In Situ Self-Growth of a ZnO Nanorod Array on Nonwoven Fabrics for Empowering Superhydrophobic and Antibacterial Features
Abstract
:1. Introduction
2. Results and Discussion
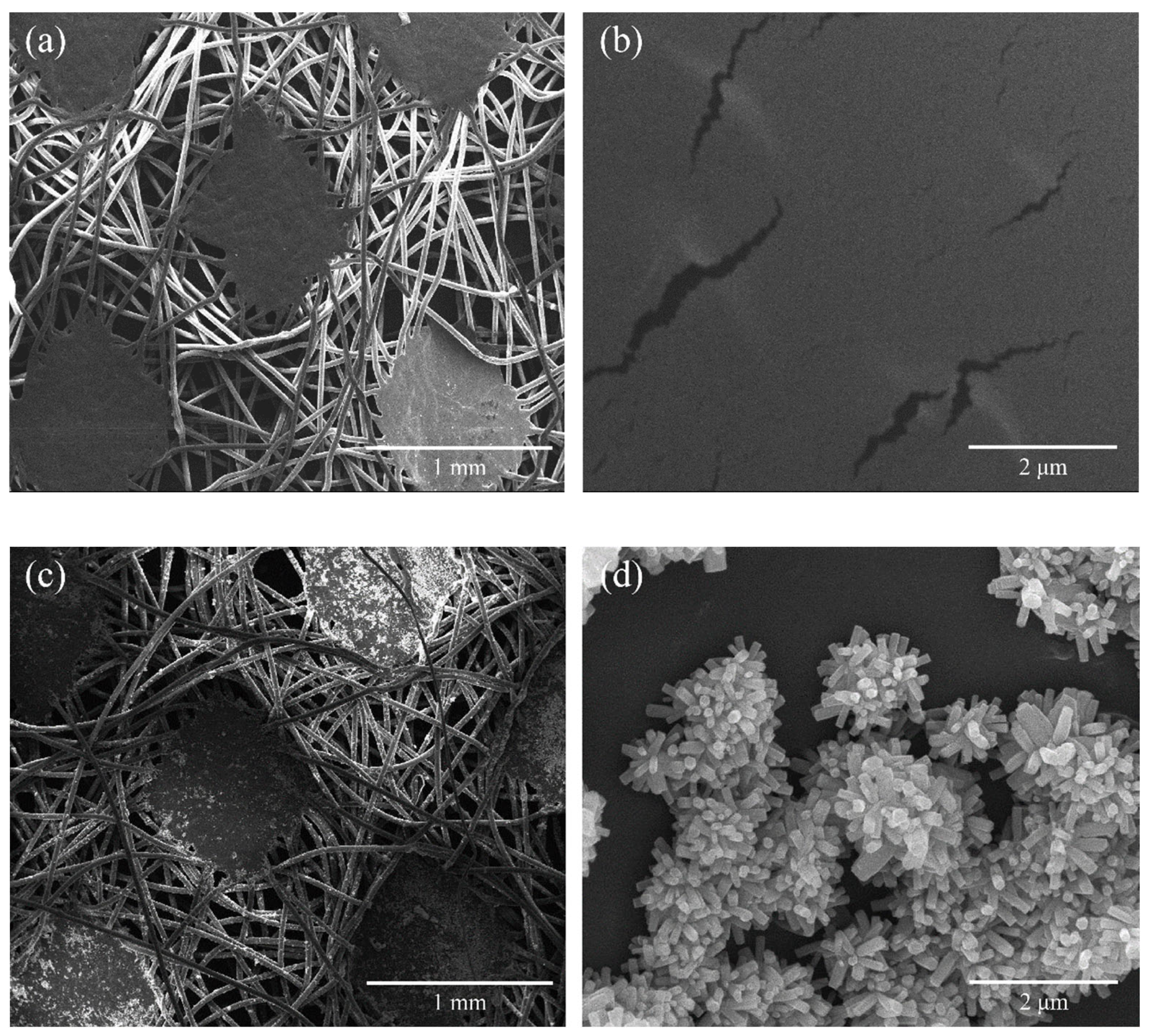
2.1. Material Characterization
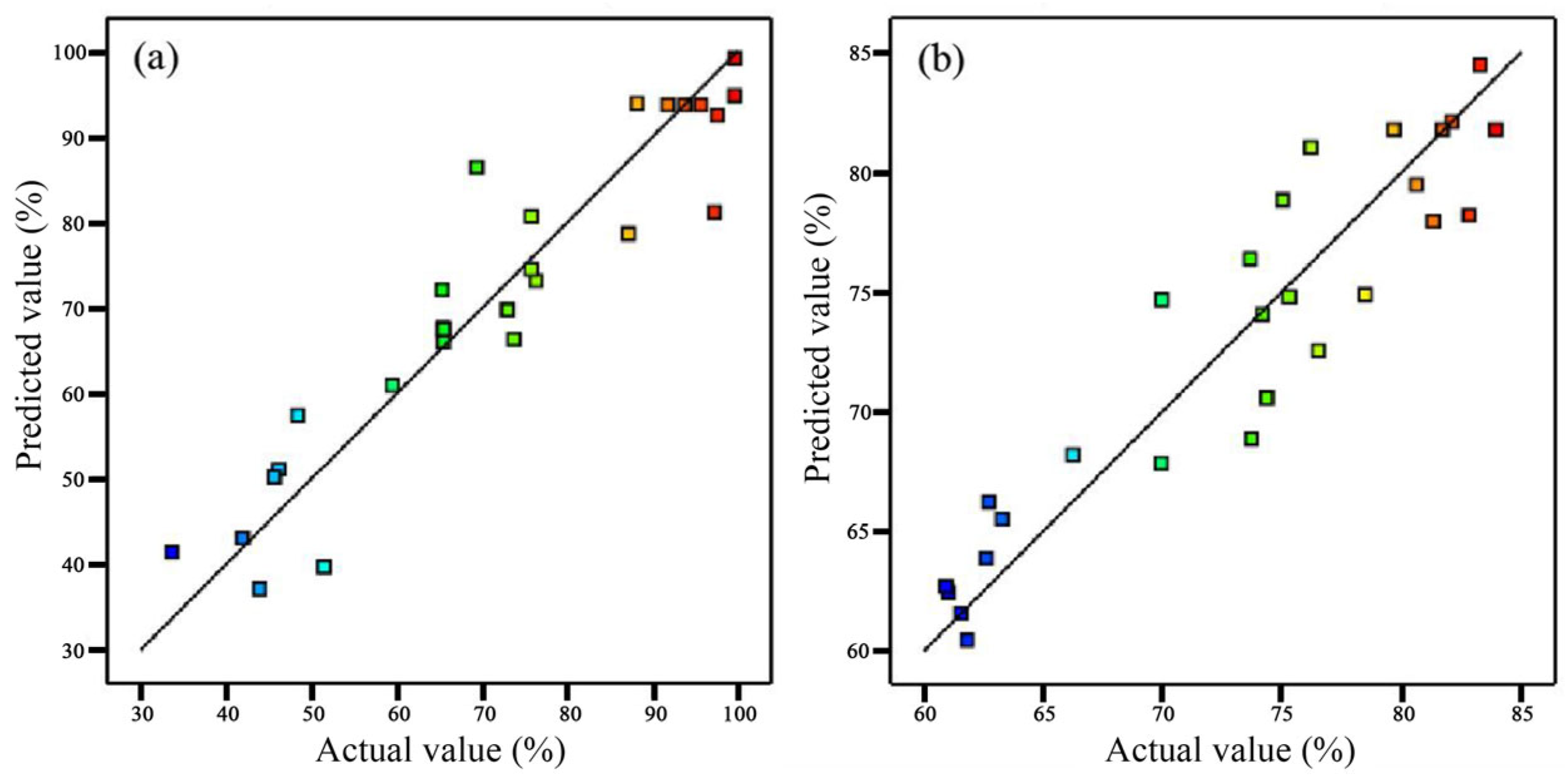
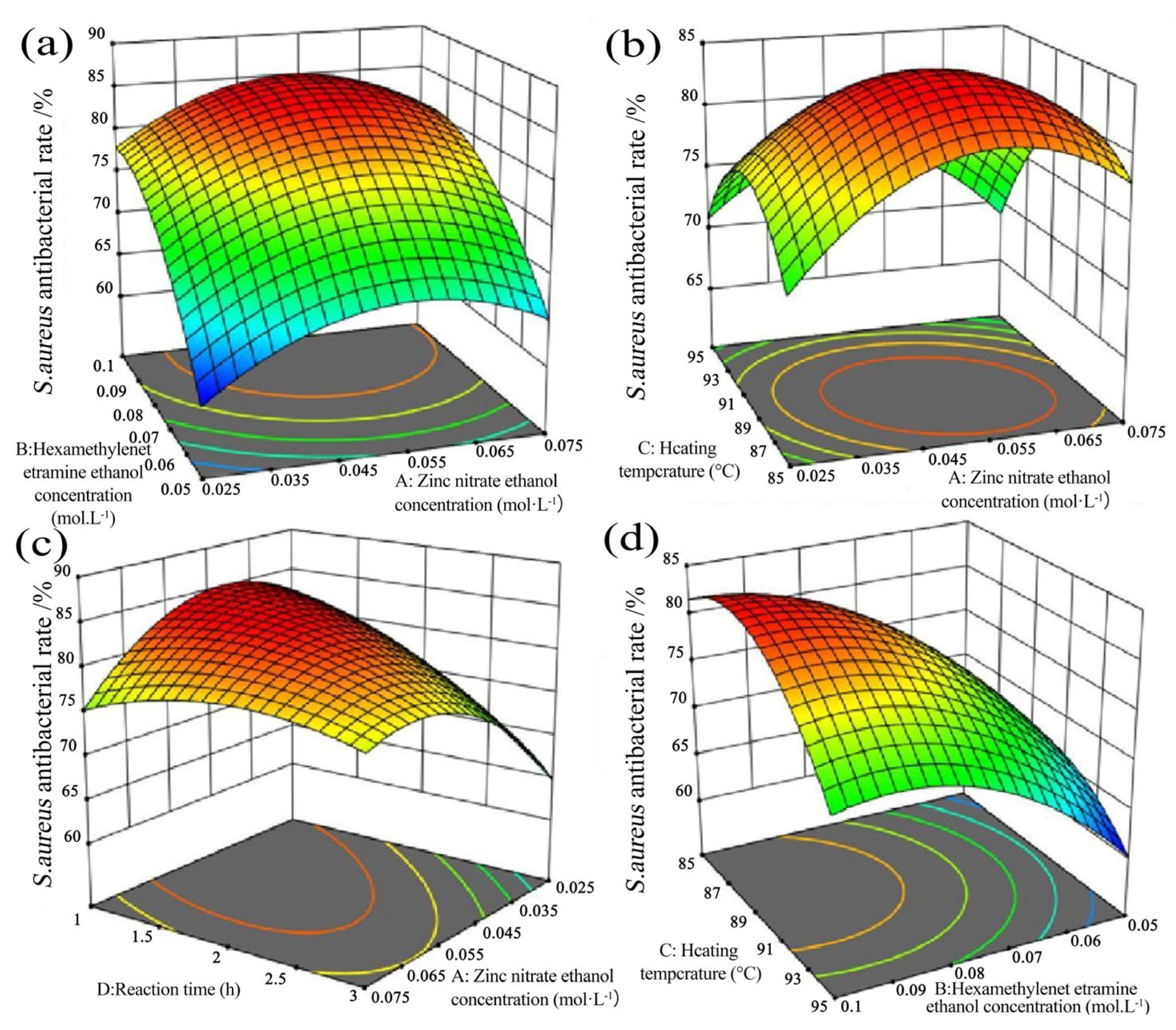
2.2. Determination of Optimal Conditions through Response Surface Methodology
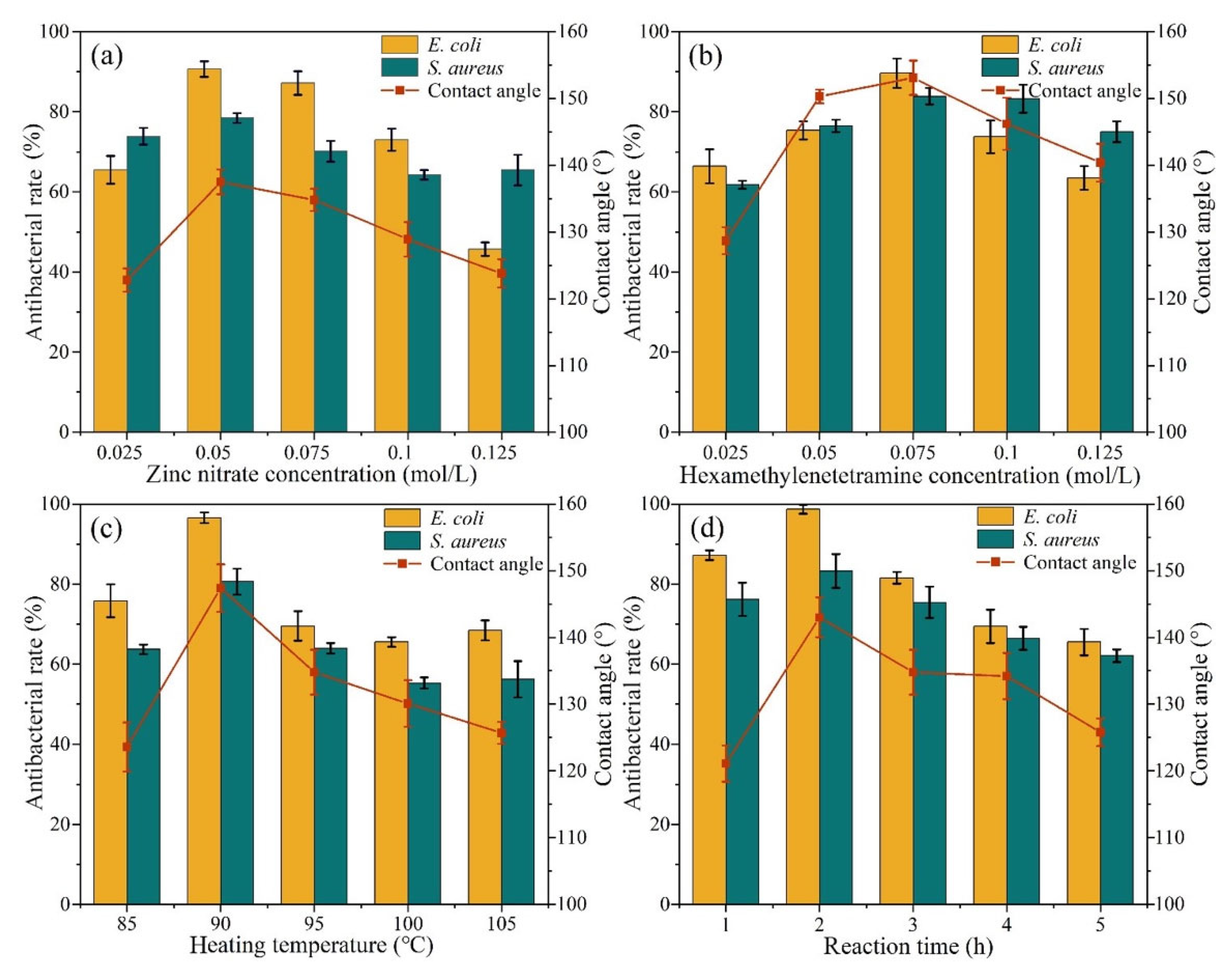
2.3. Analysis of Antibacterial and Superhydrophobic Effects of Single Factors
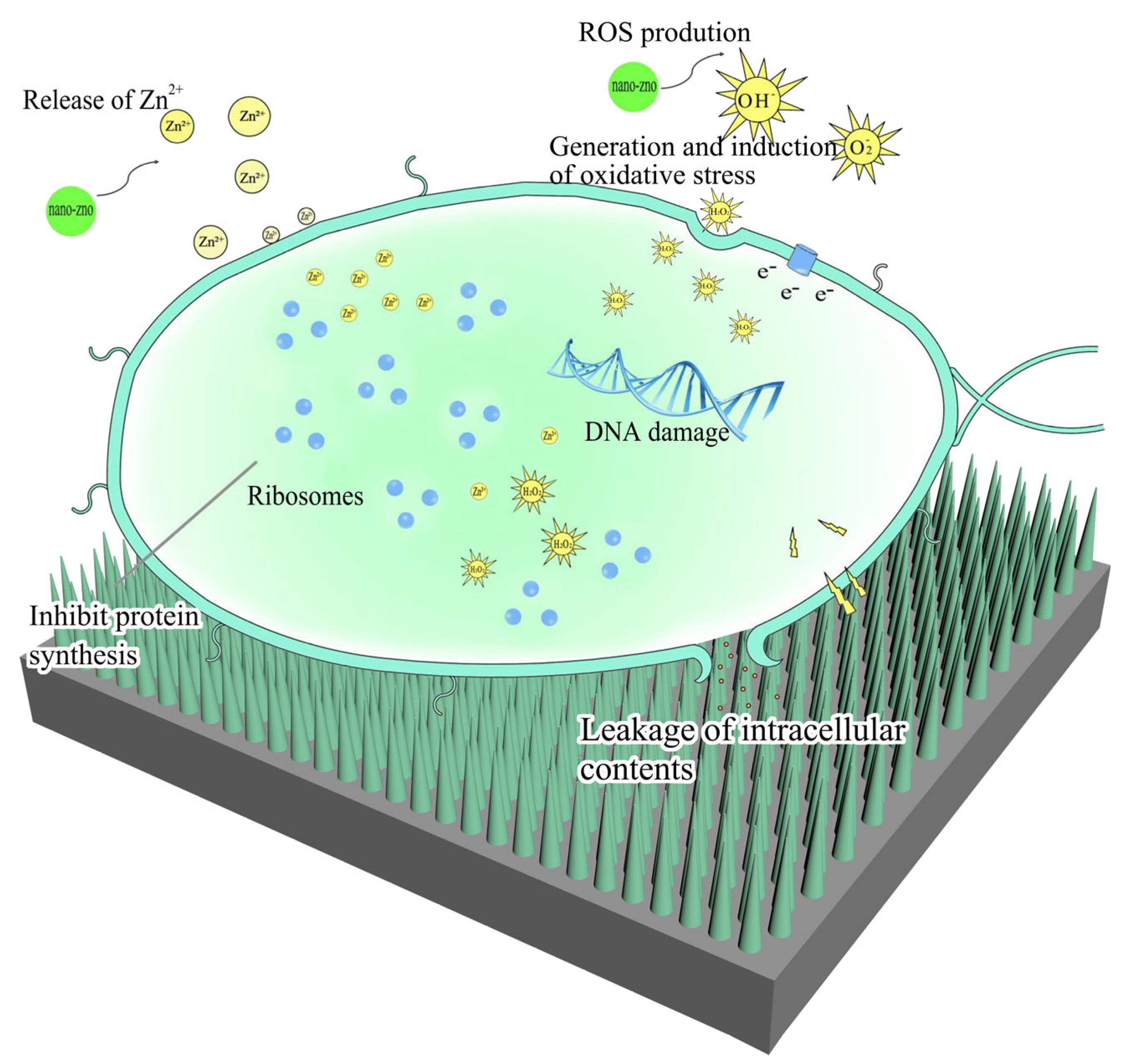
2.4. Discussion
3. Materials and Methods
3.1. Process of Material Synthesis
3.2. Examination of Antibacterial Properties
3.3. Characterization of Physicochemical Properties
3.4. Identification of Synthetic Factors
3.5. Quality Control
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohamed, A.M.; Al Sayyad, A.; Matar, E.; Isa, H.M.; Hasan, W.F.; Hashim, N.S.J.Y.; Alajaimi, B.A.; Aldolabi, Q. Factors associated with poor outcomes in patients with severe acute respiratory infections in Bahrain. Influenza Other Respir. Viruses 2023, 17, e13133. [Google Scholar] [CrossRef]
- Tang, Z.; Kong, N.; Zhang, X.; Liu, Y.; Hu, P.; Mou, S.; Liljestrom, P.; Shi, J.; Tan, W.; Kim, J.S.; et al. A materials-science perspective on tackling COVID-19. Nat. Rev. Mater. 2020, 5, 847–860. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Linklater, D.P.; Aburto-Medina, A.; Phuc, L.; Baulin, V.A.; Huu Khuong Duy, N.; Curtain, R.; Hanssen, E.; Gervinskas, G.; Ng, S.H.; et al. Antifungal versus antimicrobial defence of insect wings. J. Colloid Interface Sci. 2021, 603, 886–897. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antimicrobial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1626. [Google Scholar] [CrossRef]
- Rosenberg, M.; Visnapuu, M.; Vija, H.; Kisand, V.; Kasemets, K.; Kahru, A.; Ivask, A. Selective antibiofilm properties and biocompatibility of nano-ZnO and nano-ZnO/Ag coated surfaces. Sci. Rep. 2020, 10, 13478. [Google Scholar] [CrossRef]
- Hossain, M.I.; Edwards, J.; Tyler, J.; Anderson, J.; Bandyopadhyay, S. Antimicrobial properties of nanorods: Killing bacteria via impalement. IET Nanobiotechnol. 2017, 11, 501–505. [Google Scholar] [CrossRef]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Vi Khanh, T.; The Hong Phong, N.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; et al. Biophysical Model of Bacterial Cell Interactions with Nanopatterned Cicada Wing Surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef]
- Rizzello, L.; Sorce, B.; Sabella, S.; Vecchio, G.; Galeone, A.; Brunetti, V.; Cingolani, R.; Pompa, P.P. Impact of Nanoscale Topography on Genomics and Proteomics of Adherent Bacteria. ACS Nano 2011, 5, 1865–1876. [Google Scholar] [CrossRef]
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Xue, F.; Liu, J.; Guo, L.; Zhang, L.; Li, Q. Theoretical study on the bactericidal nature of nanopatterned surfaces. J. Theor. Biol. 2015, 385, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Shi, Y.; Pan, L.J.; Yang, M.; Peng, L.L.; Zong, S.; Shi, Y.; Yu, G.H. Multifunctional Superhydrophobic Surfaces Templated From Innately Microstructured Hydrogel Matrix. Nano Lett. 2014, 14, 4803–4809. [Google Scholar] [CrossRef]
- Huang, L.B.; Xu, S.Y.; Wang, Z.Y.; Xue, K.; Su, J.J.; Song, Y.; Chen, S.J.; Zhu, C.L.; Tang, B.Z.; Ye, R.Q. Self-Reporting and Photothermally Enhanced Rapid Bacterial Killing on a Laser-Induced Graphene Mask. ACS Nano 2020, 14, 12045–12053. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, L.; Guo, Y.; Sun, Z.; Jiang, Z.; Chen, S.; Ma, J.; Jerrams, S. Rapid fabrication of silver nanoparticle/polydopamine functionalized polyester fibers. Text. Res. J. 2019, 89, 3968–3978. [Google Scholar] [CrossRef]
- Modaresifar, K.; Azizian, S.; Ganjian, M.; Fratila-Apachitei, L.E.; Zadpoor, A.A. Bactericidal effects of nanopatterns: A systematic review. Acta Biomater. 2019, 83, 29–36. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Han, Z.; Wang, J.; Liu, S. Electrospinning of Neat Graphene Nanofibers. Fiber Mater. 2022, 4, 268–279. [Google Scholar] [CrossRef]
- Chen, D.; Chen, F.; Yuan, Y. Performance Testing and Evaluation of Novel Graphene Nonwoven Fabric for Medical Masks. China Med. Device Inf. 2022, 28, 17–20. [Google Scholar]
- Nicholas, N.J.; Franks, G.V.; Ducker, W.A. The mechanism for hydrothermal growth of zinc oxide. CrystEngComm 2012, 14, 1232–1240. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, K.; Cai, M. ZnO nanomaterials: Current advancements in antibacterial mechanisms and applications. Front. Chem. 2020, 8, 580. [Google Scholar] [CrossRef]
- Sarkar, J.; Ghosh, M.; Mukherjee, A.; Chattopadhyay, D.; Acharya, K. Biosynthesis and safety evaluation of ZnO nanoparticles. Bioprocess Biosyst. Eng. 2014, 37, 165–171. [Google Scholar] [CrossRef]
- Elbourne, A.; Chapman, J.; Gelmi, A.; Cozzolino, D.; Crawford, R.J.; Truong, V.K. Bacterial-nanostructure interactions: The role of cell elasticity and adhesion forces. J. Colloid Interface Sci. 2019, 546, 192–210. [Google Scholar] [CrossRef]
- Linklater, D.P.; Juodkazis, S.; Ivanova, E.P.J.N. Nanofabrication of mechano-bactericidal surfaces. Nanoscale 2017, 9, 16564–16585. [Google Scholar] [CrossRef]
- Bhadra, C.M.; Werner, M.; Baulin, V.A.; Truong, V.K.; Kobaisi, M.A.; Nguyen, S.H.; Balcytis, A.; Juodkazis, S.; Wang, J.Y.; Mainwaring, D.E.; et al. Subtle variations in surface properties of black silicon surfaces influence the degree of bactericidal efficiency. Nano-Micro Lett. 2018, 10, 36. [Google Scholar] [CrossRef]
- Orou, S.F.C.; Hang, K.J.; Thien, M.T.; Lee, Y.Y.; Foh, L.C.; Diem, N.D.N.; Hee, G.B.; Yong, P.S.; Fen, P.Y. Antimicrobial activity by ZnO nanorods and ZnO nanodisks: A model used to illustrate “Nanotoxicity Threshold”. J. Ind. Eng. Chem. 2018, 62, 333–340. [Google Scholar] [CrossRef]
- Zhou, H.F.; Wang, H.; Tian, X.Y.; Zheng, K.; Xu, F.; Su, Z.; Tian, K.H.; Li, Q.L.; Fang, F. Solvothermal synthesis of gallium-doped zinc oxide nanoparticles with tunable infrared absorption. Mater. Res. Express 2014, 1, 045022. [Google Scholar] [CrossRef]
- Moulahi, A.; Sediri, F. Controlled synthesis of nano-ZnO via hydro/solvothermal process and study of their optical properties. Optik 2016, 127, 7586–7593. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, J.; Guo, Z.; Liu, W. Is superhydrophobicity equal to underwater superoleophilicity? Hydrophilic wetting defects on a superhydrophobic matrix with switchable superdewetting in both air and water. J. Mater. Chem. A 2021, 9, 1471–1479. [Google Scholar] [CrossRef]
- Zhao, J.; Song, L.; Yin, J.; Ming, W.J.C.C. Anti-bioadhesion on hierarchically structured, superhydrophobic surfaces. Chem. Commun. 2013, 49, 9191–9193. [Google Scholar] [CrossRef]
- Jiang, R.; Hao, L.; Song, L.; Tian, L.; Fan, Y.; Zhao, J.; Liu, C.; Ming, W.; Ren, L.J.C.E.J. Lotus-leaf-inspired hierarchical structured surface with non-fouling and mechanical bactericidal performances. Chem. Eng. J. 2020, 398, 125609. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Jung, Y.C.; Barthlott, W.J.S.M. Fabrication of artificial Lotus leaves and significance of hierarchical structure for superhydrophobicity and low adhesion. Soft Matter 2009, 5, 1386–1393. [Google Scholar] [CrossRef]
- Dong, C.W.; Zhou, N.; Zhang, J.X.; Lai, W.A.; Xu, J.F.; Chen, J.L.; Yu, R.H.; Che, Y.P. Optimized preparation of gangue waste-based geopolymer adsorbent based on improved response surface methodology for Cd(II) removal from wastewater. Environ. Res. 2023, 221, 115246. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y.J.; Tang, X.N.; He, X.Y.; Yang, H.S. Optimised preparation and characterisation of silver-neodymium inorganic antimicrobial material through response surface methodology. Mater. Technol. 2017, 32, 210–218. [Google Scholar] [CrossRef]
- Cao, L.; Kiely, J.; Piano, M.; Luxton, R. A Copper Oxide/Zinc Oxide Composite Nano-Surface for Use in a Biosensor. Materials 2019, 12, 1126. [Google Scholar] [CrossRef]
- Xie, Y.; Wei, W.; Meng, F.; Qu, X.; Li, J.; Wang, L.; Zhou, Z. Electric-field assisted growth and mechanical bactericidal performance of ZnO nanoarrays with gradient morphologies. Nanotechnol. Rev. 2019, 8, 315–326. [Google Scholar] [CrossRef]
- Vadillo-Rodriguez, V.; Schooling, S.R.; Dutcher, J.R. In Situ Characterization of Differences in the Viscoelastic Response of Individual Gram-Negative and Gram-Positive Bacterial Cells. J. Bacteriol. 2009, 191, 5518–5525. [Google Scholar] [CrossRef]
- Mortensen, N.A.; Gonçalves, P.A.D.; Shuklin, F.A.; Cox, J.D.; Tserkezis, C.; Ichikawa, M.; Wolff, C. Surface-response functions obtained from equilibrium electron-density profiles. Nanophotonics 2021, 10, 3647–3657. [Google Scholar] [CrossRef]
- Humberg, S.; Grund, S. Response Surface Analysis with Missing Data. Multivar. Behav. Res. 2022, 57, 581–602. [Google Scholar] [CrossRef]
- Lee, D.-H.; Yang, J.-K.; Kim, K.-J. Dual-response optimization using a patient rule induction method. Qual. Eng. 2018, 30, 610–620. [Google Scholar] [CrossRef]
- Smith, J.R.; Larson, C. Statistical approaches in surface finishing. Part 3. Design-of-experiments. Trans. Inst. Met. Finish. 2019, 97, 289–294. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Hua, L.; Zhang, S.; Xu, J.; Shi, S. Optimization of preparation process of Xiaoyao powder by Orthogonal design and Box—Behnken response surface. West China J. Pharm. Sci. 2022, 37, 285–291. [Google Scholar]
- Avireddy, H.; Kannan, H.; Shankar, P.; Mani, G.K.; Kulandaisamy, A.J.; Rayappan, J.B.B. Non-mutually exclusive dual role of hexamethylenetetramine on the growth of ZnO nanostructures and their sensing footprints. Mater. Chem. Phys. 2018, 212, 394–402. [Google Scholar] [CrossRef]
- Narayanan, G.N.; Annamalai, K. Role of hexamethylenetetramine concentration on structural, morphological, optical and electrical properties of hydrothermally grown zinc oxide nanorods. J. Mater. Sci.-Mater. Electron. 2016, 27, 12209–12215. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, Y.; He, W.; Wang, Y. Optimal extraction process of Polygala tenuifolia Willd. glycoprotein by response surface experiments. China J. Tradit. Chin. Med. Pharm. 2016, 31, 4226–4229. [Google Scholar]
- Zhao, Y.; Hao, H.; Zhong, J.; Jiang, S.; Zhang, G.; Bi, J.; Yan, S.; Hou, H. Photothermocatalytic sterilization performance and mechanism of pure Nb2CTX MXenes nanosheets under infrared light irradiation. Appl. Surf. Sci. 2023, 613, 155990. [Google Scholar] [CrossRef]
- Tokunou, Y.; Toyofuku, M.; Nomura, N. Physiological Benefits of Oxygen-Terminating Extracellular Electron Transfer. mBio 2022, 13, e01957-22. [Google Scholar] [CrossRef]
- Liu, J.L.; Wang, Y.H.; Ma, J.Z.; Peng, Y.; Wang, A.Q. A review on bidirectional analogies between the photocatalysis and antimicrobial properties of ZnO. J. Alloys Compd. 2019, 783, 898–918. [Google Scholar] [CrossRef]
- Yu, J.L.; Tahmasebi, A.; Han, Y.N.; Yin, F.K.; Li, X.C. A review on water in low rank coals: The existence, interaction with coal structure and effects on coal utilization. Fuel Process. Technol. 2013, 106, 9–20. [Google Scholar] [CrossRef]
- Kwon, D.H.; Jin, E.H.; Yoo, D.H.; Roh, J.W.; Suh, D.; Commerell, W.; Huh, J.S. Analysis of the Response Characteristics of Toluene Gas Sensors with a ZnO Nanorod Structure by a Heat Treatment Process. Sensors 2022, 22, 4125. [Google Scholar] [CrossRef]
- Wei, H.; Wu, Y.; Lun, N.; Hu, C. Hydrothermal synthesis and characterization of ZnO nanorods. Mater. Sci. Eng. A 2005, 393, 80–82. [Google Scholar] [CrossRef]




| Source | E. coli Antibacterial Rate | Significance | S. aureus Antibacterial Rate | Significance | ||
|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | |||
| Model | 6.48 | 0.0013 | ** | 5.72 | 0.0022 | ** |
| A—Zn(NO3)2 | 7.89 | 0.0158 | * | 1.26 | 0.2837 | - |
| B—HMTA | 0.9064 | 0.3599 | - | 38.85 | < 0.0001 | ** |
| C—Temperature | 0.0487 | 0.8290 | - | 1.60 | 0.2297 | - |
| D—Duration | 13.70 | 0.0030 | ** | 13.32 | 0.0033 | ** |
| AB | 1.66 | 0.2214 | - | 0.0827 | 0.7786 | - |
| AC | 13.76 | 0.0030 | ** | 0.7918 | 0.3910 | - |
| AD | 0.1344 | 0.7203 | - | 5.31 | 0.0399 | * |
| BC | 9.82 | 0.0086 | ** | 1.01 | 0.3336 | - |
| BD | 5.06 | 0.0440 | * | 0.5836 | 0.4597 | - |
| CD | 7.46 | 0.0182 | * | 0.0882 | 0.7715 | - |
| A2 | 2.11 | 0.1717 | - | 10.12 | 0.0079 | ** |
| B2 | 17.89 | 0.0012 | ** | 7.01 | 0.0213 | * |
| C2 | 20.43 | 0.0007 | ** | 11.06 | 0.0060 | ** |
| D2 | 1.49 | 0.2460 | - | 2.46 | 0.1431 | - |
| Residues | 33.22 | 0.0296 | - | 4.46 | 0.1970 | - |
| Factor | Variables | Unit | Levels | ||
|---|---|---|---|---|---|
| Low | Medium | High | |||
| A | Zn(NO3)2 | mol/L | 0.025 | 0.050 | 0.075 |
| B | HMTA | mol/L | 0.050 | 0.075 | 0.100 |
| C | Temperature | °C | 85 | 90 | 95 |
| D | Duration | h | 1 | 2 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Liu, B.; Yang, A.; Zhang, P.; Li, W.; Su, Y. In Situ Self-Growth of a ZnO Nanorod Array on Nonwoven Fabrics for Empowering Superhydrophobic and Antibacterial Features. Molecules 2024, 29, 2916. https://doi.org/10.3390/molecules29122916
Yuan X, Liu B, Yang A, Zhang P, Li W, Su Y. In Situ Self-Growth of a ZnO Nanorod Array on Nonwoven Fabrics for Empowering Superhydrophobic and Antibacterial Features. Molecules. 2024; 29(12):2916. https://doi.org/10.3390/molecules29122916
Chicago/Turabian StyleYuan, Xiaoqi, Binghui Liu, Aili Yang, Peng Zhang, Wenjie Li, and Yueyu Su. 2024. "In Situ Self-Growth of a ZnO Nanorod Array on Nonwoven Fabrics for Empowering Superhydrophobic and Antibacterial Features" Molecules 29, no. 12: 2916. https://doi.org/10.3390/molecules29122916




