A Fluorescent Conjugated Polar Polymer for Probing Charge Injection in Multilayer Organic Light-Emitting Transistors
Abstract
:1. Introduction
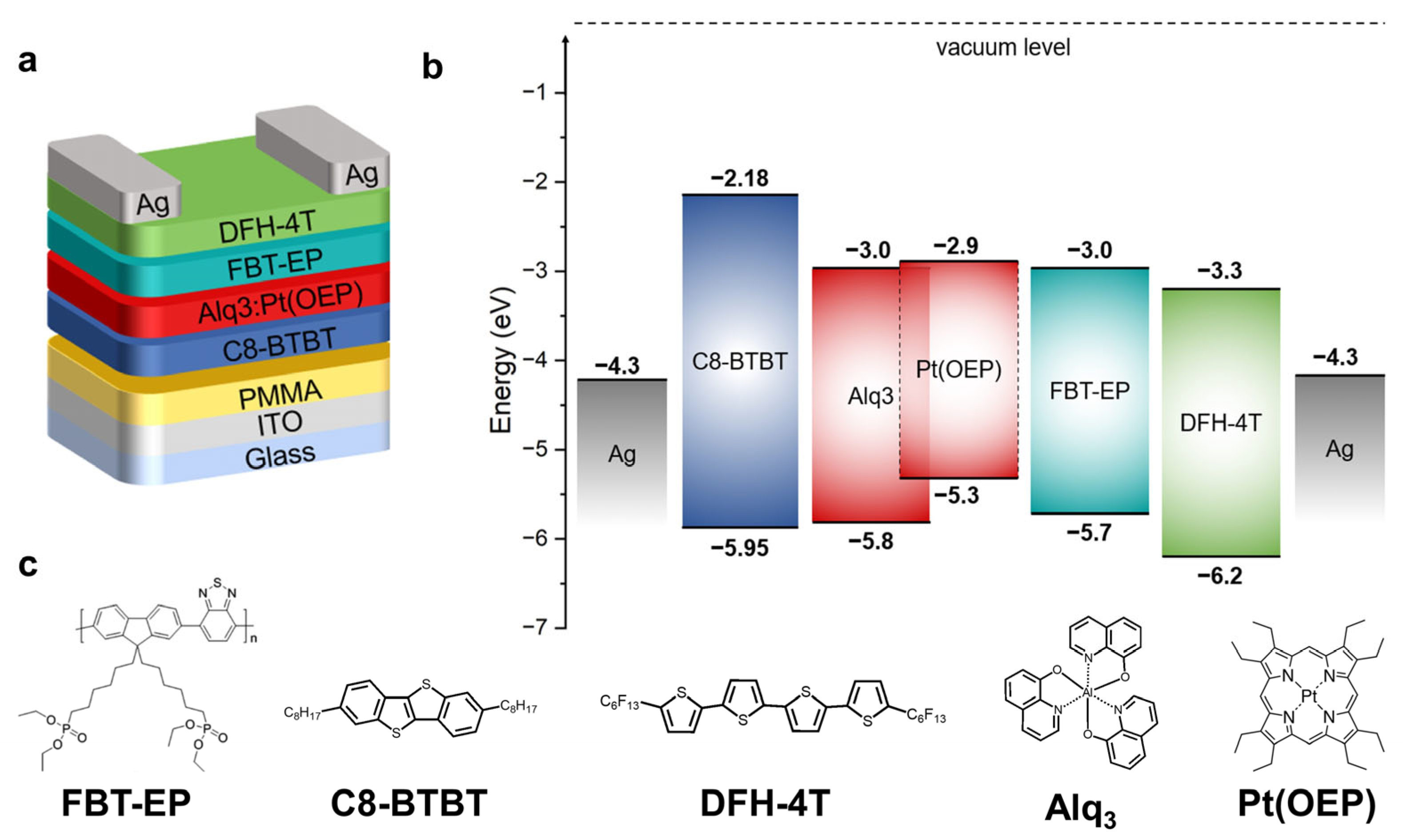
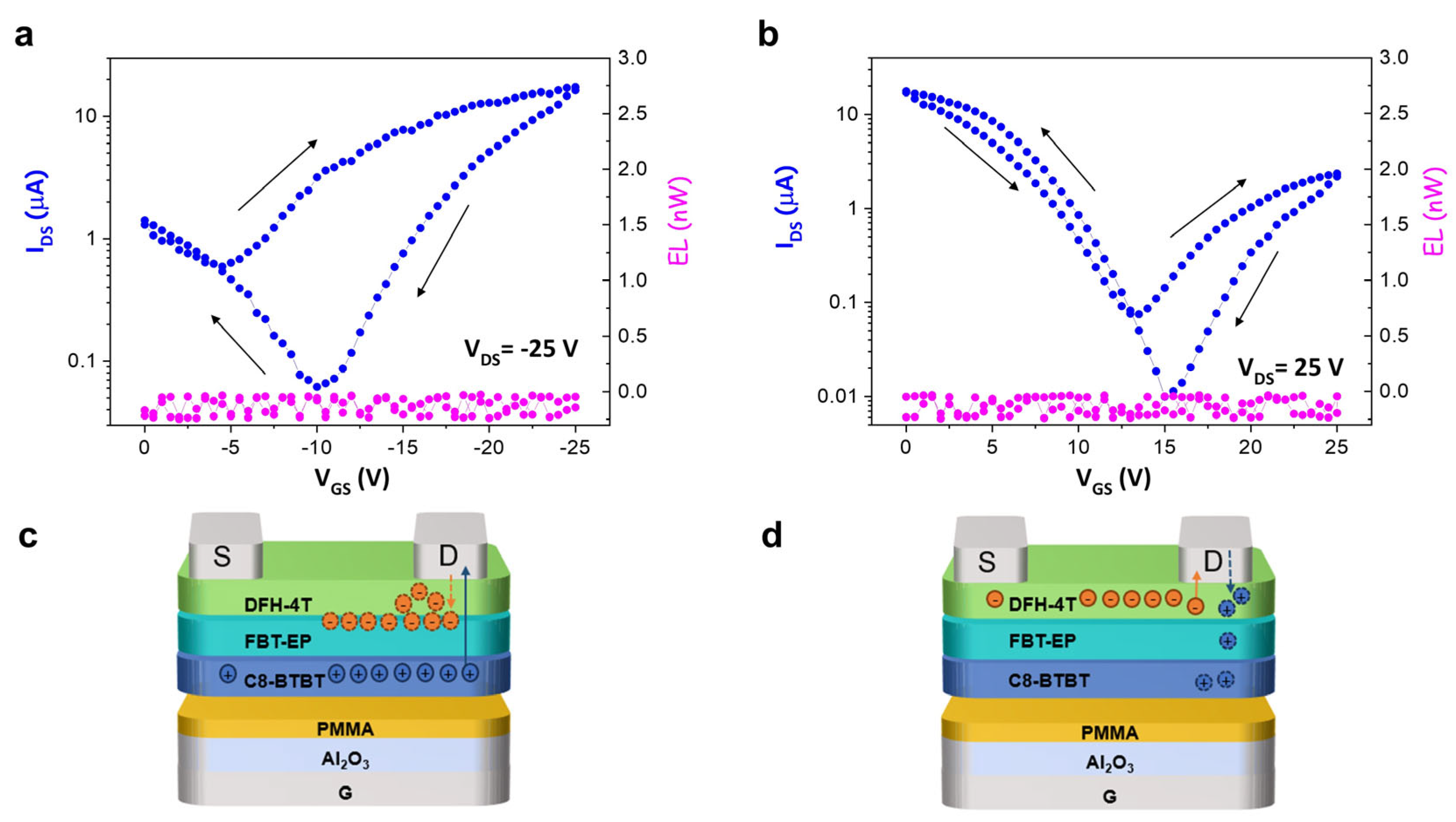
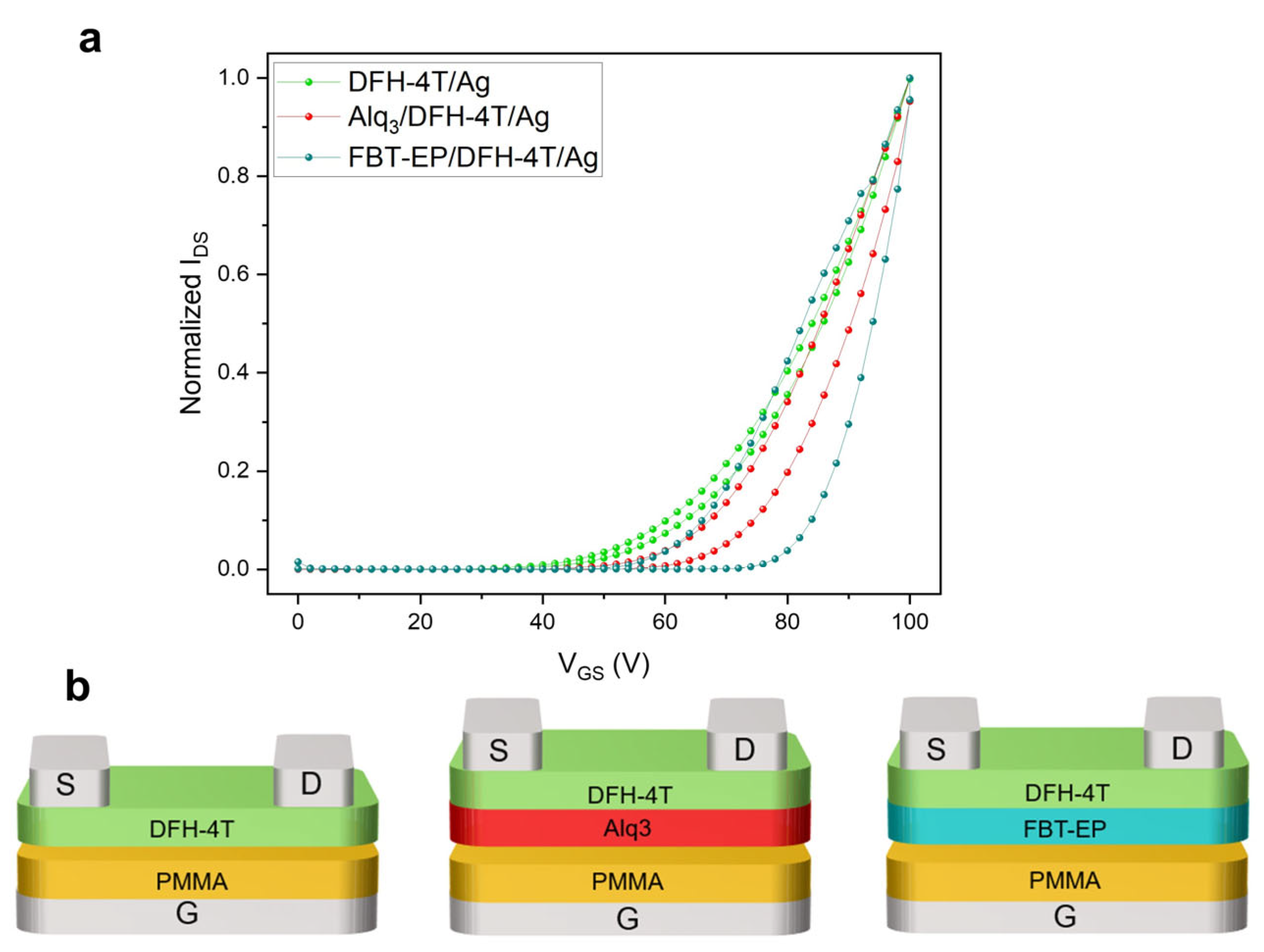
2. Results and Discussion
FBT-EP in h-OS/EML/e-OS OLET
3. Materials and Methods
3.1. Device Fabrication and Characterization
3.2. Synthesis and Characterization of FBT-EP Polymer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salehi, A.; Fu, X.; Shin, D.H.; So, F. Recent Advances in OLED Optical Design. Adv. Funct. Mater. 2019, 29, 1808803. [Google Scholar] [CrossRef]
- Zou, S.J.; Shen, Y.; Xie, F.M.; De Chen, J.; Li, Y.Q.; Tang, J.X. Recent Advances in Organic Light-Emitting Diodes: Toward Smart Lighting and Displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Sharma, K.; Abbas, B. A Study of Highly Efficient Organic Light Emitting Transistors That Outperforms Organic Light Emitting Diodes. Opt. Quant. Electron. 2023, 55, 338. [Google Scholar] [CrossRef]
- Zaumseil, J. Recent Developments and Novel Applications of Thin Film, Light-Emitting Transistors. Adv. Fun. Mat. 2020, 30, 1905269. [Google Scholar] [CrossRef]
- Wittmann, H.F.; Grüner, J.; Friend, R.H.; Spencer, G.W.C.; Moratti, S.C.; Holmes, A.B. Microcavity Effect in a Single-Layer Polymer Light-Emitting Diode. Adv. Mat. 1995, 7, 541–544. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Chen, H.; Xue, J. Transparent Electrodes for Organic Optoelectronic Devices: A Review. J. Photonics Energy 2014, 4, 040990. [Google Scholar] [CrossRef]
- Feng, J.; Liu, Y.F.; Bi, Y.G.; Sun, H.B. Light Manipulation in Organic Light-Emitting Devices by Integrating Micro/Nano Patterns. Laser Photonics Rev. 2017, 11, 1600145. [Google Scholar] [CrossRef]
- Allemeier, D.; Isenhart, B.; Dahal, E.; Tsuda, Y.; Yoshida, T.; White, M.S. Emergence and Control of Photonic Band Structure in Stacked OLED Microcavities. Nat. Comm. 2021, 12, 6111. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Liu, X.; Lai, W.Y.; Huang, W. Organic Light-Emitting Field-Effect Transistors: Device Geometries and Fabrication Techniques. Adv. Mat. 2018, 30, 1802466. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Hu, W. Organic Light-Emitting Transistors: Materials, Device Configurations, and Operations. Small 2016, 12, 1252–1294. [Google Scholar] [CrossRef]
- Qin, Z.; Gao, H.; Dong, H.; Hu, W.; Qin, Z.; Gao, H.; Dong, H.; Hu, W. Organic Light-Emitting Transistors Entering a New Development Stage. Adv. Mater. 2021, 33, 2007149. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Lai, W.Y. Chapter 15: Light-Emitting Transistors with Ambipolar Materials. In RSC Smart Materials; RSC: Stratford-upon-Avon, UK, 2020; pp. 350–374. [Google Scholar]
- Muccini, M. A Bright Future for Organic Field-Effect Transistors. Nat. Mat. 2006, 5, 605–613. [Google Scholar] [CrossRef]
- Capelli, R.; Toffanin, S.; Generali, G.; Usta, H.; Facchetti, A.; Muccini, M. Organic Light-Emitting Transistors with an Efficiency That Outperforms the Equivalent Light-Emitting Diodes. Nat. Mat. 2010, 9, 496–503. [Google Scholar] [CrossRef]
- Muccini, M.; Toffanin, S. Organic Light-Emitting Transistors: Towards the Next Generation Display Technology; Wiley: Hoboken, NJ, USA, 2016; pp. 1–281. [Google Scholar]
- Zaumseil, J.; Friend, R.H.; Sirringhaus, H. Spatial Control of the Recombination Zone in an Ambipolar Light-Emitting Organic Transistor. Nat. Mat. 2006, 5, 69–74. [Google Scholar] [CrossRef]
- Zhou, K.; Dong, H.; Zhang, H.L.; Hu, W. High Performance N-Type and Ambipolar Small Organic Semiconductors for Organic Thin Film Transistors. Phys. Chem. Chem. Phys. 2014, 16, 22448–22457. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, Y.; Liu, Y. 25th Anniversary Article: Recent Advances in n-Type and Ambipolar Organic Field-Effect Transistors. Adv. Mat. 2013, 25, 5372–5391. [Google Scholar] [CrossRef]
- Quinn, J.T.E.; Zhu, J.; Li, X.; Wang, J.; Li, Y. Recent Progress in the Development of N-Type Organic Semiconductors for Organic Field Effect Transistors. J. Mat. Chem. C 2017, 5, 8654–8681. [Google Scholar] [CrossRef]
- Yuan, D.; Sharapov, V.; Liu, X.; Yu, L. Design of High-Performance Organic Light-Emitting Transistors. ACS Omega 2020, 5, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Prosa, M.; Moschetto, S.; Benvenuti, E.; Zambianchi, M.; Muccini, M.; Melucci, M.; Toffanin, S. 2,3-Thienoimide-Ended Oligothiophenes as Ambipolar Semiconductors for Multifunctional Single-Layer Light-Emitting Transistors. J. Mat. Chem. C 2020, 8, 15048–15066. [Google Scholar] [CrossRef]
- Zaumseil, J.; Donley, C.L.; Kim, J.S.; Friend, R.H.; Sirringhaus, H. Efficient Top-Gate, Ambipolar, Light-Emitting Field-Effect Transistors Based on a Green-Light-Emitting Polyfluorene. Adv. Mat. 2006, 18, 2708–2712. [Google Scholar] [CrossRef]
- Gwinner, M.C.; Kabra, D.; Roberts, M.; Brenner, T.J.K.; Wallikewitz, B.H.; McNeill, C.R.; Friend, R.H.; Sirringhaus, H. Highly Efficient Single-Layer Polymer Ambipolar Light-Emitting Field-Effect Transistors. Adv. Mat. 2012, 24, 2728–2734. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ke, T.H.; Genoe, J.; Heremans, P.; Rolin, C. Overlapping-Gate Organic Light-Emitting Transistors. Adv. Electron. Mater. 2019, 5, 1800437. [Google Scholar] [CrossRef]
- Chen, H.; Huang, W.; Marks, T.J.; Facchetti, A.; Meng, H.; Chen, H.; Meng, H.; Huang, W.; Marks, T.J.; Facchetti, A. Recent Advances in Multi-Layer Light-Emitting Heterostructure Transistors. Small 2021, 17, 2007661. [Google Scholar] [CrossRef] [PubMed]
- Hepp, A.; Heil, H.; Weise, W.; Ahles, M.; Schmechel, R.; von Seggern, H. Light-Emitting Field-Effect Transistor Based on a Tetracene Thin Film. Phys. Rev. Lett. 2003, 91, 157406. [Google Scholar] [CrossRef] [PubMed]
- Ahles, M.; Hepp, A.; Schmechel, R.; Von Seggern, H. Light Emission from a Polymer Transistor. Appl. Phys. Lett. 2004, 84, 428–430. [Google Scholar] [CrossRef]
- Pasini, M.; Galeotti, F.; Mróz, W.; Squeo, B.M.; Luzzati, S.; Botta, C.; Scavia, G.; Giovanella, U. Synthetic Strategy for the Development of Conjugated Polyelectrolytes as Cathode Interfacial Layers: Toward Sustainable Organic Devices. Macromol. Chem. Phys. 2023, 224, 2300130. [Google Scholar] [CrossRef]
- Ohisa, S.; Kato, T.; Takahashi, T.; Suzuki, M.; Hayashi, Y.; Koganezawa, T.; McNeill, C.R.; Chiba, T.; Pu, Y.J.; Kido, J. Conjugated Polyelectrolyte Blend with Polyethyleneimine Ethoxylated for Thickness-Insensitive Electron Injection Layers in Organic Light-Emitting Devices. ACS Appl. Mater. Interfaces 2018, 10, 17318–17326. [Google Scholar] [CrossRef]
- Carulli, F.; Scavia, G.; Lassi, E.; Pasini, M.; Galeotti, F.; Brovelli, S.; Giovanella, U.; Luzzati, S. A Bifunctional Conjugated Polyelectrolyte for the Interfacial Engineering of Polymer Solar Cells. J. Colloid Interface Sci. 2019, 538, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Namdas, E.B.; Gutacker, A.; Heeger, A.J.; Bazan, G.C. Solution-Processed Organic Light-Emitting Transistors Incorporating Conjugated Polyelectrolytes. Adv. Funct. Mater. 2011, 21, 3667–3672. [Google Scholar] [CrossRef]
- Ong, G.L.; Zhang, Q.; Ong, T.S.; Kek, R.; Nee, C.H.; Tou, T.Y.; Liaw, D.J.; Yap, S.L.; Yap, S.S. Enhanced Performance of Blue OLED with Water/Alcohol Soluble Conjugated Polymer as Electron Injection Layer. Synth. Met. 2021, 272, 116658. [Google Scholar] [CrossRef]
- Seo, J.H.; Nguyen, T.-Q. Electronic Properties of Conjugated Polyelectrolyte Thin Films. J. Am. Chem. Soc. 2008, 130, 10042–10043. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Bazan, G.C. Conjugated Polyelectrolytes: Fundamentals and Applications; Wiley: Weinheim, Germany, 2013; pp. 1–410. [Google Scholar]
- Duarte, A.; Pu, K.Y.; Liu, B.; Bazan, G.C. Recent Advances in Conjugated Polyelectrolytes for Emerging Optoelectronic Applications. Chem. Mater. 2011, 23, 501–515. [Google Scholar] [CrossRef]
- Seo, J.H.; Gutacker, A.; Walker, B.; Cho, S.; Garcia, A.; Yang, R.; Nguyen, T.Q.; Heeger, A.J.; Bazan, G.C. Improved Injection in N-Type Organic Transistors with Conjugated Polyelectrolytes. J. Am. Chem. Soc. 2009, 131, 18220–18221. [Google Scholar] [CrossRef]
- Giovanella, U.; Pasini, M.; Lorenzon, M.; Galeotti, F.; Lucchi, C.; Meinardi, F.; Luzzati, S.; Dubertret, B.; Brovelli, S. Efficient Solution-Processed Nanoplatelet-Based Light-Emitting Diodes with High Operational Stability in Air. Nano Lett. 2018, 18, 3441–3448. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, I.; Suh, M.; Bailey, J.; Bradley, D.D.C.; Kim, J.-S. Optimizing Interfacial Energetics for Conjugated Polyelectrolyte Electron Injection Layers in High Efficiency and Fast Responding Polymer Light Emitting Diodes. ACS Appl. Mater. Interfaces 2022, 14, 24668–24680. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Yang, R.; Jin, Y.; Walker, B.; Nguyen, T.Q. Structure-Function Relationships of Conjugated Polyelectrolyte Electron Injection Layers in Polymer Light Emitting Diodes. App. Phys. Lett. 2007, 91, 153502. [Google Scholar] [CrossRef]
- Duan, C.; Wang, L.; Zhang, K.; Guan, X.; Huang, F.; Duan, C.; Wang, L.; Zhang, K.; Guan, X.; Huang, F. Conjugated Zwitterionic Polyelectrolytes and Their Neutral Precursor as Electron Injection Layer for High-Performance Polymer Light-Emitting Diodes. Adv. Mat. 2011, 23, 1665–1669. [Google Scholar] [CrossRef]
- Garcia, A.; Brzezinski, J.Z.; Nguyen, T.Q. Cationic Conjugated Polyelectrolyte Electron Injection Layers: Effect of Halide Counterions. J. Phys. Chem. C 2009, 113, 2950–2954. [Google Scholar] [CrossRef]
- Prosa, M.; Benvenuti, E.; Pasini, M.; Giovanella, U.; Bolognesi, M.; Meazza, L.; Galeotti, F.; Muccini, M.; Toffanin, S. Organic Light-Emitting Transistors with Simultaneous Enhancement of Optical Power and External Quantum Efficiency via Conjugated Polar Polymer Interlayers. ACS Appl. Mater. Interfaces 2018, 10, 25580–25588. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, K.; Huang, F.; Cao, Y. Water/Alcohol Soluble Conjugated Polymers for the Interface Engineering of Highly Efficient Polymer Light-Emitting Diodes and Polymer Solar Cells. Chem. Commun. 2015, 51, 5572–5585. [Google Scholar] [CrossRef]
- Moschetto, S.; Benvenuti, E.; Usta, H.; Ozdemir, R.; Facchetti, A.; Muccini, M.; Prosa, M.; Toffanin, S. Interplay between Charge Injection, Electron Transport, and Quantum Efficiency in Ambipolar Trilayer Organic Light-Emitting Transistors. Adv. Mater. Interfaces 2022, 9, 2101926. [Google Scholar] [CrossRef]
- Bao, Q.; Liu, X.; Wang, E.; Fang, J.; Gao, F.; Braun, S.; Fahlman Bao, M.Q.; Liu, X.; Braun, S.; Fahlman, M.; et al. Regular Energetics at Conjugated Electrolyte/Electrode Modifier for Organic Electronics and Their Implications on Design Rules. Adv. Mater. Interfaces 2015, 2, 1500204. [Google Scholar] [CrossRef]
- Squeo, B.M.; Carulli, F.; Lassi, E.; Galeotti, F.; Giovanella, U.; Luzzati, S.; Pasini, M. Benzothiadiazole-Based Conjugated Polyelectrolytes for Interfacial Engineering in Optoelectronic Devices. Pure Appl. Chem. 2019, 91, 477–488. [Google Scholar] [CrossRef]
- Lee, B.H.; Jung, I.H.; Woo, H.Y.; Shim, H.K.; Kim, G.; Lee, K. Multi-Charged Conjugated Polyelectrolytes as a Versatile Work Function Modifier for Organic Electronic Devices. Adv. Funct. Mater. 2014, 24, 1100–1108. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, H.; Liu, M.; Zhao, W.-M.; Su, S.-J.; Cao, C.; Chen, Y.D.; Zhou, H.; Liu, M.; Su, S.; et al. Novel Cathode Interlayers Based on Neutral Alcohol-Soluble Small Molecules with a Triphenylamine Core Featuring Polar Phosphonate Side Chains for High-Performance Polymer Light-Emitting and Photovoltaic Devices. Macromol. Rapid Commun. 2013, 34, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qin, C.; Niu, X.; Xie, Z.; Cheng, Y.; Wang, L.; Li, X. On the Origin of Efficient Electron Injection at Phosphonate-Functionalized Polyfluorene/Aluminum Interface in Efficient Polymer Light-Emitting Diodes. Appl. Phys. Lett. 2010, 97, 043506. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, C.; Ding, J.; Chen, L.; Xie, Z.; Cheng, Y.; Wang, L. High-Performance All-Polymer White-Light-Emitting Diodes Using Polyfluorene Containing Phosphonate Groups as an Efficient Electron-Injection Layer. Adv. Funct. Mater. 2010, 20, 2951–2957. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Liu, Y. High-Mobility Organic Light-Emitting Semiconductors and Its Optoelectronic Devices. Small Struct. 2021, 2, 2000083. [Google Scholar] [CrossRef]
- Abuelwafa, A.A.; Dongol, M.; El-Nahass, M.M.; Soga, T. Structural and Optical Properties of Nanocrystalline Platinum Octaethylporphyrin (PtOEP) Thin Films. J. Alloys Compd. 2016, 655, 415–422. [Google Scholar] [CrossRef]
- Borek, C.; Hanson, K.; Djurovich, P.I.; Thompson, M.E.; Aznavour, K.; Bau, R.; Sun, Y.; Forrest, S.R.; Brooks, J.; Michalski, L.; et al. Highly Efficient, near-Infrared Electrophosphorescence from a Pt-Metalloporphyrin Complex. Angew. Chem. Int. Ed. 2007, 46, 1109–1112. [Google Scholar] [CrossRef]
- Facchetti, A. Semiconductors for Organic Transistors. Mater. Today 2007, 10, 28–37. [Google Scholar] [CrossRef]
- Toffanin, S.; Capelli, R.; Koopman, W.; Generali, G.; Cavallini, S.; Stefani, A.; Saguatti, D.; Ruani, G.; Muccini, M. Organic Light-Emitting Transistors with Voltage-Tunable Lit Area and Full Channel Illumination. Laser Photonics Rev. 2013, 7, 1011–1019. [Google Scholar] [CrossRef]
- Paasch, G.; Lindner, T.; Rost-Bietsch, C.; Karg, S.; Riess, W.; Scheinert, S. Operation and Properties of Ambipolar Organic Field-Effect Transistors. J. Appl. Phys. 2005, 98, 084505. [Google Scholar] [CrossRef]
- Muccini, M.; Koopman, W.; Toffanin, S. The Photonic Perspective of Organic Light-Emitting Transistors. Laser Photonics Rev. 2012, 6, 258–275. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, H.; Jiang, L.; Zhen, Y.; Dong, H.; Hu, W. Solution-Processed Flexible Organic Ferroelectric Phototransistor. ACS Appl. Mater. Interfaces 2017, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Bangsund, J.S.; Van Sambeek, J.R.; Concannon, N.M.; Holmes, R.J. Sub-Turn-on Exciton Quenching Due to Molecular Orientation and Polarization in Organic Light-Emitting Devices. Sci. Adv. 2020, 6, eabb2659. [Google Scholar] [CrossRef] [PubMed]
- Esaki, Y.; Tanaka, M.; Matsushima, T.; Adachi, C. Active Control of Spontaneous Orientation Polarization of Tris(8-Hydroxyquinolinato)Aluminum (Alq3) Films and Its Effect on Performance of Organic Light-Emitting Diodes. Adv. Electron. Mater. 2021, 7, 2100486. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, Z.; Wang, L. Phosphonate-Functionalized Polyfluorene and Its Application in Organic Optoelectronic Devices. Polym. Bull. 2012, 68, 829–845. [Google Scholar] [CrossRef]
- Weis, M. Gradual Channel Approximation Models for Organic Field-Effect Transistors: The Space-Charge Field Effect. J. Appl. Phys. 2012, 111, 54506. [Google Scholar] [CrossRef]
- Zambianchi, M.; Benvenuti, E.; Bettini, C.; Zanardi, C.; Seeber, R.; Gentili, D.; Cavallini, M.; Muccini, M.; Biondo, V.; Soldano, C.; et al. Anthracene-Based Molecular Emitters for Non-Doped Deep-Blue Organic Light Emitting Transistors. J. Mater. Chem. C 2016, 4, 9411–9417. [Google Scholar] [CrossRef]
- Iosip, M.D.; Destri, S.; Pasini, M.; Porzio, W.; Pernstich, K.P.; Batlogg, B. New Dithieno [3,2-b:2′,3′-d]Thiophene Oligomers as Promising Materials for Organic Field-Effect Transistor Applications. Synth. Met. 2004, 146, 251–257. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moschetto, S.; Squeo, B.M.; Reginato, F.; Prosa, M.; Pasini, M.; Toffanin, S. A Fluorescent Conjugated Polar Polymer for Probing Charge Injection in Multilayer Organic Light-Emitting Transistors. Molecules 2024, 29, 3295. https://doi.org/10.3390/molecules29143295
Moschetto S, Squeo BM, Reginato F, Prosa M, Pasini M, Toffanin S. A Fluorescent Conjugated Polar Polymer for Probing Charge Injection in Multilayer Organic Light-Emitting Transistors. Molecules. 2024; 29(14):3295. https://doi.org/10.3390/molecules29143295
Chicago/Turabian StyleMoschetto, Salvatore, Benedetta Maria Squeo, Francesco Reginato, Mario Prosa, Mariacecilia Pasini, and Stefano Toffanin. 2024. "A Fluorescent Conjugated Polar Polymer for Probing Charge Injection in Multilayer Organic Light-Emitting Transistors" Molecules 29, no. 14: 3295. https://doi.org/10.3390/molecules29143295





