Stereoselective Solid-State Synthesis of Biologically Active Cyclobutane and Dicyclobutane Isomers via Conformation Blocking and Transference
Abstract
:1. Introduction
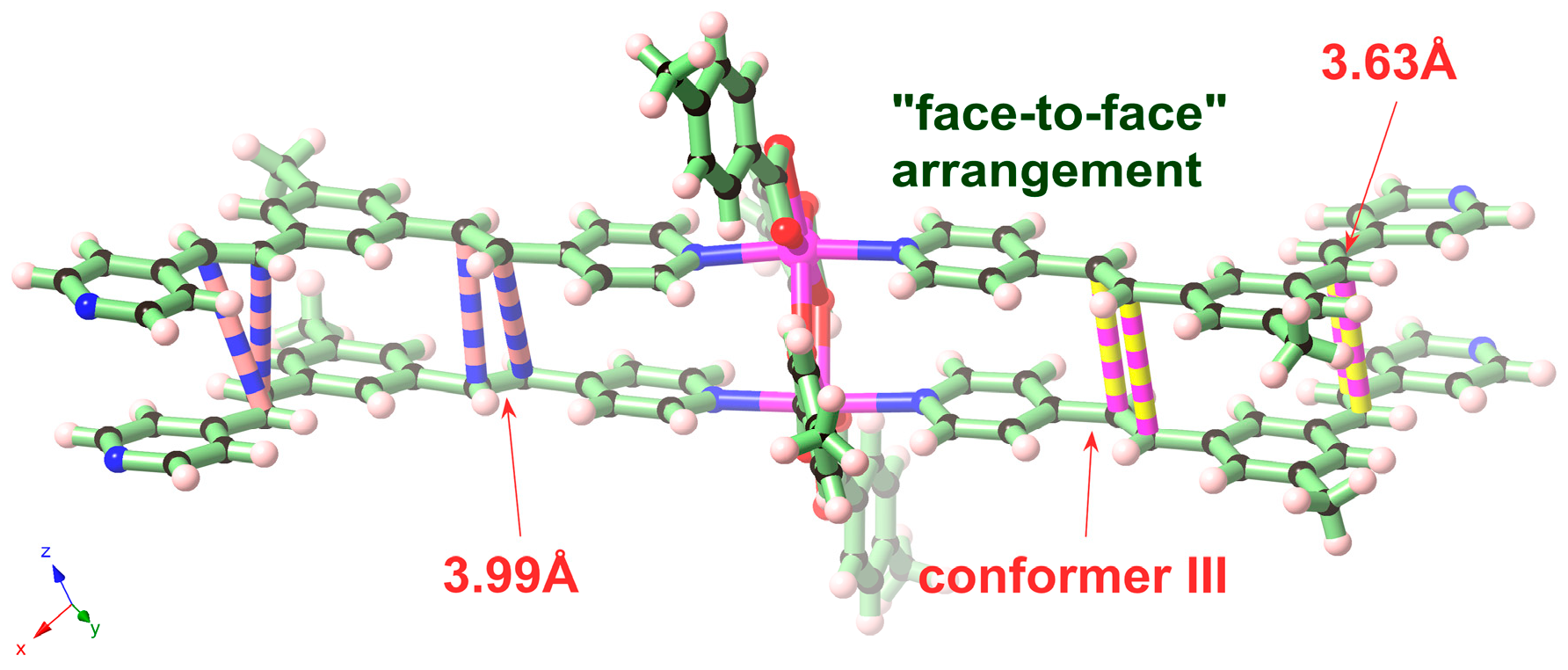
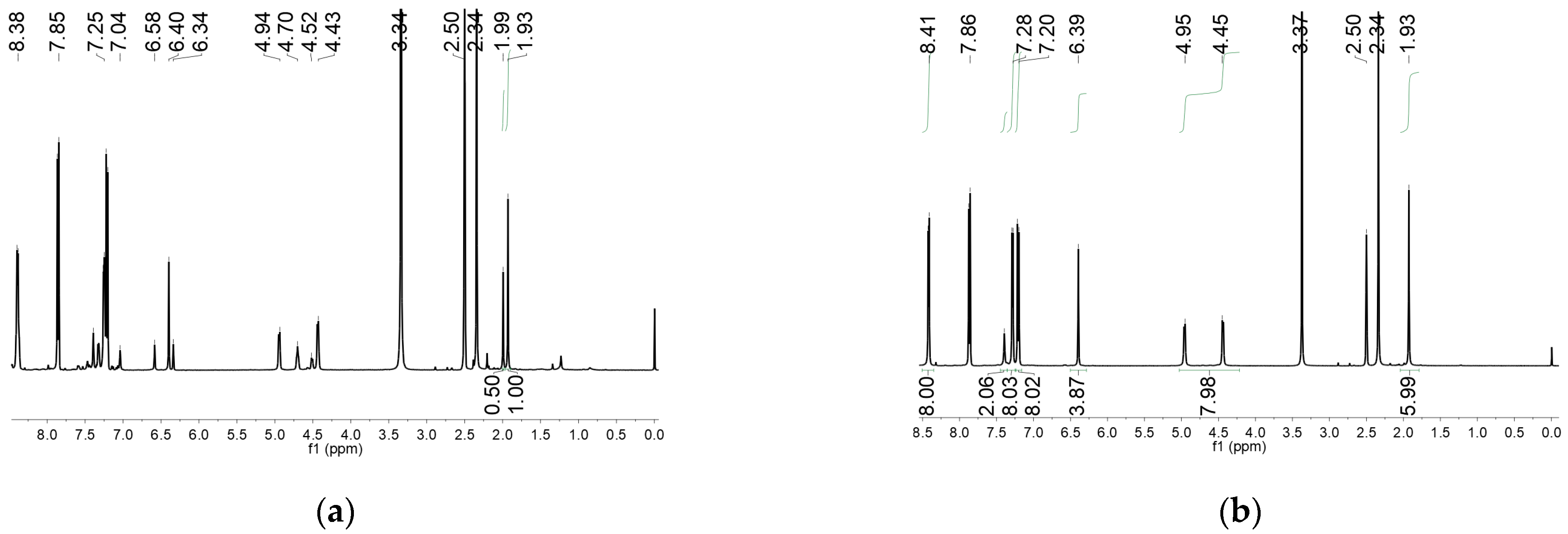
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parra, R.D.; Zeng, X.C. Staggered and eclipsed conformations of C2F6: A systematic ab initio study. J. Fluor. Chem. 1997, 83, 51–60. [Google Scholar] [CrossRef]
- Hu, F.L.; Wang, H.F.; Guo, D.; Zhang, H.; Lang, J.P.; Beves, J. Controlled formation of chiral networks and their reversible chiroptical switching behaviour by UV/microwave irradiation. Chem. Commun. 2016, 52, 7990–7993. [Google Scholar] [CrossRef]
- Wu, J.W.; Long, B.F.; Wang, M.F.; Young, D.J.; Hu, F.L.; Mi, Y.; Lang, J.P. Tunable photosalient behaviours within coordination polymers via functional molecular prearrangements. Chem. Commun. 2022, 58, 2674–2677. [Google Scholar] [CrossRef]
- MacGillivray, L.R.; Papaefstathiou, G.S.; Friscic, T.; Hamilton, T.D.; Bucar, D.K.; Chu, Q.; Varshney, D.B.; Georgiev, I.G. Supramolecular control of reactivity in the solid state: From templates to ladderanes to metal−organic frameworks. Acc. Chem. Res. 2008, 41, 280–291. [Google Scholar] [CrossRef]
- Hamilton, T.D.; Papaefstathiou, G.S.; Friscic, T.; Bucar, D.K.; MacGillivray, L.R. Onion-shell metal−organic polyhedra (MOPs): A general approach to decorate the exteriors of MOPs using principles of supramolecular chemistry. J. Am. Chem. Soc. 2008, 130, 14366–14367. [Google Scholar] [CrossRef]
- Kole, G.K.; Kojima, T.; Kawano, M.; Vittal, J.J. Reversible Single-Crystal-to-Single-Crystal Photochemical Formation and Thermal Cleavage of a Cyclobutane Ring. Angew. Chem. Int. Ed. 2014, 53, 2143–2146. [Google Scholar] [CrossRef]
- Medishetty, R.; Husain, A.; Bai, Z.; Runcevski, T.; Dinnebier, R.E.; Naumov, P.; Vittal, J.J. Single crystals pop under UV light: A photosalient effect triggered by a [2 + 2] cycloaddition reaction. Angew. Chem. Int. Ed. 2014, 53, 5907–5911. [Google Scholar] [CrossRef]
- Medishetty, R.; Koh, L.L.; Kole, G.K.; Vittal, J.J. Solid-State Structural Transformations from 2D Interdigitated Layers to 3D Interpenetrated Structures. Angew. Chem. Int. Ed. 2011, 50, 11141–11144. [Google Scholar] [CrossRef]
- Yang, S.Y.; Deng, X.L.; Jin, R.F.; Naumov, P.; Panda, M.K.; Huang, R.B.; Zheng, L.S.; Teo, B.K. Crystallographic snapshots of the interplay between reactive guest and host molecules in a porous coordination polymer: Stereochemical coupling and feedback mechanism of three photoactive centers triggered by UV-induced isomerization, dimerization, and polymerization reactions. J. Am. Chem. Soc. 2014, 136, 558–561. [Google Scholar]
- Islam, S.; Datta, J.; Maity, S.; Dutta, B.; Khan, S.; Ghosh, P.; Ray, P.P.; Mir, M.H. Photodimerization of a 1D ladder polymer through single-crystal to single-crystal transformation has an effect on electrical conductivity. Cryst. Growth Des. 2019, 19, 4057–4062. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhang, G.C.; Kwak, S.; Oh, E.J.; Yun, E.J.; Chomvong, K.; Cate, J.H.D.; Jin, Y.S. Overcoming the thermodynamic equilibrium of an isomerization reaction through oxidoreductive reactions for biotransformation. Nat. Commun. 2019, 10, 1356. [Google Scholar] [CrossRef]
- Liu, D.; Ren, Z.G.; Li, H.X.; Lang, J.P.; Li, N.Y.; Abrahams, B.F. Single-Crystal-to-Single-Crystal Transformations of Two Three-Dimensional Coordination Polymers through Regioselective [2 + 2] Photodimerization Reactions. Angew. Chem. Int. Ed. 2010, 49, 4767–4770. [Google Scholar] [CrossRef]
- Hu, F.L.; Mi, Y.; Zhu, C.; Abrahams, B.F.; Braunstein, P.; Lang, J.P. Stereoselective Solid-State Synthesis of Substituted Cyclobutanes Assisted by Pseudorotaxane-like MOFs. Angew. Chem. Int. Ed. 2018, 57, 12878–12883. [Google Scholar] [CrossRef]
- Kawamichi, T.; Kodama, T.; Kawano, M.; Fujita, M. Single-crystalline molecular flasks: Chemical transformation with bulky reagents in the pores of porous coordination networks. Angew. Chem. Int. Ed. 2008, 47, 8030–8032. [Google Scholar] [CrossRef]
- Yan, T.; Sun, L.Y.; Deng, Y.X.; Han, Y.F.; Jin, G.X. Facile Synthesis of Size-Tunable Functional Polyimidazolium Macrocycles through a Photochemical Closing Strategy. Chem. Eur. J. 2015, 21, 17610–17613. [Google Scholar] [CrossRef]
- Kato, T.; Nakamura, Y.; Morita, Y. Reactions Using Micellar Systems IV Regioselectivity in the Photodimerizations of 2-Pyridones in Micelles and Reversed Micelles. Chem. Pharm. Bull. 1983, 31, 2552–2563. [Google Scholar] [CrossRef]
- Li, N.Y.; Liu, D.; Ren, Z.G.; Lollar, C.; Lang, J.P.; Zhou, H.C. Controllable fluorescence switching of a coordination chain based on the photoinduced single-crystal-to-single-crystal reversible transformation of a syn-[2.2] metacyclophane. Inorg. Chem. 2018, 57, 849–856. [Google Scholar] [CrossRef]
- Kusaka, S.; Kiyose, A.; Sato, H.; Hijikata, Y.; Hori, A.; Ma, Y.; Matsuda, R. Dynamic topochemical reaction tuned by guest molecules in the nanospace of a metal–organic framework. J. Am. Chem. Soc. 2019, 141, 15742–15746. [Google Scholar] [CrossRef]
- Claassens, I.E.; Barbour, L.J.; Haynes, D.A. A multistimulus responsive porous coordination polymer: Temperature-mediated control of solid-state [2 + 2] cycloaddition. J. Am. Chem. Soc. 2019, 141, 11425–11429. [Google Scholar] [CrossRef]
- Brimioulle, R.; Bach, T. Enantioselective Lewis acid catalysis of intramolecular enone [2 + 2] photocycloaddition reactions. Science 2013, 342, 840–843. [Google Scholar] [CrossRef]
- Pagire, S.K.; Hossain, A.; Traub, L.; Kerres, S.; Reiser, O. Photosensitised regioselective [2 + 2]-cycloaddition of cinnamates and related alkenes. Chem. Commun. 2017, 53, 12072–12075. [Google Scholar] [CrossRef]
- Mangion, I.K.; MacMillan, D.W. Total synthesis of brasoside and littoralisone. J. Am. Chem. Soc. 2005, 127, 3696–3697. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, K.; Li, B.; Cui, L.; Li, J.; Jia, X.; Zhao, H.; Fang, J.; Li, C. Efficient separation of cis-and trans-1,2-dichloroethene isomers by adaptive biphen [3] arene crystals. Angew. Chem. Int. Ed. 2019, 131, 10387–10390. [Google Scholar] [CrossRef]
- Wei, Y.S.; Zhang, M.; Liao, P.Q.; Lin, R.B.; Li, T.Y.; Shao, G.; Zhang, J.P.; Chen, X.M. Coordination templated [2+2+2] cyclotrimerization in a porous coordination framework. Nat. Commun. 2015, 6, 8348. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Takeyama, Y.; Kusukawa, T.; Fujita, M. Cavity-directed, highly stereoselective [2 + 2] photodimerization of olefins within self-assembled coordination cages. Angew. Chem. Int. Ed. 2002, 41, 1347–1349. [Google Scholar] [CrossRef]
- Claassens, I.E.; Nikolayenko, V.I.; Haynes, D.A.; Barbour, L.J. Solvent-Mediated Synthesis of Cyclobutane Isomers in a Photoactive Cadmium (II) Porous Coordination Polymer. Angew. Chem. Int. Ed. 2018, 57, 15563–15566. [Google Scholar] [CrossRef]
- Hamilton, T.D.; Papaefstathiou, G.S.; MacGillivray, L.R. Template-controlled reactivity: Following nature’s way to design and construct metal-organic polyhedra and polygons. J. Solid State Chem. 2005, 178, 2409–2413. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Ramamurthy, V. Self-assembled coordination cage as a reaction vessel: Triplet sensitized [2 + 2] photodimerization of acenaphthylene, and [4 + 4] photodimerization of 9-anthraldehyde. Tetrahedron Lett. 2005, 46, 4495–4498. [Google Scholar] [CrossRef]
- Briceno, A.; Hill, Y.; Gonzalez, T.; Diaz, D.G. Combining hydrogen bonding and metal coordination for controlling topochemical [2 + 2] cycloaddition from multi-component assemblies. Dalton Trans. 2009, 9, 1602–1610. [Google Scholar] [CrossRef]
- Santra, R.; Biradha, K. Nitrate ion assisted argentophilic interactions as a template for solid state [2 + 2] photodimerization of pyridyl acrylic acid, its methyl ester, and acryl amide. Cryst. Growth Des. 2010, 10, 3315–3320. [Google Scholar] [CrossRef]
- Zhang, W.Z.; Han, Y.F.; Lin, Y.J.; Jin, G.X. [2 + 2] Photodimerization in the solid state aided by molecular templates of rectangular macrocycles bearing oxamidato ligands. Organometallics 2010, 29, 2842–2849. [Google Scholar] [CrossRef]
- Hu, F.L.; Wang, S.L.; Lang, J.P.; Abrahams, B.F. In-situ X-ray diffraction snapshotting: Determination of the kinetics of a photodimerization within a single crystal. Sci. Rep. 2014, 4, 6815. [Google Scholar] [CrossRef]
- Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Fujiwara, Y.I.; Kadota, K.; Nagarkar, S.S.; Tobori, N.; Kitagawa, S.; Horike, S. Synthesis of Oligodiacetylene Derivatives from Flexible Porous Coordination Frameworks. J. Am. Chem. Soc. 2017, 139, 13876–13881. [Google Scholar] [CrossRef]
- Gan, M.M.; Liu, J.Q.; Zhang, L.; Wang, Y.Y.; Hahn, F.E.; Han, Y.F. Preparation and post-assembly modification of metallosupramolecular assemblies from poly (N-heterocyclic carbene) ligands. Chem. Rev. 2018, 118, 9587–9641. [Google Scholar] [CrossRef]
- Hu, F.L.; Shi, Y.X.; Chen, H.H.; Lang, J.P. A Zn (II) coordination polymer and its photocycloaddition product: Syntheses, structures, selective luminescence sensing of iron (III) ions and selective absorption of dyes. Dalton Trans. 2015, 44, 18795–18803. [Google Scholar] [CrossRef]
- Gan, M.M.; Yu, J.G.; Wang, Y.Y.; Han, Y.F. Template-directed photochemical [2 + 2] cycloaddition in crystalline materials: A useful tool to access cyclobutane derivatives. Cryst. Growth Des. 2018, 18, 553–565. [Google Scholar] [CrossRef]
- Schmidt, G. M-J. Photodimerization in the solid state. Pure Appl. Chem. 1971, 27, 647–678. [Google Scholar] [CrossRef]
- MacGillivray, L.R.; Reid, J.L.; Ripmeester, J.A. Supramolecular control of reactivity in the solid state using linear molecular templates. J. Am. Chem. Soc. 2000, 122, 7817–7818. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Swenson, D.C.; MacGillivray, L.R. Conformational polymorphism in a heteromolecular single crystal leads to concerted movement akin to collective rack-and-pinion gears at the molecular level. Proc. Natl. Acad. Sci. USA 2008, 105, 1794–1797. [Google Scholar] [CrossRef]
- Inukai, M.; Fukushima, T.; Hijikata, Y.; Ogiwara, N.; Horike, S.; Kitagawa, S. Control of molecular rotor rotational frequencies in porous coordination polymers using a solid-solution approach. J. Am. Chem. Soc. 2015, 137, 12183–12186. [Google Scholar] [CrossRef]
- Hu, F.L.; Wang, S.L.; Abrahams, B.F.; Lang, J.P. Observance of a large conformational change associated with the rotation of the naphthyl groups during the photodimerization of criss-cross aligned C=C bonds within a 2D coordination polymer. CrystEngComm 2015, 17, 4903–4911. [Google Scholar] [CrossRef]
- Huang, Y.G.; Shiota, Y.; Su, S.Q.; Wu, S.Q.; Yao, Z.S.; Li, G.L.; Kanegawa, S.; Kang, S.; Kamachi, T.; Yoshizawa, K.; et al. Thermally Induced Intra-Carboxyl Proton Shuttle in a Molecular Rack-and-Pinion Cascade Achieving Macroscopic Crystal Deformation. Angew. Chem. Int. Ed. 2016, 55, 14628–14632. [Google Scholar] [CrossRef]
- Chiaravalloti, F.; Gross, L.; Rieder, K.H.; Stojkovic, S.; Gourdon, M.A.; Joachim, C.; Moresco, F. A rack-and-pinion device at the molecular scale. Nat. Mater. 2007, 6, 30–33. [Google Scholar] [CrossRef]
- Kelly, C.B.; Milligan, J.A.; Tilley, L.J.; Sodano, T.M. Bicyclobutanes: From curiosities to versatile reagents and covalent warheads. Chem. Sci. 2022, 13, 11721–11737. [Google Scholar] [CrossRef]
- Wang, N.; Yan, R.P.; Xiong, Y.S.; Mi, Y.; Hu, F.L.; Ge, Y.; Lang, J.P. Coordination Polymer- Mediated Molecular Surgery for Precise Interconversion of Dicyclobutane Compounds. Inorg. Chem. 2022, 61, 21016–21023. [Google Scholar] [CrossRef]
- Li, J.; Gao, K.; Bian, M.; Ding, H. Recent advances in the total synthesis of cyclobutane- containing natural products. Org. Chem. Front. 2020, 7, 136–154. [Google Scholar] [CrossRef]
- Wang, M.F.; Mi, Y.; Hu, F.L.; Niu, Z.; Yin, X.H.; Huang, Q.; Wang, H.F.; Lang, J.P. Coordination-driven stereospecific control strategy for pure cycloisomers in solid-state diene photocycloaddition. J. Am. Chem. Soc. 2019, 142, 700–704. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXLA. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- He, M.X.; Mo, Z.Y.; Wang, Z.Q.; Cheng, S.Y.; Xie, R.R.; Tang, H.T.; Pan, Y.M. Electrochemical Synthesis of 1-Naphthols by Intermolecular Annulation of Alkynes with 1,3-Dicarbonyl Compounds. Org. Lett. 2020, 22, 724–728. [Google Scholar] [CrossRef]
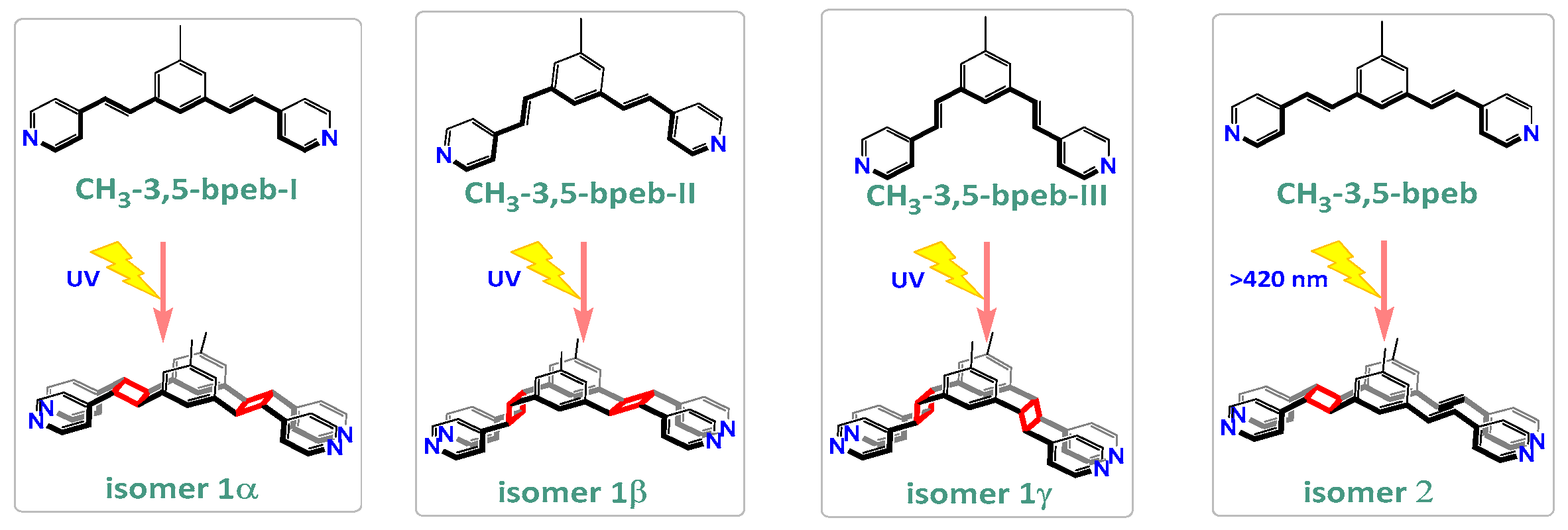

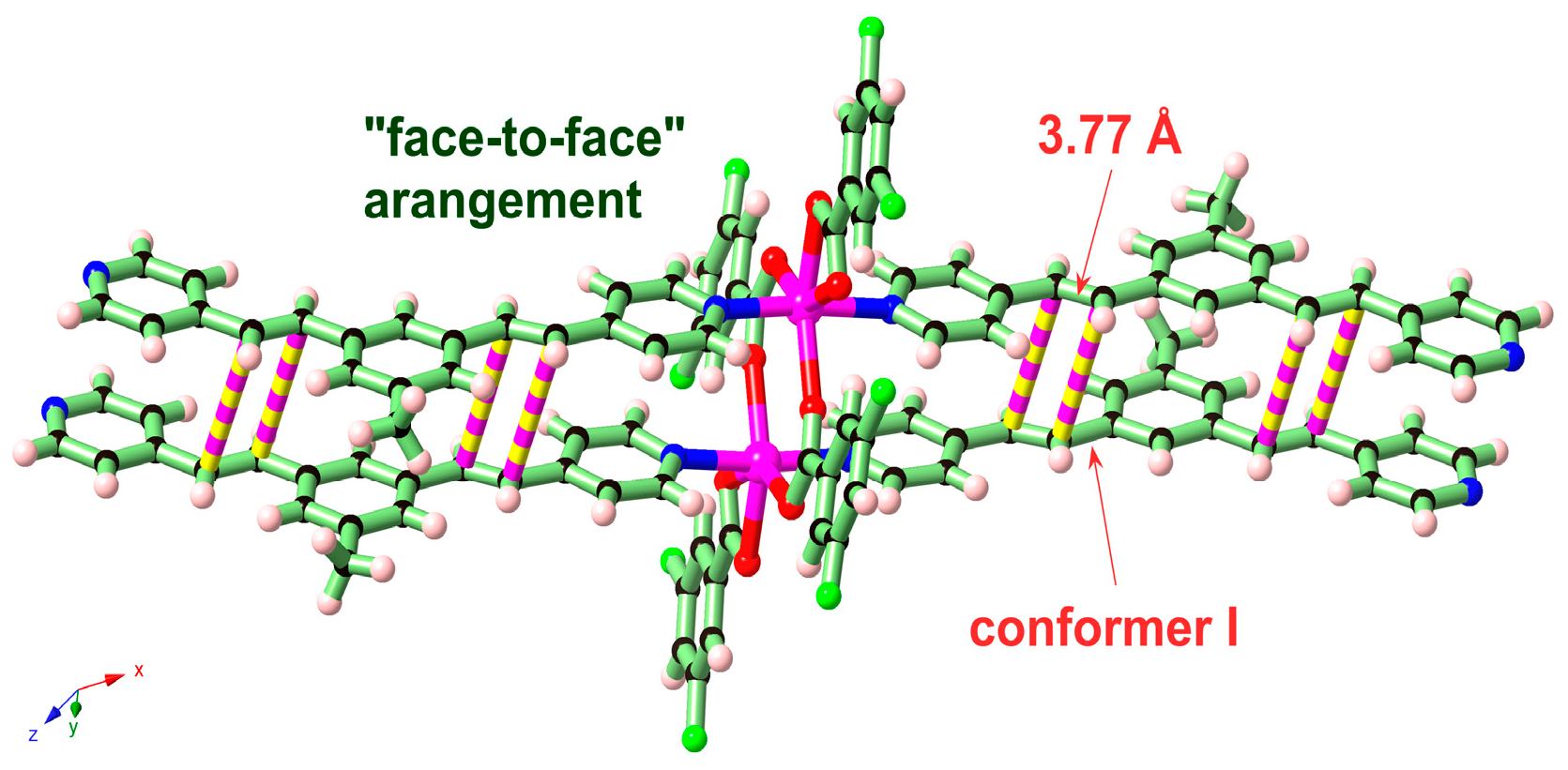

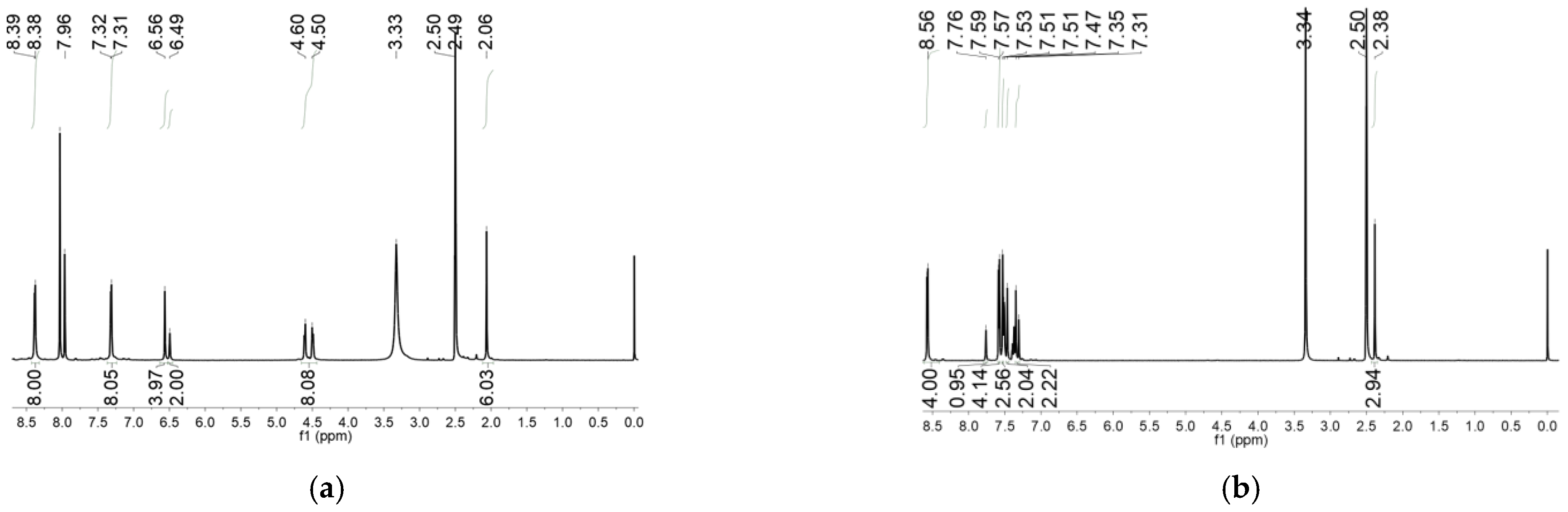

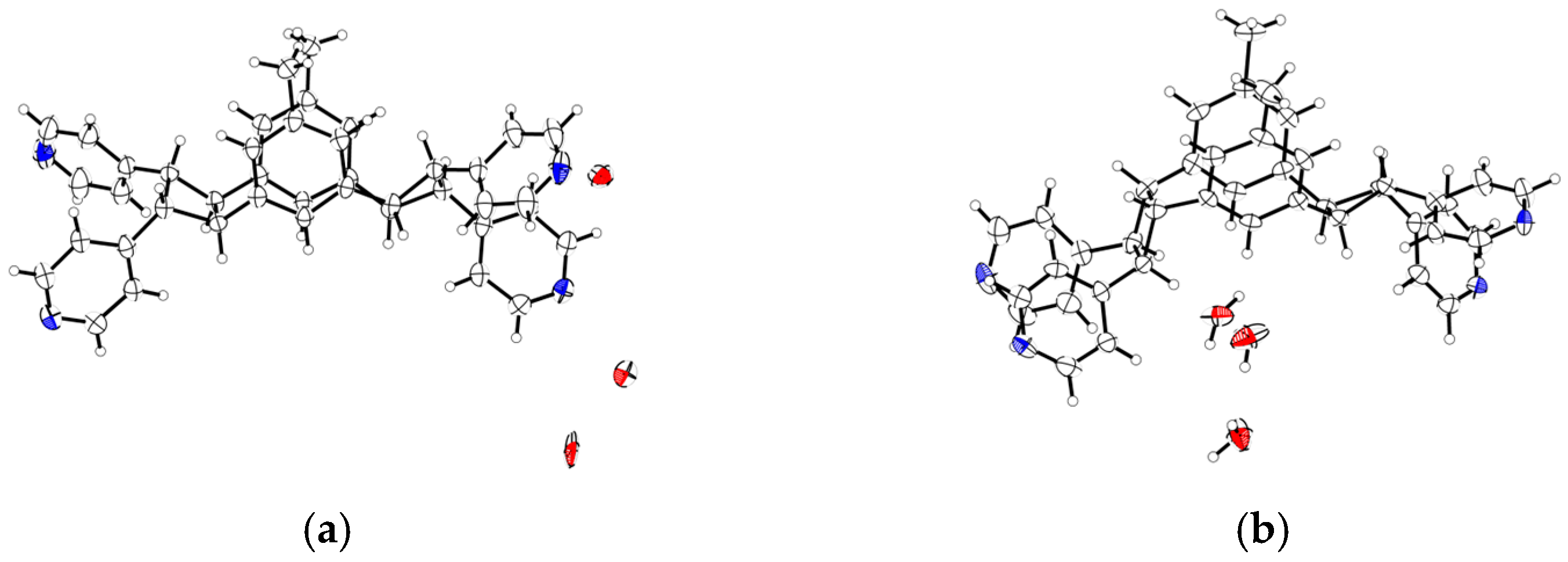
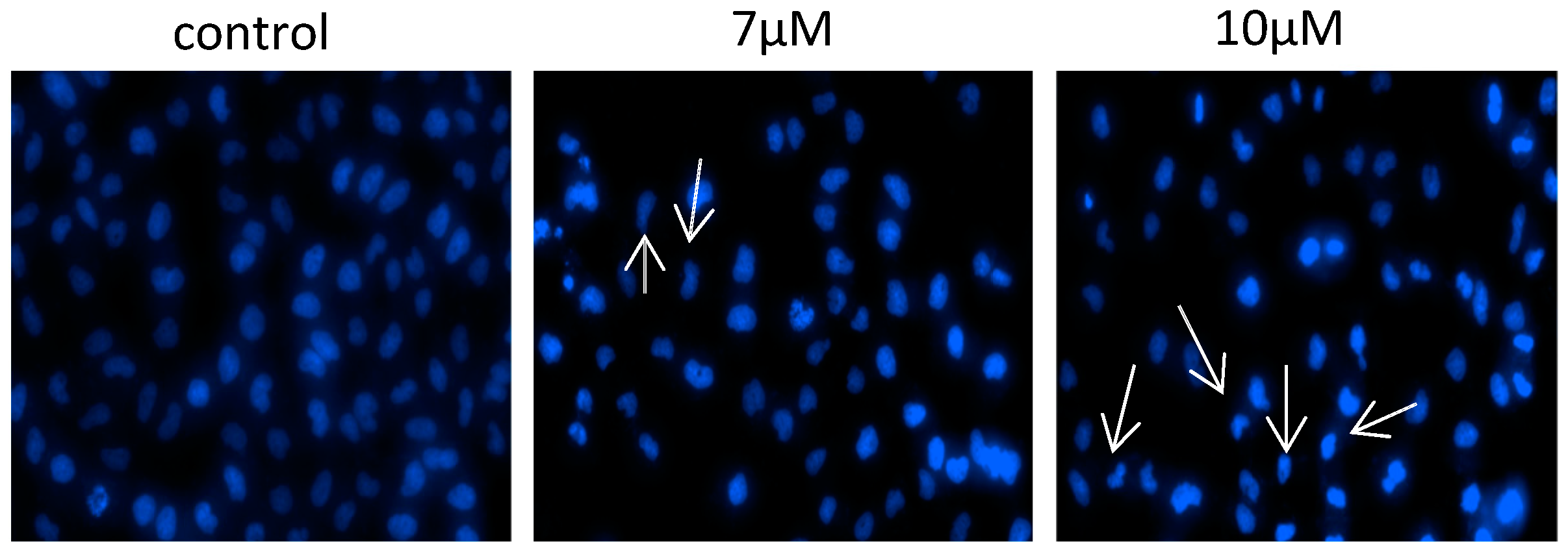

| Compounds | MGC-803 | T-24 | HepG-2 | BEL-7402 | HeLa | HL-7702 |
|---|---|---|---|---|---|---|
| Isomer 1α | >20 | >20 | >20 | >20 | >20 | >20 |
| Isomer 1β | 7.6 ± 0.2 | 7.3 ± 0.5 | 10.7 ± 0.6 | 18.8 ± 0.6 | 6.3 ± 0.3 | >20 |
| Isomer 1γ | >20 | 8.2 ± 0.9 | 10.6 ± 0.3 | 10.8 ± 0.7 | 13.3 ± 1.3 | >20 |
| Isomer 2 | >20 | 7.0 ± 0.3 | 8.2 ± 0.9 | 8.9 ± 1.2 | 6.2 ± 0.8 | >20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Z.; Gu, Y.; Young, D.; Hu, F.; Luo, Z. Stereoselective Solid-State Synthesis of Biologically Active Cyclobutane and Dicyclobutane Isomers via Conformation Blocking and Transference. Molecules 2024, 29, 2909. https://doi.org/10.3390/molecules29122909
Qin Z, Gu Y, Young D, Hu F, Luo Z. Stereoselective Solid-State Synthesis of Biologically Active Cyclobutane and Dicyclobutane Isomers via Conformation Blocking and Transference. Molecules. 2024; 29(12):2909. https://doi.org/10.3390/molecules29122909
Chicago/Turabian StyleQin, Zhen, Yunqiong Gu, Davidjames Young, Feilong Hu, and Zhirong Luo. 2024. "Stereoselective Solid-State Synthesis of Biologically Active Cyclobutane and Dicyclobutane Isomers via Conformation Blocking and Transference" Molecules 29, no. 12: 2909. https://doi.org/10.3390/molecules29122909








