Lipidomic Analysis Reveals Branched-Chain and Cyclic Fatty Acids from Angomonas deanei Grown under Different Nutritional and Physiological Conditions
Abstract
:1. Introduction
2. Results
2.1. GC-MS of Derivatizated Fatty Acids
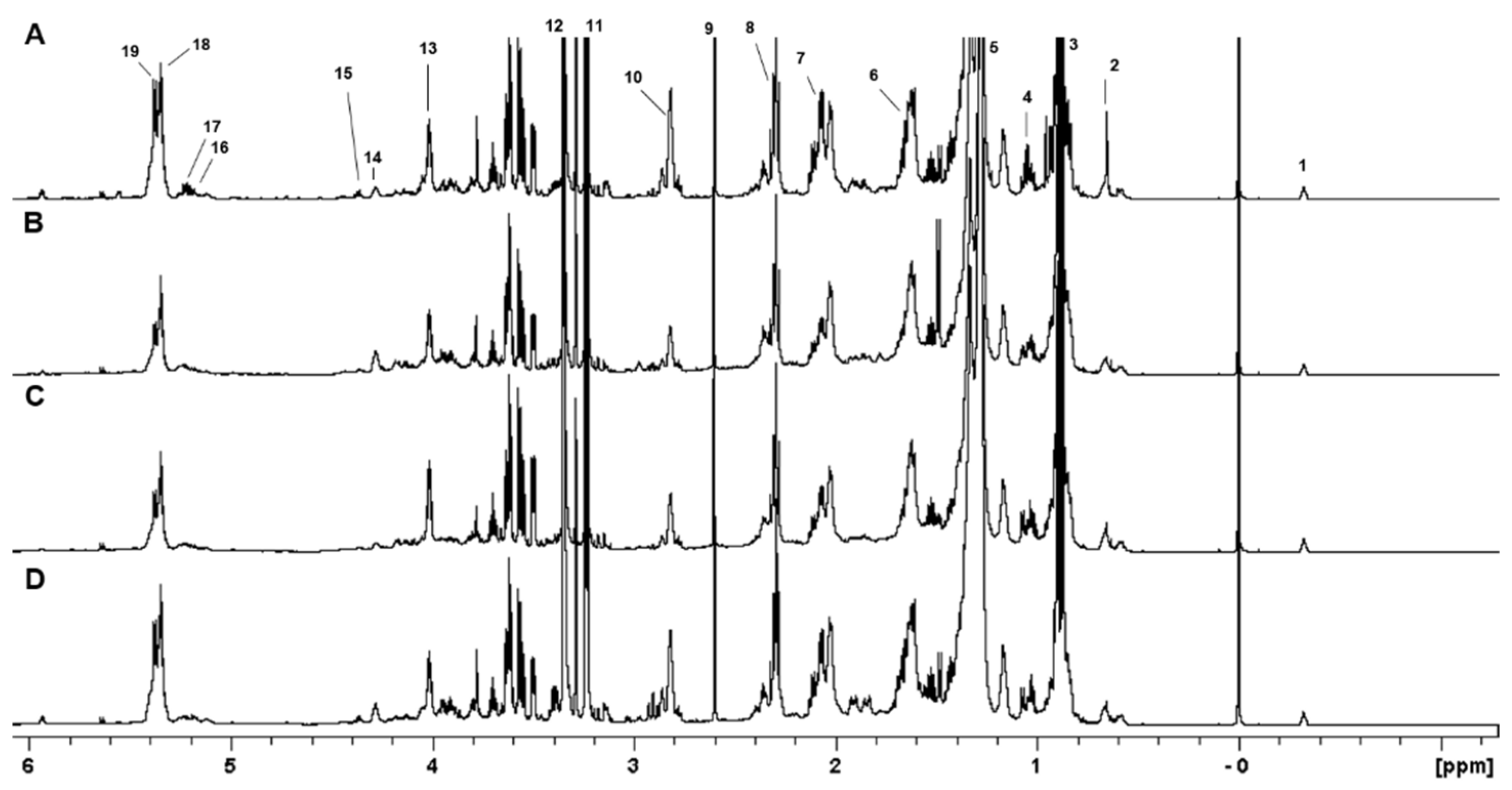
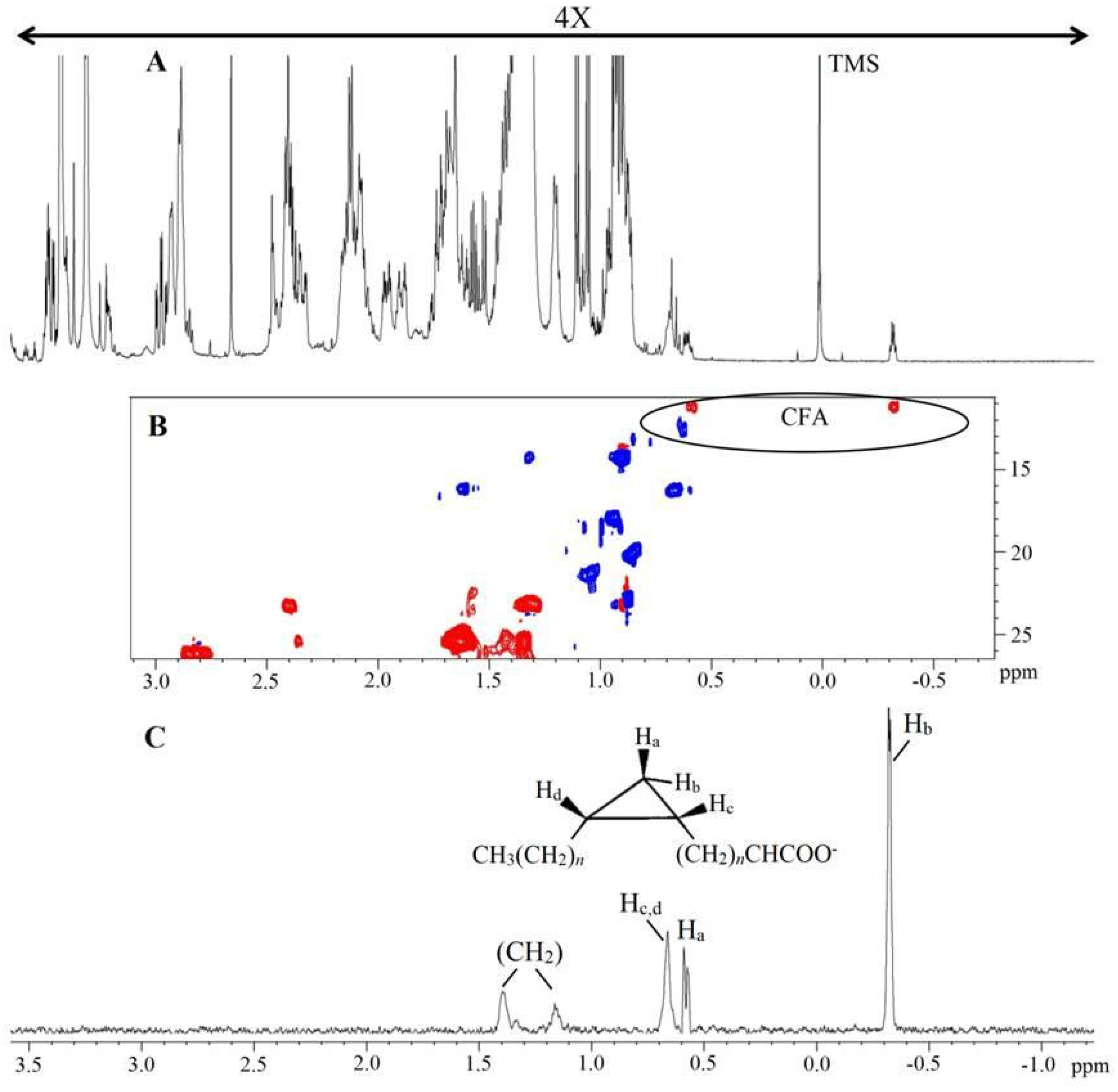
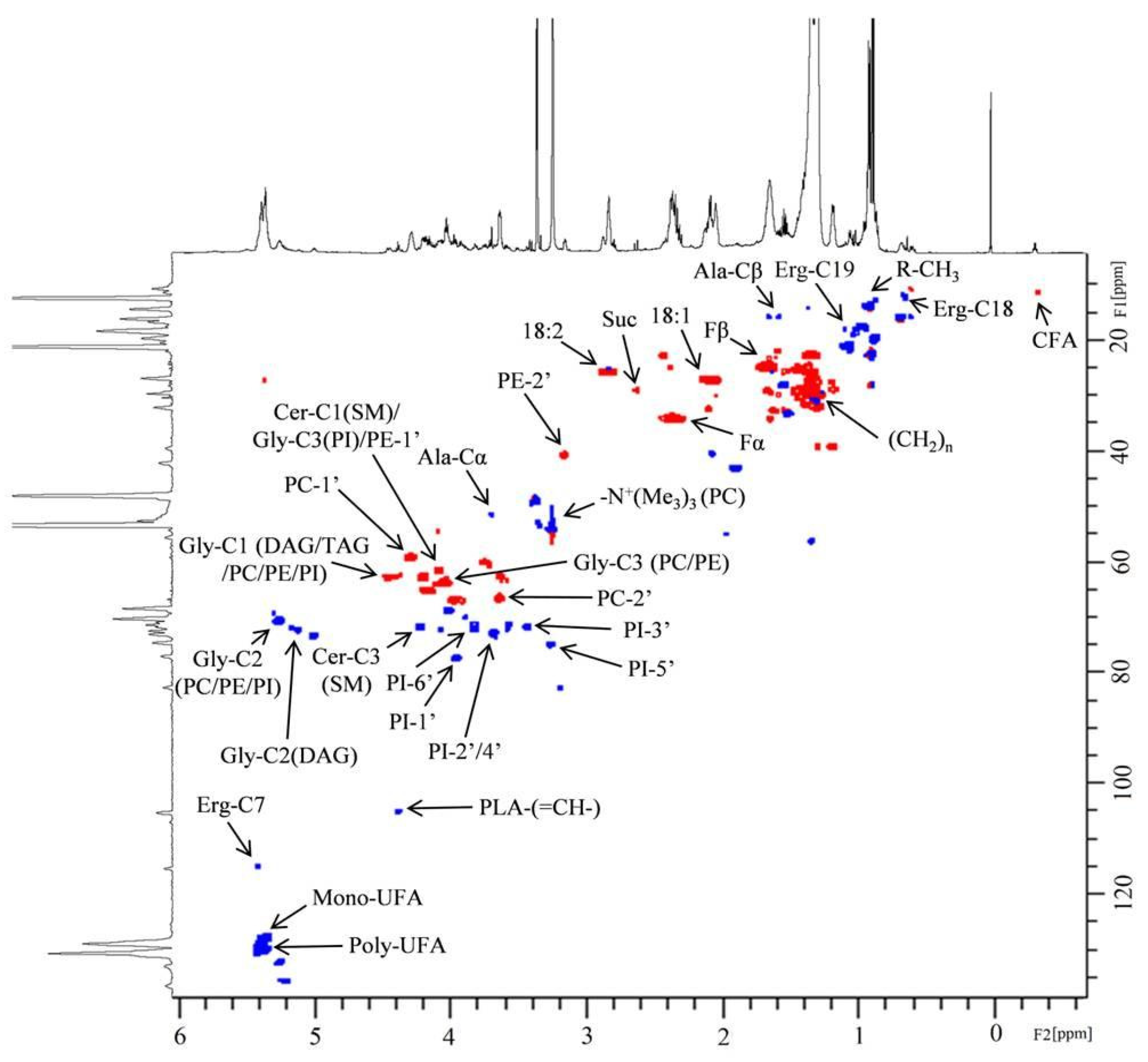
2.2. NMR Analysis of Lipid Extracts
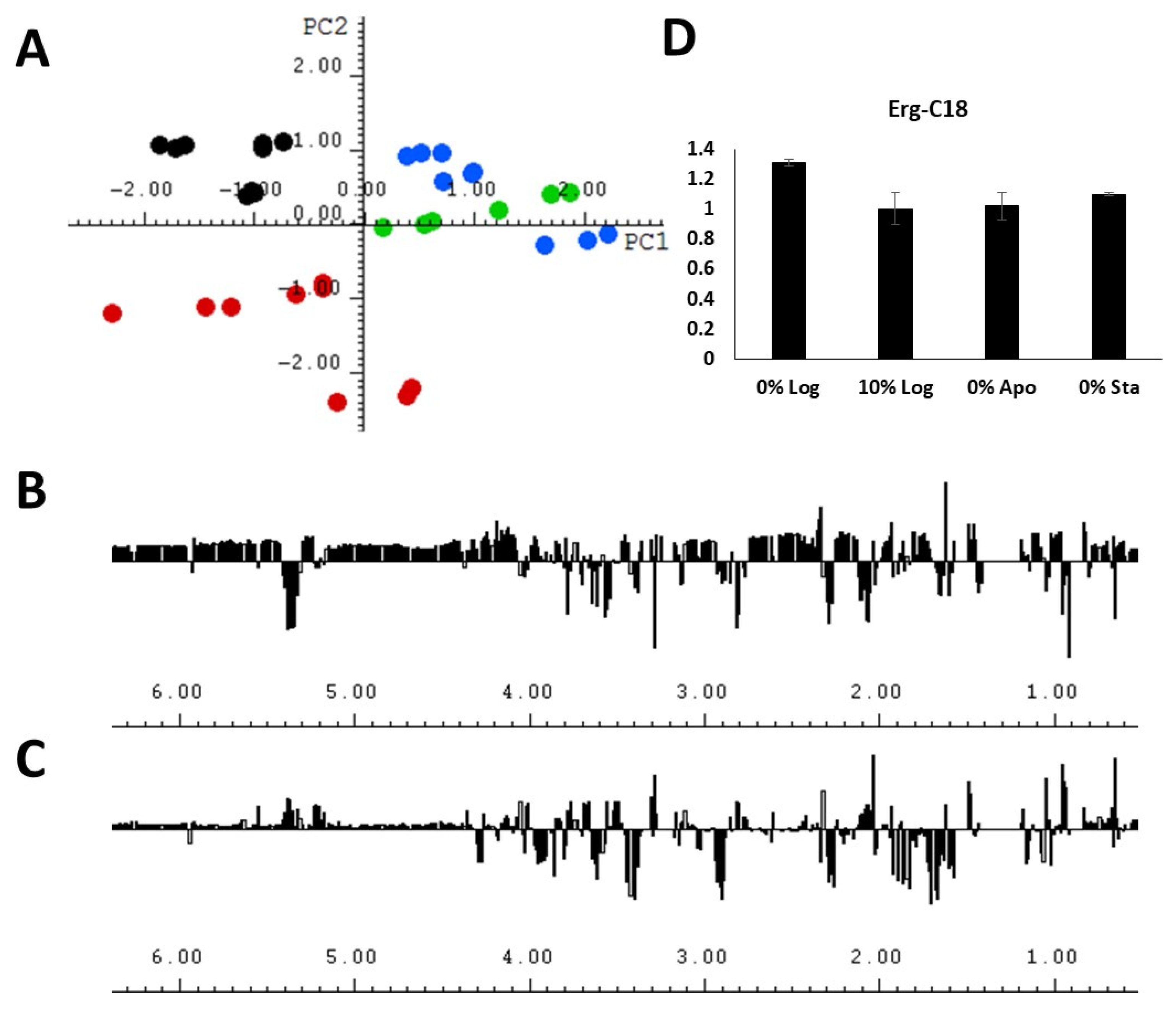
2.3. PCA
2.4. Sterol Analysis
3. Discussion
4. Materials and Methods
4.1. Parasite Culture
4.2. Lipid Extraction
4.3. Nuclear Magnetic Resonance (NMR)
4.4. Principal Component Analysis (PCA) and Data Reduction
4.5. Lipid Derivatizations
4.6. Gas Chromatography Coupled with Mass Spectrometry (GC-MS)
4.7. Cell Microscopy
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motta, M.C.M.; Catta-Preta, C.M.C.; Schenkman, S.; Martins, A.C.d.A.; Miranda, K.; de Souza, W.; Elias, M.C. The Bacterium Endosymbiont of Crithidia deanei Undergoes Coordinated Division with the Host Cell Nucleus. PLoS ONE 2010, 5, e12415. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; Borghesan, T.C.; Ferreira, R.C.; Santos, M.A.; Takata, C.S.; Campaner, M.; Nunes, V.L.; Milder, R.V.; de Souza, W.; Camargo, E.P. Phylogenetic Validation of the Genera Angomonas and Strigomonas of Trypanosomatids Harboring Bacterial Endosymbionts with the Description of New Species of Trypanosomatids and of Proteobacterial Symbionts. Protist 2011, 162, 503–524. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Ehret, G.; Poschmann, G.; Reinicke, T.; Maurya, A.K.; Kröninger, L.; Zanini, D.; Wolters, R.; Kalyanaraman, D.; Krakovka, M.; et al. Host-symbiont interactions in Angomonas deanei include the evolution of a host-derived dynamin ring around the endosymbiont division site. Curr. Biol. 2023, 33, 28–40.e7. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.S.; Catta-Preta, C.M.C.; Repolês, B.; Mottram, J.C.; De Souza, W.; Machado, C.R.; Motta, M.C.M. Importance of Angomonas Deanei KAP4 for KDNA Arrangement, Cell Division and Maintenance of the Host-Bacterium Relationship. Sci. Rep. 2021, 11, 9210. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Hoffmeister, M.; Rotte, C.; Henze, K. An Overview of Endosymbiotic Models for the Origins of Eukaryotes, Their ATP-Producing Organelles (Mitochondria and Hydrogenosomes), and Their Heterotrophic Lifestyle. Biol. Chem. 2001, 382, 1521–1539. [Google Scholar] [CrossRef]
- Du, Y.; McLaughlin, G.; Chang, K.P. 16S Ribosomal DNA Sequence Identities of β-Proteobacterial Endosymbionts in Three Crithidia Species. J. Bacteriol. 1994, 176, 3081–3084. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; A Maslov, D.; Chang, K.P. Monophyletic origin of β-division proteobacterial endosymbionts and their coevolution with insect trypanosomatid protozoa Blastocrithidia culicis and Crithidia spp. Proc. Natl. Acad. Sci. USA 1994, 91, 8437–8441. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.C.M.; Martins, A.C.d.A.; de Souza, S.S.; Catta-Preta, C.M.C.; Silva, R.; Klein, C.C.; de Almeida, L.G.P.; Cunha, O.d.L.; Ciapina, L.P.; Brocchi, M.; et al. Predicting the Proteins of Angomonas deanei, Strigomonas culicis and Their Respective Endosymbionts Reveals New Aspects of the Trypanosomatidae Family. PLoS ONE 2013, 8, e60209. [Google Scholar] [CrossRef]
- Salzman, T.A.; Batlle, A.D.C.; Angluster, J.; de Souza, W. Heme synthesis in Crithidia deanei: Influence of the endosymbiote. Int. J. Biochem. 1985, 17, 1343–1347. [Google Scholar] [CrossRef]
- Alves, J.M.P.; Voegtly, L.; Matveyev, A.V.; Lara, A.M.; da Silva, F.M.; Serrano, M.G.; Buck, G.A.; Teixeira, M.M.G.; Camargo, E.P. Identification and Phylogenetic Analysis of Heme Synthesis Genes in Trypanosomatids and Their Bacterial Endosymbionts. PLoS ONE 2011, 6, e23518. [Google Scholar] [CrossRef]
- Camargo, E.P.; Freymuller, E. Endosymbiont as supplier of ornithine carbamoyltransferase in a trypanosomatid. Nature 1977, 270, 52–53. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.P.; Chang, C.S.; Sassa, S. Heme biosynthesis in bacterium-protozoon symbioses: Enzymic defects in host hemoflagellates and complemental role of their intracellular symbiotes. Proc. Natl. Acad. Sci. USA 1975, 72, 2979–2983. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.C.M.; Leal, L.H.M.; DE Souza, W.; DE Almeida, D.F.; Ferreira, L.C.S. Detection of Penicillin-binding Proteins in the Endosymbiont of the Trypanosomatid Crithidia deanei. J. Eukaryot. Microbiol. 1997, 44, 492–496. [Google Scholar] [CrossRef]
- Palmié-Peixoto, I.V.; Rocha, M.R.; Urbina, J.A.; Souza, W.; Einicker-Lamas, M.; Motta, M.C.M. Effects of sterol biosynthesis inhibitors on endosymbiont-bearing trypanosomatids. FEMS Microbiol. Lett. 2006, 255, 33–42. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo-Martins, A.C.; Frossard, M.L.; de Souza, W.; Einicker-Lamas, M.; Motta, M.C.M. Phosphatidylcholine synthesis in Crithidia deanei: The influence of the endosymbiont. FEMS Microbiol. Lett. 2007, 275, 229–236. [Google Scholar] [CrossRef]
- De Freitas-Junior, P.R.; Catta-Preta, C.M.; Andrade, I.d.S.; Cavalcanti, D.P.; Souza, W.; Einicker-Lamas, M.; Motta, M.C.M. Effects of miltefosine on the proliferation, ultrastructure, and phospholipid composition of Angomonas deanei, a trypanosomatid protozoan that harbors a symbiotic bacterium. FEMS Microbiol. Lett. 2012, 333, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Coen, M.; Lenz, E.M.; Nicholson, J.K.; Wilson, I.D.; Pognan, F.; Lindon, J.C. An Integrated Metabonomic Investigation of Acetaminophen Toxicity in the Mouse Using NMR Spectroscopy. Chem. Res. Toxicol. 2003, 16, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.; Puppa, M.; van der Merwe, M.; Tipirneni-Sajja, A. Rapid and automated lipid profiling by nuclear magnetic resonance spectroscopy using neural networks. NMR Biomed. 2023, 36, e5010. [Google Scholar] [CrossRef] [PubMed]
- Castañé, H.; Baiges-Gaya, G.; Hernández-Aguilera, A.; Rodríguez-Tomàs, E.; Fernández-Arroyo, S.; Herrero, P.; Delpino-Rius, A.; Canela, N.; Menendez, J.A.; Camps, J.; et al. Coupling Machine Learning and Lipidomics as a Tool to Investigate Metabolic Dysfunction-Associated Fatty Liver Disease. A General Overview. Biomolecules 2021, 11, 473. [Google Scholar] [CrossRef]
- Dubois, N.; Barnathan, G.; Gouygou, J.; Bergé, J. Gas chromatographic behavior of fatty acid derivatives for mass spectrometry on low-polarity capillary columns. Eur. J. Lipid Sci. Technol. 2009, 111, 688–697. [Google Scholar] [CrossRef]
- Haken, J. Retention time relationships in the gas chromatography of the methyl esters of branched chain fatty acids. J. Chromatogr. A 1967, 26, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Hauff, S.; Vetter, W. Quantification of Branched Chain Fatty Acids in Polar and Neutral Lipids of Cheese and Fish Samples. J. Agric. Food Chem. 2010, 58, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Ran-Ressler, R.R.; Lawrence, P.; Brenna, J.T. Structural characterization of saturated branched chain fatty acid methyl esters by collisional dissociation of molecular ions generated by electron ionization. J. Lipid Res. 2012, 53, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Oudejans, R.C.H.M.; Van der Horst, D.J.; Van Dongen, J.P.C.M. Isolation and identification of cyclopropane fatty acids from the millipede Graphidostreptus tumuliporus (Myriapoda:Diplopoda). Biochemistry 1971, 10, 4938–4941. [Google Scholar] [CrossRef] [PubMed]
- Fish, W.R.; Holz, G.G.; Beach, D.H.; Owen, E.; Anekwe, G.E. The cyclopropane fatty acid of trypanosomatids. Mol. Biochem. Parasitol. 1981, 3, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.; Reiser, R. Cyclopropane fatty acid metabolism: Physical and chemical identification of propane ring metabolic products in the adipose tissue. J. Am. Oil Chem. Soc. 1965, 42, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Nádvorníková, J.; Pitthard, V.; Kurka, O.; Kučera, L.; Barták, P. Egg vs. Oil in the Cookbook of Plasters: Differentiation of Lipid Binders in Wall Paintings Using Gas Chromatography–Mass Spectrometry and Principal Component Analysis. Molecules 2024, 29, 1520. [Google Scholar] [CrossRef] [PubMed]
- de Santana-Filho, A.P.; Jacomasso, T.; Riter, D.S.; Barison, A.; Iacomini, M.; Winnischofer, S.M.B.; Sassaki, G.L. NMR metabolic fingerprints of murine melanocyte and melanoma cell lines: Application to biomarker discovery. Sci. Rep. 2017, 7, srep42324. [Google Scholar] [CrossRef]
- Benitez, D.; Pezaroglo, H.; Martínez, V.; Casanova, G.; Cabrera, G.; Galanti, N.; González, M.; Cerecetto, H. Study of Trypanosoma cruzi epimastigote cell death by NMR-visible mobile lipid analysis. Parasitology 2012, 139, 506–515. [Google Scholar] [CrossRef]
- Sanchez-Moreno, M.; Fernandez-Becerra, M.; Castilla-Calvente, J.; Osuna, A. Metabolic studies by 1H NMR of different forms of Trypanosoma cruzi as obtained by ‘in vitro’ culture. FEMS Microbiol. Lett. 1995, 133, 119–125. [Google Scholar] [CrossRef]
- Gilroy, F.V.; Edwards, M.R.; Norton, R.S.; O’Sullivan, W.J. Metabolic studies of the protozoan parasite, Crithidia luciliae, using proton nuclear magnetic resonance spectroscopy. Mol. Biochem. Parasitol. 1988, 31, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.d.L.; Buldain, G.; Frydman, B.; Cannata, J.J.B.; Cazzulo, J.J. Carbon-13 Nuclear Magnetic Resonance Analysis of [1-13C] Glucose Metabolism in Crithidia Fasciculata Evidence of CO2 Fixation by Phosphoenolpyruvate Carboxykinase. Eur. J. Biochem. 1985, 149, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Frydman, B.; Santos, C.d.L.; Cannata, J.J.B.; Cazzulo, J.J. Carbon-13 Nuclear Magnetic Resonance Analysis of [1-13C]Glucose Metabolism in Trypanosoma Cruzi Evidence of the Presence of Two Alanine Pools and of Two CO2 Fixation Reactions. Eur. J. Biochem. 1990, 192, 363–368. [Google Scholar] [CrossRef]
- Penin, P.; Sanchez-Moreno, M.; de Diego, J. Proton nuclear magnetic resonance analysis of metabolic end products of the Bolivia strain of Trypanosoma cruzi and three of its clones. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Olson, C.L.; Engman, D.M.; Ames, J.B. NMR structure of the calflagin Tb24 flagellar calcium binding protein of Trypanosoma brucei. Protein Sci. 2012, 21, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Miralles, D.; Marın, C.; Magán, R.; Fernández-Ramos, C.; Entrala, E.; Cordova, O.; Vargas, F.; Sánchez-Moreno, M. In vitro culture and biochemical characterization of six trypanosome isolates from Peru and Brazil. Exp. Parasitol. 2002, 102, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Moreno, B.; Urbina, J.A.; Oldfield, E.; Bailey, B.N.; Rodrigues, C.O.; Docampo, R. 31P NMR Spectroscopy of Trypanosoma Brucei, Trypanosoma Cruzi, and Leishmania Major. Evidence for High Levels of Condensed Inorganic Phosphates. J. Biol. Chem. 2000, 275, 28356–28362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Utzinger, J.; Saric, J.; Li, J.V.; Burckhardt, J.; Dirnhofer, S.; Nicholson, J.K.; Singer, B.H.; Brun, R.; Holmes, E. Global metabolic responses of mice to Trypanosoma brucei brucei infection. Proc. Natl. Acad. Sci. USA 2008, 105, 6127–6132. [Google Scholar] [CrossRef] [PubMed]
- Mundim, M.H.; Roitman, I.; Hermans, M.A.; Kitajima, E.W. Simple Nutrition of Crithidia deanei, a Reduviid Trypanosomatid with an Endosymbiont. J. Protozool. 1974, 21, 518–521. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, G.L.; Wood, D.L.; Cain, G.D. Lipids and carbohydrates in symbiotic and aposymbiotic Crithidia oncopelti and Blastocrithidia culicis. Comp. Biochem. Physiol. Part B Comp. Biochem. 1983, 76, 143–152. [Google Scholar] [CrossRef]
- Bronia, D.H.; Aguerri, A.M.; Bertetto, S.T.; Broña, D.H.; Aguerri, A.M.; Bertetto, A. Trypanosoma cruzi: Changes in lipid composition during aging in culture. Exp. Parasitol. 1986, 61, 151–159. [Google Scholar] [CrossRef]
- Korn, E.D.; Greenblatt, C.L.; Lees, A.M. Synthesis of unsaturated fatty acids in the slime mold Physarum polycephalum and the zooflagellates Leishmania tarentolae, Trypanosoma lewisi, and Crithidia sp.: A comparative study. J. Lipid Res. 1965, 6, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Meyers, H.; Holz, G.G. Biosynthesis of Lipids by Kinetoplastid Flagellates. J. Biol. Chem. 1966, 241, 5000–5007. [Google Scholar] [CrossRef]
- Kaneda, Y.; Nagakura, K.; Goutsu, T. Lipid composition of three morphological stages of Trypanosoma cruzi. Comp. Biochem. Physiol. Part B Comp. Biochem. 1986, 83, 533–536. [Google Scholar] [CrossRef]
- Kaneda, T. Iso-and Anteiso-Fatty Acids in Bacteria: Biosynthesis, Function, and Taxonomic Significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar] [CrossRef]
- Mansilla, M.C.; Cybulski, L.E.; Albanesi, D.; De Mendoza, D. Control of Membrane Lipid Fluidity by Molecular Thermosensors Temperature-Dependent Changes in the Content of UFAS of Glycerophospholipids In E. coli. J. Bacteriol. 2004, 186, 6681–6688. [Google Scholar] [CrossRef] [PubMed]
- Giotis, E.S.; McDowell, D.A.; Blair, I.S.; Wilkinson, B.J. Role of Branched-Chain Fatty Acids in pH Stress Tolerance in Listeria monocytogenes. Appl. Environ. Microbiol. 2007, 73, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Kniazeva, M.; Crawford, Q.T.; Seiber, M.; Wang, C.-Y.; Han, M. Monomethyl Branched-Chain Fatty Acids Play an Essential Role in Caenorhabditis elegans Development. PLoS Biol. 2004, 2, e257. [Google Scholar] [CrossRef]
- Holz, G.G.; Beach, D.H.; Singh, B.N.; Fish, W.R. Biosynthesis of the novel fatty acid, 17-methyl-cis-9,10-methyleneoctadecanoic acid, by the parasitic protozoan, Herpetomonas megaseliae. Lipids 1983, 18, 607–610. [Google Scholar] [CrossRef]
- Muñoz-Rojas, J.; Bernal, P.; Duque, E.; Godoy, P.; Segura, A.; Ramos, J.-L. Involvement of Cyclopropane Fatty Acids in the Response of Pseudomonas putida KT2440 to Freeze-Drying. Appl. Environ. Microbiol. 2006, 72, 472–477. [Google Scholar] [CrossRef]
- Macdonald, P.M.; Sykes, B.D.; McElhaney, R.N. Fluorine-19 nuclear magnetic resonance studies of lipid fatty acyl chain order and dynamics in Acholeplasma laidlawii B membranes. A direct comparison of the effects of cis- and trans-cyclopropane ring and double-bond substituents on orientational order. Biochemistry 1985, 24, 4651–4659. [Google Scholar] [CrossRef] [PubMed]
- Taylor, F.; E Cronan, J. Selection and properties of Escherichia coli mutants defective in the synthesis of cyclopropane fatty acids. J. Bacteriol. 1976, 125, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Korn, E.D.; Von Brand, T.; Tobie, E.J. The sterols of Trypanosoma cruzi and Crithidia fasciculata. Comp. Biochem. Physiol. 1969, 30, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.G.; Nakayasu, E.S.; Sant’Anna, C.; De Cicco, N.N.T.; Atella, G.C.; de Souza, W.; Almeida, I.C.; Cunha-E-Silva, N. Trypanosoma cruzi Epimastigotes Are Able to Store and Mobilize High Amounts of Cholesterol in Reservosome Lipid Inclusions. PLoS ONE 2011, 6, e22359. [Google Scholar] [CrossRef] [PubMed]
- Dufourc, E.J. Sterols and membrane dynamics. J. Chem. Biol. 2008, 1, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Camargo, E.P. Growth and Differentiation in Trypanosoma cruzi. I. Origin of Metacyclic Trypanosomes in Liquid Media. Rev. Inst. Med. Trop. Sao Paulo 1964, 6, 93–100. [Google Scholar] [PubMed]
- Cabrini, L.; Landi, L.; Stefanelli, C.; Barzanti, V.; Maria, S.A. Extraction of lipids and lipophilic antioxidants from fish tissues: A comparison among different methods. Comp. Biochem. Physiol. Part B Comp. Biochem. 1992, 101, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Liu, L.-Y.; Yang, M.-H.; Lee, M.-H. Ethyl Acetate/Ethyl Alcohol Mixtures as an Alternative to Folch Reagent for Extracting Animal Lipids. J. Agric. Food Chem. 2004, 52, 4984–4986. [Google Scholar] [CrossRef] [PubMed]
- Sassaki, G.L.; Riter, D.S.; Filho, A.P.S.; Guerrini, M.; Lima, M.A.; Cosentino, C.; Souza, L.M.; Cipriani, T.R.; Rudd, T.R.; Nader, H.B.; et al. A robust method to quantify low molecular weight contaminants in heparin: Detection of tris(2-n-butoxyethyl) phosphate. Analyst 2011, 136, 2330–2338. [Google Scholar] [CrossRef]
- Doyle, P.S.; Chen, C.-K.; Johnston, J.B.; Hopkins, S.D.; Leung, S.S.F.; Jacobson, M.P.; Engel, J.C.; McKerrow, J.H.; Podust, L.M. A Nonazole CYP51 Inhibitor Cures Chagas’ Disease in a Mouse Model of Acute Infection. Antimicrob. Agents Chemother. 2010, 54, 2480–2488. [Google Scholar] [CrossRef]
- Sassaki, G.L.; Souza, L.M.; Serrato, R.V.; Cipriani, T.R.; Gorin, P.A.; Iacomini, M. Application of acetate derivatives for gas chromatography–mass spectrometry: Novel approaches on carbohydrates, lipids and amino acids analysis. J. Chromatogr. A 2008, 1208, 215–222. [Google Scholar] [CrossRef]
| Fatty Acid Class | 0% Log | 10% Log | 0% Apo | 0% Sta |
|---|---|---|---|---|
| SFA | 31.67 | 31.54 | 31.86 | 30.12 |
| UFA | 68.33 | 68.46 | 68.14 | 69.88 |
| MUFA | 31.07 | 35.16 | 34.40 | 30.90 |
| PUFA | 28.69 | 25.65 | 25.64 | 30.26 |
| BFA | 11.30 | 12.82 | 11.69 | 12.39 |
| CFA | 6.53 | 7.07 | 7.72 | 8.67 |
| UFA/SFA | 2.16 | 2.17 | 2.14 | 2.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana-Filho, A.P.; Pereira, A.J.; Laibida, L.A.; Souza-Melo, N.; DaRocha, W.D.; Sassaki, G.L. Lipidomic Analysis Reveals Branched-Chain and Cyclic Fatty Acids from Angomonas deanei Grown under Different Nutritional and Physiological Conditions. Molecules 2024, 29, 3352. https://doi.org/10.3390/molecules29143352
Santana-Filho AP, Pereira AJ, Laibida LA, Souza-Melo N, DaRocha WD, Sassaki GL. Lipidomic Analysis Reveals Branched-Chain and Cyclic Fatty Acids from Angomonas deanei Grown under Different Nutritional and Physiological Conditions. Molecules. 2024; 29(14):3352. https://doi.org/10.3390/molecules29143352
Chicago/Turabian StyleSantana-Filho, Arquimedes Paixão, Aramís José Pereira, Letícia Adejani Laibida, Normanda Souza-Melo, Wanderson Duarte DaRocha, and Guilherme Lanzi Sassaki. 2024. "Lipidomic Analysis Reveals Branched-Chain and Cyclic Fatty Acids from Angomonas deanei Grown under Different Nutritional and Physiological Conditions" Molecules 29, no. 14: 3352. https://doi.org/10.3390/molecules29143352
APA StyleSantana-Filho, A. P., Pereira, A. J., Laibida, L. A., Souza-Melo, N., DaRocha, W. D., & Sassaki, G. L. (2024). Lipidomic Analysis Reveals Branched-Chain and Cyclic Fatty Acids from Angomonas deanei Grown under Different Nutritional and Physiological Conditions. Molecules, 29(14), 3352. https://doi.org/10.3390/molecules29143352









