Effect of Encapsulated Phenolic Compounds of Cocoa on Growth of Lactic Acid Bacteria and Antioxidant Activity of Fortified Drinking Yogurt
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of Cocoa Phenolic Compounds Preparations
2.2. Number of Lactic Acid Bacteria in Yogurts
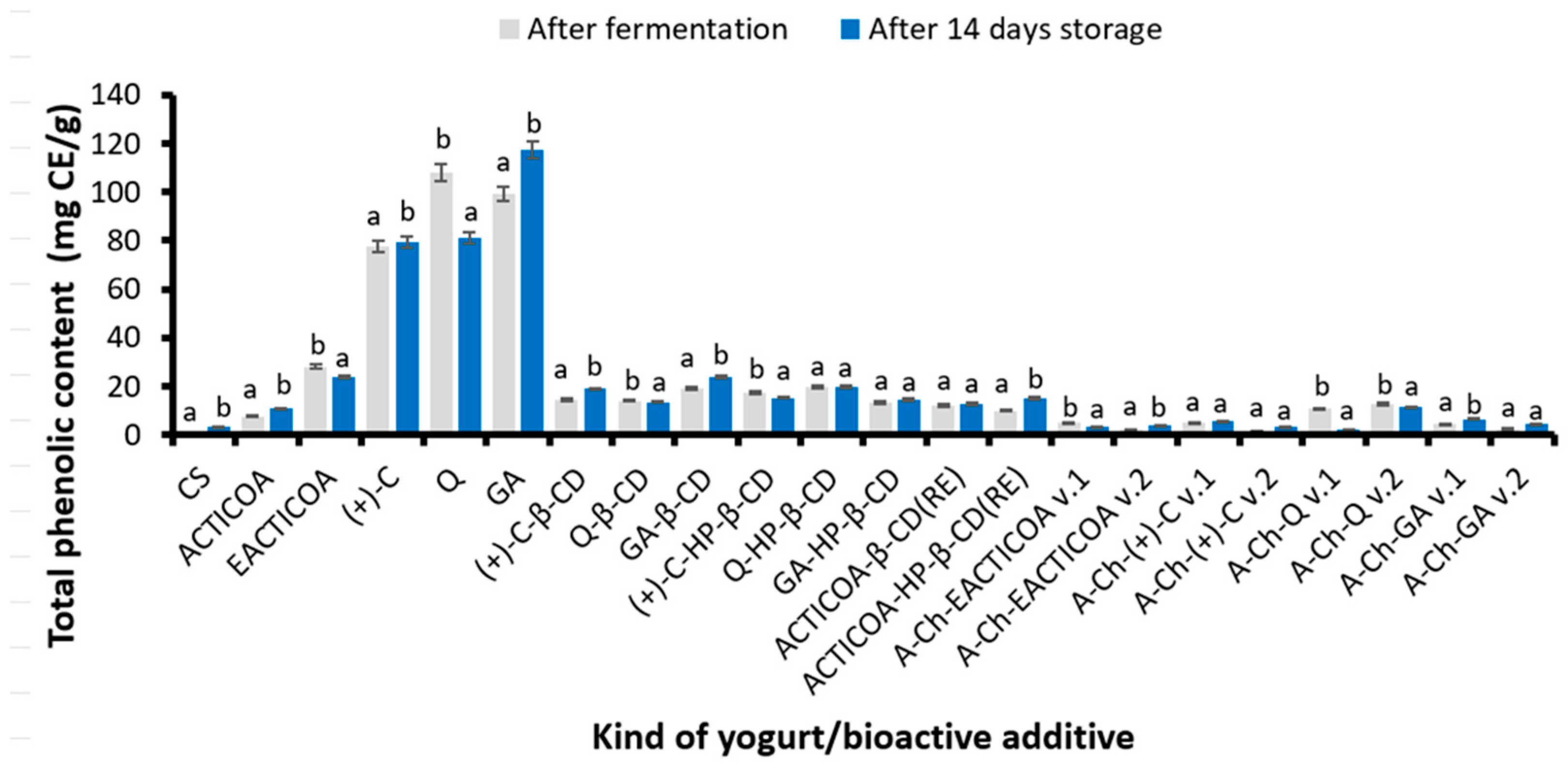
2.3. Total Phenolic Content
2.4. Antioxidant Activity
2.4.1. Free Radical Scavenging Capacity (DPPH)
2.4.2. ABTS Cation Radical Scavenging Ability
2.4.3. Ferric Reducing Ability (FRAP)
3. Materials and Methods
3.1. Materials
Chemical Reagents
3.2. Preparation of Cocoa Phenolic Compounds Encapsulates
3.2.1. ACTICOA Extract Preparation
3.2.2. Synthesis of Inclusion Complexes of Phenolic Compounds with β-CD or HP-β-CD Using Classical Method
3.2.3. Synthesis of ACTICOA Inclusion Complexes with β-CD or HP-β-CD Using Reactive Extraction
3.2.4. Preparation of Alginate–Chitosan Capsules Using Method 1 or Method 2
3.3. Preparation of Drinking Yogurt
3.4. Adding of Bioactive Preparations to Yogurt
3.5. Methods
3.5.1. Microbiological Analysis of Yogurt
3.5.2. UHPLC-DAD-ESI–MS/MS Analysis of Phenolic Compounds
3.5.3. Efficiency of Encapsulate Obtaining
3.5.4. Total Phenolic Content Determination
3.5.5. Antioxidant Activity Determination
3.5.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corrieu, G.; Bèal, C. Yogurt: The product and its manufacture. Encycl. Food Health 2016, 5, 617–624. [Google Scholar] [CrossRef]
- Georgakouli, K.; Mpesios, A.; Kouretas, D.; Petrotos, K.; Mitsagga, C.; Giavasis, I.; Jamurtas, A.Z. The effects of an olive fruit polyphenol-enriched yogurt on body composition, blood redox status, physiological and metabolic parameters and yogurt microflora. Nutrients 2016, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, C.I.; Kurilshikov, A.; Leeming, E.R.; Visconti, A.; Bowyer, R.C.E.; Menni, C.; Falchi, M.; Koutnikova, H.; Veiga, P.; Zhernakova, A.; et al. Yoghurt consumption is associated with changes in the composition of the human gut microbiome and metabolome. BMC Microbiol. 2022, 22, 39. [Google Scholar] [CrossRef]
- O’Connor, L.M.; Lentjes, M.A.H.; Luben, R.N.; Khaw, K.-T.; Wareham, N.J.; Forouhi, N.G. Dietary dairy product intake and incident type 2 diabetes: A prospective study using dietary data from a 7-day food diary. Diabetologia 2014, 57, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Livingston, K.A.; Fox, C.S.; Meigs, J.B.; Jacques, P.F. Yogurt consumption is associated with better diet quality and metabolic profile in American men and women. Nutr. Res. 2013, 33, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Marx, W.; Rocks, T.; Loughman, A.; Chandrasekaran, V.; Ruusunen, A.; Dawson, S.L.; West, M.; Mullarkey, E.; Pasco, J.A.; et al. The effects of dairy and dairy derivatives on the gut microbiota: A systematic literature review. Gut Microbes 2020, 12, 1799533. [Google Scholar] [CrossRef] [PubMed]
- Poon, T.; Juana, J.; Noori, D.; Jeansen, S.; Pierucci-Lagha, A.; Musa-Veloso, K. Effects of a fermented dairy drink containing Lacticaseibacillus paracasei subsp. paracasei CNCM I-1518 (Lactobacillus casei CNCM I-1518) and the standard yogurt cultures on the incidence, duration, and severity of common infectious diseases: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2020, 12, 3443. [Google Scholar] [CrossRef] [PubMed]
- Fiore, G.; Di Profio, E.; Sculati, M.; Verduci, E.; Zuccotti, G.V. Health effects of yogurt consumption during paediatric age: A narrative review. Int. J. Food Sci. Nutr. 2022, 73, 738–759. [Google Scholar] [CrossRef] [PubMed]
- Petrotos, K.B.; Karkanta, F.K.; Gkoutsidis, P.E.; Giavasis, I.; Papatheodorou, K.N.; Ntontos, A.C. Production of novel bioactive yogurt enriched with olive fruit polyphenols. World Acad. Sci. Eng. Technol. 2012, 64, 867–872. [Google Scholar]
- Sun-Waterhouse, D.; Zhou, J.; Wadhwa, S.S. Effects of adding apple polyphenols before and after fermentation on the properties of drinking yoghurt. Food Bioprocess Technol. 2011, 5, 2674–2686. [Google Scholar] [CrossRef]
- WeiDong, W.; Hao, H.; Jie, Q.; JiaYu, C.; Yuee, S. Research on preparation of yoghurt solidified with plant polyphenols. China Dairy Ind. 2017, 45, 53–56. [Google Scholar]
- Karaaslan, M.; Ozden, M.; Vardin, H.; Turkoglu, H. Phenolic fortification of yogurt using grape and callus extracts. LWT—Food Sci. Technol. 2011, 44, 1065–1072. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Zhou, J.; Wadhwa, S.S. Drinking yoghurts with berry polyphenols added before and after fermentation. Food Control 2013, 32, 450–460. [Google Scholar] [CrossRef]
- Raikos, V.; Ni, H.; Hayes, H.; Ranawana, V. Antioxidant properties of a yogurt beverage enriched with salal (Gaultheria shallon) berries and blackcurrant (Ribes nigrum) pomace during cold storage. Beverages 2019, 5, 2. [Google Scholar] [CrossRef]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Cyclodextrin encapsulated catechin: Effect of pH, relative humidity and various food models on antioxidant stability. LWT—Food Sci. Technol. 2017, 85, 232–239. [Google Scholar] [CrossRef]
- Ho, S.; Thoo, Y.Y.; Young, D.J.; Siow, L.F. Stability and recovery of cyclodextrin encapsulated catechin in various food matrices. Food Chem. 2019, 275, 594–599. [Google Scholar] [CrossRef]
- Aree, T. Understanding structures and thermodynamics of β-cyclodextrin encapsulation of chlorogenic, caffeic and quinic acids: Implications for enriching antioxidant capacity and masking bitterness in coffee. Food Chem. 2019, 293, 550–560. [Google Scholar] [CrossRef]
- Garrido, E.M.P.; Cerqueira, A.S.; Chavarria, D.; Silva, T.; Borges, F.; Garrido, J.M. Microencapsulation of caffeic acid phenethyl ester and caffeic acid phenethyl amide by inclusion in hydroxypropyl-β-cyclodextrin. Food Chem. 2018, 254, 260–265. [Google Scholar] [CrossRef]
- El Darra, N.; Rajha, H.N.; Debs, E.; Saleh, F.; El-Ghazzawi, I.; Louka, N.; Maroun, R.G. Comparative study between ethanolic and β-cyclodextrin assisted extraction of polyphenols from peach pomace. Int. J. Food Sci. 2018, 8, 9491681. [Google Scholar] [CrossRef]
- Gracia, M.R.; Serrano-Martínez, A.; Hernández-Sánchez, P.; Guardiola, L.; Núñez-Delicado, E.; López-Miranda, S. Effect of cyclodextrin and extraction method on extraction of phenolic compounds extraction from red wine pomace. J. Int. Soc. Antioxid. 2016, 4, 1–5. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Pałecz, B.; Rachwał-Rosiak, D.; Hodurek, P.; Miśkiewicz, K.; Oracz, J.; Żyżelewicz, D. Inclusion complexes of β-cyclodextrin with chlorogenic acids (CHAs) from crude and purified aqueous extracts of green Robusta coffee beans (Coffea canephora L.). Food Res. Int. 2014, 61, 202–213. [Google Scholar] [CrossRef]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Ma, L.L.; Shi, H.T.; Zhu, J.B.; Wu, J.; Ding, Z.W.; An, Y.; Zou, Y.Z.; Ge, J.B. Alginate oligosaccharide prevents acute doxorubicin cardiotoxicity by suppressing oxidative stress and endoplasmic reticulum-mediated apoptosis. Mar. Drugs 2016, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector—Biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Tusi, S.K.; Khalaj, L.; Ashabi, G.; Kiaei, M.; Khodagholi, F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 2011, 32, 5438–5458. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Shi, X.; Gao, Y.; Cai, N.; Jiang, Z.; Xu, X. Anti-inflammatory activity of guluronate oligosaccharides obtained by oxidative degradation from alginate in lipopolysaccharide-activated murine macrophage RAW 264.7 cells. J. Agric. Food Chem. 2015, 63, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wu, L.; Jia, L.; Li, X.; Sun, Y. Alginate—Chitosan microspheres for controlled release of tea polyphenol. Adv. Mater. Res. 2011, 152–153, 1726–1729. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, M.; Scher, L.J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–483. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, X.; Guo, Y.; Han, K.; Huo, N. Preparation and in vitro delivery performance of chitosan–alginate microcapsule for IgG. Food Agric. Immunol. 2016, 28, 1–13. [Google Scholar] [CrossRef]
- Anbinder, P.S.; Deladino, L.; Navarro, A.S.; Amalvy, J.I.; Martino, M.N. Yerba mate extract encapsulation with alginate and chitosan systems: Interactions between active compound encapsulation polymers. J. Encapsulation Adsorpt. Sci. 2011, 1, 80–87. [Google Scholar] [CrossRef]
- Kanokpanont, S.; Yamdech, R.; Aramwit, P. Stability enhancement of mulberry-extracted anthocyanin using alginate/chitosan microencapsulation for food supplement application. Artif. Cells Nanomed. Biotechnol. 2018, 46, 773–782. [Google Scholar] [CrossRef]
- Bulatao, R.; Samin, J.; Salazar, J.; Monserate, J. Encapsulation of anthocyanins from black rice (Oryza sativa L.) bran extract using chitosan-alginate nanoparticles. J. Food Res. 2017, 6, 40–47. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Oracz, J.; Kaczmarska, M.; Budryn, G.; Grzelczyk, J. Preparation and characterization of inclusion complex of (+)-catechin with β-cyclodextrin. Food Res. Int. 2018, 113, 263–268. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Hydroxypropyl-β-Cyclodextrin as a Green Co-Solvent in the Aqueous Extraction of Polyphenols from Waste Orange Peels. Beverages 2020, 6, 50. [Google Scholar] [CrossRef]
- Bozinou, E.; Lakka, A.; Poulianiti, K.; Lalas, S.; Makris, D.P. Cyclodextrins as high-performance green co-solvents in the aqueous extraction of polyphenols and anthocyanin pigments from solid onion waste. Eur. Food Res. Technol. 2021, 247, 2831–2845. [Google Scholar] [CrossRef]
- Grigorakis, S.; Benchennouf, A.; Halahlah, A.; Makris, D.P. High-Performance Green Extraction of Polyphenolic Antioxidants from Salvia fruticosa Using Cyclodextrins: Optimization, Kinetics, and Composition. Appl. Sci. 2020, 10, 3447. [Google Scholar] [CrossRef]
- Chakroun, D.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Enhanced-performance extraction of olive (Olea europaea) leaf polyphenols using L-lactic acid/ammonium acetate deep eutectic solvent combined with β-cyclodextrin: Screening, optimisation, temperature effects and stability. Biomass Convers. Biorefinery 2021, 11, 1125–1136. [Google Scholar] [CrossRef]
- Carrasco-Sandoval, J.; Aranda-Bustos, M.; Henríquez-Aedo, K.; López-Rubio, A.; Fabra, M.J. Bioaccessibility of different types of phenolic compounds co-encapsulated in alginate/chitosan-coated zein nanoparticles. LWT—Food Sci. Technol. 2021, 149, 112024. [Google Scholar] [CrossRef]
- Abril-Sánchez, C.; Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Evaluation of the properties of the essential oil citronellal nanoencapsulated by cyclodextrins. Chem. Phys. Lipids 2019, 219, 72–78. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Jia, W.; Chao, J.; Zhang, L. Theoretical and experimental study of the inclusion complexes of ferulic acid with cyclodextrins. Supramol. Chem. 2009, 21, 597–602. [Google Scholar] [CrossRef]
- Tomren, M.A.; Másson, M.; Loftsson, T.; Tønnesen, H.H. Studies on curcumin and curcuminoids: XXXI. Symmetric and asymmetric curcuminoids: Stability, activity and complexation with cyclodextrin. Int. J. Pharm. 2007, 338, 27–34. [Google Scholar] [CrossRef]
- Haładyn, K.; Tkacz, K.; Wojdyło, A.; Nowicka, P. The types of polysaccharide coatings and their mixtures as a factor affecting the stability of bioactive compounds and health-promoting properties expressed as the ability to inhibit the α-amylase and α-glucosidase of chokeberry extracts in the microencapsulation process. Foods 2021, 10, 1994. [Google Scholar] [CrossRef]
- Filannino, P.; Gobbetti, M.; De Angelis, M.; Di Cagno, R. Hydroxycinnamic acids used as external acceptors of electrons: An energetic advantage for strictly heterofermentative lactic acid bacteria. Appl. Environ. Microbiol. 2014, 80, 7574–7582. [Google Scholar] [CrossRef]
- Piekarska-Radzik, L.; Klewicka, E. Mutual influence of polyphenols and Lactobacillus spp. bacteria in food: A review. Eur. Food Res. Technol. 2021, 247, 9–24. [Google Scholar] [CrossRef]
- Jakubowska, M.; Karamucki, T. The effect of storage time and temperature on the quality of natural yoghurt. Acta Sci. Pol. Zootech. 2019, 18, 29–38. [Google Scholar] [CrossRef]
- Romero, M.L.M.; von Staszewski, M.; Martínez, M.J. The effect of green tea polyphenols addition on the physicochemical, microbiological and bioactive characteristics of yogurt. Br. Food J. 2021, 123, 2380–2397. [Google Scholar] [CrossRef]
- Tepe, B.; Sokmen, M.; Akpulat, H.A.; Sokmen, A. In vitro antioxidant activities of the methanol extracts of five Allium species from Turkey. Food Chem. 2005, 92, 89–92. [Google Scholar] [CrossRef]
- Feng, Y.; Niu, L.; Li, D.; Zeng, Z.; Sun, C.; Xiao, J. Effect of calcium alginate/collagen hydrolysates beads encapsulating high-content tea polyphenols on quality characteristics of set yogurt during cold storage. LWT—Food Sci. Technol. 2024, 191, 115608. [Google Scholar] [CrossRef]
- Codex Stan 243-2003; Codex Alimentarius. Standard for Fermented Milks. Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; p. 4.
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How does the phenol structure influence the results of the Folin-Ciocalteu assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef]
- Damin, M.R.; Alcântara, M.R.; Nunes, A.P.; Oliveira, M.N. Effects of milk supplementation with skim milk powder, whey protein concentrate and sodium caseinate on acidification kinetics, rheological properties and structure of nonfat stirred yogurt. LWT—Food Sci. Technol. 2009, 42, 1744–1750. [Google Scholar] [CrossRef]
- Amirdivani, S.; Baba, A.S.H. Green tea yogurt: Major phenolic compounds and microbial growth. J. Food Sci. Technol. 2015, 52, 4652–4660. [Google Scholar] [CrossRef]
- Kim, D.; Cho, W.; Yeon, S.; Choi, S.; Lee, C. Effects of Lotus (Nelumbo nucifera) leaf on quality and antioxidant activity of yogurt during refrigerated storage. Food Sci. Anim. Resour. 2019, 39, 792–803. [Google Scholar] [CrossRef]
- Chen, J.; Qin, X.; Zhong, S.; Chen, S.; Su, W.; Liu, Y. Characterization of curcumin/cyclodextrin polymer inclusion complex and investigation on its antioxidant and antiproliferative activities. Molecules 2018, 23, 1179. [Google Scholar] [CrossRef]
- Rajha, H.N.; Chacar, S.; Aff, C.; Vorobiev, E.; Louka, N.; Maroun, R.G. β-cyclodextrin-assisted extraction of polyphenols from vine shoot cultivars. J. Agric. Food Chem. 2015, 63, 3387–3393. [Google Scholar] [CrossRef]
- Arana-Sánchez, A.; Estarrón-Espinosa, M.; Obledo-Vázquez, E.N.; Padilla-Camberos, E.; Silva-Vázquez, R.; Lugo-Cervantes, E. Antimicrobial and antioxidant activities of Mexican oregano essential oils (Lippia graveolens H. B. K.) with different composition when microencapsulated in β-cyclodextrin. Lett. Appl. Microbiol. 2010, 50, 585–590. [Google Scholar] [CrossRef]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, E. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. Antioxidant activity of yogurt made from milk characterized by different casein haplotypes and fortified with chestnut and sulla honeys. J. Dairy Sci. 2014, 97, 6662–6670. [Google Scholar] [CrossRef]
- Najgebauer-Lejko, D.; Sady, M.; Grega, T.; Walczycka, M. The impact of tea supplementation on microflora, pH and antioxidant capacity of yoghurt. Int. Dairy J. 2011, 21, 568–574. [Google Scholar] [CrossRef]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT—Food Sci. Technol. 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Pandey, P.; Grover, K.; Dhillon, T.S.; Kaur, A.; Javed, M. Evaluation of polyphenols enriched dairy products developed by incorporating black carrot (Daucus carota L.) concentrate. Heliyon 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Nguyen, L.; Hwang, E. Quality characteristics and antioxidant activity of yogurt supplemented with aronia (Aronia melanocarpa) juice. Prev. Nutr. Food Sci. 2016, 21, 330–337. [Google Scholar] [CrossRef]
- Amirdivani, S.; Baba, A.S. Changes in yogurt fermentation characteristics, and antioxidant potential and in vitro inhibition of angiotensin-1 converting enzyme upon the inclusion of peppermint, dill and basil. LWT-Food Sci. Technol. 2011, 44, 1458–1464. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D. Impact of in-vitro gastro-pancreatic digestion on polyphenols and cinnamaldehyde bioaccessibility and antioxidant activity in stirred cinnamon-fortified yogurt. LWT—Food Sci. Technol. 2018, 89, 164–170. [Google Scholar] [CrossRef]
- Illupapalayam, V.V.; Smith, S.C.; Gamlath, S. Consumer acceptability and antioxidant potential of probiotic-yogurt with spices. LWT—Food Sci. Technol. 2014, 55, 255–262. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Development of a Low-Temperature and High-Performance Green Extraction Process for the Recovery of Polyphenolic Phytochemicals from Waste Potato Peels Using Hydroxypropyl β-Cyclodextrin. Appl. Sci. 2020, 10, 3611. [Google Scholar] [CrossRef]
- Hubert, J.; Berger, M.; Nepveu, F.; Paul, F.; Daydé, J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008, 109, 709–721. [Google Scholar] [CrossRef]
- Żyżelewicz, D.; Budryn, G.; Oracz, J.; Antolak, H.; Kręgiel, D.; Kaczmarska, M. The effect on bioactive components and characteristics of chocolate by functionalization with raw cocoa beans. Food Res. Int. 2018, 113, 234–244. [Google Scholar] [CrossRef]
- Pasukamonset, P.; Kwon, O.; Adisakwattana, S. Alginate-based encapsulation of polyphenols from Clitoria ternatea petal flower extract enhances stability and biological activity under simulated gastrointestinal conditions. Food Hydrocoll. 2016, 61, 772–779. [Google Scholar] [CrossRef]
- Belščak, A.; Komes, D.; Horžić, D.; Kovačević Ganić, K. Comparative study of commercially available cocoa products in terms of their bioactive composition. Food Res. Int. 2009, 42, 707–716. [Google Scholar] [CrossRef]
- Schinella, G.; Mosca, S.; Cienfuegos-Jovellanos, E.; Ángeles Pasamar, M.; Muguerza, B.; Ramón, D.; Ríos, J.L. Antioxidant properties of polyphenol-rich cocoa products industrially processed. Food Res. Int. 2010, 43, 1614–1623. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Bassoli, D.; Benassi, M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]



| Tested Parameter | ACTICOA | EACTICOA |
|---|---|---|
| (+)–Catechin | 3.47 ± 0.03 a | 17.33 ± 0.12 b |
| (−)–Epicatechin | 18.59 ± 0.12 a | 107.42 ± 0.13 b |
| Procyanidin B2 | 7.56 ± 0.10 a | 46.94 ± 0.05 b |
| Procyanidin C1 | 5.88 ± 0.05 a | 29.72 ± 0.08 b |
| Total flavan-3-ols | 35.49 ± 0.21 a | 201.39 ± 0.15 b |
| Gallic acid | 0.11 ± 0.01 a | 0.63 ± 0.03 b |
| Protocatechuic acid | 0.11 ± 0.02 a | 0.74 ± 0.02 b |
| Caffeic acid aspartate | 1.47 ± 0.13 a | 6.72 ± 0.05 b |
| p-Coumaric acid aspartate | 0.04 ± 0.01 a | 0.18 ± 0.02 b |
| Total phenolic acids | 1.72 ± 0.06 a | 8.26 ± 0.10 b |
| Total phenolic compounds | 37.21 ± 0.74 a | 209.65 ± 0.70 b |
| TPC (mg CE/g) | 228.85 ± 0.15 a | 435.14 ± 0.18 b |
| Tested Parameter | Type of Encapsulate | |||||||
|---|---|---|---|---|---|---|---|---|
| (+)-C-β-CD | Q-β-CD | GA-β-CD | (+)-C-HP-β-CD | Q-HP-β-CD | GA-HP-β-CD | ACTICOA-β-CD(RE) | ACTICOA-HP-β-CD(RE) | |
| Obtaining efficiency (%) | 67.81 ± 0.79 f | 55.75 ± 0.67 c | 67.54 ± 0.54 e | 98.90 ± 1.10 h | 62.46 ± 1.54 d | 98.20 ± 1.34 g | 42.97 ± 4.01 a | 50.60 ± 1.50 b |
| Concentration of phenolic compounds in the encapsulate (mg/g) | ||||||||
| Flavonoids | ||||||||
| (+)–Catechin | 155.68 ± 0.23 c | nd | nd | 184.87 ± 0.31 d | nd | nd | 1.15 ± 0.01 b | 0.63 ± 0.02 a |
| (−)–Epicatechin | nd | nd | nd | nd | nd | nd | 8.71 ± 0.04 b | 5.45 ± 0.06 a |
| Procyanidin B2 | nd | nd | nd | nd | nd | nd | 3.14 ± 0.03 b | 1.82 ± 0.01 a |
| Procyanidin C1 | nd | nd | nd | nd | nd | nd | 4.62 ± 0.01 b | 2.74 ± 0.06 a |
| Quercetin | nd | 135.98 ± 0.21 a | nd | nd | 178.98 ± 0.28 b | nd | nd | nd |
| Total flavonoids | 155.68 ± 0.23 d | 135.98 ± 0.21 c | nd | 184.87 ± 0.31 f | 178.98 ± 0.28 e | nd | 17.62 ± 0.08 b | 10.64 ± 0.12 a |
| Phenolic acids | ||||||||
| Gallic acid | nd | nd | 245.09 ± 0.25 a | nd | nd | 252.09 ± 0.31 b | nd | nd |
| Protocatechuic acid | nd | nd | nd | nd | nd | nd | nd | nd |
| Caffeic acid aspartate | nd | nd | nd | nd | nd | nd | 0.07 ± 0.02 b | 0.04 ± 0.02 a |
| p-Coumaric acid aspartate | nd | nd | nd | nd | nd | nd | 0.02 ± 0.01 a | 0.01 ± 0.01 a |
| Total phenolic acids | nd | nd | 245.09 ± 0.25 c | nd | nd | 252.09 ± 0.31 d | 0.09 ± 0.03 b | 0.06 ± 0.0 a |
| Total phenolic compounds | 155.68 ± 0.23 d | 135.98 ± 0.21 c | 245.09 ± 0.25 g | 184.87 ± 0.31 f | 178.98 ± 0.28 e | 252.09 ± 0.31 b | 17.79 ± 0.15 b | 10.70 ± 0.12 a |
| TPC (mg CE/g) | 202.26 ± 0.18 e | 211.53 ± 0.13 f | 287.09 ± 0.16 g | 165.03 ± 0.19 c | 294.89 ± 0.23 h | 197.94 ± 0.17 d | 109.81 ± 0.19 b | 101.06 ± 0.16 a |
| Tested Parameter | Type of Encapsulate | |||||||
|---|---|---|---|---|---|---|---|---|
| A-Ch-EACTICOA v.1 | A-Ch-EACTICOA v.2 | A-Ch-(+)-C v.1 | A-Ch-(+)-C v.2 | A-Ch-Q v.1 | A-Ch-Q v.2 | A-Ch-GA v.1 | A-Ch-GA v.2 | |
| Obtaining efficiency (%) | 95.67 ± 0.38 b | 94.78 ± 0.29 a | 98.34 ± 0.23 d | 99.45 ± 0.25 g | 99.87 ± 0.23 h | 97.67 ± 0.78 c | 98.64 ± 0.56 e | 98.89 ± 0.29 f |
| Concentration of phenolic compounds in the encapsulate (mg/g) | ||||||||
| Flavonoids | ||||||||
| (+)–Catechin | 1.12 ± 0.03 b | 1.06 ± 0.01 a | 53.42 ± 0.10 c | 54.10 ± 0.12 d | nd | nd | nd | nd |
| (−)–Epicatechin | 5.91 ± 0.03 b | 5.80 ± 0.03 a | nd | nd | nd | nd | nd | nd |
| Procyanidin B2 | 3.22 ± 0.02 a | 3.21 ± 0.01 a | nd | nd | nd | nd | nd | nd |
| Procyanidin C1 | 1.61 ± 0.01 a | 1.62 ± 0.02 a | nd | nd | nd | nd | nd | nd |
| Quercetin | nd | nd | nd | nd | 62.98 ± 0.16 b | 57.54 ± 0.27 a | nd | nd |
| Total flavonoids | 11.86 ± 0.09 a | 11.69 ± 0.07 b | 53.42 ± 0.10 c | 54.10 ± 0.12 d | 62.98 ± 0.16 f | 57.54 ± 0.27 e | nd | nd |
| Phenolic acids | ||||||||
| Gallic acid | nd | nd | nd | nd | nd | nd | 55.09 ± 0.10 b | 45.37 ± 0.13 a |
| Caffeic acid aspartate | 0.06 ± 0.01 a | 0.06 ± 0.01 a | nd | nd | nd | nd | nd | nd |
| p-Coumaric acid aspartate | 0.02 ± 0.01 a | 0.02 ± 0.01 a | nd | nd | nd | nd | nd | nd |
| Total phenolic acids | 0.08 ± 0.02 a | 0.08 ± 0.02 a | nd | nd | nd | nd | nd | nd |
| Total phenolic compounds | 11.94 ± 0.11 b | 11.77 ± 0.10 a | 53.42 ± 0.10 d | 54.10 ± 0.12 e | 62.98 ± 0.16 h | 57.54 ± 0.27 g | 55.09 ± 0.10 f | 45.37 ± 0.13 c |
| TPC (mg CE/g) | 32.46 ± 0.09 c | 29.23 ± 0.08 b | 73.50 ± 0.16 f | 25.69 ± 0.05 a | 189.63 ± 0.19 h | 159.71 ± 0.08 g | 69.40 ± 0.11 e | 35.73 ± 0.04 d |
| Preparation | DPPH EC50 (mg/mg DPPH) | ABTS EC50 (mg/mg ABTS) | FRAP (µM Trolox/g) |
|---|---|---|---|
| ACTICOA | 0.55 ± 0.02 c | 0.32 ± 0.03 f | 1105.32 ± 1.06 p |
| EACTICOA | 0.20 ± 0.01 b | 0.15 ± 0.02 c | 2804.29 ± 1.12 r |
| (+)-C | 0.23 ± 0.01 b | 0.05 ± 0.01 a | 4801.51 ± 1.09 s |
| Q | 0.21 ± 0.01 b | 0.12 ± 0.02 b | 6616.17 ± 1.16 t |
| GA | 0.15 ± 0.01 a | 0.15 ± 0.01 c | 7204.89 ± 1.08 u |
| (+)-C-β-CD | 2.09 ± 0.02 g | 0.48 ± 0.02 h | 477.49 ± 2.21 g |
| Q-β-CD | 2.07 ± 0.01 g | 0.35 ± 0.01 g | 596.78 ± 1.45 i |
| GA-β-CD | 1.56 ± 0.01 d | 0.21 ± 0.02 e | 778.56 ± 1.26 m |
| (+)-C-HP-β-CD | 4.01 ± 0.03 j | 0.22 ± 0.01 e | 580.83 ± 1.98 h |
| Q-HP-β-CD | 2.56 ± 0.02 i | 0.20 ± 0.02 d | 657.98 ± 1.18 j |
| GA-HP-β-CD | 1.89 ± 0.01 f | 0.18 ± 0.01 d | 845.74 ± 1.07 o |
| ACTICOA-β-CD(RE) | 1.65 ± 0.01 e | 1.08 ± 0.01 i | 819.72 ± 1.21 n |
| ACTICOA-HP-β-CD(RE) | 2.21 ± 0.02 h | 1.45 ± 0.02 j | 1128.2 ± 1.08 p |
| A-Ch-EACTICOA v.1 | 12.08 ± 0.09 | 9.89 ± 0.03 s | 200.52 ± 1.06 c |
| A-Ch-EACTICOA v.2 | 9.92 ± 0.07 o | 6.67 ± 0.03 p | 180.75 ± 1.09 a |
| A-Ch-(+)-C v.1 | 9.98 ± 0.05 n | 6.75 ± 0.01 r | 729.42 ± 1.11 l |
| A-Ch-(+)-C v.2 | 9.09 ± 0.08 m | 5.60 ± 0.02 o | 459.73 ± 1.05 f |
| A-Ch-Q v.1 | 8.66 ± 0.06 l | 2.25 ± 0.01 l | 337.95 ± 1.01 e |
| A-Ch-Q v.2 | 7.68 ± 0.04 k | 1.87 ± 0.02 k | 675.89 ± 1.12 k |
| A-Ch-GA v.1 | 10.98 ± 0.07 p | 3.38 ± 0.02 n | 206.54 ± 1.07 d |
| A-Ch-GA v.2 | 13.64 ± 0.05 r | 2.80 ± 0.01 m | 186.17 ± 1.04 b |
| Kind of Yogurt/Bioactive Additive | Lactobacillus spp. Log (cfu/mL) ± SD | Streptococcus spp. Log (cfu/mL) ± SD |
|---|---|---|
| CS after fermentation | 7.7 ± 0.43 a | 9.3± 0.07 e |
| CS after 14 days storage | 6.5 ± 0.76 | 8.8± 0.23 * |
| Yogurts with additions after 14 days of storage | ||
| ACTICOA | 5.4 ± 0.33 b | 7.6 ± 0.00 d# |
| EACTICOA | 5.9 ± 0.68 b | 8.0 ± 0.11 d |
| (+)-C | 5.7 ± 0.43 b | 5.8 ± 0.00 d# |
| Q | 7.0 ± 0.66 | 7.4 ± 0.40 d |
| GA | 3.5 ± 1.33 c | 5.2 ± 0.00 d# |
| (+)-C-β-CD | 5.5 ± 0.18 b | 7.5 ± 0.99 |
| Q-β-CD | 6.7 ± 0.57 | 8.1 ± 0.11 d |
| GA-β-CD | 6.9 ± 0.27 | 8.1 ± 0.04 d |
| (+)-C-HP-β-CD | 5.4 ± 0.18 b | 6.5 ± 0.50 d# |
| Q-HP-β-CD | 5.6 ± 0.00 b | 5.7 ± 0.24 d# |
| GA-HP-β-CD | 5.3 ± 0.00 b | 7.8 ± 0.13 d |
| ACTICOA-β-CD(RE) | 5.6 ± 0.60 | 7.5 ± 0.00 d# |
| ACTICOA-HP-β-CD(RE) | 6.3 ± 1.0 | 7.7 ± 0.93 |
| A-Ch-EACTICOA v.1 | 5.7 ± 0.00 b | 8.2 ± 0.04 d |
| A-Ch-EACTICOA v.2 | 5.5 ± 0.28 b | 8.2 ± 0.15 d |
| A-Ch-(+)-C v.1 | 6.0 ± 0.54 b | 8.2 ± 0.43 |
| A-Ch-(+)-C v.2 | 7.2 ± 0.23 | 7.6 ± 1.34 |
| A-Ch-Q v.1 | 7.5 ± 0.04 | 8.7 ± 0.39 |
| A-Ch-Q v.2 | 5.7 ± 0.48 | 8.1 ± 0.36 |
| A-Ch-GA v.1 | 7.0 ± 0.73 | 8.5 ± 0.25 |
| A-Ch-GA v.2 | 6.7 ± 0.11 | 8.3 ± 0.12 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogalska, M.; Oracz, J.; Klewicka, E.; Żyżelewicz, D. Effect of Encapsulated Phenolic Compounds of Cocoa on Growth of Lactic Acid Bacteria and Antioxidant Activity of Fortified Drinking Yogurt. Molecules 2024, 29, 3344. https://doi.org/10.3390/molecules29143344
Rogalska M, Oracz J, Klewicka E, Żyżelewicz D. Effect of Encapsulated Phenolic Compounds of Cocoa on Growth of Lactic Acid Bacteria and Antioxidant Activity of Fortified Drinking Yogurt. Molecules. 2024; 29(14):3344. https://doi.org/10.3390/molecules29143344
Chicago/Turabian StyleRogalska, Milena, Joanna Oracz, Elżbieta Klewicka, and Dorota Żyżelewicz. 2024. "Effect of Encapsulated Phenolic Compounds of Cocoa on Growth of Lactic Acid Bacteria and Antioxidant Activity of Fortified Drinking Yogurt" Molecules 29, no. 14: 3344. https://doi.org/10.3390/molecules29143344
APA StyleRogalska, M., Oracz, J., Klewicka, E., & Żyżelewicz, D. (2024). Effect of Encapsulated Phenolic Compounds of Cocoa on Growth of Lactic Acid Bacteria and Antioxidant Activity of Fortified Drinking Yogurt. Molecules, 29(14), 3344. https://doi.org/10.3390/molecules29143344









