [CrossRef]
- Zhu, T.; Kang, W.; Yang, H.; Li, Z.; Zhou, B.; He, Y.; Wang, J.; Aidarova, S.; Sarsenbekuly, B. Advances of Microemulsion and Its Applications for Improved Oil Recovery. Adv. Colloid Interface Sci. 2022, 299, 102527. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, D.; Wang, D.; Zhang, C.; Yang, Z. Experiment Investigation of Microemulsion Enhanced Oil Recovery in Low Permeability Reservoir. J. Mater. Res. Technol. 2020, 9, 8306–8313. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Q.; Tian, M.; Li, Y.; Han, X.; Guo, R. Review: Microemulsions for the Sustainable Development of EOR. Sustainability 2024, 16, 629. [Google Scholar] [CrossRef]
- Slomkowski, S.; Alemán, J.V.; Gilbert, R.G.; Hess, M.; Horie, K.; Jones, R.G.; Kubisa, P.; Meisel, I.; Mormann, W.; Penczek, S.; et al. Terminology of Polymers and Polymerization Processes in Dispersed Systems (Iupac Recommendations 2011). Pure Appl. Chem. 2011, 83, 2229–2259. [Google Scholar] [CrossRef]
- Wang, D.-Q.; Yin, D.-Y.; Wang, J.-D.; Zhou, Y.-Z.; Zhang, C.-L. Prediction and Programming of Microemulsion Phase Behavior Simulation. Pet. Sci. 2022, 19, 1401–1410. [Google Scholar] [CrossRef]
- Lu, M.; Lindman, B.; Holmberg, K. Effect of Polymer Addition on the Phase Behavior of Oil–Water–Surfactant Systems of Winsor Iii Type. Phys. Chem. Chem. Phys. 2024, 26, 3699–3710. [Google Scholar] [CrossRef] [PubMed]
- Alzahid, Y.A.; Mostaghimi, P.; Walsh, S.D.C.; Armstrong, R.T. Flow Regimes during Surfactant Flooding: The Influence of Phase Behaviour. Fuel 2019, 236, 851–860. [Google Scholar] [CrossRef]
- Chai, J.L.; Liu, N.; Bai, T.T.; Zhang, H.M.; Liu, N.N.; Wang, D. Compositions and Physicochemical Properties of Tween Type Surfactants-Based Microemulsions. J. Dispers. Sci. Technol. 2014, 35, 441–447. [Google Scholar] [CrossRef]
- Suhail, N.; Alzahrani, A.K.; Basha, W.J.; Kizilbash, N.; Zaidi, A.; Ambreen, J.; Khachfe, H.M. Microemulsions: Unique Properties, Pharmacological Applications, and Targeted Drug Delivery. Front. Nanotechnol. 2021, 3, 754889. [Google Scholar] [CrossRef]
- Bera, A.; Mandal, A. Microemulsions: A Novel Approach to Enhanced Oil Recovery: A Review. J. Pet. Explor. Prod. Technol. 2015, 5, 255–268. [Google Scholar] [CrossRef]
- Prince, L.M. A Theory of Aqueous Emulsions. J. Colloid Interface Sci. 1969, 29, 216–221. [Google Scholar] [CrossRef]
- Schulman, J.H.; Riley, D.P. X-ray Investigation of the Structure of Transparent Oil-Water Disperse Systems. I. J. Colloid Sci. 1948, 3, 383–405. [Google Scholar] [CrossRef]
- Bowcott, J.E.; Schulman, J.H. Emulsions control of droplet size and phase continuity in transparent oil-water dispersions stabilized with soap and alcohol. Z. Elektrochem. Berichte Bunsenges. Phys. Chem. 1955, 59, 283–290. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of Self-Assembly of Hydrocarbon Amphiphiles Into Micelles and Bilayers. J. Chem. Soc. Faraday Trans. 2 1976, 72, 1525. [Google Scholar] [CrossRef]
- Schechter, R.S.; Bourrel, M. Microemulsions and Related Systems: Formulation, Solvency, and Physical Properties; M. Dekker: New York, NY, USA, 1988. [Google Scholar]
- Qu, J.; Wan, Y.; Tian, M.; Lv, W. Microemulsions Based on Diverse Surfactant Molecular Structure: Comparative Analysis and Mechanistic Study. Processes 2023, 11, 3409. [Google Scholar] [CrossRef]
- Shinoda, K.; Friberg, S. Microemulsions: Colloidal Aspects. Adv. Colloid Interface Sci. 1975, 4, 281–300. [Google Scholar] [CrossRef]
- Zhao, X. Micellar solubilization of petroleum fractions by heavy alkylbenzene sulfonate surfactant. J. Mol. Liq. 2021, 329, 115519. [Google Scholar] [CrossRef]
- Cheng, C.-H.D. Molecular Association Behaviors of Anionic Surfactants in Aqueous Solutions (Micelles, Microemulsions, Light Scattering); University of Michigan: Ann Arbor, MI, USA, 1985. [Google Scholar]
- Ridley, R.E.; Fathi-Kelly, H.; Kelly, J.P.; Vasquez, V.R.; Graeve, O.A. Predicting Destabilization in Salt-Containing Aqueous Reverse Micellar Colloidal Systems. ACS Earth Space Chem. 2021, 5, 2223–2232. [Google Scholar] [CrossRef]
- Bonto, M.; Eftekhari, A.A.; Nick, H.M. An Overview of the Oil-Brine Interfacial Behavior and a New Surface Complexation Model. Sci. Rep. 2019, 9, 6072. [Google Scholar] [CrossRef] [PubMed]
- Hirasaki, G.J.; Lawson, J.B. An Electrostatic Approach to the Association of Sodium and Calcium with Surfactant Micelles. SPE Reserv. Eng. 1986, 1, 119–130. [Google Scholar] [CrossRef]
- Eastoe, J.; Tabor, R.F. Surfactants and Nanoscience. In Colloidal Foundations of Nanoscience; Berti, D., Palazzo, G., Eds.; Jai-Elsevier Science Inc.: Oxford, UK, 2014; pp. 135–157. ISBN 978-0-444-59541-6. [Google Scholar]
- Holmberg, K.; Jönsson, B.; Kronberg, B.; Lindman, B. Surfactant Micellization. In Surfactants and Polymers in Aqueous Solution; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; pp. 39–66. ISBN 978-0-470-85642-0. [Google Scholar]
- Tartaro, G.; Mateos, H.; Schirone, D.; Angelico, R.; Palazzo, G. Microemulsion Microstructure(S): A Tutorial Review. Nanomaterials 2020, 10, 1657. [Google Scholar] [CrossRef]
- Hyde, S. The Language of Shape: The Role of Curvature in Condensed Matter: Physics, Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Rokade, A.; Kakade, S. Review on microemulsion: Theory, methods of preparation. Int. J. Emerg. Technol. Innov. Res. 2023, 10, h501–h512. [Google Scholar]
- Zhang, X.; Liao, X.; Gong, Z.; Li, X.; Jia, C. Formation of Fatty Acid Methyl Ester Based Microemulsion and Removal Mechanism of Pahs from Contaminated Soils. J. Hazard. Mater. 2021, 413, 125460. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ma, Q.; Dou, T.; Jin, S.; Jiang, L. Raman Spectroscopy of Emulsions and Emulsion Chemistry. Crit. Rev. Anal. Chem. 2023, 2, 1–13. [Google Scholar] [CrossRef]
- Hufnagel, T.; Rädle, M.; Karbstein, H.P. Influence of Refractive Index Differences on the Signal Strength for Raman-Spectroscopic Measurements of Double Emulsion Droplets. Appl. Sci. 2022, 12, 9056. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Vimalathithan, R.M.; Ponnusamy, V. Synthesis and Characterization of Baso4 Nano-Particles Using Micro-Emulsion Technique. Adv. Appl. Sci. Res. 2010, 1, 197–204. [Google Scholar]
- Zang, J.; Feng, M.; Zhao, J.; Wang, J. Micellar and Bicontinuous Microemulsion Structures Show Different Solute–Solvent Interactions: A Case Study Using Ultrafast Nonlinear Infrared Spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 19938–19949. [Google Scholar] [CrossRef]
- Do Nascimento, D.S.; Volpe, V.; Fernandez, C.J.; Oresti, G.M.; Ashton, L.; Grünhut, M. Confocal Raman Spectroscopy Assisted by Chemometric Tools: A Green Approach for Classification and Quantification of Octyl P-Methoxycinnamate in Oil-in-Water Microemulsions. Microchem. J. 2023, 184, 108151. [Google Scholar] [CrossRef]
- Li, Y.; Driver, M.; Winuprasith, T.; Zheng, J.; Mcclements, D.J.; He, L. In Situ Sers Detection of Emulsifiers at Lipid Interfaces Using Label-Free Amphiphilic Gold Nanoparticles. Analyst 2014, 139, 5075–5078. [Google Scholar] [CrossRef]
- Hess, S.T.; Huang, S.; Heikal, A.A.; Webb, W.W. Biological and Chemical Applications of Fluorescence Correlation Spectroscopy: A Review. Biochemistry 2002, 41, 697–705. [Google Scholar] [CrossRef]
- Tcherniak, A.; Reznik, C.; Link, S.; Landes, C.F. Fluorescence Correlation Spectroscopy: Criteria for Analysis in Complex Systems. Anal. Chem. 2009, 81, 746–754. [Google Scholar] [CrossRef]
- Sankaran, J.; Wohland, T. Current Capabilities and Future Perspectives of Fcs: Super-Resolution Microscopy, Machine Learning, and In Vivo Applications. Commun. Biol. 2023, 6, 699. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.P.; Hartley, P.G. Progress in Microemulsion Characterization. Curr. Opin. Colloid Interface Sci. 2012, 17, 274–280. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Li, X.; Cao, X.; Ma, C. The Phase Behavior and Microstructures of Tween 80/Short-Chainedalcohol/Ethyl Butyrate/Water Microemulsion System. J. Chin. Inst. Food Sci. Technol. 2022, 22, 77–87. [Google Scholar] [CrossRef]
- Nyden, M.; Soederman, O. Structures and Emulsification Failure In the Microemulsion Phase in the Didodecyldimethylammonium Sulfate/Hydrocarbon/Water System. A Self-Diffusion NMR Study. Langmuir 1995, 11, 1537–1545. [Google Scholar] [CrossRef]
- Han, K.S.; Bazak, J.D.; Chen, Y.; Graham, T.R.; Washton, N.M.; Hu, J.Z.; Murugesan, V.; Mueller, K.T. Pulsed Field Gradient Nuclear Magnetic Resonance and Diffusion Analysis in Battery Research. Chem. Mater. 2021, 33, 8562–8590. [Google Scholar] [CrossRef]
- Rigby, S.P.; Daut, S. A Statistical Model for the Heterogeneous Structure of Porous Catalyst Pellets. Adv. Colloid Interface Sci. 2002, 98, 87–119. [Google Scholar] [CrossRef]
- Ruzicka, E.; Pellechia, P.; Benicewicz, B.C. Polymer Molecular Weights via DOSY NMR. Anal. Chem. 2023, 95, 7849–7854. [Google Scholar] [CrossRef] [PubMed]
- Peat, G.; Boaler, P.J.; Dickson, C.L.; Lloyd-Jones, G.C.; Uhrín, D. Sharper-Dosy: Sensitivity Enhanced Diffusion-Ordered NMR Spectroscopy. Nat. Commun. 2023, 14, 4410. [Google Scholar] [CrossRef]
- Mishra, R.; Dumez, J.-N. Theoretical Analysis of Flow Effects In Spatially Encoded Diffusion NMR. J. Chem. Phys. 2023, 158, 014204. [Google Scholar] [CrossRef]
- Söderman, O.; Olsson, U. Dynamics of Amphiphilic Systems Studied Using NMR Relaxation and Pulsed Field Gradient Experiments. Curr. Opin. Colloid Interface Sci. 1997, 2, 131–136. [Google Scholar] [CrossRef]
- Colafemmina, G.; Mateos, H.; Palazzo, G. Diffusion NMR Studies of Complex Liquid Formulations. Curr. Opin. Colloid Interface Sci. 2020, 48, 109–120. [Google Scholar] [CrossRef]
- Besghini, D.; Mauri, M.; Simonutti, R. Time Domain NMR in Polymer Science: From the Laboratory to the Industry. Appl. Sci. 2019, 9, 1801. [Google Scholar] [CrossRef]
- Gradišek, A.; Cifelli, M.; Wojcik, M.; Apih, T.; Dvinskikh, S.V.; Gorecka, E.; Domenici, V. Study of Liquid Crystals Showing Two Isotropic Phases by 1H NMR Diffusometry and 1H NMR Relaxometry. Crystals 2019, 9, 178. [Google Scholar] [CrossRef]
- Kärger, J.; Avramovska, M.; Freude, D.; Haase, J.; Hwang, S.; Valiullin, R. Pulsed Field Gradient NMR Diffusion Measurement In Nanoporous Materials. Adsorption 2021, 27, 453–484. [Google Scholar] [CrossRef]
- Price, W.S. (Ed.) Setup and Analysis of Pgse Experiments. In NMR Studies of Translational Motion: Principles and Applications; Cambridge Molecular Science; Cambridge University Press: Cambridge, UK, 2009; pp. 198–220. ISBN 978-0-521-80696-1. [Google Scholar]
- Yamashita, Y.; Sakamoto, K. Chapter 38-Structural Analysis of Formulations. In Cosmetic Science and Technology; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 635–655. ISBN 978-0-12-802005-0. [Google Scholar]
- Lucas, L.H.; Otto, W.H.; Larive, C.K. The 2d-J-Dosy Experiment: Resolving Diffusion Coefficients in Mixtures. J. Magn. Reson. 2002, 156, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Pagès, G.; Gilard, V.; Martino, R.; Malet-Martino, M. Pulsed-Field Gradient Nuclear Magnetic Resonance Measurements (Pfg Nmr) for Diffusion Ordered Spectroscopy (Dosy) Mapping. Analyst 2017, 142, 3771–3796. [Google Scholar] [CrossRef] [PubMed]
- Valentini, M.; Vaccaro, A.; Rehor, A.; Napoli, A.; Hubbell, J.A.; Tirelli, N. Diffusion NMR Spectroscopy for the Characterization of the Size and Interactions of Colloidal Matter: The Case of Vesicles and Nanoparticles. J. Am. Chem. Soc. 2004, 126, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, C.; Hoffman, R.E.; Aserin, A.; Garti, N. NMR Chromatography Using Microemulsion Systems. Langmuir 2011, 27, 4497–4504. [Google Scholar] [CrossRef] [PubMed]
- Chatzidaki, M.D.; Demisli, S.; Zingkou, E.; Liggri, P.G.V.; Papachristos, D.P.; Balatsos, G.; Karras, V.; Nallet, F.; Michaelakis, A.; Sotiropoulou, G.; et al. Essential Oil-in-Water Microemulsions for Topical Application: Structural Study, Cytotoxic Effect and Insect Repelling Activity. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130159. [Google Scholar] [CrossRef]
- Herrera, D.; Chevalier, T.; Frot, D.; Barré, L.; Drelich, A.; Pezron, I.; Dalmazzone, C. Monitoring the Formation Kinetics of a Bicontinuous Microemulsion. J. Colloid Interface Sci. 2022, 609, 200–211. [Google Scholar] [CrossRef]
- Awad, T.S.; Asker, D.; Romsted, L.S. Evidence of Coexisting Microemulsion Droplets in Oil-in-Water Emulsions Revealed By 2d Dosy 1h NMR. J. Colloid Interface Sci. 2018, 514, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, M.; Poggi, G.; Ciolli, G.; Fratini, E.; Giorgi, R.; Baglioni, P. A Triton X-100-Based Microemulsion for the Removal of Hydrophobic Materials from Works of Art: Saxs Characterization and Application. Materials 2018, 11, 1144. [Google Scholar] [CrossRef] [PubMed]
- Stribeck, N. X-ray Scattering of Soft Matter; Springer Laboratory; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-69855-5. [Google Scholar]
- Walenta, E. Small Angle X-ray Scattering. Von O. Glatter Und O. Kratky. London: Academic Press Inc. Ltd. 1982. ISBN 0-12-286280-5. X, 515 Seiten, Geb. £ 43.60; Us $ 81.00. Acta Polym. 1985, 36, 296. [Google Scholar] [CrossRef]
- Sakuragi, M. Evaluation of the Supramolecular Structure of Drug Delivery Carriers Using Synchrotron X-ray Scattering. Polym. J. 2021, 53, 1335–1344. [Google Scholar] [CrossRef]
- Giri Rachman Putra, E.; Bharoto; Santoso, E.; Mulyana, Y.A. Scientific Review: Small Angle Neutron Scattering Spectrometer (Smarter) for Nanostructure Studies of Soft Condensed Matter. Neutron News 2007, 18, 23–29. [Google Scholar] [CrossRef]
- Sunaina; Mehta, S.K.; Ganguli, A.K.; Vaidya, S. Small-Angle X-ray Scattering as an Effective Tool to Understand the Structure and Rigidity of the Reverse Micelles with the Variation of Surfactant. J. Mol. Liq. 2021, 326, 115302. [Google Scholar] [CrossRef]
- Brunner-Popela, J.; Glatter, O. Small-Angle Scattering of Interacting Particles. I. Basic Principles of a Global Evaluation Technique. J. Appl. Cryst. 1997, 30, 431–442. [Google Scholar] [CrossRef]
- Schmidt, R.F.; Prause, A.; Prévost, S.; Doutch, J.; Gradzielski, M. Phase Behavior and Structure of a Biocompatible Microemulsion Based on Tween 20, 2-Ethylhexylglycerine and Isopropyl Palmitate in Water. Colloid Polym. Sci. 2023, 301, 753–762. [Google Scholar] [CrossRef]
- Nakamura, E.; Iwase, H.; Arima-Osonoi, H.; Sakuragi, M. Effect of Water Content on Stratum Corneum Penetration Mechanism of W/O Type Microemulsions. RSC Adv. 2023, 13, 17742–17749. [Google Scholar] [CrossRef]
- Lipfert, F.; Kerscher, M.; Mattauch, S.; Frielinghaus, H. Stability of Near-Surface Ordering of Bicontinuous Microemulsions in External Shear-Fields. J. Colloid Interface Sci. 2019, 534, 31–36. [Google Scholar] [CrossRef]
- Deng, L.; Kang, X.; Que, F.; Zhang, H. Structure-Activity Relationship of Thymol Microemulsion Inhibiting Candida Albicans. J. Chin. Inst. Food Sci. Technol. 2018, 18, 86–92. [Google Scholar] [CrossRef]
- Wang, R.; Huang, X. Anionic Surfactant-Stabilized Hydrophobic Ionic Liquid-Based Bicontinuous Microemulsion: Formulation, Microstructure and Laccase Kinetics. J. Mol. Liq. 2019, 292, 111404. [Google Scholar] [CrossRef]
- Söderman, O.; Nydén, M. NMR In Microemulsions. NMR Translational Diffusion Studies of A Model Microemulsion. Colloids Surf. A Physicochem. Eng. Asp. 1999, 158, 273–280. [Google Scholar] [CrossRef]
- Murgia, S.; Palazzo, G.; Mamusa, M.; Lampis, S.; Monduzzi, M. Aerosol-Ot in Water Forms Fully-Branched Cylindrical Direct Micelles in the Presence of the Ionic Liquid 1-Butyl-3-Methylimidazolium Bromide. Phys. Chem. Chem. Phys. 2011, 13, 9238–9245. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Wennerstroem, H. Self-Diffusion in Bicontinuous Cubic Phases, L3 Phases, and Microemulsions. J. Phys. Chem. 1990, 94, 8683–8694. [Google Scholar] [CrossRef]
- Palazzo, G.; Berti, D. Chapter 9-Diffusion and Aggregation. In Colloidal Foundations of Nanoscience; Berti, D., Palazzo, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 199–231. ISBN 978-0-444-59541-6. [Google Scholar]
- Wu, J.; Mei, P.; Lai, L. Microemulsion and Interfacial Properties of Anionic/Nonionic Surfactant Mixtures Based on Sulfonate Surfactants: The Influence of Alcohol. J. Mol. Liq. 2023, 371, 120814. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Yang, W. Preparation, In Vitro and In Vivo Evaluation of a Novel Mitiglinide Microemulsions. Saudi Pharm. J. 2024, 32, 101919. [Google Scholar] [CrossRef] [PubMed]
- Giustini, M.; Palazzo, G.; Colafemmina, G.; Della Monica, M.; Giomini, M.; Ceglie, A. Microstructure and Dynamics of the Water-in-Oil Ctab/N-Pentanol/N-Hexane/Water Microemulsion: A Spectroscopic and Conductivity Study. J. Phys. Chem. 1996, 100, 3190–3198. [Google Scholar] [CrossRef]
- Olsson, U.; Schurtenberger, P. Structure, Interactions, and Diffusion in a Ternary Nonionic Microemulsion Near Emulsification Failure. Langmuir 1993, 9, 3389–3394. [Google Scholar] [CrossRef]
- Cazabat, A.M.; Langevin, D. Diffusion of Interacting Particles: Light Scattering Study of Microemulsions. J. Chem. Phys. 1981, 74, 3148–3158. [Google Scholar] [CrossRef]
- Annese, C.; D’accolti, L.; Fusco, C.; Mele, G.; Giorgio, G.; Colafemmina, G.; Murgia, S.; Palazzo, G. Oxidation-Proof Microemulsions: Microstructure and Reactivity in the Presence of Dioxiranes. J. Colloid Interface Sci. 2013, 408, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Galantini, L.; Giampaolo, S.M.; Mannina, L.; Pavel, N.V.; Viel, S. Study of Intermicellar Interactions and Micellar Sizes in Ionic Micelle Solutions by Comparing Collective Diffusion and Self-Diffusion Coefficients. J. Phys. Chem. B 2004, 108, 4799–4805. [Google Scholar] [CrossRef]
- Ruf, H. Treatment of Contributions of Dust to Dynamic Light Scattering Data. Langmuir 2002, 18, 3804–3814. [Google Scholar] [CrossRef]
- Egelhaaf, S.; Olsson, U.; Schurtenberger, P.; Morris, J.; Wennerström, H. Quantitative Measurements of Ostwald Ripening Using Time-Resolved Small-Angle Neutron Scattering. Phys. Rev. E Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 1999, 60, 5681–5684. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Keire, D.A.; Chen, K. Comparison of NMR and Dynamic Light Scattering for Measuring Diffusion Coefficients of Formulated Insulin: Implications for Particle Size Distribution Measurements in Drug Products. AAPS J. 2017, 19, 1760–1766. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.; Dev Verma, S.; Singh, M.K.; Sen, S. Fluorescence Correlation Spectroscopy: An Efficient Tool for Measuring Size, Size-Distribution and Polydispersity of Microemulsion Droplets in Solution. Anal. Chem. 2011, 83, 7736–7744. [Google Scholar] [CrossRef]
- Wang, W.; Wei, H.; Du, Z.; Tai, X.; Wang, G. Formation and Characterization of Fully Dilutable Microemulsion with Fatty Acid Methyl Esters as Oil Phase. ACS Sustain. Chem. Eng. 2015, 3, 443–450. [Google Scholar] [CrossRef]
- Belkoura, L.; Stubenrauch, C.; Strey, R. Freeze Fracture Direct Imaging: A New Freeze Fracture Method for Specimen Preparation in Cryo-Transmission Electron Microscopy. Langmuir 2004, 20, 4391–4399. [Google Scholar] [CrossRef]
- Davidovich, I.; Issman, L.; De Paula, C.; Ben-Barak, I.; Talmon, Y. A Cryogenic-Electron Microscopy Study of the one-Phase Corridor in the Phase Diagram of a Nonionic Surfactant-Based Microemulsion System. Colloid Polym. Sci. 2015, 293, 3189–3197. [Google Scholar] [CrossRef]
- Ten Klooster, S.; Takeuchi, M.; Schroën, K.; Tuinier, R.; Joosten, R.; Friedrich, H.; Berton-Carabin, C. Tiny, Yet Impactful: Detection and Oxidative Stability of Very Small Oil Droplets in Surfactant-Stabilized Emulsions. J. Colloid Interface Sci. 2023, 652, 1994–2004. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Chapter 3-Characterization of Nanoparticles. In Advances in Nanotechnology-Based Drug Delivery Systems; Das Talukdar, A., Dey Sarker, S., Patra, J.K., Eds.; Nanotechnology in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2022; pp. 45–82. ISBN 978-0-323-88450-1. [Google Scholar]
- Hodoroaba, V.-D.; Motzkus, C.; Macé, T.; Vaslin-Reimann, S. Performance of High-Resolution Sem/Edx Systems Equipped with Transmission Mode (Tsem) for Imaging and Measurement of Size and Size Distribution of Spherical Nanoparticles. Microsc. Microanal. 2014, 20, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Morrison, E.D.; Frethem, C.D.; Zasadzinski, J.A.; Mccormick, A.V. Cryogenic Electron Microscopy Study of Nanoemulsion Formation from Microemulsions. Langmuir 2014, 30, 10826–10833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, M.; Chai, J.; Cui, X.; Wang, J. Preparation, Characterization and Application of a Surfactant-Free Microemulsion Containing 1-Octen-3-Ol, Ethanol, and Water. J. Mol. Liq. 2020, 300, 112278. [Google Scholar] [CrossRef]
- Vratsanos, M.A.; Gianneschi, N.C. Direct Observation of Emulsion Morphology, Dynamics, and Demulsification. ACS Nano 2022, 16, 7783–7793. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Valenta, C.; Matsko, N.B. Electron Microscopy of Pharmaceutical Systems. Micron 2013, 44, 45–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhan, F.; Liao, G.; Liu, W.; Su, X.; Feng, Y. In Situ Micro-Emulsification During Surfactant Enhanced Oil Recovery: A Microfluidic Study. J. Colloid Interface Sci. 2022, 620, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Z.; Chen, Z. Depiction of a Non-Aqueous Ionic Liquid Surfactant-Free Microemulsion by Dielectric Relaxation Spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129482. [Google Scholar] [CrossRef]
- Ja’afar, S.M.; Mokhtar, W.N.A.W.; Ramli, S.; Khalid, R.M.; Othaman, R. Coconut Oil Based Microemulsion Formulations for Hair Care Product Application. JSM 2019, 48, 599–605. [Google Scholar] [CrossRef]
- Mo, C.; Li, X. Microstructure and Structural Transition in Palm-Kernel Oil Microemulsion Using 1.5-Order Differential Electroanalysis. Colloid Polym. Sci. 2007, 285, 1361–1367. [Google Scholar] [CrossRef]
- Qi, Y.; Zhou, Y.; Yang, X.; Li, J.; Bai, L.; Wu, Z.; Qin, Z. Formulation and Stability Study of Vitamin E Microemulsion with Green Surfactant. Sustain. Chem. Pharm. 2023, 36, 101334. [Google Scholar] [CrossRef]
- Kumar, N.; Mandal, A. Surfactant Stabilized Oil-in-Water Nanoemulsion: Stability, Interfacial Tension, and Rheology Study for Enhanced Oil Recovery Application. Energy Fuels 2018, 32, 6452–6466. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and Stability of Nano-Emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.R. Phase Transitions and Critical Phenomena. In Modern Thermodynamics and Statistical Mechanics: A Comprehensive Foundation; Puri, R.R., Ed.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 297–315. ISBN 978-3-031-54310-4. [Google Scholar]
- Cai, H.; An, X.; Shen, W. Effects of the Dispersed Droplet Sizes on the Critical Behavior of Pseudobinary Microemulsion. Chin. Sci. Bull. 2007, 52, 1139–1141. [Google Scholar] [CrossRef]
- Su, W.; Gao, L.; Wang, L.; Zhi, H. Calibration of Differential Scanning Calorimeter (DSC) for Thermal Properties Analysis of Phase Change Material. J. Therm. Anal. Calorim. 2021, 143, 2995–3002. [Google Scholar] [CrossRef]
- Graciaa, A.; Lachaise, J.; Sayous, J.G.; Grenier, P.; Yiv, S.; Schechter, R.S.; Wade, W.H. The Partitioning of Complex Surfactant Mixtures Between Oil/Water/Microemulsion Phases at High Surfactant Concentrations. J. Colloid Interface Sci. 1983, 93, 474–486. [Google Scholar] [CrossRef]
- Podlogar, F.; Sibanc, R.; Gasperlin, M. Evolutionary Artificial Neural Networks as Tools for Predicting the Internal Structure of Microemulsions. J. Pharm. Pharm. Sci. 2008, 11, 67–76. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Qu, J.; Liu, W.; Peng, B.; Cong, S.; Yu, H.; Zhang, B.; Li, Y. Advancements in Characterization Techniques for Microemulsions: From Molecular Insights to Macroscopic Phenomena. Molecules 2024, 29, 2901. https://doi.org/10.3390/molecules29122901
Li L, Qu J, Liu W, Peng B, Cong S, Yu H, Zhang B, Li Y. Advancements in Characterization Techniques for Microemulsions: From Molecular Insights to Macroscopic Phenomena. Molecules. 2024; 29(12):2901. https://doi.org/10.3390/molecules29122901
Chicago/Turabian StyleLi, Longfei, Jiepeng Qu, Weidong Liu, Baoliang Peng, Sunan Cong, Haobo Yu, Biao Zhang, and Yingying Li. 2024. "Advancements in Characterization Techniques for Microemulsions: From Molecular Insights to Macroscopic Phenomena" Molecules 29, no. 12: 2901. https://doi.org/10.3390/molecules29122901






