KCNT1 Channel Blockers: A Medicinal Chemistry Perspective
Abstract
1. Introduction
2. Potassium Channels: General Aspects and Involvement in Epilepsy
- Inwardly rectifying channels (2TM/P);
- Voltage-gated and ligand-gated channels (6TM/P);
- Hybrid channels made from the two previously mentioned classes (8TM/2P);
- Tandem pore domain channels (4TM/2P).

3. Medicinal Chemistry Strategies for Identification of KCNT1 Blockers
3.1. Drug Repurposing
3.2. High-Throughput and Virtual Screening Approaches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Epilepsy: A Public Health Imperative. Available online: https://www.ilae.org/files/dmfile/19053_Epilepsy_A-public-health-imperative-For-Web.pdf (accessed on 4 May 2024).
- Fisher, R.S.; Boas, W.v.E.; Blume, W.; Elger, C.; Genton, P.; Lee, P.; Engel, J. Epileptic Seizures and epilepsy: Definitions proposed by the international league against epilepsy (ILAE) and the international bureau for epilepsy (IBE). Epilepsia 2005, 46, 470–472. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; Van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W.; et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010, 51, 676–685. [Google Scholar] [CrossRef]
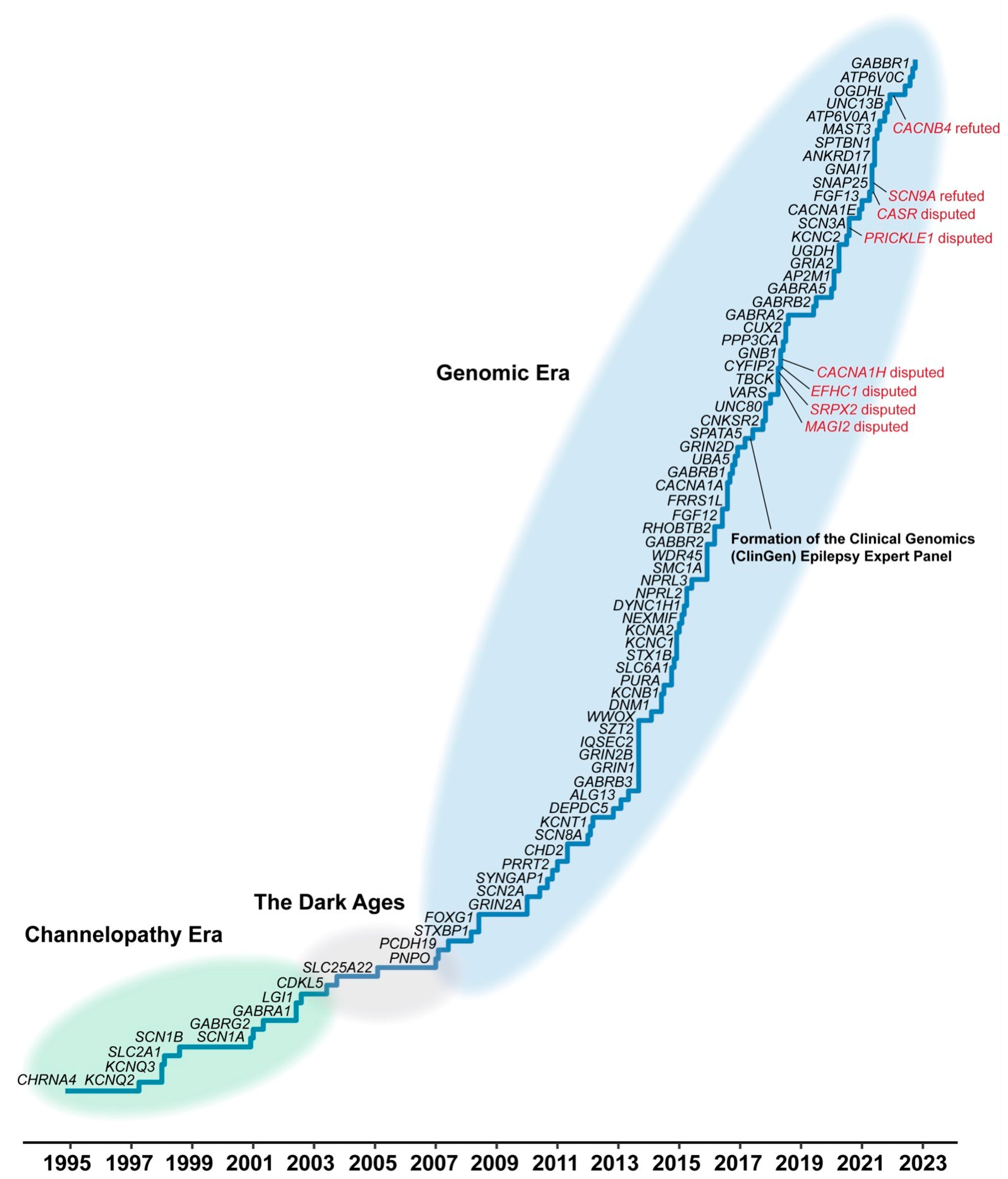
- Bonardi, C.M.; Heyne, H.O.; Fiannacca, M.; Fitzgerald, M.P.; Gardella, E.; Gunning, B.; Olofsson, K.; Lesca, G.; Verbeek, N.; Stamberger, H.; et al. KCNT1-related epilepsies and epileptic encephalopathies: Phenotypic and mutational spectrum. Brain 2021, 144, 3635–3650. [Google Scholar] [CrossRef]
- Rosati, A.; De Masi, S.; Guerrini, R. Antiepileptic drug treatment in children with epilepsy. CNS Drugs 2015, 29, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.H.; Guerrini, R. The epileptic encephalopathies. Handb. Clin. Neurol. 2013, 111, 619–626. [Google Scholar] [PubMed]
- Raga, S.; Specchio, N.; Rheims, S.; Wilmshurst, J.M. Developmental and epileptic encephalopathies: Recognition and approaches to care. Epileptic Disord. 2021, 23, 40–52. [Google Scholar] [CrossRef]
- Steinlein, O.K.; Mulley, J.C.; Propping, P.; Wallace, R.H.; Phillips, H.A.; Sutherland, G.R.; Scheffer, I.E.; Berkovic, S.F. A missense mutation in the neuronal nicotinic acetylcholine receptor α4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat. Genet. 1995, 11, 201–203. [Google Scholar] [CrossRef]
- Lopez-Santiago, L.; Isom, L.L. Dravet syndrome: A developmental and epileptic encephalopathy. Epilepsy Curr. 2019, 19, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Scala, M.; Efthymiou, S.; Sultan, T.; De Waele, J.; Panciroli, M.; Salpietro, V.; Maroofian, R.; Striano, P.; Van Petegem, F.; Houlden, H.; et al. Homozygous SCN1B variants causing early infantile epileptic encephalopathy 52 affect voltage-gated sodium channel function. Epilepsia 2021, 62, e82–e87. [Google Scholar] [CrossRef]
- Singh, N.A.; Charlier, C.; Stauffer, D.; DuPont, B.R.; Leach, R.J.; Melis, R.; Ronen, G.M.; Bjerre, I.; Quattlebaum, T.; Murphy, J.V.; et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat. Genet. 1998, 18, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Singh, N.A.; Ryan, S.G.; Lewis, T.B.; Reus, B.E.; Leach, R.J.; Leppert, M. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat. Genet. 1998, 18, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Cohen, J.; Pevsner, J.; Aradhya, S.; McKnight, D.; Butler, E.; Johnston, M.; Fatemi, A. A novel variant in GABRB2 associated with intellectual disability and epilepsy. Am. J. Med. Genet. Part A 2014, 164, 2914–2921. [Google Scholar] [CrossRef]
- Wallace, R.H.; Wang, D.W.; Singh, R.; Scheffer, I.E.; George, A.L.; Phillips, H.A.; Saar, K.; Reis, A.; Johnson, E.W.; Sutherland, G.R.; et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel ß1 subunit gene SCN1B. Nat. Genet. 1998, 19, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.A.; Berkovic, S.F.; Petrou, S. Mechanisms of human inherited epilepsies. Prog. Neurobiol. 2009, 87, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Helbig, I.; Xian, J.; Ruggiero, S. Beyond the Ion Channel. The ILAE Genetics Commission Blog. Available online: http://epilepsygenetics.net/2023/01/18/the-history-of-epilepsy-genetics/ (accessed on 4 May 2024).
- Oyrer, J.; Maljevic, S.; Scheffer, I.E.; Berkovic, S.F.; Petrou, S.; Reid, C.A.; Sexton, P.M. Ion channels in genetic epilepsy: From genes and mechanisms to disease-targeted therapies. Pharmacol. Rev. 2017, 70, 142–173. [Google Scholar] [CrossRef] [PubMed]
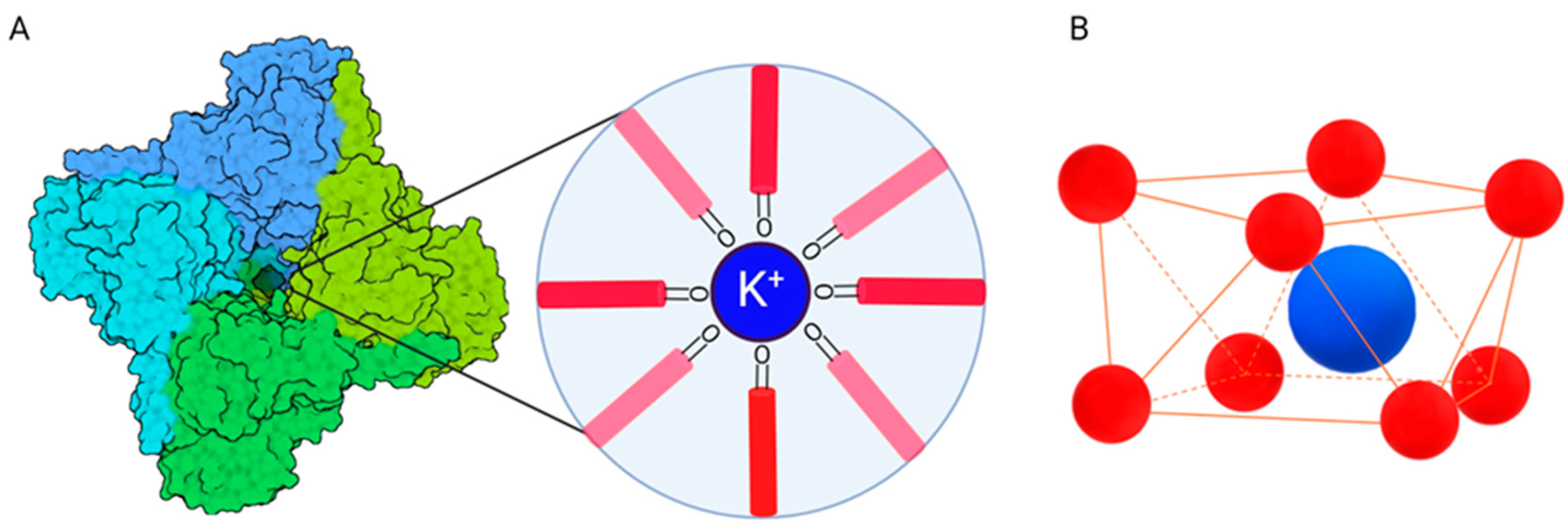
- MacKinnon, R. Potassium channels. FEBS Lett. 2003, 555, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.M.C.; Haynes, W.J.; Loukin, S.H.; Kung, C.; Saimi, Y. Prokaryotic K+ channels: From crystal structures to diversity. FEMS Microbiol. Rev. 2005, 29, 961–985. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Chaturvedi, P.; Sahu, P.; Ludhiadch, A.; Singh, P.; Singh, G.; Munshi, A. Role of potassium ion channels in epilepsy: Focus on current therapeutic strategies. CNS Neurol. Disord. Drug Targets 2024, 23, 67–87. [Google Scholar] [CrossRef]
- Kuang, Q.; Purhonen, P.; Hebert, H. Structure of potassium channels. Cell. Mol. Life Sci. 2015, 72, 3677–3693. [Google Scholar] [CrossRef]
- Doyle, D.A.; Cabral, J.o.M.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Choe, S. Potassium channel structures. Nat. Rev. Neurosci. 2002, 3, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Fowler, P.W.; Tai, K.; Sansom, M.S.P. The selectivity of K+ ion channels: Testing the hypotheses. Biophys. J. 2008, 95, 5062–5072. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Combi, R. Potassium channels and human epileptic phenotypes: An updated overview. Front. Cell. Neurosci. 2016, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Helbig, I.; Ellis, C.A. Personalized medicine in genetic epilepsies—Possibilities, challenges, and new frontiers. Neuropharmacology 2020, 172, 107970. [Google Scholar] [CrossRef] [PubMed]
- Soldovieri, M.V.; Ambrosino, P.; Mosca, I.; Miceli, F.; Franco, C.; Canzoniero, L.M.T.; Kline-Fath, B.; Cooper, E.C.; Venkatesan, C.; Taglialatela, M. Epileptic encephalopathy In a patient with a novel variant in the Kv7.2 S2 transmembrane segment: Clinical, genetic, and functional features. Int. J. Mol. Sci. 2019, 20, 3382. [Google Scholar] [CrossRef] [PubMed]
- Dilena, R.; Di Francesco, J.C.; Soldovieri, M.V.; Giacobbe, A.; Ambrosino, P.; Mosca, I.; Galli, M.A.; Guez, S.; Fumagalli, M.; Miceli, F.; et al. Early Treatment with quinidine in 2 patients with epilepsy of infancy with migrating focal seizures (EIMFS) due to Gain-of-Function KCNT1 mutations: Functional studies, clinical responses, and critical issues for personalized therapy. Neurotherapeutics 2018, 15, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Nappi, P.; Miceli, F.; Soldovieri, M.V.; Ambrosino, P.; Barrese, V.; Taglialatela, M. Epileptic channelopathies caused by neuronal Kv7 (KCNQ) channel dysfunction. Pflügers Arch. Eur. J. Physiol. 2020, 472, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Xu, H.Y.; Hu, H.N.; Tian, F.Y.; Chen, F.; Liu, H.N.; Zhan, L.; Pi, X.P.; Liu, J.; Gao, Z.B.; et al. Discovery of HN37 as a Potent and Chemically Stable Antiepileptic Drug Candidate. J. Med. Chem. 2021, 64, 5816–5837. [Google Scholar] [CrossRef] [PubMed]
- Musella, S.; Carotenuto, L.; Iraci, N.; Baroli, G.; Ciaglia, T.; Nappi, P.; Basilicata, M.G.; Salviati, E.; Barrese, V.; Vestuto, V.; et al. Beyond retigabine: Design, synthesis, and pharmacological characterization of a potent and chemically stable neuronal Kv7 channel activator with anticonvulsant activity. J. Med. Chem. 2022, 65, 11340–11364. [Google Scholar] [CrossRef]
- Kalappa, B.I.; Soh, H.; Duignan, K.M.; Furuya, T.; Edwards, S.; Tzingounis, A.V.; Tzounopoulos, T. Potent KCNQ2/3-specific channel activator suppresses in vivo epileptic activity and prevents the development of tinnitus. J. Neurosci. 2015, 35, 8829–8842. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Reed, N.; Liu, R.; Aizenman, E.; Wipf, P.; Tzounopoulos, T. Synthesis and evaluation of potent KCNQ2/3-specific channel activators. Mol. Pharmacol. 2016, 89, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.; Surur, A.S.; Beirow, K.; Kindermann, M.K.; Schulig, L.; Bodtke, A.; Bednarski, P.J.; Link, A. Sulfide analogues of flupirtine and retigabine with nanomolar KV7.2/KV7.3 channel opening activity. ChemMedChem 2019, 14, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Ostacolo, C.; Miceli, F.; Di Sarno, V.; Nappi, P.; Iraci, N.; Soldovieri, M.V.; Ciaglia, T.; Ambrosino, P.; Vestuto, V.; Lauritano, A.; et al. Synthesis and pharmacological characterization of conformationally restricted retigabine analogues as novel neuronal Kv7 channel activators. J. Med. Chem. 2019, 63, 163–185. [Google Scholar] [CrossRef] [PubMed]
- Awsare, B.; Lerner, J.; Ashbrenner, E.; Sevinsky, H.; Bozik, M.; Dworetzky, S.; Donahue, L.; Killingsworth, R.; Francoeur, B.; Qureshi, I. Phase 1 study evaluating the safety and tolerability of BHV-7000, a novel, selective Kv7.2/7.3 potassium channel activator, in healthy adults (P8-1.007). Neurology 2024, 102. [Google Scholar] [CrossRef]
- Kameyama, M.; Kakei, M.; Sato, R.; Shibasaki, T.; Matsuda, H.; Irisawa, H. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature 1984, 309, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Bader, C.R.; Bernheim, L.; Bertrand, D. Sodium-activated potassium current in cultured avian neurones. Nature 1985, 317, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Dryer, S.E.; Fujii, J.T.; Martin, A.R. A Na+-activated K+ current in cultured brain stem neurones from chicks. J. Physiol. 1989, 410, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Sun, L.; Wang, Y.; Jia, M.; Wang, Q.; Cai, X.; Wu, J. Double-edged role of KNa channels in brain tuning: Identifying epileptogenic network micro-macro disconnection. Curr. Neuropharmacol. 2022, 20, 916–928. [Google Scholar] [CrossRef]
- Hite, R.K.; Yuan, P.; Li, Z.; Hsuing, Y.; Walz, T.; MacKinnon, R. Cryo-electron microscopy structure of the Slo2.2 Na+-activated K+ channel. Nature 2015, 527, 198–203. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; von Hehn, C.A.; Mei, X.; Kaczmarek, L.K. Localization of the Na+-activated K+ channel Slick in the rat central nervous system. J. Comp. Neurol. 2005, 484, 80–92. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Gan, L.; Kaczmarek, L.K. Localization of the Slack potassium channel in the rat central nervous system. J. Comp. Neurol. 2002, 454, 241–254. [Google Scholar] [CrossRef]
- Joiner, W.J.; Tang, M.D.; Wang, L.Y.; Dworetzky, S.I.; Boissard, C.G.; Gan, L.; Gribkoff, V.K.; Kaczmarek, L.K. Formation of intermediate-conductance calcium-activated potassium channels by interaction of Slack and Slo subunits. Nat. Neurosci. 1998, 1, 462–469. [Google Scholar] [CrossRef]
- Gururaj, S.; Palmer, E.E.; Sheehan, G.D.; Kandula, T.; Macintosh, R.; Ying, K.; Morris, P.; Tao, J.; Dias, K.-R.; Zhu, Y.; et al. A de novo mutation in the sodium-activated potassium channel KCNT2 alters ion selectivity and causes epileptic encephalopathy. Cell Rep. 2017, 21, 926–933. [Google Scholar] [CrossRef]
- Ambrosino, P.; Soldovieri, M.V.; Bast, T.; Turnpenny, P.D.; Uhrig, S.; Biskup, S.; Döcker, M.; Fleck, T.; Mosca, I.; Manocchio, L.; et al. De novo gain-of-function variants in KCNT2 as a novel cause of developmental and epileptic encephalopathy. Ann. Neurol. 2018, 83, 1198–1204. [Google Scholar] [CrossRef]
- Cioclu, M.C.; Mosca, I.; Ambrosino, P.; Puzo, D.; Bayat, A.; Wortmann, S.B.; Koch, J.; Strehlow, V.; Shirai, K.; Matsumoto, N.; et al. KCNT2-related disorders: Phenotypes, functional, and pharmacological properties. Ann. Neurol. 2023, 94, 332–349. [Google Scholar] [CrossRef]
- Barcia, G.; Fleming, M.R.; Deligniere, A.; Gazula, V.-R.; Brown, M.R.; Langouet, M.; Chen, H.; Kronengold, J.; Abhyankar, A.; Cilio, R.; et al. De novo gain-of-function KCNT1 channel mutations cause malignant migrating partial seizures of infancy. Nat. Genet. 2012, 44, 1255–1259. [Google Scholar] [CrossRef]
- Heron, S.E.; Smith, K.R.; Bahlo, M.; Nobili, L.; Kahana, E.; Licchetta, L.; Oliver, K.L.; Mazarib, A.; Afawi, Z.; Korczyn, A.; et al. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat. Genet. 2012, 44, 1188–1190. [Google Scholar] [CrossRef]
- Rubboli, G.; Plazzi, G.; Picard, F.; Nobili, L.; Hirsch, E.; Chelly, J.; Prayson, R.A.; Boutonnat, J.; Bramerio, M.; Kahane, P.; et al. Mild malformations of cortical development in sleep-related hypermotor epilepsy due to KCNT1 mutations. Ann. Clin. Transl. Neurol. 2018, 6, 386–391. [Google Scholar] [CrossRef]
- Martin, H.C.; Kim, G.E.; Pagnamenta, A.T.; Murakami, Y.; Carvill, G.L.; Meyer, E.; Copley, R.R.; Rimmer, A.; Barcia, G.; Fleming, M.R.; et al. Clinical whole-genome sequencing in severe early-onset epilepsy reveals new genes and improves molecular diagnosis. Hum. Mol. Genet. 2014, 23, 3200–3211. [Google Scholar] [CrossRef]
- Ohba, C.; Kato, M.; Takahashi, N.; Osaka, H.; Shiihara, T.; Tohyama, J.; Nabatame, S.; Azuma, J.; Fujii, Y.; Hara, M.; et al. De novo KCNT1 mutations in early-onset epileptic encephalopathy. Epilepsia 2015, 56, e121–e128. [Google Scholar] [CrossRef]
- Lim, C.X.; Ricos, M.G.; Dibbens, L.M.; Heron, S.E. KCNT1 mutations in seizure disorders: The phenotypic spectrum and functional effects. J. Med. Genet. 2016, 53, 217–225. [Google Scholar] [CrossRef]
- Hinckley, C.A.; Zhu, Z.; Chu, J.H.; Gubbels, C.; Danker, T.; Cherry, J.J.; Whelan, C.D.; Engle, S.J.; Nguyen, V. Functional evaluation of epilepsy-associated KCNT1 variants in multiple cellular systems reveals a predominant gain of function impact on channel properties. Epilepsia 2023, 64, 2126–2136. [Google Scholar] [CrossRef]
- Kim, G.E.; Kronengold, J.; Barcia, G.; Quraishi, I.H.; Martin, H.C.; Blair, E.; Taylor, J.C.; Dulac, O.; Colleaux, L.; Nabbout, R.; et al. Human Slack potassium channel mutations increase positive cooperativity between individual channels. Cell Rep. 2014, 9, 1661–1672. [Google Scholar] [CrossRef]
- Fleming, M.R.; Brown, M.R.; Kronengold, J.; Zhang, Y.; Jenkins, D.P.; Barcia, G.; Nabbout, R.; Bausch, A.E.; Ruth, P.; Lukowski, R.; et al. Stimulation of Slack K+ channels alters mass at the plasma membrane by triggering dissociation of a phosphatase-regulatory complex. Cell Rep. 2016, 16, 2281–2288. [Google Scholar] [CrossRef]
- Zhang, Y.; Brown, M.R.; Hyland, C.; Chen, Y.; Kronengold, J.; Fleming, M.R.; Kohn, A.B.; Moroz, L.L.; Kaczmarek, L.K. Regulation of neuronal excitability by interaction of fragile X mental retardation protein with Slack potassium channels. J. Neurosci. 2012, 32, 15318–15327. [Google Scholar] [CrossRef]
- Milligan, C.J.; Li, M.; Gazina, E.V.; Heron, S.E.; Nair, U.; Trager, C.; Reid, C.A.; Venkat, A.; Younkin, D.P.; Dlugos, D.J.; et al. KCNT1 gain of function in 2 epilepsy phenotypes is reversed by quinidine. Ann. Neurol. 2014, 75, 581–590. [Google Scholar] [CrossRef]
- Bearden, D.; Strong, A.; Ehnot, J.; DiGiovine, M.; Dlugos, D.; Goldberg, E.M. Targeted treatment of migrating partial seizures of infancy with quinidine. Ann. Neurol. 2014, 76, 457–461. [Google Scholar] [CrossRef]
- Mikati, M.A.; Jiang, Y.H.; Carboni, M.; Shashi, V.; Petrovski, S.; Spillmann, R.; Milligan, C.J.; Li, M.; Grefe, A.; McConkie, A.; et al. Quinidine in the treatment of KCNT1-positive epilepsies. Ann. Neurol. 2015, 78, 995–999. [Google Scholar] [CrossRef]
- Xu, D.; Chen, S.; Yang, J.; Wang, X.; Fang, Z.; Li, M. Precision therapy with quinidine of KCNT1-related epileptic disorders: A systematic review. Br. J. Clin. Pharmacol. 2022, 88, 5096–5112. [Google Scholar] [CrossRef]
- Rizzo, F.; Ambrosino, P.; Guacci, A.; Chetta, M.; Marchese, G.; Rocco, T.; Soldovieri, M.V.; Manocchio, L.; Mosca, I.; Casara, G.; et al. Characterization of two de novo KCNT1 mutations in children with malignant migrating partial seizures in infancy. Mol. Cell. Neurosci. 2016, 72, 54–63. [Google Scholar] [CrossRef]
- Yang, B.; Gribkoff, V.; Pan, J.; Damagnez, V.; Dworetzky, S.; Boissard, C.; Bhattacharjee, A.; Yan, Y.; Sigworth, F.; Kaczmarek, L. Pharmacological activation and inhibition of Slack (Slo2.2) channels. Neuropharmacology 2006, 51, 896–906. [Google Scholar] [CrossRef]
- Conrad, K.A.; Molk, B.L.; Chidsey, C.A. Pharmacokinetic studies of quinidine in patients with arrhythmias. Circulation 1977, 55, 1–7. [Google Scholar] [CrossRef]
- Kusuhara, H.; Suzuki, H.; Terasaki, T.; Kakee, A.; Lemaire, M.; Sugiyama, Y. P-Glycoprotein mediates the efflux of quinidine across the blood-brain barrier. J. Pharmacol. Exp. Ther. 1997, 283, 574–580. [Google Scholar]
- Nenov, N.I.; Crumb, W.J.; Pigott, J.D.; Harrison, L.H.; Clarkson, C.W. Quinidine interactions with human atrial potassium channels. Circ. Res. 1998, 83, 1224–1231. [Google Scholar] [CrossRef]
- Po, S.S.; Wang, D.W.; Yang, I.C.H.; Johnson, J.P.; Nie, L.; Bennett, P.B. Modulation of HERG potassium channels by extracellular magnesium and quinidine. J. Cardiovasc. Pharmacol. 1999, 33, 181–185. [Google Scholar] [CrossRef]
- Paul, A.A.; Witchel, H.J.; Hancox, J.C. Inhibition of the current of heterologously expressed HERG potassium channels by flecainide and comparison with quinidine, propafenone and lignocaine. Br. J. Pharmacol. 2009, 136, 717–729. [Google Scholar] [CrossRef]
- Finlayson, K.; Witchel, H.J.; McCulloch, J.; Sharkey, J. Acquired QT interval prolongation and HERG: Implications for drug discovery and development. Eur. J. Pharmacol. 2004, 500, 129–142. [Google Scholar] [CrossRef]
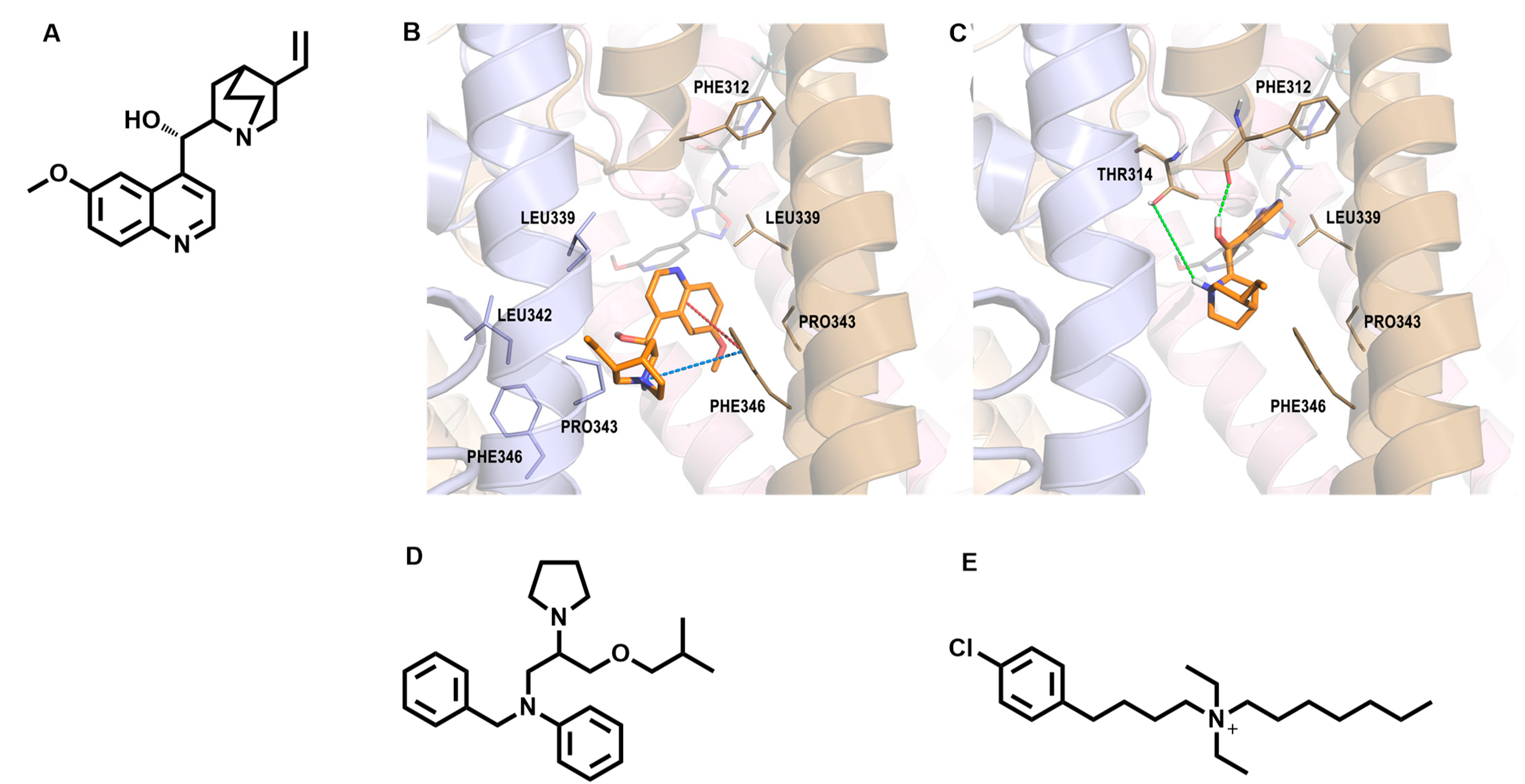
- Cole, B.A.; Johnson, R.M.; Dejakaisaya, H.; Pilati, N.; Fishwick, C.W.G.; Muench, S.P.; Lippiat, J.D. Structure-based identification and characterization of inhibitors of the epilepsy-associated KNa1.1 (KCNT1) potassium channel. iScience 2020, 23, 101100. [Google Scholar] [CrossRef]
- McTague, A.; Nair, U.; Malhotra, S.; Meyer, E.; Trump, N.; Gazina, E.V.; Papandreou, A.; Ngoh, A.; Ackermann, S.; Ambegaonkar, G.; et al. Clinical and molecular characterization of KCNT1-related severe early-onset epilepsy. Neurology 2018, 90, e55–e66. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Fan, J.; Yan, R.; Huang, B.; Zhou, F.; Yuan, T.; Gong, J.; Huang, Z.; Jiang, D. Structural basis of human Slo2.2 channel gating and modulation. Cell Rep. 2023, 42, 112858. [Google Scholar] [CrossRef]
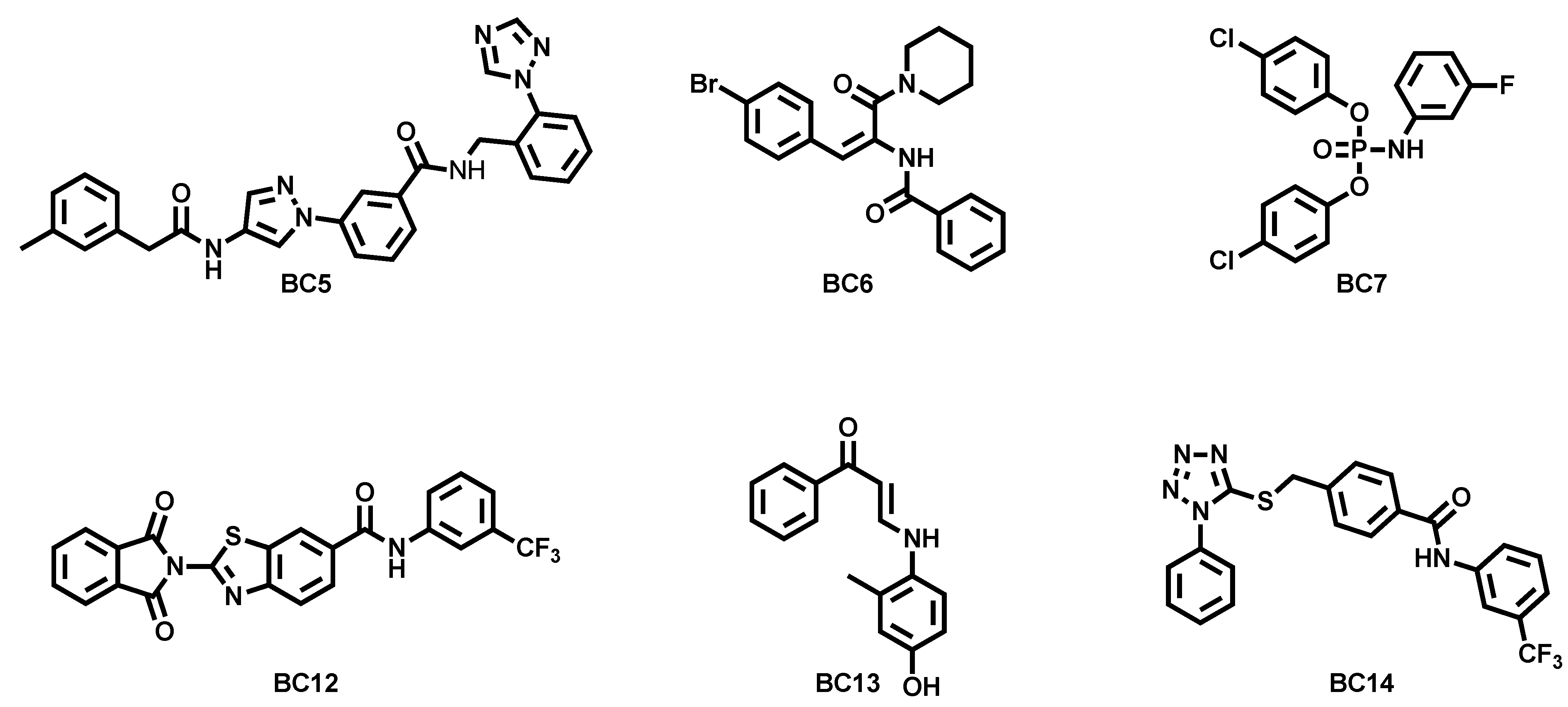
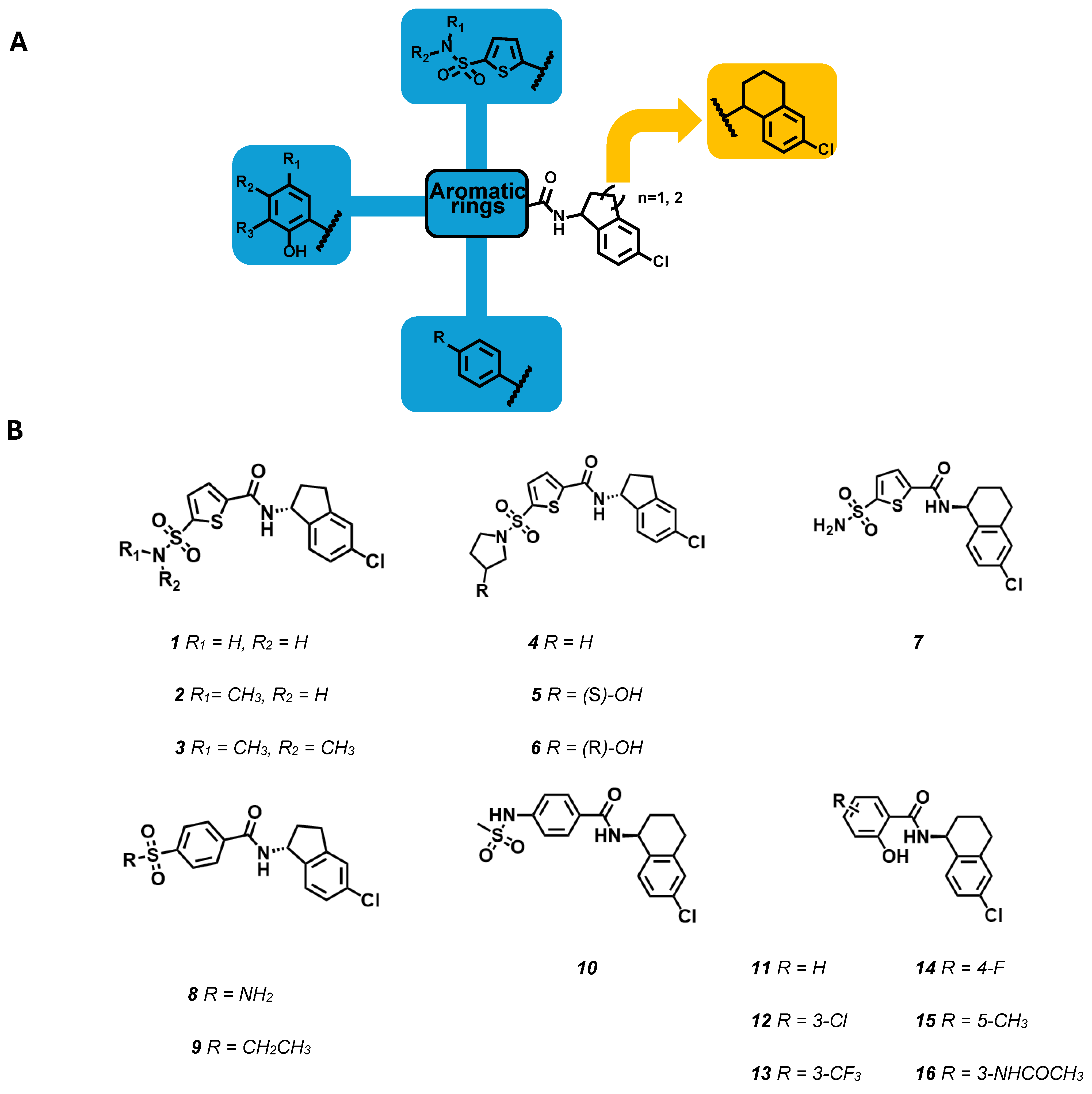
- Iraci, N.; Carotenuto, L.; Ciaglia, T.; Belperio, G.; Di Matteo, F.; Mosca, I.; Carleo, G.; Basilicata, M.G.; Ambrosino, P.; Turcio, R.; et al. In silico assisted identification, synthesis and in vitro pharmacological characterization of potent and selective blockers of the epilepsy-associated KCNT1 channel. J. Med. Chem. 2024, 67, 9124–9149. [Google Scholar] [CrossRef]
- Hollingshead, L.M.; Faulds, D.; Fitton, A. Bepridil. Drugs 1992, 44, 835–857. [Google Scholar] [CrossRef]
- Li, Y.; Sato, T.; Arita, M. Bepridil blunts the shortening of action potential duration caused by metabolic inhibition via blockade of ATP-sensitive K(+) channels and Na(+)-activated K(+) channels. J. Pharmacol. Exp. Ther. 1999, 291, 562–568. [Google Scholar]
- Șterbuleac, D.; Maniu, C.L. An antiarrhythmic agent as a promising lead compound for targeting the hEAG1 ion channel in cancer therapy: Insights from molecular dynamics simulations. Chem. Biol. Drug Des. 2016, 88, 683–689. [Google Scholar] [CrossRef]
- de Los Angeles Tejada, M.; Stolpe, K.; Meinild, A.K.; Klaerke, D.A. Clofilium inhibits Slick and Slack potassium channels. Biol. Targets Ther. 2012, 2012, 465–470. [Google Scholar]
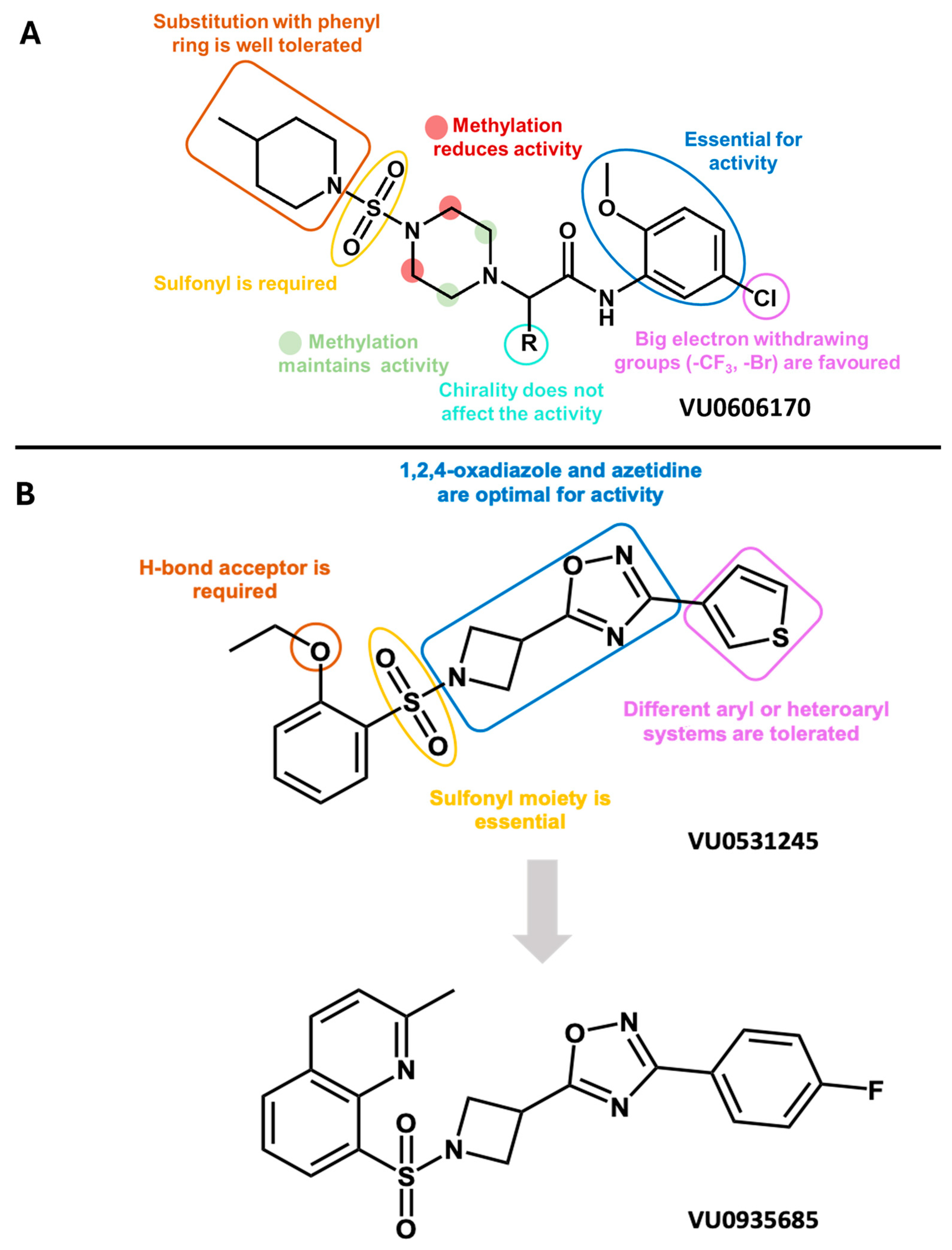
- Spitznagel, B.D.; Mishra, N.M.; Qunies, A.M.; Prael, F.J.; Du, Y.; Kozek, K.A.; Lazarenko, R.M.; Denton, J.S.; Emmitte, K.A.; Weaver, C.D. VU0606170, a selective Slack channels inhibitor, decreases calcium oscillations in cultured cortical neurons. ACS Chem. Neurosci. 2020, 11, 3658–3671. [Google Scholar] [CrossRef]
- Qunies, A.M.; Mishra, N.M.; Spitznagel, B.D.; Du, Y.; Acuña, V.S.; David Weaver, C.; Emmitte, K.A. Structure–activity relationship studies in a new series of 2-amino-N-phenylacetamide inhibitors of Slack potassium channels. Bioorganic Med. Chem. Lett. 2022, 76, 129013. [Google Scholar] [CrossRef]
- Qunies, A.M.; Spitznagel, B.D.; Du, Y.; David, W.C.; Emmitte, K.A. Design, synthesis, and biological evaluation of a novel series of 1,2,4-oxadiazole inhibitors of SLACK potassium channels: Identification of in vitro tool VU0935685. Bioorganic Med. Chem. 2023, 95, 117487. [Google Scholar] [CrossRef]
- Qunies, A.M.; Emmitte, K. Small-molecule inhibitors of Slack potassium channels as potential therapeutics for childhood epilepsies. Pharm. Pat. Anal. 2022, 11, 45–56. [Google Scholar] [CrossRef]
- Martinez-Botella, G.; Charifson, P.; Reddy, K.; Kahlig, M.K.; Marron, B. Kcnt1 Inhibitors and Methods of Use. Patent WO2020227097A1, 1 May 2020. [Google Scholar]
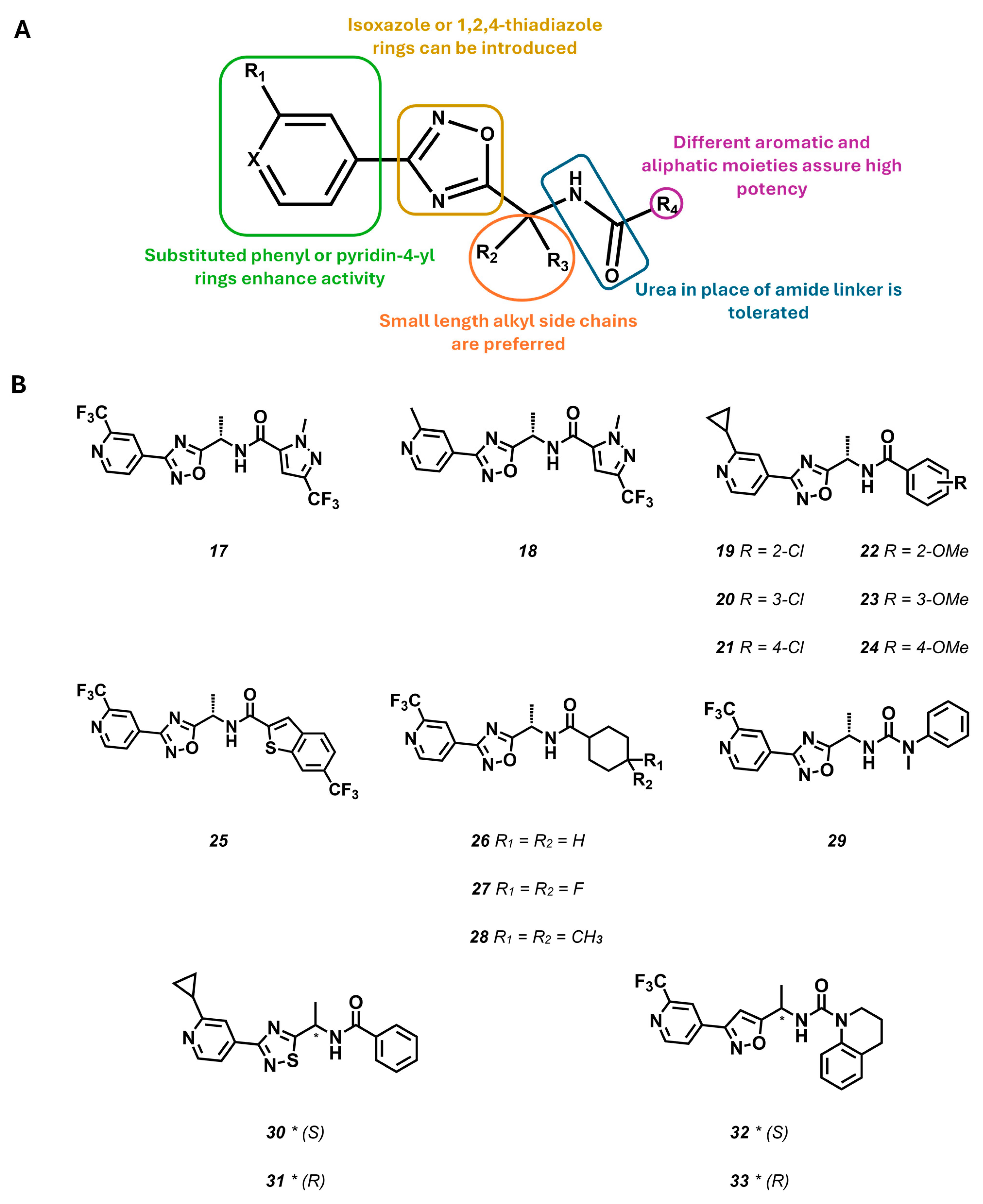
- Griffin, A.M.; Kahlig, K.M.; Hatch, R.J.; Hughes, Z.A.; Chapman, M.L.; Antonio, B.; Marron, B.E.; Wittmann, M.; Martinez-Botella, G. Discovery of the first orally available, selective KNa1.1 inhibitor: In vitro and in vivo activity of an oxadiazole series. ACS Med. Chem. Lett. 2021, 12, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Burbano, L.E.; Li, M.; Jancovski, N.; Jafar-Nejad, P.; Richards, K.; Sedo, A.; Soriano, A.; Rollo, B.; Jia, L.; Gazina, E.V.; et al. Antisense oligonucleotide therapy for KCNT1 encephalopathy. JCI Insight 2022, 7, e146090. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Matteo, F.; Mancuso, F.; Turcio, R.; Ciaglia, T.; Stagno, C.; Di Chio, C.; Campiglia, P.; Bertamino, A.; Giofrè, S.V.; Ostacolo, C.; et al. KCNT1 Channel Blockers: A Medicinal Chemistry Perspective. Molecules 2024, 29, 2940. https://doi.org/10.3390/molecules29122940
Di Matteo F, Mancuso F, Turcio R, Ciaglia T, Stagno C, Di Chio C, Campiglia P, Bertamino A, Giofrè SV, Ostacolo C, et al. KCNT1 Channel Blockers: A Medicinal Chemistry Perspective. Molecules. 2024; 29(12):2940. https://doi.org/10.3390/molecules29122940
Chicago/Turabian StyleDi Matteo, Francesca, Francesca Mancuso, Rita Turcio, Tania Ciaglia, Claudio Stagno, Carla Di Chio, Pietro Campiglia, Alessia Bertamino, Salvatore Vincenzo Giofrè, Carmine Ostacolo, and et al. 2024. "KCNT1 Channel Blockers: A Medicinal Chemistry Perspective" Molecules 29, no. 12: 2940. https://doi.org/10.3390/molecules29122940
APA StyleDi Matteo, F., Mancuso, F., Turcio, R., Ciaglia, T., Stagno, C., Di Chio, C., Campiglia, P., Bertamino, A., Giofrè, S. V., Ostacolo, C., & Iraci, N. (2024). KCNT1 Channel Blockers: A Medicinal Chemistry Perspective. Molecules, 29(12), 2940. https://doi.org/10.3390/molecules29122940