Research on Food Preservation Based on Antibacterial Technology: Progress and Future Prospects
Abstract
:1. Introduction
2. Antibacterial and Food Preservation Based on aPDT
2.1. Mechanism of aPDT
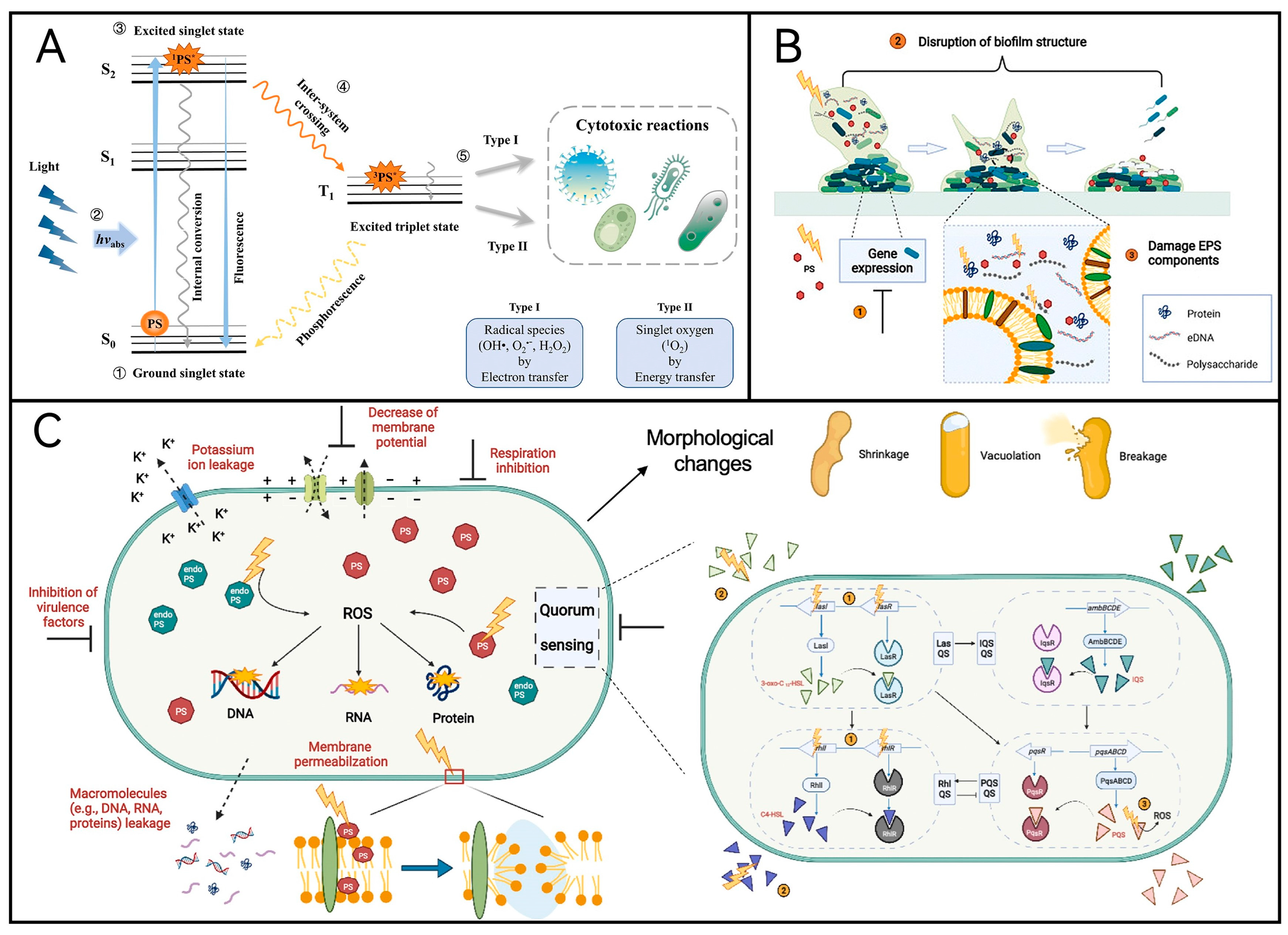
2.2. Photosensitizer

2.3. Application of aPDT in Different Food Matrices
2.3.1. Fruit and Vegetables
2.3.2. Seafood
2.3.3. Dairy Products
2.3.4. Meat Products
3. Antibacterial and Food Preservation Based on Ionizing Radiation
3.1. Mechanism of Ionizing Radiation
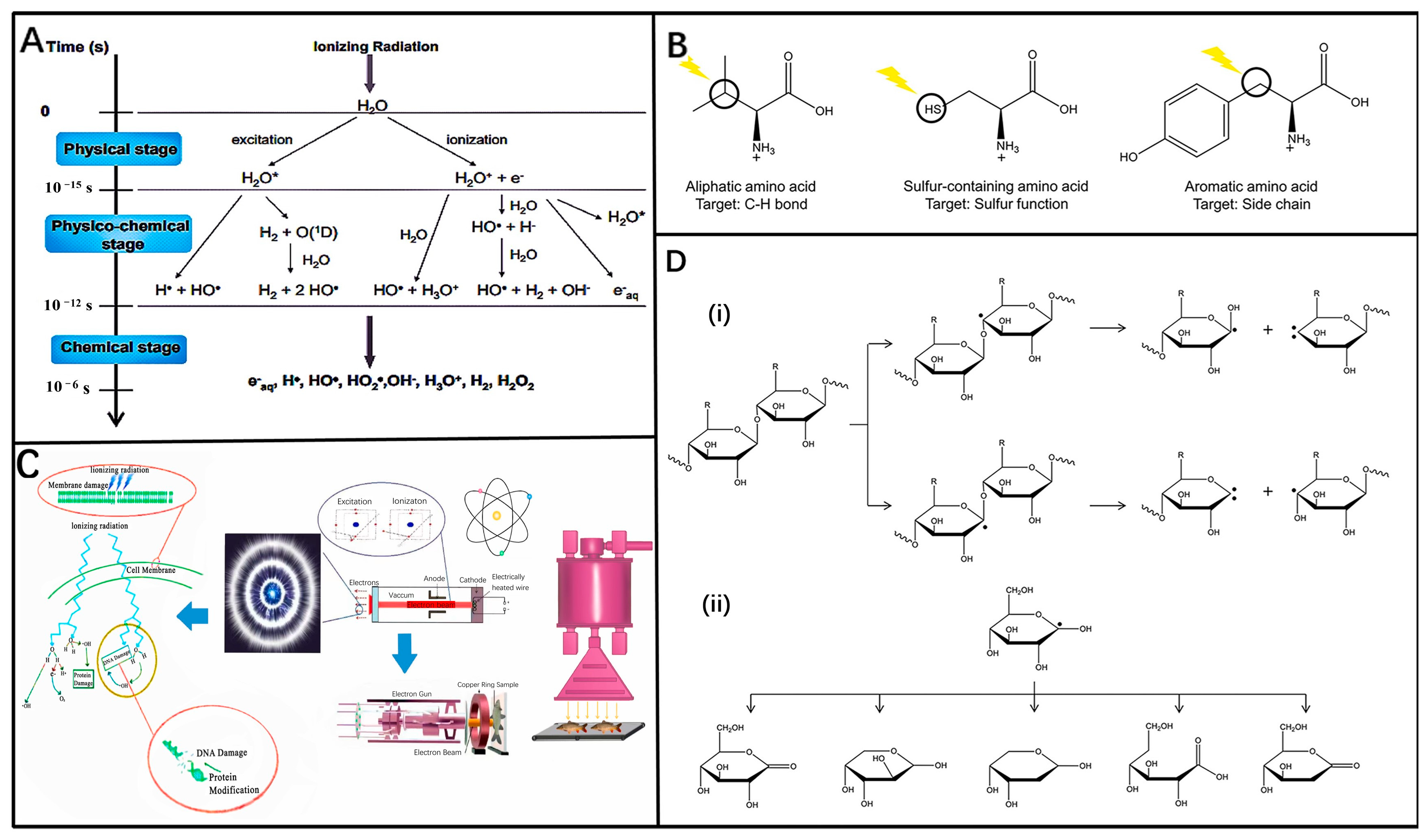
3.2. Applications of Ionizing Radiation in Different Food

4. Antibacterial and Food Preservation Based on AMPs
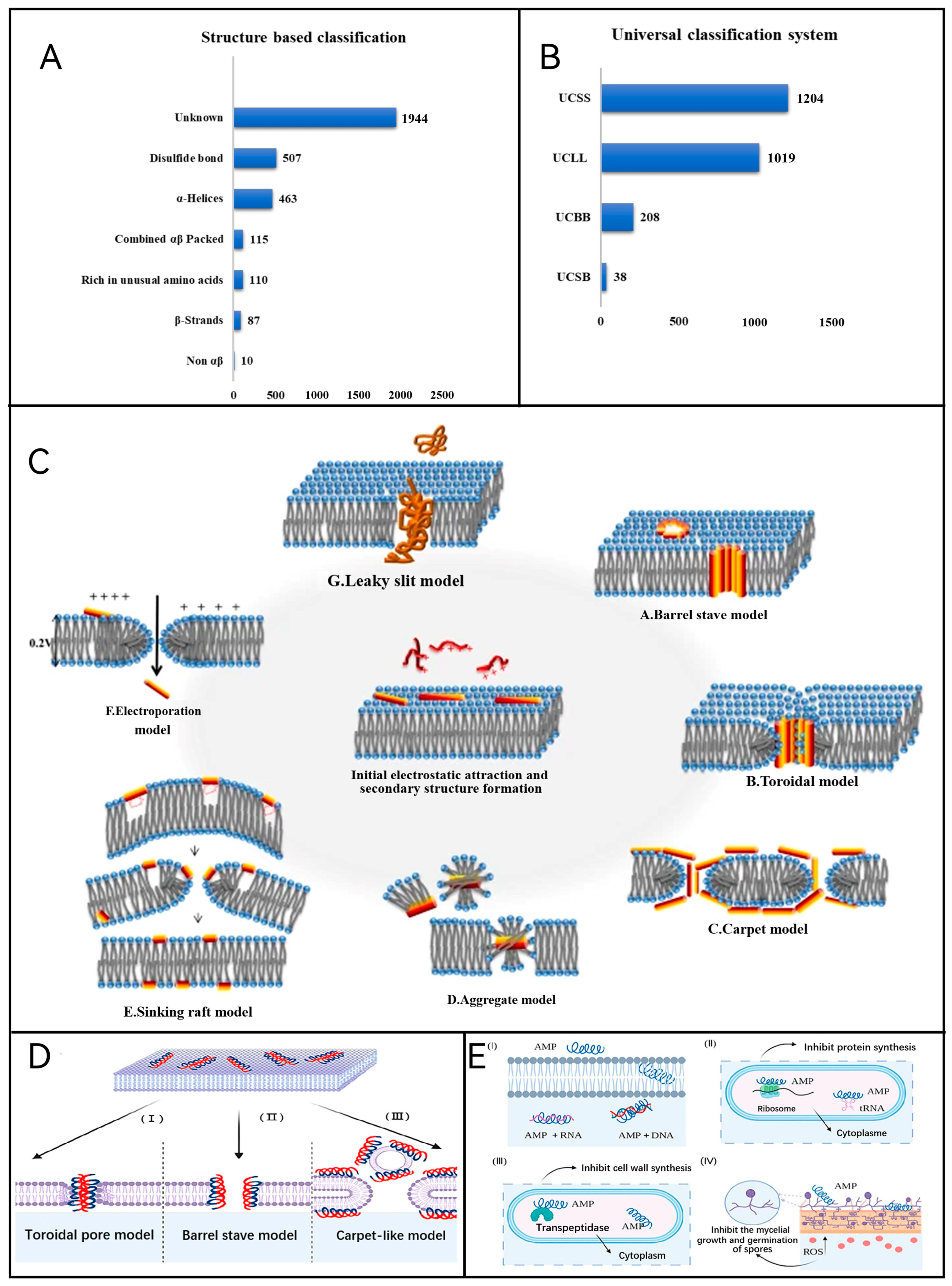
4.1. Sources of Antimicrobial Peptides
4.1.1. Naturally Occurring
4.1.2. Molecular Modification
4.1.3. Biosynthesis by Using Eukaryotic and Prokaryotic Expression Systems
4.2. Specific Applications of Typical AMPs
4.2.1. Nisin
4.2.2. Pediocin
4.2.3. Cecropins
5. Other Technical Approaches
6. Limitations and Disadvantages
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviation
| aPDT | antimicrobial photodynamic therapy | TAB | total asparagus bread powder |
| IR | ionizing radiation | ARP | actin-related protein |
| AMPs | antimicrobial peptides | MMb | 3-methoxy-3-methyl-1-butanol |
| PS | polymeric substance | DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EPS | extracellular polymeric substance | LEEB | low-energy electron beams |
| DNA | deoxyribo nucleic acid | NEB | nanosecond electron beams |
| UVA | ultraviolet radiation A | ALF | anti-lipopolysaccharide factors |
| UVC | ultraviolet radiation C | MGD | mytilus galloprovincialis defensin |
| SOC | spin-orbit coupling | HPLC | high-resolution liquid chromatography |
| LED | light-emitting diode | LC-MS | liquid chromatograph-mass spectrometer |
| ROS | reactive oxygen species | LL37 | antibacterial protein LL-37 |
| Curs | curcumins | PET-28(+) | PET-28A(+) plasmid |
| CS | corn starch | ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| NBs | nano gas bubbles | hRBCs | human red blood cells |
| Oxy | oxygen | MRSA | methicillin-resistant staphylococcus aureus |
| FMN- | flavin mononucleotide | NPM | pectin microencapsulation |
| CDs | carbon dots | TA | titratable acidity |
| KGM | konjac glucomannan | HPP | high-pressure processing |
| MICs | minimal inhibitory concentrations | UHP | ultrahigh-pressure |
| SOD | superoxide dismutase | ZEO | Zataria multiflora Boiss essential oil |
| POD | polyphenol oxidase | SLNS | solid lipid nanoparticles |
| FDA | Food and Drug Administration | β-CD | β-cyclodextrin |
| EB | erythrosine B |
References
- Fuenmayor, C.A.; Licciardello, F. The challenges of food preservation materials and technologies for a more sustainable world. Food Packag. Shelf Life 2024, 43, 101273. [Google Scholar] [CrossRef]
- Muthuvelu, K.S.; Ethiraj, B.; Pramnik, S.; Raj, N.K.; Venkataraman, S.; Rajendran, D.S.; Bharathi, P.; Palanisamy, E.; Narayanan, A.S.; Vaidyanathan, V.K.; et al. Biopreservative technologies of food: An alternative to chemical preservation and recent developments. Food Sci. Biotechnol. 2023, 32, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Ghate, V.S.; Zhou, W.; Yuk, H. Perspectives and Trends in the Application of Photodynamic Inactivation for Microbiological Food Safety. Compr. Rev. Food Sci. Food Saf. 2019, 18, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Khezerlou, A.; Zolfaghari, H.; Forghani, S.; Abedi-Firoozjah, R.; Sani, M.A.; Negahdari, B.; Jalalvand, M.; Ehsani, A.; McClements, D.J. Combining Non-Thermal Processing Techniques with Edible Coating Materials: An Innovative Approach to Food Preservation. Coatings 2023, 13, 830. [Google Scholar] [CrossRef]
- Liu, D.; Gu, W.; Wang, L.; Sun, J. Photodynamic inactivation and its application in food preservation. Crit. Rev. Food Sci. Nutr. 2021, 63, 11–15. [Google Scholar] [CrossRef]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, J.; Zhong, J.; Deng, W.; Zhang, Z.; Wu, Y.; Zheng, Q. Antifungal efficacy of LEDs against major postharvest pathogens of litchi fruit in vitro and in vivo. Food Control 2023, 154, 110019. [Google Scholar] [CrossRef]
- Ritacca, A.G.; Prejanò, M.; Alberto, M.E.; Marino, T.; Toscano, M.; Russo, N. On the antibacterial photodynamic inactivation mechanism of Emodin and Dermocybin natural photosensitizers: A theoretical investigation. J. Comput. Chem. 2024, 45, 1254–1260. [Google Scholar] [CrossRef]
- Peleg, M. Selected challenges to modeling the kinetics of microbial inactivation and chemical reactions during food preservation. Curr. Opin. Food Sci. 2023, 51, 101029. [Google Scholar] [CrossRef]
- Yu, X.; Zou, Y.; Zhang, Z.; Wei, T.; Ye, Z.; Yuk, H.-G.; Zheng, Q. Recent advances in antimicrobial applications of curcumin-mediated photodynamic inactivation in foods. Food Control 2022, 138, 108986. [Google Scholar] [CrossRef]
- Sheng, L.; Li, X.; Wang, L. Photodynamic inactivation in food systems: A review of its application, mechanisms, and future perspective. Trends Food Sci. Technol. 2022, 124, 167–181. [Google Scholar] [CrossRef]
- Hoenes, K.; Bauer, R.; Spellerberg, B.; Hessling, M. Microbial photoinactivation by visible light results in limited loss of mem-brane integrity. Antibiotics 2021, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Nguyen, T.Y.N.; Vo, N.K.M.; Le, C.T.; Bui, X.T.; Pham, T.T. Blue light-emitting diode as the promising photodynamic method for the inactivation of Staphylococcus aureus. Syst. Microbiol. Biomanufacturing 2023, 4, 223–229. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Q.; Huang, Y.; Qi, M.; Yan, H.; Li, W.; Zhuang, H. Antibacterial Efficacy and Mechanisms of Curcumin-Based Photodynamic Treatment against Staphylococcus aureus and Its Application in Juices. Molecules 2022, 27, 7136. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Zhang, M.; Mujumdar, A.S. Phototreatment (below 1100 nm) improving quality attributes of fresh-cut fruits and vegetables: A review. Food Res. Int. 2023, 163, 112252. [Google Scholar] [CrossRef] [PubMed]
- Lena, A.; Marino, M.; Manzano, M.; Comuzzi, C.; Maifreni, M. An Overview of the Application of Blue Light-Emitting Diodes as a Non-Thermic Green Technology for Microbial Inactivation in the Food Sector. Food Eng. Rev. 2023, 16, 59–84. [Google Scholar] [CrossRef]
- Cossu, M.; Ledda, L.; Cossu, A. Emerging trends in the photodynamic inactivation (PDI) applied to the food decontamination. Food Res. Int. 2021, 144, 110358. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; He, X.; Feng, M.; Xu, X.; Chen, J.; Wang, Y. Flavin mononucleotide in visible light photoinitiating systems for multiple-photocrosslinking and photoencapsulation strategies. Acta Biomater. 2023, 172, 272–279. [Google Scholar] [CrossRef]
- Gonçalves, L.C.P. Photophysical properties and therapeutic use of natural photosensitizers. J. Photochem. Photobiol. 2021, 7, 100052. [Google Scholar] [CrossRef]
- Munir, Z.; Molinar, C.; Banche, G.; Argenziano, M.; Magnano, G.; Cavallo, L.; Mandras, N.; Cavalli, R.; Guiot, C. Encapsulation in Oxygen-Loaded Nanobubbles Enhances the Antimicrobial Effectiveness of Photoactivated Curcumin. Int. J. Mol. Sci. 2023, 24, 15595. [Google Scholar] [CrossRef]
- Wen, F.; Li, P.; Yan, H.; Su, W. Turmeric carbon quantum dots enhanced chitosan nanocomposite films based on photodynamic inactivation technology for antibacterial food packaging. Carbohydr. Polym. 2023, 311, 120784. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.P.S.; Faustino, M.A.F.; Almeida, A.; Lourenço, L.M.O. The Antimicrobial Photoinactivation Effect on Escherichia coli through the Action of Inverted Cationic Porphyrin–Cyclodextrin Conjugates. Microorganisms 2022, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.D.; Corrêa, T.Q.; Bagnato, V.S. Cooperative and competitive antimicrobial photodynamic effects induced by a combination of methylene blue and curcumin. Laser Phys. Lett. 2021, 18, 075601. [Google Scholar] [CrossRef]
- Li, N.; Yang, X.; Lin, D. Development of bacterial cellulose nanofibers/konjac glucomannan-based intelligent films loaded with curcumin for the fresh-keeping and freshness monitoring of fresh beef. Food Packag. Shelf Life 2022, 34, 100989. [Google Scholar] [CrossRef]
- Wang, J.J.; He, T.; Chen, L.; Xu, G.; Dong, S.; Zhao, Y.; Zheng, H.; Liu, Y.; Zeng, Q. Antibacterial efficiency of the curcumin-mediated photodynamic inactivation coupled with L-arginine against Vibrio parahaemolyticus and its application on shrimp. Int. J. Food Microbiol. 2024, 411, 110539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, S.; Wang, W.; Sun, J.; Chen, Z.; Wang, J.; Ma, Q. Enhanced photodynamic inactivation against Escherichia coli O157:H7 provided by chitosan/curcumin coating and its application in food contact surfaces. Carbohydr. Polym. 2024, 337, 122160. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, B.B.; Campanholi, K.d.S.S.; Machado, R.R.B.; Nakamura, C.V.; Silva, A.A.; Caetano, W.; Pozza, M.S.d.S. Reducing Pseudomonas fluorescens in milk through photodynamic inactivation using riboflavin and curcumin with 450 nm blue light-emitting diode. Int. Dairy J. 2024, 148, 105787. [Google Scholar] [CrossRef]
- Chen, H.-M.; Zhou, Q.; Huang, L.-J.; Lin, J.; Liu, J.-F.; Huang, Z.-Y.; Zhang, R.-L.; Wang, J.-J.; Zhao, Y.; Wu, Y.-N.; et al. Curcumin-mediated photodynamic treatment extends the shelf life of salmon (Salmo salar) sashimi during chilled storage: Comparisons of preservation effects with five natural preservatives. Food Res. Int. 2023, 173, 113325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Wu, J.; Qi, G.; Chen, G.; Li, H.; Wang, H. Photodynamic Inactivation Mediated by Curcumin Solid Lipid Nanoparticles on Bacteria and Its Application for Fresh Carrot Juice. Food Bioprocess Technol. 2023, 17, 1294–1308. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Xu, F.; Zhou, A.; Zeng, S.; Zheng, B.; Lin, S. Effective Preservation of Chilled Pork Using Photodynamic Antibacterial Film Based on Curcumin-β-Cyclodextrin Complex. Polymers 2023, 15, 1023. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Z.; Shao, C.; Xie, J. Chlorophyllin-Based 405 nm Light Photodynamic Improved Fresh-Cut Pakchoi Quality at Postharvest and Inhibited the Formation of Biofilm. Foods 2022, 11, 2541. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-H.; Kim, S.-H.; Kang, D.-H. Quercetin mediated antimicrobial photodynamic treatment using blue light on Escherichia coli O157:H7 and Listeria monocytogenes. Curr. Res. Food Sci. 2023, 6, 100428. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-H.; Cho, E.-R.; Kang, D.-H. The effect of quercetin mediated photodynamic inactivation on apple juice properties at different temperature and its bactericidal mechanism. Food Control 2023, 144, 109362. [Google Scholar] [CrossRef]
- Zhu, S.; Ukwatta, R.H.; Cai, X.; Zheng, Y.; Xue, F.; Li, C.; Wang, L. The physiochemical and photodynamic inactivation properties of corn starch/erythrosine B composite film and its application on pork preservation. Int. J. Biol. Macromol. 2023, 225, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; He, T.; Li, H.; Dong, H.; Liu, Y.; Zeng, Q.; Zhao, Y. Enhancement of riboflavin-mediated photodynamic inactivation against Salmonella on tuna fillets coupled with slightly basic electrolyzed water. Food Control 2024, 162, 110441. [Google Scholar] [CrossRef]
- Pei, J.; Zhu, S.; Liu, Y.; Song, Y.; Xue, F.; Xiong, X.; Li, C. Photodynamic Effect of Riboflavin on Chitosan Coatings and the Application in Pork Preservation. Molecules 2022, 27, 1355. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Pang, Y.; Liu, D.; Sun, J.; Bai, W. Photodynamic Inactivation of Staphylococcus aureus Using Aloe-emodin as Photosensitizer. Food Res. Int. 2024, 178, 113959. [Google Scholar] [CrossRef] [PubMed]
- Mikulich, A.V.; Plavskii, V.Y.; Tretyakova, A.I.; Nahorny, R.K.; Sobchuk, A.N.; Dudchik, N.V.; Emeliyanova, O.A.; Zhabrouskaya, A.I.; Plavskaya, L.G.; Ananich, T.S.; et al. Potential of using medicinal plant extracts as photosensitizers for antimicrobial photodynamic therapy. Photochem. Photobiol. 2024, 384, 56366. [Google Scholar] [CrossRef] [PubMed]
- Stura, I.; Munir, Z.; Cavallo, L.; Torri, L.; Mandras, N.; Banche, G.; Spagnolo, R.; Pertusio, R.; Cavalli, R.; Guiot, C. Combining Blue Light and Yellow Curcumin to Obtain a Green Tool for Berry Preservation against Bacterial Contamination: A Preliminary Investigation. Foods 2023, 12, 2038. [Google Scholar] [CrossRef]
- Du, T.; Li, X.; Wang, S.; Su, Z.; Sun, H.; Wang, J.; Zhang, W. Phytochemicals-based edible coating for photodynamic preservation of fresh-cut apples. Food Res. Int. 2023, 163, 112293. [Google Scholar] [CrossRef]
- Lv, Y.; Li, P.; Cen, L.; Wen, F.; Su, R.; Cai, J.; Chen, J.; Su, W. Gelatin/carboxymethylcellulose composite film combined with photodynamic antibacterial: New prospect for fruit preservation. Int. J. Biol. Macromol. 2024, 257, 128643. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.-H.; Hu, D.; Wang, R.-H.; Li, Y.; Wen, Y.; Xiao, H.; Zhao, Y.; Wang, J.J. Curcumin-loaded emulsions stabilized by the succinylated Antarctic krill proteins: Establishment of photodynamic inactivation to preserve salmon. LWT 2023, 178, 114613. [Google Scholar] [CrossRef]
- Huang, J.; Chen, B.; Zeng, Q.-H.; Liu, Y.; Liu, H.; Zhao, Y.; Wang, J.J. Application of the curcumin-mediated photodynamic inactivation for preserving the storage quality of salmon contaminated with L. monocytogenes. Food Chem. 2021, 359, 129974. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Shi, Q.; Du, Y.; Zeng, Q.; Liu, H.; Zhang, Z.; Zheng, H.; Wang, J.J. Preservation effects of photodynamic inactivation-mediated antibacterial film on storage quality of salmon fillets: Insights into protein quality. Food Chem. 2024, 444, 138685. [Google Scholar] [CrossRef] [PubMed]
- Miazaki, J.B.; dos Santos, A.R.; de Freitas, C.F.; Stafussa, A.P.; Mikcha, J.M.G.; Bergamasco, R.d.C.; Tonon, L.A.C.; Madrona, G.S.; Caetano, W.; da Silva, L.H.; et al. Edible coatings and application of photodynamics in ricotta cheese preservation. LWT 2022, 165, 113697. [Google Scholar] [CrossRef]
- Saraiva, B.B.; Rodrigues, B.M.; Junior, R.C.d.S.; Scapim, M.R.d.S.; Lancheros, C.A.C.; Nakamura, C.V.; Caetano, W.; Pereira, P.C.d.S.; de Santana, E.H.W.; Pozza, M.S.d.S. Photodynamic inactivation of Pseudomonas fluorescens in Minas Frescal cheese using curcumin as a photosensitizer. LWT 2021, 151, 112143. [Google Scholar] [CrossRef]
- Munir, M.T.; Federighi, M. Control of Foodborne Biological Hazards by Ionizing Radiations. Foods 2020, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Akhila, P.P.; Sunooj, K.V.; Aaliya, B.; Navaf, M.; Sudheesh, C.; Sabu, S.; Sasidharan, A.; Mir, S.A.; George, J.; Khaneghah, A.M. Application of electromagnetic radiations for decontamination of fungi and mycotoxins in food products: A comprehensive review. Trends Food Sci. Technol. 2021, 114, 399–409. [Google Scholar] [CrossRef]
- Bisht, B.; Bhatnagar, P.; Gururani, P.; Kumar, V.; Tomar, M.S.; Sinhmar, R.; Rathi, N.; Kumar, S. Food irradiation: Effect of ionizing and non-ionizing radiations on preservation of fruits and vegetables—A review. Trends Food Sci. Technol. 2021, 114, 372–385. [Google Scholar] [CrossRef]
- Ben Ghorbal, S.; Werhani, R.; Abdelwaheb, C. Effects of certain physical stresses on the composition of the membrane of bacteria implicated in food and environmental contamination. Int. J. Environ. Health Res. 2024, 34, 408–418. [Google Scholar] [CrossRef]
- Kroc, T.K. Monte Carlo simulations demonstrating physics of equivalency of gamma, electron-beam, and X-ray for radiation sterilization. Radiat. Phys. Chem. 2023, 204, 110702. [Google Scholar] [CrossRef]
- Rafiepour, P.; Sina, S.; Mortazavi, S.M.J. A multiscale Monte Carlo simulation of irradiating a typical-size apple by low-energy X-rays and electron beams. Radiat. Phys. Chem. 2023, 212, 111016. [Google Scholar] [CrossRef]
- El-Nour, S.A.A.; Hammad, A.A.; Fathy, R.; Eid, A.S. Application of coliphage as biocontrol agent in combination with gamma irradiation to eliminate multi-drug-resistant E. coli in minimally processed vegetables. Environ. Sci. Pollut. Res. Int. 2023, 30, 123907–123924. [Google Scholar] [CrossRef] [PubMed]
- Son, N.A.; Ha, N.T.N.; Sang, N.T.M.; Duc, L.D.D.; Trieu, L.N. Effects of low energy (160 keV) X-ray on microbial inactivation, sprouting inhibition and genetic variation in potato. Food Biosci. 2022, 47, 101555. [Google Scholar] [CrossRef]
- Mazhar, A.; El-Hansi, N.; Shafaa, M.W.; Shalaby, M. Radiation sterilization of liposomes: A literature review. Radiat. Phys. Chem. 2024, 218, 111592. [Google Scholar] [CrossRef]
- Song, H.-Y.; Kim, K.-I.; Han, J.M.; Park, W.Y.; Seo, H.S.; Lim, S.; Byun, E.-B. Ionizing radiation technology to improve the physicochemical and biological properties of natural compounds by molecular modification: A review. Radiat. Phys. Chem. 2022, 194, 110013. [Google Scholar] [CrossRef]
- Wei, Q.; Mei, J.; Xie, J. Application of electron beam irradiation as a non-thermal technology in seafood preservation. LWT 2022, 169, 113994. [Google Scholar] [CrossRef]
- Lim, J.-S.; Ha, J.-W. Growth-Inhibitory Effect of X-ray Irradiation on Gram-Negative and Gram-Positive Pathogens in Apple, Orange, and Tomato Juices. Food Bioprocess Technol. 2021, 14, 1909–1919. [Google Scholar] [CrossRef]
- Noor, M.M.; Arshad, M.S.; Ahmad, R.S.; Imran, A.; Khalid, W.; Suleria, H.A.R. Stability and quality improvement of shrimp patties by Asparagus racemosus and gamma irradiation. Int. J. Food Prop. 2022, 25, 1328–1342. [Google Scholar] [CrossRef]
- Li, H.-L.; Huang, J.-J.; Li, M.-J.; Chen, Y.-N.; Xiong, G.-Q.; Cai, J.; Zu, X.-Y. Effects of cobalt-sourced γ-irradiation on the meat quality and storage stability of crayfishes (Procambarus clarkii). Food Sci. Technol. 2023, 43, E104222. [Google Scholar] [CrossRef]
- Wang, F.; Zhong, H.; Cheng, J.-H. Comprehensive Analysis of the Structure and Allergenicity Changes of Seafood Allergens Induced by Non-Thermal Processing: A Review. Molecules 2022, 27, 5857. [Google Scholar] [CrossRef] [PubMed]
- Indiarto, R.; Irawan, A.N.; Subroto, E. Meat Irradiation: A Comprehensive Review of Its Impact on Food Quality and Safety. Foods 2023, 12, 1845. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.; Khorvash, R.; Goli, M.; Ranjbaran, S.M.; Najarian, A.; Nafchi, A.M. Review of proposed different irradiation methods to inactivate food-processing viruses and microorganisms. Food Sci. Nutr. 2021, 9, 5883–5896. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Chang, G.; Liu, Y.; Ni, K.; Zhou, T.; Lv, X.; Yu, J.; Bai, J.; Wang, X. Inactivation of suspended cells and biofilms of the gram-negative bacteria by electron beam irradiation and possible mechanisms of action. LWT 2022, 172, 114171. [Google Scholar] [CrossRef]
- Błaszak, M.; Jakubowska, B.; Lachowicz-Wiśniewska, S.; Migdał, W.; Gryczka, U.; Ochmian, I. Effectiveness of E-Beam Radiation against Saccharomyces cerevisiae, Brettanomyces bruxellensis, and Wild Yeast and Their Influence on Wine Quality. Molecules 2023, 28, 4867. [Google Scholar] [CrossRef] [PubMed]
- Darré, M.; Vicente, A.R.; Cisneros-Zevallos, L.; Artés-Hernández, F. Postharvest Ultraviolet Radiation in Fruit and Vegetables: Applications and Factors Modulating Its Efficacy on Bioactive Compounds and Microbial Growth. Foods 2022, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Berrios-Rodriguez, A.; Olanya, O.M.; Niemira, B.A.; Ukuku, D.O.; Mukhopadhyay, S.; Orellana, L.E. Gamma radiation treatment of postharvest produce for Salmonella enterica reduction on baby carrot and grape tomato. J. Food Saf. 2021, 42, e12951. [Google Scholar] [CrossRef]
- Berrios-Rodriguez, A.; Olanya, O.; Ukuku, D.; Niemira, B.; Orellana, L.; Mukhopadhyay, S.; Cassidy, J.; Boyd, G. Inactivation of Listeria monocytogenes on post-harvest carrot and tomato by gamma radiation, sanitizer, biocontrol treatments and their combinations. LWT 2020, 118, 108805. [Google Scholar] [CrossRef]
- Mladenova, R.B.; Solakov, N.Y.; Loginovska, K.K. Evaluation of gamma irradiation effects on antioxidant capacity of propolis. Appl. Radiat. Isot. 2024, 207, 111254. [Google Scholar] [CrossRef]
- Boshevska, M.; Sandeva, I.; Verde, S.C.; Spasevska, H.; Jankuloski, Z. Effects of different irradiation doses and storage period on microbiological characteristics of wheat (Triticum aestivum L.). Food Control 2024, 158, 110201. [Google Scholar] [CrossRef]
- Vazirov, R.; Sokovnin, S.Y.; Krivonogova, A.; Isaeva, A. Radiation surface antimicrobial processing of poultry meat and by-products using the nanosecond low-energy electron beam. Radiat. Phys. Chem. 2024, 217, 111528. [Google Scholar] [CrossRef]
- Damdam, A.N.; Alzahrani, A.; Salah, L.; Salama, K.N. Effects of UV-C Irradiation and Vacuum Sealing on the Shelf-Life of Beef, Chicken and Salmon Fillets. Foods 2023, 12, 606. [Google Scholar] [CrossRef]
- Singh, A.; Duche, R.T.; Wandhare, A.G.; Sian, J.K.; Singh, B.P.; Sihag, M.K.; Singh, K.S.; Sangwan, V.; Talan, S.; Panwar, H. Milk-Derived Antimicrobial Peptides: Overview, Applications, and Future Perspectives. Probiotics Antimicrob. Proteins 2022, 15, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Bin Hafeez, A.; Jiang, X.; Bergen, P.J.; Zhu, Y. Antimicrobial Peptides: An Update on Classifications and Databases. Int. J. Mol. Sci. 2021, 22, 11691. [Google Scholar] [CrossRef]
- Mondal, R.; Chakraborty, T.; Ghosh, S. Review on Application of Antibacterial Peptides in Food Preservation. Int. J. Pharm. Rev. Res. 2023, 82, 85–88. [Google Scholar] [CrossRef]
- Elbediwi, M.; Rolff, J. Metabolic pathways and antimicrobial peptide resistance in bacteria. J. Antimicrob. Chemother. 2024, 79, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef]
- Brzoza, P.; Godlewska, U.; Borek, A.; Morytko, A.; Zegar, A.; Kwiecinska, P.; Zabel, B.A.; Osyczka, A.; Kwitniewski, M.; Cichy, J. Redox active anti-microbial peptides in controlling growth of microorganisms at bodybarriers. Antioxidants 2021, 10, 446. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, L.; Sun, X.; Ma, A. Bioactive Peptides: A Promising Alternative to Chemical Preservatives for Food Preservation. J. Agric. Food Chem. 2021, 69, 12369–12384. [Google Scholar] [CrossRef]
- Tong, J.; Zhang, Z.; Wu, Q.; Huang, Z.; Malakar, P.K.; Chen, L.; Liu, H.; Pan, Y.; Zhao, Y. Antibacterial peptides from seafood: A promising weapon to combat bacterial hazards in food. Food Control 2021, 125, 108004. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Chen, Z.; Xue, Z.; Jia, Y.; Guo, Q.; Ma, Q.; Zhang, M.; Chen, H. Identification and antimicrobial activity evaluation of three peptides from laba garlic and the related mechanism. Food Funct. 2019, 10, 4486–4496. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, H.; Fang, L.; Liu, D.; Liu, J.; Su, M.; Fang, Z.; Ren, W.; Jiao, H. The Modification and Design of Antimicrobial Peptide. Curr. Pharm. Des. 2018, 24, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tie, K.; Zhang, Y.; Feng, X.; Cao, Y.; Han, W. Design, expression, and characterization of the hybrid antimicrobial peptide T-catesbeianin-1 based on FyuA. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2018, 24, e3059. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Sun, C.; Wu, T.; Wang, J.; Wang, B.; Du, W. Expression and purification of ShLysG in Escherichia coli and initial characterization of its antimicrobial, antioxidant and anti-inflammatory activities. Process Biochem. 2020, 99, 70–78. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, X. Biosynthesis, Bioactivity, Biosafety and Applications of Antimicrobial Peptides for Human Health. Biosaf. Health 2022, 4, 118–134. [Google Scholar] [CrossRef]
- Li, M.; Zhou, R.; Wang, Y.; Lu, Y.; Chu, X.; Dong, C. Heterologous Expression of Frog Antimicrobial Peptide Odorranain-C1 in Pichia pastoris: Biological Characteristics and Its Application in Food Preservation. J. Biotechnol. 2024, 390, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, H.; Fu, Y.; Chang, C.; Wu, J. Sodium alginate/gum arabic/glycerol multicomponent edible films loaded with natamycin: Study on physicochemical, antibacterial, and sweet potatoes preservation properties. Int. J. Biol. Macromol. 2022, 213, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Madrazo, A.L.; Campos, M.R.S. Antibacterial properties of peptides from chia (Salvia hispanica L.) applied to pork meat preservation. J. Food Sci. 2023, 88, 4194–4217. [Google Scholar] [CrossRef] [PubMed]
- Olivos, A.E.C.; Linares-Castañeda, A.; Corzo-Ríos, L.J.; Márquez-Lemus, M.; Jiménez-Martínez, C. Nisin as a food preservative: Physicochemical, sensory properties and antimicrobial activity in Mexican tomato sauce. Int. J. Food Sci. Technol. 2024, 59, 3020–3030. [Google Scholar] [CrossRef]
- Elsherif, W.M.; Hassanien, A.A.; Zayed, G.M.; Kamal, S.M. Natural approach of using nisin and its nanoform as food bio-preservatives against methicillin resistant Staphylococcus aureus and E. coli O157:H7 in yoghurt. BMC Vet. Res. 2024, 20, 192. [Google Scholar] [CrossRef]
- Liu, W.; Huang, K.; Tan, Z.; Wang, C.; Wen, T.; Huang, L.; Hang, F.; Xie, C.; Wang, S.; Li, K. Application of nisin-embedded pectin microcapsules for ‘Guiqi’ mango fruit postharvest preservation. Food Packag. Shelf Life 2024, 42, 101261. [Google Scholar] [CrossRef]
- Kuniyoshi, T.M.; O’Connor, P.M.; Lawton, E.; Thapa, D.; Mesa-Pereira, B.; Abulu, S.; Hill, C.; Ross, R.P.; Oliveira, R.P.; Cotter, P.D. An oxidation resistant pediocin PA-1 derivative and penocin A display effective anti-Listeria activity in a model human gut environment. Gut Microbes 2022, 14, 2004071. [Google Scholar] [CrossRef]
- Eghbal, N.; Viton, C.; Gharsallaoui, A. Nano and microencapsulation of bacteriocins for food applications: A review. Food Biosci. 2022, 50, 102173. [Google Scholar] [CrossRef]
- Komora, N.; Maciel, C.; Isidro, J.; Pinto, C.A.; Fortunato, G.; Saraiva, J.M.A.; Teixeira, P. The Impact of HPP-Assisted Biocontrol Approach on the Bacterial Communities’ Dynamics and Quality Parameters of a Fermented Meat Sausage Model. Biology 2023, 12, 1212. [Google Scholar] [CrossRef]
- Guo, L.; Tang, M.; Luo, S.; Zhou, X. Screening and Functional Analyses of Novel Cecropins from Insect Transcriptome. Insects 2023, 14, 794. [Google Scholar] [CrossRef] [PubMed]
- Steiner, H.; Hultmark, D.; Engström, A.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef]
- Makwana, P.; Rahul, K.; Ito, K.; Subhadra, B. Diversity of Antimicrobial Peptides in Silkworm. Life 2023, 13, 1161. [Google Scholar] [CrossRef]
- Mehni, A.M.; Rahnamaeian, M.; Hassanzadeh, N.; Far, H.F. Pseudomonas tolaasii, the causal agent of mushroom brown blotch, is susceptible to insect cecropins. J. Plant Pathol. 2023, 105, 817–824. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Moradi, B. Cecropins Activity against Bacterial Pathogens. Infect. Dis. Clin. Pract. 2021, 29, e6–e12. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Luo, G.; Ren, D.; Wu, X.; Xu, D. Polylactic acid-based microcapsules for moisture-triggered release of chlorine dioxide and its application in cherry tomatoes preservation. Int. J. Biol. Macromol. 2024, 258, 128662. [Google Scholar] [CrossRef]
- Silva, F.V.M.; Sulaiman, A. Control of Enzymatic Browning in Strawberry, Apple, and Pear by Physical Food Preservation Methods: Comparing Ultrasound and High-Pressure Inactivation of Polyphenoloxidase. Foods 2022, 11, 1942. [Google Scholar] [CrossRef] [PubMed]
- Laein, S.S.; Mohajer, F.; Khanzadi, A.; Gheybi, F.; Azizzadeh, M.; Noori, S.M.A.; Mollaei, F.; Hashemi, M. Effect of alginate coating activated by solid lipid nanoparticles containing Zataria multiflora essential oil on chicken fillet’s preservation. Food Chem. 2024, 446, 138816. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Yeo, D.; Wang, Z.; Woo, S.; Seo, Y.; Hossain, I.; Choi, C. Viability of SARS-CoV-2 on lettuce, chicken, and salmon and its inactivation by peracetic acid, ethanol, and chlorine dioxide. Food Microbiol. 2023, 110, 104164. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Chen, Z.; Zhao, Y.; An, J.; Huang, H.; Liu, R.; Huang, C. Polyvinyl alcohol film with chlorine dioxide microcapsules can be used for blueberry preservation by slow-release of chlorine dioxide gas. Front. Nutr. 2023, 10, 1177950. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z. Application of chlorine dioxide-based hurdle technology to improve microbial food safety—A review. Int. J. Food Microbiol. 2022, 379, 109848. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.S.; Zhou, M.; Zhou, D.; Ashokkumar, M.; Martin, G.J. The formation of double emulsions in skim milk using minimal food-grade emulsifiers—A comparison between ultrasonic and high pressure homogenisation efficiencies. J. Food Eng. 2018, 219, 81–92. [Google Scholar] [CrossRef]
- Meza-Velázquez, J.A.; Aguilera-Ortiz, M.; Ragazzo-Sanchez, J.A.; León, J.A.R.-D.; Minjares-Fuentes, J.R.; Luna-Zapién, E.A. Combined application of high pressure and ultrasound in fig paste: Effect on bioactive and volatile compounds. Food Sci. Biotechnol. 2023, 33, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Merlino, M.; Condurso, C.; Cincotta, F.; Nalbone, L.; Ziino, G.; Verzera, A. Essential Oil Emulsion from Caper (Capparis spinosa L.) Leaves: Exploration of Its Antibacterial and Antioxidant Properties for Possible Application as a Natural Food Preservative. Antioxidants 2024, 13, 718. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.M.; Abdelsalam, E.; Mohammed, R.S.; Ashour, W.E.S.; Vilas-Boas, A.A.; Pintado, M.; El Habbasha, E.S. Polyphenol-Rich Extracts and Essential Oil from Egyptian Grapefruit Peel as Potential Antioxidant, Antimicrobial, and Anti-Inflammatory Food Additives. Appl. Sci. 2024, 14, 2776. [Google Scholar] [CrossRef]
- Wang, N.; Fan, H.; Wang, J.; Wang, H.; Liu, T.; Wang, N.; Fan, H.; Wang, J.; Wang, H.; Liu, T. Fabrication and characterization of curcumin-loaded composite nanoparticles based on high-hydrostatic-pressure-treated zein and pectin: Interaction mechanism, stability, and bioaccessibility. Food Chem. 2024, 446, 138286. [Google Scholar] [CrossRef]
| PS | Matrix | Photosensitizer and Usage | Light | Cultivation Condition | Bacteriostatic Effect |
|---|---|---|---|---|---|
| Curcumin | Shrimp | 8 μM Soak 20 min | 455–460 nm 1.2 J/cm 2 | Storage at 10 °C for a certain period of time | Quantity of Vibrio parahaemolyticus after 6 days storage < 2.0 log10 CFU/g |
| Curcumin | Food contact surface | 15 mg/L Soak | 420 nm 20 mW/cm 2 (5 min) | Incubate at 30 °C for 30 min | Escherichia coli O157 H7 CFUs reduced by 0.22 log10 CFU/mL |
| Curcumin + Riboflavin | Milk | 250 μg/mL Solution mixing | 450 nm 2.7 mW/cm 2 (5 min) | Incubation at 25 °C for 48 h | The average bacterial count of riboflavin is 6.95 log10 cfu/mL, while curcumin is 6.73 log10 CFU/mL |
| Curcumin | Sashimi salmon | 500 μmol/L Soak 20 min | 445–460 nm 3.80 mW/cm2 (60 min) | Below 4 °C, measured every two days | The counts of Psychrophilic bacteria, Pseudomonas, Enterobacteriaceae, and Hydrogen sulfide bacteria were 3.34, 2.38, 2.75, and 2.47 log10 CFU/g |
| Curcumin | Carrot juice | 100 μM Solution mixing | 455–450 nm 0.9 W/cm2 (30 min) | Incubated at 37 °C for 1 h | Escherichia coli and Staphylococcus aureus decreased by 2.36 log10 CFU/mL and 6.60 log10 CFU/mL |
| Curcumin-β-cyclodextrin | Chilled pork | Supernatant after separation of 400 mg β-cyclodextrin and curcumin solution | 425 nm (45 min) | 4 ± 1 °C temperature; 5565% humidity; samples are collected every two days | TVC 5.78 ± 0.17 log10 CFU/g |
| Curcumin | Fruit juice | 10 μM Solution mixing | 440 nm 3.6 × 10−3 W/cm2 (6 min) | Incubation at 37 °C for 24 h | Staphylococcus aureus in mango juice and pineapple juice decreased by 1.8 and 3.5 log10 CFU/mL |
| Chlorophyll | Fresh-cut pakchoi | 1 × 10−5 mol/L Surface spraying | 405 nm 22.27 J/cm2/d (12 h every day) | 4 °C, Light for 12 h a day, measured every two days | The colony count decreased by 74.23% |
| Quercetin | Milk | 75 μM Solution mixing | 405 nm 80 and 120 J/cm2 (the former is Escherichia coli and the latter is Listeria monocytogenes) | Incubated in the dark at 37 °C for 24–48 h | Escherichia coli O157:H7 and Listeria monocytogenes decreased by 5.01 log10 CFU/mL and 1.93 log10 CFU/mL |
| Quercetin | White grape juice | 75 μM Solution mixing | 405 nm 20 and 40 J/cm2 (The former is Escherichia coli and the latter is Listeria monocytogenes) | Incubated in the dark at 37 °C for 24–48 h | Escherichia coli O157:H7 and Listeria monocytogenes decreased by 5.46 log10 CFU/mL and 5.98 log10 CFU/mL |
| Quercetin | Apple juice | 50 μM Solution mixing | 405 nm 19.2 mW/cm2; 60 J/cm2 | Incubated at 37 °C for 24–48 h | Escherichia coli O157:H7 cells decreased by about 4.90 logarithms, but no cells were detected by Listeria monocytogenes (more than 6.73 log10 arithmic decrease) |
| Erythrin B | Pork | 0.06 g/mL Film wrapping | 400–800 nm 3.6 J/cm2 (30 min) | Handle for 60 min under dark and light. | Growth inhibition of bacteria 2.4 log10 CFU/m |
| Riboflavin | Tuna Fillet | 80 μM Solution mixing | 455 nm 5.2 mW/cm2 30 min) | Incubate overnight at 37 °C | The maximum reduction of salmonella cell population was 5.02 log10 CFU/mL |
| Riboflavin | Fresh pork nuggets | Add an appropriate amount of riboflavin to 2% w/w chitosan solution; soak for 30 s | 360 nm 15 w | Sealed in disposable Petri dishes and stored in a 4 °C refrigerator | The inhibition zones of Escherichia coli and Staphylococcus aureus coating were 6.37 ± 0.15 mm and 7.61 ± 0.32 mm |
| Aloe emodin | Apple juice | 1 μg/mL; solution mixing | 450–460 nm 40 mW/cm2 | Incubated on solid Agar medium at 37 °C for 12 h | The survival rate of bacteria significantly decreased to about 13% |
| Item | Advantages | Disadvantages |
|---|---|---|
| Chlorine dioxide |
|
|
| Combination of ultrasound and ultra-high-pressure technology |
|
|
| Essential oil |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Z.; Wang, H.; Dong, B. Research on Food Preservation Based on Antibacterial Technology: Progress and Future Prospects. Molecules 2024, 29, 3318. https://doi.org/10.3390/molecules29143318
Chu Z, Wang H, Dong B. Research on Food Preservation Based on Antibacterial Technology: Progress and Future Prospects. Molecules. 2024; 29(14):3318. https://doi.org/10.3390/molecules29143318
Chicago/Turabian StyleChu, Zejing, Hongsu Wang, and Biao Dong. 2024. "Research on Food Preservation Based on Antibacterial Technology: Progress and Future Prospects" Molecules 29, no. 14: 3318. https://doi.org/10.3390/molecules29143318
APA StyleChu, Z., Wang, H., & Dong, B. (2024). Research on Food Preservation Based on Antibacterial Technology: Progress and Future Prospects. Molecules, 29(14), 3318. https://doi.org/10.3390/molecules29143318








