Extraction Optimization of Phenolic Compounds from Triadica sebifera Leaves: Identification, Characterization and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Extraction Parameters on the Extraction Yield
2.2. Model Fitting and Analysis
2.3. Verification of Optimal Extraction Conditions and Extraction Yield
2.4. Composition Analysis of Phenolic Compounds
2.5. In Vitro Antioxidant Activity
2.6. Correlation Analysis
2.7. Analysis of SEM
2.8. FTIR Analysis
3. Materials and Methods
3.1. Plant Materials and Reagents
3.2. Single-Factor Experiments
3.3. Response Surface Design
3.4. Determination of Phenolic Compound Content
3.5. Validation of the Model
3.6. High-Performance Liquid Chromatography (HPLC) Analysis
3.7. Leaf Extract Preparation and Drying Process
3.8. In Vitro Antioxidant Activity
3.8.1. DPPH Radical Scavenging Capacity
3.8.2. ABTS Radical Scavenging Capacity
3.9. Scanning Electron Microscopy (SEM) Analysis
3.10. Fourier Transform Infrared Spectroscopy (FTIR)
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, D.; Zhou, B.; Jiang, Y.; Tan, X.; Yuan, D.; Zhang, L. Enhancing Freezing Tolerance of Brassica Napus L. by Overexpression of a Stearoyl-Acyl Carrier Protein Desaturase Gene (SAD) from Sapium sebiferum (L.) Roxb. Plant Sci. 2018, 272, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Mao, Y.; Su, P.; Wang, D.; Chen, X.; Huang, S.; Ni, J.; Zhao, W.; Wu, L. A High Throughput Plant Regeneration System from Shoot Stems of Sapium sebiferum Roxb., a Potential Multipurpose Bioenergy Tree. Ind. Crops Prod. 2020, 154, 112653. [Google Scholar] [CrossRef]
- Wang, R.; Hanna, M.A.; Zhou, W.-W.; Bhadury, P.S.; Chen, Q.; Song, B.-A.; Yang, S. Production and Selected Fuel Properties of Biodiesel from Promising Non-Edible Oils: Euphorbia lathyris L., Sapium sebiferum L. and Jatropha curcas L. Bioresour. Technol. 2011, 102, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Fei, W.; Yang, S.; Yang, F.; Qu, G.; Tang, W.; Ou, J.; Peng, D. Alteration of the Fatty Acid Composition of Brassica Napus L. via Overexpression of Phospholipid: Diacylglycerol Acyltransferase 1 from Sapium sebiferum (L.) Roxb. Plant Sci. 2020, 298, 110562. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Y. Transcriptomics of Chinese Sapium sebiferum (L.) Roxb Seed to Reveal Key Enzymes Involved in Oil Accumulation. Oil Crop Sci. 2020, 5, 107–113. [Google Scholar] [CrossRef]
- Ma, M.; Li, X.; Liang, H.; Jiang, H.; Cui, H. Chemical Constituents Variation of Seed Oil of the Chinese Tallow Tree (Sapium sebiferum (L.) Roxb) at Different Harvesting Time. Ind. Crops Prod. 2023, 202, 117061. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, Y.; Peng, T.; Guo, Y.; Chen, F. Phenolic Composition and Effects on Allergic Contact Dermatitis of Phenolic Extracts Sapium sebiferum (L.) Roxb. Leaves. J. Ethnopharmacol. 2015, 162, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Zhang, Y.-T.; Guo, Y.-R.; Huang, Q.-L.; Peng, T.; Xu, Y.; Tang, L.; Chen, F. Antioxidant and Anti-Inflammatory Activities of the Phenolic Extracts of Sapium sebiferum (L.) Roxb. Leaves. J. Ethnopharmacol. 2013, 147, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Shen, Q.; Wang, S.; Song, G. Discovery, Disassembly, Depolymerization and Derivatization of Catechyl Lignin in Chinese Tallow Seed Coats. Int. J. Biol. Macromol. 2023, 239, 124256. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, Y.; Guo, Y.; Chen, F. Chemical Composition, Antioxidant and Antimicrobial Activity of Chinese Tallow Tree Leaves. Ind. Crops Prod. 2015, 76, 374–377. [Google Scholar] [CrossRef]
- Jiang, J.; Qian, S.; Song, T.; Lu, X.; Zhan, D.; Zhang, H.; Liu, J. Food-Packaging Applications and Mechanism of Polysaccharides and Polyphenols in Multicomponent Protein Complex System: A Review. Int. J. Biol. Macromol. 2024, 270, 132513. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and Their Potential Role to Fight Viral Diseases: An Overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef] [PubMed]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-Assisted Extractions of Polyphenols—A Comprehensive Review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Shang, H.; Zhou, H.; Duan, M.; Li, R.; Wu, H.; Lou, Y. Extraction Condition Optimization and Effects of Drying Methods on Physicochemical Properties and Antioxidant Activities of Polysaccharides from Comfrey (Symphytum officinale L.) Root. Int. J. Biol. Macromol. 2018, 112, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Duarah, P.; Joardar, S.; Debnath, B.; Purkait, M.K. Optimized Extraction of Polyphenols from Tea Factory Waste and Cost-Effective Drying Methods for Sustainable Utilization. Bioresour. Technol. Rep. 2024, 26, 101833. [Google Scholar] [CrossRef]
- Rubel, I.A.; Iraporda, C.; Novosad, R.; Cabrera, F.A.; Genovese, D.B.; Manrique, G.D. Inulin Rich Carbohydrates Extraction from Jerusalem Artichoke (Helianthus tuberosus L.) Tubers and Application of Different Drying Methods. Food Res. Int. 2018, 103, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Sukadeetad, K.; Nakbanpote, W.; Heinrich, M.; Nuengchamnong, N. Effect of Drying Methods and Solvent Extraction on the Phenolic Compounds of Gynura pseudochina (L.) DC. Leaf Extracts and Their Anti-Psoriatic Property. Ind. Crops Prod. 2018, 120, 34–46. [Google Scholar] [CrossRef]
- Ben Mabrouk, A.; Putaux, J.-L.; Boufi, S. Valorization of Olive Leaf Waste as a New Source of Fractions Containing Cellulose Nanomaterials. Ind. Crops Prod. 2023, 202, 116996. [Google Scholar] [CrossRef]
- Danise, T.; Innangi, M.; Curcio, E.; Piccolella, S.; Fioretto, A.; Pacifico, S. White Poplar (Populus alba L.) Leaf Waste Recovery and Intercropping Outcome on Its Polyphenols. Ind. Crops Prod. 2021, 171, 113866. [Google Scholar] [CrossRef]
- Andreou, V.; Psarianos, M.; Dimopoulos, G.; Tsimogiannis, D.; Taoukis, P. Effect of Pulsed Electric Fields and High Pressure on Improved Recovery of High-added-value Compounds from Olive Pomace. J. Food Sci. 2020, 85, 1500–1512. [Google Scholar] [CrossRef]
- Metrouh-Amir, H.; Duarte, C.M.M.; Maiza, F. Solvent Effect on Total Phenolic Contents, Antioxidant, and Antibacterial Activities of Matricaria pubescens. Ind. Crops Prod. 2015, 67, 249–256. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, H.; Huang, J.; Chen, Q.; Li, X.; Chen, X.; Liang, J.; Wang, L. Ultrasound-Assisted Extraction of Polyphenols from Pine Needles (Pinus elliottii): Comprehensive Insights from RSM Optimization, Antioxidant Activity, UHPLC-Q-Exactive Orbitrap MS/MS Analysis and Kinetic Model. Ultrason. Sonochem. 2024, 102, 106742. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, W.; Zhang, W.; Hu, Q.; Zeng, X. Optimizing the Extraction of Phenolic Antioxidants from Kudingcha Made Frrom Ilex Kudingcha C.J. Tseng by Using Response Surface Methodology. Sep. Purif. Technol. 2011, 78, 311–320. [Google Scholar] [CrossRef]
- Xu, D.-P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.-B. Ultrasound-Assisted Extraction of Natural Antioxidants from the Flower of Limonium sinuatum: Optimization and Comparison with Conventional Methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.-S.; Dangles, O.; Chemat, F. Ultrasound-Assisted Extraction of Polyphenols (Flavanone Glycosides) from Orange (Citrus sinensis L.) Peel. Food Chem. 2010, 119, 851–858. [Google Scholar] [CrossRef]
- Umego, E.C.; Barry-Ryan, C. Optimisation of Polyphenol Extraction for the Valorisation of Spent Gin Botanicals. LWT 2024, 199, 116114. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Manikandan, S.; Vigna Nivetha, C.; Dinesh, R. Ultrasound Assisted Extraction of Bioactive Compounds from Nephelium Lappaceum L. Fruit Peel Using Central Composite Face Centered Response Surface Design. Arab. J. Chem. 2017, 10, S1145–S1157. [Google Scholar] [CrossRef]
- Vo, T.P.; Nguyen, L.N.H.; Le, N.P.T.; Mai, T.P.; Nguyen, D.Q. Optimization of the Ultrasonic-Assisted Extraction Process to Obtain Total Phenolic and Flavonoid Compounds from Watermelon (Citrullus lanatus) Rind. Curr. Res. Food Sci. 2022, 5, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Z.; Shi, H.; Yu, J.; Huang, G.; Huang, H. Ultrasound-Assisted Extraction and Properties of Polysaccharide from Ginkgo Biloba Leaves. Ultrason. Sonochem. 2023, 93, 106295. [Google Scholar] [CrossRef]
- Luo, S.; Zeng, C.; Luo, F.; Li, M.; Feng, S.; Zhou, L.; Chen, T.; Yuan, M.; Huang, Y.; Ding, C. Optimization of Ultrasound-Assisted Extraction of Triterpenes from Bergenia emeiensis Leaves and Inhibition Effect on the Growth of Hela Cells. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100266. [Google Scholar] [CrossRef]
- Rao, M.V.; Sengar, A.S.; Sunil, C.K.; Rawson, A. Ultrasonication—A Green Technology Extraction Technique for Spices: A Review. Trends Food Sci. Technol. 2021, 116, 975–991. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Yang, S.; Zhang, G.; Li, A.; Du, P.; Liu, L.; Li, C. Optimization of Ultrasonic-Assisted Extraction of Flavonoids, Polysaccharides, and Eleutherosides from Acanthopanax senticosus Using Response Surface Methodology in Development of Health Wine. LWT 2022, 165, 113725. [Google Scholar] [CrossRef]
- Ciric, A.; Krajnc, B.; Heath, D.; Ogrinc, N. Response Surface Methodology and Artificial Neural Network Approach for the Optimization of Ultrasound-Assisted Extraction of Polyphenols from Garlic. Food Chem. Toxicol. 2020, 135, 110976. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.R.; Chew, K.W.; Leong, H.Y.; Manickam, S.; Show, P.L.; Nguyen, T.H.P. Sonoprocessing-Assisted Solvent Extraction for the Recovery of Pigment-Protein Complex from Spirulina platensis. Chem. Eng. J. 2020, 398, 125613. [Google Scholar] [CrossRef]
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of Low Power Ultrasound-Assisted Extraction of Phenolic Compounds from Mandarin (Citrus reticulata Blanco Cv. Sainampueng) Peel. Food Chem. 2018, 241, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Pressurized Liquid Extraction of Phenolic Compounds from Rice (Oryza sativa) Grains. Food Chem. 2016, 192, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken Design: An Alternative for the Optimization of Analytical Methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, J.; Li, J.; Li, P.; Zhao, M.; Xia, G. Optimization of Ultrasonic-Assisted Extraction of Polyphenol from Areca Nut (Areca catechu L.) Seeds Using Response Surface Methodology and Its Effects on Osteogenic Activity. Ultrason. Sonochem. 2023, 98, 106511. [Google Scholar] [CrossRef]
- He, Q.; Zhang, L.; Li, T.; Li, C.; Song, H.; Fan, P. Genus sapium (Euphorbiaceae): A Review on Traditional Uses, Phytochemistry, and Pharmacology. J. Ethnopharmacol. 2021, 277, 114206. [Google Scholar] [CrossRef]
- de Lima, D.P.; dos Santos Pinto Júnior, E.; de Menezes, A.V.; de Souza, D.A.; de São José, V.P.B.; da Silva, B.P.; de Almeida, A.Q.; de Carvalho, I.M.M. Chemical Composition, Minerals Concentration, Total Phenolic Compounds, Flavonoids Content and Antioxidant Capacity in Organic and Conventional Vegetables. Food Res. Int. 2024, 175, 113684. [Google Scholar] [CrossRef]
- Teixeira, F.; Silva, A.M.; Sut, S.; Dall’Acqua, S.; Ramos, O.L.; Ribeiro, A.B.; Ferraz, R.; Delerue-Matos, C.; Rodrigues, F. Ultrasound-Assisted Extraction of Bioactive Compounds from Goji Berries: Optimization, Bioactivity, and Intestinal Permeability Assessment. Food Res. Int. 2024, 188, 114502. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, Z.; Wang, L.; Tian, S. Optimization of Ultrasonic-Assisted Extraction of Polyphenols from Salvia deserta Schang Flowers Based on Response Surface Methodology and Deep Neural Network and Analysis of Its in Vitro Antioxidant Activities. Ind. Crops Prod. 2024, 213, 118389. [Google Scholar] [CrossRef]
- Chen, X.; He, Y.; Liu, Z.; Huang, Z.; Xu, C.; Liu, Y.; Haran, Y.; Nisar, W.; Yan, S.; Li, J. Ultrasound-Assisted Extraction of Polyphenols from Lotus Rhizome Epidermis by Alcohol/Salt-Based Aqueous Two-Phase System: Optimization, Extraction Mechanism and Antioxidant Activities. Food Chem. 2024, 453, 139620. [Google Scholar] [CrossRef] [PubMed]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Vittori, S.; Sagratini, G. Determination of Fourteen Polyphenols in Pulses by High Performance Liquid Chromatography-Diode Array Detection (HPLC-DAD) and Correlation Study with Antioxidant Activity and Colour. Food Chem. 2017, 221, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Mahato, P.L.; Weatherby, T.; Ewell, K.; Jha, R.; Mishra, B. Scanning Electron Microscope-Based Evaluation of Eggshell Quality. Poult. Sci. 2024, 103, 103428. [Google Scholar] [CrossRef] [PubMed]
- Budnimath, S.H.; Bhuvaneshwari, G.; Ganiger, V.M.; Jagadeesh, S.L.; Goudar, G.; Patil, S.N.; Chandrashekar, V.M. Physical, Reconstitution and Phenolic Properties of Instant Drink Mix Prepared with Moringa Oleifera Leaf, Raw Banana and Whey Protein Concentrate. Meas. Food 2023, 11, 100108. [Google Scholar] [CrossRef]
- Lv, Y.; Cai, X.; Shi, N.; Gao, H.; Zhang, Z.; Yan, M.; Li, Y. Emulsification Performance and Stabilization Mechanism of Okra Polysaccharides with Different Structural Properties. Food Hydrocoll. 2024, 153, 109997. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Mancini, M.C.; de Oliveira, F.C.S.; Passos, T.M.; Quilty, B.; Thiré, R.M.d.S.M.; McGuinness, G.B. FTIR Analysis and Quantification of Phenols and Flavonoids of Five Commercially Available Plants Extracts Used in Wound Healing. Matér. Rio J. 2016, 21, 767–779. [Google Scholar] [CrossRef]
- Cao, Y.; Song, Z.; Dong, C.; Ni, W.; Xin, K.; Yu, Q.; Han, L. Green Ultrasound-Assisted Natural Deep Eutectic Solvent Extraction of Phenolic Compounds from Waste Broccoli Leaves: Optimization, Identification, Biological Activity, and Structural Characterization. LWT 2023, 190, 115407. [Google Scholar] [CrossRef]
- Iftikhar, M.; Zhang, H.; Iftikhar, A.; Raza, A.; Begum, N.; Tahamina, A.; Syed, H.; Khan, M.; Wang, J. Study on Optimization of Ultrasonic Assisted Extraction of Phenolic Compounds from Rye Bran. LWT 2020, 134, 110243. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, S.; Wang, H.; Yang, J.; Li, L.; Zhu, J.; Liu, Y. Ultrasound-Alkaline Combined Extraction Improves the Release of Bound Polyphenols from Pitahaya (Hylocereus undatus ‘Foo-Lon’) Peel: Composition, Antioxidant Activities and Enzyme Inhibitory Activity. Ultrason. Sonochem. 2022, 90, 106213. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Shi, N.; Zhang, Z.; Chen, Y.; Yan, M.; Li, Y. Response Surface Methodology Optimization and HPLC-ESI-QTOF-MS/MS Analysis on Ultrasonic-Assisted Extraction of Phenolic Compounds from Okra (Abelmoschus esculentus) and Their Antioxidant Activity. Food Chem. 2023, 405, 134966. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.-Y.; Qian, W.-Z.; Yi, L.; Ye, Y.-L.; Gu, T.; Gao, S.; Cao, G.-X. Nutrient Composition and Antioxidant Activity of Cercis Chinensis Flower in Response to Different Development Stages. Horticulturae 2023, 9, 961. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Kan, H.; Hao, J.; Hu, Q.; Lu, B.; Liu, Y. Comparison of Different Drying Techniques for Zanthoxylum Bungeanum Leaves: Changes in Color, Microstructure, Antioxidant Capacities, and Volatile Components. LWT 2023, 188, 115469. [Google Scholar] [CrossRef]
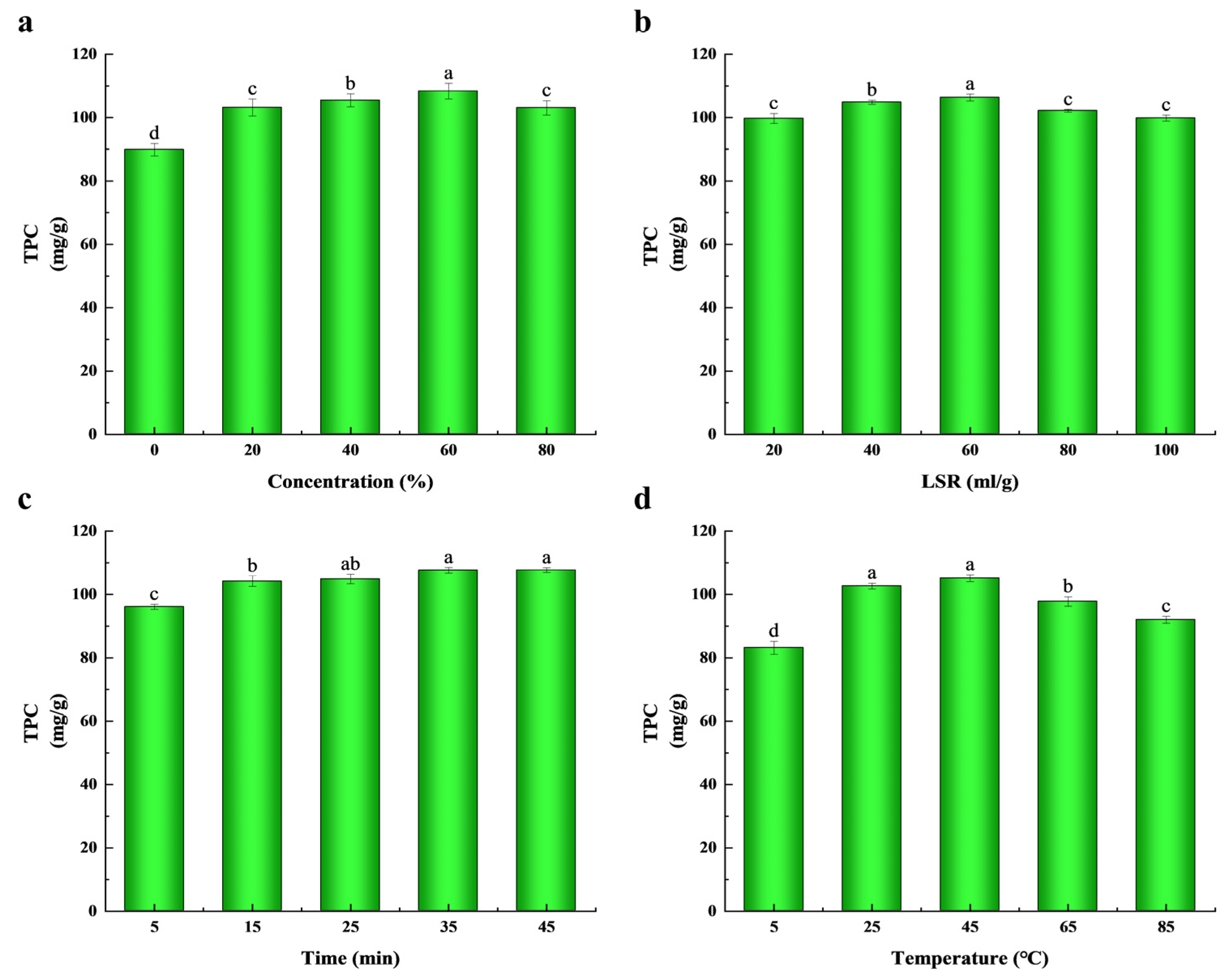
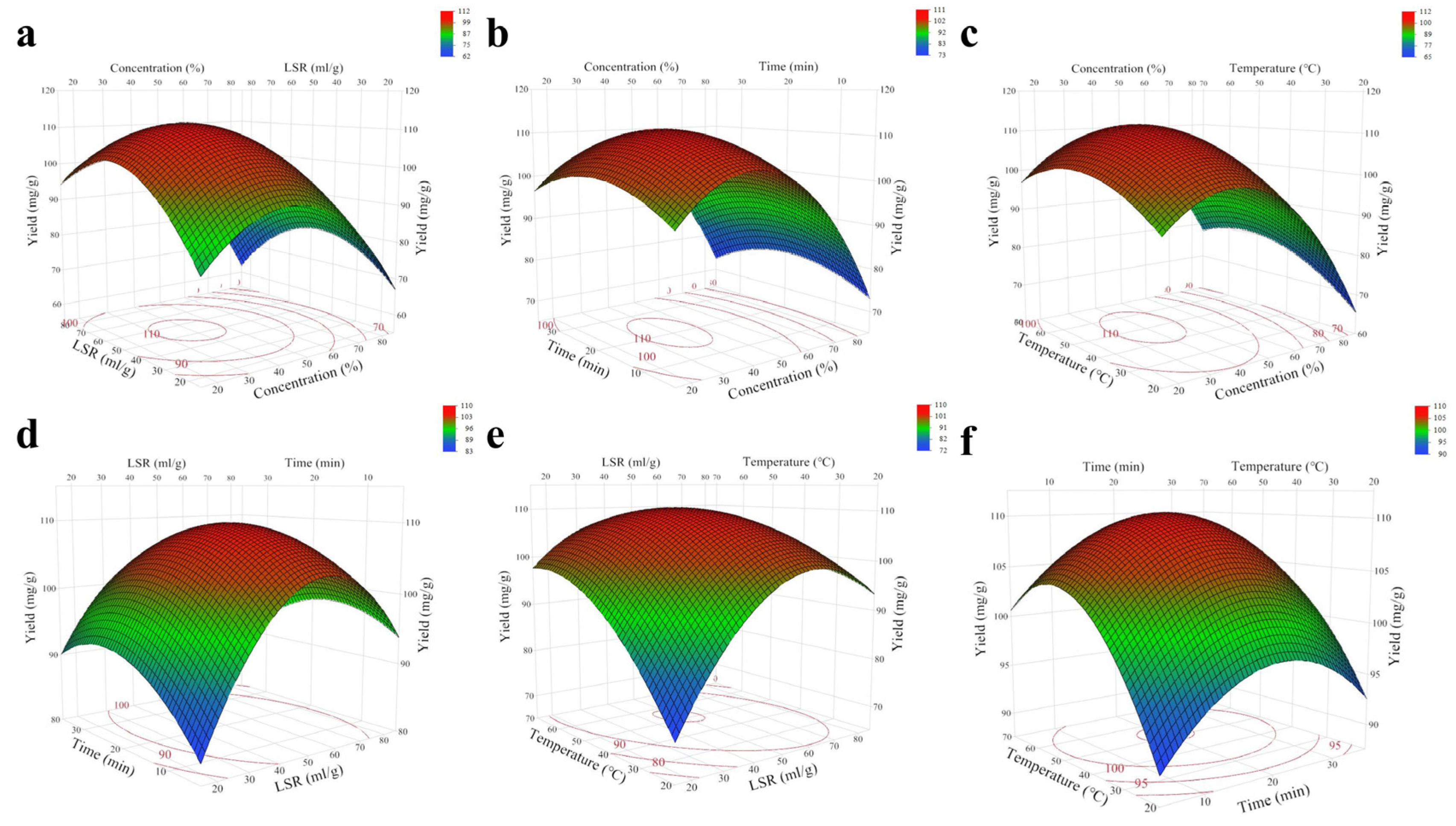

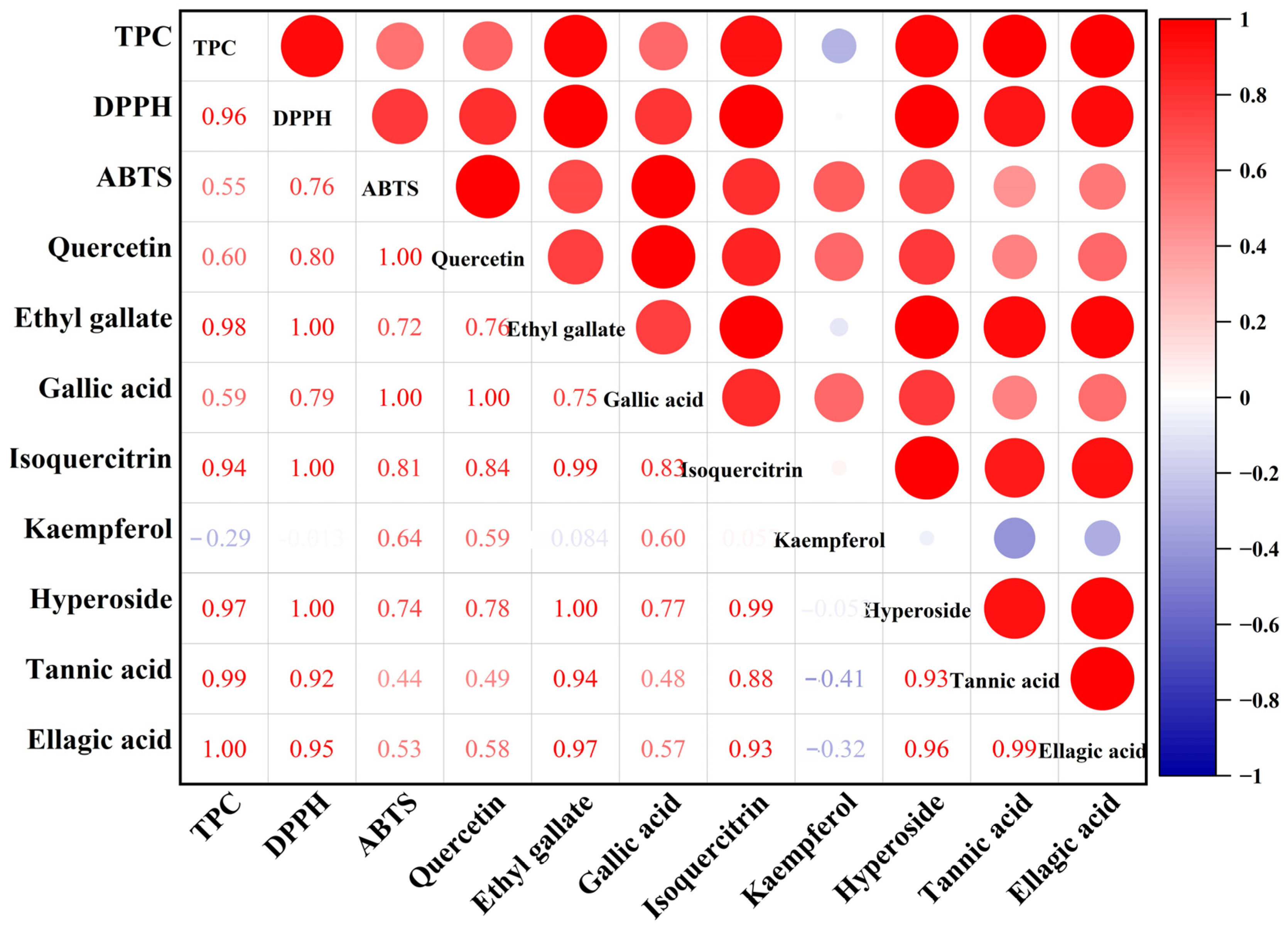
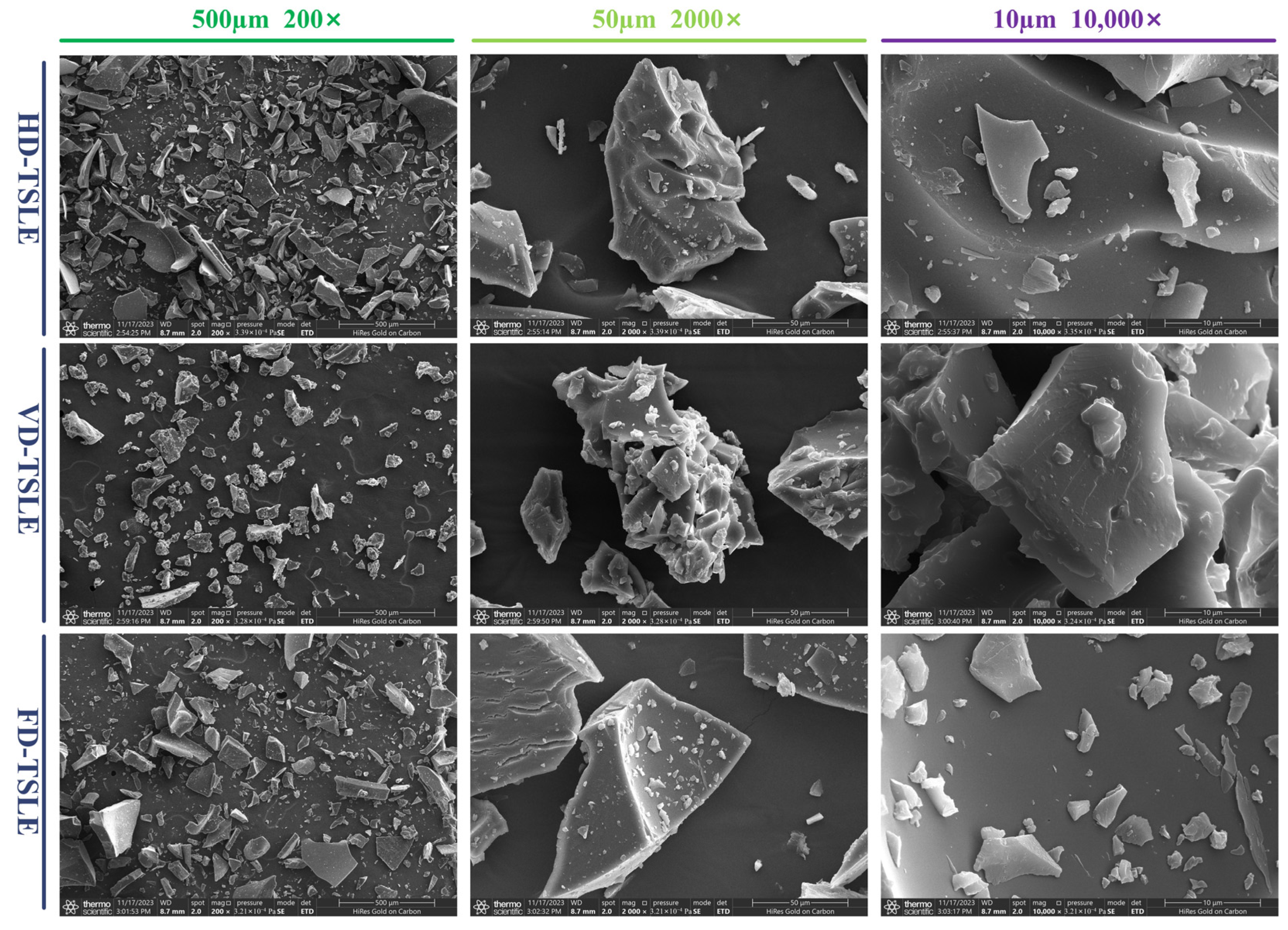

| Run | X1: Ethanol Concentration (%) | X2: LSR (mL/g) | X3: Time (min) | X4: Temperature (°C) | TPC (mg/g) * |
|---|---|---|---|---|---|
| 1 | −1(20) | −1(20) | 0(20) | 0(45) | 88.34 |
| 2 | −1(20) | 0(50) | 0(20) | −1(25) | 94.98 |
| 3 | −1(20) | 0(50) | −1(5) | 0(45) | 99.89 |
| 4 | −1(20) | 0(50) | 1(35) | 0(45) | 104.93 |
| 5 | −1(20) | 0(50) | 0(20) | 1(65) | 101.75 |
| 6 | −1(20) | 1(80) | 0(20) | 0(45) | 99.65 |
| 7 | 0(50) | −1(20) | 0(20) | −1(25) | 84.98 |
| 8 | 0(50) | −1(20) | −1(5) | 0(45) | 86.98 |
| 9 | 0(50) | −1(20) | 1(35) | 0(45) | 91.34 |
| 10 | 0(50) | −1(20) | 0(20) | 1(65) | 103.33 |
| 11 | 0(50) | 0(50) | −1(5) | −1(25) | 97.04 |
| 12 | 0(50) | 0(50) | 1(35) | −1(25) | 97.12 |
| 13 | 0(50) | 0(50) | 0(20) | 0(45) | 111.02 |
| 14 | 0(50) | 0(50) | 0(20) | 0(45) | 109.98 |
| 15 | 0(50) | 0(50) | 0(20) | 0(45) | 108.16 |
| 16 | 0(50) | 0(50) | −1(5) | 1(65) | 103.33 |
| 17 | 0(50) | 0(50) | 1(35) | 1(65) | 100.98 |
| 18 | 0(50) | 1(80) | 0(20) | −1(25) | 98.67 |
| 19 | 0(50) | 1(80) | −1(5) | 0(45) | 97.12 |
| 20 | 0(50) | 1(80) | 1(35) | 0(45) | 91.03 |
| 21 | 0(50) | 1(80) | 0(20) | 1(65) | 94.51 |
| 22 | 1(80) | −1(20) | 0(20) | 0(45) | 77.68 |
| 23 | 1(80) | 0(50) | 0(20) | −1(25) | 73.93 |
| 24 | 1(80) | 0(50) | −1(5) | 0(45) | 78.82 |
| 25 | 1(80) | 0(50) | 1(35) | 0(45) | 82.54 |
| 26 | 1(80) | 0(50) | 0(20) | 1(65) | 85.46 |
| 27 | 1(80) | 1(80) | 0(20) | 0(45) | 75.09 |
| Source | df | Sum of Squares | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Model | 14 | 2821.1521 | 201.511 | 28.4210 | <0.0001 *** |
| X1-Concentration (%) | 1 | 1121.7200 | 1121.7200 | 158.2068 | <0.0001 *** |
| X2-LSR (mL/g) | 1 | 45.7080 | 45.7080 | 6.4466 | 0.0260 * |
| X3-Time (min) | 1 | 1.8881 | 1.8881 | 0.2663 | 0.6152 |
| X4-Temperature (°C) | 1 | 151.5141 | 151.5141 | 21.3695 | 0.0006 *** |
| X1X2 | 1 | 48.3025 | 48.3025 | 6.8126 | 0.0228 * |
| X1X3 | 1 | 0.4356 | 0.4356 | 0.0614 | 0.8084 |
| X1X4 | 1 | 27.3006 | 27.3006 | 3.8505 | 0.0733 |
| X2X3 | 1 | 5.6644 | 5.6644 | 0.7989 | 0.3890 |
| X2X4 | 1 | 126.6750 | 126.6750 | 17.8662 | 0.0012 ** |
| X3X4 | 1 | 1.4762 | 1.4762 | 0.2082 | 0.6563 |
| X12 | 1 | 1051.2528 | 1051.2528 | 148.2682 | <0.0001 *** |
| X22 | 1 | 625.7815 | 625.7815 | 88.2599 | <0.0001 *** |
| X32 | 1 | 163.2210 | 163.2210 | 23.0206 | 0.0004 *** |
| X42 | 1 | 128.6857 | 128.6857 | 18.1498 | 0.0011 ** |
| Residual | 12 | 85.0826 | 7.079 | ||
| Lack of Fit | 10 | 80.8914 | 8.0891 | 3.8601 | 0.2232 |
| Pure Error | 2 | 4.1912 | 2.0956 | ||
| Cor Total | 12 | 84.0825 | |||
| R2 | 0.9707 | ||||
| Adj R2 | 0.9366 |
| ID | Compounds | Formula | Regression Equation | Linear Range (μg/mL) | R2 | Rt (min) | Concentrations (μg/g) |
|---|---|---|---|---|---|---|---|
| 1 | Quercetin | C15H10O7 | y = 7.1294x − 0.1801 | 0.1–40 | 0.9999 | 18.667 | 177.98 ± 26.03 |
| 2 | Ethyl gallate | C9H10O5 | y = 18.5491x − 1.4280 | 0.1–100 | 0.9999 | 23.121 | 3469.99 ± 79.19 |
| 3 | Gallic acid | C7H6O5 | y = 22.3831x − 0.6774 | 0.1–40 | 0.9998 | 7.793 | 651.27 ± 22.73 |
| 4 | Isoquercitrin | C21H20O12 | y = 11.1103x − 4.0684 | 1–200 | 0.9999 | 8.244 | 9905.99 ± 328.13 |
| 5 | Kaempferol | C15H10O6 | y = 8.8767x − 0.3402 | 0.4–100 | 0.9999 | 20.468 | 51.11 ± 0.77 |
| 6 | Hyperoside | C21H20O12 | y = 9.3525x − 0.5628 | 0.4–100 | 0.9999 | 7.933 | 958.33 ± 69.13 |
| 7 | Tannic acid | C76H52O46 | y = 1.4179x − 4.3884 | 1–1000 | 0.9999 | 4.33 | 13,988.12 ± 531.46 |
| 8 | Ellagic acid | C14H6O8 | y = 46.5936x − 1.4697 | 0.4–200 | 0.9999 | 7.302 | 5161.07 ± 512.88 |
| Single Factor | Ethanol Concentration (%) | Liquid–Solid Ratio (mL/g) | Time (min) | Temperature (°C) |
|---|---|---|---|---|
| Ethanol concentration | 0,20,40,60,80 | 40 | 40 | 40 |
| Liquid–solid ratio | 40 | 20,40,60,80,100 | 40 | 40 |
| Extraction Time | 20 | 20 | 5,15,25,35,45 | 20 |
| Temperature | 25 | 25 | 25 | 5,25,45,65,85 |
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1: Ethanol concentration (%) | 20 | 50 | 80 |
| X2: Liquid–solid ratio (mL/g) | 20 | 50 | 80 |
| X3: Extraction time (min) | 5 | 20 | 35 |
| X4: Extraction temperature (°C) | 25 | 45 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.-J.; Zhang, X.-Y.; Cheng, Y.; Qiu, Y.-X.; Hu, Y.-Y.; Yu, T.; Qian, W.-Z.; Zhang, D.-J.; Gao, S. Extraction Optimization of Phenolic Compounds from Triadica sebifera Leaves: Identification, Characterization and Antioxidant Activity. Molecules 2024, 29, 3266. https://doi.org/10.3390/molecules29143266
Fan S-J, Zhang X-Y, Cheng Y, Qiu Y-X, Hu Y-Y, Yu T, Qian W-Z, Zhang D-J, Gao S. Extraction Optimization of Phenolic Compounds from Triadica sebifera Leaves: Identification, Characterization and Antioxidant Activity. Molecules. 2024; 29(14):3266. https://doi.org/10.3390/molecules29143266
Chicago/Turabian StyleFan, Shao-Jun, Xin-Yue Zhang, Yu Cheng, Yu-Xian Qiu, Yun-Yi Hu, Ting Yu, Wen-Zhang Qian, Dan-Ju Zhang, and Shun Gao. 2024. "Extraction Optimization of Phenolic Compounds from Triadica sebifera Leaves: Identification, Characterization and Antioxidant Activity" Molecules 29, no. 14: 3266. https://doi.org/10.3390/molecules29143266







