Abstract
Nickel-based catalysts are regarded as the most excellent urea oxidation reaction (UOR) catalysts in alkaline media. Whatever kind of nickel-based catalysts is utilized to catalyze UOR, it is widely believed that the in situ-formed Ni3+ moieties are the true active sites and the as-utilized nickel-based catalysts just serve as pre-catalysts. Digging the pre-catalyst effect on the activity of Ni3+ moieties helps to better design nickel-based catalysts. Herein, five different anions of OH−, CO32−, SiO32−, MoO42−, and WO42− were used to bond with Ni2+ to fabricate the pre-catalysts β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4. It is found that the true active sites of the five as-fabricated catalysts are the same in situ-formed Ni3+ moieties and the five as-fabricated catalysts demonstrate different UOR activity. Although the as-synthesized five catalysts just serve as the pre-catalysts, they determine the quantity of active sites and activity per active site, thus determining the catalytic activity of the catalysts. Among the five catalysts, the amorphous nickel tungstate exhibits the most superior activity per active site and can catalyze UOR to reach 158.10 mA·cm–2 at 1.6 V, exceeding the majority of catalysts. This work makes for a deeper understanding of the pre-catalyst effect on UOR activity and helps to better design nickel-based UOR catalysts.
1. Introduction
Substituting renewable energies for fossil fuels is an effective method to conquer energy and environment problems at the same time, which is an urgent need for our society [1,2,3,4,5,6]. However, renewable energies like solar and wind have drawbacks such as intermittency and transportation difficulties. Water electrolysis powered by renewable energies can transform these renewable energies into hydrogen molecules which can solve the drawbacks of renewable energies [7,8,9,10,11,12]. Water electrolysis is composed of the hydrogen evolution reaction (HER) at the anode and oxygen evolution reaction (OER) at the cathode [13,14,15]. Compared with HER, OER makes up the majority of energy waste in water electrolysis due to the inherent sluggish kinetics [16,17,18,19,20]. Changing the OER by the urea oxidation reaction (UOR) can extremely decrease the overpotential of overall water electrolysis, thus improving the utilization of renewable energies [1,3,10,19,20]. Therefore, the design of cheap and efficient UOR catalysts has received much attention from scientists.
Since the discovery of urea electrolysis, platinum and rhodium have been regarded as the most superior UOR catalysts [21]. The rare reserves and high cost of platinum and rhodium prompt the scientists to design new catalysts. Nickel-based catalysts bio-inspired from urease have received much attention since their discovery and they exhibit comparable UOR performance to precious metals platinum and rhodium [7,22,23,24,25]. In order to obtain more active nickel-based catalysts, various types of nickel-based catalysts were developed to catalyze UOR mainly spanning from nickel oxides [17,24,26,27,28,29,30], nickel X-ides (X refers to C, N, P, S, Se, Te, etc.) [3,11,22,25,31], nickel-based composites [9,23,32], etc. For nickel oxide-related catalysts, Chen’s group added Rh nanoparticles to NiO nanosheets which outperformed the counter NiO nanosheets [27]. Rh and NiO are both UOR active components, and the addition of Rh nanoparticles endows the NiO nanosheet with a superior electronic structure. For nickel X-ide-related catalysts, Zhang’s group supported nickel nitride nanospheres on nickel foam, which can efficiently catalyze the hydrogen generation and urea decomposition [31]. The superior electron conductivity of the support nickel foam brought out the catalytic performance of nickel nitride. For nickel-based composite-related catalysts, Hameed’s group developed a type of NiO/graphene composite by a facile coprecipitation and calcination method [33]. The introduction of graphene improves the conductivity of NiO and alters the electronic structure of NiO, and the NiO/graphene composite demonstrates more enhanced UOR activity than NiO. Through lots of in situ experimental research by advanced instruments and many theoretical investigations, although various types of nickel-based UOR catalysts are synthesized and demonstrate different UOR performances, it is widely believed that the as-synthesized catalysts just serve as the pre-catalysts and the in situ-formed Ni3+ moieties are the true active sites [24,25,34]. Since the true active sites are all Ni3+ moieties, we question why different nickel-based catalysts demonstrate different UOR activity and what the significance of synthesizing different types of nickel-based catalysts is Systematic investigation about the effect of pre-catalysts on UOR activity helps to understand the meaning of synthesizing different kinds of catalysts and will lay a great foundation for further enhancing the UOR performance of nickel-based catalysts.
In this work, five catalysts were synthesized by bonding Ni2+ with OH−, CO32−, SiO32−, MoO42− and WO42− anions, respectively. Modern characterization techniques were adopted to analyze the catalyst structure and lots of electrochemical methods were utilized to uncover the UOR mechanism. It is found that the as-fabricated five catalysts just serve as the pre-catalysts and the true active sites are the in situ-formed Ni3+ moieties. The pre-catalysts determine the UOR catalytic activity by deciding the quantity of active sites and activity per active site. The as-prepared amorphous nickel tungstate exhibits the most superior activity per active site and demonstrates superior UOR activity, selectivity, and durability. This work helps to better understand the UOR mechanism.
2. Results and Discussion
2.1. Structure Characterization
β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 catalysts were fabricated according to Scheme 1, which is detailed in the Materials and Methods section. β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 were obtained by mixing the Ni2+ solution with OH−, CO32−, SiO32−, MoO42−, and WO42− solutions, respectively, followed by ultrasonication, centrifugation, and lyophilization.
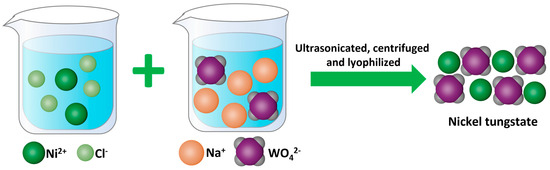
Scheme 1.
Synthetic scheme of Ni-WO4.
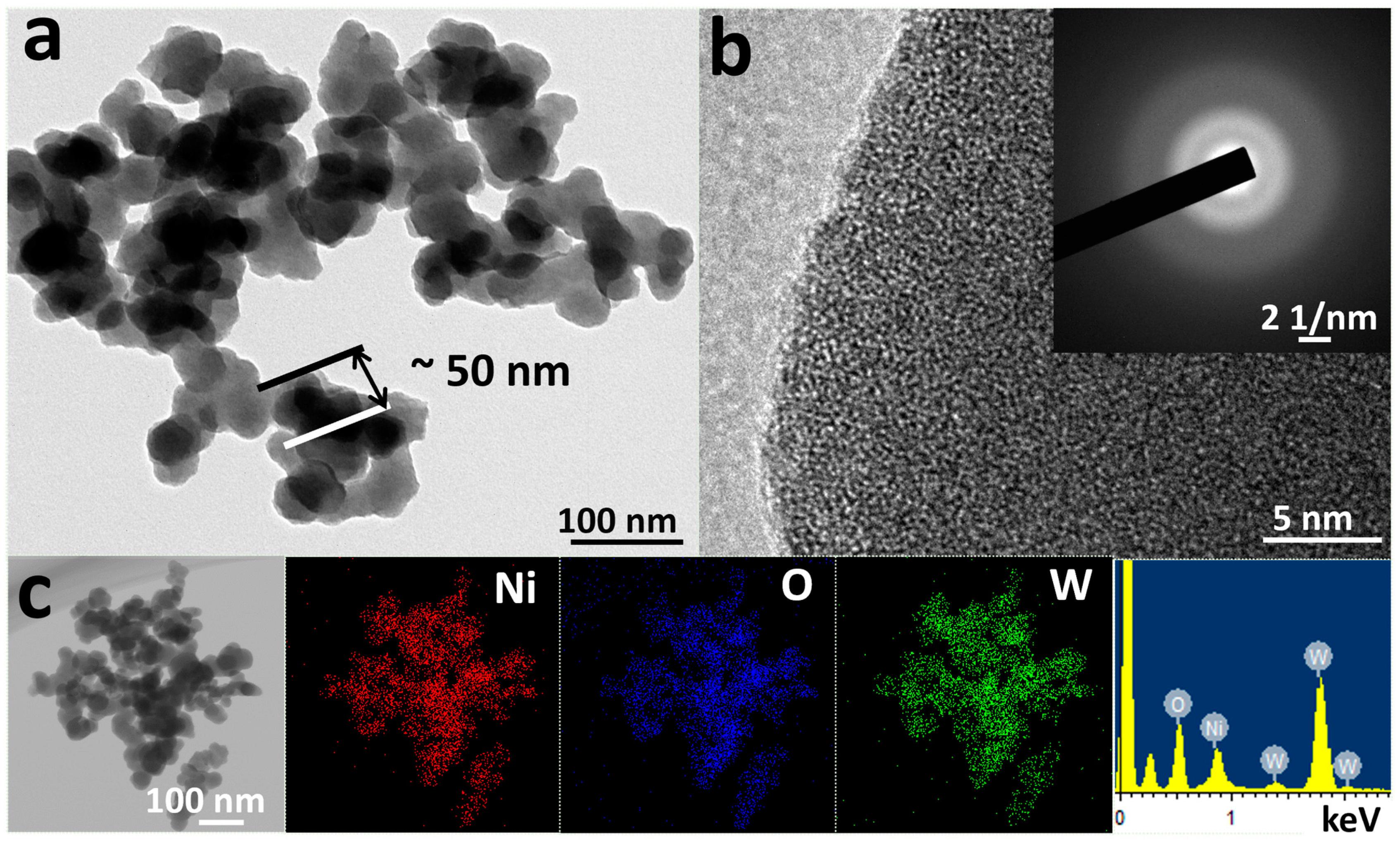
XRD was adopted to analyze the catalyst structure shown in Figure S1. β-Ni(OH)2 was poorly crystallized and typical peaks of (001), (100), and (011) facets located at 19.2, 33.2, and 38.6° were indexed to PDF#73–1520 [30]. XRD patterns of Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 all demonstrated amorphous hump peaks and the amorphous features were verified by their corresponding HRTEM images. TEM was used to observe the catalyst morphologies and analyze the detailed catalyst phase structure. β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 all display nanoparticle morphologies (Figure 1a and Figure S2a–d). A (011) facet of 0.21 nm is observed in the HRTEM image of β-Ni(OH)2 and the selected area electron diffraction (SAED) pattern demonstrates typical polycrystalline diffraction ring feature (Figure S2e) [32,35,36]. No lattice planes can be detected in the HRTEM images of Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4, and their corresponding SAED patterns all demonstrate amorphous halo features (Figure S2f–h and Figure 1b). Uniform distribution of elements Ni, O, and W can be seen in the EDS mapping images of Ni-WO4 and signals of Ni, O, and W are clearly detected in the EDS diagram of Ni-WO4 (Figure 1c). XRD and TEM results illustrate that β-Ni(OH)2 is a poorly crystallized nanoparticle, and Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 are amorphous nanoparticles.
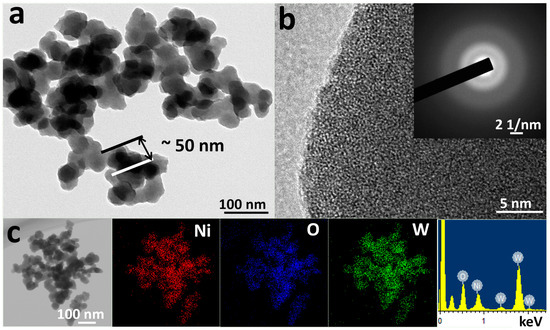
Figure 1.
(a) TEM image, (b) HRTEM image, inset is SAED pattern, and (c) EDS mapping images and EDS diagram of Ni-WO4.
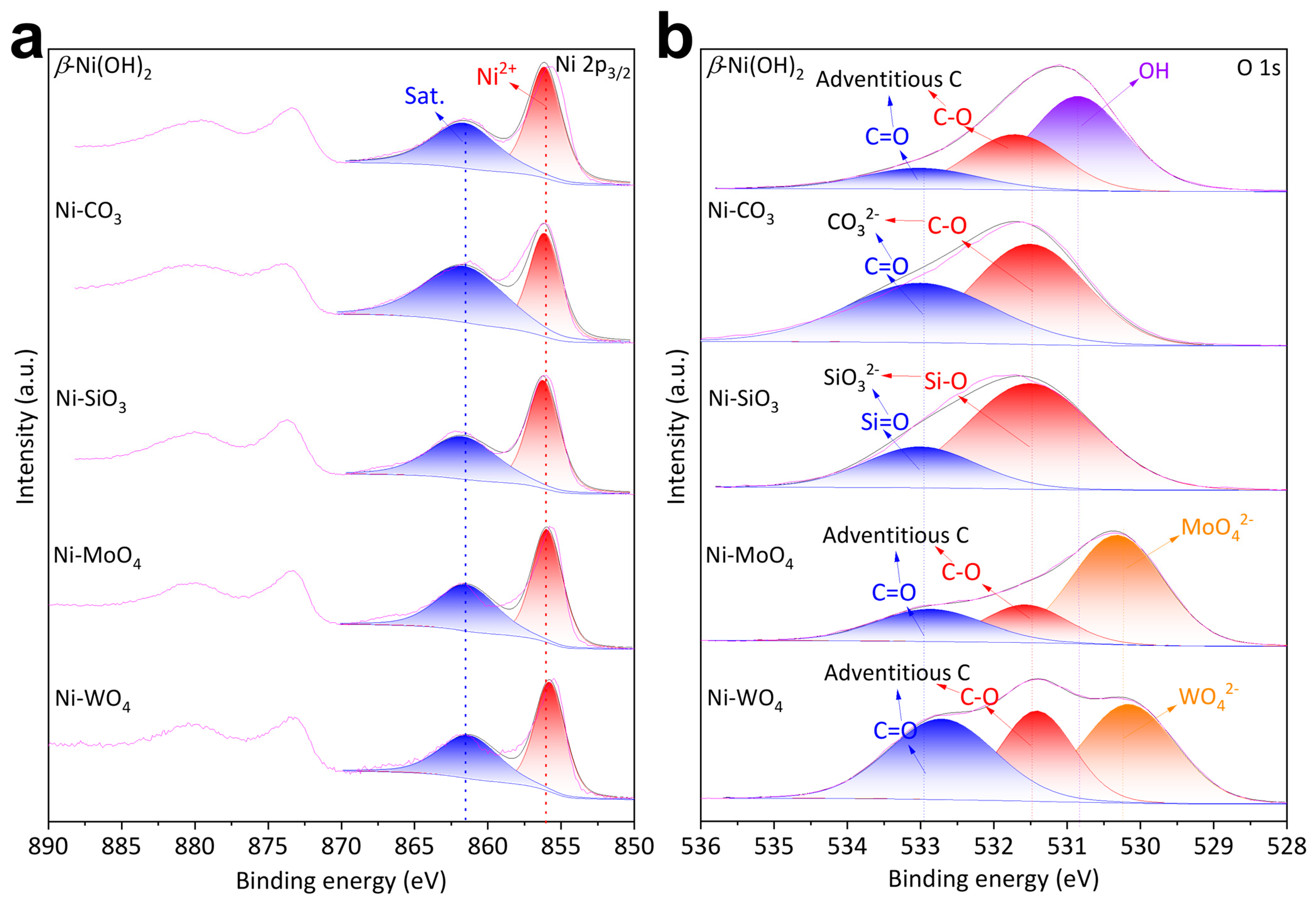
Element chemical environments were characterized by XPS. Ni 2p3/2 XPS of β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 were fitted by the moiety of Ni2+ (856.10 eV), followed by a satellite (861.60 eV) at a higher binding energy (Figure 2a) [12,36,37]. The fittings of O 1s XPS are different (Figure 2b). C-O moieties (531.70 eV) and C=O moieties (533.00 eV) from adventitious carbon and OH− moieties (530.85 eV) from β-Ni(OH)2 structure were detected on β-Ni(OH)2 [30,36]. C-O moieties (531.50 eV) and C=O moieties (533.00 eV) from nickel carbonate structure were detected on Ni-CO3 [36,38,39]. Si-O moieties (531.50 eV) and Si=O moieties (533.00 eV) from nickel metasilicate structure were detected on Ni-SiO3 [40,41]. C-O moieties (531.70 eV) and C=O moieties (533.00 eV) from adventitious carbon and Mo-O moieties (530.31 eV) from nickel molybdate structures were detected on Ni-MoO4 [42,43]. C-O moieties (531.70 eV) and C=O moieties (533.00 eV) from adventitious carbon and W-O moieties (530.31 eV) from nickel tungstate structures were detected on Ni-WO4 [7,43]. Si 2p3/2 (102.45 eV) and 2p1/2 (103.06 eV) were detected on Ni-SiO3 (Figure S3a) [41]. Mo 3d5/2 (232.13 eV) and 3d3/2 (235.26 eV) were detected on Ni-MoO4 (Figure S3b) [42,44].
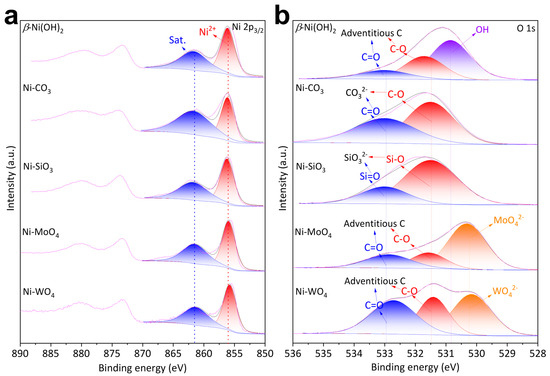
Figure 2.
(a) Ni 2p XPS and (b) O 1s XPS of β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4.
2.2. Electrochemical Analysis
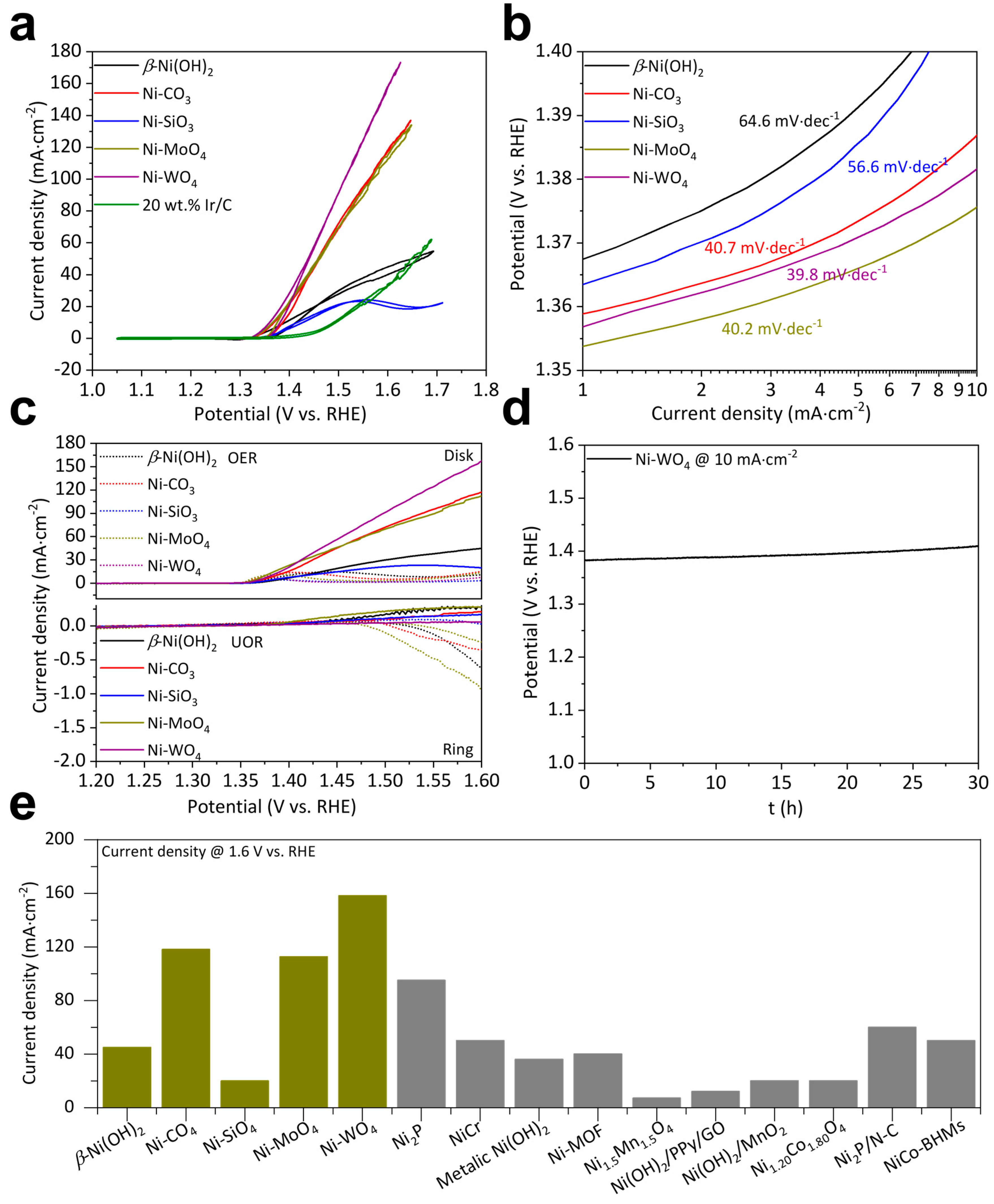
Electrochemical measurements were conducted on electrochemical workstations. UOR CV curves of β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 all demonstrate a more negative overpotential than those of OER at a fixed current density, while 20 wt.% Ir/C does not (Figure S4a). Therefore, the as-fabricated five catalysts are all applicable to UOR catalysis. β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 start to catalyze UOR at a potential of 1.35, 1.34, 1.35, 1.35, and 1.36 V, respectively. β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 deliver 10 mA·cm–2 at 1.41, 1.39, 1.42, 1.38, and 1.38 V, respectively. β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 catalyze UOR at 1.6 V reaching 44.87, 117.69, 19.86, 112.60, and 158.10 mA·cm–2, respectively. Onset potentials and overpotentials at 10 mA·cm–2 do not show significant differences. But Ni-WO4 can catalyze UOR to reach an extremely higher current density at 1.6 V than β-Ni(OH)2, Ni-CO3, Ni-SiO3, and Ni-MoO4. UOR kinetics were examined by Tafel slope values. Ni-WO4 exhibits a lower value of 39.8 mV·dec–1 than β-Ni(OH)2 (64.6 mV·dec–1), Ni-CO3 (40.7 mV·dec–1), Ni-SiO3 (56.6 mV·dec–1), and Ni-MoO4 (40.2 mV·dec–1) (Figure 3b). UOR selectivity was performed on an RRDE equipment. The ring electrode demonstrates negligible current of the UOR RRDE curves, while the ring electrode exhibits obvious current of the OER RRDE curves, suggesting that O2 was produced during the OER test and no O2 was produced during the UOR test. Therefore, we can conclude that β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 can effectively oxidize urea molecules in the tested solution. The UOR stability of Ni-WO4 was examined by the CP method. Ni-WO4 can catalyze the UOR for at least 30 h at 10 mA·cm–2 (Figure 3d). Catalyst support can effectively impact the catalyst activity. Therefore, UOR activity of the as-fabricated five catalysts is compared to catalysts decorated on the same support in previous publications. Impressively, the UOR activity of Ni-WO4 exceeds the majority of the superior catalysts (Figure 3e) [17,18,24,25,26,32,45,46,47,48].
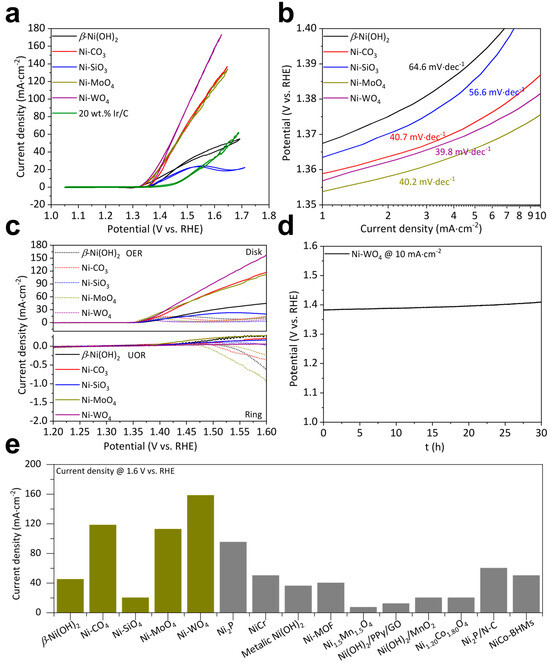
Figure 3.
(a) UOR CV curves, (b) UOR Tafel slopes, and (c) OER and UOR RRDE polarization curves of β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4. (d) UOR durability of Ni-WO4. (e) Comparison of UOR performance of catalysts decorated on glassy carbon electrode.
2.3. Determining Factors of UOR Activity
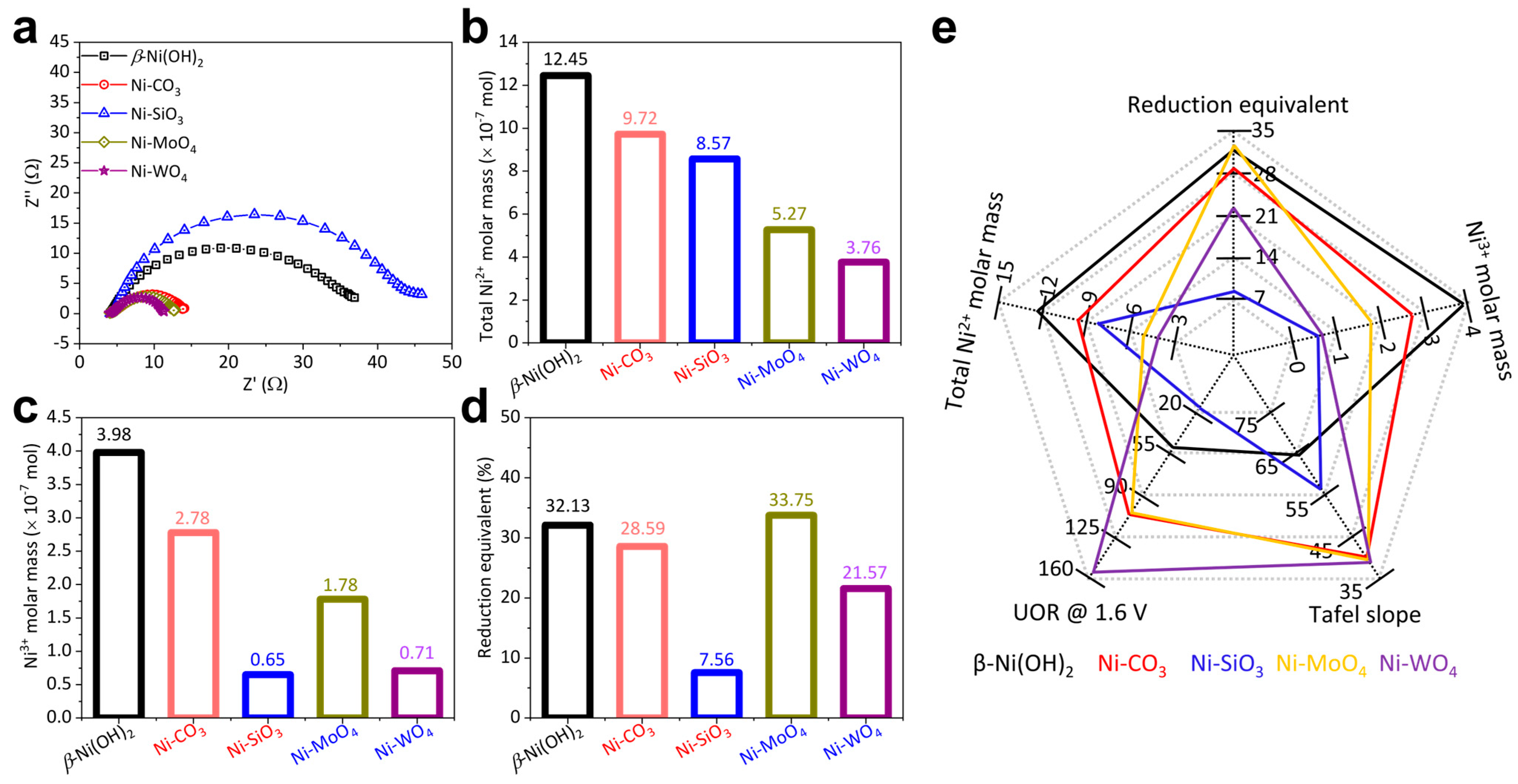
Catalytic activity is the sum of activity per active site. Therefore, the activity per active site and the quantity of active sites co-determine the catalyst activity [49]. Charge transfer resistance reflecting the UOR barrier was evaluated by EIS [38]. The UOR EIS diameter follows this sequence: Ni-SiO3 < β-Ni(OH)2 < Ni-MoO4 < Ni-CO3 < Ni-WO4 (Figure 4a), suggesting that the UOR process proceeds more efficiently on Ni-WO4 than Ni-SiO3, Ni-MoO4, and Ni-CO3.
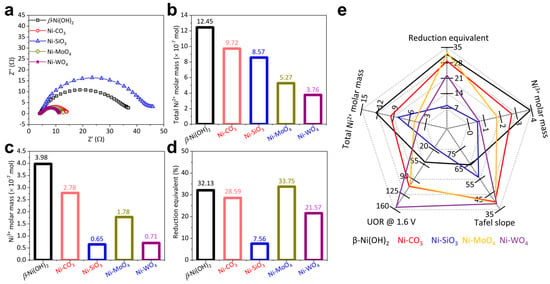
Figure 4.
(a) UOR EIS, (b) total Ni2+ molar mass, (c) Ni3+ molar mass transformed from Ni2+, (d) reduction equivalent, and (e) summary of UOR performance of β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4.
The quantity of active sites: It is widely believed that Ni3+ moieties are the true active sites and the pre-catalysts just serve as the precursors during UOR catalysis [11,25,31,50]. It is crucial to untangle the relationship among the Ni2+ molar mass, the Ni3+ molar mass, the reduction equivalent (the capacity to accumulate Ni3+) and UOR activity. The total Ni2+ molar mass (calculated from the catalyst loading on glass carbon electrode) follows this sequence: β-Ni(OH)2 > Ni-CO3 > Ni-SiO3 > Ni-MoO4 > Ni-WO4 (Figure 4b). The Ni3+ molar mass (calculated from the reduction peak in Figure S4b) follows this sequence: β-Ni(OH)2 > Ni-CO3 > Ni-MoO4 > Ni-WO4 > Ni-SiO3 (Figure 4c). The reduction equivalent follows this sequence: Ni-MoO4 > β-Ni(OH)2 > Ni-CO3 > Ni-WO4 > Ni-SiO3 (Figure 4d). The summary of the relationship among the Ni2+ molar mass, the Ni3+ molar mass, reduction equivalent and UOR activity is shown in Figure 4e. The Ni2+ molar mass, Ni3+ molar mass, reduction equivalent and UOR activity do not exhibit a reasonable logic relationship, indicating that the quantity of active sites cannot solely decide the UOR activity.
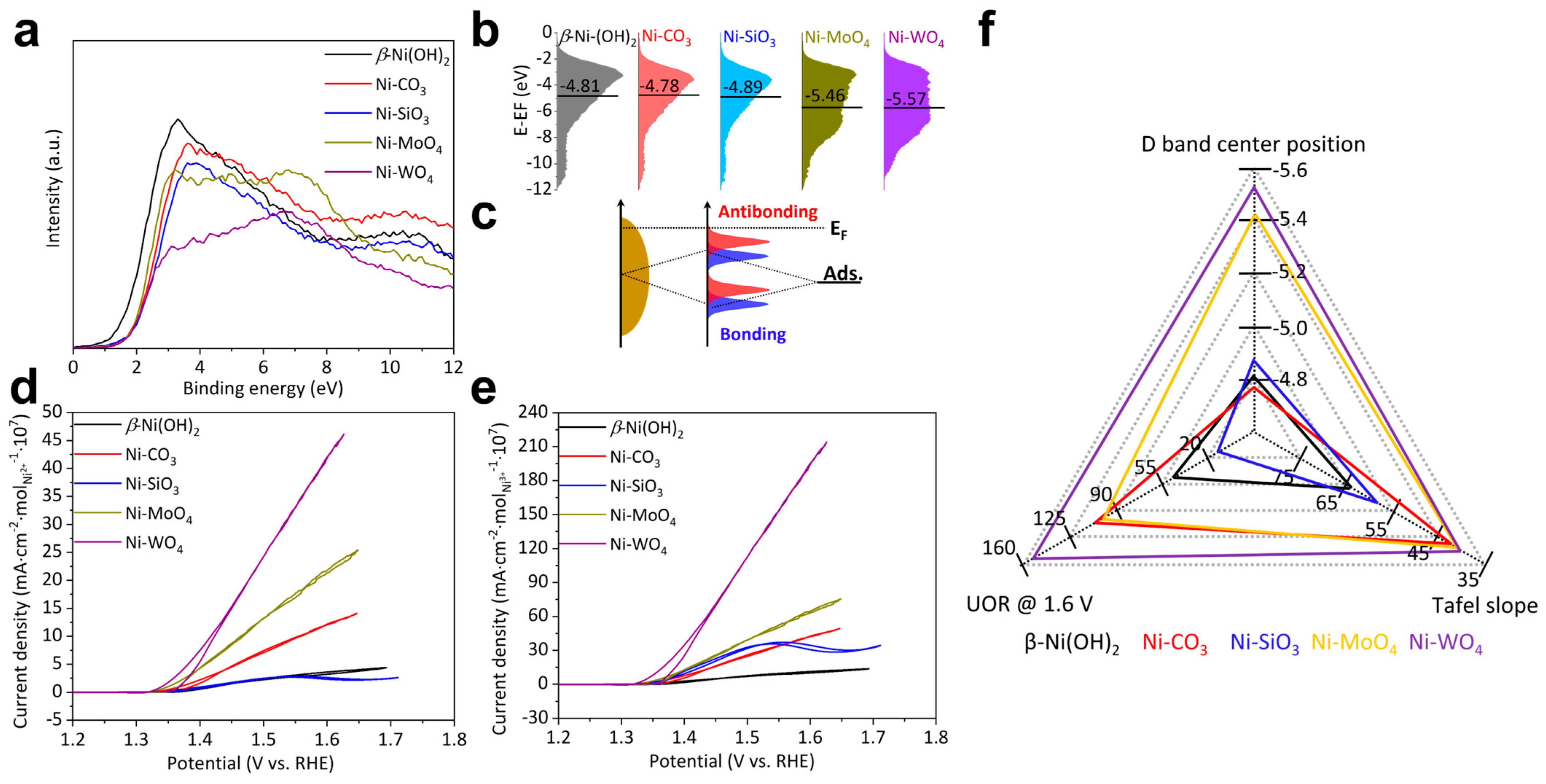
The activity per active site: The activity per active site is determined by the electronic structure of catalysts. The electronic structure was analyzed by valence band (VB) spectra shown in Figure 5a. VB spectra of β-Ni(OH)2, Ni-CO3, and Ni-SiO3 demonstrate similar shapes and are just located at slightly different binding energies. VB spectra of Ni-MoO4 and Ni-WO4 exhibit similar shapes but significantly differ from those of β-Ni(OH)2, Ni-CO3, and Ni-SiO3 especially below 6 eV. This indicates that Ni-MoO4 and Ni-WO4 possess significantly different electronic structures from β-Ni(OH)2, Ni-CO3, and Ni-SiO3 [7]. This phenomenon is due to the fact that transition metals mainly contribute below 6 eV in VB spectra [51]. D band centers were calculated from VB spectra (Figure 5b). D band center positions follow this sequence: Ni-CO3 (−4.78 eV) > β-Ni(OH)2 (−4.81 eV) > Ni-SiO3 (−4.89 eV) > Ni-MoO4 (−5.46 eV) > Ni-WO4 (−5.57 eV). The lower the d band center position, the weaker the bonding strength between the catalyst and intermediates. The bonding strength between the catalyst and intermediates subject to d band center position [51,52] and the bonding scheme are depicted in Figure 5c. The desorption of the UOR intermediates is the rate-controlling step and Ni-WO4 demonstrates the lowest d band center, which facilitates the desorption of the UOR intermediates. Therefore, Ni-WO4 exhibits the highest activity per active site. For the direct analysis of the activity per active site, the UOR CV curves were normalized by the Ni2+ and Ni3+ molar mass. The UOR activity per Ni2+ molar mass follows this sequence: β-Ni(OH)2 ≈ Ni-CO3 < Ni-SiO3 < Ni-MoO4 < Ni-WO4 (Figure 5d). The UOR activity per Ni3+ molar mass follows this sequence: Ni-CO3 < Ni-SiO3 < β-Ni(OH)2 < Ni-MoO4 < Ni-WO4 (Figure 5e). The summary of the total Ni2+ molar mass, Ni3+ molar mass, and UOR activity does not exhibit a reasonable logic relationship, suggesting that the activity per active site cannot solely determine the UOR activity as well (Figure 5f).
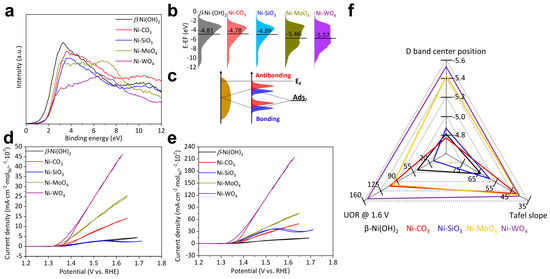
Figure 5.
(a) Valence band spectra, (b) d band models derived from valence band spectra, (c) bond formation scheme, (d) UOR CV curves normalized by the total Ni2+ molar mass, (e) UOR CV curves normalized by Ni3+ molar mass, and (f) summary of UOR performance of β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4.
The activity per active site and the quantity of active sites co-determines the UOR activity of β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4. Ni-WO4 with the lowest d band center position demonstrates the highest activity per active site and possesses slightly fewer active sites compared to Ni-CO3 with the maximum quantity of active sites. Therefore, Ni-WO4 exhibits the highest UOR activity.
2.4. Effect of the Pre-Catalyst Catalyst on UOR Activity
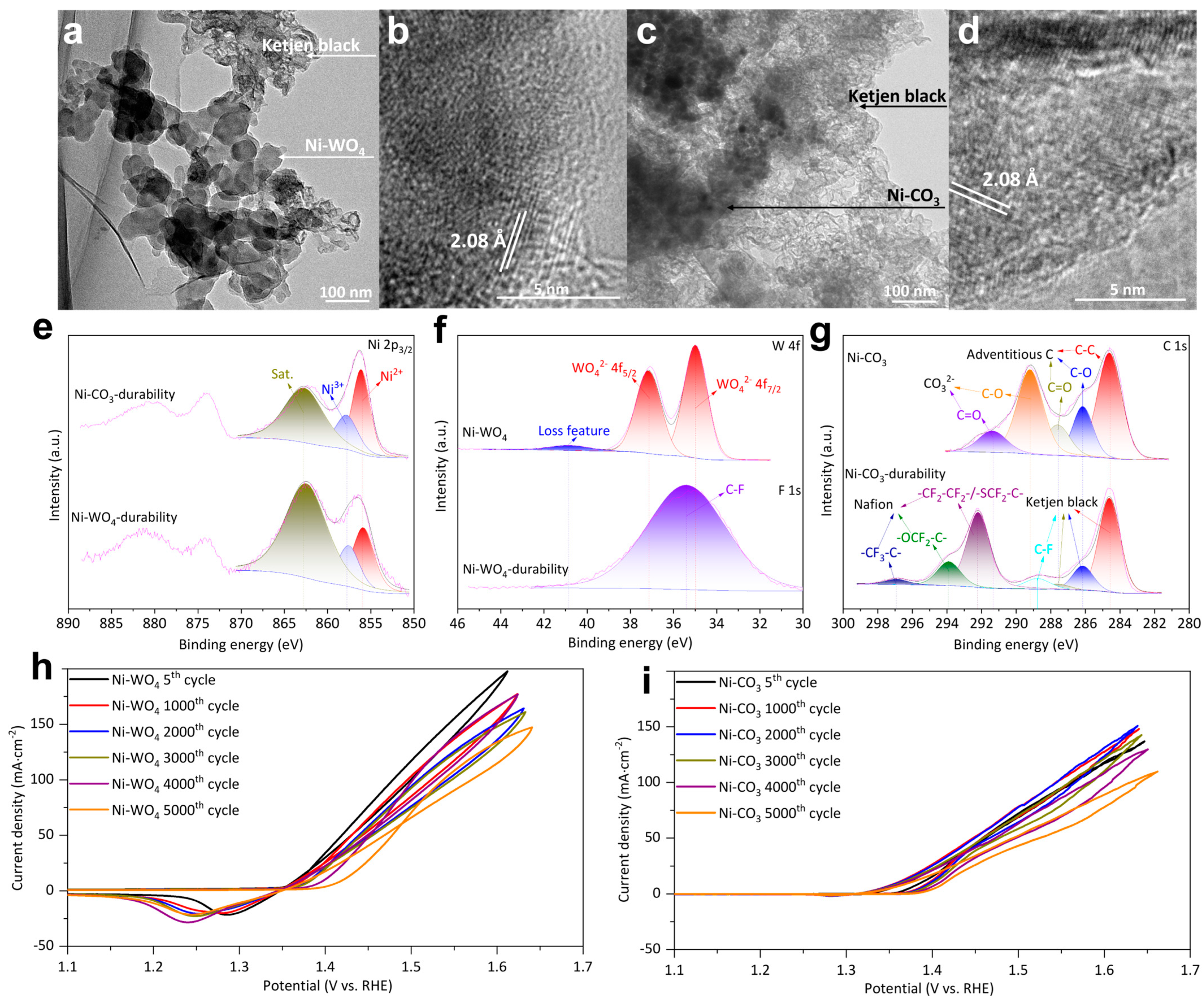
To verify if the true active sites are Ni3+ moieties for Ni-WO4 and Ni-CO3, TEM and XPS were adopted to examine Ni-WO4 durability and Ni-CO3 durability. Catalyst nanoparticles and Ketjen black are observed in TEM images of Ni-WO4 durability and Ni-CO3 durability (Figure 6a,c). HRTEM images of Ni-WO4 durability and Ni-CO3 durability both demonstrate a facet with a width of 2.08 Å ascribed to the (200) facet of nickel (oxy)hydroxide (Figure 6b,d) [22,35]. Ni 2p3/2 XPS of Ni-WO4 durability and Ni-CO3 durability are fitted with Ni2+ (856.10 eV) and Ni3+ (857.80 eV), followed by a satellite (862.70 eV) [36], confirming the formation of Ni3+ moieties. W 4f XPS of Ni-WO4 possesses three peaks of 4f7/2 (34.98 eV), 4f5/2 (37.16 eV), and tungsten oxide loss feature (40.81 eV) (Figure 6f) [53,54]. W signal cannot be detected in the corresponding binding energy range for Ni-WO4 durability, suggesting that element W on the Ni-WO4 surface was completely dissolved during UOR catalysis. F 1s XPS of Ni-WO4 durability demonstrates one peak of C-F moieties located at 35.39 eV which results from the Nafion molecules (Figure 6f) [55]. C-C (284.60 eV), C-O (286.14 eV), C=O (287.60 eV), C-O (289.29), and C=O (291.46) are detected in C 1s XPS of Ni-CO3 (Figure 6g) [55,56]. C-C (284.60 eV), C-O (286.14 eV), C=O (287.60 eV), C-F (288.77 eV), -CF2-CF2-/-SCF2-C- (292.19 eV), -OCF2-C- (293.88 eV), and -CF3-C- (296.89 eV) are detected in C 1s XPS of Ni-CO3 durability (Figure 6g) [55]. The comparison of C 1s and W 4f fitting results of Ni-CO3 and Ni-WO4 before and after UOR durability tests (Table S1). The signal of nickel carbonate structure in C 1s XPS spectrum of Ni-CO3 durability disappeared, indicating the complete dissolution of nickel carbonate on Ni-CO3 surface during UOR catalysis. The UOR activity of both Ni-WO4 and Ni-CO3 just slightly fluctuates during the continuous 5000 cycles (Figure 6h,i). Therefore, we can infer that the WO42− and CO32− do not directly participate in the UOR catalysis and true active sites are Ni3+ moieties. The higher UOR activity of Ni-WO4 than Ni-CO3 for all the 5000 cycles indicates that the pre-catalysts are not the true active sites but determine the final UOR activity.
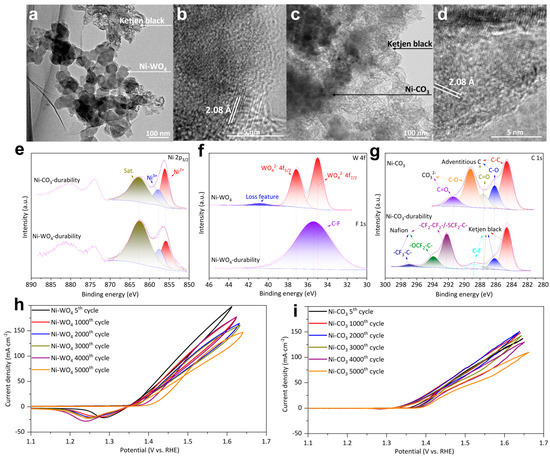
Figure 6.
(a) TEM image and (b) HRTEM image of Ni-WO4 durability. (c) TEM image and (d) HRTEM image of Ni-CO3 durability. (e) Ni 2p XPS of Ni-CO3 durability and Ni-WO4 durability. (f) W 4f XPS of Ni-WO4 and F 1s XPS of Ni-WO4 durability. (g) C 1s XPS of Ni-CO3 and Ni-CO3 durability. (h) UOR CV curves of Ni-WO4. (i) UOR CV curves of Ni-CO3.
3. Materials and Methods
3.1. Fabrication of β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4
1 M Ni2+, OH−, CO32−, SiO32−, MoO42−, and WO42− solutions were prepared by dispersing the salts of NiCl2·6H2O, NaOH, Na2CO3, Na2SiO3, Na2MoO4·2H2O, and Na2WO4·2H2O in deionized water, respectively. β-Ni(OH)2 was obtained by mixing Ni2+ solution with OH− solution with a volume ratio of 1:2. Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 were prepared by mixing Ni2+ solution with CO32−, SiO32−, MoO42−, and WO42− solutions with a volume ratio of 1:1, respectively. After being thoroughly washed, centrifugated, and lyophilized, β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 were obtained.
3.2. Structure Characterization
An X-ray diffractometer (XRD, DX–2700BH, Dandong Haoyuan instrument, Dandong, China) was used to collect XRD data with Cu Kα radiation at a scan rate of 2°·min−1. A transmission electron microscope (TEM, JEOL JEM–2100F, Japan Electronics Co., Ltd, Showima City, Tokyo, Japan) was used to observe catalysts’ morphologies with an accelerating voltage of 200 kV. An X-ray photoelectron spectroscope (XPS, Axis Supra, Shimadzu, Japan) was used to analyze element chemical states. A Shirley background was applied to all the XPS spectra and binding energies of all XPS spectra were charge referenced to 284.8 eV. The equation ∫N(ε)εdε/∫N(ε)dε was applied to calculate the d band center, where N(ε) represents the density of states.
3.3. Electrochemcial Analysis
The UOR performance was evaluated in 1 M KOH with 0.33 M urea. The as-synthesized catalysts, a Hg/HgO electrode and a carbon rod, were used as the working, reference, and counter electrodes. Working electrodes were prepared by casting 30 μL catalyst inks onto a glass carbon electrode polished by a polishing cloth using 50 nm Al2O3. Catalysts (4 mg) and ketjen black (0.5 mg) dispersed into the mixed solution of ethanol (1000 μL) and Nafion (40 μL) were used as the catalyst ink. Cyclic voltammetry (CV) curves scanned at 5 mV·s–1, chronopotentiometry (CP) curves measured at 10 mA·cm–1, electrochemical impedance spectroscopy (EIS) tested at 1.4 V from 100 mHz to 100 kHz, and the rotating ring-disk electrode (RRDE) method were used to analyze the UOR performance. The electrochemical data were collected after 10 cycles of CV curves in 1 M KOH. All the potentials were calibrated by the equation ERHE = EHg/HgO + 0.098 + 0.059 × pH. The reduction equivalent was calculated by the equation RE = n(Ni3+)/n(Ni2+).
4. Conclusions
In summary, β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, and Ni-WO4 were fabricated by bonding Ni2+ with OH−, CO32−, SiO32−, MoO42−, and WO42− anions, respectively. Through systematic investigations about the catalysts structure and UOR mechanism, the five as-fabricated catalysts are found to serve as pre-catalysts and the true active sites are the in situ-formed Ni3+ moieties. The pre-catalyst has effects on the quantity of active sites and the activity per active site to determine the UOR activity. The as-fabricated amorphous nickel tungstate possesses the most superior activity per active site and a relatively high number of active sites, thus demonstrating excellent UOR activity to catalyze the UOR reaching 158.10 mA·cm–2 at 1.6 V, exceeding the majority of the reported catalysts. This work provides a deeper understanding of the pre-catalyst effect on UOR activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29143321/s1, Figure S1: XRD patterns of β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4 and Ni-WO4; Figure S2: (a–d) TEM images of β-Ni(OH)2, Ni-CO3, Ni-SiO3 and Ni-MoO4. (e–h) HRTEM images of β-Ni(OH)2, Ni-CO3, Ni-SiO3 and Ni-MoO4, insets are the corresponding SAED patterns; Figure S3: (a) Si 2p XPS of Ni-SiO3. (b) Mo 3d XPS of Ni-MoO4; Figure S4: (a) OER and UOR CV curves of β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, Ni-WO4 and 20 wt.% Ir/C. (b) OER CV curves of β-Ni(OH)2, Ni-CO3, Ni-SiO3, Ni-MoO4, Ni-WO4 and 20 wt.% Ir/C; Table S1: C 1s and W 4f XPS fitting results of Ni-CO3 and Ni-WO4.
Author Contributions
Conceptualization, H.L. and L.C.; methodology, H.L.; software, P.W. and X.Q.; validation, H.L., L.C., S.Y. and J.W.; formal analysis, H.L., L.C., S.Y. and J.W.; investigation, A.Y., Y.W., Y.Y., J.L. and Z.R.; resources, H.L., L.C., S.Y. and J.W.; data curation, P.W. and X.Q.; writing—original draft preparation, H.L.; writing—review and editing, H.L., L.C., S.Y. and J.W.; visualization, H.L. and L.C.; supervision, L.C., S.Y. and J.W.; project administration, J.W.; funding acquisition, H.L. and J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (52201257), Shenzhen Science and Technology Program (KQTD20200820113045083 and JCYJ20220818102403007), Newly Introduced Scientific Research Start-up Funds for High-tech Talents (DD11409024) and Shenzhen Research Fund for Returned Scholars (DD11409017 and AK29100016).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ren, L.; Yang, D.; Li, J.; Li, H.; Yang, J.-H. Nitrogen doped carbon fiber supported nickel phosphide for efficient electrocatalytic overall urea splitting. Appl. Surf. Sci. 2023, 624, 157173. [Google Scholar] [CrossRef]
- Wei, T.; Meng, G.; Zhou, Y.; Wang, Z.; Liu, Q.; Luo, J.; Liu, X. Amorphous Fe-Co oxide as an active and durable bifunctional catalyst for the urea-assisted H2 evolution reaction in seawater. Chem. Commun. 2023, 59, 9992–9995. [Google Scholar] [CrossRef]
- Xu, X.; Xu, L.; Wei, X.; Huang, M.; Toghan, A. Unravelling the mechanistic pathway of the Ni5P4/NiSe heterojunction for catalyzing the urea-rich water oxidation. Mater. Today Phys. 2023, 36, 101148. [Google Scholar] [CrossRef]
- Soltani, M.; Amin, H.M.A.; Cebe, A.; Ayata, S.; Baltruschat, H. Metal-Supported Perovskite as an Efficient Bifunctional Electrocatalyst for Oxygen Reduction and Evolution: Substrate Effect. J. Electrochem. Soc. 2021, 168, 034504. [Google Scholar] [CrossRef]
- Saeed, M.; Munir, M.; Intisar, A.; Waseem, A. Facile Synthesis of a Novel Ni-WO3@g-C3N4 Nanocomposite for Efficient Oxidative Desulfurization of Both Model and Real Fuel. ACS Omega 2022, 7, 15809–15820. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Amin, H.M.A.; Peng, Y.; Corva, M.; Pentcheva, R.; Tschulik, K. Facet-Dependent Intrinsic Activity of Single Co3O4 Nanoparticles for Oxygen Evolution Reaction. Adv. Funct. Mater. 2022, 33, 2210945. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Wen, Y.; Li, S.; Cui, C.; Ni, F.; Liu, Y.; Lin, H.; Li, Y.; Peng, H.; et al. Regulating the Local Charge Distribution of Ni Active Sites for the Urea Oxidation Reaction. Angew. Chem. Int. Ed. Engl. 2021, 60, 10577–10582. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Ye, Y.; Dai, X.; Yin, X.; Cao, Y.; Cai, R.; Feng, B.; Wang, Q.; Zhang, X. La and S Co-Doping Induced the Synergism of Multiphase Nickel-Iron Nanosheets with Rich Oxygen Vacancies to Trigger Large-Current-Density Oxygen Evolution and Urea Oxidation Reactions. Small 2023, 19, e2303250. [Google Scholar] [CrossRef]
- Kim, M.; Min, K.; Ko, D.; Seong, H.; Eun Shim, S.; Baeck, S.H. Regulating the electronic structure of Ni2P by one-step Co, N dual-doping for boosting electrocatalytic performance toward oxygen evolution reaction and urea oxidation reaction. J. Colloid Interface Sci. 2023, 650 Pt B, 1851–1861. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Huang, Y.; Huang, Q.; Li, F.; Tian, S. Surface Engineering over Metal-Organic Framework Nanoarray to Realize Boosted and Sustained Urea Oxidation. Small 2023, 19, e2305585. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, Y.; Li, L.; Luo, S.; Qing, Y.; Tian, C.; Xu, H.; Zhang, J.; Wu, Y. Ultrafine Homologous Ni2P–Co2P Heterostructures via Space-Confined Topological Transformation for Superior Urea Electrolysis. Adv. Funct. Mater. 2023, 33, 2303300. [Google Scholar] [CrossRef]
- Liu, X.; Lu, H.; Zhu, S.; Cui, Z.; Li, Z.; Wu, S.; Xu, W.; Liang, Y.; Long, G.; Jiang, H. Alloying-Triggered Phase Engineering of NiFe System via Laser-Assisted Al Incorporation for Full Water Splitting. Angew. Chem. Int. Ed. Engl. 2023, 62, e202300800. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, F.; Graham, N.; Yu, W. Engineering Morphology and Electron Redistribution of a Ni/WO3 Mott-Schottky Bifunctional Electrocatalyst for Efficient Alkaline Urea Splitting. ACS Appl. Mater. Interfaces 2023, 15, 50116–50125. [Google Scholar] [CrossRef]
- Zan, L.; Amin, H.M.A.; Mostafa, E.; Abd-El-Latif, A.A.; Iqbal, S.; Baltruschat, H. Electrodeposited Cobalt Nanosheets on Smooth Silver as a Bifunctional Catalyst for OER and ORR: In Situ Structural and Catalytic Characterization. ACS Appl. Mater. Interfaces 2022, 14, 55458–55470. [Google Scholar] [CrossRef]
- Liu, Z.; Corva, M.; Amin, H.M.A.; Blanc, N.; Linnemann, J.; Tschulik, K. Single Co3O4 Nanocubes Electrocatalyzing the Oxygen Evolution Reaction: Nano-Impact Insights into Intrinsic Activity and Support Effects. Int. J. Mol. Sci. 2021, 22, 13137. [Google Scholar] [CrossRef]
- Veettil, V.T.; Vijayakumar, A.U.; Ashdot, A.; Zitoun, D. Precious-Group-Metal-Free Energy-Efficient Urea Electrolysis: Membrane Electrode Assembly Cell Using Ni3N Nanoparticles as Catalyst. ACS Appl. Energy Mater. 2022, 5, 1397–1402. [Google Scholar] [CrossRef]
- Wang, X.-H.; Hong, Q.-L.; Zhang, Z.-N.; Ge, Z.-X.; Zhai, Q.-G.; Jiang, Y.-C.; Chen, Y.; Li, S.-N. Two-dimensional nickel–cobalt bimetallic hydroxides towards urea electrooxidation. Appl. Surf. Sci. 2022, 604, 154484. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, G.; Shui, L.; Zhou, G.; Wang, X. Urea electrooxidation-boosted hydrogen production on nitrogen-doped porous carbon nanorod-supported nickel phosphide nanoparticles. J. Energy Chem. 2022, 72, 88–96. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, H.; Tan, H.; Wu, X.; Kang, Y.; Dong, Y.; Li, X.; Jin, S.; Chang, X. Deciphering the active origin for urea oxidation reaction over nitrogen penetrated nickel nanoparticles embedded in carbon nanotubes. J. Colloid Interface Sci. 2022, 626, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, H.; Miao, J.; Hu, F.; Wang, L.; Tang, Y.; Qiao, M.; Guo, C. Strategies for designing more efficient electrocatalysts towards the urea oxidation reaction. J. Mater. Chem. A 2022, 10, 3296–3313. [Google Scholar] [CrossRef]
- Chang, J.; Lv, Q.; Li, G.; Ge, J.; Liu, C.; Xing, W. Core-shell structured Ni12P5/Ni3(PO4)2 hollow spheres as difunctional and efficient electrocatalysts for overall water electrolysis. Appl. Catal. B-Environ. 2017, 204, 486–496. [Google Scholar] [CrossRef]
- He, M.; Feng, C.; Liao, T.; Hu, S.; Wu, H.; Sun, Z. Low-Cost Ni2P/Ni0.96S Heterostructured Bifunctional Electrocatalyst toward Highly Efficient Overall Urea-Water Electrolysis. ACS Appl. Mater. Interfaces 2020, 12, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, S.; Feng, C.; Wu, H.; Zhang, J.; Mei, H. Novel MOF-Derived Nickel Nitride as High-Performance Bifunctional Electrocatalysts for Hydrogen Evolution and Urea Oxidation. ACS Sustain. Chem. Eng. 2020, 8, 7414–7422. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Cui, Z.; Zhu, S.; Li, Z.; Wu, S.; Ma, L.; Han, X.; Liang, Y. Spin State Tuning of the Octahedral Sites in Ni–Co-Based Spinel toward Highly Efficient Urea Oxidation Reaction. J. Phys. Chem. C 2021, 125, 9190–9199. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, S.; Cui, Z.; Li, Z.; Wu, S.; Liang, Y. Ni2P nanoflakes for the high-performing urea oxidation reaction: Linking active sites to a UOR mechanism. Nanoscale 2021, 13, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Chernev, P.; Mohammadi, M.R.; Klingan, K.; Loos, S.; Pasquini, C.; Kubella, P.; Jiang, S.; Yang, X.; Cui, Z.; et al. Self-supported Ni(OH)2/MnO2 on CFP as a flexible anode towards electrocatalytic urea conversion: The role of composition on activity, redox states and reaction dynamics. Electrochim. Acta 2019, 318, 32–41. [Google Scholar] [CrossRef]
- Ma, G.; Xue, Q.; Zhu, J.; Zhang, X.; Wang, X.; Yao, H.; Zhou, G.; Chen, Y. Ultrafine Rh nanocrystals decorated ultrathin NiO nanosheets for urea electro-oxidation. Appl. Catal. B-Environ. 2020, 265, 118567. [Google Scholar] [CrossRef]
- Yang, J.-H.; Chen, M.; Xu, X.; Jiang, S.; Zhang, Y.; Wang, Y.; Li, Y.; Zhang, J.; Yang, D. CuO-Ni(OH)2 nanosheets as effective electro-catalysts for urea oxidation. Appl. Surf. Sci. 2021, 560, 150009. [Google Scholar] [CrossRef]
- Chen, F.; Yang, F.; Sheng, C.; Li, J.; Xu, H.; Qing, Y.; Chen, S.; Wu, Y.; Lu, X. Electronic structure modulation of nickel hydroxide porous nanowire arrays via manganese doping for urea-assisted energy-efficient hydrogen generation. J. Colloid Interface Sci. 2022, 626, 445–452. [Google Scholar] [CrossRef]
- Yang, W.; Yang, X.; Hou, C.; Li, B.; Gao, H.; Lin, J.; Luo, X. Rapid room-temperature fabrication of ultrathin Ni(OH)2 nanoflakes with abundant edge sites for efficient urea oxidation. Appl. Catal. B-Environ. 2019, 259, 118020. [Google Scholar] [CrossRef]
- Hu, S.; Feng, C.; Wang, S.; Liu, J.; Wu, H.; Zhang, L.; Zhang, J. Ni3N/NF as Bifunctional Catalysts for Both Hydrogen Generation and Urea Decomposition. ACS Appl. Mater. Interfaces 2019, 11, 13168–13175. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Mao, H.; Guo, X.; Sun, D.; Sun, Z.; Wang, B.; Zhang, Y.; Song, X.-M. Hierarchical Ni(OH)2/Polypyrrole/Graphene Oxide Nanosheets as Excellent Electrocatalysts for the Oxidation of Urea. ACS Sustain. Chem. Eng. 2018, 6, 15570–15581. [Google Scholar] [CrossRef]
- Abdel Hameed, R.M.; Medany, S.S. NiO nanoparticles on graphene nanosheets at different calcination temperatures as effective electrocatalysts for urea electro-oxidation in alkaline medium. J. Colloid Interface Sci. 2017, 508, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Stoch, J.; Gablankowska-Kukucz, J. The Effect of Carbonate Contaminations on the XPS O 1s Band Structure in Metal Oxides. Surf. Interface Anal. 1991, 17, 165–167. [Google Scholar] [CrossRef]
- Barwe, S.; Weidner, J.; Cychy, S.; Morales, D.M.; Dieckhofer, S.; Hiltrop, D.; Masa, J.; Muhler, M.; Schuhmann, W. Electrocatalytic Oxidation of 5-(Hydroxymethyl)furfural Using High-Surface-Area Nickel Boride. Angew. Chem. Int. Ed. Engl. 2018, 57, 11460–11464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zou, Y.; Tao, L.; Chen, W.; Zhou, L.; Liu, Z.; Zhou, B.; Huang, G.; Lin, H.; Wang, S. Electrochemical Oxidation of 5-Hydroxymethylfurfural on Nickel Nitride/Carbon Nanosheets: Reaction Pathway Determined by In Situ Sum Frequency Generation Vibrational Spectroscopy. Angew. Chem. Int. Ed. Engl. 2019, 58, 15895–15903. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.M.A.; Attia, M.; Tetzlaff, D.; Apfel, U.P. Tailoring the Electrocatalytic Activity of Pentlandite FexNi9-XS8 Nanoparticles via Variation of the Fe:Ni Ratio for Enhanced Water Oxidation. ChemElectroChem 2021, 8, 3863–3874. [Google Scholar] [CrossRef]
- Guo, F.; Ye, K.; Du, M.; Huang, X.; Cheng, K.; Wang, G.; Cao, D. Electrochemical impedance analysis of urea electro-oxidation mechanism on nickel catalyst in alkaline medium. Electrochim. Acta 2016, 210, 474–482. [Google Scholar] [CrossRef]
- Ni, M.; Ratner, B.D. Differentiation of Calcium Carbonate Polymorphs by Surface Analysis Techniques—An XPS and TOF-SIMS study. Surf. Interface Anal. 2008, 40, 1356–1361. [Google Scholar] [CrossRef]
- Buchner, S.; Radtke, C.; Balzaretti, N.M. Densification of Lithium Disilicate under High Pressure Investigated by XPS. Open J. Inorg. Non-Met. Mater. 2013, 3, 15–21. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Bancroft, G.M.; Henderson, G.S.; Ho, R.; Dalby, K.N.; Huang, Y.; Yan, Z. Bridging, non-bridging and free (O2–) oxygen in Na2O-SiO2 glasses: An X-ray Photoelectron Spectroscopic (XPS) and Nuclear Magnetic Resonance (NMR) study. J. Non-Cryst. Solids 2011, 357, 170–180. [Google Scholar] [CrossRef]
- Yao, J.; Zhou, Y.; Yan, J.M.; Jiang, Q. Regulating Fe2(MoO4)3 by Au Nanoparticles for Efficient N2 Electroreduction under Ambient Conditions. Adv. Energy Mater. 2021, 11, 2003701. [Google Scholar] [CrossRef]
- Xie, F.Y.; Gong, L.; Liu, X.; Tao, Y.T.; Zhang, W.H.; Chen, S.H.; Meng, H.; Chen, J. XPS studies on surface reduction of tungsten oxide nanowire film by Ar+ bombardment. J. Electron Spectrosc. Relat. Phenom. 2012, 185, 112–118. [Google Scholar] [CrossRef]
- Haetge, J.; Djerdj, I.; Brezesinski, T. Nanocrystalline NiMoO4 with an ordered mesoporous morphology as potential material for rechargeable thin film lithium batteries. Chem. Commun. 2012, 48, 6726–6728. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, S.; Subramanian, P.; Levi, E.; Aurbach, D.; Gedanken, A.; Schechter, A. Exceptionally Active and Stable Spinel Nickel Manganese Oxide Electrocatalysts for Urea Oxidation Reaction. ACS Appl. Mater. Interfaces 2016, 8, 12176–12185. [Google Scholar] [CrossRef]
- Singh, R.K.; Schechter, A. Electroactivity of NiCr Catalysts for Urea Oxidation in Alkaline Electrolyte. ChemCatChem 2017, 9, 3374–3379. [Google Scholar] [CrossRef]
- Zhu, D.; Guo, C.; Liu, J.; Wang, L.; Du, Y.; Qiao, S.Z. Two-dimensional metal-organic frameworks with high oxidation states for efficient electrocatalytic urea oxidation. Chem. Commun. 2017, 53, 10906–10909. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Dou, X.; Dai, J.; An, X.; Guo, Y.; Zhang, L.; Tao, S.; Zhao, J.; Chu, W.; Zeng, X.C.; et al. Metallic Nickel Hydroxide Nanosheets Give Superior Electrocatalytic Oxidation of Urea for Fuel Cells. Angew. Chem. Int. Ed. Engl. 2016, 55, 12465–12469. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Wygant, B.R.; Kawashima, K.; Mullins, C.B. Catalyst or Precatalyst? The Effect of Oxidation on Transition Metal Carbide, Pnictide, and Chalcogenide Oxygen Evolution Catalysts. ACS Energy Lett. 2018, 3, 2956–2966. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Shen, R.; Ma, R.; Liu, Q.; Cao, G.; Wang, J. Correlating electrocatalytic oxygen reduction activity with d-band centers of metallic nanoparticles. Energy Storage Mater. 2018, 13, 189–198. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Cai, J.; Zheng, X.; Han, D.; Wu, Y.; Zang, Y.; Niu, S.; Liu, Y.; Zhu, J.; et al. Tailoring the d-Band Centers Enables Co4-N Nanosheets to Be Highly Active for Hydrogen Evolution Catalysis. Angew. Chem. Int. Ed. Engl. 2018, 57, 5076–5080. [Google Scholar] [CrossRef]
- Babar, P.; Patil, K.; Lee, D.M.; Karade, V.; Gour, K.; Pawar, S.; Kim, J.H. Cost-effective and efficient water and urea oxidation catalysis using nickel-iron oxyhydroxide nanosheets synthesized by an ultrafast method. J. Colloid Interface Sci. 2021, 584, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Li, Z.; Xu, Y.; Sun, J.; Hong, W.; Liu, X.; Wang, J.; Yang, S. Simple synthesis of amorphous NiWO4 nanostructure and its application as a novel cathode material for asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 8044–8052. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, M.; Bhuvaneswari, M.S.; Nachimuthu, P.; Schwenzer, B.; Kim, S.; Yang, Z.; Liu, J.; Graff, G.L.; Thevuthasan, S.; Hu, J. Spectroscopic investigations of the fouling process on Nafion membranes in vanadium redox flow batteries. J. Membr. Sci. 2011, 366, 325–334. [Google Scholar] [CrossRef]
- Jia, Y.; Luo, T.; Yu, X.-Y.; Liu, J.-H.; Huang, X.-J. Surfactant-free preparation of nickel carbonate hydroxide in aqueous solution and its toxic ion-exchange properties. New J. Chem. 2013, 37, 534–539. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).