Synergistic Leaching of Titanium, Aluminum, and Magnesium Components during Dilute Acid Pressure Treatment of High-Titanium Blast Furnace Slag
Abstract
:1. Introduction
2. Results and Discussions
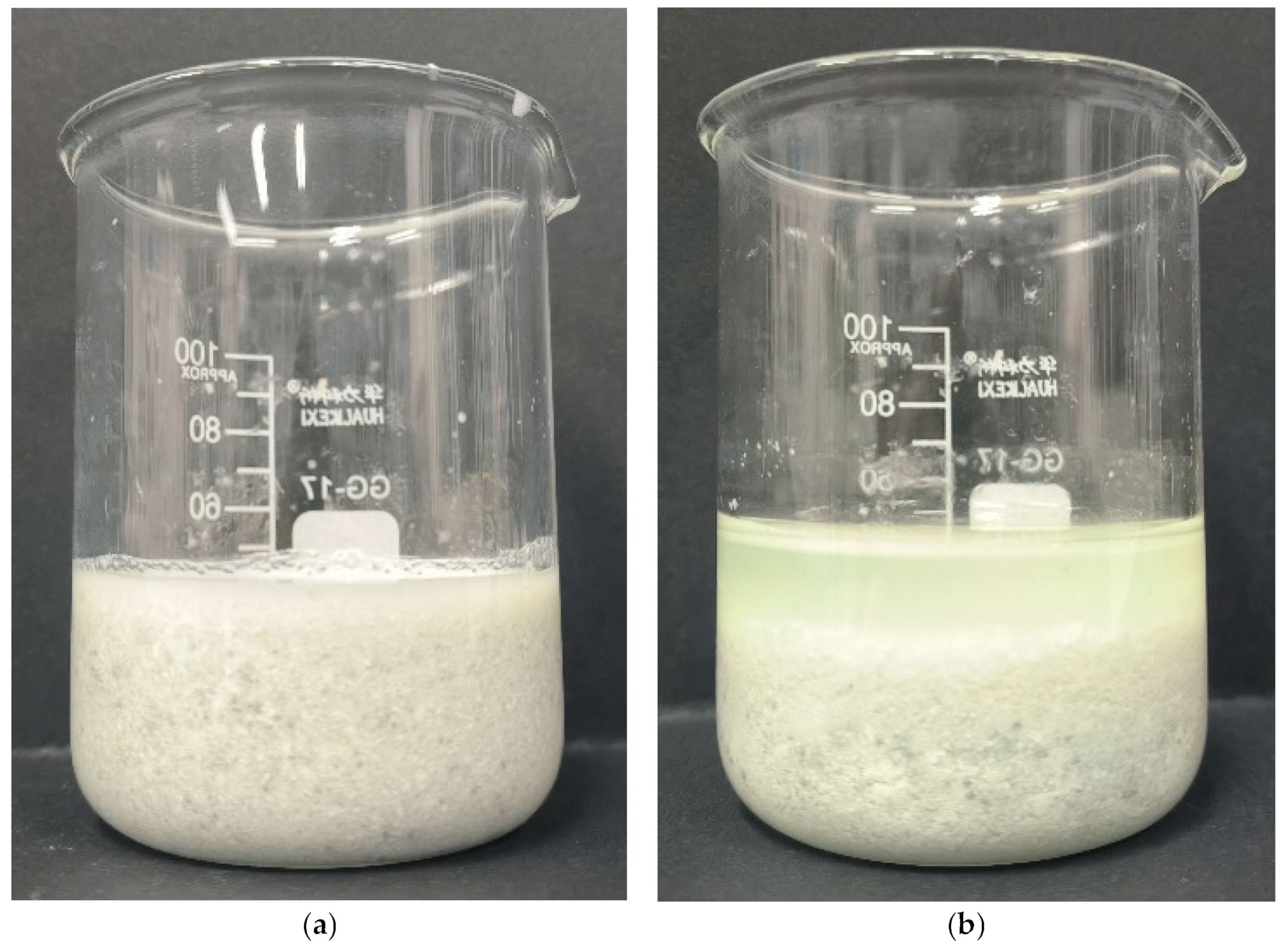
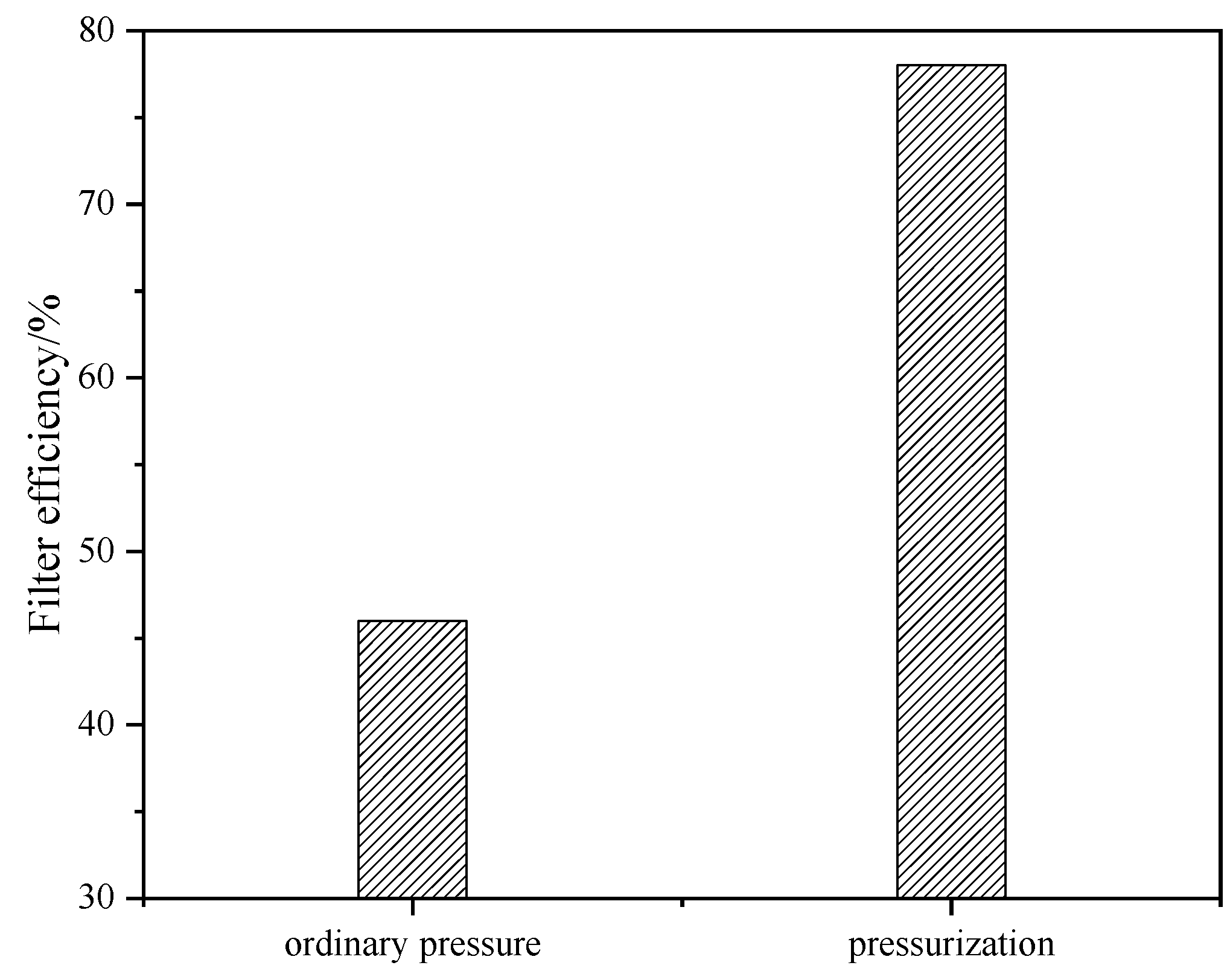
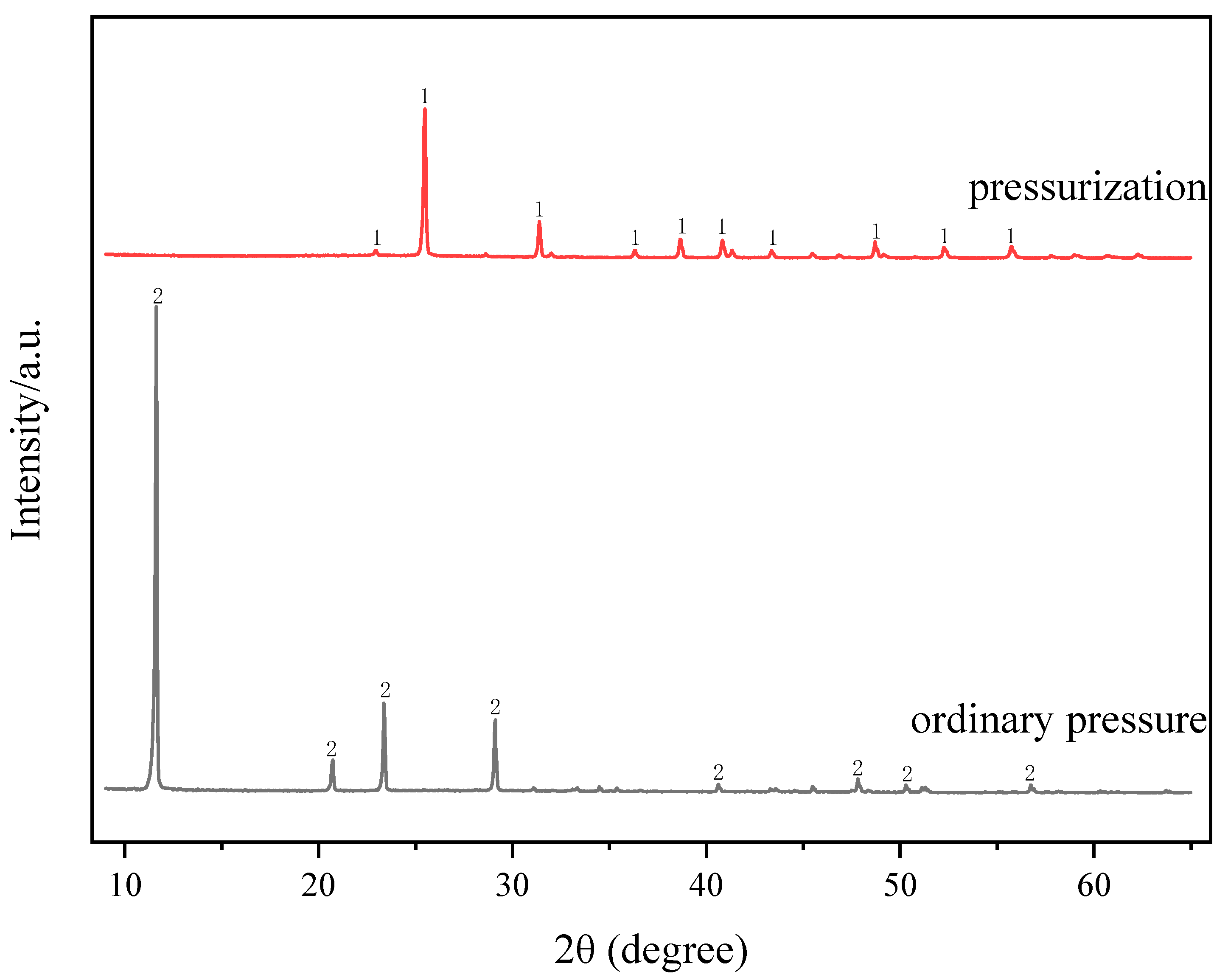
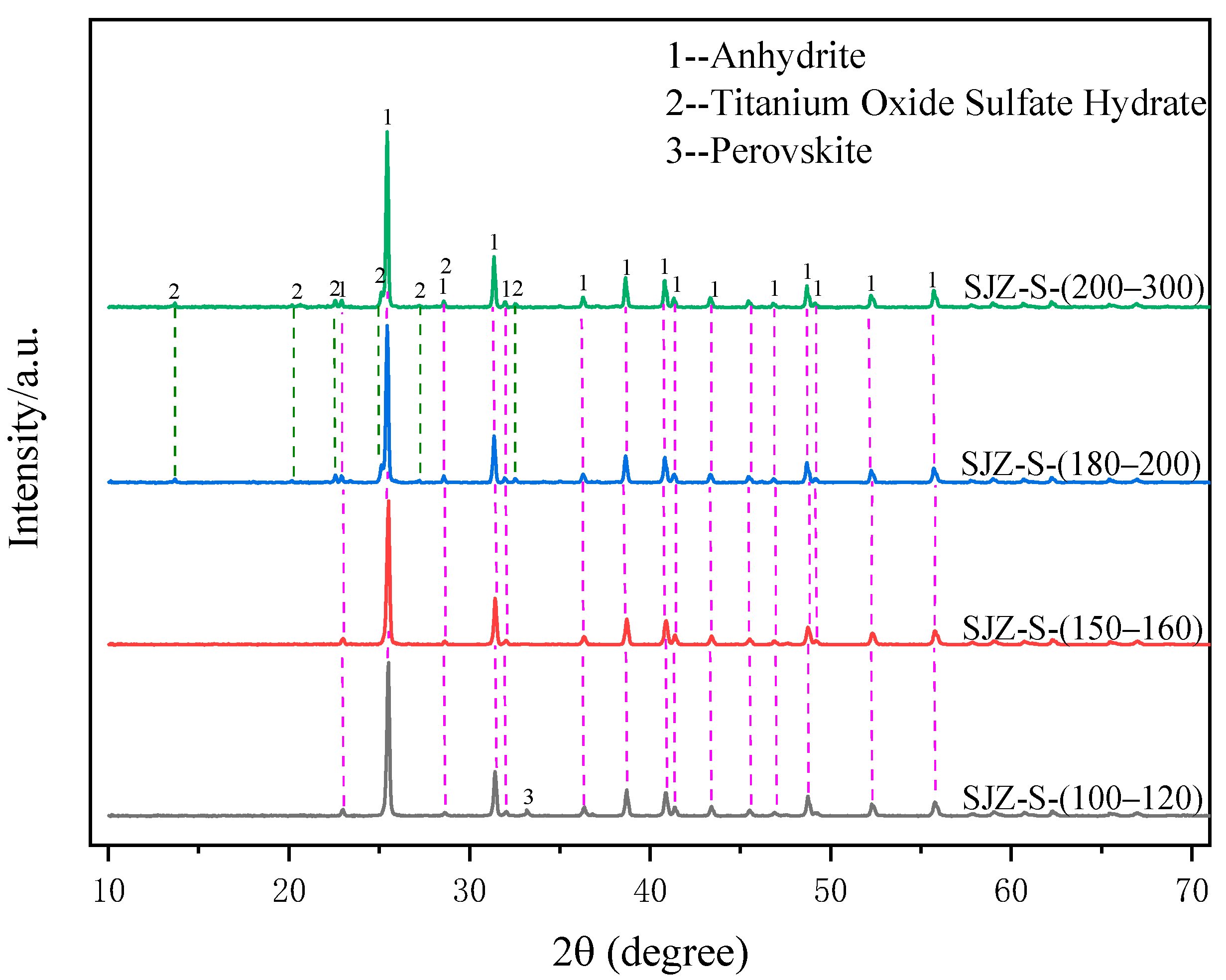
2.1. Effect of Pressure on Filtration Performance of Acid-Leached Slurry
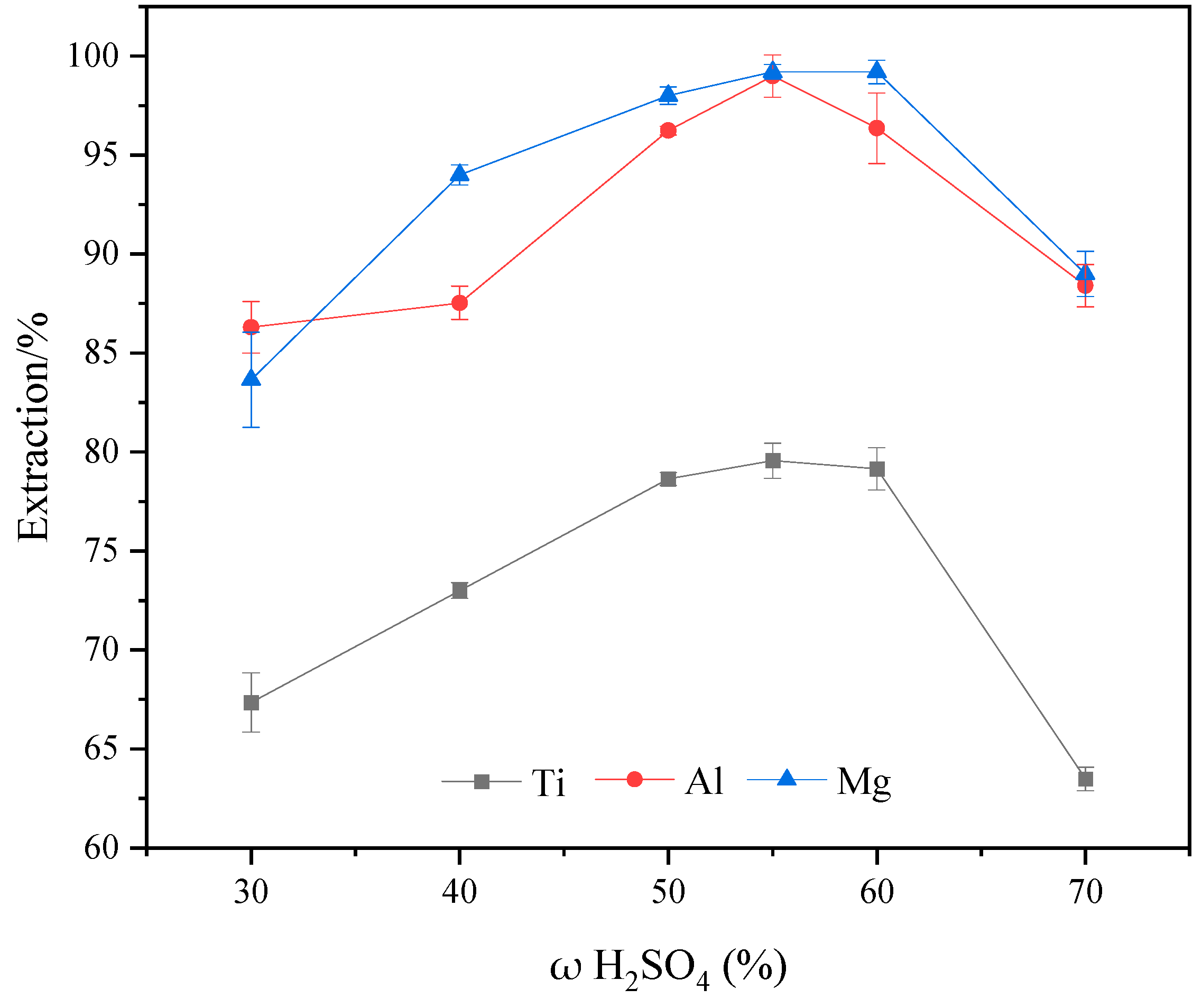
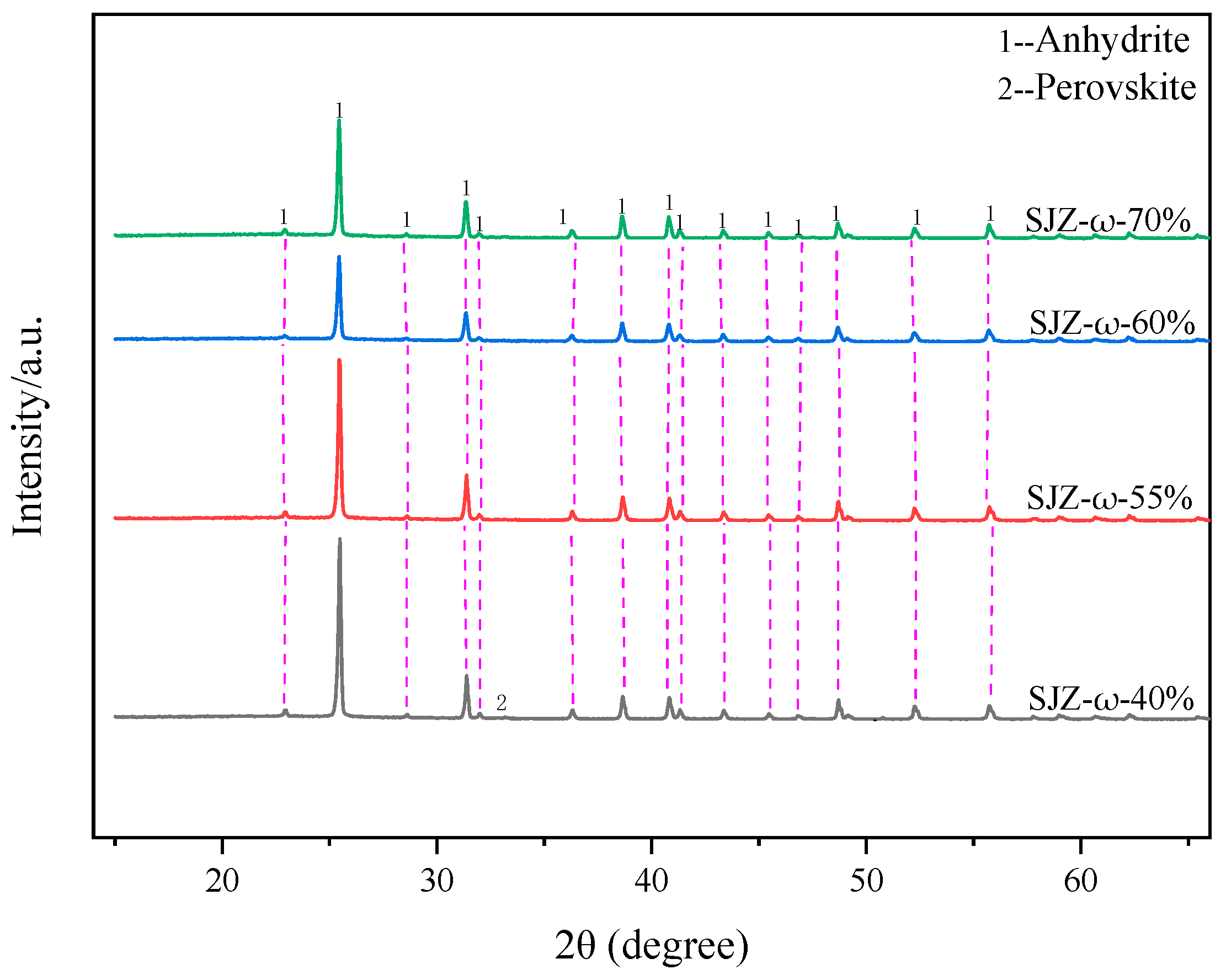
2.2. Effect of Sulfuric Acid Concentration on Ti, Al, and Mg Extraction Efficiency
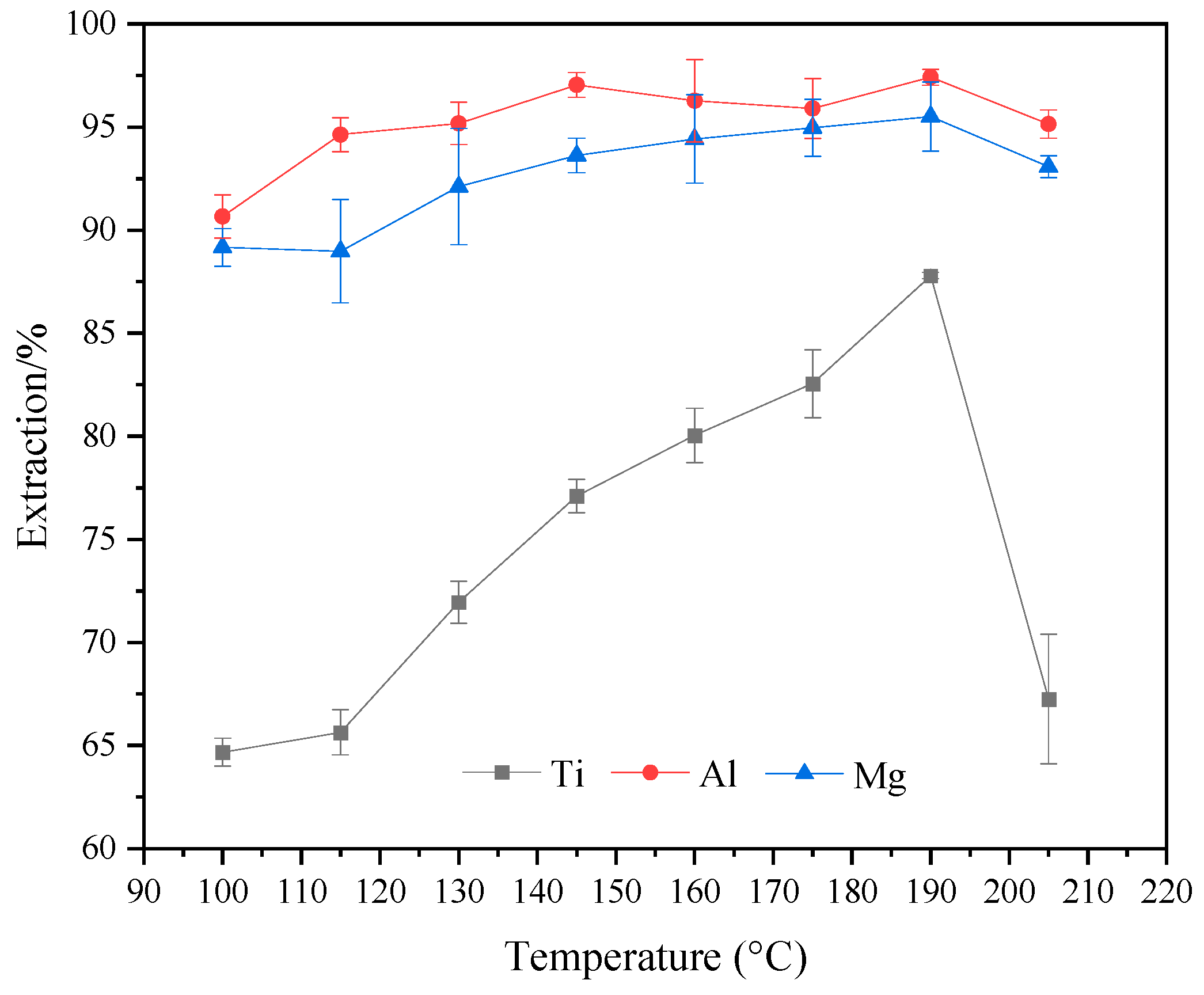
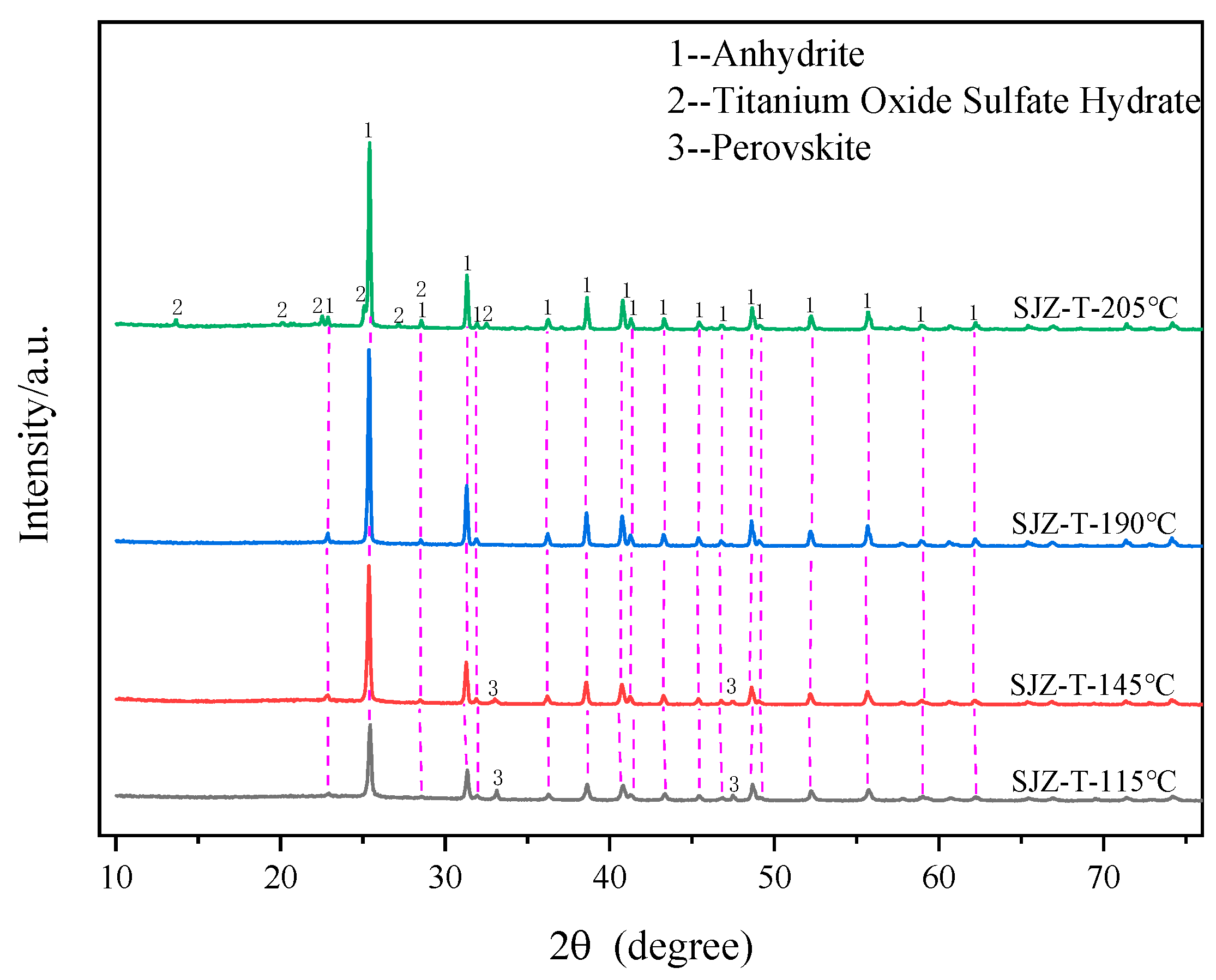
2.3. Effect of Reaction Temperature on the Extraction Rate of Ti, Al, and Mg
2.4. Effect of TBFS Particle Size on Extraction Rates of Ti, Al, and Mg
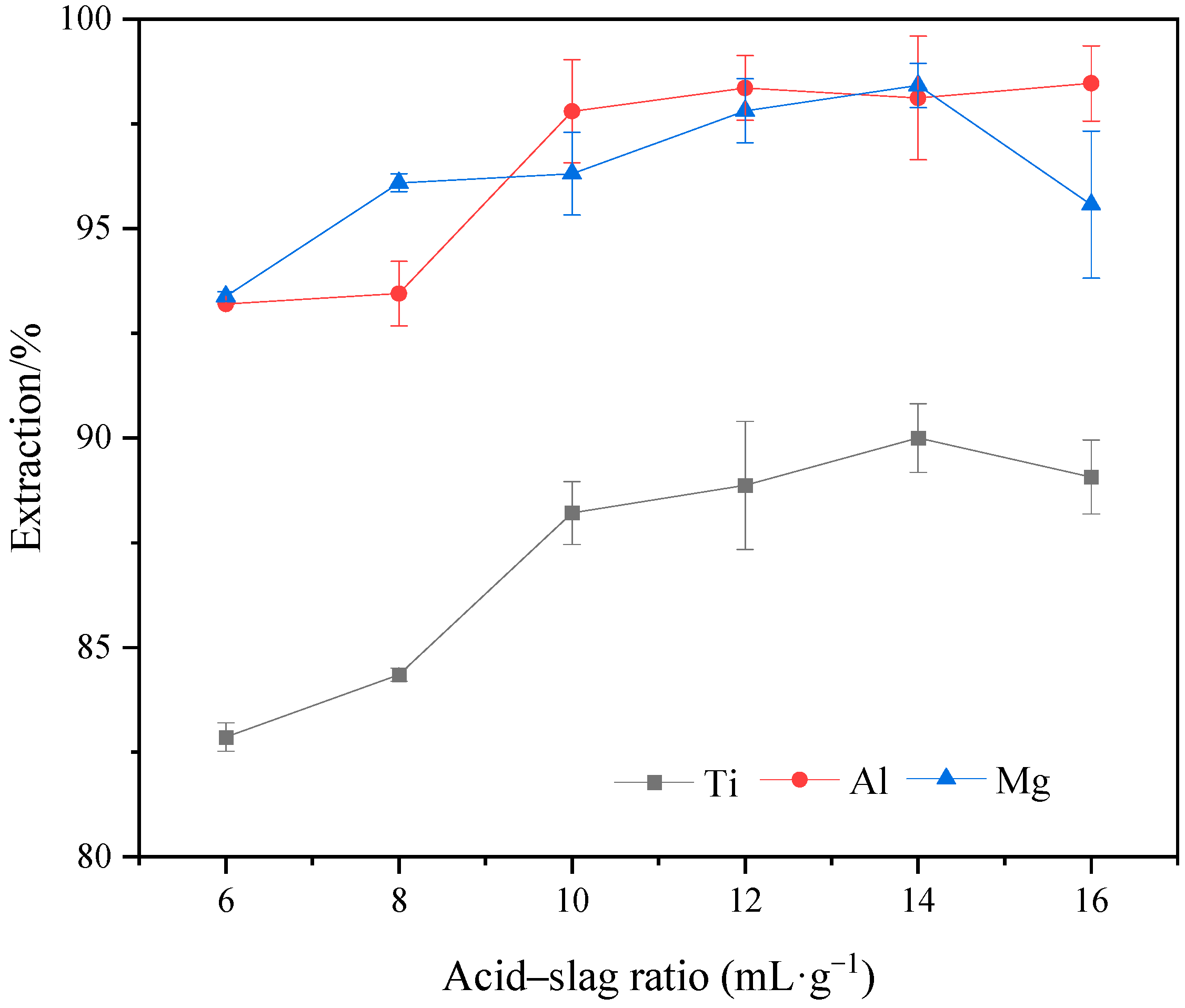
2.5. Effect of Acid–Slag Ratio on the Extraction Rate of Ti, Al, and Mg
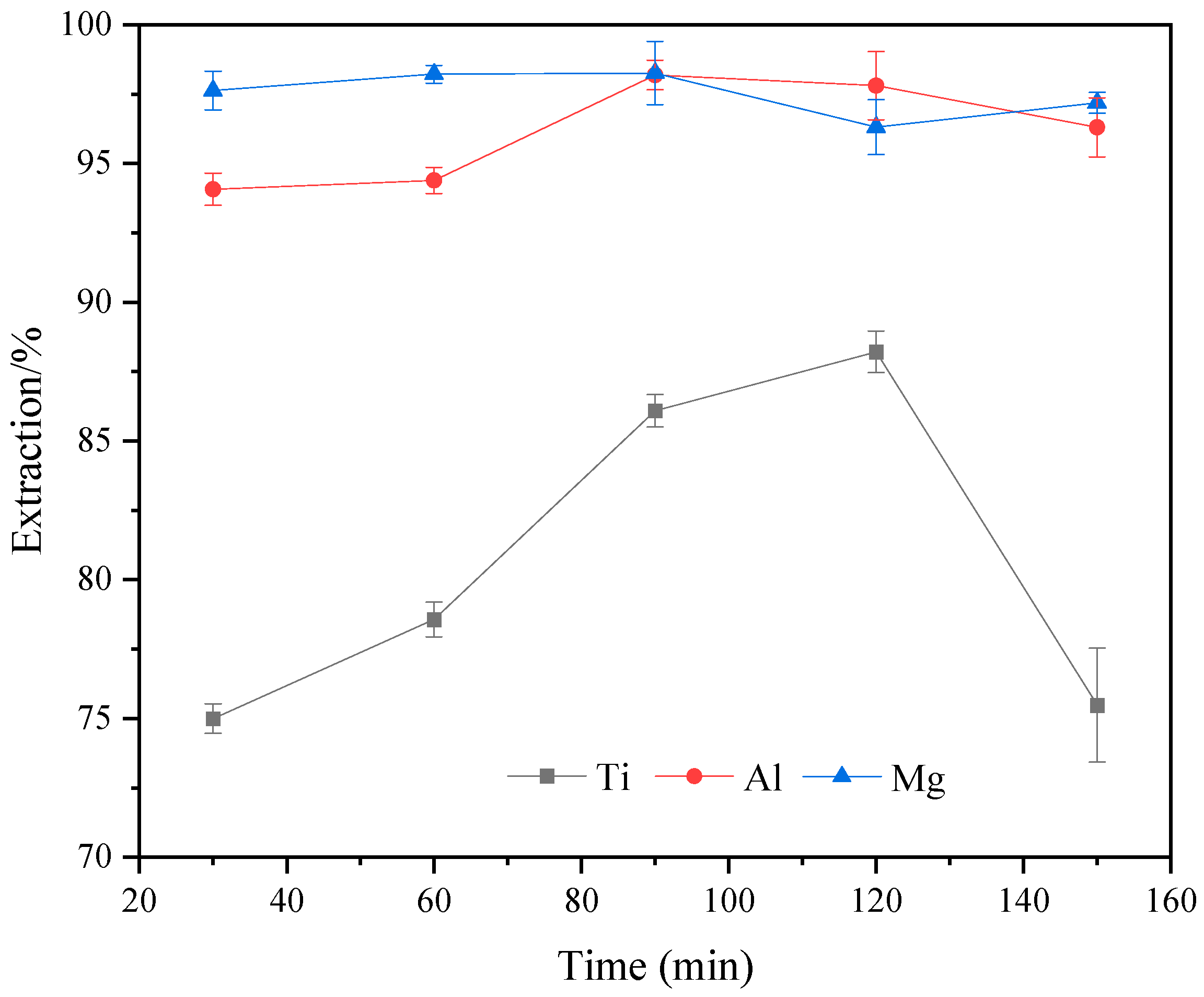
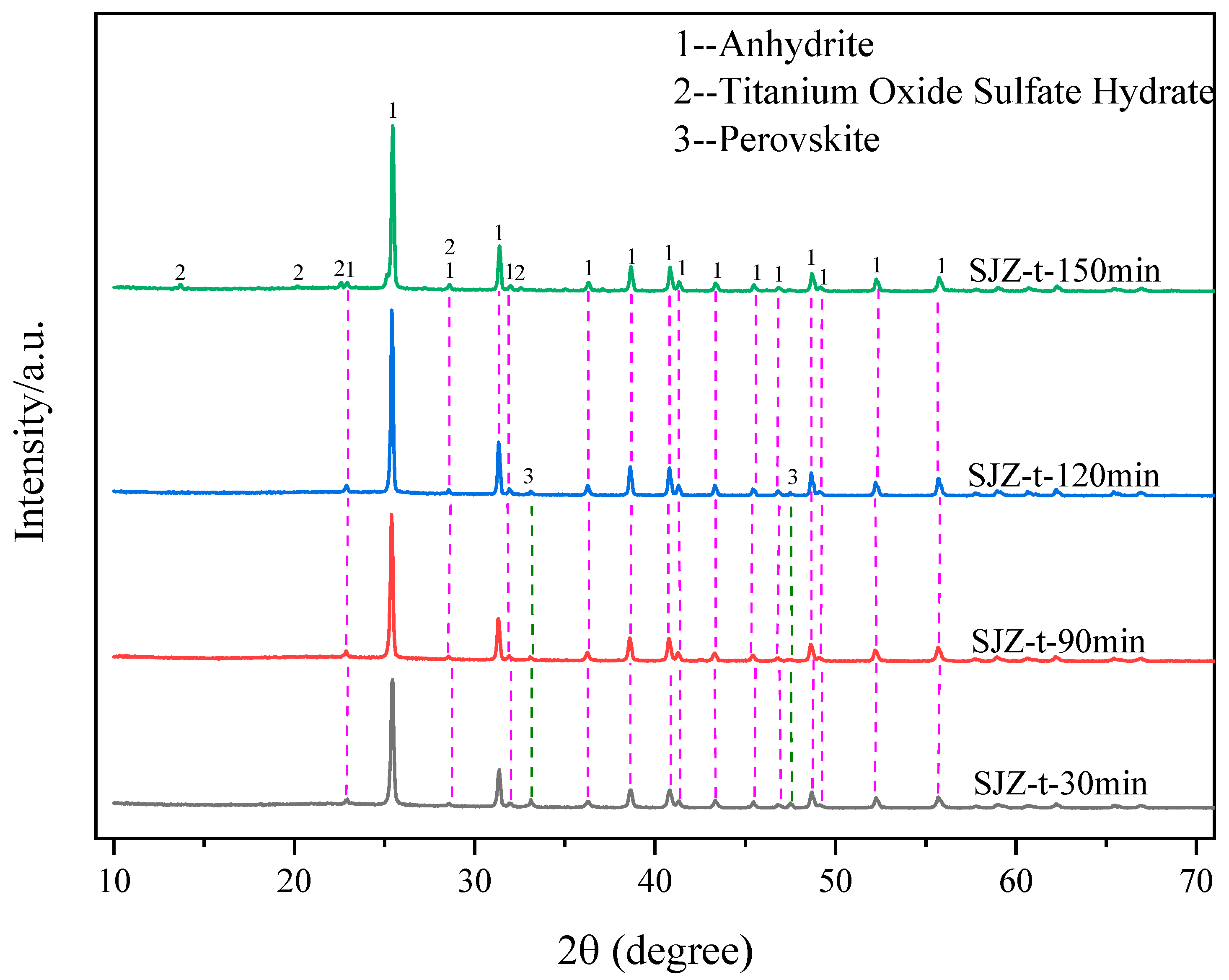
2.6. Effect of Reaction Time on the Extraction Rate of Ti, Al, and Mg
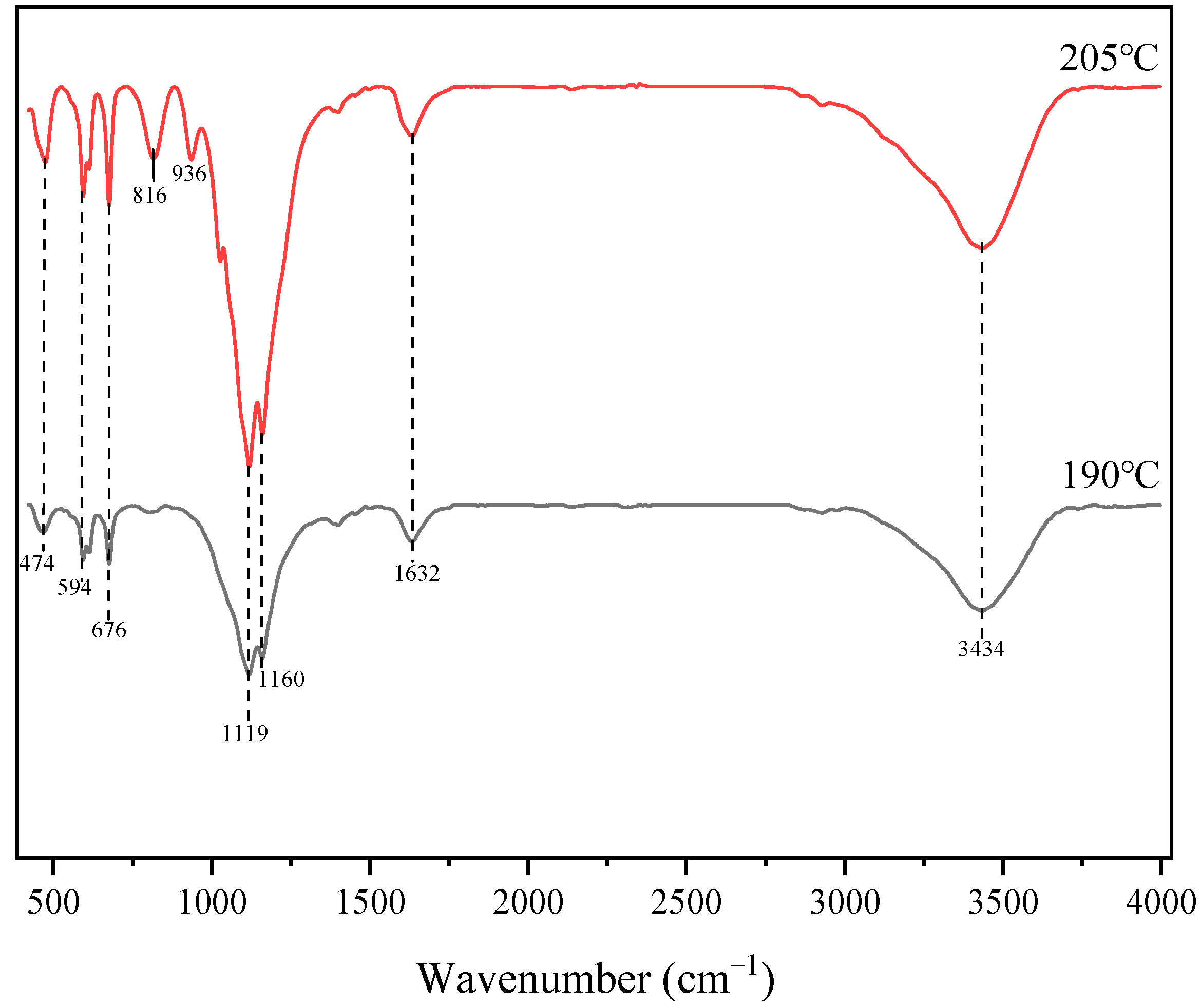
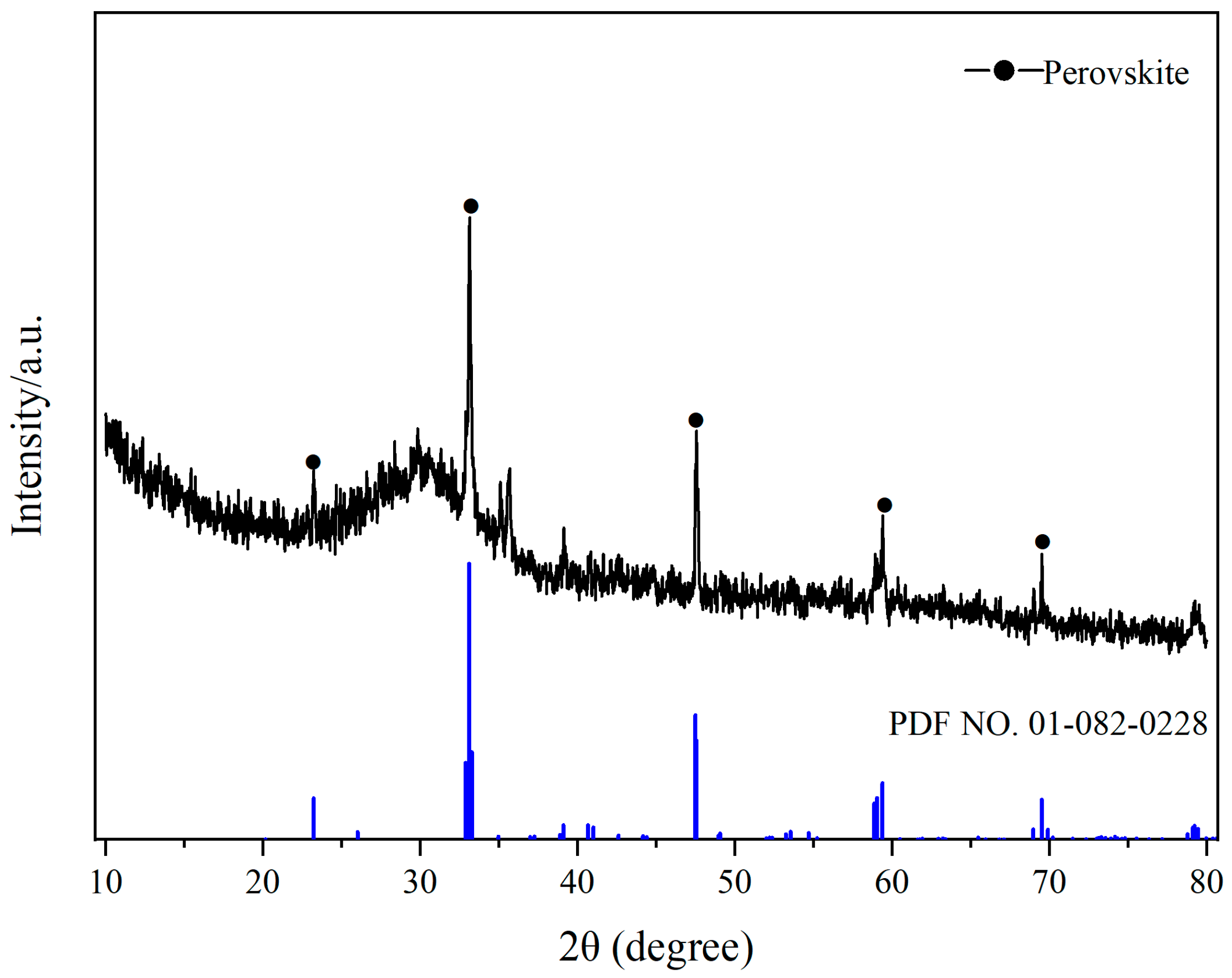
2.7. FTIR Spectroscopy Analysis
2.8. The Benefits of Economy and Environment
3. Materials and Methods
3.1. Experimental Materials
3.2. Experimental Principles
3.3. Experimental Procedures
3.4. Content Analysis Method
3.4.1. Testing Principle of Titanium Content
3.4.2. Testing Principle of Aluminum Content
3.4.3. Testing Principle of Magnesium Content
3.5. Sample Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.; Li, H.Y.; Yuan, Y.H.; Huang, J.; Diao, J.; Li, G. Microemulsion leaching of vanadium from sodium-roasted vanadium slag by fusion of leaching and extraction processes. Int. J. Miner. Metall. Mater. 2021, 28, 974–980. [Google Scholar] [CrossRef]
- Shi, J.; Qiu, Y.; Yu, B.; Dong, J.; Hou, C.; Li, J.; Liu, C. Titanium extraction from titania-bearing blast furnace slag: A review. JOM 2022, 74, 654–667. [Google Scholar] [CrossRef]
- Lin, H.F.; Jiang, Y.; Qiu, Z.Q.; Wang, K. Research on the leaching characteristics of Ca, Mg, Al elements in high titanium blast furnace slag. Iron Steel Vanadium Titan. 2023, 44, 18–24. [Google Scholar]
- Cai, Y.; Song, N.; Yang, Y.; Sun, L.; Hu, P.; Wang, J. Recent progress of efficient utilization of titanium-bearing blast furnace slag. Int. J. Miner. Metall. Mater. 2022, 29, 22–31. [Google Scholar] [CrossRef]
- Li, Z.; Lei, Y.; Ma, W.; Zhang, Y.; Wang, C. Preparation of high-purity TiSi2 and eutectic Si–Ti alloy by separation of Si–Ti alloy for clean utilization of Ti-bearing blast furnace slag. Sep. Purif. Technol. 2021, 265, 118473. [Google Scholar] [CrossRef]
- Zou, J.; Liu, Z.; Guo, Q. Comprehensive utilisation of blast furnace slag. Can. Metall. Q. 2023, 63, 1–8. [Google Scholar] [CrossRef]
- Proctor, D.M.; Fehling, K.A.; Shay, E.C.; Wittenborn, J.L.; Green, J.J.; Avent, C.; Bigham, R.D.; Connolly, M.; Lee, B.; Shepker, T.O.; et al. Physical and chemical characteristics of blast furnace, basic oxygen furnace, and electric arc furnace steel industry slags. Environ. Sci. Technol. 2000, 34, 1576–1582. [Google Scholar] [CrossRef]
- Li, S.Q.; Hua, S.G.; Li, X.M.; Pei, D.J.; Wang, D.Y. Study on preparation of high strength ceramic materials from Titanium-bearing blast furnace Slag. Multipurp. Util. Miner. Resour. 2024, 29, 22–31. [Google Scholar]
- Ha, N.S.; Marundrury, S.S.; Pham, T.M.; Pournasiri, E.; Shi, F.; Hao, H. Effect of grounded blast furnace slag and rice husk ash on performance of ultra-high-performance concrete (UHPC) subjected to impact loading. Constr. Build. Mater. 2022, 329, 127213. [Google Scholar] [CrossRef]
- Zhou, H.L.; Feng, K.Q.; Chen, C.H.; Yan, Z.D. Influence of CeO2 addition on the preparation of foamed glass-ceramics from high-titanium blast furnace slag. Int. J. Miner. Metall. Mater. 2018, 25, 689–695. [Google Scholar] [CrossRef]
- Linghu, R.; Liu, Y.; Zhang, Y.; Zhang, Y.; Gao, J.; Zhong, Y. Characterization of delayed coke and fluid coke gasification using blast furnace slag as a disposable catalyst. Energy Fuels 2019, 33, 6734–6741. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Xue, X.X. Study on compound fertilizer from Titanium-bearing blast furnace slag and its use in soybean cultivation. Environ. Eng. 2012, 30, 96–99. [Google Scholar]
- Huang, R.; Bai, C.; Lv, X.; Liu, S. Preparation of titanium alloy from Titania-bearing blast furnace slag. In Proceedings of the 3rd International Symposium on High-Temperature Metallurgical Processing, Orlando, FL, USA, 11–15 March 2012; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 75–83. [Google Scholar]
- Lei, Y.; Sun, L.; Ma, W.; Ma, X.; Wu, J.; Li, S.; Morita, K. An approach to employ titanium-bearing blast-furnace slag to prepare Ti and Al–Si alloys. J. Alloys Compd. 2018, 769, 983–990. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Liu, W.Z.; Zhang, G.Q.; Tang, S.W.; Yue, H.R.; Liang, B.; Luo, D.M. Recover titanium, aluminum, magnesium and separating silicon from titanium-bearing blast furnace slag by sulfuric acid curing-leaching. Int. J. Miner. Metall. Mater. 2022, 29, 1705–1714. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, T.; Zhang, Y. A novel preparation of titanium dioxide from titanium slag. Hydrometallurgy 2009, 96, 52–56. [Google Scholar] [CrossRef]
- Zhang, L.B.; Chen, G.; Peng, J.H.; Chen, J.; Guo, S.H.; Duan, X.H. Microwave absorbing properties of high titanium slag. J. Cent. South Univ. Technol. 2009, 16, 588–593. [Google Scholar] [CrossRef]
- Koike, R.; Sugiura, Y. Metal powder bed fusion in high gravity. CIRP Ann. 2021, 70, 191–194. [Google Scholar] [CrossRef]
- Sun, Q.; Hua, L. Micro texture of titanium alloys excited nonlinearly by electromagnetic pulse. Scr. Mater. 2021, 200, 113828. [Google Scholar] [CrossRef]
- He, S.Q. Study on Principle and Chemical Kinetics of Valuable Components Extraction and Separation from Panzhihua High Titanium-Blast Furnace Slag. Ph.D. Thesis, Doctor of Engineering, Southwest University of Science and Technology, Mianyang, China, 2020. [Google Scholar]
- Jiang, T.; Dong, H.G.; Guo, Y.F.; Li, G.H.; Yang, Y.B. Study on leaching Ti from Ti bearing blast furnace slag by sulphuric acid. Miner. Process. Extr. Metall. 2010, 119, 33–38. [Google Scholar] [CrossRef]
- Yan, F.; Li, C.; Liang, B. A two-step sulfuric acid leaching process of Ti-bearing blast furnace slag. Chin. J. Process Eng. 2006, 3, 413–417. [Google Scholar]
- Wang, W.; Zeng, D.; Chen, Q.; Yin, X. Experimental determination and modeling of gypsum and insoluble anhydrite solubility in the system CaSO4–H2SO4–H2O. Chem. Eng. Sci. 2013, 101, 120–129. [Google Scholar] [CrossRef]
- Bao, Z.J.; Li, J.L.; Wang, W.W. Solubility of calcium sulfate in distiller liquor. Chem. Eng. 1995, 23, 21–25+2. [Google Scholar]
- Li, J.H. Study on the Separation and Extraction of Titanium Components from Ti-Bearing Blast Furnace Slag. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2010. [Google Scholar]
- Komaraiah, D.; Radha, E.; Kalarikkal, N.; Sivakumar, J.; Reddy, M.R.; Sayanna, R. Structural, optical and photoluminescence studies of sol-gel synthesized pure and iron doped TiO2 photocatalysts. Ceram. Int. 2019, 45, 25060–25068. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, C.; Liang, B.; Xie, J. Leaching behavior of air-cooled Ti-bearing blast-furnace slag in hydrochloric acid. Chin. J. Nonferrous Met. 2008, 18, 557–563. [Google Scholar]
- Liu, X.H.; Sui, Z.T. The behaviors of the impurities in Ti-bearing slag during leaching process. Multipurp. Util. Miner. Resour. 2005, 5, 3–6. [Google Scholar]
- Dong, L.Y.; Li, X. Research on synthesis of solid TiOSO4. Jiangsu Ceram. 2011, 44, 11–12. [Google Scholar]
- Xu, C.H. Study on the New Preparation of High-Titanium Slag from Blast Furnace Slag. Master’s Thesis, Sichuan University, Chengdu, China, 2007. [Google Scholar]
- Mang, G.Q.; Cheng, M. Research of Al and Fe leaching rate in the process of slag leaching by hydrochloric acid. Appl. Mech. Mater. 2014, 675, 1417–1420. [Google Scholar] [CrossRef]
- He, S.; Wang, Y. Extraction of valuable components from ti-bearing blast furnace slag using sulfuric acid calcination process. JOM 2023, 75, 392–399. [Google Scholar] [CrossRef]
- Perez, S.P.M.; Mamani, S.C.; Zapata, L.I.V.; Piedra, J.L.L.; Ayasta, S.G.; Lafitte, E.D.R.; Aparicio Roque, F.G.; Coronado Zuloeta, O. Influence of rice husk ash (rha) with gypsum and ichu fibers in the processing of geopolymers. Innov. Infrastruct. Solut. 2023, 8, 211. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S. Modification of phase evolution in alkali-activated blast furnace slag by the incorpora-tion of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Yin, Y.-S.; Yin, J.; Zhang, W.; Tian, H.; Hu, Z.-M.; Feng, L.-H.; Chen, D.-L. Characterization of mineral matter in coal ashes with infrared and Raman spectroscopy. Spectrosc. Spectr. Anal. 2018, 38, 789–793. [Google Scholar]
- Lei, X.F.; Xue, X.X.; Yang, H.; Zhu, X.J.; Li, Z.P. Effects of leaching concentration on photocatalytic activity of acid leaching residue of high titanium slag. Chin. J. Nonferrous Met. 2015, 25, 1640–1647. [Google Scholar]
- Jiao, K.X.; Zhang, J.L.; Wang, Z.Y.; Chen, C.L.; Liu, Y.X. Effect of TiO2 and FeO on the viscosity and structure of blast furnace primary slags. Steel Res. Int. 2017, 88, 1600296. [Google Scholar] [CrossRef]
- Kalantari, K.; Kalbasi, M.; Sohrabi, M.; Royaee, S.J. Enhancing the photocatalytic oxida-tion of dibenzothiophene using visible light responsive Fe and N co-doped TiO2 nano-particles. Ceram. Int. 2017, 43, 973–981. [Google Scholar] [CrossRef]
- Hou, X.; Wang, D.; Shi, Y.; Guo, H.; He, Y. Hydraulic activity and microstructure analysis of high-titanium slag. Materials 2020, 13, 1239. [Google Scholar] [CrossRef]
- Liu, X.; Liebermann, R.C. X-ray powder diffraction study of CaTiO3 perovskite at high temperatures. Phys. Chem. Miner. 1993, 20, 171. [Google Scholar] [CrossRef]
- GB/T 4701.1-2009.2009; Ferrotitanium–Determination of Titanium Content–The Ammonium Ferric Sulfate Titration Method. General Administration of Quality Supervision. Inspection and Quarantine of the People‘s Republic of China: Beijing, China, 2009.
- GB/T 4701.6-2008.2008; Ferrotitanium–Determination of Aluminum Content–EDTA Titrimetric Method. Codeofchina Inc.: Beijing, China, 2008.
- GB/T 1511-2016.2016; Manganese Ores–Determination of Calcium and Magnesium Contents–EDTA Titrimetric Method. NBCHAO: Foshan, China, 2016.


| Component | CaO | SiO2 | TiO2 | Al2O3 | MgO | SO3 | Fe2O3 |
| Content | 28.08 | 26.74 | 19.65 | 13.86 | 7.64 | 1.05 | 0.79 |
| Component | K2O | MnO | Na2O | F | BaO | SrO | ZrO2 |
| Content | 0.72 | 0.64 | 0.53 | 0.17 | 0.07 | 0.04 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, K.; He, S.; Yu, B.; Qian, S.; Wu, X.; Li, W.; Zhao, C. Synergistic Leaching of Titanium, Aluminum, and Magnesium Components during Dilute Acid Pressure Treatment of High-Titanium Blast Furnace Slag. Molecules 2024, 29, 3336. https://doi.org/10.3390/molecules29143336
Yuan K, He S, Yu B, Qian S, Wu X, Li W, Zhao C. Synergistic Leaching of Titanium, Aluminum, and Magnesium Components during Dilute Acid Pressure Treatment of High-Titanium Blast Furnace Slag. Molecules. 2024; 29(14):3336. https://doi.org/10.3390/molecules29143336
Chicago/Turabian StyleYuan, Ke, Siqi He, Bo Yu, Shiyi Qian, Xueyu Wu, Wenyi Li, and Chunmeng Zhao. 2024. "Synergistic Leaching of Titanium, Aluminum, and Magnesium Components during Dilute Acid Pressure Treatment of High-Titanium Blast Furnace Slag" Molecules 29, no. 14: 3336. https://doi.org/10.3390/molecules29143336




