Phytochemistry and Biological Profile of the Chinese Endemic Herb Genus Notopterygium
Abstract
:1. Introduction
2. Botanical Description
2.1. Botanical Systematics
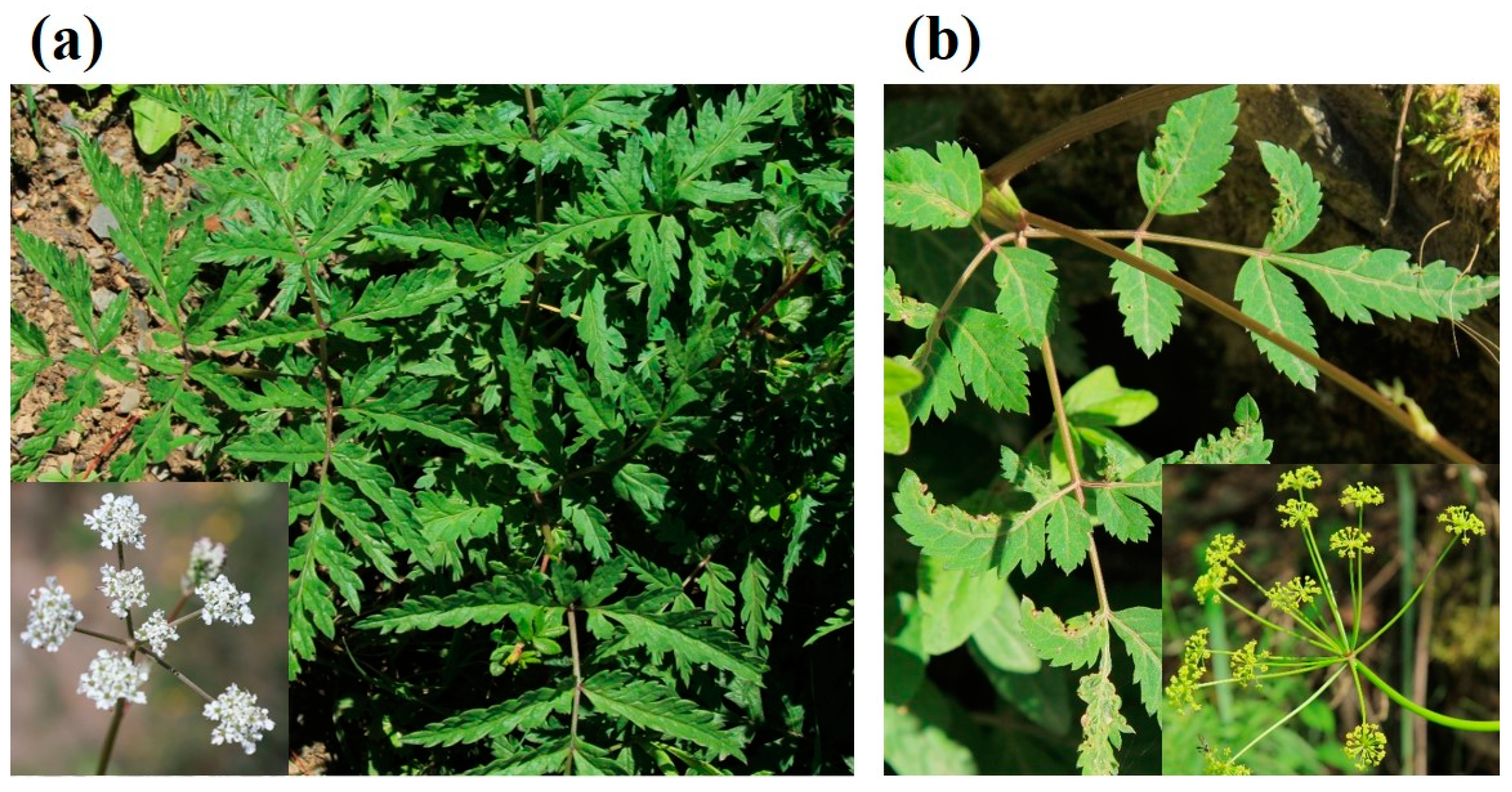
2.2. Botanical Characteristics
3. Phytochemical Composition
3.1. Volatile Oils
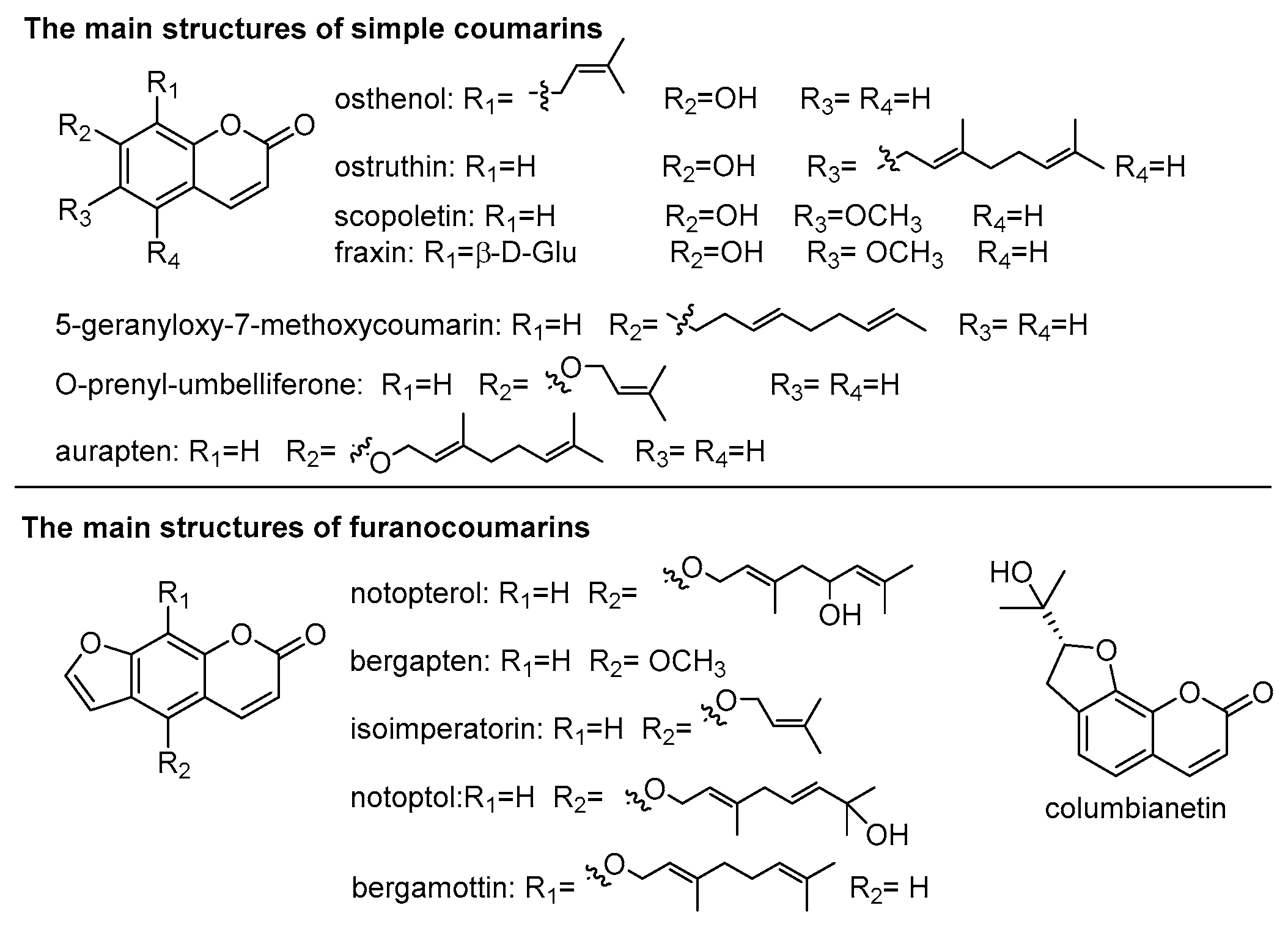
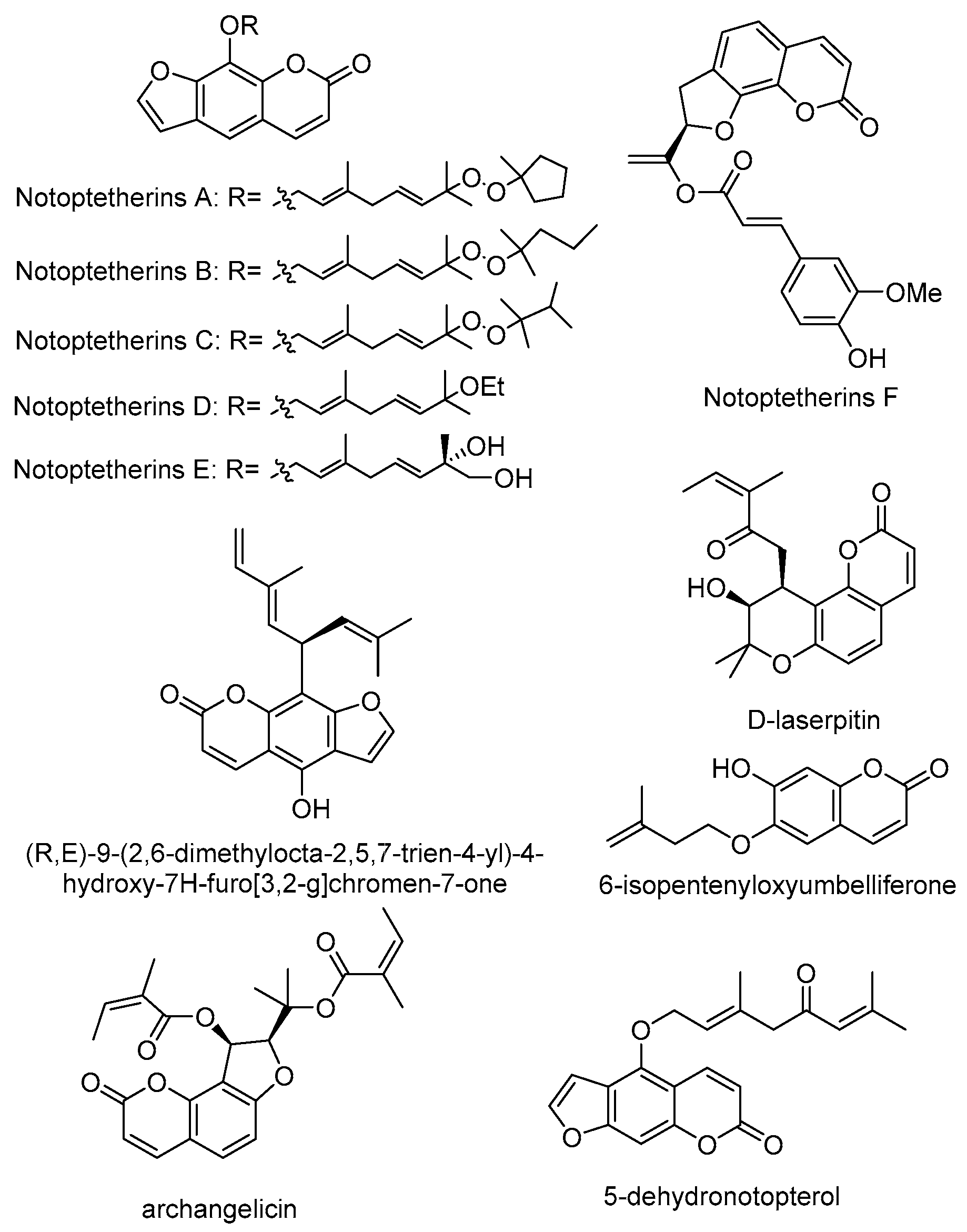
3.2. Coumarins
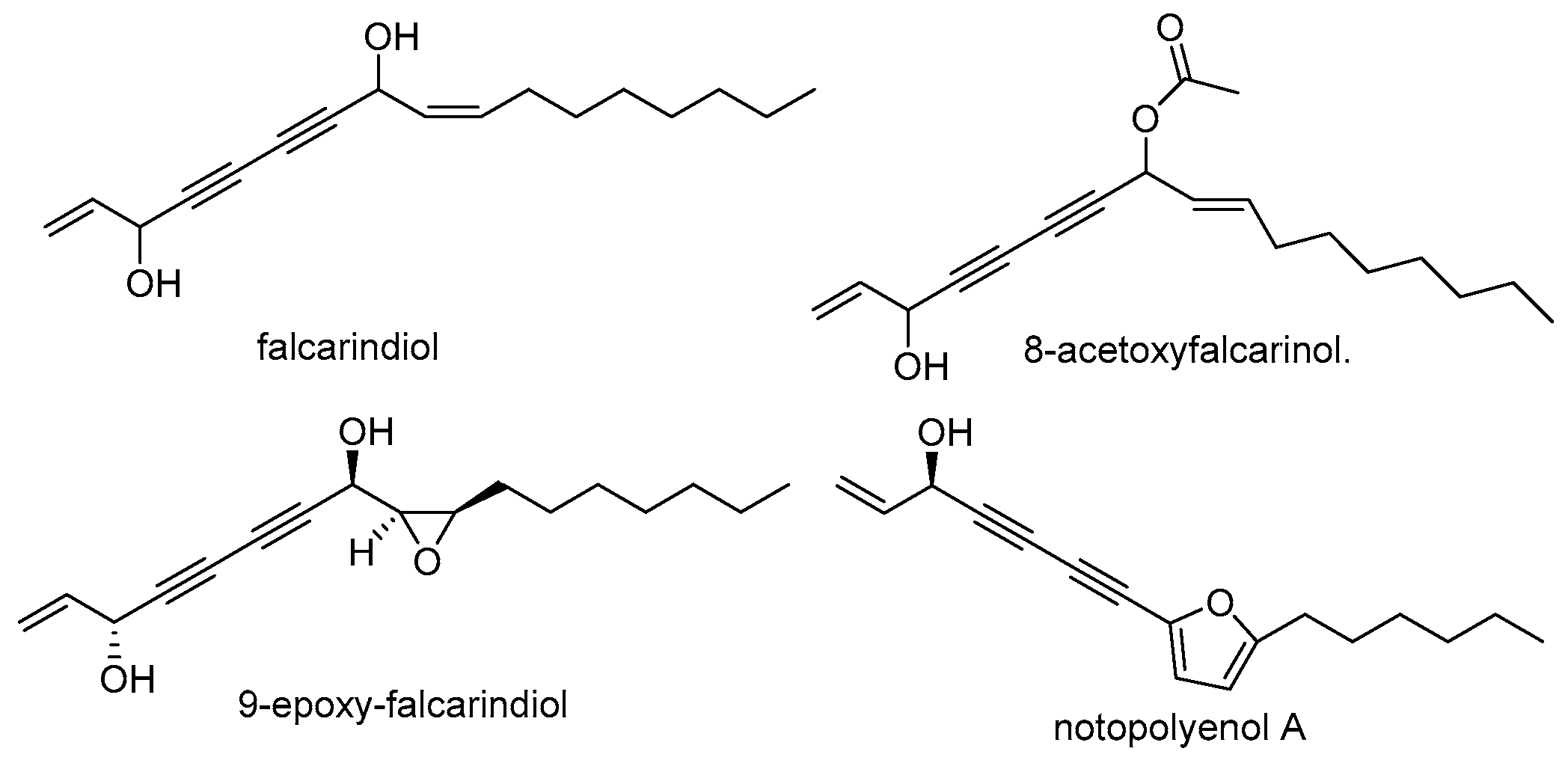
3.3. Polyene-Alkyne
3.4. Phenolics and Flavonoids
3.5. Others
4. Biological Activity
4.1. Anti-Inflammatory
4.2. Anti-Tumor
4.3. Antibacterial and Antifungal
4.4. Other Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chinese Pharmacopoeia Communication. Pharmacopoeia of the People’s Republic of China, 2020th ed.; People’s Medical Publishing House: Beijing, China, 2020. [Google Scholar]
- Ma, X.; Wu, Y.; Li, Y.; Huang, Y.; Liu, Y.; Luo, P.; Zhang, Z. Rapid Discrimination of Notopterygium incisum and Notopterygium franchetii Based on Characteristic Compound Profiles Detected by UHPLC-QTOF-MS/MS Coupled with Multivariate Analysis. Phytochem. Anal. 2020, 31, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Azietaku, J.T.; Ma, H.; Yu, X.; Li, J.; Oppong, M.B.; Cao, J.; An, M.; Chang, Y. A Review of the Ethnopharmacology, Phytochemistry and Pharmacology of Notopterygium incisum. J. Ethnopharmacol. 2017, 202, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-R.; Tang, L.-H.; Chen, Y.-Y.; Shu, L.-X.; Xu, Y.-Y.; Yao, Y.-Q.; Li, Y.-B. Systematic Characterization of the Chemical Constituents in Vitro and in Vivo of Qianghuo by UPLC-Q-TOF-MS/MS. Fitoterapia 2024, 172, 105758. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, Y.; Li, J.; Li, M. The Pharmacological Mechanism Analysis of Qianghuo Shengshi Tang in the Treatment of Ankylosing Spondylitis. Minerva Surg. 2024, 79, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Liu, M.-L.; López-Pujol, J.; Jia, R.-W.; Kou, Y.-X.; Yue, M.; Guan, T.-X.; Li, Z.-H. The Hybridization Origin of the Chinese Endemic Herb Genus Notopterygium (Apiaceae): Evidence from Population Genomics and Ecological Niche Analysis. Mol. Phylogenet. Evol. 2023, 182, 107736. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y.; Raven, P.H.; Hong, D.Y. Flora of China Volume 14: Apiaceae through Ericaceae; Science Press: Beijing, China, 2005; ISBN 1-930723-41-5. [Google Scholar]
- Liu, M.-L.; He, Y.-L.; López-Pujol, J.; Jia, Y.; Li, Z.-H. Complex Population Evolutionary History of Four Cold-Tolerant Notopterygium Herb Species in the Qinghai-Tibetan Plateau and Adjacent Areas. Heredity 2019, 123, 242–263. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Feng, L.; Yue, M.; He, Y.-L.; Zhao, G.-F.; Li, Z.-H. Species Delimitation and Interspecific Relationships of the Endangered Herb Genus Notopterygium Inferred from Multilocus Variations. Mol. Phylogenet. Evol. 2019, 133, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-L.; Shang, Q.-H.; Cheng, Y.-J.; Wang, N.; Sa, W.; Li, B.-G.; Li, Z.-H. Drivers of Intraspecific Differentiation of an Alpine Cold-Tolerant Herb, Notopterygium Oviforme: Roles of Isolation by Distance and Ecological Factors. J. Syst. Evol. 2023, 61, 383–398. [Google Scholar] [CrossRef]
- Namba, T.; Gu, Z.M.; Zhou, G.C.; Wang, T.Z.; Huo, M.; Komatsu, K. Pharmacognostical Study on the Chinese “Qiang-Huo”: On the Anatomical Characteristics of the Underground Parts of Notopterygium incisum and N. forbesii. Nat. Med. 1995, 49, 409–417. [Google Scholar]
- Qiu, Y.; Lu, X.; Pang, T.; Zhu, S.; Kong, H.; Xu, G. Study of Traditional Chinese Medicine Volatile Oils from Different Geographical Origins by Comprehensive Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry (GCxGC-TOFMS) in Combination with Multivariate Analysis. J. Pharm. Biomed. Anal. 2007, 43, 1721–1727. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, J.; Huo, G.; Ding, L.; Chen, L.; Wang, X.; Wang, B.; Wu, J.; Wang, R. Constituents, Antibacterial Effect, and Cytotoxicity of Essential Oil from Aerial Parts of Notopterygium incisum. Curr. Microbiol. 2023, 80, 243. [Google Scholar] [CrossRef] [PubMed]
- Wedge, D.; Gao, Z.; Tabanca, N.; Demirci, B.; Baser, K.; Pridgeon, J.; Becnel, J.; Sampson, B.; Werle, C. The Chemical Composition and Biological Activities of Notopterygium incisum and Notopterygium Forbesii Essential Oils from China. Planta Med. 2009, 75, 1089–1093. [Google Scholar] [CrossRef]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical Fluid Extraction of Seed Oils—A Short Review of Current Trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Manjare, S.D.; Dhingra, K. Supercritical Fluids in Separation and Purification: A Review. Mater. Sci. Energy Technol. 2019, 2, 463–484. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, S.-Y.; Sun, H.; Yang, A.-D.; Ma, Y.; Ma, X.-J.; Wu, R. Quantitative analysis of volatile oils and isoimperatorin in rhizoma et Radix notopterygii. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2007, 32, 566–569. [Google Scholar]
- Xi, X.; Yin, H.; Jin, X.; He, Y. Extraction of Notopterygium Forbesii by Surercritical CO2 Extraction. Chin. J. Pharm. Anal. 2009, 29, 2093–2101. [Google Scholar]
- Poumale, H.M.P.; Hamm, R.; Zang, Y.; Shiono, Y.; Kuete, V. 8—Coumarins and Related Compounds from the Medicinal Plants of Africa. In Medicinal Plant Research in Africa; Kuete, V., Ed.; Elsevier: Oxford, UK, 2013; pp. 261–300. ISBN 978-0-12-405927-6. [Google Scholar]
- Martin, A.L.A.R.; Pereira, R.L.S.; Rocha, J.E.; Farias, P.A.M.; Freitas, T.S.; de Lemos Caldas, F.R.; Figueredo, F.G.; Sampaio, N.F.L.; de Morais Oliveira-Tintino, C.D.; Tintino, S.R.; et al. Unlocking Bacterial Defense: Exploring the Potent Inhibition of NorA Efflux Pump by Coumarin Derivatives in Staphylococcus Aureus. Microb. Pathog. 2024, 190, 106608. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Yang, B.; Zhou, X.; Wang, J.; Yang, J.; Liu, Y. Two New Isocoumarins Isolated from the Marine-Sponge-Derived Fungus Setosphaeria sp. SCSIO41009. Chem. Biodivers. 2024, 21, e202302069. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Singh, M.; Sharma, P.; Sharma, S.C.; Mujwar, S.; Kapoor, M.; Mishra, K.K.; Wani, T.A. Design, Synthesis, and Biological Evaluation of Novel Coumarin Analogs Targeted against SARS-CoV-2. Molecules 2024, 29, 1406. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hussain, S.A.; Luo, M. Columbianadin Ameliorates Experimental Acute Reflux Esophagitis in Rats via Suppression of NF-κB Pathway. Acta Cir. Bras. 2024, 39, e391824. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Küpeli Akkol, E.; Capasso, R. Anticancer Potential of Furanocoumarins: Mechanistic and Therapeutic Aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef] [PubMed]
- Ganjeh, M.S.; Mazlomifar, A.; Shahvelayti, A.S.; Moghaddam, S.K. Coumarin Linked to 2-Phenylbenzimidazole Derivatives as Potent α-Glucosidase Inhibitors. Sci. Rep. 2024, 14, 7408. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, G.; Wang, T.; Hou, Y.; Hou, B.; Li, X.; Wang, C.; Sun, M.; Su, M.; Guo, Z.; et al. Angelica Keiskei Water Extract Mitigates Age-Associated Physiological Decline in Mice. Redox Rep. Commun. Free Radic. Res. 2024, 29, 2305036. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.-F.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxid. Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Tian, X.; Han, L.; Li, Y.; Peng, Y.; Zheng, J. Mechanism-Based Inactivation of Cytochrome P450 2D6 by Notopterol. Chem. Biol. Interact. 2020, 322, 109053. [Google Scholar] [CrossRef] [PubMed]
- Kozawa, M.; Fukumoto, M.; Matsuyama, Y.; Baba, K. Chemical Studies on the Constituents of the Chinese Crude Drug “Quiang Huo”. Chem. Pharm. Bull. 1983, 31, 2712–2717. [Google Scholar] [CrossRef]
- Wu, X.-W.; Zhang, Y.-B.; Zhang, L.; Yang, X.-W. Simultaneous Quantification of 33 Active Components in Notopterygii Rhizoma et Radix Using Ultra High Performance Liquid Chromatography with Tandem Mass Spectrometry. J. Chromatogr. B 2018, 1092, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Okamura, Y.; Dibwe, D.F.; Awale, S.; Kadota, S.; Tezuka, Y. Anti-Austerity Agents from Rhizoma et Radix Notopterygii (Qianghuo). Planta Med. 2012, 78, 796–799. [Google Scholar] [CrossRef]
- Wu, S.-B.; Zhao, Y.; Fan, H.; Hu, Y.-H.; Hamann, M.T.; Peng, J.-N.; Starks, C.M.; O’Neil-Johnson, M.; Hu, J.-F. New Guaiane Sesquiterpenes and Furanocoumarins from Notopterygium incisum. Planta Med. 2008, 74, 1812–1817. [Google Scholar] [CrossRef]
- You, M.; Xiong, J.; Zhao, Y.; Cao, L.; Wu, S.-B.; Xia, G.; Hu, J.-F. Glycosides from the Methanol Extract of Notopterygium incisum. Planta Med. 2011, 77, 1939–1943. [Google Scholar] [CrossRef]
- Xu, K.; Jiang, S.; Zhou, Y.; Zhang, Y.; Xia, B.; Xu, X.; Zhou, Y.; Li, Y.; Wang, M.; Ding, L. Discrimination of the Seeds of Notopterygium incisum and Notopterygium franchetii by Validated HPLC-DAD–ESI-MS Method and Principal Component Analysis. J. Pharm. Biomed. Anal. 2011, 56, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, Y.; Ma, X.; Zhang, C.; Xu, Z.; Jiang, Y.; Tu, P. Nitric Oxide Inhibitory Coumarins from the Roots and Rhizomes of Notopterygium incisum. Fitoterapia 2018, 131, 65–72. [Google Scholar] [CrossRef]
- Río, J.A.D.; Díaz, L.; García-Bernal, D.; Blanquer, M.; Ortuño, A.; Correal, E.; Moraleda, J.M. Chapter 5—Furanocoumarins: Biomolecules of Therapeutic Interest. In Studies in Natural Products Chemistry; Atta-ur-Rahman, F.R.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 43, pp. 145–195. [Google Scholar]
- Ma, Z.; Xu, W.; Liu-Chen, L.-Y.; Lee, D.Y.W. Novel Coumarin Glycoside and Phenethyl Vanillate from Notopterygium Forbesii and Their Binding Affinities for Opioid and Dopamine Receptors. Bioorg. Med. Chem. 2008, 16, 3218–3223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, S.; Xu, K.; Shi, H.; Zhou, Y.; Deng, W.; Ding, L.; Peng, S. Chemical constituents contained in seeds of Notopterygium franchetii. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2012, 37, 941–945. [Google Scholar]
- Xiao, Y.; Sun, Y.; Liu, X. Studies on the Chemical Constituents of Notopterygium incisum Ting. Zhongguo Zhong Yao Za Zhi 1994, 19, 421–422+447. [Google Scholar]
- Gu, Z.M.; Zhang, D.X.; Yang, X.W.; Hattori, M.; Namba, T. Isolation of Two New Coumarin Glycosides from Notopterygium forbesii and Evaluation of a Chinese Crude Drug, Qiang-Huo, the Underground Parts of N. incisum and N. forbesii, by High-Performance Liquid Chromatography. Chem. Pharm. Bull. 1990, 38, 2498–2502. [Google Scholar] [CrossRef]
- Qian, G.-S.; Wang, Q.; Leung, K.S.-Y.; Qin, Y.; Zhao, Z.; Jiang, Z.-H. Quality Assessment of Rhizoma et Radix Notopterygii by HPTLC and HPLC Fingerprinting and HPLC Quantitative Analysis. J. Pharm. Biomed. Anal. 2007, 44, 812–817. [Google Scholar] [CrossRef]
- Xin, L.; Ling, L. Studies on the chemical constituents of not opterygium forbesii boiss. J. Integr. Plant Biol. 1988, 30, 562–564. [Google Scholar]
- Hou, Y.; Lian, Y.; Wu, H.; Li, M.; Hao, Y.; Li, W.; Li, Z. Quality Control of Notopterygii rhizoma et Radix Using near Infrared Spectroscopy and Chemometrics. Vib. Spectrosc. 2020, 111, 103181. [Google Scholar] [CrossRef]
- Su, X.; Wu, Y.; Li, Y.; Huang, Y.; Liu, Y.; Luo, P.; Zhang, Z. Effect of Different Post-Harvest Processing Methods on the Chemical Constituents of Notopterygium franchetii by an UHPLC-QTOF-MS-MS Metabolomics Approach. Molecules 2019, 24, 3188. [Google Scholar] [CrossRef]
- Wu, X.Y. Coumarins from Notopterygium incisum and Their Inhibitory Effect against Lipopolysaccharide-Induced Nitric Oxide Production in RAW 264.7 Macrophage Cells. Chin. Tradit. Herb. Drugs 2020, 51, 3383–3392. [Google Scholar]
- Xiao, L.; Zhou, Y.-M.; Zhang, X.-F.; Du, F.-Y. Notopterygium incisum Extract and Associated Secondary Metabolites Inhibit Apple Fruit Fungal Pathogens. Pestic. Biochem. Physiol. 2018, 150, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, X.; Zhang, S.; Zhu, Y.; Zhao, Q. Chemical constituents of coumarins compounds from Notopterygium incisum and their anti-oxidant activity. Chin. Tradit. Herb. Drugs 2019, 50, 1310–1315. [Google Scholar]
- Zhang, S.; You, J.; Zhou, G.; Li, C.; Suo, Y. Analysis of Free Fatty Acids in Notopterygium forbesii Boiss by a Novel HPLC Method with Fluorescence Detection. Talanta 2012, 98, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lv, J.-M.; Hu, D.; Abe, I. Biosynthesis of Alkyne-Containing Natural Products. RSC Chem. Biol. 2021, 2, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.-Y.; Yang, Z.; Lin, H.-W.; Han, B.-N. Alkynyl-Containing Peptides of Marine Origin: A Review. Mar. Drugs 2016, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Blunder, M.; Fakhrudin, N.; Liu, X.; Noha, S.M.; Malainer, C.; Kramer, M.P.; Cocic, A.; Kunert, O.; Schinkovitz, A.; et al. Polyacetylenes from Notopterygium incisum—New Selective Partial Agonists of Peroxisome Proliferator-Activated Receptor-Gamma. PLoS ONE 2013, 8, e61755. [Google Scholar] [CrossRef]
- Liu, X.; Kunert, O.; Blunder, M.; Fakhrudin, N.; Noha, S.M.; Malainer, C.; Schinkovitz, A.; Heiss, E.H.; Atanasov, A.G.; Kollroser, M.; et al. Polyyne Hybrid Compounds from Notopterygium incisum with Peroxisome Proliferator-Activated Receptor Gamma Agonistic Effects. J. Nat. Prod. 2014, 77, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zheng, X.; Zhang, C.; Zhang, Q.; Jiang, Y.; Tu, P. Cytotoxic Polyacetylenes Isolated from the Roots and Rhizomes of Notopterygium incisum. Chin. Chem. Lett. 2019, 30, 428–430. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Med. Basel Switz. 2018, 5, 93. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-Inflammatory and Anti-Allergic Potential of Dietary Flavonoids: A Review. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef]
- Zheng, X.; Wen, R.; Liu, Y.; Gan, L.; Zhang, Q.; Jiang, Y.; Tu, P. Nitric Oxide Inhibitory Phenolic Constituents Isolated from the Roots and Rhizomes of Notopterygium incisum. Bioorg. Chem. 2022, 128, 106060. [Google Scholar] [CrossRef]
- Xu, K.; Jiang, S.; Sun, H.; Zhou, Y.; Xu, X.; Peng, S.; Ding, L. New Alkaloids from the Seeds of Notopterygium incisum. Nat. Prod. Res. 2012, 26, 1898–1903. [Google Scholar] [CrossRef]
- Okuyama, T.; Takata, M.; Nishino, H.; Nishino, A.; Takayasu, J.; Iwashima, A. Studies on the Antitumor-Promoting Activity of Naturally Occurring Substances. II. Inhibition of Tumor-Promoter-Enhanced Phospholipid Metabolism by Umbelliferous Materials. Chem. Pharm. Bull. 1990, 38, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-S.; Tan, Q.-W.; Guan, L.-P. Antioxidant, Anti-Inflammatory, Antibacterial, and Analgesic Activities and Mechanisms of Quinolines, Indoles and Related Derivatives. Mini Rev. Med. Chem. 2021, 21, 2261–2275. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Beiranvand, M. A Review of the Most Common in Vivo Models of Stomach Ulcers and Natural and Synthetic Anti-Ulcer Compounds: A Comparative Systematic Study. Phytomedicine Plus 2022, 2, 100264. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the Immune System in Cancer: From Tumor Initiation to Metastatic Progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Okuyama, E.; Nishimura, S.; Ohmori, S.; Ozaki, Y.; Satake, M.; Yamazaki, M. Analgesic Component of Notopterygium incisum Ting. Chem. Pharm. Bull. 1993, 41, 926–929. [Google Scholar] [CrossRef]
- Orellana-Urzúa, S.; Briones-Valdivieso, C.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Potential Role of Natural Antioxidants in Countering Reperfusion Injury in Acute Myocardial Infarction and Ischemic Stroke. Antioxidants 2023, 12, 1760. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-A.; Li, J.; Azietaku, J.T.; Liu, W.; He, J.; Chang, Y.-X. A Single Standard to Determine Multi-Components Method Coupled with Chemometric Methods for the Quantification, Evaluation and Classification of Notopterygii Rhizoma et Radix from Different Regions. Molecules 2019, 24, 3574. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-S.; Xu, X.-X.; Shi, Y.-Y.; Chen, Y.; Li, Y.-Q.; Jiang, S.-Q.; Wang, T.; Li, P.; Li, F. System Pharmacology Analysis to Decipher the Effect and Mechanism of Active Ingredients Combination from Herb Couple on Rheumatoid Arthritis in Rats. J. Ethnopharmacol. 2022, 288, 114969. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Qiu, L.; Qiu, H.; Shen, Y.; Tang, M.; Huang, Y.; Peng, Y.; Wang, J.; Fu, Q. Notopterol Alleviates the Progression of Osteoarthritis: An In Vitro and In Vivo Study. Cytokine 2023, 169, 156309. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shi, P.; Ma, R.; Xie, X.; Zhao, L.; Wang, J. Notopterol Inhibits the NF-κB Pathway and Activates the PI3K/Akt/Nrf2 Pathway in Periodontal Tissue. J. Immunol. 2023, 211, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, H.; Huang, S.; Wang, S.; Liu, Q.; Luo, L.; Gan, S.; Fu, G.; Zou, P.; Chen, G.; et al. Notopterol Attenuates Monocrotaline-Induced Pulmonary Arterial Hypertension in Rat. Front. Cardiovasc. Med. 2022, 9, 859422. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-T.; Yeh, C.-T.; Yadav, V.K.; Pikatan, N.W.; Fong, I.-H.; Lee, W.-H.; Chiu, Y.-S. Notopterol Mitigates IL-1β-Triggered Pyroptosis by Blocking NLRP3 Inflammasome via the JAK2/NF-kB/Hsa-miR-4282 Route in Osteoarthritis. Heliyon 2024, 10, e28094. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhang, X.; Huang, H.; Ding, N.; Zhang, S.; Hutchinson, S.Z.; Zhang, X. Adiponectin Protects Rat Myocardium against Chronic Intermittent Hypoxia-Induced Injury via Inhibition of Endoplasmic Reticulum Stress. PLoS ONE 2014, 9, e94545. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-W.; Wei, W.; Yang, X.-W.; Zhang, Y.-B.; Xu, W.; Yang, Y.-F.; Zhong, G.-Y.; Liu, H.-N.; Yang, S.-L. Anti-Inflammatory Phenolic Acid Esters from the Roots and Rhizomes of Notopterygium Incisium and Their Permeability in the Human Caco-2 Monolayer Cell Model. Molecules 2017, 22, 935. [Google Scholar] [CrossRef]
- Blunder, M.; Liu, X.; Kunert, O.; Winkler, N.A.; Schinkovitz, A.; Schmiderer, C.; Novak, J.; Bauer, R. Polyacetylenes from Radix et Rhizoma Notopterygii Incisi with an Inhibitory Effect on Nitric Oxide Production In Vitro. Planta Med. 2014, 80, 415–418. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Y.; Zeng, J.; Li, S. Nodakenetin Alleviates Inflammatory Pain Hypersensitivity by Suppressing NF-κB Signal Pathway. Neuroimmunomodulation 2022, 29, 486–492. [Google Scholar] [CrossRef]
- Ohnuma, T.; Komatsu, T.; Nakayama, S.; Nishiyama, T.; Ogura, K.; Hiratsuka, A. Induction of Antioxidant and Phase 2 Drug-Metabolizing Enzymes by Falcarindiol Isolated from Notopterygium incisum Extract, Which Activates the Nrf2/ARE Pathway, Leads to Cytoprotection against Oxidative and Electrophilic Stress. Arch. Biochem. Biophys. 2009, 488, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.; Liu, C.; Lu, X.; Yang, C.; Qiu, S. Distributive Differences of P2Xs between the Forelimb and Hind Limb of Adjuvant Arthritis Rats and Intervention by Notopterygh Rhizoma et Radix. Pharm. Biol. 2019, 57, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Zhang, C.; Huang, M.; Yu, H.; Wang, Y.; Wang, Y. Mitochondrion-NLRP3 Inflammasome Activation in Macrophages: A Novel Mechanism of the Anti-Inflammatory Effect of Notopterygium in Rheumatoid Arthritis Treatment. Biomed. Pharmacother. Biomedecine Pharmacother. 2023, 167, 115560. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Gao, Y.; Lu, H.; Liu, W.; Xu, X.; Xing, B.; Liang, X.; Wang, N.; Jiang, X.; Zhao, Q. Notopterygium incisum Root Extract (NRE) Alleviates Epileptiform Symptoms in PTZ-Induced Acute Seizure Mice. CNS Neurol. Disord. Drug Targets 2023, 22, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Cheng, T.-F.; Jia, Y.-R.; Li, P.; Li, F. Anti-Rheumatoid Arthritis Effects of Traditional Chinese Herb Couple in Adjuvant-Induced Arthritis in Rats. J. Ethnopharmacol. 2017, 205, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Chen, Y.; Zhao, R.; Ma, D.; Zhao, Y.; Zhang, Y.; Jiang, J.; Yu, W. Application of Omics Technology to Investigate the Mechanism Underlying the Role of San Hua Tang in Regulating Microglia Polarization and Blood-Brain Barrier Protection Following Ischemic Stroke. J. Ethnopharmacol. 2023, 314, 116640. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-B.; Pang, F.; Wen, Y.; Zhang, H.-F.; Zhao, Z.; Hu, J.-F. Antiproliferative and Apoptotic Activities of Linear Furocoumarins from Notopterygium incisum on Cancer Cell Lines. Planta Med. 2010, 76, 82–85. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Yang, C.-K.; Chen, M.-Y.; Yadav, V.K.; Fong, I.-H.; Yeh, C.-T.; Cherng, Y.-G. Furanocoumarin Notopterol: Inhibition of Hepatocellular Carcinogenesis through Suppression of Cancer Stemness Signaling and Induction of Oxidative Stress-Associated Cell Death. Nutrients 2023, 15, 2447. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, X.; Yang, L.; Zhao, Y.; Chew, Z.; Xiao, J.; Liu, C.; Zheng, X.; Zheng, Y.; Shi, Q.; et al. The Natural Compound Notopterol Binds and Targets JAK2/3 to Ameliorate Inflammation and Arthritis. Cell Rep. 2020, 32, 108158. [Google Scholar] [CrossRef]
- Inthanon, S.; Dejkriengkraikul, P.; Yodkeeree, S. Notopterol Suppresses IL-17-Induced Proliferation and Invasion of A549 Lung Adenocarcinoma Cells via Modulation of STAT3, NF-κB, and AP-1 Activation. Int. J. Mol. Sci. 2023, 24, 15057. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, Y.; Huang, Z.; Wang, M.; Jiang, J.; Yan, M.; Xiang, W.; Li, S.; Yu, Y.; Chen, L.; et al. Notopterol Improves Cognitive Dysfunction and Depression-like Behavior via Inhibiting STAT3/NF-ĸB Pathway Mediated Inflammation in Glioma-Bearing Mice. Int. Immunopharmacol. 2023, 118, 110041. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Ojika, M.; Sakagami, Y. Differentiation in a Rat PC12 Cell Line Induced by Ostruthin and (-)-Bornyl Ferulate, Constituents of a Chinese Herbal Medicine. Biosci. Biotechnol. Biochem. 1999, 63, 1501–1502. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, W.; Wu, Y.; Wu, Q.; Lu, H.; Xu, Z.; Gao, H.; Zhao, Q. Notopterygium incisum Extract (NRE) Rescues Cognitive Deficits in APP/PS1 Alzhneimer’s Disease Mice by Attenuating Amyloid-Beta, Tau, and Neuroinflammation Pathology. J. Ethnopharmacol. 2020, 249, 112433. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lai, D.; Liu, Q.Z.; Zhou, L.; Liu, Z.L. Identification of Nematicidal Constituents of Notopterygium incisum Rhizomes against Bursaphelenchus Xylophilus and Meloidogyne Incognita. Molecules 2016, 21, 1276. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chang, W.; Yao, J.; Liu, F.; Zeng, Q.; He, Z. Anti-Osteoporosis Effect of Notopterygium incisum Ting Ex H. T. Chang Extract in Rats. Trop. J. Pharm. Res. 2021, 18, 2051–2055. [Google Scholar] [CrossRef]
- Wu, C.-X.; Liu, Y.; Zhang, J.-C. Chronic Intermittent Hypoxia and Hypertension: A Review of Systemic Inflammation and Chinese Medicine. Chin. J. Integr. Med. 2013, 19, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Guo, S.; Guo, Y.-F.; Zahoor, A.; Shaukat, A.; Chen, Y.; Umar, T.; Deng, P.G.; Guo, M. Upregulated-Gene Expression of pro-Inflammatory Cytokines (TNF-α, IL-1β and IL-6) via TLRs Following NF-κB and MAPKs in Bovine Mastitis. Acta Trop. 2020, 207, 105458. [Google Scholar] [CrossRef] [PubMed]
- Hua, P.; Liang, R.; Tu, Y.; Yin, Y.; Law, M.-K.; Chen, M. Reactive Oxygen Species and Nitric Oxide Scavenging Nanoparticles Alleviating Rheumatoid Arthritis through Adjusting the Seeds and Growing Soils. Acta Pharm. Sin. B 2023, 13, 5016–5029. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J. Efficient Extraction, Antioxidant Activities and Anti-Inflammation of Polysaccharides from Notopterygium franchetii Boiss. Carbohydr. Polym. 2020, 248, 116783. [Google Scholar] [CrossRef]
- Tian, X.-Y.; Xie, L.; Wang, W.-Y.; Zou, X.-S.; Zhao, G.-Y.; Chen, M.-H. Pomelo Peel Volatile Oil Alleviates Neuroinflammation on Focal Cerebral Ischemia Reperfusion Injury Rats via Inhibiting TLR4/NF-κB Signaling Pathway. Curr. Pharm. Biotechnol. 2021, 22, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xu, J.; Huang, Q.; Han, J.; Duan, L.; Fan, J.; Lv, Z.; Guo, M.; Hu, G.; Chen, L.; et al. The Role of Interleukin-17 in Lung Cancer. Mediat. Inflamm. 2016, 2016, 8494079. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Xiong, C.; Li, J.; Zhang, D.; Shi, Y.; Sun, W.; Duan, X. Species Quantification in Complex Herbal Formulas-Vector Control Quantitative Analysis as a New Method. Front. Pharmacol. 2020, 11, 488193. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jin, X.; Ji, H.; Zhu, C.; Yang, Y.; Zhou, Y.; Yu, G.; Wang, C.; Tang, Z. Water Extract of Notopterygium incisum Alleviates Cold Allodynia in Neuropathic Pain by Regulation of TRPA1. J. Ethnopharmacol. 2023, 305, 116065. [Google Scholar] [CrossRef] [PubMed]
- Nazari-Khanamiri, F.; Ghasemnejad-Berenji, M. A Hypothesis That Notopterol May Be Effective in COVID-19 via JAK/STAT and Other Signaling Pathways. J. Basic Clin. Physiol. Pharmacol. 2023, 34, 405–407. [Google Scholar] [CrossRef]
- Sarma, S.; Dowerah, D.; Basumatary, M.; Phonglo, A.; Deka, R.C. Inhibitory Potential of Furanocoumarins against Cyclin Dependent Kinase 4 Using Integrated Docking, Molecular Dynamics and ONIOM Methods. J. Biomol. Struct. Dyn. 2024, 1–30. [Google Scholar] [CrossRef]
| Biological Activity | Component/Compound | Results | References |
|---|---|---|---|
| Anti-inflammatory | Notopterol | Down-regulated the hypersecretion of inflammatory mediators and alleviated the degradation of the extracellular matrix. | [68] |
| Inhibited synthesis of inflammatory mediators such as IL-1β, IL-32, and IL-8 in LPS-stimulated human gingival fibroblasts. | [69] | ||
| Enhanced the survival rate and improved the functioning of the right ventricle. | [70] | ||
| Acted as therapeutic agent in the treatment of osteoarthritis (OA.) | [71] | ||
| Pterostilbene and notopterol | Exhibited potential therapeutic effects on obstructive sleep apnea syndrome (OSAS). | [72] | |
| 4-methyl-3-trans-hexenylferulate, (-)-boroylferulate, 4-methoxyphenyl ferulate, and phenylyl ferulate | Inhibited NO production in RAW 264.7 cells. | [73] | |
| 3-hydroxy allyl polyacetylenes | [74] | ||
| Notoflavinols A and B, and (2R)-5,4′-dihydroxy-7-O-[(E)-3,7-dimethyl-2,6-octadienyl]flavanone | [35,57] | ||
| Nodakenetin | Reduced CFA-induced inflammatory pain, but did not have a substantial therapeutic impact. | [75] | |
| Falcarindiol | Activated the Nrf2/ARE pathway and induced cytoprotective enzymes. | [76] | |
| Polyacetylenes | Acted as selective partial PPARγ agonist in the luciferase reporter model. | [51] | |
| Volatile oils | Exhibited strong antioxidant activity in vitro and potent anti-inflammatory activity in zebrafish embryos. | [13] | |
| Extract of NI or the formula containing NI | Down-regulated P2X1, P2X3, P2X4, P2X5, and P2X7 to inhibit FCA-induced RA in rats. | [77] | |
| Regulated NLRP3, pro-Caspase-1, Caspase-1, and CD11b in the ankle joint of AA rats. | [78] | ||
| Improved cognitive dysfunction in Alzheimer’s disease (AD) mice. | [79] | ||
| Reduced levels of TNF-α, IL-6, and VEGF—possessed evident anti-arthritic effects in AIA rats. | [80] | ||
| Regulated gut microbiota, inhibited pro-inflammatory factors in rats with IS. | [81] | ||
| Anti-tumor | Notopol, notopterol, 5-[(2 E,5 Z)-7-hydroxy-3,7-dimethyl-2,5-octadienoxy]psoralene, and 5-[(2,5)-epoxy-3-hydroxy-3,7-dimethyl-6-octenoxy]psoralene | Exhibited antiproliferative activity against hepg-2 and C6 cancer cell lines. | [82] |
| Notopterol | Suppressed the viability, migration, and invasion capacity of the human HCC hepj5 and Mahlavu cell lines. | [83] | |
| Bound Janus kinase (JAK)2 and JAK3 kinase domains to inhibit JAK/signal transducers and activators of transcription (JAK-STAT) activation. | [84] | ||
| Suppressed IL-17-induced proliferation and invasion of A549 lung adenocarcinoma cells via modulation of STAT3, NF-κB, and AP-1 activation. | [85] | ||
| Exhibited anti-glioma effects and alleviated neuropsychiatric symptoms. | [86] | ||
| Strustin | Exhibited activity on PANC-1 (PC50 = 7.2 μmol/L) and PSN-1 (PC50 = 7.8 μmol/L). | [87] | |
| Ostruthin and (-)-bornyl ferulate | Induced comparable neurite-like structures in twenty percent of rat PC12 cells at 2 mg/mL, and showed cytotoxicity at concentrations higher than 3 mg/mL. | [87] | |
| Antibacterial | Volatile oils | The diameters of inhibition zone (dizs) of NI-EO against E. coli and S. aureus were 14.63 and 11.25 mm and the minimum inhibitory concentrations were 3.75 and 7.5 μL/mL, respectively. | [13] |
| Antifungal | Ethyl acetate extract of NI | Exhibited antifungal activities against apple fruit pathogens of Colletotrichum gloeosporioides and Botryosphaeria dothidea with MIC values ranging from 8 to 250 mg L−1. | [46] |
| The others | Extract of NI | Showed potential therapeutic effects on ovariectomy-induced osteoporotic and thrombus rats. | [88] |
| Columbianetin, falcarindiol, falcarinol, and isoimperatorin | Showed strong nematicidal activity against the two species of nematodes. | [89] | |
| Extract of NI | Mitigated ovariectomy-induced osteoporosis in rats. | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Zheng, R.; Chen, P.; Li, L. Phytochemistry and Biological Profile of the Chinese Endemic Herb Genus Notopterygium. Molecules 2024, 29, 3252. https://doi.org/10.3390/molecules29143252
Tang Z, Zheng R, Chen P, Li L. Phytochemistry and Biological Profile of the Chinese Endemic Herb Genus Notopterygium. Molecules. 2024; 29(14):3252. https://doi.org/10.3390/molecules29143252
Chicago/Turabian StyleTang, Zhikang, Renlin Zheng, Ping Chen, and Liangchun Li. 2024. "Phytochemistry and Biological Profile of the Chinese Endemic Herb Genus Notopterygium" Molecules 29, no. 14: 3252. https://doi.org/10.3390/molecules29143252





