Characterization of Low-Molecular-Weight Dissolved Organic Matter Using Optional Dialysis and Orbitrap Mass Spectrometry
Abstract
1. Introduction
2. Results
2.1. DOC Concentrations and UV−Vis Characteristics of the Retentate or Dialysate
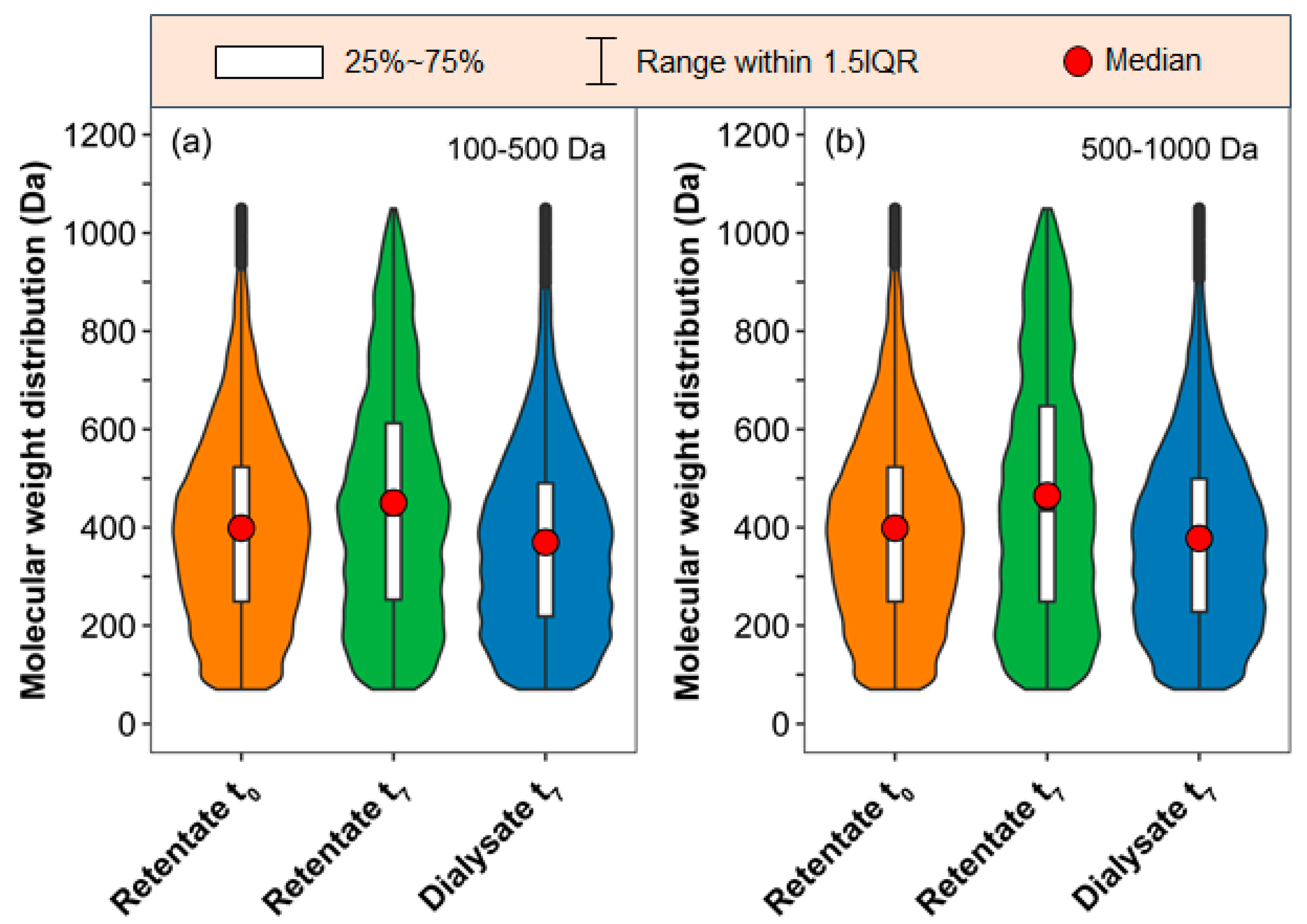
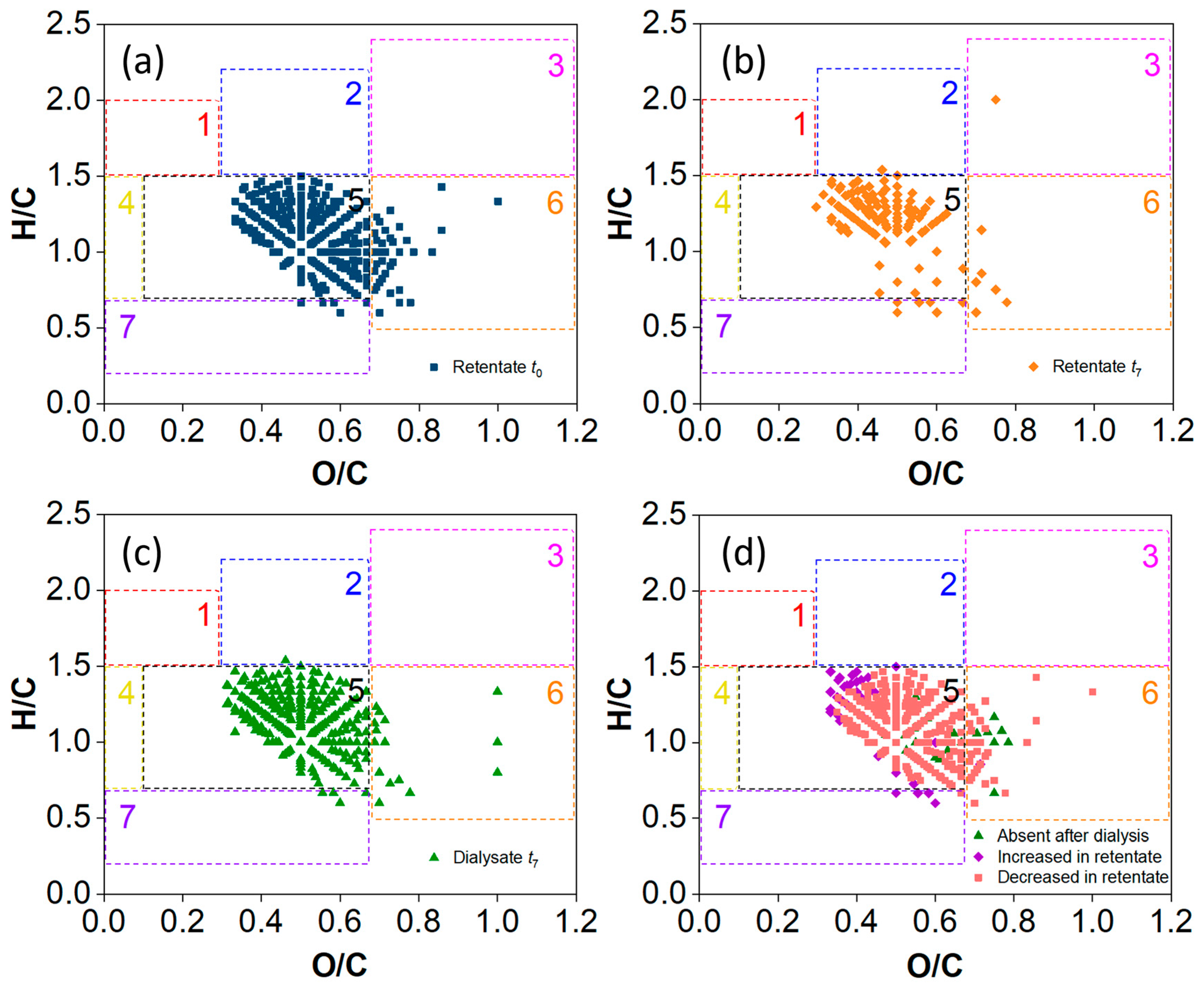
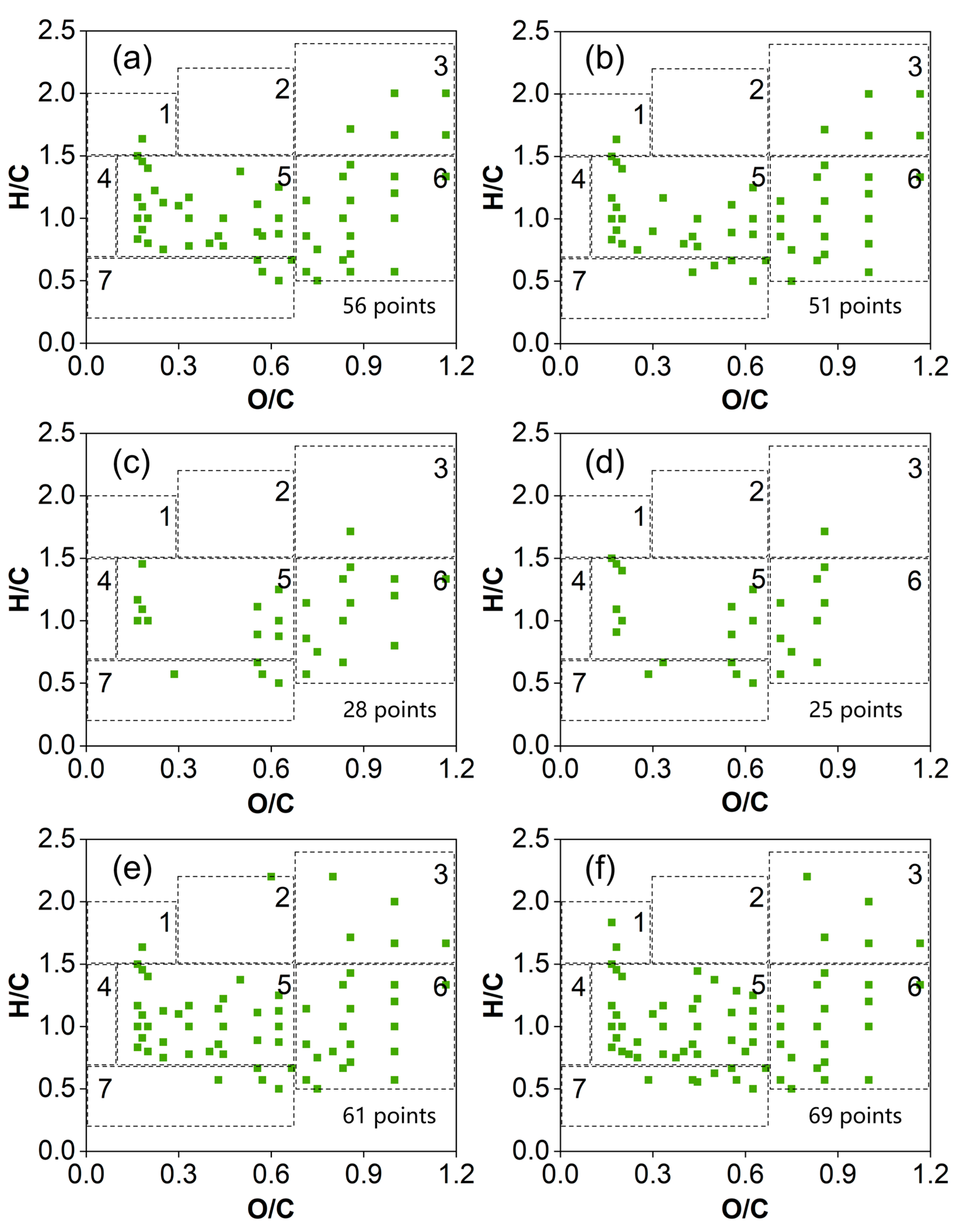
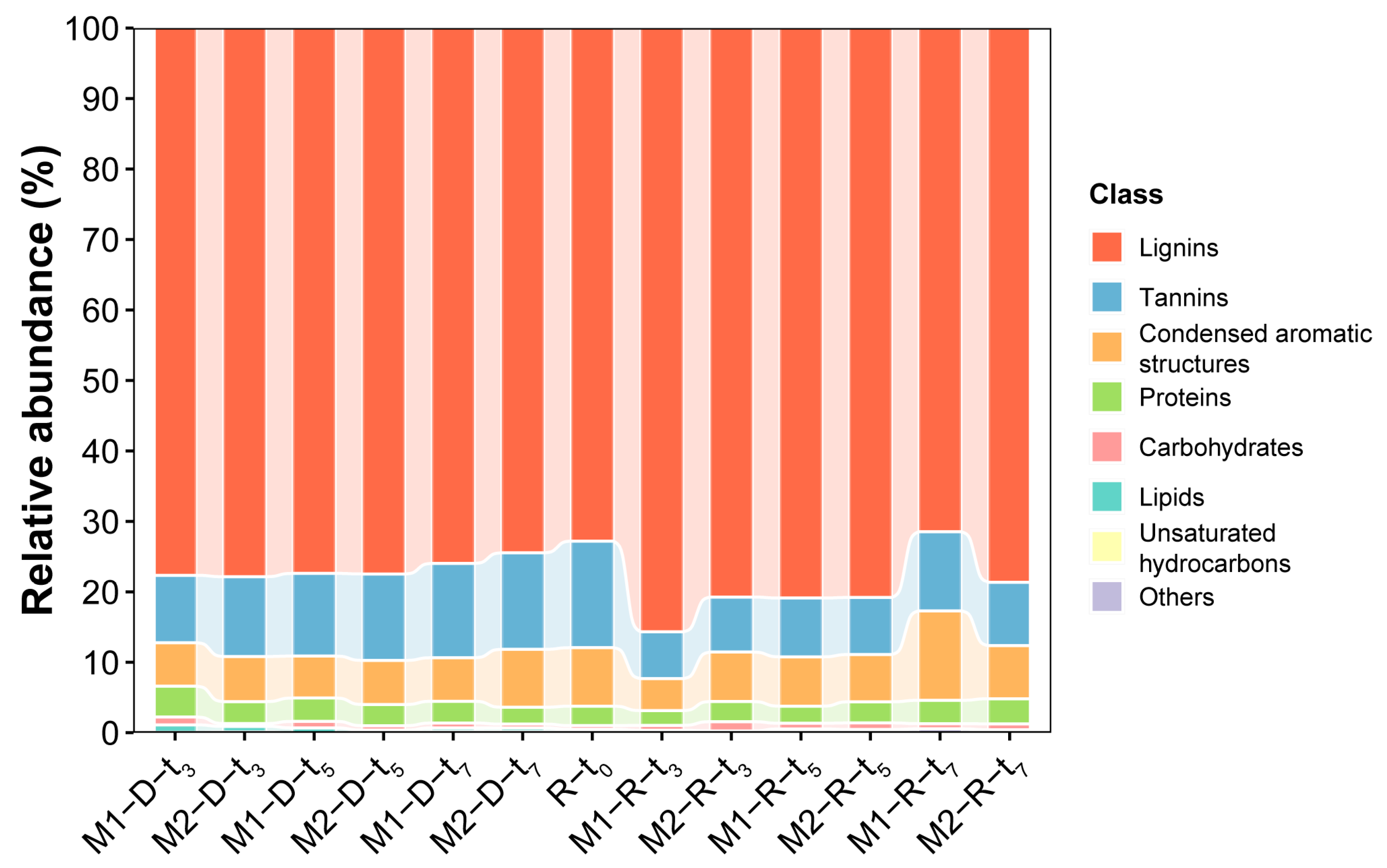
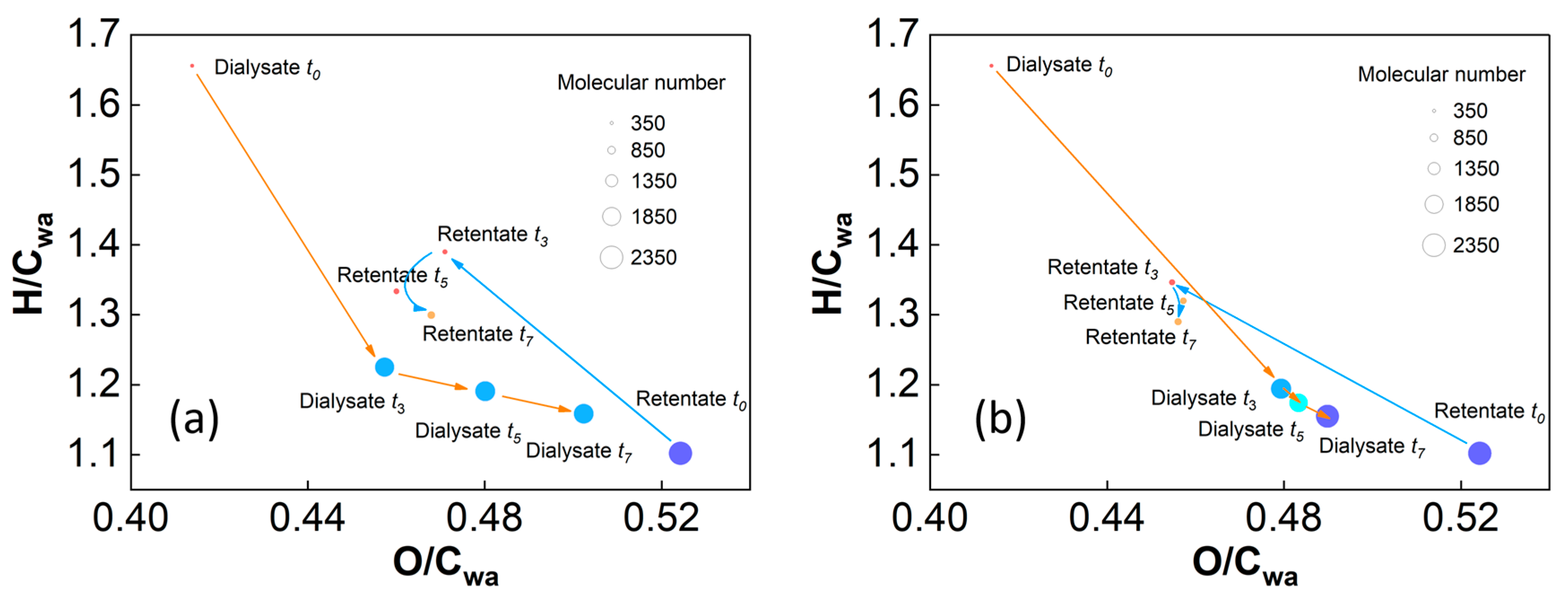
2.2. MS and Van Krevelen Plots of the SRFA Retentate and Dialysate
3. Discussion
3.1. Variations in the DOC Concentrations and Optical Parameters of the UV–Vis Spectra in the Retentate and Dialysate
3.2. Comparison of the Mass Spectral Characteristics of the SRFA Retentate and Dialysate
3.3. Characterization of LMW DOMs by Combining Membrane Dialysis and Orbitrap MS Analysis
4. Materials and Methods
4.1. Samples and Reagents
4.2. Membrane Dialysis
4.3. Determination of Dissolved Organic Carbon (DOC)
4.4. Ultraviolet−Visible Absorption Spectra
4.5. Mass Spectrometry Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, Z.; Shang, Y.; Song, K.; Liu, G.; Hou, J.; Lyu, L.; Tao, H.; Li, S.; He, C.; Shi, Q.; et al. Composition of dissolved organic matter (DOM) in lakes responds to the trophic state and phytoplankton community succession. Water Res. 2022, 224, 119073. [Google Scholar] [CrossRef]
- Kellerman, A.M.; Dittmar, T.; Kothawala, D.N.; Tranvik, L.J. Chemodiversity of dissolved organic matter in lakes driven by climate and hydrology. Nat. Commun. 2014, 5, 3804. [Google Scholar] [CrossRef] [PubMed]
- Brailsford, F.L.; Glanville, H.C.; Marshall, M.R.; Yates, C.A.; Owen, A.T.; Golyshin, P.N.; Johnes, P.J.; Jones, D.L. Land cover and nutrient enrichment regulates low-molecular weight dissolved organic matter turnover in freshwater ecosystems. Limnol. Oceanogr. 2021, 66, 2979–2987. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Huang, X.; Liu, Y.; Guan, M.; Tian, Y. Hydrological conditions can change the effects of major nutrients and dissolved organic matter on phytoplankton community dynamics in a eutrophic river. J. Hydrol. 2024, 628, 130503. [Google Scholar] [CrossRef]
- Shang, Y.; Wen, Z.; Song, K.; Liu, G.; Lai, F.; Lyu, L.; Li, S.; Tao, H.; Hou, J.; Fang, C.; et al. Natural versus anthropogenic controls on the dissolved organic matter chemistry in lakes across China: Insights from optical and molecular level analyses. Water Res. 2022, 221, 118779. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Wen, Z.; Shang, Y.; Yang, H.; Lyu, L.; Liu, G.; Fang, C.; Du, J.; Zhao, Y. Quantification of dissolved organic carbon (DOC) storage in lakes and reservoirs of mainland China. J. Environ. Manag. 2018, 217, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Brailsford, F.; Glanville, H.; Marshall, M.; Golyshin, P.; Johnes, P.; Yates, C.A.; Owen, A.; Jones, D.L. Microbial use of low molecular weight DOM in filtered and unfiltered freshwater: Role of ultra-small microorganisms and implications for water quality monitoring. Sci. Total Environ. 2017, 598, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Jang, K.-S.; Tanentzap, A.J.; Zhao, W.; Lennon, J.T.; Liu, J.; Li, M.; Stegen, J.; Choi, M.; Lu, Y.; et al. Thermal responses of dissolved organic matter under global change. Nat. Commun. 2024, 15, 576. [Google Scholar] [CrossRef] [PubMed]
- Tipping, E.; Boyle, J.F.; Schillereff, D.N.; Spears, B.M.; Phillips, G. Macronutrient processing by temperate lakes: A dynamic model for long-term, large-scale application. Sci. Total Environ. 2016, 572, 1573–1585. [Google Scholar] [CrossRef]
- Jia, L.; Yang, Q.; Cui, H. Insight into the dynamics of dissolved organic matter components under latitude change perturbation. Ecotoxicol. Environ. Saf. 2024, 269, 115734. [Google Scholar] [CrossRef]
- Remucal, C.K.; Cory, R.M.; Sander, M.; McNeill, K. Low Molecular Weight Components in an Aquatic Humic Substance As Characterized by Membrane Dialysis and Orbitrap Mass Spectrometry. Environ. Sci. Technol. 2012, 46, 9350–9359. [Google Scholar] [CrossRef] [PubMed]
- Belzile, C.; Guo, L. Optical properties of low molecular weight and colloidal organic matter: Application of the ultrafiltration permeation model to DOM absorption and fluorescence. Mar. Chem. 2006, 98, 183–196. [Google Scholar] [CrossRef]
- Cui, X.; Choo, K.H. Granular iron oxide adsorbents to control natural organic matter and membrane fouling in ultrafiltration water treatment. Water Res. 2013, 47, 4227–4237. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, M.; Li, T.; Dumbrell, A.J.; Saleem, M.; Kuang, L.; Luan, L.; Wang, S.; Li, Z.; Jiang, J. Molecular weight of dissolved organic matter determines its interactions with microbes and its assembly processes in soils. Soil Biol. Biochem. 2023, 184, 109117. [Google Scholar] [CrossRef]
- Guo, L.; Hunt, B.J.; Santschi, P.H.; Ray, S.M. Effect of dissolved organic matter on the uptake of trace metals by American oysters. Environ. Sci. Technol. 2001, 35, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.C.; Li, X.M.; Guo, M.J.; Li, F.F.; Yang, K.L.; Liu, X. Dissolved organic matters with low molecular weight fractions exhibit high photochemical potential for reactive oxygen formation. Chemosphere 2022, 305, 135542. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.; Sposito, G. Molecular structure in soil humic substances: The new view. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q. The hydrophobic effects: Our current understanding. Molecules 2022, 27, 7009. [Google Scholar] [CrossRef]
- Bowen, J.C.; Kaplan, L.A.; Cory, R.M. Photodegradation disproportionately impacts biodegradation of semi-labile DOM in streams. Limnol. Oceanogr. 2020, 65, 13–26. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Shi, Q.; Ren, S.; Yu, J.; Ji, F.; Luo, W.; Yang, M. Characterization of low molecular weight dissolved natural organic matter along the treatment trait of a waterworks using Fourier transform ion cyclotron resonance mass spectrometry. Water Res. 2012, 46, 5197–5204. [Google Scholar] [CrossRef]
- Oleinikova, O.V.; Drozdova, O.Y.; Lapitskiy, S.A.; Demin, V.V.; Bychkov, A.Y.; Pokrovsky, O.S. Dissolved organic matter degradation by sunlight coagulates organo-mineral colloids and produces low-molecular weight fraction of metals in boreal humic waters. Geochim. Et Cosmochim. Acta 2017, 211, 97–114. [Google Scholar] [CrossRef]
- Wünsch, U.J.; Stedmon, C.A.; Tranvik, L.J.; Guillemette, F. Unraveling the size-dependent optical properties of dissolved organic matter. Limnol. Oceanogr. 2018, 63, 588–601. [Google Scholar] [CrossRef]
- Xu, H.C.; Guo, L.D. Molecular size-dependent abundance and composition of dissolved organic matter in river, lake and sea waters. Water Res. 2017, 117, 115–126. [Google Scholar] [CrossRef]
- Cortés-Francisco, N.; Caixach, J. Molecular characterization of dissolved organic matter through a desalination process by high resolution mass spectrometry. Environ. Sci. Technol. 2013, 47, 9619–9627. [Google Scholar] [CrossRef]
- Hawkes, J.A.; Dittmar, T.; Patriarca, C.; Tranvik, L.; Bergquist, J. Evaluation of the Orbitrap mass spectrometer for the molecular fingerprinting analysis of natural dissolved organic matter. Anal. Chem. 2016, 88, 7698–7704. [Google Scholar] [CrossRef]
- Mangal, V.; Stock, N.L.; Guéguen, C. Molecular characterization of phytoplankton dissolved organic matter (DOM) and sulfur components using high resolution Orbitrap mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cooper, W.J.; Jung, J.; Song, W. Photosensitized degradation of amoxicillin in natural organic matter isolate solutions. Water Res. 2011, 45, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Driver, S.J.; Perdue, E.M. Acidic functional groups of Suwannee River natural organic matter, humic acids, and fulvic acids. In Advances in the Physicochemical Characterization of Dissolved Organic Matter: Impact on Natural and Engineered Systems; ACS Publications: Washington, DC, USA, 2014; Volume 1160, pp. 75–86. [Google Scholar]
- He, Z.; Ohno, T.; Wu, F.; Olk, D.C.; Honeycutt, C.W.; Olanya, M. Capillary Electrophoresis and Fluorescence Excitation-Emission Matrix Spectroscopy for Characterization of Humic Substances. Soil Sci. Soc. Am. J. 2008, 72, 1248–1255. [Google Scholar] [CrossRef]
- Ghabbour, E.A.; Davies, G. Spectrophotometric analysis of fulvic acid solutions—A second look. Ann. Environ. Sci. 2009, 3, 131–138. [Google Scholar]
- Guéguen, C.; Cuss, C.W. Characterization of aquatic dissolved organic matter by asymmetrical flow field-flow fractionation coupled to UV–Visible diode array and excitation emission matrix fluorescence. J. Chromatogr. A 2011, 1218, 4188–4198. [Google Scholar] [CrossRef]
- Nwosu, U.G.; Cook, R.L. 13C nuclear magnetic resonance and electron paramagnetic spectroscopic comparison of hydrophobic acid, Transphilic acid, and reverse osmosis may 2012 isolates of organic matter from the Suwannee River. Environ. Eng. Sci. 2015, 32, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.M.; Barbier, P.F. Oxidation kinetics of natural organic matter by sonolysis and ozone. Water Res. 1994, 28, 1383–1391. [Google Scholar] [CrossRef]
- Xu, B.; Li, D.-P.; Li, W.; Xia, S.-J.; Lin, Y.-L.; Hu, C.-Y.; Zhang, C.-J.; Gao, N.-Y. Measurements of dissolved organic nitrogen (DON) in water samples with nanofiltration pretreatment. Water Res. 2010, 44, 5376–5384. [Google Scholar] [CrossRef]
- Lee, W.; Westerhoff, P. Dissolved organic nitrogen measurement using dialysis pretreatment. Environ. Sci. Technol. 2005, 39, 879–884. [Google Scholar] [CrossRef]
- Wang, Q.; Hua, B.; Yang, J.; Liu, F.; Zhu, G.; Deng, B. Dialysis pretreatment for dissolved organic nitrogen analysis in freshwaters. J. Chem. 2015, 2015, 629794. [Google Scholar] [CrossRef][Green Version]
- Jing, W.; Runyu, Z.; Liying, W.; Zhi, Z.; Haijun, Y. Multidimensional features and sources apportionment of dissolved organic matter from Endmember Samples in the Karst Area. Acta Sci. Circumstantiae 2022, 42, 425–437. [Google Scholar]
- Li, P.; Hur, J. Utilization of UV-Vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 131–154. [Google Scholar] [CrossRef]
- Lee, M.-H.; Osburn, C.L.; Shin, K.-H.; Hur, J. New insight into the applicability of spectroscopic indices for dissolved organic matter (DOM) source discrimination in aquatic systems affected by biogeochemical processes. Water Res. 2018, 147, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-P.; Aiken, G.; O’Loughlin, E. Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ. Sci. Technol. 1994, 28, 1853–1858. [Google Scholar] [CrossRef]
- Chen, Y.; Senesi, N.; Schnitzer, M. Information provided on humic substances by E4/E6 ratios. Soil Sci. Soc. Am. J. 1977, 41, 352–358. [Google Scholar] [CrossRef]
- Hawkes, J.A.; d’Andrilli, J.; Agar, J.N.; Barrow, M.P.; Berg, S.M.; Catalán, N.; Chen, H.; Chu, R.K.; Cole, R.B.; Dittmar, T. An international laboratory comparison of dissolved organic matter composition by high resolution mass spectrometry: Are we getting the same answer? Limnol. Oceanogr. Methods 2020, 18, 235–258. [Google Scholar] [CrossRef]
- Pan, Q.; Zhuo, X.; He, C.; Zhang, Y.; Shi, Q. Validation and evaluation of high-resolution orbitrap mass spectrometry on molecular characterization of dissolved organic matter. ACS Omega 2020, 5, 5372–5379. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Wang, Q.; Huang, Q.; Fu, Q.; Liu, Y.; Wang, J.; Hu, S.; Mašek, O.; Wang, L.; Zhang, J. Effect of pyrolysis temperature on the characterisation of dissolved organic matter from pyroligneous acid. Molecules 2021, 26, 3416. [Google Scholar] [CrossRef] [PubMed]
- Wan Mohd Zamri, W.M.I.; Sjahrir, F.; Yaacob, N.S.; Dzulkafli, N.F.; Ahmad, M.F.; Abdullah, H.; Maniyam, M.N.; Hashim, E.F.; Kawasaki, N.; Komatsu, K.; et al. Assessment of Aqueous Extraction Methods on Extractable Organic Matter and Hydrophobic/Hydrophilic Fractions of Virgin Forest Soils. Molecules 2021, 26, 2480. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Xu, L.; Li, G.; Gao, X.; Ma, H. Characterization of Dissolved Organic Matter Released from Aged Biochar: A Comparative Study of Two Feedstocks and Multiple Aging Approaches. Molecules 2023, 28, 4558. [Google Scholar] [CrossRef] [PubMed]
- Altare, N.; Vione, D. Photochemical Implications of Changes in the Spectral Properties of Chromophoric Dissolved Organic Matter: A Model Assessment for Surface Waters. Molecules 2023, 28, 2664. [Google Scholar] [CrossRef]
- Ge, J.; Qi, Y.; Yao, W.; Yuan, D.; Hu, Q.; Ma, C.; Volmer, D.A.; Liu, C.-Q. Identification of Trace Components in Sauce-Flavor Baijiu by High-Resolution Mass Spectrometry. Molecules 2023, 28, 1273. [Google Scholar] [CrossRef]
- Yuthawong, V.; Kasuga, I.; Kurisu, F.; Furumai, H. Molecular-level changes in dissolved organic matter compositions in Lake Inba water during KMnO4 oxidation: Assessment by Orbitrap mass spectrometry. J. Water Environ. Technol. 2019, 17, 27–39. [Google Scholar] [CrossRef]
- Cortés-Francisco, N.; Caixach, J. Fragmentation studies for the structural characterization of marine dissolved organic matter. Anal. Bioanal. Chem. 2015, 407, 2455–2462. [Google Scholar] [CrossRef]
- Kerner, M.; Hohenberg, H.; Ertl, S.; Reckermann, M.; Spitzy, A. Self-organization of dissolved organic matter to micelle-like microparticles in river water. Nature 2003, 422, 150–154. [Google Scholar] [CrossRef]
- He, Z.; Ohno, T.; Cade-Menun, B.J.; Erich, M.S.; Honeycutt, C.W. Spectral and chemical characterization of phosphates associated with humic substances. Soil Sci. Soc. Am. J. 2006, 70, 1741–1751. [Google Scholar] [CrossRef]
- He, Z.; Liu, S.; Nam, S.; Klasson, K.T.; Cheng, H.N. Molecular level characterization of the effect of roasting on the extractable components of glandless cottonseed by Fourier transform ion cyclotron resonance mass spectrometry. Food Chem. 2023, 403, 134404. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Muller-Karger, F.E.; Zepp, R.G. Absorbance, absorption coefficient, and apparent quantum yield: A comment on common ambiguity in the use of these optical concepts. Limnol. Oceanogr. 2002, 47, 1261–1267. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef]
- Lee, Y.K.; Hong, S.; Hur, J. A fluorescence indicator for source discrimination between microplastic-derived dissolved organic matter and aquatic natural organic matter. Water Res. 2021, 207, 117833. [Google Scholar] [CrossRef]
- Liying, W.; Fengchang, W.; Zhang, R.; Wen, L.; Haiqing, L. Characterization of dissolved organic matter fractions from Lake Hongfeng, Southwestern China Plateau. J. Environ. Sci. 2009, 21, 581–588. [Google Scholar]
- Merder, J.; Freund, J.A.; Feudel, U.; Hansen, C.T.; Hawkes, J.A.; Jacob, B.; Klaproth, K.; Niggemann, J.; Noriega-Ortega, B.E.; Osterholz, H.; et al. ICBM-OCEAN: Processing ultrahigh-resolution mass spectrometry data of complex molecular mixtures. Anal. Chem. 2020, 92, 6832–6838. [Google Scholar] [CrossRef]
- Guo, M.; Li, X.; Wang, Y.; Zhang, Y.; Fu, Q.; Huguet, A.; Liu, G. New insights into the mechanism of phosphate release during particulate organic matter photodegradation based on optical and molecular signatures. Water Res. 2023, 236, 119954. [Google Scholar] [CrossRef]
- Yuthawong, V.; Kasuga, I.; Kurisu, F.; Furumai, H. Comparison of Low Molecular Weight Dissolved Organic Matter Compositions in Lake Inba and Kashima River by Orbitrap Mass Spectrometry. J. Water Environ. Technol. 2017, 15, 12–21. [Google Scholar] [CrossRef]


| c (mg·L−1) | n | m/zwa | AImod,wa | DBEwa | H /Cwa | O /Cwa | CHO | CHON | CHONS | CHOS | Instrument Parameters | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | R (e4) | m/z | ||||||||||||
| 20 | 3675 | 472.28 | 0.40 | 12.15 | 1.00 | 0.49 | 3141 | 495 | 0 | 39 | OE | 14 | 150–1000 | [46] |
| 20 | 3412 | 457.91 | 0.40 | 11.65 | 1.00 | 0.51 | 2696 | 539 | 0 | 177 | OV | 14 | ||
| 20 | 5036 | 455.78 | 0.41 | 11.99 | 0.97 | 0.50 | 3490 | 1114 | 0 | 432 | OV | 48 | ||
| 20 | 2280 | 407.92 | 0.42 | 10.84 | 1.00 | 0.45 | 1822 | 347 | 0 | 111 | QE | 24 | ||
| 20 | 3487 | 454.77 | 0.39 | 11.46 | 1.01 | 0.50 | 2602 | 663 | 0 | 222 | OV | 24 | ||
| 20 | 1870 | 437.64 | 0.41 | 11.47 | 0.97 | 0.53 | 1705 | 127 | 0 | 38 | QE | 14 | ||
| 20 | 3301 | 495.45 | 0.41 | 12.97 | 0.97 | 0.50 | 2913 | 339 | 0 | 49 | OE | NG | ||
| 50 | 4350–7029 | 368.69–454.48 | 0.36–0.42 | 9.58–12.01 | 0.92–1.04 | 0.57–0.65 | NG | NG | NG | NG | OF | 50 | 150–800 | [47] |
| 67 | 3935 | 355.19 | 0.35 | 8.60 | 1.09 | 0.53 | 2689 | 913 | 44 | 289 | QE | 14 | 70–1050 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Zhang, R.; Huang, G.; Yuan, H.; Wang, L.; Xu, S. Characterization of Low-Molecular-Weight Dissolved Organic Matter Using Optional Dialysis and Orbitrap Mass Spectrometry. Molecules 2024, 29, 3370. https://doi.org/10.3390/molecules29143370
Li Q, Zhang R, Huang G, Yuan H, Wang L, Xu S. Characterization of Low-Molecular-Weight Dissolved Organic Matter Using Optional Dialysis and Orbitrap Mass Spectrometry. Molecules. 2024; 29(14):3370. https://doi.org/10.3390/molecules29143370
Chicago/Turabian StyleLi, Qiuxing, Runyu Zhang, Guopei Huang, Haijun Yuan, Liying Wang, and Shuxia Xu. 2024. "Characterization of Low-Molecular-Weight Dissolved Organic Matter Using Optional Dialysis and Orbitrap Mass Spectrometry" Molecules 29, no. 14: 3370. https://doi.org/10.3390/molecules29143370
APA StyleLi, Q., Zhang, R., Huang, G., Yuan, H., Wang, L., & Xu, S. (2024). Characterization of Low-Molecular-Weight Dissolved Organic Matter Using Optional Dialysis and Orbitrap Mass Spectrometry. Molecules, 29(14), 3370. https://doi.org/10.3390/molecules29143370







